Abstract
A 52-year-old man presented with palatine tonsillar swelling caused by follicular lymphoma. His tumor burden was low, but exacerbation of snoring and dysphagia was observed. Considering the first wave of coronavirus disease 2019 (COVID-19) pandemic, he received palliative 4-Gy irradiation to the tonsils in 2 fractions, which induced partial regression of tonsillar swellings and eradication of the circulating lymphoma cells. We suggest that low-dose radiotherapy triggered an abscopal effect of lymphoma, which allowed the patient time to receive COVID-19 vaccination before starting immunosuppressive chemo-immunotherapy.
Keywords: low-dose radiotherapy, abscopal effect, CD5, follicular lymphoma
Introduction
Follicular lymphoma (FL) is the most common indolent B cell lymphoma. Although more than 90% of patients have advanced-stage disease at the diagnosis, most FL patients do not require immediate treatment unless they have symptomatic disease. At the time of treatment indication, rituximab-containing chemoimmunotherapy is usually recommended (1). However, therapy-induced impaired immunity can render patients vulnerable to infections, especially during the coronavirus disease 2019 (COVID-19) pandemic (2). As indolent B cell lymphoma, including FL, is extremely responsive to radiation therapy, there has been a recent trend in treating indolent lymphomas with low-dose radiotherapy of 4 Gy (2 Gy ×2) (3,4).
Distant effects of radiotherapy at non-irradiated sites were reported anecdotally before the development of imaging equipment and were known as ‘abscopal effects’ (5,6). The term ‘abscopal’ is derived from the Latin ‘ab’ for ‘away from’ and ‘scopus’ for ‘target’. Recently, the increased utilization of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has revealed the more frequent occurrence of abscopal regression, especially in FL (7), which has been recognized as an immunogenic tumor (5).
We herein report a case of palatine tonsil CD5+, CD10-, MUM1-, Ki-67low FL treated with low-dose radiotherapy that showed not only effective local control but also distant circulating lymphoma cell clearance. Since CD5+, CD10-, MUM1-, Ki-67low FL is rarely reported, we also discuss the characteristics of this unusual subtype of FL.
Case Report
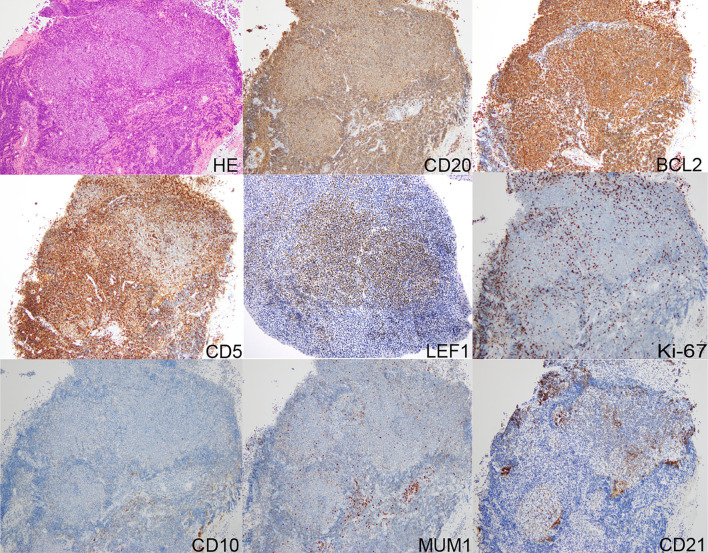
In July 2020, a 52-year-old man was referred to us presenting with cough, snoring, and dysphagia. A physical examination revealed bilateral tonsillar enlargement. A blood count showed a normal hemoglobin (15.3 g/dL) level and platelet count (234×109/L) and mild leukocytosis (11.9×109/L) with 25% abnormal large lymphocytes (Fig. 1) and an immunophenotype of CD5+, CD10-, CD11c-, CD19+, CD20+, CD23-, CD25+, Igk+, CD200+, and ROR1-. A tonsillar biopsy revealed nodular proliferation of large lymphoid cells. Immunohistochemistry results indicated that the tumor cells were CD3-, CD5+, CD10-, CD20+, CD25+, BCL2+, BCL6+/-, MUM1-, cyclinD1-, SOX11-, and LEF1+, and the Ki-67 labeling index was low (10%) (Fig. 2). Interphase fluorescent in situ hybridization revealed that the cells were negative for IGH/BCL2, IGH/CCND1, or BIRC3(API2)/MALT1 fusion genes. A diagnosis of CD5+, CD10-, MUM1-, Ki-67low FL grade 3A was made based on the pathological findings in the CD21-positive follicular dendritic meshworks within the follicular areas.
