Abstract
Background and Objective
Little is known about trajectories of recovery 12 months after hospitalization for severe COVID-19.
Methods
We conducted a prospective, longitudinal cohort study of patients with and without neurologic complications during index hospitalization for COVID-19 from March 10, 2020, to May 20, 2020. Phone follow-up batteries were performed at 6 and 12 months after COVID-19 onset. The primary 12-month outcome was the modified Rankin Scale (mRS) score comparing patients with or without neurologic complications using multivariable ordinal analysis. Secondary outcomes included activities of daily living (Barthel Index), telephone Montreal Cognitive Assessment (t-MoCA), and Quality of Life in Neurologic Disorders (Neuro-QoL) batteries for anxiety, depression, fatigue, and sleep. Changes in outcome scores from 6 to 12 months were compared using nonparametric paired-samples sign test.
Results
Twelve-month follow-up was completed in 242 patients (median age 65 years, 64% male, 34% intubated during hospitalization) and 174 completed both 6- and 12-month follow-up. At 12 months, 197/227 (87%) had ≥1 abnormal metric: mRS >0 (75%), Barthel Index <100 (64%), t-MoCA ≤18 (50%), high anxiety (7%), depression (4%), fatigue (9%), or poor sleep (10%). Twelve-month mRS scores did not differ significantly among those with (n = 113) or without (n = 129) neurologic complications during hospitalization after adjusting for age, sex, race, pre–COVID-19 mRS, and intubation status (adjusted OR 1.4, 95% CI 0.8–2.5), although those with neurologic complications had higher fatigue scores (T score 47 vs 44; p = 0.037). Significant improvements in outcome trajectories from 6 to 12 months were observed in t-MoCA scores (56% improved, median difference 1 point; p = 0.002) and Neuro-QoL anxiety scores (45% improved; p = 0.003). Nonsignificant improvements occurred in fatigue, sleep, and depression scores in 48%, 48%, and 38% of patients, respectively. Barthel Index and mRS scores remained unchanged between 6 and 12 months in >50% of patients.
Discussion
At 12 months after hospitalization for severe COVID-19, 87% of patients had ongoing abnormalities in functional, cognitive, or Neuro-QoL metrics and abnormal cognition persisted in 50% of patients without a history of dementia/cognitive abnormality. Only fatigue severity differed significantly between patients with or without neurologic complications during index hospitalization. However, significant improvements in cognitive (t-MoCA) and anxiety (Neuro-QoL) scores occurred in 56% and 45% of patients, respectively, between 6 and 12 months. These results may not be generalizable to those with mild or moderate COVID-19.
Neurologic events in the context of acute SARS-CoV-2 infection have been reported in 12%–80% of hospitalized patients1-3 and contribute to worse in-hospital1-3 and early postdischarge outcomes.4 In a prospective study of 4,491 consecutive patients hospitalized with COVID-19, we previously reported a 38% increased hazard of in-hospital death and a decreased likelihood of discharge home among patients diagnosed with a neurologic disorder by a board-certified neurologist compared with contemporaneous controls with COVID-19 without a neurologic event.1 We then followed patients with neurologic events postdischarge along with a propensity score–matched contemporaneous COVID-19 control group without neurologic events hospitalized during the same time frame. We performed 6-month structured follow-up interviews, which included standardized functional, cognitive, and neuropsychiatric batteries, and identified persistent deficits in >90% of patients. Patients with neurologic complications during index hospitalization had significantly worse 6-month functional outcomes, more severely impaired activities of daily living, and reduced ability to return to work compared with those without neurologic complications.4 Other larger COVID-19 cohorts have demonstrated similar degrees of disability up to 6 months after discharge,5,6 but there is a paucity of data examining long-term functional and cognitive outcomes or trajectories of recovery. Studies that have assessed 12-month outcomes after COVID-19 have focused primarily on subjective symptoms7,8 rather than using objective, standardized outcome batteries. Given that the acute COVID-19 illness is protracted in many patients, measurement of outcomes at 6 months may be too soon to adequately capture the full recovery potential of these patients.
In this prospective, longitudinal study of 12-month outcomes after severe COVID-19, we aimed to evaluate objective measures of functional status (modified Rankin Scale [mRS], Barthel Index of activities of daily living) and cognition (telephone Montreal Cognitive Assessment [t-MoCA]) as well as patient-reported quantified psychiatric metrics (Quality of Life in Neurologic Disorders [Neuro-QoL] anxiety, depression, fatigue, and sleep). Our primary outcome of interest was a comparison of mRS scores between patients with and without neurologic events during index hospitalization. In addition to comparing secondary 12-month outcomes between these groups, we also aimed to assess trajectories of recovery over time in a subset of patients who had testing at both 6 and 12 months after COVID-19. We hypothesized that those with neurologic events would continue to do worse than those without at 12 months but that there would be an overall improvement across metric scores.
