Abstract
The application of large-scale metabolomic profiling provides new opportunities for realizing the potential of omics-based precision medicine for asthma. By leveraging data from over 14,000 individuals in four distinct cohorts, this study identifies and independently replicates seventeen steroid metabolites whose levels were significantly reduced in individuals with prevalent asthma. Although steroid levels were reduced among all asthma cases regardless of medication use, the largest reductions were associated with inhaled corticosteroid (ICS) use, as confirmed in a four-year low-dose ICS clinical trial. Effects of ICS use on steroid levels were dose-dependent, however, significant reductions also occurred with low-dose ICS use. Using information from electronic medical records (EMR), we found that cortisol levels were substantially reduced throughout the entire 24-hour daily period in asthma patients treated with ICS compared with those untreated and non-asthma patients. Moreover, asthma patients treated with ICS showed significant increases in fatigue and anemia as compared to those without ICS use. Adrenal suppression in asthma patients treated with ICS may therefore represent a larger public health problem than previously recognized. Regular cortisol monitoring of asthma patients treated with ICS is needed to provide the optimal balance between minimizing adverse effects of adrenal suppression while capitalizing on the established benefits of ICS treatment.
Keywords: asthma, metabolomics, precision medicine, biobanks, inhaled corticosteroids, steroid resistance, adrenal suppression
Asthma imparts a tremendous global health and economic burden, affecting over 350 million people worldwide1-4, with annual asthma-related costs surpassing $80 billion/year in the USA. Any improvement in biologic understanding or treatment will therefore have significant public health ramifications. While several genetic variants have been determined to influence an individual’s asthma liability5-8, asthma also has substantial environmental triggers9 and the majority of cases arise from complex interactions between both factors.
Metabolomics, the systematic analysis of small molecules in a biological sample, provides an integrated profile of biological status, reflecting the ‘net results’ of genetic, transcriptomic, proteomic, and environmental interactions, making it ideally suited to the study of asthma. The clinical potential of asthma metabolomics is well-demonstrated through the improvement in predictive accuracy of asthma phenotypes and the improved understanding of underlying biology it has provided relative to genetics or environmental factors alone8. While a number of metabolomic signatures of asthma-relevant phenotypes have been reported to date8, there are several drawbacks of these studies that have limited their overall clinical impact, including a lack of independent replication10-13 and small sample sizes11. The identification and validation of metabolic signatures for asthma phenotypes using multiple large, well-characterized epidemiological cohorts and clinical trials with comprehensive coverage of the global metabolome is imperative for the translational potential of metabolomics to be fully realized11,13-18.
Inhaled corticosteroid (ICS) use has formed the foundation of care for individuals with moderate to severe asthma19 with tremendous benefit; however, the impact of ICS use on adrenal insufficiency has been a persistent concern20-24. While multiple studies have examined the effect of ICS use on adrenal suppression, significant limitations in study design have limited their overall utility21,25-31, resulting in conflicting findings32. To add further complexity, not only are there genetic variants that influence asthma liability5-8 but also those that influence steroid response33-38 and susceptibility to adrenal suppression39. As a composite measure that captures both the influence of these genetic susceptibilities together with the impact of ICS use, the metabolome is ideally suited to inform our understanding of the biological impact of these ICS medications while simultaneously incorporating individual genetic variation. The effect of ICS use on individuals with asthma is imperative to understand, particularly given the recent changes to the Global Initiative for Asthma (GINA) guidelines1 that now recommend “as needed” low dose ICS use for the preferred reliever option among adolescents and adults.
Metabolomic findings from epidemiological studies may be further enhanced for clinical translation by integrating them with Electronic Medical Records (EMR)40, as many established clinical biomarkers and tests are directly related or are metabolites themselves41,42. In this study, we utilized metabolomics to investigate prevalent asthma and the impact of ICS use, leveraging multiple cohorts in combination with clinical tests available via EMR to explore a metabolomic-driven precision medicine approach for optimized asthma management.
