Abstract
Limb muscles are remarkably complex and evolutionarily labile. Although their anatomy is of great interest for studies of the evolution of form and function, their homologies among major amniote clades have remained obscure. Studies of adult musculature are inconclusive owing to the highly derived morphology of modern amniote limbs, but correspondences become increasingly evident earlier in ontogeny. We followed the embryonic development of forelimb musculature in representatives of six major amniote clades and found, contrary to current consensus, that these early splitting patterns are highly conserved across Amniota. Muscle mass cleavage patterns and topology are highly conserved in reptiles including birds, irrespective of their skeletal modifications: the avian flight apparatus results from slight early topological modifications that are exaggerated during ontogeny. Therian mammals, while conservative in their cleavage patterns, depart drastically from the ancestral amniote musculoskeletal organization in terms of topology. These topological changes occur through extension, translocation, and displacement of muscle groups later in development. Overall, the simplicity underlying the apparent complexity of forelimb muscle development allows us to resolve conflicting hypotheses about homology and to trace the history of each individual forelimb muscle throughout the amniote radiations.
Limb muscles enable highly variable and diverse locomotor capabilities, perhaps making them more susceptible to evolutionary change than most other muscle groups. Different schemes for limb muscle homology have been proposed using various criteria, including innervation, topology and function1–14(For a recent overview of the disparate nomenclature authors have applied to individual forelimb muscles see11). The “embryonic origin” criterion of homology is often referenced, but the vast majority of embryonic anatomical information for amniotes comes from a few studies on a handful of species, dating from the first half of the 20th century and limited to the proximal portion of the arm15–21, as well as a handful of recent studies focusing on birds and mammals22–28. Far more is known about the cellular and molecular processes involved in muscle development: unlike the skeleton and connective tissue of the limbs, limb muscles derive from cells that arise from the dorsal portion of the somite, the dermomyotome, that delaminate, migrate, and invade the limb bud. Here they form two opposing masses of differentiated myotubules. Each mass later cleaves, forming smaller divisions and subdivisions that eventually split into individualized recognizable muscles of the adult29,30. Muscles are referred to as being Dorsal or Ventral, on the basis of their putative embryonic origin and their topographic relationship with the nerves of the brachial plexus innervating them. Branches of the Dorsal and Ventral cords of the plexus typically innervate Dorsal and Ventral muscles, respectively4,13,31,32. This distinction has been used as a tool for further fine-tuning homology hypotheses based on other criteria, with the idea that Dorsal muscles of one species cannot be homologous to Ventral muscles of another. Molecular markers can be used to distinguish between dorsal and ventral muscle precursors33,34; however, no known genes show patterns of expression specific to individual muscles derived from subsequent divisions. Embryonic assessments of homology must therefore center on topology and cleavage pattern. Owing to assumptions regarding the embryonic origins and relationships among adult muscles, the evolutionary history of amniote forelimb musculature has been portrayed as involving a series of transitions or modifications, the more notable of these being an expansion of the deltoid musculature to form scapulohumeral muscles in reptiles and the humeroradialis muscle in archosaurs11,32; a shift from ventral to dorsal of the supracoracoid musculature of mammals to form the “neomorphic” muscles associated with the scapula9,11,35, and a shift of the dorsal hand muscles of mammals towards the forearm to become the distal forearm extensors of the digits6,11,12.
We followed the development of forelimb musculature in embryos of six species representing major clades of reptiles (birds, crocodylians, turtles and lizards) and therian mammals (marsupials and placentals) by means of whole-mount immunostaining, confocal imaging and 3D volume segmentation (Fig.1). We examined muscle development and cleavage by following the staining pattern of the MF-20 antibody (DSHB), targeting the Myosin heavy chain protein expressed in mature myocytes — an antibody that has been used for the study of muscle development in amphibians and amniotes36–39. Previous studies have analyzed the development of limb muscles by means of in situ hybridization of markers like MyoD22,24, which label more immature muscle progenitors like myoblasts and could allow visualization of earlier morphogenetic events. However, we find that the MF-20 antibody staining pattern shows the same anatomy that MyoD in situs in stages beyond HH28/E11 of birds and mouse, respectively (comparing with, for example, C-D, F-I of Figure 1 from24), and that the earliest expression pattern of MyoD, labelling immature cells not stained by MF-20, shows the simplest anatomy of the opposing dorsal and ventral pre-muscles masses (A-B, E of Fig. 1 from24) but does not provide extra information regarding the splitting patterns that are the focus of this study. Based on this, MF-20 was used to label mature muscle cells in all stages of development and all species, even if other antibodies or molecular markers can provide more information regarding previous developmental processes, such as migration of somitic cells or the localization of pre-muscular progenitors.
Figure 1:

Raw data visualization and 3D segmentation. A, B: individual z-slices obtained from zeiss LSM880 imaging of a PO20 Paroedura pictus embryo, visualized with FiJi. C: 3D maximum projection of the entire z-stack, generated with FiJI. D, E: Individual z-slices visualized in VGStudio Max3.4. Regions of Interest (ROI) are selected in each z-slice and the reconstructed 3D volume can be visualized as the rendered volume (F). Scale bars A, B, C and F 500 μm. D and E show 650 μm scalebar of VGStudio.
Current consensus is that early muscle splitting patterns are different among the major amniote clades17–20 in the number of divisions emerging from the Dorsal and Ventral muscle masses, and in the ultimate derivation of adult muscles from those early subdivisions. In contrast to this accepted notion of disparity, we found a common and stereotyped set of divisions across all Amniota (Fig.2): the dorsal mass gives rise to one intrinsic (located within the arm) division, and three extrinsic (located “outside” of the arm) divisions. The extrinsic divisions include the Deltoid division anteriorly, the Latissimus division posteriorly, and the Subscapular division deep to the Latissimus division. The ventral muscle mass gives rise to two extrinsic divisions — the anterior Supracoracoideus division and posterior Pectoral division — and one intrinsic division. Both intrinsic divisions later separate proximodistally into three subdivisions associated with the three developmental segments of the arm: proximal zeugopod (upper arm), intermediate stylopod (forearm), and distal autopod (wrist and hand). This contradicts the previous hypothesis of independent Elbow and Wrist forearm matrices6(see below). The Intrinsic dorsal division forms, from proximal to distal, the Triceps subdivision, Extensor subdivision, and Hand extensor subdivision. The Intrinsic ventral division forms the Brachial subdivision, Flexor subdivision, and Hand flexor subdivision. In all species studied, these conserved early muscle divisions produce six dorsal and five ventral anatomical units (Fig.3). The only exception to this pattern appears to be the therian condition, in which the hand extensor subdivision is conspicuously absent. Therians therefore apparently lack dorsal hand muscles entirely, in contrast to current consensus that these muscles are present but shifted proximally along the forearm6,9. The highly conserved splitting patterns we found among arm muscles make it possible to determine most of their homologies across amniotes based on development alone (Extended data Figs.1, 2), and to challenge some assumed homologies and some proposed evolutionary transitions invoked to explain the origin of particular morphologies. Surprisingly, even turtles and birds, with their extremely modified anatomy, maintain the ancestral pattern of muscle division. Therian mammals have the most transformed forelimb musculature; however, their unique characters derive not from modification of these early cleavage patterns but from changes in position, translocation and extension of the resulting muscle divisions.
Figure 2:

Stereotypical early cleavage pattern of the forelimb musculature in amniote embryos. In all species studied, the Dorsal and Ventral muscle masses cleave into the same distinct anatomical units, originating the individual muscles of the arm. The only exception to this sequence and arrangement of divisions seems to be the failure to develop of the Hand extensor subdivision (HE) in therian mammals (only the mouse is shown). All scale bars are 500 μm.
Figure 3:

Diagram representing the stereotyped cleavage pattern of the amniote forelimb musculature.