Methods
Study Design and Patient Cohort
We conducted a prospective, observational outcome study of consecutive patients with COVID-19 hospitalized at 4 New York City area hospitals within the same hospital system between March 10, 2020, and May 20, 2020 (Study of Neurologic and Psychiatric Events in Acute COVID-19 [SNaP Acute COVID-19]). Detailed enrollment, methodology, and outcomes at hospital discharge and at 6 months post–COVID-19 onset have been reported.1,4 Patients were prospectively evaluated by a team of neurologists for development of new neurologic disorders during acute COVID-19 hospitalization. Recrudescence of old neurologic deficits in the context of acute infection were not counted as new neurologic events. A total of 4,491 patients were included in SNaP Acute COVID-19 (606 with new neurologic events and 3,885 without new neurologic events).1 Patients with new neurologic disorders who survived to discharge were then propensity score matched by age, sex, and severity of illness (intubation status) to patients with COVID-19 without neurologic events hospitalized during the same period and 6- and 12-month interviews were conducted. Inclusion criteria were age ≥18 years, hospital admission, reverse transcriptase PCR (RT-PCR)–positive SARS-CoV-2 infection from nasopharyngeal sampling, survival to discharge, and consent to participate in a follow-up interview. Surrogate consent was allowed. Exclusion criteria were negative or missing SARS-CoV-2 RT-PCR test or evaluation in an outpatient or emergency department setting only.
Neurologic Diagnoses and Severity of Illness Scales
Neurologic diagnoses—including toxic-metabolic encephalopathy, hypoxic-ischemic encephalopathy, stroke (ischemic or hemorrhagic), seizure, neuropathy, myopathy, movement disorder, encephalitis/meningitis, myelopathy, and myelitis—followed established criteria.9-18 A second review of neurologic diagnoses was performed by relevant subspecialty coauthors (e.g., epilepsy, stroke, neurocritical care subspecialists). Patients could be coded for more than 1 neurologic complication.
Demographic data, medical/neurologic history, clinical course, and hospital outcomes (e.g., discharge disposition, hospital length of stay) were collected. Severity of illness during hospitalization was assessed using the sequential organ failure assessment (SOFA) score19 and requirement for intubation. Pre–COVID-19 baseline functional status was assessed with mRS scores as reported by patients or their surrogate.
Study Outcomes
Longitudinal follow-up assessments were conducted by telephone interview among case and control hospital survivors or their surrogates who consented to participate. Contact was attempted at 6 months (±1 month) and 12 months (±2 months) from the onset of neurologic symptoms among cases or from the onset of COVID-19 symptoms among controls. As previously published, the median time from general COVID-19 symptom onset to neurologic complication in this cohort was 2 days.1 Three attempts to contact were required before patients/surrogates were coded as “unreachable.” We have previously reported outcomes at 6 months4 and here present 12-month outcomes and trajectories of metrics over time.
The primary outcome was the mRS (0 = no symptoms, 6 = dead),20 analyzed using ordinal logistic regression. Secondary outcomes included the Barthel Index21 of activities of daily living (0 = completely dependent, 100 = independent for all activities), the t-MoCA (22 = perfect score; ≤18 = abnormal cognition),22 and Neuro-QoL23 short form self-reported health measures of anxiety, depression, fatigue, and sleep. Patients with fewer than 13 years of education received an additional point when scoring the t-MoCA.24 Neuro-QoL raw scores were converted into T scores with a mean of 50 and SD of 10 in a reference population (US general population or clinical sample).25 Higher T scores indicate worse self-reported health for the anxiety, depression, fatigue, and sleep metrics. Neuro-QoL T scores were considered abnormal if they were >1 SD above the mean (i.e., T score >60). All of the above batteries have been validated for surrogate completion with the exception of the t-MoCA, which was only scored if the patient was able to complete the assessment. Incomplete or partial responses to a given metric were excluded from analysis. Patients were assessed as being abnormal on at least 1 metric if any of the metrics measured were abnormal (as defined above). If all of the metrics were in normal range, but some scales were not completed, the patient was excluded from coding for this measure.