RESULTS
Cohorts studied
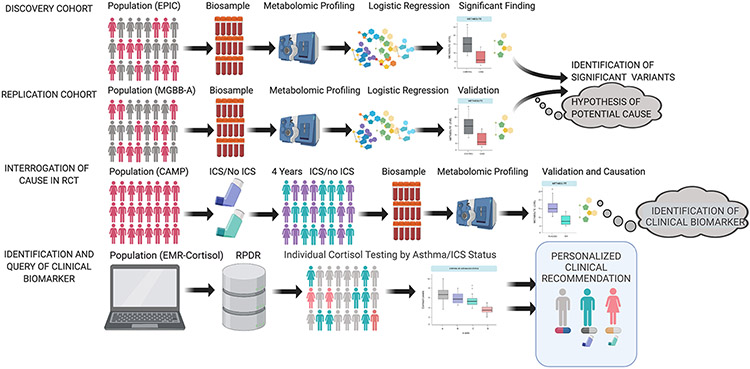
We applied an analytic framework leveraging over 14,000 individuals from four epidemiological studies to discover and validate asthma associated metabolites and provide clinical recommendations that optimize ICS treatment recommendations (Fig. 1): 1) Discovery cohort: European Prospective Investigation of Cancer (EPIC-Norfolk, n=10,754); 2) Replication cohort: Mass General Brigham Biobank-Asthma (MGBB-Asthma, n=610); 3) Validation of ICS effect in a randomized clinical trial: Childhood Asthma Management Program (CAMP, n=1,120); and 4) Assessment of ICS effect using clinical cortisol measurements extracted from EMRs: (EMR-Cortisol, n=2,235). Clinical characteristics of the EPIC-Norfolk, MGBB and CAMP subjects are summarized in Table 1. EPIC-Norfolk asthma cases (N=661) and controls (N=10,093) differed significantly by smoking status (P=0.003) and marginally by sex (P=0.08). Asthma cases (N=287) and controls (N=323) in MGBB-Asthma significantly differed by sex (P<0.001), race (P=0.002), ICS treatment (P<0.001) and body mass index (BMI) (P<0.001). Asthma cases (N=383) and controls (N=1852) in EMR-Cortisol significantly differed by sex (P=3.9x10−14), race (P=1.4x10−3), ICS treatment (P<2.2x10−16) and adrenal insufficiency diagnosis (P=0.03). All CAMP subjects had asthma.
Fig. 1. Overall study design.
The study incorporates four independent cohorts: the EPIC Norfolk cohort, the Mass General Brigham Biobank (MGBB)-Asthma cohort, the CAMP randomized controlled trial (RCT) and the EMR-Cortisol cohort. The cohorts were utilized sequentially to identify prevalent asthma metabolites, validate significant metabolites, assess the effect of inhaled corticosteroids (ICS) on the steroid metabolites, and evaluate the utility of cortisol as a biomarker of adrenal suppression among asthmatics on ICS. The details of our approach for each cohort are illustrated.
Abbreviations: MGBB-A, Mass General Brigham Biobank (MGBB)-Asthma; RCT, Randomized Controlled Trial; CAMP, Childhood Asthma Management Program; ICS, inhaled corticosteroids; EMR, Electronic Medical health Record; RPDR, Research Patient Data Registry
Table 1.
Clinical characteristics of subjects in the large discovery epidemiologic cohort (EPIC-Norfolk), in the biobank and electronic medical record (EMR)-based replication cohorts (MGBB-Asthma, EMR-Cortisol) and in an inhaled corticosteroid (ICS)-randomized clinical trial (Childhood Asthma Management Program, CAMP)
| Discovery cohort: EPIC-Norfolk | ||||
|---|---|---|---|---|
| Number of subjects | All subjects (n = 10,754) |
Asthma (n = 661) |
No asthma (n = 10,093) |
P-value* |
| Age, mean (SD) | 59.73 (9.0) | 59.24 (9.1) | 59.76 (8.9) | 0.14 |
| BMI kg/m2, mean (SD) | 26.19 (3.7) | 26.10 (3.9) | 26.20 (3.7) | 0.53 |
| Sex, n (%) | 0.08 | |||
| Female | 5,759 (53.6) | 376 (56.9) | 5,383 (53.3) | |
| Male | 4,995 (46.4) | 285 (43.1) | 4,710 (46.7) | |
| Race, n (%) | 0.76 | |||
