Abstract
Background and Objectives
Narcolepsy and idiopathic hypersomnia usually begin in early adolescence, but diagnostic delays ranging from 5 to 10 years are common, affecting disease burden. To improve early identification of these treatable conditions, we developed and validated the Pediatric Hypersomnolence Survey (PHS).
Methods
Content was developed through literature review, patient focus groups, interviews with experts in the field, and field testing. We then validated the 14-item self-reported survey across 3 hospitals and web recruitment from patient groups. In the validation phase, we recruited a total of 331 participants (patients with narcolepsy type 1 [n = 64], narcolepsy type 2 [n = 34], idiopathic hypersomnia [n = 36], and other sleep disorders [n = 97] and healthy controls [n = 100], ages 8–18 years) to complete the survey. We assessed a range of psychometric properties, including discriminant diagnostic validity for CNS disorders of hypersomnolence using receiver operating characteristic curve analysis and reliability across a 1-week period.
Results
Confirmatory factor analysis indicated a 4-domain solution with good reliability expressed by satisfactory omega values. Across groups, the PHS total score showed appropriate positive correlations with other validated surveys of sleepiness (r = 0.65–0.78, p < 0.001) and negative correlations with multiple sleep latency test measures (mean sleep latency: r = −0.27, p = 0.006; number of sleep-onset REM periods: r = 0.26, p = 0.007). Compared to controls and patients with other sleep disorders, the area under the curve for participants with narcolepsy or idiopathic hypersomnia was 0.87 (standard error 0.02, 95% CI 0.83–0.91) with high sensitivity (81.3, 95% CI 73.7%–87.5%) and specificity (81.2%, 95 CI 75.1%–86.4%). Test-retest reliability was r = 0.87.
Discussion
The PHS is a valid and reliable tool for clinicians to identify pediatric patients with narcolepsy and idiopathic hypersomnia. Implemented in clinical practice, the PHS will potentially decrease diagnostic delays and time to treatment, ultimately reducing disease burden for these debilitating conditions.
Classification of Evidence
This study provides Class III evidence that the PHS accurately identifies patients with central disorders of hypersomnolence.
Narcolepsy and idiopathic hypersomnia (IH) are chronic, debilitating neurologic disorders that typically begin between 10 and 15 years of age, yet diagnosis is often delayed in adults and children by 5 to 15 years1,2 Narcolepsy is categorized as narcolepsy type 1 (NT1; narcolepsy with cataplexy) and narcolepsy type 2 (NT2; narcolepsy without cataplexy); both conditions present with excessive daytime sleepiness, sleep-related hallucinations (hypnagogic hallucinations, hypnopompic hallucinations), disrupted nighttime sleep, and sleep paralysis. The symptoms of IH overlap with those of narcolepsy, but patients typically have longer sleep times, less severe daytime sleepiness, more fatigue complaints, and significant difficulty waking in the morning (sleep inertia).3,4 Delays in narcolepsy diagnosis are attributed largely to lack of awareness of the condition. In a survey including 300 primary care physicians and 100 sleep medicine specialists, only 22% of sleep specialists and 7% of the primary care physicians identified all key narcolepsy symptoms.5 The odds of a delayed narcolepsy diagnosis doubles if symptoms begin before 18 years of age.6 Less is known about diagnostic delays in IH, but in an unpublished poll of 305 health care providers, more than half (57%) said they had misdiagnosed IH.7
Untreated symptoms of narcolepsy and IH can have several negative consequences. For example, chronic excessive daytime sleepiness is associated with lower academic functioning, including poor grades, increased tardiness and absenteeism, and behavioral issues that negatively affect school performance such as disruptive behavior and conduct problems.2 Chronic sleepiness can also result in feelings of low self-worth because patients are often perceived as lazy or unmotivated.4 Experimental studies show that excessive daytime sleepiness increases inattention and impulsivity, thus posing safety concerns.8-10
Unfortunately, there is a lack of disease-specific, validated, and reliable diagnostic screening tools for pediatric IH and narcolepsy.11 Both the Epworth Sleepiness Scale for Children and Adolescents (ESS-CHAD) and the Pediatric Daytime Sleepiness Scale (PDSS) for children and adolescents are clinically available to assess 1 factor of pediatric IH and narcolepsy-sleepiness severity. The ESS-CHAD diagnostic accuracy for pediatric narcolepsy or IH has yet to be assessed,12 and only a Chinese version of the PDSS has been validated in a study including n = 31 participants with pediatric narcolepsy living in China.13 To address the critical need to expedite the accurate identification narcolepsy and IH on the basis of >1 disease symptom, our research intention was to develop and validate the Pediatric Hypersomnolence Survey (PHS). Our objective was to develop and validate a brief but comprehensive instrument for use by health care providers, school professionals, and caregivers to evaluate symptoms of excessive daytime sleepiness and to identify those children and adolescents who then warrant further diagnostic evaluation and testing for pediatric IH and narcolepsy. This article describes the psychometric properties, validity, and reliability of the PHS among patients with narcolepsy or IH vs controls (healthy community controls and patients with other sleep disorders) 8 to 18 years of age.