**: This subdivision fails to develop in therian mammals.
THE SHOULDER AND CHEST MUSCULATURE
Although the pectoral girdles of turtles and crocodylians are differently and peculiarly modified from the superficially lizard-like ancestral reptilian skeletal architecture32,4041, we find their pattern of muscle development is not different to that of geckoes, which are more anatomically conservative: ventrally, the Pectoralis division forms the broad M. pectoralis, as previously described17,18 and anterior to it, the Supracoracoideus division forms only M. supracoracoideus (Extended data Fig.3), in agreement with17, but contradicting18. Dorsally, the Latissimus division gives rise to the large, fan-shaped M. latissimus dorsi and deep to it, the Subscapular division divides into M. subscapularis and M. scapulohumeralis, while the Deltoid division cleaves early in development into two conspicuous lobes that become the clavicular and scapular deltoids (Extended data Figs.4). The cleavage of the Dorsal mass into three divisions (Latissimus, Subscapular and Deltoid) in the proximal aspect of the arm was previously described mammals and birds19,20 and in squamates by42, though this last was ignored by Romer17. Here we show this is also the case in non-avian reptiles, contrary to the conclusions drawn by previous studies concerning lizards and turtles17,18. Of the variations on this general pattern, the most remarkable is the development of “M. teres major” from the Latissimus division in crocodylians, as reported previously in turtles18 but not in lizards (see below for mammals). As describe below, this means that turtles, crocodylians, and birds all have divided latissimi, which may be a rare morphological apomorphy of the proposed clade Archelosauria.
In birds, the evolution of the flying apparatus resulted in a highly distinctive and modified pectoral girdle. The main ventral muscle of birds remains M. pectoralis. The Supracoracoid division shifts from its original anterior position to lay deep to the Pectoralis division and then extends posteriorly along the coracoid and sternum and anteriorly along the coracoid plate, where it curves at the level of the shoulder to sit along the proximal border of the humerus (Extended data Fig.3) in agreement with previous work20. In contrast to this previous work, however, we find that this distal portion gives rise to M. tensor propatagialis, an avian muscle essential for the performance of sophisticated flight, which thus is not homologous to M. humeroradialis of crocodylians as was previously assumed (see below)32. The curved and elongated portion corresponding to M. supracoracoideus passes through a space in between the scapula, coracoid and humerus (the foramen triosseum) and attaches to the humerus dorsally, forming a pulley that raises the arm during the upstroke despite the ventral position of the muscle. As in alligator and turtle the Latissimus division of the quail also divides into two muscles, Mm. latissimus dorsi anterior and posterior. Many birds possess these two latissimus muscles and it is possible the anterior portion of the avian latissimus dorsi muscle of birds is homologous to the region that becomes the so-called M. teres major of crocodylians and turtles. As noted above, this latissimus division may be an apomorphy uniting these clades as the proposed clade Archelosauria, for which there is abundant molecular but scant morphological evidence.
The Deltoid division separates early into two muscle portions, as observed in most species studied. In non-avian reptiles, however, these portions generally associate with the scapula and clavicle and correspond to the Mm. deltoideus scapularis and clavicularis, respectively, while in birds they originate largely from proximal humerus, and have been referred to as Mm. deltoideus major and minor, respectively. Previous embryonic work20 misidentified the anterior portion, M. deltoideus minor, as M. tensor propatagii, which forms anterior and deep to the Deltoid division from the extension of the Supracoracoideus division, as described above. We find that in reptiles, including birds, muscles that have been proposed to be evolutionarily derived from the Deltoid division on the basis of adult anatomy (M. subcoracosapularis and M. scapulohumeralis posterior of11) are derived instead from the Subscapular division. This is in agreement with some earlier embryological accounts20 (Extended data Fig.1). As the scapula becomes thinner and longer, mirroring the evolutionary transition in bird-line archosaurs, the derivatives of the avian Subscapular division remained attached to its postero-lateral surface, forming a large muscle accompanied by a smaller one. These correspond to M. subscapularis and M. scapulohumeralis posterior of other reptiles13,32,40. In birds, these muscles have historically been called M. scapulohumeralis caudalis/posterior and M. scapulohumeralis cranialis, respectively43,44. The so-called “M. subscapularis” of birds is a small muscle originating on the proximal portion of the scapula, unlike M. subscapularis of other amniotes. Its homologies are unclear.
The most drastic transformation of shoulder and chest developmental pattern and anatomy among amniotes is observed in therian mammals. The coracoid is reduced to a small process fused to the scapula and the sternum is braced solely by ribs and the clavicle. The scapula is broad, and its lateral surface is bisected by a ridge (the scapular spine), forming a supra- and infraspinous fossa. The supracoracoid musculature has been suggested to have shifted either partially12,19,45 or entirely11,35 to the dorsal aspect of the scapula, and the pectoral musculature is regarded as broadly homologous to the reptilian pectoralis, having expanded over the chest region and separated into two main portions with several subdivisions of debated homologies9. In contrast to that of reptiles, the Pectoralis division of therians forms only M. pectoralis minor and the deeper portion of M. pectoralis major (in addition to the “skin muscle” M. panniculus carnosus) (Extended data Fig.5) while a large portion of M. pectoralis major is in fact of Supracoracoideus-division origin, thus being non-homologous to the reptilian pectoralis it so resembles. The Supracoracoideus division of therians extends dorsally and fully invades the lateral surface of the scapula, bifurcating around the sides of the scapular spine, giving rise to Mm. supraspinatus and infraspinatus, occupying the supra and infraspinous fossae, respectively (Extended data Fig.5). On the ventral aspect of the shoulder the Supracoracoideus division gives rise to the superficial portion of M. pectoralis major as noted above, and the therian “M. deltoideus clavicularis.” Therefore, contrary to previous assumptions, the therian supracoracoideus division does not translocate dorsally, but rather expands over the shoulder while still forming muscles of the chest. The Deltoid division of the mouse, like those of other amniotes studied, separates into two muscular portions early in development; however, both remain associated with the scapula, likely owing to exclusion from the clavicle region by the precocious expansion of the Supracoracoideus division. The most anterior muscle, termed “M. deltoideus scapularis”, likely corresponds to the muscle portion that becomes the clavicular deltoid in reptiles, while the posterior portion, termed M. teres minor, corresponds to that which becomes the scapular deltoid of reptiles (Extended data Fig.6). M. teres minor was not observed in the short-tailed opossum; however, it is present in other marsupials45,46, and derives from the Deltoid division in a similar fashion to what we observe in the mouse19. The Subscapular division of therians expands occupying the broad medial surface of the scapula (forming M. subscapularis) and extends over its posterior edge to cover part of its postero-lateral surface; this portion separates forming M. teres major (Extended data Fig.6). Note that the so-called Teres major muscle of turtles and crocodylians derives from the Latissimus division (Extended data Fig.4), while the mammalian homonym derives from the Subscapular division, showing these muscles are not truly homologues, contrary to previous assumptions9,11.
THE ARM MUSCULATURE
The Triceps and Biceps subdivisions form muscle complexes with origin on the girdles or proximal humerus. They respectively serve largely to extend and flex the forearm. We find the Triceps subdivision in all amniotes extends well beyond the elbow along the posterior margin of the dorsal forearm, where it forms the posterior-most forearm extensor muscle. Although this muscle has received various names11,20,32,40,43, its identity as a Triceps division derivative and homology across all taxa is clear based on our data (Extended data Fig.7). We also corroborate that M. dorsoepitrochlearis, an exclusively mammalian muscle, derives from the Triceps and not from the Latissimus division11. The Biceps division gives rise to the M. biceps brachii and Mm. coracobrachiales (Extended data Fig.8). While we find M. brachialis of the Alligator also derives from the Biceps division, it is not homologous to the brachialis muscles of other amniotes (see below).