| African American | 9 (0.1) | 1 (0.2) | 8 (0.1) | |
| White | 10,684 (99.7) | 655 (99.5) | 10,029 (99.8) | |
| Asian | 9 (0.1) | 1 (0.2) | 8 (0.1) | |
| Other | 9 (0.1) | 1 (0.2) | 8 (0.1) | |
| Smoking, n (%) | ||||
| Never | 5,012 (46.6) | 308 (46.6) | 4,704 (46.6) | 0.003 |
| Former | 4,573 (42.5) | 306 (46.3) | 4,267 (42.3) | |
| Current | 1,169(10.9) | 47(7.1) | 1,122(11.1) | |
| Replication cohort: MGBB-Asthma | ||||
| Number of subjects | All subjects (n = 610) |
Asthma (n = 287) |
No asthma (n = 323) |
P-value* |
| Age, mean (SD) | 32.7 (5.3) | 33.1 (6.6) | 32.4 (3.7) | 0.11 |
| BMI kg/m2, mean (SD) | 25.6 (6.5) | 28.3 (8.0) | 23.2 (3.1) | <2.2x10−16 2x10−16 |
| Sex, n (%) | ||||
| Female | 359 (58.9) | 208 (72.5) | 151 (46.7) | |
| Male | 251 (41.1) | 79 (27.5) | 172 (53.3) | |
| Race, n (%) | 9.8x10−3 | |||
| African American | 51 (8.4) | 34 (11.8) | 17 (5.3) | |
| White | 475 (77.9) | 222 (77.4) | 253 (78.3) | |
| Asian | 28 (4.6) | 10 (3.5) | 18 (5.6) | |
| Other | 56 (9.2) | 21 (7.3) | 35 (10.8) | |
| Inhaled corticosteroid intake, n (%) | <2.2x10−16 | |||
| No | 413 (67.7) | 90 (31.4) | 323 (100) | |
| Yes | 172 (28.2) | 172 (59.9) | 0 (0) | |
| Oral corticosteroid intake, n (%) | ||||
| No | 265 (43.4) | 265 (92.3) | NA | NA |
| Yes | 17 (2.8) | 17 (5.9) | NA | NA |
| Asthma severity scale | ||||
| No mild, moderate or severe asthma | 72 (11.8) | 72 (25.1) | NA | NA |
| Mild intermittent or persistent | 146 (23.9) | 146 (50.9) | NA | NA |
| Moderate persistent | 50 (8.2) | 50 (17.4) | NA | NA |
| Severe persistent | 19 (3.1) | 19 (6.6) | NA | NA |
| Smoking, n (%) | 1.2x10−3 | |||
| No | 482 (79) | 210 (73.2) | 272 (84.2) | |
| Yes | 128 (21) | 77 (26.8) | 51 (15.8) | |
| Validation cohort: Randomized clinical trial of ICS use - CAMP | ||||
| Number of subjects | All subjects (n = 1041) |
Baseline | End of trial | P-value* |
| (n = 560 at both time points) | ||||
| Age, mean (SD) | 8.8 (2.1) | 8.8 (2.1) | 12.8 (2.1) | <2.2x10−16 |
| BMI kg/m2, mean (SD) | 18.1 (3.5) | 18.0 (3.3) | 21.2 (4.5) | <2.2x10−16 |
| Sex, n (%) | NA | |||
| Female | 420 (40.3) | 201 (35.9) | 201 (35.9) | |
| Male | 621 (59.7) | 359 (64.1) | 359 (64.1) | |
| Race, n (%) | NA | |||
| African American | 138 (13.3) | 82 (14.6) | 82 (14.6) | |
| White | 711 (68.3) | 395 (70.5) | 395 (70.5) | |
| Hispanic | 98 (9.4) | 56 (10) | 56 (10) | |
| Other | 94 (9.0) | 27 (4.8) | 27 (4.8) | |
| Treatment, Steroid use (%) | NA | |||
| Budesonide | 311 (29.9) | 151 (27) | 151 (27) | |
| Nedocromil + Placebo | 730 (70.1) | 409 (73) | 409 (73) | |
| Validation cohort: Assessment of ICS effect in a clinical cohort EMR-Cortisol | ||||
| Number of subjects | All subjects (n = 2,235) |
Asthma (n = 383) |
No asthma (n = 1,852) |
P-value* |
| Age, mean (SD) | 56.1 (16.2) | 55.2 (16) | 56.3 (16.3) | 0.20 |
| Sex, n (%) | 3.9x10−14 | |||
| Female | 1377 (61.6) | 302 (78.9) | 1075 (58.0) | |
| Male | 858 (38.4) | 81 (21.1) | 777 (42.0) | |
| Race, n (%) | 1.4x10−3 | |||