Methods
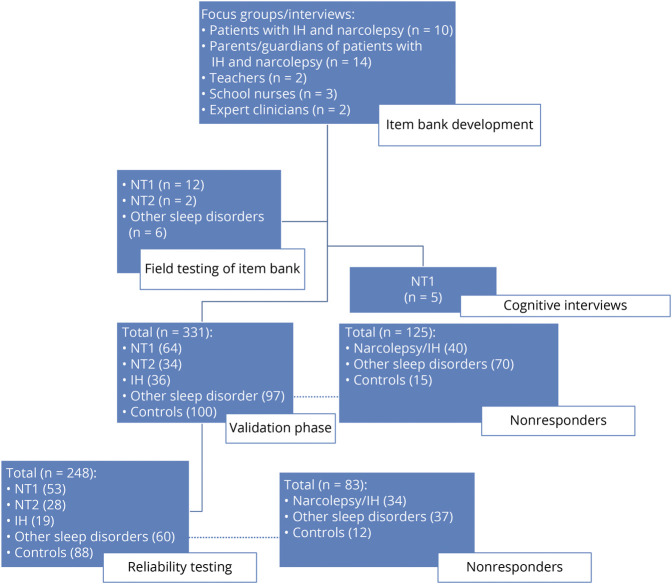
An overview of the study development and validation is presented in Figure 1.
Figure 1. Protocol and Study Enrollment by Phase.
IH = idiopathic hypersomnia; NT1 = narcolepsy type 1; NT2 = narcolepsy type 2.
Part 1: Development of the PHS
Defining Constructs and Item Pool
We identified key domains and specific item content pertinent to the PHS through a literature review. We reviewed literature with PubMed search terms pediatric narcolepsy, diagnosis, screening, and symptoms on July 15, 2016, and reviewed n = 134 publications (limited to <18 years of age, English language) to determine content areas. We also reviewed existing validated narcolepsy symptom measures for adults (Swiss Narcolepsy Scale,14 Ullanlinna Narcolepsy Scale15) and children (modified ESS,16 Cleveland Adolescent Sleepiness Survey,17 PDSS18) and diagnostic criteria from International Classification of Sleep Disorders version 3 (ICSD 3).4
The developmental phase of the PHS involved conducting focus groups and phone interviews using semistructured interviews with open-ended questions. Participants 8 to 18 years of age with either narcolepsy or IH (n = 6 with NT1, n = 2 with NT2, and n = 2 with IH) diagnosed by ICSD 3 criteria4 took part in a 1-hour focus group conducted at Boston Children's Hospital, with groups divided by age (8–12 and 13–18 years). The diagnoses of 2 participants with IH were based on a mean sleep latency value ≤8 minutes.4 We also conducted a separate 1-hour focus group of primary caregivers (n = 14) of patients with narcolepsy or IH. Last, we conducted semistructured phone interviews of teachers (n = 3) and school nurses (n = 2) who had encountered students with recent diagnoses of narcolepsy or IH. The interviewer asked participants with narcolepsy and IH to reflect on early presenting symptoms and symptoms they recognized to be abnormal from peers. Parents/guardians and teachers were asked about other observable symptoms of narcolepsy and IH that presented in the home or school setting. The interviewer audio-recorded the focus group sessions and phone interviews and then transcribed content. A research assistant used Open Code 4.019 to code the content, to extract common themes and terminology, and to assign the text to a domain. After the first round of focus groups, we reached saturation, and no further sessions were conducted. All participants received a $50 gift card for participating.
Item Development
From the symptom concepts elicited from literature review and focus groups, we generated 57 items and transformed them into questionnaire items with Likert scales response options (“often”, “sometimes”, “never”, and “do not know”). We then reviewed our item bank with 2 sleep medicine experts at Boston Children's Hospital (J.O. and T.S.) for further feedback on the appropriateness of content and wording.
Cognitive Interviews
We conducted cognitive interviews with 5 participants with narcolepsy to gauge understanding of the question, item relevance, and frequency of symptom. A research assistant (E.S.) took notes during these interviews, and wording of the questions was revised accordingly.
Field Testing
We administered the 57 questions to a separate group of patients with narcolepsy (n = 12 with NT1, n = 2 with NT2, and n = 6 with other sleep disorders) who were 8 to 18 years of age to obtain data to reduce the number of items. We used the Cronbach coefficient α to assess convergent validity and retained items with scores ≥0.89. This reduced items to 39 questions, and we then conducted Spearman correlation testing between individual questions within domains. If domain items had correlations >0.8, we selected the best item according to face validity. In this way, we reduced the PHS to 14 items. Participants were provided a $10 gift card for completing surveys.
Part 2: PHS Survey Validation
Participants
The validation study was conducted between March 2017 and July 2020. A separate group of participants with IH and narcolepsy who did not participate in development of the PHS were recruited from either rosters of patients awaiting a polysomnogram (PSG)/multiple sleep latency test (MSLT) for the diagnosis of a hypersomnia disorder or from sleep clinics with established diagnosis of narcolepsy or IH provided within 1 year before recruitment. Due to the relatively small number of patients per year diagnosed with a central hypersomnia in any given institution, participants were recruited across 3 sites (Boston Children's Hospital, King's Daughter Hospital, Geisinger Medical Center). Diagnoses of patients were based on evaluation by board-certified pediatric sleep specialists using ICSD 3 criteria.4 As comparison groups, we also recruited healthy controls and patients with other sleep disorders. An additional group of participants with narcolepsy (n = 30) or IH (n = 8) were recruited from the patient support group Wake Up Narcolepsy via web advertisement. For these web participants, their parent or guardian reported that the diagnosis was determined by a physician, but confirmatory sleep studies or laboratory testing was not obtained.
Participants With Narcolepsy and IH
Diagnoses of patients were based on ICSD 3 criteria4 from board-certified pediatric sleep specialists. The eMethods section (links.lww.com/WNL/B891) provides a review of the diagnostic criteria.
We recruited healthy controls from the BCH Research Patient Registry, a database of healthy children interested in participating in research. These participants are typically recruited from the community, web advertisements, general pediatric clinics, and health fairs.