Figure 1.
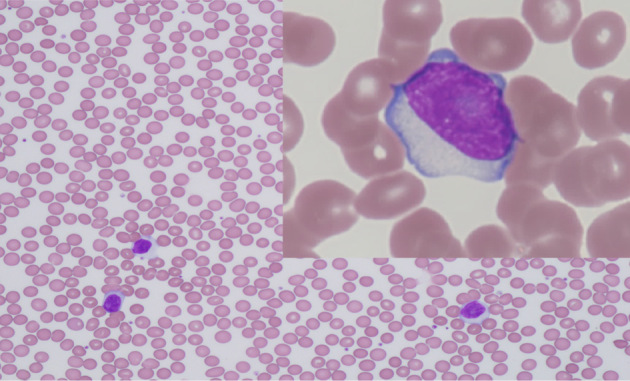
Peripheral blood smear showed circulating lymphoma cells.
Figure 2.
A biopsy specimen of the tonsil showed follicle-like architecture with lymphoma infiltration. The cells were positive for CD20, BCL2, CD5, and LEF1; Ki-67low; and negative for CD10 and MUM1. CD21-positive follicular dendritic meshworks were observed in follicular areas. HE: Hematoxylin and Eosin staining
Both the Follicular Lymphoma International Prognostic Index (FLIPI) and tumor burden status by Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria were low, but snoring and dysphagia worsened during the two months of observation. After an exhaustive discussion concerning the risk of severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) infection under the first wave of the COVID-19 pandemic, he received palliative 4-Gy irradiation to the tonsils in 2 fractions (2 Gy ×2) to avoid the use of immunosuppressive chemotherapy. His tonsillar swelling partially regressed over three months, leading to marked relief of his symptoms. Interestingly, a delayed decrease in circulating lymphoma cells was observed one month after irradiation, with the cells temporarily disappearing altogether five months later (Fig. 3).
Figure 3.
Clinical course of the patient. COVID-19: coronavirus disease 2019
Three months after irradiation, he developed SARS-CoV2 infection (Fig. 3). His clinical course of COVID-19 was generally mild and did not require medical intervention. Since tonsillar swelling and circulating lymphoma cells reappeared six months after irradiation, we are planning to administer chemoimmunotherapy after he completes COVID-19 vaccination.
Discussion
We diagnosed this case as FL based on the finding of a follicular growth pattern, comprising CD20-positive monoclonal B cells, despite an atypical immunophenotype and cytogenetics, including CD5+ CD10- immunophenotype, and the absence of t(14;18)(q32;q21). CD5-positive FL has been reported in several case reports (8-10). LEF1, a widely used marker for chronic lymphocytic leukemia/small lymphocytic lymphoma, was positive in our case. Interestingly, LEF1 has been reported to be positive in some patients with CD5+ FLs (11,12). A large-scale series from MD Anderson Cancer Center revealed a 2.7% (88/3,286) incidence of CD5+ FL (9). In these previously reported cases of CD5+ FL, the characteristics included male predominance, Grade 3 histology, leukemic manifestation, expression of CD25, and absence of t(14;18)(q32;q21) (8-10), all of which were seen in our case. However, in contrast to these previous studies, our case was CD10-, MUM1-, and Ki-67low, representing low-grade FL (13) despite a Grade 3 morphology. These clinical low-grade features may have afforded our patient time to achieve local and abscopal regression.
FL is extremely susceptible to radiation therapy, low-dose radiotherapy (2 Gy ×2) can be used for the palliation of patients who have symptoms related to a single disease site, with 57% Complete remission (CR) rates and an 82% overall response rate (ORR) (14). Due to concerns regarding immunosuppression during the COVID-19 pandemic, we used palliative low-dose radiotherapy (2 Gy ×2) for his symptomatic tonsillar disease. Although the response is not usually sustained, low-dose radiotherapy has been reported as to have a high local control rate with few toxicities (15). There has been a recent trend in treating indolent lymphomas with low-dose radiotherapy because of the high radiosensitivity and tolerability (3,4).