| African American | 122 (5.5) | 34 (8.9) | 88 (4.8) | |
| White | 1959 (87.7) | 317 (82.8) | 1642 (88.7) | |
| Asian | 52 (2.3) | 7 (1.8) | 45 (2.4) | |
| Other | 102 (4.6) | 25 (6.5) | 77 (4.2 | |
| Inhaled steroid intake, n (%) | <2.2x10−16 | |||
| No | 2079 (93.0) | 268 (70) | 1811 (97.8) | |
| Yes | 156 (7.0) | 115 (30) | 41 (2.2) | |
| Adrenal Insufficiency diagnosis, n (%) | 0.03 | |||
| No | 1640 (73.4) | 263 (68.7) | 1377 (74.4) | |
| Yes | 595 (26.6) | 120 (31.3) | 475 (25.6) | |
Missing data: In MGBB-Asthma, BMI was missing for seven subjects; inhaled steroid intake was missing for 25 subjects; oral corticosteroid intake (OCS) and asthma severity were only extracted for asthma cases (therefore corresponding P-values cannot be determined) and OCS was not available for five subjects. Data on inhaled and oral medications were not available in EPIC-Norfolk cohort.
Significance of difference was evaluated using chi-squared test for categorical variables and two-sample t-test for continuous variables. In CAMP, the categorical variables sex, race and treatment did not change with time point, therefore NA is indicated for P-values.
Abbreviations: BMI, body mass index; SD, standard deviation; CAMP, Childhood Asthma Management Program
Global metabolomic profiling and replication of findings
Nine-hundred and seventy-three metabolites remained for analysis after quality control. In EPIC-Norfolk, 35 (3.6%) were significantly associated with prevalent asthma after multiple testing corrections using Bonferroni threshold (Supplementary Table 1). Thirty-four of those were significantly reduced in asthma cases compared with controls (range of ORs=0.65-0.81; range of P-values=1.4x10−27−1.5x10−7) and were annotated to canonical curated pathways for corticosteroids, pregnenolone, and androgenic steroids (Extended Data Fig. 1A). Importantly, while only 40 of the 973 (4.1%) metabolites on the global profiling platform were annotated to steroid sub-pathways, 34 of these metabolites (85% of the total steroids measured) reached stringent Bonferroni statistical significance. Only one other metabolite, Glycosyl-N-palmitoyl-sphingosine, a sphingolipid, reached statistical significance and was associated with increased levels in asthma cases (OR=1.19, P=7.6x10−6) (Supplementary Table 1, Extended Data Fig. 1B).
Twenty-five of the 35 significant metabolites in EPIC were quantified and available for analysis in MGBB-Asthma. Seventeen of the 25 metabolite associations replicated in MGBB-Asthma with a consistent direction of effect at an FDR threshold of 5%; 15 of which also met the more stringent Bonferroni threshold (Table 2, Fig. 2A). All 17 were annotated to major steroid hormone biosynthesis sub-pathways, specifically corticosteroid, pregnenolone, and androgenic steroid pathways with their identity confirmed by Metabolon (Table 2, Extended Data Fig. 2).
Table 2.
Plasma metabolites significantly associated with asthma in EPIC-Norfolk with replication in MGBB-Asthma.