The forearm musculature was classically proposed (without embryological evidence) to derive from dorsal and ventral Elbow and Wrist matrices2,8, respectively giving rise to muscles connecting the humerus to the antebrachium and the antebrachium to the hand. In contrast, we find the Extensor and Flexor divisions cleave into three superficial and one deep lobes. Individual muscles derive from each lobe, instead of arising from proximal and distal forearm muscle divisions. The Flexor division cleaves into three superficial lobes: one clearly associated with the ulna, another with the radius, and a third, central lobe in between (Extended data Fig.9). The three Extensor lobes, in contrast, are shifted anteriorly, as the ulnar region of the dorsal forearm is occupied by the distal extension of the Triceps division mentioned earlier. Consequently, the posterior-most lobe is “central”, the middle lobe is radial and the most-anterior lobe extends proximally along the humerus. M. brachialis of mammals, birds, lizards and turtles was previously interpreted as being derived from the same region as M. biceps brachii; however, we find this muscle derives from the anterior-most portion of the Extensor lobe, as does the muscle called Humeroradialis of crocodylians, which is homologous to the brachialis of other amniotes (Extended data Fig.8). The Central lobes of both Extensor and Flexor divisions form the major muscles with actions on the digits or the whole hand (Extensor digitorum and Flexor digitorum11), and in some cases secondary muscles that can associate with muscles from a neighboring lobe, sharing a tendon or forming a muscle complex (Extended data Fig.9). The deep flexor lobe is present in all species and gives rise to a variable number and type of muscles. Its distal portion extends into the hand in reptiles, deep to the Hand flexor division muscles, where it cleaves into variable individual units that aggregate into compound muscles (the ulnar and carpal heads of M. flexor digitorum longus in Alligator, palmar head of M. flexor digitorum longus in Paroedura, are examples).
The mammalian Flexor division is slightly different from the reptilian one: instead of running parallel to each other, the superficial ulnar and radial lobes and their muscle fibers diverge with respect to the central lobe. This is possibly a consequence of the torsion of the radius associated with the change in elbow orientation that occurs in mammalian development and evolution. We find the muscle referred to as M. flexor carpi radialis derives from the central lobe, making it homologous to the reptilian M. flexor digitorum longus, not to the identically named radial flexors of other amniotes as is the current consensus11. Previous work has highlighted the possibility that muscles designated as M. palmaris longus are not homologous across amniotes11. We find that the mammalian M. palmaris longus derives from the most anterior portion of the ulnar lobe, confirming it is not a homologue to the muscle so-named in turtles, which instead comes from the central lobe and is homologous to other reptiles’ M. flexor digitorum longus. M. epitrochleoanconeus, a unique mammalian muscle, develops from the proximal end of the ulnar lobe in both Mus and Monodelphis, as has also been shown previously in humans27.
THE HAND MUSCULATURE
The muscles of the hand have generally been treated as an anatomical unit9,11; however, during development they derive from two distinct regions. The most distal portions of the Extensor and Flexor divisions separate, forming the Hand extensor (HE) and Hand flexor (HF) subdivisions, respectively. Both regions separate into distinctive layers during development. Avian hand elements are greatly transformed47; accompanying this skeletal transformation, the hand musculature of birds is also highly simplified. This extreme reduction of the number of hand intrinsic muscles observed in birds does not derive from heavily reduced muscle precursors or absence of an entire division as seen in mammals (see below). In early stages the precursor regions of these muscles look normal, and it is later in development that both dorsal and ventral avian hand divisions fail to separate into the layers observed in other amniotes and ultimately form a significantly smaller number of superficial muscles. Trying to establish one-to-one homologies in this case, between a handful of individual muscles in birds and more than fifty in other reptiles, is extremely difficult. However, as the avian hand muscles show an absence of stratification into layers in both dorsal and ventral muscle divisions, they are most likely at least partially homologous to some of the most-superficial hand extensor and flexor muscles of other amniotes, as those superficial muscles are the first to differentiate in other species studied and the portion originating the deeper ones forms and individualizes later.
The hand extensor subdivision of non-avian reptiles separates distinctly into two layers, which form the Mm. extensores digitorum breves superficiales and Mm. extensor digitorum breves profundus, respectively. In contrast, the entire Hand extensor subdivision of therians fails to develop, and we find that therian hands bear no trace of the ancestral extensor musculature, contradicting the current hypothesis that these dorsal muscles shifted proximally to become extensors in the distal portion of the forearm6,9. All therian hand muscles derive from the hand flexor subdivision alone. The hand flexor subdivision of reptiles cleaves into separate layers (Extended data Fig.10). The most superficial (ventral) layer appears to be itself stratified. Its more superficial portion gives rise to muscles of varying robusticity serving the first and fifth digits, as well as a variable number of muscles serving the other individual digits, while its deeper portion gives rise to muscles that have either been lumped together as part of the most superficially derived Mm. flexor digitorum breves superficialis or identified as separate muscles. Originating from the most-superficial portion, Mm. flexores digitorum breves superficialis of digits II-IV of the mouse undergo a radical translocation towards the proximal end of the forearm and fuse to form M. flexor digitorum superficialis as previously described25. We find that in perinatal stages of the short-tailed opossum these muscles also begin elongating towards the proximal forearm (Extended data Fig.10), indicating this process might be ancestral for therian mammals. The therian M. lumbricales and crocodylian M. flexores digitorum breves intermedius and profundus appear to derive from the layer deep to this portion. The following layers seems to become the reptilian Mm. lumbricales (possibly the therian M. contrahentes) and a deeper layer gives rise to Mm. interossei (the therian M. flexores breves profundi) (Extended data Fig.2). In perinatal stages of Monodelphis, we observed muscles derived from the hand flexor subdivision extending towards the dorsal aspect of the hand, squeezing in between the metacarpals (Extended data Fig.10), similar to the pattern described for M. flexores breves profundi of humans48. Based on this and the complete absence of dorsal muscle progenitors (hand extensor subdivision), the ventral and dorsal Interossei/Intermetacarpales seem to derive in their totality from the ventral musculature. In reptiles we were not capable of observing the individualization or time of appearance of these deep muscles; thus, our data do not allow us to determine whether Mm. interossei dorsales in other amniotes also correspond to dorsally translocated ventral muscles or whether they are, in turn, dorsal muscles not homologous to the mammalian homonyms. As mentioned before, the ventral origin of all hand muscles of mammals, including those with a more dorsal location, and the complete absence of a dorsal hand extensor subdivision contradicts the notion that the dorsal hand musculature observed in reptiles migrated proximally into the dorsal forearm in mammal evolution6,9. Rather, this points to the possibility that independent muscles deriving from the Extensor subdivision co-opted the tendinous framework left unused by the disappearance of the dorsal muscles of the hand, facilitated by the modular nature of tendon development as was shown by49.
DISCUSSION
Our data show that the muscular masses that give rise to the forelimb musculature cleave in a highly stereotyped pattern across amniotes, most similar to that described by Sullivan in chicken embryos20. The patterns previously described for non-avian reptiles (turtles and lizards) differ in greater measure from our own descriptions, likely owing more to the availability of material and the state of the art of the techniques used than to interspecific differences in development among turtles and lizards.
The development of the resulting divisions remains conservative in later stages of muscle division. Even the forelimb muscles of birds, which appear highly transformed, have largely arisen through simple stretching and translation of muscle divisions that look essentially as those of other reptiles. While the different adult anatomies exhibited by reptiles derive from these similar embryonic patterns, the origin of the unique adult musculature of therian mammals results from a rearrangement of the shoulder musculature, complete loss of the dorsal hand musculature and translocation and reorganization of whole individual flexor muscles (Fig.4) — all nevertheless achieved by modifying the stereotyped amniote trajectory. The adult anatomy of stem and early amniotes, including those ancestors of mammals, can be readily compared to that of non-avian reptiles41,50, suggesting the ancestral pattern of amniote forelimb muscle development was like that of reptiles and that the events observed in therian embryos represent unique therian or mammal developmental innovations leading to unique adult anatomies. Monotremes, whose embryos are not readily available, represent a mosaic of “reptilian” and “therian” musculoskeletal anatomy5,51–53, and information on their embryology could be vital to understand the developmental basis of mammal evolutionary history. Interestingly, the pattern of muscle development here described looks very different from that described for amphibian species37,54, in which individual muscles seem to form without continuity with other muscles or derivation from a muscle mass.