Outcome Trajectories
mRS, Barthel Index, and t-MoCA scores were coded as unchanged if scores were the same at 6 and 12 months and worse or improved if scores differed at all. Anxiety, depression, fatigue, and sleep Neuro-QoL T scores were coded as worse if there was a ≥1 point increase or improved if there was a ≥1 point decrease in scores between 6- and 12-month measurements.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the NYU Grossman School of Medicine Institutional Review Board. All patients or their surrogates provided consent for participation.
Statistical Analyses
Demographic variables, medical history, clinical course, and in-hospital outcomes were compared between patients with COVID-19 with and without a new neurologic event using Mann-Whitney U tests for non-normally distributed continuous variables and χ2 or Fisher exact tests for categorical values, as appropriate. For patients who died, an mRS score of 6 was assigned, but no other outcome variables were scored.
Multivariable ordinal logistic regression models predicting 12-month mRS scores were constructed to determine the effect of neurologic complications during COVID-19 hospitalization, adjusting for previously identified, clinically relevant demographic and clinical covariates.4 Secondary outcomes were compared between those with or without neurologic disorders using Mann-Whitney U (Wilcoxon rank-sum) or χ2 tests as appropriate. Multivariable logistic regression models adjusting for relevant covariates and respecting the 1 in 10 rule to avoid overfitting the models were constructed to identify the effect of neurologic complications on dichotomized secondary outcomes when univariate analysis yielded a p < 0.05. Kaplan-Meier survival analysis was conducted to compare differences in survival distributions between hospital discharge and 12 months follow-up among those with and without neurologic events. Dates of death were collected by chart review and surrogate interview.
Next, among a subgroup of patients who completed both 6- and 12-month follow-up interviews, nonparametric paired samples sign tests were used to compare continuous, non-normally distributed, nonsymmetrical metric scores over time. Similarly, nonparametric related-samples McNemar tests were used to compare dichotomous outcomes over time. Correlations between different outcome measures were assessed using Spearman correlation coefficients. All analyses were conducted using IBM SPSS Statistics for Macintosh version 25 (IBM Corp.).
Results
Enrollment Characteristics
Of 715 patients eligible for 12-month follow-up, contact was attempted with 590 patients. A total of 242/590 (41%) completed the 12-month follow-up interview (Figure 1). Calls were not attempted in 125 patients due to language barrier, missing or defunct contact information, or indication at the 6-month call that the respondent did not wish to be recontacted. Of the 348 calls that were attempted but not completed, 270 were unreachable after 3 phone calls, 7 were unable to understand the consent process, and 67 refused participation. In the entire cohort, the median age was 65 years (IQR 53–73) and 155/241 (64%) were male. Of the 242 included patients, 113 (47%) had neurologic complications during index hospitalization and 129 (53%) had no neurologic complications during hospitalization. The median time from neurologic symptom onset after SARS-CoV-2 infection (or COVID-19 symptom onset for controls) to follow-up interview was 393 days (IQR 375–409 days). Among those able to participate in outcome assessments, direct patient responses were obtained from 84/113 (74%) neurologic patients and 110/129 (85%) controls, while surrogate responses were utilized in 29/113 (26%) neurologic patients and 19/129 (15%) controls (p = 0.199). Because some interviews were completed by surrogates, and some patients were too impaired to participate in cognitive or Neuro-QoL testing, not all metrics were completed by all patients: 235/242 (97%) completed mRS scoring, 236/242 (98%) completed the Barthel Index, 170/242 (70%) completed the t-MoCA, 223/242 (92%) completed the fatigue Neuro-QoL, 221/242 (91%) completed the sleep Neuro-QoL, 225/242 (93%) completed the anxiety Neuro-QoL, and 225/242 (93%) completed the depression Neuro-QoL.
Figure 1. Study Enrollment.
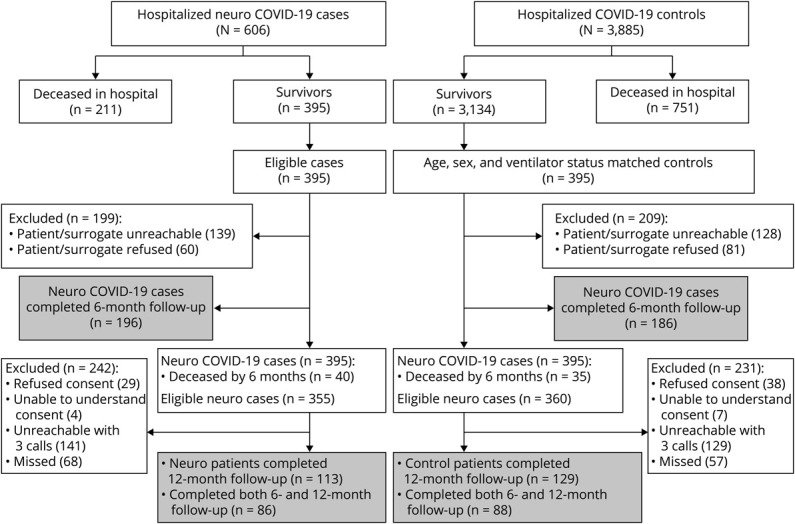
Enrollment flow chart identifying inclusion and exclusion of patients with or without neurologic complications from index COVID-19 hospitalization to 6-month and 12-month follow-up. A total of 395 COVID-19 patients with neurologic complications who survived index hospitalization were propensity score matched by age, sex, and intubation status to 395 patients with COVID-19 hospitalized during the same time frame and without neurologic complications who survived to discharge.