| Metabolite | Metabolite Subclass |
EPIC-Norfolk (N=10,754) | MGBB-Asthma (N=610) | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value‡ | OR | 95% CI | P-value‡ | ||
| Dehydroisoandrosterone sulfate (DHEA-S) | Androgenic Steroid | 0.65 | 0.60, 0.70 | 1.4x10−27 | 0.38 | 0.25, 0.55 | 1.1x10−6 |
| Steroid conjugate* | Corticosteroid | 0.68 | 0.61, 0.72 | 2.9x10−21 | 0.60 | 0.44, 0.81 | 1.1x10−3 |
| Cortisone | Corticosteroid | 0.72 | 0.67, 0.77 | 7.8x10−20 | 0.46 | 0.31, 0.66 | 6.2x10−5 |
| 21-hydroxypregnenolone disulfate | Pregnenolone Steroid | 0.71 | 0.65, 0.77 | 4.5x10−16 | 0.47 | 0.33, 0.63 | 2.9x10−6 |
| Epiandrosterone sulfate | Androgenic Steroid | 0.74 | 0.69, 0.80 | 1.1x10−13 | 0.41 | 0.29, 0.57 | 1.0x10−7 |
| 16a-hydroxy DHEA 3-sulfate | Androgenic Steroid | 0.81 | 0.66, 0.79 | 2.5x10−5 | 0.64 | 0.48, 0.85 | 1.9x10−3 |
| Pregnenolone sulfate | Pregnenolone Steroid | 0.73 | 0.66, 0.79 | 3.2x10−12 | 0.38 | 0.27, 0.52 | 3.4x10−9 |
| Androsterone sulfate | Androstane Steroid | 0.77 | 0.72, 0.83 | 2.1x10−11 | 0.45 | 0.33, 0.62 | 1.1x10−6 |
| Cortisol | Corticosteroid | 0.78 | 0.72, 0.84 | 3.3x10−11 | 0.53 | 0.37, 0.74 | 2.9x10−4 |
| pregnenetriol disulfate* | Pregnenolone Steroid | 0.73 | 0.69, 0.82 | 3.2x10−12 | 0.60 | 0.45, 0.79 | 4.6x10−4 |
| 17-hydroxypregnenolone sulfate* | Pregnenolone Steroid | 0.74 | 0.68, 0.82 | 7.4x10−10 | 0.48 | 0.36, 0.63 | 1.9x10−7 |
| etiocholanolone glucuronide | Androstane Steroid | 0.79 | 0.71, 0.84 | 1.5x10−7 | 0.49 | 0.37, 0.63 | 1.7x10−7 |
| 5-androstenetriol disulfate* | Androstane Steroid | 0.79 | 0.72, 0.86 | 1.5x10−7 | 0.50 | 0.38, 0.65 | 3.1x10−7 |
| Tetrahydrocorticosterone glucuronide‡‡ | Corticosteroid | 0.81 | 0.74, 0.88 | 9.9x10−7 | 0.74 | 0.58, 0.94 | 0.015 |
| Steroid conjugate*‡‡ | Corticosteroid | 0.68 | 0.75, 0.90 | 2.9x10−21 | 0.73 | 0.55, 0.95 | 0.020 |
| 5alpha-androstan-3beta, 17alpha-diol disulfate | Androgenic Steroid | 0.81 | 0.74, 0.89 | 1.8x10−5 | 0.56 | 0.44, 0.71 | 1.1x10−6 |
| 16a-hydroxy-DHEA-disulfate* | Androgenic Steroid | 0.81 | 0.73, 0.89 | 2.5x10−5 | 0.54 | 0.42, 0.70 | 2.6x10−6 |
Generalized linear model was used to compare asthma cases with controls; Statistical significance was determined using a bonferroni threshold and false discovery rate of 0.05.
The P-value thresholds for declaring significance was Bonferroni in both EPIC-Norfolk (P<5.14x10−5) and MGBB-Asthma (P<2x10−3)
Replicated metabolites in MGBB-Asthma at FDR threshold only
Abbreviations: MGBB: Mass General Brigham Biobank
Metabolite names with an asterisk * indicate that the identity of the metabolite has not been confirmed based on an analytical standard
Fig. 2. Plasma steroid metabolite associations in EPIC-Norfolk cohort and Mass General Brigham Biobank (MGBB)-Asthma cohort.
A. Plasma metabolites significantly associated with asthma in the EPIC-Norfolk cohort and replicated in the MGBB-Asthma cohort (n=17 metabolites). The dotted line indicates the cut off for odds ratio (OR) < or >1 for direction of effect. Metabolite names with an asterisk * indicate that the metabolite has not been officially confirmed based on a standard. B. Steroid metabolite associations between asthma cases and controls; asthma cases with ICS treatment and controls; asthma cases not treated with ICS and controls; and asthma cases with ICS treatment and not treated with ICS in the MGBB-Asthma cohort. The dotted line indicates the cut off for OR< or >1 for direction of effect. Individual values shown for each metabolite as odds ratios along with their lower and upper 95% confidence intervals in A and B. The legend key color shows the sub-pathway they belong to (A, B). Generalized linear model was used to compare groups (A, B); Statistical significance was determined using a bonferroni threshold and false discovery rate of 0.05 (A, B).