Figure 4:

Unique aspects of mammal anatomy derive from unique aspects of embryology. While reptile musculoskeletal anatomy can vary drastically, these different anatomies derive from fairly similar and conservative developmental steps. In contrast, the unique aspects of mammal adult anatomy departing drastically from the ancestral amniote pattern (highlighted in yellow) derive from embryological processes likely unique to mammals as are the expansion of a broad scapula accomodating muscles of ventral origin extending towards the dorsal aspect of the shoulder, the displacement of the Deltoid musculature by the Supracoracoideus division, or the absence of intrinsic extensor musculature on the dorsal aspect of the hand, leading to the re-arrangement of the forearm extensor musculature and the hand tendinous anatomy. ⴕ denote extinct fossil taxa: Captorhinus, Dimetrodon, Ophiacodon. Drawings modified from Romer, 1922, Holmes1977, Romer 1950 and Haines 1939.
It is important to note that the cleavage pattern of muscles masses and even the distinctive morphology of individual muscles precede their association with bones. The association between muscle and bone is established well after the formation of the muscle and the cartilage predecessors of bones, as noted by55. A long-standing, implicit assumption is that homology of skeletal attachment sites is indicative of homology of muscles, an idea reformulated in more modern developmental terms as the muscle scaffold theory56. Although skeletal elements as points of origin and attachment for muscles are important to function, and although attachments are often conserved, the early division of, for example, the Deltoid division into two lobes relies neither on the presence of the clavicle nor the scapula; indeed, we find that muscles such as deltoids can become associated with different bones than in the ancestral condition while maintaining their topology relative to other muscles, as in mammals. This suggests that the notion that a muscle with the same embryonic origin but different attachments cannot be homologous is unfounded. Therefore, for instance, the clavicular deltoid of conservative amniotes, which is attached to the scapula, and the mammalian so-called “scapular deltoid”, which is not, appear nonetheless to be homologues. A view from early muscle development has thus allowed us to resolve the evolutionary history and identities of several problematic amniote arm muscles, including the homology of the archelosaurian teres major muscle, the embryonic origin of the avian tensor propatagii muscle, the complex re-arrangement of the pectoral and supracoracoideus musculature in therian mammals, the identity of the crocodylian humeroradialis muscle and its homology to the brachialis muscle of other amniotes, the non-homology of muscles like M. teres major, flexor carpi radialis, palmaris longus and deltoideus clavicularis across amniotes.
The factors involved in the patterning and instruction of muscle cleavage, growth, attachment and individualization are not yet fully understood. Current evidence suggests that external cues from the developing connective tissue57–59, tendons21, vasculature60 and nerves61 provide information for the proper development of limb muscle architecture62. Interestingly, in the earliest stages of muscle mass splitting, the nerves of the brachial plexus are observed in tight connection with the developing muscle masses; they may in fact contribute to the physical separation of the divisions from each other (Fig.5). Despite the dramatic variety of amniote locomotor anatomy, the initial steps of development and structural arrangement of the musculoskeletal elements have fundamental, and often highly conserved, influences on the adult anatomy of forelimb muscles. It is tempting to imagine the developing nervous system, extracellular connective tissue and tendinous system as agents involved in the cleavage of the early muscle masses, providing not only molecular cues but a physical scaffold exerting mechanical influence, directing and constraining muscle division patterns; however, more research is needed in order to understand how these developing anatomical structures can physically influence each other’s development. More research on these developmental processes across different clades is needed, also, in order to understand not only basic evolutionary correspondence but also the intricate interactions established among developing tissues, and the role of those interactions as both effectors in development and constraints in evolution.
Figure 5:

The developing Brachial plexus and forelimb musculature in Alligator mississipiensis. The influence and instructing interactions of surrounding tissues is relevant for the developmental pattern of forelimb musculature. As observed in other species (not shown) the nerves of the brachial plexus (SN VII to XI in Alligator) extend in between the Divisions of the forelimb developing musculature, being possibly even responsible or in part involved in the separation. In early stages, the proximal base of the Dorsal cord separates the deep Subscapular division from the rest of the Dorsal muscle mass, and ventrally, the Ventral intrinsic division is separated from the Pectoralis and Supracoracoideus divisions that remain together. In later stages the nerves also extend to lie in between the separated Deltoid, Latissimus and Dorsal intrinsic divisions dorsally and separating the Pecotralis and Supracoracoideus divisions. The correspondence between the passage of the nerves and the separation of the muscle masses is stronger in earlier stages, and in older embryos the nerves associate less intimately with them, indicating that probably in later stages the separation of individual muscles relies on different cues than in the earlier cleavage steps. Scale bars 500 μm.
METHODS
All methods were approved and comply with all ethical regulations of Yale University’s Office of Animal Research Support (OARS) IACUC protocol 2017–20153.
Embryo collection
Mouse (Mus musculus) timed matings were established between B6SJLF1/J males (obtained from the Jackson Laboratory, Bar Harbor, Maine) and Swiss Webster outbred females (obtained from Taconic BioSciences, Albany, New York). Noon of the day of plug was recorded as embryonic E0.5. The uterus from pregnant females was isolated and embryos dissected into phosphate buffered saline (PBS). Grey short-tailed opossum (Monodelphis domestica) embryos were collected from a colony housed at Yale University. Males and females were paired and filmed to determine the time of copulation63,64. Embryos were collected at different days after copulation and stages were determined following65. Fertilized japanese quail (Coturnix japonica) eggs were purchased from Stromberg’s Chicken and Game Birds Unlimited and incubated at 37.5°C at 70% humidity until the desired stage66. American alligator (Alligator mississipiensis) eggs were obtained from the Rockefeller Wildlife Refuge, Louisiana Department of Wildlife and Fisheries, and incubated at 32°C and 90% humidity until the desired stage67. Pictus geckos (Paroedura picta) eggs were obtained from our breeding colony at Yale University and incubated at 28°C and 50–60% humidity until the desired stage68. Common musk turtle (Sternotherus odoratus) eggs were purchased from Alabama Turtle Farmer farm (https://www.alabamaturtlefarmer.com/), incubated at 28°C and 90% humidity, and collected every three days between incubation days 14 and 2669. All embryos were collected and dissected in cold PBS, then fixed in 4% Paraformaldehyde (PFA) on a shaker at 4°C for 7 days, then dehydrated 3–4 times with 15 minutes washes of 100% methanol and stored at −20°C until further processing.