Demographic and Clinical Differences Between Groups
At 12 months, compared with controls without neurologic complications, those with neurologic events during hospitalization more often had a history of seizure or dementia, but were otherwise similar in age, sex, race/ethnicity, and medical comorbidity rates (Table 1). Most neurologic events occurred in patients without a history of similar events. For example, none of the patients diagnosed with ischemic or hemorrhagic stroke had a history of stroke, and only 1 of 10 patients with a newly diagnosed movement disorder had a movement disorder history. However, 6 of 12 patients who developed seizure in the context of acute COVID-19 had a seizure disorder. Markers of severity of COVID-19 illness during index hospitalization, including intubation (33% in neurologic complication patients vs 34% in controls; p = 0.822), SOFA scores, minimum oxygen saturation, and minimum blood pressure, did not differ significantly between groups. Rate of discharge to a nursing home was slightly higher among patients with neurologic events, whereas more patients were discharged to long-term acute care hospitals in the control group.
Table 1.
Demographics, Hospital Course, and Discharge Disposition Among Patients With and Without Neurologic Events During Index COVID-19 Hospitalization
Primary Outcome: mRS
At 12 months, 26/110 (24%) patients with neurologic complications had no disability or mild symptoms that did not interfere with activities (mRS 0–1) compared with 49/125 (39%) patients without neurologic complications (p = 0.011). There was a suggestion of worse mRS scores in those with neurologic complications (median mRS 3 [IQR 2–4]) compared with controls (median mRS 2 [IQR 0–4]; univariate ordinal logistic regression odds ratio [OR] 1.54, 95% CI 0.98–2.43, Wald χ2 [1] = 3.46; p = 0.063). However, in multivariable ordinal logistic regression analysis after adjusting for age, sex, race, pre–COVID-19 mRS, and intubation status, there was no significant difference in 12-month mRS scores between patients with or without neurologic complications during index hospitalization (adjusted OR for worse mRS scores 1.4, 95% CI 0.8–2.5; p = 0.258; Figure 2). Additional sensitivity analyses using models adjusting for past neurologic history did not change these results.
Figure 2. Ordinal Analysis of 12-Month mRS Scores.
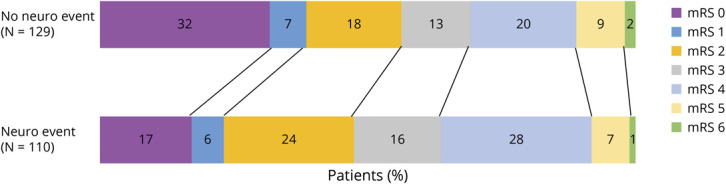
Modified Rankin Scale (mRS) scores 12 months after COVID-19 symptom onset in patients with and without neurologic complications during index COVID-19 hospitalization. There was no significant difference between groups in mRS scores using multivariable ordinal logistic regression analysis adjusting for age, sex, race, pre–COVID-19 mRS, and intubation status (adjusted odds ratio for worse mRS scores 1.4, 95% CI 0.8–2.5; p = 0.258). mRS 0 = no symptoms or disability; 1 = symptoms, but no disability; 2 = slight disability, unable to carry out all previous activities, but able to function without assistance; 3 = moderate disability, requiring help, but able to walk without assistance; 4 = moderately severe disability, unable to walk or attend to bodily needs without assistance; 5 = severe disability, bedbound, incontinent, requiring constant care; 6 = dead.
Between 6 and 12 months, 1/113 (1%) patients with neurologic events and 2/129 (2%) controls died (p = 1.00). A total of 451 patients completed 6- or 12-month follow-up interviews. Of these, 78/451 (17%) died between hospital admission and 6- or 12-month follow-up (41/220 [19%] with neurologic events and 37/231 [16%] without neurologic events; Kaplan-Meier log rank p = 0.379; eFigure 1 [links.lww.com/WNL/B895]).