At Boston Children's Hospital, we recruited consecutive patients with other sleep disorders who reported daytime sleepiness or fatigue. Some of these patients had PSG testing alone or PSG/MSLT testing as part of their diagnostic evaluation. The specific sleep disorders (reported in Results) were diagnosed by board-certified sleep physicians.
Procedure
We administered the PHS to participants along with other validated measures (detailed below) through the REDCap survey system and collected the site participants' sleep study and medication data through chart review or their medical history form. Participants with IH or narcolepsy diagnosed within 1 year of survey administration were asked to recall the presence and frequency of symptoms before diagnosis. Otherwise, participants were asked to answer survey questions based on the last 1 week. All participants received 3 reminder emails to complete their survey over the course of 2 weeks. Participants received a second PHS 1 week after completion of the first PHS via REDCap to assess reliability of their responses. Participants who completed all surveys received a $10 gift card.
Measures
Pediatric Hypersomnolence Survey
The PHS is a 14-item self-reported questionnaire with rating scale option of “often” = 3 points, “sometimes” = 2 points, “never” = 1 point, and “do not know” = not scored for a total PHS score. The decision to retain a “do not know” response was 2-fold. First, symptoms of narcolepsy such as cataplexy are difficult to differentiate from normal weakness that can occur with laughter.20 Second, clinical experience shows that children often lack insight into the presence/severity of their symptoms. Thus, the intention of retaining the “do not know” option was to prompt further assessment of a sleepy child by a health care provider rather than force a categorical response. The sum of questions 1 through 5 and question 12 made up a sleepiness subscale score. The total PHS score is the sum of all items 1 through 14.
Pediatric Daytime Sleepiness Scale
The PDSS is an 8-item self-reported questionnaire that assesses sleepiness among children 5 to 17 years of age.18
Epworth Sleepiness Scale for Children and Adolescents
This 8-item questionnaire assesses self-reported propensity to fall asleep during daytime situations and daytime activities. It has been validated in children 6 to 16 years of age.16,21 Values of 0 to 10 are considered normal range of sleepiness in adults.21
Swiss Narcolepsy Scale
This 5-item questionnaire assesses frequency of core narcolepsy symptoms currently validated in adult patients with narcolepsy.14 A score <0 has a reported sensitivity of 89% and specificity of 88% for the NT1 diagnosis in adults.22
PSG and MSLT
In brief, the MSLT occurs the day after an overnight PSG and consists of a series of five 20-minute daytime naps at 2-hour intervals. A mean sleep latency ≤8 minutes is considered objective evidence of sleepiness. The PSG and MSLT are also used to detect the presence of inappropriate REM sleep intrusions according to the appearance of sleep-onset REM periods (SOREMPs) during daytime naps.
Medical Information Form
Parents/guardians completed a 20-item demographic and medical information form. Questions included ethnicity, symptom duration, currently used medications, medical history, habitual sleep times, and snoring history.
Data Analysis/Power Calculations
Construct Validity
We used a confirmatory factor analysis to assess construct validity. Confirmatory factor analysis models use a simultaneous equation approach to define unobservable latent factors with observable indicators. We evaluated model fit by means of an omnibus asymptotic χ2 test that tests the hypothesis that [Σ(θ) = Σ]; support of the null model is desired. Power was estimated with R (R Foundation for Statistical Computing, Vienna, Austria) and with a proposed sample size of n = 200 total participants for an α nominal level of α = 5%, df = 65, root mean square error difference = 0.05 to 0.08, and power = 85%.
Internal Consistency Reliability
We report reliability using omega and maximal reliability,23 estimated within the structural equation modeling procedure.
Convergent Validity
The optimal model fit was contrasted with that of a nested model in which the correlation between a domain and all other domains was constrained to be equal to zero (pointing to nonconvergence).24 We ran 4 such models with respective constraints for each one of the latent dimensions and contrasted the best fitted model in which constructs were allowed to freely correlate.
Convergent validity was also established with Pearson correlation testing among PHS total, ESS-CHAD, PDSS, and Swiss Narcolepsy Scale scores across all participants and PSG/MSLT measures (mean sleep latency time, SOREMPs) when available.
Discriminative Validity
Group comparisons on the PHS were performed used the Tukey method in analysis of variance tests. We used analysis of covariance to test whether the CNS hypersomnia group participants (narcolepsy or IH) have different total scores and sleepiness subscores on the PHS than healthy controls and those with other sleep disorders controlling for age at time of diagnosis, site or web-based recruitment, medication use that could affect sleep and wake states (stimulants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, tricyclic agents, sleep aids), race, and sex. We also used receiver operating characteristic (ROC) curves from signal detection theory and report area under the curve (AUC) with standard error and 95% CIs. Power was estimated for 2 groups having the same ratio of negative/positive cases, an AUC of 0.70, which signals nonchance classification25 compared to a chance classification (0.500), a nominal α level of 5%, and a type II error of 20%. Results indicated that n = 62 participants (31 in CNS hypersomnia group and 31 healthy controls) would suffice to identify as significant an AUC ≥0.700.
Temporal Stability
We evaluated test-retest stability across the 1-week period using the Pearson correlation coefficient. Furthermore, we created a difference score by subtracting time 2 scores from time 1 scores and used a 1-sample t test with a reference value of zero. A nonsignificant statistic would indicate that on average the scores do not change by a significant margin.