Polymerization and Immunostaining
Dehydrated embryos were bleached in a solution of 4:1:1 Methanol:DMSO:H2O2 placed under a light source overnight. Bleached embryos were washed 2 times in 100% methanol for 15 minutes and rehydrated with a series of decreasing methanol solutions (100%, 75%, 50%, 25% methanol in dH2O) and then washed twice in PBS. Rehydrated embryos were incubated in a polymerization solution with 4% acrylamide, and 0.25% VA-044 initiator in PBS overnight on a rocker at 4°C70, but without PFA. Air on the tubes containing the embryos was taken out with vacuum and replaced by N2 and incubated on a rocker at 37°C for 4 hours. Polymerized embryos were washed twice for 15 minutes in PBS at 37°C and then placed in a solution of sodium dodecyl sulfate (SDS) 4%, boric bcid 200mM, pH8.5 on a rocker at 37°C until they became transparent. Transparent embryos were washed twice in PBS at 37°C for 30 minutes and 4 times in PBSt (PBS +1% Triton-X100, Sigma-Aldrich) for 1 hour at room temperature. Embryos were incubated overnight in a Permeabilization solution of PBSt 20% DMSO, Glycine 0.3M after which they were incubated overnight in a Blocking solution of PBSt 10% DMSO and 10% Horse Normal Serum (HNS)71. Embryos were incubated in PBSt 5% DSMO, 5% (HNS), 0.1% sodium azide and primary antibodies against Myosin heavy chain (MF-20, Developmental Studies Hybridoma Bank, DSHB) at 1:40 dilution or Myosin heavy chain (MYH3, AB124205, ABCAM) 1:1000 in some Monodelphis embryos, plus combinations of antibodies targeting Neurofilament (3A10, DSHB) 1:50, Collagen type II (II-II6B3, DSHB) or Sox9 (anti-Sox9, AB5535 ABCAM) 1:1000 for 3–4 days on a rocker at 37°C. After this, embryos were washed in PBSt for 1 hour at 37°C and 5 times for 1 hour in PBSt at room temperature. Anti-mouse secondary antibodies (Alexa Fluor Goat anti-IgG1 488, Goat anti-IgG2b 555, Goat anti-rabbit 647 Invitrogen) were used at a dilution of 1:500 in PBSt 5% DMSO, 5% HNS, 0.1% sodium azide and incubated for 3–4 days on a rocker at 37°C inside a dark box. Embryos were then washed in PBSt for 1 hour at 37°C and 5 times for 1 hour in PBSt at room temperature in a dark box. At least 2 (for Monodelphis) or 3 embryos were initially stained per each individual embryonic stage from around the forelimb paddle-stage to interdigital membrane thinning or disappearance stages, for each combination of antibodies. Upon examination of their anatomy, more embryos were stained in similar stages with intermediate external morphology to bridge the gaps of anatomical development progression when needed.
Imaging and processing
Immunostained embryos were transferred to RIMS (Refractive Index Matching Solution72) and allowed to settle for 3–4 days. After this, embryos were mounted on Clear Wall glass bottom dishes (PELCO) in liquid 1% low melting point agarose made on RIMS and left in the dark at room temperature to solidify. Embryos were then imaged using a Carl Zeiss LSM880 Confocal Microscope collecting multiple tiles of Z-stacks, according to the size of the embryo. Files were opened with Fiji (ImageJ)(Fig.1A–C)73 and each channel converted into .jpg file series to be imported as separated Volumes into VGStudioMax 3.0 for digital segmentation where individual muscles or portions of muscles can be segmented using a combination of the volumetric brush and region-growing tools (Fig.1 D–E). No signal was excluded during the process, and only objects that were obviously separate spatially were made into individual regions of interest. VGStudio segmentation is lossless in that the segments created using these tools are voxel objects instead of surfaces and therefore retain the full resolution, internal structure, and grayscale fidelity of the original data. Rendering was performed in VGStudio directly from the lossless volume objects such that the 3D images generated reflect the entirety of the regional fluorescent signal.
Muscles considered
A number of muscles (Serratus muscles, M. levator scapularis, M. sternocoracoideus, M. costocoracoideus, M. trapezius (derived from the cucullaris muscle), Rhomboid muscles) attach to and effect their action on the pectoral girdle. However, they do not derive from the migratory cells forming the limb Dorsal and Ventral muscle masses, but instead originate directly from the somites23,24,26 or the lateral plate mesoderm in the case of the cucullaris muscle and its derivatives74. These muscles were not considered in this study, which only focuses on those muscles derived from the forelimb muscles masses.
Extended Data
Extended Data Fig. 1. Homologies of the Dorsal musculature of the forelimb in Amniota.

Homologies of the Dorsal musculature of the forelimb in Amniota. Muscles in individual rows are inferred to be homologous across clades based on their embryological origin and cleavage pattern from the divisions and subdivisions identified in the species studied. Rows containing more than one muscle per taxa reference cases in which the distinction of the muscles are not clear or individual portions of one muscle can be homologized to individual muscles of another group. Each muscle is named according to the nomenclature proposed by the authors listed under the clade names. Names proposed for reptilian musculature by Abdala and Diogo, 2010, are listed below if the nomenclature is not the same as in the other studies or they refer to a muscle complex.
Extended Data Fig. 2. Homologies of the Ventral musculature of the forelimb in Amniota.

Homologies of the Ventral musculature of the forelimb in Amniota. Muscles in individual rows are inferred to be homologous across clades based on their embryological origin and cleavage pattern from the divisions and subdivisions identified in the species studied. Rows containing more than one muscle per taxa reference cases in which the distinction of the muscles are not clear or individual portions of one muscle can be homologized to individual muscles of another group. Each muscle is named according to the nomenclature proposed by the authors listed under the clade names. Names proposed for reptilian musculature by Abdala and Diogo, 2010, are listed below if the nomenclature is not the same as in the other studies or they refer to a muscle complex.
Extended Data Fig. 3. Development of the forelimb musculature of reptiles, with focus on the ventral extrinsic musculature.

Development of the forelimb musculature of reptiles, with focus on the ventral extrinsic musculature derived from the Pectoralis (dark green) and Supracoracoideus (light green) divisions. All views ventral, except Paroedura PO 22 that is lateral and Coturnix HH33–34 that is a medial view. The Supracoracoideus division usually lays anterior to the Pectoralis division; in Coturnix in turn, it has shifted deep to it and extends into the humerus forming a pronounced curve. The proximal portion forms M. supracoracoideus (empty light green arrow) while the distal portion, beyond the curve, forms M. tensor propatagialis (light green arrow). PD: Pectoralis division, sc: M. supracoracoideus, SCD: Supracoracoid division, tpp: M. tensor propatagialis. All scale bars are 500 μm.
Extended Data Fig. 4. Development of the forelimb musculature of reptiles, with focus on the dorsal extrinsic musculature.

Development of the forelimb musculature of reptiles, with focus on the dorsal extrinsic musculature derived from the Latissimus (dark red), Deltoid (red) and Subscapular (magenta) divisions. All images show lateral view, except for the second row of Coturnix showing medial views of the forelimb musculature. The empty red arrows and the red arrows point at the development of the scapular and clavicular lobes of the Deltoid division respectively. In Sternotherus, like in some other turtles, the scapular portion does not form. The empty dark-red arrow points at the anterior lobe formed from the Latissimus division. In Alligator, this gives rise to the Teres major muscle (tm). In Chrisemys, as described by Walker 1947, this also gives rise to M. teres major. In Sternotherus, this muscle does not seem to develop, though in stage 16 an incipient anterior lobe of the Latissimus division, comparable to that of Alligator can be observed (tm?). In Coturnix, the Latissimus division divides (although slightly later) into two lobes, giving rise to the anterior (tm?) and posterior heads of the latissimus muscle. This sort of lobation is not observed in Paroedura and no comparable muscle develops in lizards. The empty magenta arrow points at M. subscapularis (M. scapulohumeralis caudalis of birds). dc: M. deltoideus clavicularis, DD: Deltoid division, ds: M. deltoideus scapularis, ld: M. latissimus dorsii, ldp: M. latissimus dorsii posterior, LD: Latissimus division, shc: M. scapulohumeralis caudalis, sbcc: M. subcoracoideus, sbsc: M. subscapularis, SSD: Subscapular division, tm: M. teres major, *: M. scapulohumeralis anterior. All scale bars are 500 μm.
Extended Data Fig. 5. Development of the forelimb musculature of therian mammals with focus on the ventral extrinsic musculature.