Secondary Outcomes
Overall, 197/227 (87%) patients who completed all 12-month follow-up batteries had at least 1 abnormal metric (Table 2): 176/235 (75%) had mRS > 0, 150/236 (64%) had Barthel Index of activities of daily living < 100, and 80/161 (50%) patients without a history of dementia/cognitive abnormalities had an abnormal T-MoCA score (≤18; eTable 1, links.lww.com/WNL/B895). Neuro-QoL scores >1 SD above the mean occurred in 16/225 (7%) for anxiety scores, 9/225 (4%) for depression scores, 20/223 (9%) for fatigue scores and 22/221 (10%) for sleep scores. Excluding patients with a pre–COVID-19 mRS > 0, 107/130 (82%) patients had at least 1 abnormal metric at 12 months.
Table 2.
Secondary Outcomes at 12 Months After COVID-19 Symptom Onset Compared Between Patients With or Without Neurologic Events During Index COVID-19 Hospitalization
There were significant correlations between t-MoCA scores and depression T scores (Spearman ρ −0.187; p = 0.016), anxiety and depression T scores (Spearman ρ 0.716; p < 0.001), anxiety and fatigue T scores (Spearman ρ 0.674; p < 0.001), anxiety and sleep T scores (Spearman ρ 0.655; p < 0.001), depression and fatigue T scores (Spearman ρ 0.596; p < 0.001), depression and sleep T scores (Spearman ρ 0.624; p < 0.001) and sleep and fatigue T scores (Spearman ρ 0.736; p < 0.001).
Comparing those with vs without neurologic complications, only fatigue scores differed significantly in univariate analysis (14% of neurologic patients had T scores >60 compared with 4% of patients without neurologic complications; p = 0.010). In multivariable logistic regression analysis adjusting for age, ventilator status and baseline mRS, patients with neurologic complications more often had severe fatigue than controls (adjusted OR 3.18, 95% CI 1.1–9.4; p = 0.037). Of the 15 patients with neurologic deficits and severe fatigue, 7/15 (47%) had toxic-metabolic encephalopathy, 4/15 (27%) had seizure, 2/15 (13%) had movement disorders, 1/15 (7%) had Guillain-Barré syndrome, and 1/15 (7%) had neuropathy. More patients with neurologic events had at least 1 abnormal metric (92%) compared with controls (82%; p = 0.039).
Outcome Trajectories From 6 to 12 Months After COVID-19 Onset
Next, we evaluated changes in outcome metrics over time. Overall, 174 patients completed both 6- and 12-month follow-up interviews (86 with neurologic events and 88 controls). Raw scores for patients who completed functional, cognitive, and Neuro-QoL metrics at both 6 and 12 months are reported in Table 3, and scores for all patients at each time frame are shown in eTable 1 (links.lww.com/WNL/B895). At 6 months, 150/171 (88%) had at least 1 abnormal metric compared with 141/168 (84%) patients at 12 months (paired-samples McNemar test p = 0.230). When excluding patients with pre–COVID-19 baseline mRS > 0, results were similar (83% had at least 1 abnormal metric at 6 months vs 80% at 12 months; McNemar test p = 0.503).
Table 3.
Functional, Cognitive, and Patient-Reported Outcome Metrics Among Patients Who Completed Both 6-Month and 12-Month Post–COVID-19 Interviews (n = 174)
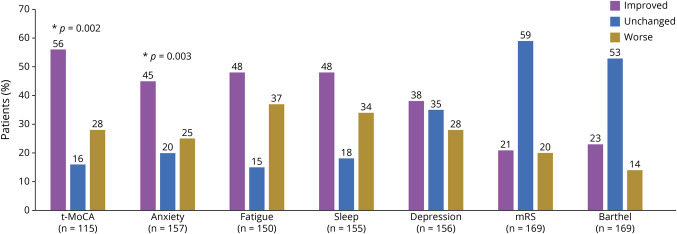
A nonparametric, paired-sample sign test with continuity correction was conducted to evaluate changes in outcome metrics from 6 to 12 months follow-up. There was a statistically significant median improvement in t-MoCA scores (+1 point) from 6 months (median score 18) to 12 months (median score 19) (z = −2.437, p = 0.002) and a statistically significant improvement in Neuro-QoL anxiety scores from 6 months (median score 49.5) to 12 months (median score 47.3) (z = −3.164, p = 0.002). Overall, 56% of patients had improved t-MoCA scores over time and 45% had improved anxiety scores (Figure 3). Although there were no statistically significant changes, improvements were seen in 48% of fatigue scores, 48% of sleep scores, and 38% of depression scores. Conversely, the majority of patients showed no changes in mRS or Barthel Index scores between 6 and 12 months follow-up.