For all tests, we took significance at p < 0.05. The statistical software used for the analysis was Mplus 8.6 (Muthén & Muthén, Los Angeles, CA).26
Standard Protocol, Approvals, Registrations, and Patient Consents
We obtained documented informed consent from participants or a parent/guardian of the participants and assent from participants 7 to 17 years old. We obtained Institutional Review Board approval from all sites, and appropriate data-sharing agreements were initiated and maintained by Boston Children's Hospital Clinical Trials Business Office.
Results
Participant Characteristics
Total recruitment data are reported in Figure 1, and demographics and clinical information about study participants are detailed Table 1. The PHS study response rate was 72.5%. Of the 331 participants who completed the first PHS, n = 248 (74.9%) completed a retest of the PHS for reliability assessment (time 2).
Table 1.
Demographic and Clinical Characteristics of Participants
Participants included 64 participants with NT1, 34 with NT2, 36 with IH, 97 with other sleep disorders, and 100 controls. The diagnosis of 2 participants with NT1 was based on CSF hypocretin levels ≤110 pg/mL, and the rest met sleep study diagnostic criteria.4 Among the patients with IH, 16 were diagnosed with the mean sleep latency test (MSLT) score ≤ 8 minutes with <2 SOREMPs, and 12 were diagnosed by other ICSD 3 criteria.
Among those with other sleep disorders, n = 23 participants had PSG testing alone as part of their diagnostic workup, and n = 9 were found to have obstructive sleep apnea (mean obstructive apnea-hypopnea index was 10.3/h [13.6]) on their PSGs. PSG/MSLT testing was normal in 22 participants, and the cause of their subjective sleepiness was reported as unknown. Additional participants in the other sleep disorders group included n = 19 with delayed sleep-phase disorder, n = 6 with parasomnias, n = 32 with behaviorally insufficient sleep disorder, n = 2 with restless leg syndrome, and n = 7 with chronic fatigue.
Participants selected the “do not know” responses on individual questions ranging in frequency from 1.2% to 11.1%. The question with the highest such response (11.2%) was question 7: “My voice slurs when I laugh hard.”
Construct Validity
Confirmatory Factor Analysis
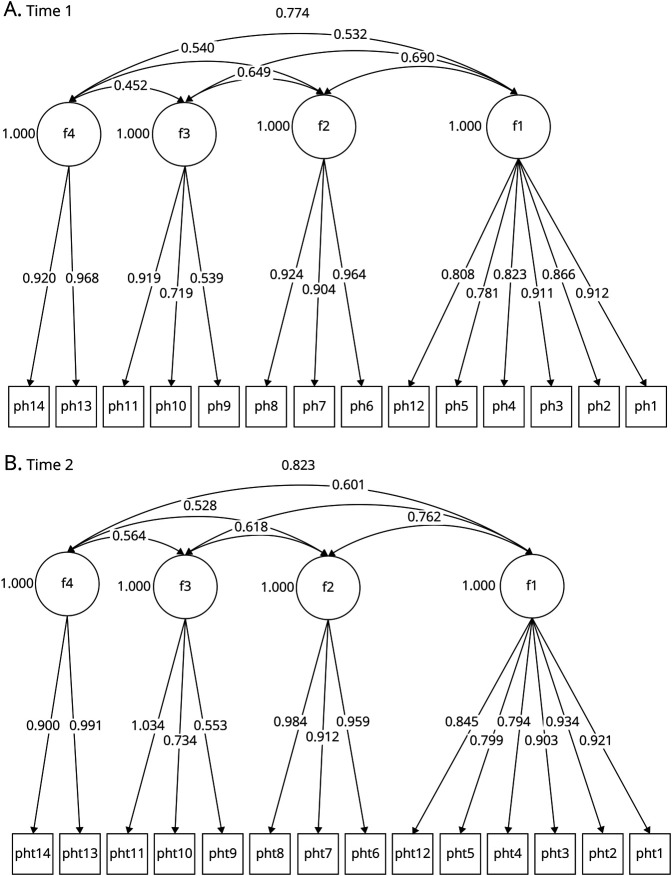
Three competing models were tested: a unidimensional model, a 3-factor model using items 1 through 12 (containing the latent factors F1, excessive daytime sleepiness; F2, cataplexy; and F3, REM-related phenomenon), and a 4-factor model (containing the latent domain F1, excessive daytime sleepiness; F2, cataplexy; F3, REM-related phenomenon; and F4, fatigue) using items 1 through 14. Results are shown in eTable 1 (links.lww.com/WNL/B891) across 2 time points. Specifically, the 4-factor solution proved to be superior to the 3-factor solution (change χ2 [3] = 81.53, p < 0.001) and the unidimensional model at time 1 (change χ2 [3] = 353.73, p < 0.001). These results were also confirmed with the use of time 2 data confirming the superiority of the 4-factor solution over the 3-factor model (change χ2 [3] = 74.74, p < 0.001) and the unidimensional structure (change χ2 [3] = 296.56, p < 0.001). Figure 2 displays the 4-factor structures using the 2 measurements over time with standardized estimates. Correlations between the PHS factors were within acceptable ranges for survey development at 0.45 and 0.77 at time 1 and 0.53 and 0.76 at time 2.
Figure 2. Optimal Factor Solution of PHS Over Time.
The 4 latent factors were as follows: F1, excessive daytime sleepiness; F2, cataplexy; F3, REM-related phenomenon; and F4, fatigue. PHS = Pediatric Hypersomnolence Survey. (A) Time 1 and (B) time 2.
Internal Consistency
As shown in eTable 2 (links.lww.com/WNL/B891), reliability estimates for each PHS factors were acceptable, ranging between 0.79 and 0.95 at time point 1 and between 0.85 and 0.97 at time point 2.