Abbreviations: Ast, asthma cases; ICS, inhaled corticosteroids
Discovery and Replication for Metabolite-Asthma Associations
The 34 corticosteroid, androgenic and pregnenolone steroid metabolites that were measured in MGBB-Asthma, were at lower levels in both asthma cases on and off ICS when compared with controls (Supplementary Table 2, Fig. 2B), with the largest decreases observed in asthma cases on ICS. There was also a nominally significant decrease in 12 out of 34 (35%) of steroids metabolites when comparing asthma cases with ICS treatment to those not treated with ICS (nominal p-value<0.05) (Supplementary Table 2). While progestin steroids also demonstrated these general trends, the reductions were was not as robust (Supplementary Table 2).
Two steroid metabolites, cortisol and cortisone, were measured in CAMP. While there was no significant difference in cortisol or cortisone between the treatment groups at baseline (cortisol: β=0.09; 95% CI=−0.37, 0.55; P=0.70 and cortisone: β=0.19; 95% CI=−0.30, 0.68; P=0.45), at the end of the four year clinical trial, both metabolites were associated with reduced levels in subjects randomized to low dose ICS (Budesonide) intake compared to those who received nedocromil or placebo (cortisol: β=−0.87; 95% CI=−1.50, −0.24; P=6.8x103 and cortisone: β=−0.60; 95% CI=−1.20, −0.01; P=0.04). These associations remained significant after extensive interrogation of potential confounding by asthma severity (Supplementary Table 3).
In MGBB-Asthma, significant inverse associations were observed between cortisol levels and fluticasone ICS dose measured continuously (β=−0.004; 95% CI=−0.007, −0.001; P=3.0e-03), ordinally (β=−0.29; 95% CI=−0.44, −0.14; P=2.0e-04), and in dose categories. Levels were lower in individuals on “low dose” (44-200 mcg, β=−0.22; 95% CI=−0.43, −0.01; P=0.04) and “moderate to high dose” (>200 mcg, β=−0.64; 95% CI=−0.97, −0.32; P=1.5e-04) ICS relative to those with “no ICS use”. Furthermore, the observed reductions in cortisol and cortisone observed in CAMP subjects were also a result of low-dose ICS.
Quantification of cortisol and assessment of adrenal insufficiency in the EMR
The EMR-Cortisol subjects (N=2,235) are summarized in Table 1. The asthma cases and controls differed by sex (P=3.9x10−14), race (P=1.4x10−5), ICS treatment (P<2.2x10−16) and adrenal insufficiency diagnosis (P=0.03). We determined that asthma cases on ICS treatment had the lowest minimum cortisol levels throughout a 24-hour daily period, compared to asthma cases without ICS treatment (β=−1.74 mcg/dL; 95% CI=−2.90, −0.59; P=3.3x10−3) and controls not treated with ICS (β=−2.86 mcg/dL; 95% CI=−3.96, −1.76; P=3.7x10−7). The largest differences occurred in early morning, the time when individuals are most vulnerable to asthma attacks43,44 (Fig. 3). There was no difference between asthma cases on ICS and controls on ICS (P=0.11); however, ICS controls may also have other comorbid and confounding diagnoses that make this relationship difficult to assess.
Fig. 3. Quantification of Cortisol in the EMR-Cortisol cohort.
Top, histogram showing the frequency of subjects during the indicated times of sample collection (24-hour daily period) on the x-axis of the bottom graph. Bottom, graph showing the aggregated minimum cortisol levels for four groups of subjects during the indicated times of sample collection on the x-axis. Subjects were binned into three time categories (04:00-12:00, 12:00-18:00, 18:00-04:00) based on the availability of subjects and smoothed using loess curve fitting. Legend label is colored based on four categories: Ast_ICS: asthma cases with ICS treatment (Average=4.2 mcg/dL; SD=5.2); Ast_No_ICS: Asthma cases without ICS treatment (Average=6.03 mcg/dL; SD=5.1); Controls_ICS: Controls with ICS treatment (Average=5.58 mcg/dL; SD=4.6); Controls_No_ICS: Controls without ICS treatment (Average=7.02 mcg/dL; SD=5.8). P-value for asthma cases with ICS treatment compared to controls without ICS treatment (Mean difference=−2.80 mcg/dL; 95% CI=−1.4, −4.2; P=1.9x10−6). Tukey’s HSD test was used to identify significant differences between the asthma/ICS subgroups. Pairwise comparisons between the subgroups were also performed using generalized linear models, adjusted for collection time, age, gender and race.