Development of the forelimb musculature of therian mammals, Mus and Monodelphis, with focus on the ventral extrinsic musculature derived from the Pectoral (dark green) and Supracoracoideus (light green) divisions. The Pectoralis division originates M. panniculus carnosus (white bordered dark green arrow), M. pectoralis minor (empty dark green arrow) and the deep portion of M. pectoralis major (dark green arrow). The ventral portion of the Supracoracoideus division forms the superficial portion of M. pectoralis major (empty light green arrow) and the clavicular deltoid (light green arrow). The dorsal extension of the Supracoracoideus division invades the dorsal aspect of the scapula (*) and bifurcates around the scapular spine (**) originating M. supraspinatus (yellow bordered light green arrow) and M. infraspinatus (red bordered light green arrow). The upper series of mouse embryos depicts a medial view of stage E12.0, a lateral view of stage E12.5 and ventral views of E13.5 and 14.5. Bottom series of Monodelphis and Mus show the developing embryos with all muscle groups except for those deriving from the Pectoral and Supracoracoideus divisions removed, and the developing skeleton stained with either Sox9 or Col II antibodies. M. panniculus carnosus was removed from Monodelphis MC 33 and 33+ and Mus E14.5. dc: M. deltoideus clavicularis, isp: M. infraspinatus, pc: M. panniculus carnosus, PD: Pectoralis division, pmi: M. pectoralis minor, pmp: M. pectoralis major profundus, pms: M. pectoralis major superficialis, ssp: M. supraspinatus. All scale bars are 500 μm.
Extended Data Fig. 6. Development of the forelimb musculature of therian mammals with focus on the dorsal extrinsic musculature.

Development of the forelimb musculature of therian mammals, Mus and Monodelhpis, with focus on the dorsal extrinsic musculature derived from the Latissimus (dark red), Deltoid (bright red) and Subscapular (magenta) divisions. As in reptiles, the Deltoid division of mice cleaves early into two lobes, forming M. scapulodeltoid (red arrow) and M. teres minor (empty red arrow). In Monodelphis a Teres minor muscle was not observed. The posterior portion of the Subscapular division extends along the posterior margin and lateral surface of the scapula (*), forming M. teres major (empty magenta arrow). The upper series shows Mus embryos between E12.0 and 14.5, all in lateral view. The bottom series of Monodelphis and Mus show the development of the muscles and skeleton in lateral view. M. latissimus dorsii is not shown in mice E14.5. DD: Deltoid division, ds: M. deltoideus scapularis, ld: M. latissimus dorsii, LD: Latissimus division, ssc: M. subscapularis, SSD: Subscapular division, tm: M. teres major, tmi: M. teres minor. All scale bars are 500 μm.
Extended Data Fig. 7. Development of the forelimb musculature of amniotes, with focus on the dorsal intrinsic musculature.

Development of the forelimb musculature of amniotes, with focus on the dorsal intrinsic musculature derived from the Triceps (light yellow) and Extensor (yellow) divisions. Note the distal extension of the Triceps division along the ulna, originating the posterior most extensor muscle of the forearm (empty light-yellow arrow), and the humeral lobe of the Extensor division, extending proximally along the humerus (yellow arrow) and forming M. brachialis of lizards, turtles, birds and mammals, homologous to M. humeroradialis of crocodylians. Mm. dorsoepitrochlearis (light yellow arrow) of mammals derives from the Triceps subdivision. c: central lobe (posterior-most lobe) of the Extensor division, dep: M. dorsoepitrochlearis, h: humeral lobe of the Extensor subdivision, r: radial lobe of the Extensor subdivision, u: “ulnar” lobe of the forearm musculature, derived from the Triceps subdivision. All scale bars are 500 μm.
Extended Data Fig. 8. Development of the forelimb musculature of amniotes, with focus on the brachial muscles that act as flexors of the forearm.
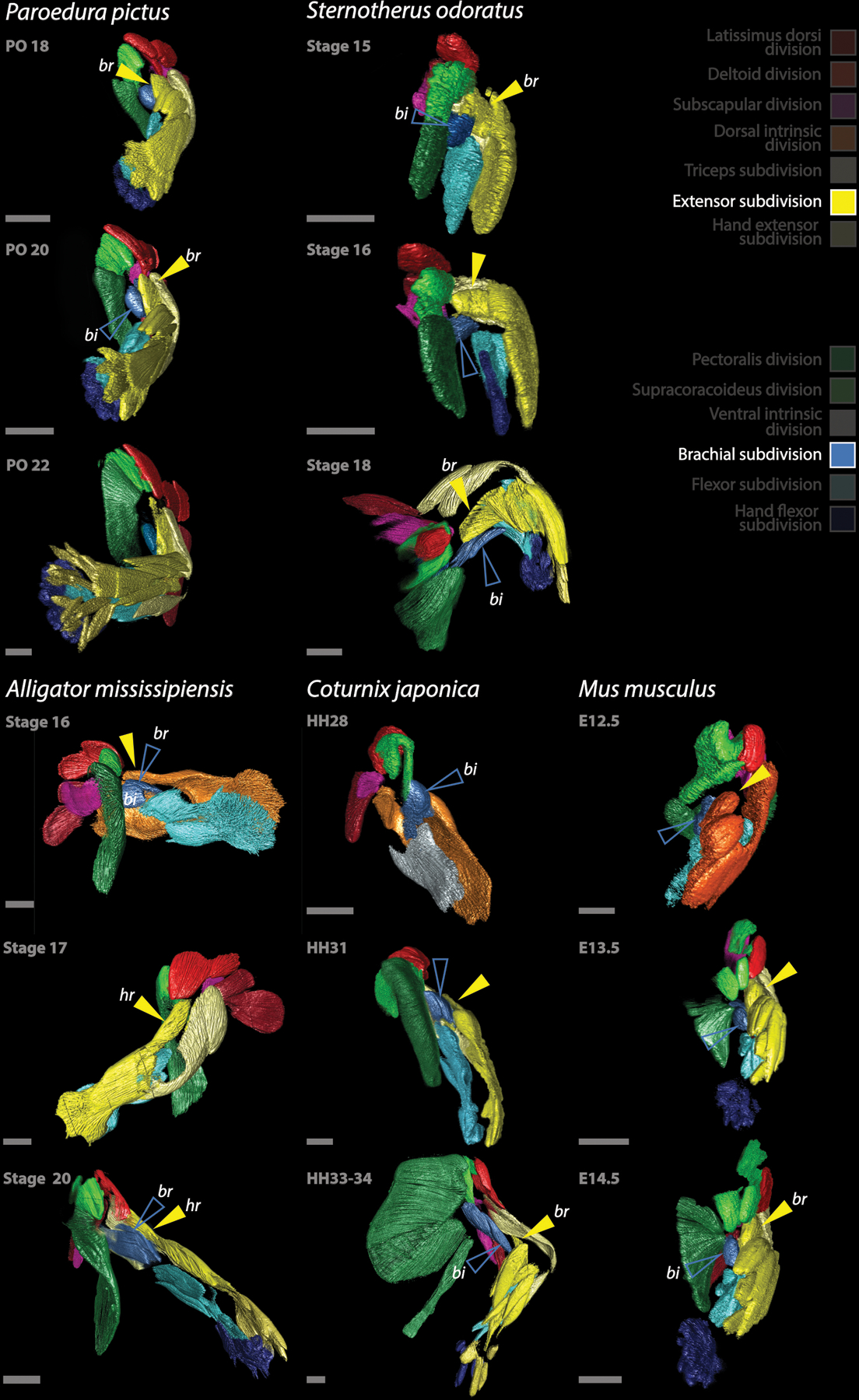
Development of the forelimb musculature of amniotes, with focus on the brachial muscles that act as flexors of the forearm. From the Extensor division, on the dorsal forearm, a muscle extends proximally over the humerus (yellow arrow) forming M. brachialis of Paroedura, Sternotherus, Coturnix and Mus, homologous to M. humeroradialis of Alligator. On the other hand, the biceps muscle and the crocodylians M. brachialis derive from the Brachial subdivision (empty blue arrow). bi: M. biceps brachii, br: M. brachialis (note that the muscle termed “brachialis” derive from different origins than in the other species in Alligator), hr: M. humeroradialis (note that the humeroradialis muscle derives from the same portion of the Brachial subdivision than the brachialis muscles of the other species). All scale bars are 500 μm.