Convergent Validity
Results indicated significant misfit when constraining excessive daytime sleepiness to have a zero relationship with all other constructs (change χ2 [3] = 1,633.40, p < 0.001), suggesting convergence. Similarly, a significant misfit was observed when constraining the relationship between cataplexy and all other constructs to be null (change χ2 [3] = 1,110.90, p < 0.001) and similarly for REM-related phenomenon (change χ2 [3] = 500.37, p < 0.001) and fatigue (change χ2 [3] = 1,396.12, p < 0.001). Consequently, the results suggest that all constructs converged toward assessing the total PHS score.
Across all participants, the total PHS total score correlated in expected directions with the ESS-CHAD (r = 0.78, p < 0.001), Swiss Narcolepsy Scale (n = 331, r = −0.52, p < 0.001), and PDSS (n = 331, r = 0.65, p < 0.001) scores. Among the 104 participants who had PSG/MSLT testing, the PHS showed correlations with mean sleep latency (n = 104, r = −0.27, p = 0.01) and number of daytime SOREMPs (n = 104 r, = 0.26, p = 0.01).
Discriminant Validity
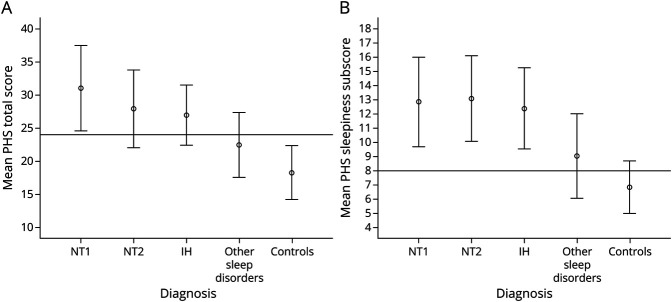
The mean (SD) PHS total score and PHS sleepiness subscore for each diagnostic group are presented in Figure 3, and group comparisons reported in Table 2. According to analysis of covariance testing, the IH and narcolepsy group had higher PHS total scores than healthy controls (B = 8.70, 95% CI 7.05–10.35, p < 0.001) and individuals with other sleep disorders (B = 5.03, 95% CI 3.60–6.46, p < 0.001), controlling for age at time of diagnosis, hospital site or web-based recruitment, medication use that could affect wake/sleep, race, and sex. Similarly, the IH and narcolepsy group had higher PHS sleepiness subscores than healthy controls (B = 5.10, 95% CI 4.19–6.00, p < 0.001) and participants with other sleep disorders (B = 3.09, 95% CI 2.31–3.88, p < 0.001), controlling for the same variables.
Figure 3. PHS Total and Subscale Scores.
(A) Mean Pediatric Hypersomnolence Survey (PHS) total score by diagnostic group. Cutoff line drawn at score of 24. (B) Mean PHS sleepiness subscale score by diagnostic group. Cutoff line drawn at score of 8. Error bars represent 1 SD. IH = idiopathic hypersomnia; NT1 = narcolepsy type 1; NT2 = narcolepsy type 2.
Table 2.
Group Differences on the PHS Scores
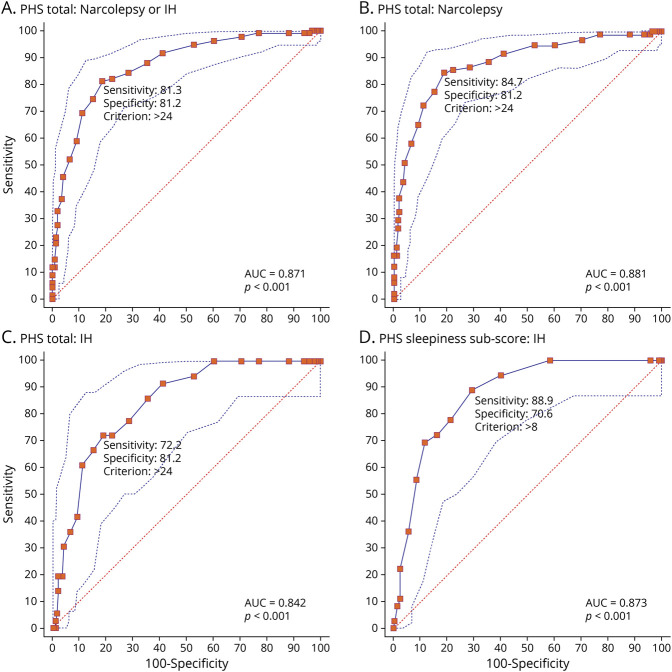
ROC curves for the PHS total score are presented in Figure 4. With a Youden score of J = 0.63, a total PHS score of 24 was the best cutoff value for CNS disorders of hypersomnolence (narcolepsy and IH) vs healthy controls and individuals with other sleep disorders (AUC = 0.87 [0.02], 95% CI 0.83–0.91, p < 0.001, Figure 4A). At this cutoff score, the PHS total score had a sensitivity of 81.3% (95% CI 73.7%–87.5%) and specificity of 81.2% (95% CI 75.10%–86.4%) for these CNS disorders of hypersomnolence. The false positives (18.8%) were made up of 34 participants with other sleep disorders and 10 community controls. Within the group of other sleep disorders who had a PHS total score of >24, 12 participants had PSG/MSLT testing with mean sleep latency of 17.3 (2.0) and mean of 0.08 SOREMPs (0.29). Overall, positive predictive power and negative predictive power were equal to 74.7% and 86.5%, respectively.
Figure 4. ROC Curves for Total PHS Scores.