Abbreviations: Ast, Asthma cases; ICS, inhaled corticosteroids
Further within the EMR, there were 755 mild asthma cases with adrenal insufficiency testing, including 200 asthma cases with and 555 asthma cases without ICS use.
Of the four adrenal insufficiency symptoms tested, we observed significant increases among asthma cases with ICS use compared to those without for symptoms of fatigue (OR=2.27; 95% CI=1.61, 3.22; p=3.2x10−6) and anemia (OR=2.28; 95% CI=1.57, 3.35; p=2.0x10−5). No significant differences were observed for weight loss or hyperpigmentation (Supplementary Table 4).
DISCUSSION
In this study, we used four independent cohorts to demonstrate the translational utility of metabolomics for asthma in a precision medicine framework. There were several key findings from this work. First, substantial reductions in seventeen endogenous steroid metabolites were associated with prevalent asthma, including two primary hypothalamic-pituitary-adrenal axis (HPA) steroid hormones that are biomarkers for adrenal suppression45, DHEA-S and cortisol. Further interrogation demonstrated that this reduction was strongly associated with ICS use among asthma cases. In addition, EMR-extracted measures of cortisol demonstrated a global reduction among asthma cases with ICS use so pronounced that throughout the entire 24-hour period, the average peak cortisol levels among this group did not even attain the average lowest cortisol levels among all other groups. Second, we observed a dose-effect relationship with steroids and ICS use, and for the first time, we robustly identified a significant association between cortisol and low dose ICS use. Third, we observed that while this reduction in steroids was primarily driven by ICS use, a portion of the observed reduction was independent of ICS use, suggesting that the reduced steroid levels also represent a fundamental characteristic of pathophysiology of asthma as a disease. Finally, for asthma cases, we identified significant associations between adrenal insufficiency symptoms and ICS use.
To date, multiple studies have investigated the potential adverse side effects of ICS use for asthma; however, the overall utility of these studies to assess adrenal suppression has been hampered by poor study design, with the vast majority being case reports20,21,25-29,31, having small to modest samples sizes30, short trial periods46-48 or a limited range of ICS dose46,49. A more comprehensive interrogation is therefore recommended32. Studies focused on adrenal insufficiency and ICS use, both within asthma, and more broadly, are often limited in statistical power30 due to the low prevalence of adrenal suppression diagnoses, but have identified associations with high dose ICS use23. Taken together, while acknowledged as a potential harmful side effect, clinical suppression of the HPA-axis from ICS therapy, particularly at low doses has been considered to have minimal long-term systemic ramifications on adrenal function21,46 and while there is an acknowledged potential effect, it has been largely with regard to high dose ICS therapy23,47,50,51. Using multiple well-powered cohorts, designed to address a series of scientific inquiries, this study clarifies existing confusion and expands understanding regarding the effect of ICS therapy for asthma in multiple domains. Most importantly, we demonstrate robust evidence of the broad-based reduction in endogenous steroids and that this reduction is present even at low ICS doses. This study also considered the impact of long-term ICS use including 25 years of EMR data and a four-year clinical trial. While long-term ICS use remains a primary treatment recommendation52,53, until now, few studies have examined the impact of ICS treatment on steroid levels over this length of time, particularly in the context of both a both a rigorous RCT and with real life EMR data. Finally, this study also considered the impact of ICS on the 24-hour daily diurnal variation of cortisol by extracting cortisol measurements from baseline adrenal insufficiency testing, something that has not yet been described using large scale real-life populations.