Figure 3. Percentage of Patients With Improved, Worse, or Same Outcome Scores Between 6 and 12 Months After COVID-19 Hospitalization (N = 174).
mRS = modified Rankin Scale; t-MoCA = telephone Montreal Cognitive Assessment.
Discussion
In this prospective cohort study, we found that at 12 months after severe COVID-19 onset, fewer than 20% of all patients scored within the normal range on all of the assessed metrics. Patients with neurologic complications fared worse, as only 8% of neurologic patients scored in the normal range on all metrics, compared with 18% of control patients. Although we did not find a significant effect of neurologic events during index COVID-19 hospitalization on the primary mRS outcome at 12 months, there was a greater than 3-fold significantly increased odds of severe fatigue among neurologic patients, even after adjusting for confounders. In addition, abnormal scores on cognitive testing persisted in 50% of patients without a pre–COVID-19 history of cognitive abnormalities, irrespective of the presence or absence of a neurologic complication during hospitalization. Indeed, rates of abnormal cognition were substantially higher than rates of abnormalities in other domains such as activities of daily living, anxiety, depression, fatigue, or sleep, indicating that cognition should be an area of focus for post–acute COVID-19 study.
When examining trajectories of recovery from 6 to 12 months, the majority of patients did not have improvements in functional status (mRS) or activities of daily living (Barthel Index), but there were significant improvements in cognition and anxiety scores between 6 and 12 months follow-up, and substantial proportions of patients had nonsignificant improvements in fatigue, sleep, and depression scores. Whereas some studies have reported that 45%–77% of patients have at least 1 symptom 12 months after COVID-19 hospitalization,7,8,26 we identified higher rates of objective abnormalities in functional, cognitive, and self-reported patient-centered outcomes. In contrast to subjective symptoms-based data, our findings highlight that standardized metrics may unmask disability or psychiatric symptoms of which patients may not even be cognizant. A smaller longitudinal study of 51 patients assessed at 12 months after COVID-19 adult respiratory distress syndrome utilized standardized batteries and identified severe fatigue in 26% of patients and abnormal 6-Minute Walk Test in 36% of patients,26 although only 7/51 (16%) patients had an abnormal MoCA score (<25). Because this study included only patients who were capable of in-person follow-up, the most severely affected patients were likely not captured. In addition, although metrics were assessed at 3, 6, and 12 months after COVID-19, the authors were only able to report aggregate findings, rather than assess improvements over time, because few patients had more than 1 visit. To our knowledge, our study represents the largest prospective cohort study to report functional, cognitive, and psychiatric metrics in patients with severe COVID-19 at 12 months after infection, and the first to report repeated assessments and trajectories of recovery over time. Other strengths of our study include its prospective ascertainment of in-hospital new neurologic complications diagnosed by neurologists, and multidomain, long-term serial follow-up structure.
Whereas we previously reported higher in-hospital mortality rates1 and worse 6-month mRS scores4 for patients with neurologic complications compared with contemporaneous patients with COVID-19 without neurologic events, the difference in mRS scores between groups was no longer significant at 12 months in multivariable analysis. This finding may be attributable to neurologic improvement over time or the smaller number of patients who completed 12-month follow-up, which would mean less power to detect small differences. Because there were fewer neurologic patients who completed the 12-month interview than controls, it is possible that some of the most debilitated neurologic patients were unable to complete follow-up or their surrogates were unwilling to participate. Because a higher proportion of patients with neurologic complications died in-hospital compared with those without,1 our findings represent a more mild spectrum of sequelae following neurologic events in COVID-19, and we may be underestimating the effect of neurologic injury.
The finding of higher rates of severe fatigue among patients with COVID-19 with neurologic events is not entirely surprising given the high rates of chronic fatigue observed in a variety of neurologic disorders including stroke, multiple sclerosis, dysautonomia, traumatic brain injury, and neuromuscular disorders.27 The underlying pathophysiology of fatigue may be related to diencephalic, hypothalamic/pituitary lesions, neurotransmitter imbalances, or interruption of cortical and basal ganglia circuits.27 Proinflammatory cytokines have also been implicated in the pathogenesis of fatigue28 and COVID-19–related hyperinflammation coupled with concomitant neurologic injury may exacerbate fatigue symptoms. Interestingly, we did not observe differences in sleep metrics between the 2 groups, although sleep and fatigue T scores were very highly correlated among individuals.