Receiver operating characteristic (ROC) curves for total Pediatric Hypersomnolence Survey (PHS) scoresfor (A) CNS hypersomnia disorders (narcolepsy or idiopathic hypersomnia [IH]), (B) narcolepsy (narcolepsy types 1 and 2), and (C) IH. (D) Sleepiness subscale score of PHS for idiopathic hypersomnia. All comparisons are made against controls and participants with other sleep disorders. AUC = area under the curve.
ROC analyses showed that the accuracy of PHS total score for narcolepsy diagnosis was AUC of 0.88 (0.02, 95% CI 0.84–0.92, p < 0.001, Figure 4B). With a cutoff score of 24 (Youden J = 0.65), the sensitivity and specificity for narcolepsy were 84.7% (95% CI 76.0%–91.2%) and 81.2% (95% CI 75.1%–86.4%), respectively. Positive predictive power and negative predictive power were 69.2% and 91.4%, respectively.
Among participants with IH, ROC analysis of the PHS total score showed high diagnostic accuracy for IH (AUC 0.84 [0.03], 95% CI 0.77–0.89, p < 0.001, Figure 4C). A PHS total cutoff score of 24 (Youden J = 0.53) yielded a sensitivity of 72.2% (95% CI 54.8%–85.8%) and specificity of 81.2 (95% CI 75.1%–86.4%). Positive predictive power and negative predictive power were 41.3% and 94.1%, respectively.
Given that domains of cataplexy and REM-related symptoms are more specific to narcolepsy, we calculated items relating only to the PHS sleepiness subscore to test accuracy for IH diagnosis, which yielded an AUC of 0.87 (0.03, 95% CI 0.82–0.91, p < 0.001, Figure 4D). A cutoff score of 8 on the PHS sleepiness subscore had a sensitivity of 88.9% (95% CI 73.9%–96.1%) and specificity of 70.6% (95% CI 63.7%–76.8%) for IH. Positive predictive power and negative predictive power were 35.6% and 97.2%, respectively.
The PHS total score had an accuracy for pediatric CNS disorders of hypersomnolence (narcolepsy and IH) similar to the ESS-CHAD (AUC 0.87 [0.02], 95% CI 0.82–0.90) but higher than the PDSS (0.74 [0.03] 95% CI 0.69–0.80]. Given the similar accuracy compared to the PHS, we further studied the sensitivity and specificity of the ESS-CHAD in our sample. To reach sensitivity >80% for detection of CNS disorders of hypersomnolence in our population, the optimal cutoff on the on the ESS-CHAD was identified as 9. With this cutoff, the sensitivity was 90.5% and specificity was 67.7%. The positive predictive value of the ESS-CHAD in our study population was 65.5%, and the negative predictive value was 91.2%.
Temporal Stability
eFigure 1 (links.lww.com/WNL/B891) displays the relationship between 2 measurements of PHS total score using the same sample of participants (n = 167). The correlation between the 2 measurements was r = 0.87 (p < 0.001), demonstrating strong similarity in responses. Furthermore, a difference score was created by subtracting time 2 scores from time 1 scores and was subjected to a 1-sample t test using a reference value of zero. Our results showed stability of the change scores over time with a mean estimate of 0.24, which was not significantly different from zero (t [247] = 1.06, p = 0.29).
The PHS instrument is presented in Figure 5.
Figure 5. PHS Is a 14-Item Scale.
Scoring is “often” = 3 points, “sometimes” = 2 points, “never” = 1 point, and “do not know” = not scored. Total score is the sum of all 14 items, and cutoff score for narcolepsy/idiopathic hypersomnia (IH) is >24. Sleepiness subscore is the sum of items 1 through 5 and 12. Cutoff score for the sleepiness subscore is >8 for IH. PHS = Pediatric Hypersomnolence Survey.
Class of Evidence
This study provides Class III evidence that the PHS accurately identifies patients with central disorders of hypersomnolence.
Discussion
We developed the PHS to screen for narcolepsy and IH in children and adolescents, and it shows robust psychometric properties. The PHS content reflects extensive literature review and input from hypersomnia experts and stakeholders, including patients, parents/guardians, and school professionals. The PHS shows excellent validity using gold-standard assessments (MSLT values) and reliability. In addition to healthy controls, we included patients with other sleep disorders as a comparator group because narcolepsy is commonly misdiagnosed as other sleep problems.27 It is important to note that the PHS identifies narcolepsy and IH with both sensitivity and specificity >80%. The PHS shows greater accuracy than the PDSS and comparable accuracy to the ESS-CHAD in our study sample. While the ESS-CHAD has higher sensitivity (90.5%) than the PHS total score, the PHS has higher specificity than the unidimensional ESS-CHAD (67.6%). At least in clinical practice, higher specificity may be useful in making decisions about which patients require further diagnostic testing. Overall, we believe our data support use of the PHS as a screening tool for narcolepsy and IH.