Extended Data Fig. 9. Development of the forelimb musculature of amniotes, with focus on the intrinsic musculature deriving from the Flexor division.

Development of the forelimb musculature of amniotes, with focus on the intrinsic musculature deriving from the Flexor division (light blue). The Flexor division forms a deep lobe, and superficially a central lobe (empty light blue arrow), flanked by an ulnar lobe on the ulnar side of the forearm (bottom portion of each arm in the images) and a radial lobe on the radial side (top). Note that the central lobe of the Flexor subdivision (pointed at by the empty light-blue arrow and originating M. flexor digitorum longus/communis) is the source of the so-called M. flexor carpi radialis of mice, otherwise originated from the radial lobe in other species. M. palmaris longus of Sternotherus, derives from the central lobe too, while its homonym derives from the ulnar lobe in mice. c: central lobe of the Flexor subdivision, d: deep lobe of the Flexor subdivision, epa: M. epitrochleoanconeus, fcra: M. flexor carpi radialis, fcu: M. flexor carpi ulnaris, fdc: M. flexor digitorum comunis, fdl: M. flexor digitorum longus, pal: M. palmaris longus, prop: M. pronator profundus, pros: M. pronator superficialis, prot: M. pronator teres, r: radial lobe of the Flexor subdivision, u: ulnar lobe of the Flexor subdivision. All scale bars are 500 μm.
Extended Data Fig. 10. Development of the forelimb musculature of amniotes, with focus on the ventral hand musculature deriving from the Hand flexor division.

Development of the forelimb musculature of amniotes, with focus on the ventral hand musculature deriving from the Hand flexor division (dark blue). The hand flexor musculature derives from the distal portion of the Flexor division and stratifies into layers; a superficial one (white bordered blue arrow) forms two layers of muscles, usually grouped as the Mm. flexores digitorum breves. M. lumbricales (*) of the mouse seem to develop from this portion of the Hand flexor division. A deeper layer (light blue bordered dark blue arrow), also stratified, forms the deepest sets of muscles, termed lumbricales and interossei dorsales and ventrales. In the mouse, the most-superficial set of hand flexor muscles (colored white in E15.5) elongates and translocates proximally into the forearm to form M. flexor digitorum superficialis. Deriving from the HF, a group of muscles extends dorsally in between the metacarpals in Monodelphis stages MC33+ and 34 (red bordered blue arrows), likely precursors of M. flexor digitorum breves profundi and/or M. intermetacarpales. Note that the Hand extensor musculature fails to develop as observed in a dorsal view of the mouse forearm in E14.5 and of the short-tailed opossum MC stages 33+ and 34. Also, both the dorsal and ventral hand musculature is conspicuously reduced in Coturnix, developing from a non-stratified muscle division and forming considerable fewer muscles than in other reptiles. DL: deep layer of the Hand flexor subdivision, fbp: M. flexores digitroum breves profundi, fdl: humeral head of M. flexor digitorum longus, fdlu: ulnar head of M. flexor digitorum longus, fds: M. flexor digitorum superficialis, SL: superficial layer of Hand flexor subdivision, *: M. lumbricales. All scale bars are 500 μm.
Acknowledgements
We would like to thank Ruth Elsey for her help in collecting alligator eggs and Matthew Bradford for his help in obtaining musk turtle eggs. We also want to thank Yale’s Institute for Biospheric Studies (YIBS) and the Yale Peabody Museum of Natural History for funding this research.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Data availability
Confocal files have been deposited in Dryad (doi:10.5061/dryad.r2280gbd8) and will be available after publication.
REFERENCES
- 1.Howell AB Morphogenesis of the shoulder architecture. Part IV. Reptilia. The Quarterly Review of Biology 11, 183–208 (1936). [Google Scholar]
- 2.Howell AB Phylogeny of the distal musculature of the pectoral appendage. Journal of Morphology 60, 287–315 (1936). [Google Scholar]
- 3.Howell AB Morphogenesis of the shoulder architecture: Aves. The Auk 54, 364–375 (1937). [Google Scholar]
- 4.Howell AB Morphogenesis of the shoulder architecture. Part VI. Therian Mammalia. The Quarterly Review of Biology 12, 440–463 (1937). [Google Scholar]
- 5.Howell AB Morphogenesis of the shoulder architecture. Part V. Monotremata. The Quarterly Review of Biology 12, 191–205 (1937). [Google Scholar]
- 6.Haines RW A revision of the extensor muscles of the forearm in tetrapods. Journal of Anatomy 73, 211 (1939). [PMC free article] [PubMed] [Google Scholar]
- 7.Haines RW The flexor muscles of the forearm and hand in lizards and mammals. Journal of Anatomy 84, 13 (1950). [PMC free article] [PubMed] [Google Scholar]
- 8.Straus WL Jr The homologies of the forearm flexors: urodeles, lizards, mammals. American Journal of Anatomy 70, 281–316 (1942). [Google Scholar]
- 9.Diogo R, Abdala V, Aziz M, Lonergan N & Wood B From fish to modern humans–comparative anatomy, homologies and evolution of the pectoral and forelimb musculature. Journal of Anatomy 214, 694–716 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diogo R & Abdala V Muscles of vertebrates: comparative anatomy, evolution, homologies and development. (CRC Press, 2010). [Google Scholar]
- 11.Abdala V & Diogo R Comparative anatomy, homologies and evolution of the pectoral and forelimb musculature of tetrapods with special attention to extant limbed amphibians and reptiles. Journal of Anatomy 217, 536–573 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diogo R, Bello‐Hellegouarch G, Kohlsdorf T, Esteve‐Altava B & Molnar JL Comparative myology and evolution of marsupials and other vertebrates, with notes on complexity, Bauplan, and “scala naturae”. The Anatomical Record 299, 1224–1255 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Russell A & Bauer AM in Biology of the Reptilia Vol. 20 (ed Gaunt A & Adler K Gans C) (Society for the Study of Amphibians and Reptiles, 2008). [Google Scholar]
- 14.Romer AS Vertebrate body. (1970).