Improvements in t-MoCA scores over time should generate a degree of optimism regarding recovery from long COVID-19, particularly because “brain fog,” confusion, and dysexecutive function appear to be common protracted postacute sequelae.29-31 The pathogenesis of postacute cognitive dysfunction is likely multifactorial, but possibilities include posthypoxic brain injury, blood–brain barrier disruption with ongoing inflammation, autoimmune mechanisms, or neurodegenerative disease.32 Indeed, significant decay in premorbid to post–COVID-19 MoCA scores has been documented in >20% of patients with only mild symptomatic COVID-19. In this study, patients with COVID-19 were found to have an 18-fold increased odds of developing cognitive decline compared with seronegative controls.33 Although we found a median of only 1 point improvement in t-MoCA scores over a 6-month interval (between 6 and 12 months follow-up), this likely represents a clinically significant change based on data from the 30-item standard MoCA, which indicates that a 1.7-point change over a much longer 3.5 years of follow-up represents a clinically meaningful difference.34
Similarly, post–COVID-19 psychiatric sequelae—particularly anxiety disorders—are common and occur at a higher frequency than has been observed in individuals with influenza or other respiratory tract infections.35 The improvements we observed in Neuro-QoL anxiety scores were smaller but significant and may be related to improvements in or acclimation to pandemic-related stressors, therapeutic intervention, or biological recovery. There is likely an interplay between psychiatric well-being and cognitive function, as evidenced by the significant correlations we observed between t-MoCA scores and depression T scores. However, we did not detect significant improvements in depression scores over time, which implies that improvements in depression are not likely the driving factor for cognitive improvements.
There are limitations to this study. First, it is possible that we observed a practice effect with the t-MoCA, rather than a true cognitive improvement; however, test–retest reliability studies evaluating the full 30-item MoCA at a shorter time frame (1 month) demonstrated a <1 point mean change in scores.36 In addition, the t-MoCA is a screening test, is not as sensitive as formal neuropsychometric testing, and may be biased by race or social determinants of health,37,38 although we adjusted scores for education level. Although we performed a sensitivity analysis excluding patients with a pre–COVID-19 history of dementia or cognitive impairment, we did not have pre–COVID-19 t-MoCA scores to assess the degree of change after COVID-19 hospitalization. Future studies utilizing longitudinal cohorts with pre–COVID-19 data may be able to more precisely assess the cognitive effect of severe COVID-19. Second, because we allowed for surrogate responses, not all metrics were completed on all patients, which may have limited our power to detect small differences. In addition, outcomes may be worse than we estimated as the sickest patients were not able to participate in some of the testing and family members of patients who did poorly may have been less motivated to participate in research. Third, we did not have a SARS-CoV2–negative control group. It is possible that pandemic-related societal, economic, and environmental stressors may have contributed to certain outcomes (notably psychiatric sequelae) rather than being biologically driven by SARS-CoV-2. Last, we may not have captured all neurologic complications during hospitalization, particularly among the sickest patients who could not tolerate being off sedation for assessment. Hence, we may have underestimated the effect of neurologic complications on long-term outcomes.
At 12 months after severe COVID-19, >80% of patients who had no baseline functional abnormalities (mRS 0) had at least 1 abnormal measure of functional, cognitive, or neuropsychiatric outcome. Abnormal cognitive testing results were found in 50% of patients who did not have a pre–COVID-19 history of dementia or cognitive abnormalities. We did not detect an effect of neurologic events during index COVID-19 hospitalization on the primary outcome of mRS scores, although patients with neurologic complications had significantly higher rates of severe fatigue compared with controls. Among the subset of patients who underwent serial assessments at 6 and 12 months after COVID-19, there were significant improvements in cognition and anxiety scores. Given the high morbidity burden at 1 year after COVID-19 onset, research focusing on therapeutic interventions is warranted.
Acknowledgment
The authors thank the patients and families who participated in this study.
Glossary
- IQR
interquartile range
- mRS
modified Rankin Scale
- Neuro-QoL
Quality of Life in Neurologic Disorders
- OR
odds ratio
- QoL
quality of life
- RT-PCR
reverse transcriptase PCR
- SNaP Acute COVID-19
Study of Neurologic and Psychiatric Events in Acute COVID-19
- SOFA
sequential organ failure assessment
- t-MoCA
telephone Montreal Cognitive Assessment
Appendix. Authors

Footnotes
COVID-19 Resources: NPub.org/COVID19
CME Course: NPub.org/cmelist
Study Funding
The authors report no targeted funding.