The cause of excessive daytime sleepiness in children and adolescents is broad and ranges from behavioral, neurologic, circadian, and respiratory disorders to use of sedating medications/substances and poor sleep hygiene.4,28 Among these etiologies, insufficient sleep is highly prevalent among school-aged children/adolescents, with short sleep reported in 57.8% of middle school students and 72.7% of high school students on the 2015 Youth Risk Behavior Survey.29 Daytime sleepiness severity due to insufficient sleep varies with the extent and chronicity of sleep deprivation.30 Still, it is important for parents/guardians, school professionals, and health care providers concerned about a child/adolescent with excessive daytime sleepiness to know optimal sleep durations for age,31 to inquire about habitual sleep duration (weekdays and weekends), and to reassess sleepiness complaints when sleep extension is achieved. The PHS was not validated against a group solely with insufficient sleep, plausibly limiting the generalizability of our findings. In individuals with unexplained chronic daytime sleepiness, the PHS could reduce unnecessary sleep study testing when the total score is ≤24 or sleepiness subscore is ≤8. Conversely, if thresholds are met, use of the PHS may help identify those with CNS disorders of hypersomnolence more rapidly and, we hope, reduce diagnostic delays and disease burden.32,33
The PHS has an ≈19% false-negative and 19% false-positive rate based on the total PHS score for narcolepsy or IH. False negatives could result from recall bias among those diagnosed before survey administration or lower accuracy of the PHS for pediatric NT2 and IH, conditions without cataplexy symptoms (1 domain of the PHS). False positives in our healthy control population are higher than the community rate of narcolepsy or IH (estimated <1%).34 Most false positives occurred in the other sleep disorders group. If the PHS score is elevated, clinicians should take further history on hypersomnia disorder symptoms and pursue diagnostic testing outlined in the ICSD 34 as needed.
Our study has several limitations. First, because pediatric narcolepsy and IH are rare disorders, we included recently diagnosed participants (≤1 year from survey date) treated with medications. We asked patients to report symptoms before treatment; thus, recall bias is possible. While we controlled for medication use in our regression model of PHS scores, a larger multisite study of undiagnosed narcolepsy and IH patients awaiting confirmatory diagnostic testing would offer better PHS validation. Second, we recruited a subset of pediatric patients with narcolepsy from patient advocacy websites and did not obtain their confirmatory diagnostic tests to verify diagnoses. It is reassuring that site of recruitment (websites vs hospital sites) did not affect the significance of group differences. Third, the majority of patients in the focus groups had narcolepsy, and no patients with IH participated in field testing or cognitive interviews. The PHS total scores were highest in the NT1 cohort, driving higher accuracy of the PHS for narcolepsy. To optimize IH screening, we encourage use of the total PHS score and the PHS sleepiness subscore (items 1–5 and 12) because the latter has a higher IH sensitivity. Fourth, IH symptoms are quite heterogeneous, with some patients reporting long sleep times and more profound sleep inertia and others reporting normal sleep duration and severe daytime sleepiness.4 We tried to capture participants with IH who represent this heterogeneity for PHS generalizability, but such variability may reduce detection of group differences. Last, the instructions and questions relate to in-person schooling; thus, the utility of the PHS during vacation and remote schooling is unknown. Because most participants in our focus group noted narcolepsy/IH symptoms in school setting, many PHS questions pertain to this environment.
Narcolepsy and IH are underrecognized in the pediatric population, resulting in long diagnostic and treatment delays. It is our hope that the PHS will used by health care providers, school professionals, and even parents/guardians to screen for narcolepsy and IH in children and adolescents with chronic daytime sleepiness. This simple assessment should help direct these patients quickly to providers familiar with CNS hypersomnia for definitive diagnosis and treatment.35 Future research is needed (1) to prospectively assess the PHS accuracy in larger cohorts of patients, (2) to determine accuracy for pediatric patients with hypersomnia due to other medical conditions (such as secondary narcolepsy in Prader-Willi syndrome and myotonic dystrophy), (3) to further refine the PHS for better pediatric IH specificity, and (4) to test the PHS against other conditions such as attention-deficit/hyperactivity disorder or depression (nonsleep disorders that patients with IH and narcolepsy are commonly misdiagnosed as having).7,36
Acknowledgment
The authors acknowledge Monica Gow, founder and executive director of Wake Up Narcolepsy Inc, for her expertise and assistance in developing this study.
Glossary
- AUC
area under the curve
- ESS-CHAD
Epworth Sleepiness Scale for Children and Adolescents
- ICSD 3
International Classification of Sleep Disorders version 3
- IH
idiopathic hypersomnia
- MSLT
multiple sleep latency test
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- PDSS
Pediatric Daytime Sleepiness Scale
- PHS
Pediatric Hypersomnolence Survey
- PSG
polysomnogram
- ROC
receiver operating characteristic
- SOREMP
sleep-onset REM period
Appendix. Authors

Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was supported by an investigator-initiated grant to K.M. from the American Sleep Medicine Foundation, Coverys Healthcare Foundation, and Wake Up Narcolepsy, Inc. K.M. also receives grant support from NIH grant K23 NS104267.