- 15.Romer AS The development of the thigh musculature of the chick twelve figures. Journal of Morphology 43, 347–385 (1927). [Google Scholar]
- 16.Romer AS The development of tetrapod limb musculature—the thigh of Lacerta. Journal of Morphology 71, 251–298 (1942). [Google Scholar]
- 17.Romer AS The development of tetrapod limb musculature—the shoulder region of Lacerta. Journal of Morphology 74, 1–41 (1944). [Google Scholar]
- 18.Walker WF The development of the shoulder region of the turtle, Chrysemys picta marginata, with special reference to the primary musculature. Journal of morphology 80, 195–249 (1947). [DOI] [PubMed] [Google Scholar]
- 19.Cheng CC The development of the shoulder region of the opossum, Didelphys virginiana, with special reference to the musculature. Journal of Morphology 97, 415–471 (1955). [Google Scholar]
- 20.Sullivan G Anatomy and embryology of the wing musculature of the domestic fowl (Gallus). Australian Journal of Zoology 10, 458–518 (1962). [Google Scholar]
- 21.Kardon G Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019–4032 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Valasek P, Evans DJ, Maina F, Grim M & Patel K A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development 132, 447–458 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Valasek P et al. Somitic origin of the medial border of the mammalian scapula and its homology to the avian scapula blade. Journal of anatomy 216, 482–488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valasek P et al. Cellular and molecular investigations into the development of the pectoral girdle. Developmental biology 357, 108–116 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Huang AH et al. Repositioning forelimb superficialis muscles: tendon attachment and muscle activity enable active relocation of functional myofibers. Developmental cell 26, 544–551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pu Q, Huang R & Brand‐Saberi B Development of the shoulder girdle musculature. Developmental Dynamics 245, 342–350 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Diogo R, Siomava N & Gitton Y Development of human limb muscles based on whole-mount immunostaining and the links between ontogeny and evolution. Development 146, dev180349 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Watson SS, Riordan TJ, Pryce BA & Schweitzer R Tendons and muscles of the mouse forelimb during embryonic development. Developmental dynamics: an official publication of the American Association of Anatomists 238, 693–700 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christ B & Brand-Saberi B Limb muscle development. International Journal of Developmental Biology 46, 905–914 (2004). [PubMed] [Google Scholar]
- 30.Chevallier A, Kieny M & Mauger A Limb-somite relationship: origin of the limb musculature. Development 41, 245–258 (1977). [PubMed] [Google Scholar]
- 31.Fürbringer M Untersuchungen zur Morphologie und Systematik der Vögel: zugleich ein Beitrag zur Anatomie der Stütz-und Bewegungsorgane. Vol. 15 (van Holkema T, 1888). [Google Scholar]
- 32.Meers MB Crocodylian forelimb musculature and its relevance to Archosauria. The anatomical record 274, 891–916 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Vogel A, Rodriguez C, Warnken W & Belmonte JCI Dorsal cell fate specified by chick Lmxl during vertebrate limb development. Nature 378, 716–720 (1995). [DOI] [PubMed] [Google Scholar]
- 34.Schäfer K & Braun T Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nature genetics 23, 213–216 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Kardong KV Vertebrates: comparative anatomy, function, evolution. (McGraw-Hill New York, 2006). [Google Scholar]
- 36.Ziermann JM & Diogo R Cranial muscle development in frogs with different developmental modes: direct development versus biphasic development. Journal of Morphology 275, 398–413 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Diogo R & Ziermann JM Development of fore‐and hindlimb muscles in frogs: morphogenesis, homeotic transformations, digit reduction, and the forelimb–hindlimb enigma. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 322, 86–105 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Nagashima H et al. Evolution of the turtle body plan by the folding and creation of new muscle connections. Science 325, 193–196 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Botelho JF, Smith-Paredes D, Nuñez-Leon D, Soto-Acuna S & Vargas AO The developmental origin of zygodactyl feet and its possible loss in the evolution of Passeriformes. Proceedings of the Royal Society B: Biological Sciences 281, 20140765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker W Jr The locomotor apparatus of Testudines. Biology of Reptilia 4, 1–100 (1973). [Google Scholar]
- 41.Romer AS The locomotor apparatus of certain primitive and mammal-like reptiles. Bulletin of American Museum of Natural History 46, 517–606 (1922). [Google Scholar]
- 42.Sewertzoff A Studien über die Entwickelung der Muskeln, Nerven und des Skeletts der Extremitäten der niederen Tetrapoda: Beiträge zu einer Theorie der pentadactylen Extremität der Wirbeltiere. (Typo-lithogr. de la Société JN Kouchnéreff, 1908). [Google Scholar]
- 43.George JC & Berger AJ Avian myology. (1966).
- 44.Van den Berge J Myologia. Handbook of avian anatomy: Nomina anatomica avium, 189–247 (1993). [Google Scholar]
- 45.Warburton N Functional morphology and evolution of marsupial moles (Marsupialia: Notoryctemorphia; ). (2003). [Google Scholar]
- 46.Jenkins PA & Weijs W The functional anatomy of the shoulder in the Virginia opossum (Didelphis virginiana). Journal of Zoology 188, 379–410 (1979). [Google Scholar]
- 47.Botelho JF et al. New developmental evidence clarifies the evolution of wrist bones in the dinosaur–bird transition. PLoS biology 12, e1001957 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cihak R Ontogenesis of the skeleton and intrinsic muscles of the human hand and foot. (Springer Science & Business Media, 2013). [PubMed] [Google Scholar]
- 49.Huang AH et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development 142, 2431–2441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes R The osteology and musculature of the pectoral limb of small captorhinids. Journal of Morphology 152, 101–140 (1977). [DOI] [PubMed] [Google Scholar]
- 51.McKay WJS The morphology of the muscles of the shoulder-girdle in monotremes. (1894).
- 52.Shrivastava R The deltoid musculature of the Monotremata. American Midland Naturalist, 434–440 (1962). [Google Scholar]
- 53.Regnault S, Fahn-Lai P, Norris RM & Pierce SE Shoulder Muscle Architecture in the Echidna (Monotremata: Tachyglossus aculeatus) Indicates Conserved Functional Properties. Journal of Mammalian Evolution, 1–13 (2020). [Google Scholar]
- 54.Diogo R & Tanaka EM Development of fore‐and hindlimb muscles in GFP‐transgenic axolotls: Morphogenesis, the tetrapod bauplan, and new insights on the Forelimb‐Hindlimb Enigma. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 322, 106–127 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Diogo R, Walsh S, Smith C, Ziermann JM & Abdala V Towards the resolution of a long‐standing evolutionary question: muscle identity and attachments are mainly related to topological position and not to primordium or homeotic identity of digits. Journal of anatomy 226, 523–529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuoka T et al. Neural crest origins of the neck and shoulder. Nature 436, 347–355 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chevallier A & Kieny M On the role of the connective tissue in the patterning of the chick limb musculature. Wilhelm Roux’s archives of developmental biology 191, 277–280 (1982). [DOI] [PubMed] [Google Scholar]
- 58.Mauger A, Kieny M, Hedayat I & Goetinck PF Tissue interactions in the organization and maintenance of the muscle pattern in the chick limb. Development 76, 199–215 (1983). [PubMed] [Google Scholar]
- 59.Kardon G, Harfe BD & Tabin CJ A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Developmental cell 5, 937–944 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Tozer S et al. Involvement of vessels and PDGFB in muscle splitting during chick limb development. Development 134, 2579–2591 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Hurren B, Collins JJ, Duxson MJ & Deries M First neuromuscular contact correlates with onset of primary myogenesis in rat and mouse limb muscles. PloS one 10, e0133811 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deries M & Thorsteinsdóttir S Axial and limb muscle development: dialogue with the neighbourhood. Cellular and molecular life sciences 73, 4415–4431 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kin K, Maziarz J & Wagner GP Immunohistological study of the endometrial stromal fibroblasts in the opossum, Monodelphis domestica: evidence for homology with eutherian stromal fibroblasts. Biology of reproduction 90, 111, 111–112 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Griffith OW et al. Endometrial recognition of pregnancy occurs in the grey short-tailed opossum (Monodelphis domestica). Proceedings of the Royal Society B 286, 20190691 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCrady E Embryology of the opossum. (1938).
- 66.Ainsworth SJ, Stanley RL & Evans DJ Developmental stages of the Japanese quail. Journal of Anatomy 216, 3–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson MW The reproductive biology and embryology of the crocodilians. Biology of the Reptilia 12, 330–491 (1981). [Google Scholar]
- 68.Noro M, Uejima A, Abe G, Manabe M & Tamura K Normal developmental stages of the Madagascar ground gecko Paroedura pictus with special reference to limb morphogenesis. Developmental dynamics: an official publication of the American Association of Anatomists 238, 100–109 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Smith‐Paredes D, Lord A, Meyer D & Bhullar BAS A developmental staging system and musculoskeletal development sequence of the Common Musk turtle (Sternotherus odoratus). Developmental Dynamics (2020). [DOI] [PubMed] [Google Scholar]
- 70.Zheng H & Rinaman L Simplified CLARITY for visualizing immunofluorescence labeling in the developing rat brain. Brain Structure and Function 221, 2375–2383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Renier N et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Yang B et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945–958 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schindelin J et al. Fiji: an open-source platform for biological-image analysis. Nature methods 9, 676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theis S et al. The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137, 2961–2971 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Confocal files have been deposited in Dryad (doi: 10.5061/dryad.r2280gbd8) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Confocal files have been deposited in Dryad (doi:10.5061/dryad.r2280gbd8) and will be available after publication.