Disclosure
J.A. Frontera receives/received funding for the following COVID-19–related grants: NIH/NINDS 3U24NS11384401S1, NIH/NHLBI 1OT2HL161847-01, and NIH/NIA 3P30AG066512-01. A.B. Troxel receives funding for the following COVID-19–related grants: NIH/NINDS 3U24NS11384401S1 and NIH/NHLBI 1OT2HL161847-01. S.B. Meropol and S. Yaghi receive funding for the following COVID-19–related grant: NIH/NINDS 3U24NS11384401S1. S. Thawani, L. Hasanaj, and L.J. Balcer receive funding for the following COVID-19–related grant: NIH/NHLBI 1OT2HL161847-01. T. Wisniewski received funding for the following COVID-19–related grant: NIH/NIA 3P30AG066512-01. The remaining authors report no disclosures relevant to this article. Go to Neurology.org/N for full disclosures.
References
- 1.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96(4):e575-e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskandar EN, Altschul DJ, de la Garza Ramos R, et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 2021;96(11):e1527-e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou SH, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19: a report for the GCS-NeuroCOVID consortium and the ENERGY. Consortium JAMA Netw Open. 2021;4:e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frontera JA, Yang D, Lewis A, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4(9):e2127403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seessle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. Epub 2021 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47(3):303-327. [DOI] [PubMed] [Google Scholar]
- 10.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267-1284. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34-e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott TF, Frohman EM, De Seze J, Gronseth GS, Weinshenker BG. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;77(24):2128-2134. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barre syndrome and validation of Brighton criteria. Brain. 2014;137:33-43. [DOI] [PubMed] [Google Scholar]
- 16.Kalita J, Misra UK, Das M. Neurophysiological criteria in the diagnosis of different clinical types of Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2008;79:289-293. [DOI] [PubMed] [Google Scholar]
- 17.Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599-612. [DOI] [PubMed] [Google Scholar]
- 18.Frontera J, Mainali S, Fink EL, et al. Global consortium study of neurological dysfunction in COVID-19 (GCS-NeuroCOVID): study design and rationale. Neurocrit Care. 2020;33(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. on behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intens Care Med. 1996;22(7):707-710. [DOI] [PubMed] [Google Scholar]
- 20.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604-607. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61-65. [PubMed] [Google Scholar]
- 22.Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal Cognitive Assessment versus face-to-face Montreal Cognitive Assessment and neuropsychological battery. Stroke. 2013;44(1):227-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77(13):1272-1275. [DOI] [PubMed] [Google Scholar]
- 25.Neuro-QoL. Neuro-QoL reference populations. Accessed November 2021. healthmeasures.net/score-and-interpret/interpret-scores/neuro-qol/reference-populations
- 26.Latronico N, Peli E, Calza S, et al. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax. 2022;77(3):300-303. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363(9413):978-988. [DOI] [PubMed] [Google Scholar]
- 28.Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279-292. [DOI] [PubMed] [Google Scholar]
- 29.Frontera JA, Lewis A, Melmed K, et al. Prevalence and predictors of prolonged cognitive and psychological symptoms following COVID-19 in the United States. Front Aging Neurosci. Epub 2021. Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asadi-Pooya AA, Akbari A, Emami A, et al. Long COVID-19 syndrome-associated brain fog. J Med Virol. Epub 2021 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarker A, Ge Y. Mining long-COVID-19 symptoms from Reddit: characterizing post-COVID-19 syndrome from patient reports. JAMA Open. 2021;4(3):ooab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontera JA, Boutajangout A, Masurkar AV, et al. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID-19 subjects with normal cognition, mild cognitive impairment or Alzheimer’s dementia. Alzheimers Dement. Epub 2022 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Brutto OH, Wu S, Mera RM, Costa AF, Recalde BY, Issa NP. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: a longitudinal prospective study nested to a population cohort. Eur J Neurol. 2021;28:3245-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan K, Rossetti H, Hynan LS, et al. Changes in Montreal Cognitive Assessment scores over time. Assessment. 2017;24(6):772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 37.Milani SA, Marsiske M, Cottler LB, Chen X, Striley CW. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimer Dement. 2018;10:773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valdes E, Fuchs B, Morrison C, et al. Demographic and social determinants of cognitive dysfunction following hospitalization for COVID-19. J Neurol Sci. 2022:120146. [DOI] [PMC free article] [PubMed] [Google Scholar]