Disclosure
K. Maski has received consulting fees and grant funding from Jazz Pharmaceuticals and consulting fees from Harmony Biosciences, KemPharm Inc, Takeda Pharmaceuticals, and Alkermes. T. Scammell has received consulting fees from Accelerator, Alkermes, Avadel, Axsome, Harmony Biosciences, Jazz Pharmaceuticals, Merck, Suven, and Takeda. L. Jesteadt is employed by the nonprofit agency Wake Up Narcolepsy, Inc. C. Crisp is employed by the nonprofit agency Wake Up Narcolepsy, Inc. J. Worhach, E. Steinhart, M. Boduch, A. Morse, M. Strunc, J. Owens, D. Williams, and G. Sideridis report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15(5):502-507. [DOI] [PubMed] [Google Scholar]
- 2.Luca G, Haba-Rubio J, Dauvilliers Y, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22(5):482-495. [DOI] [PubMed] [Google Scholar]
- 3.Trotti LM. Waking up is the hardest thing I do all day: sleep inertia and sleep drunkenness. Sleep Med Rev. 2017;35:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine. International Classification of Sleep Disorders–Third Edition (ICSD-3). American Academy of Sleep Medicine; 2014. [Google Scholar]
- 5.Rosenberg R, Kim AY. The AWAKEN survey: knowledge of narcolepsy among physicians and the general population. Postgrad Med. 2014;126(1):78-86. [DOI] [PubMed] [Google Scholar]
- 6.Maski K, Steinhart E, Williams D, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med. 2017;13:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavidia M. Idiopathic hypersomnia campaign seeks to spread awareness, empower patients.Accessed January 6, 2021. ajmc.com/view/idiopathic-hypersomnia-campaign-seeks-to-spread-awareness-empower-patients
- 8.Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4(S2):4-14. [DOI] [PubMed] [Google Scholar]
- 9.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann NY Acad Sci. 2008;1129:305-322. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrova A, Fronczek R, Van der Ploeg J, et al. Reward-seeking behavior in human narcolepsy. J Clin Sleep Med. 2011;7:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang H, Gao X, Zhang J. Symptom measures in pediatric narcolepsy patients: a review. Ital J Pediatr. 2021;47(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benmedjahed K, Wang YG, Lambert J, et al. Assessing sleepiness and cataplexy in children and adolescents with narcolepsy: a review of current patient-reported measures. Sleep Med. 2017;32:143-149. [DOI] [PubMed] [Google Scholar]
- 13.Yang CM, Huang YS, Song YC. Clinical utility of the Chinese version of the Pediatric Daytime Sleepiness Scale in children with obstructive sleep apnea syndrome and narcolepsy. Psychiatry Clin Neurosci. 2010;64(2):134-140. [DOI] [PubMed] [Google Scholar]
- 14.Sturzenegger C, Bassetti CL. The clinical spectrum of narcolepsy with cataplexy: a reappraisal. J Sleep Res. 2004;13(4):395-406. [DOI] [PubMed] [Google Scholar]
- 15.Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkilä K. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3:52-59. [DOI] [PubMed] [Google Scholar]
- 16.Janssen KC, Phillipson S, O'Connor J, Johns MW. Validation of the Epworth Sleepiness Scale for Children and Adolescents using Rasch analysis. Sleep Med. 2017;33:30-35. [DOI] [PubMed] [Google Scholar]
- 17.Spilsbury JC, Drotar D, Rosen CL, Redline S. The Cleveland Adolescent Sleepiness Questionnaire: a new measure to assess excessive daytime sleepiness in adolescents. J Clin Sleep Med. 2007;3:603-612. [PMC free article] [PubMed] [Google Scholar]
- 18.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The Pediatric Daytime Sleepiness Scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26(4):455-458. [PubMed] [Google Scholar]
- 19.ICT Services and System Development and Division of Epidemiology and Global Health. OpenCode 4.0. Accessed January 9, 2019. umu.se/en/department-of-epidemiology-and-global-health/research/open-code2/
- 20.Overeem S, Lammers GJ, van Dijk JG. Weak with laughter. Lancet. 1999;354(9181):838. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540-545. [DOI] [PubMed] [Google Scholar]
- 22.Sturzenegger CBCLG, Kallweit U, van der Zande W, Bassetti C. Swiss Narcolepsy Scale: a simple screening tool for hypocretin-deficient narcolepsy with cataplexy. Clin Translational Neurosci. 2018;2:1-5. [Google Scholar]
- 23.Raykov T. Estimation of composite reliability for congeneric measures. Appl Psychol Meas. 1997;21:173-184. [Google Scholar]
- 24.Gorsuch R. Factor analysis. Lawrence Erlbaum; 1983. [Google Scholar]
- 25.Hsu LM. Diagnostic validity statistics and the MCMI-III. Psychol Assess. 2002;14(4):410-422. [PubMed] [Google Scholar]
- 26.Muthén LK, Muthén BO. Mplus User's Guide, 6 ed. Muthén & Muthén; 2021. [Google Scholar]
- 27.Dunne L, Patel P, Maschauer EL, Morrison I, Riha RL. Misdiagnosis of narcolepsy. Sleep Breath 2016;20:1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owens JA, Babcock D, Weiss M. Evaluation and treatment of children and adolescents with excessive daytime sleepiness. Clin Pediatr. 2020;59(4-5):340-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheaton AG, Jones SE, Cooper AC, Croft JB. Short sleep duration among middle school and high school students–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(3):85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens J; Adolescent Sleep Working Group, Committee on Adolescence. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921-e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paruthi S, Brooks LJ, D'Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taddei RN, Werth E, Poryazova R, Baumann CR, Valko PO. Diagnostic delay in narcolepsy type 1: combining the patients' and the doctors' perspectives. J Sleep Res. 2016;25(6):709-715. [DOI] [PubMed] [Google Scholar]
- 33.Thorpy M, Morse AM. Reducing the clinical and socioeconomic burden of narcolepsy by earlier diagnosis and effective treatment. Sleep Med Clin. 2017;12(1):61-71. [DOI] [PubMed] [Google Scholar]
- 34.Longstreth WT Jr, Ton TG, Koepsell T, Gersuk VH, Hendrickson A, Velde S. Prevalence of narcolepsy in King County, Washington, USA. Sleep Med. 2009;10:422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter LP, Acebo C, Kim A. Patients' journeys to a narcolepsy diagnosis: a physician survey and retrospective chart review. Postgrad Med. 2014;126(3):216-224. [DOI] [PubMed] [Google Scholar]
- 36.Wilenius L, Partinen M. Attention-deficit/hyperactivity disorder patients may have undiagnosed narcolepsy. Cureus. 2020;12(6):e8436. [DOI] [PMC free article] [PubMed] [Google Scholar]