Abscopal regression of malignancy including FL after localized radiation therapy is defined as the disappearance or a reduction in the volume of neoplastic lesions located outside the irradiated volume in the absence of any other anticancer treatment (5,6). In the instance of splenic irradiation for chronic leukemia, the abscopal effect in systemic bone marrow and blood smears may be explained by the direct radiation effect on circulating neoplastic cells passing through the irradiated spleen (6). Although this case seems consistent with circulating leukemic reduction after splenic irradiation, the long-lasting gradual reduction in circulating lymphocytes likely indicates the distant effects of radiation therapy rather than direct radiation damage to lymphoma cells passing through the tonsils during radiation, as radiation-induced direct cell killing occurs within hours or days and does not last for months (16,17). In addition, tumor antigens or cytokines released from the irradiated tonsils may have elicited an anti-lymphoma immune response lasting for months (18-20). Finally, we cannot exclude the possibility that the circulating lymphoma clearance seen in our case represented coincidental spontaneous regression, as spontaneous regression occasionally occurs in FL (7,21,22).
Since hemato-oncology patients have been reported to have not only an increased risk of mortality from COVID-19 but also a diminished vaccine response (2,23), low-dose radiotherapy may be an acceptable treatment choice to afford time for a patient to generate a response to COVID-19 vaccination.
A written informed consent was obtained from the patient for the publication.
Author's disclosure of potential Conflicts of Interest (COI).
Kensuke Kojima: Research funding, Eisai, Daiichi-Sankyo, Kyowa-Kirin, Otsuka Pharmaceutical and Asahi Kasei; Honoraria, Janssen Pharmaceutical, AstraZeneca and AbbVie.
References
- 1.Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol 95: 316-327, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Shah V, Ko Ko T, Zuckerman M, et al. Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with haematological malignancies; King's College Hospital experience. Br J Haematol 190: e279-e282, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerrato M, Orlandi E, Vella A, et al. Efficacy of low-dose radiotherapy (2 Gy ×2) in the treatment of marginal zone and mucosa-associated lymphoid tissue lymphomas. Br J Radiol 94: 20210012, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleh K, Michot JM, Schernberg A, et al. Repeated courses of low-dose 2×2 Gy radiation therapy in patients with indolent B-cell non-Hodgkin lymphomas. Cancer Med 9: 3725-3732, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 40: 25-37, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 356: 82-90, 2015. [DOI] [PubMed] [Google Scholar]
- 7.MacManus MP, Hofman MS, Hicks RJ, et al. Abscopal regressions of lymphoma after involved-site radiation therapy confirmed by positron emission tomography. Int J Radiation Oncol Biol Phys 108: 204-211, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi H, Sato K, Yoshida M, et al. CD5-positive follicular lymphoma characterized by CD25, MUM1, low frequency of t(14;18) and poor prognosis. Pathol Int 64: 95-103, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Hu S, Zuo Z, et al. CD5-positive follicular lymphoma: clinicopathologic correlations and outcome in 88 cases. Mod Pathol 28: 787-798, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Sekiguchi Y, Imai H, Wakabayashi M, et al. CD5-positive follicular lymphoma: a case report and literature review. Intern Med 50: 899-904, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Patel N, Durkin L, Bodo J, Hsi ED. Immunohistochemical expression of lymphoid enhancer binding factor 1 in CD5-positive marginal zone, lymphoplasmacytic, and follicular lymphomas. Am J Clin Pathol 153: 646-655, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Menter T, Trivedi P, Ahmad R, et al. Diagnostic utility of lymphoid enhancer binding factor 1 immunohistochemistry in small B-cell lymphomas. Am J Clin Pathol 147: 292-300, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Naresh KN. MUM1 expression dichotomises follicular lymphoma into predominantly, MUM1-negative low-grade and MUM1-positive high-grade subtypes. Haematologica 92: 267-268, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Russo AL, Chen YH, Martin NE, et al. Low-dose involved-field radiation in the treatment of non-Hodgkin lymphoma: predictors of response and treatment failure. Int J Radiat Oncol Biol Phys 86: 121-127, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Hoskin P, Popova B, Schofield O, et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol 22: 332-340, 2021. [DOI] [PubMed] [Google Scholar]
- 16.Matt S, Hofmann TG. The DNA damage-induced cell death response: a roadmap to kill cancer cells. Cell Mol Life Sci 73: 2829-2850, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood 106: 3150-3159, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoops L, Haas RLM, de Kemp S, et al. In vivo p53 response and immune reaction underlie highly effective low-dose radiotherapy in follicular lymphoma. Blood 110: 1116-1122, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Walle T, Monge RM, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol 10: 1-27, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res 76: 5994-6005, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Rees GJ. Abscopal regression in lymphoma: a mechanism in common with total body irradiation? Clin Radiol 32: 475-480, 1981. [DOI] [PubMed] [Google Scholar]
- 22.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol 15: 424-435, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Ollila TA, Lu S, Masel R, et al. Antibody Response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol 7: 1714-1716, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]