To date, large-scale precision medicine initiatives have primarily focused on other omic data types, particularly genetics. This study clearly demonstrates the potential that metabolomics offers in the realm of precision medicine initiatives. Starting with global metabolomic profiling in large scale and well-powered epidemiological cohorts, we identified and validated a robust metabolic signature with endogenous steroid metabolites. While the following steps often necessitate additional laboratory work, in several cases, as is demonstrated here with cortisol, a clinically relevant biomarker may be immediately obvious, expediting the steps toward clinical translation. As the extent of clinically relevant metabolites, including both clinical biomarkers and drugs, identified via global metabolomic profiling continues to increase, the clinical potential of metabolomics should not go underappreciated. When considering precision medicine approaches, additional incorporation of other relevant omic findings, such as underlying genetic predisposition, should also be considered. Existing evidence suggests that the impact of ICS use may be even more extreme for individuals with genetic susceptibility to corticosteroid-induced adrenal suppression39. Identifying such at-risk individuals and considering specific treatment course modifications that are most efficacious to this group may build on the current findings to further define personalized approaches for asthma treatment specific to ICS use and may motivate future scientific research in this area. More in-depth research is suggested54 and merited and in future studies. As the heterogeneity of asthma as a disease is well established, both from clinical classifications and underlying genetic susceptibility55, analogous precision medicines recommendations may continue to optimize treatment approaches for specific groups. A central point relevant to precision medicine that may be gleaned from these findings is that metabolomics has tremendous potential to be utilized as an initial step towards precision medicine that may be further informed by other omic data types.
Treatment with ICS has formed the foundation of care for individuals with moderate to severe asthma since they were introduced into the standard management of asthma over 30 years ago19. They have been instrumental in reducing asthma exacerbations and improving overall quality of life56. Yet, while the effectiveness of ICS as a treatment for asthma should not go underappreciated, the risks of ICS use that have been further clarified and substantiated through this study, should also be considered. These findings are of particular interest given recent changes to the GINA guidelines1. In 2021, GINA released updated treatment recommendations for asthma in adolescents and adults that recommended two alternate tracks for treatment. Track 1 recommends “as needed” low dose ICS as the preferred reliever approach while Track 2 recommends short acting beta agonist (SABA) as the alternate reliever approach. The updated recommendations make a notable shift toward increased ICS use and away from SABA1, which has been the primary reliever recommendation for the last 50 years. Our findings not only found a dose effect relationship with ICS use and cortisol, but also demonstrated a significant decrease in cortisol at low ICS doses. This highlights the urgent need to quantify the impact of low dose ICS use on adrenal suppression more carefully. Such information may have a substantial impact on these updated treatment recommendations, and an overall shift in mindset towards an increased awareness of these adverse side effects and more stringent indications needed to prescribe ICS, particularly when considering higher ICS dosages and frequencies.
It is important, however, to note that simple measures can be implemented to mitigate the systemic side effects of ICS use. Clinical recommendations for regular monitoring of adrenal hormones, particularly morning cortisol measurements51,57, among individuals with ICS use is one clear and inexpensive clinical recommendation that may identify at-risk individuals and enable treatment modifications prior to significant and potentially permanent long-term complications. Critical evaluation of the prescribed ICS dose is also imperative, with a focus on identifying the minimal dose for overall symptom control. This is even more relevant as prior ICS dose research reported that in cases where maximal efficacy was achieved at a dose of 500 mcg/day, 90% of the overall benefit was achieved at doses of 100 to 250 mcg/day58,59, suggesting that for most individuals, substantial reductions in ICS dose may have minimal clinical impact while simultaneously substantially reducing the overall extent of adrenal suppression. As such, when considering the clinical implications of these findings, for asthma cases that require ICS use, regular cortisol monitoring while simultaneously utilizing the minimal ICS dose for overall symptom control, will likely provide the optimal balance between minimizing adverse adrenal suppression and capitalizing on the established benefits of ICS treatment.
Despite the strengths of the reported findings, several limitations should be noted. First, our discovery cohort (EPIC-Norfolk) did not have information on ICS use. We therefore created a robust binary variable for ICS use in MGBB-Asthma using information on prescription counts. While we did not have complete information on the specific details of ICS prescription lengths that likely leads to some misclassification; this misclassification would result in a bias towards the null hypothesis and the effects we identified would remain highly significant. We also recognize that ICS use in inherently confounded with asthma severity. We have addressed this by adjusting for measures of asthma severity in some analyses and by restricting our analysis to mild asthma cases in others. While the approaches implemented can account for part of this confounding, ultimately the use of ICS RCTs or rigorous pharmacoepidemiologic studies are required to disentangle the effects of ICS use and asthma severity more clearly. Second, we validated our findings in using the CAMP RCT, at which time the participant were adolescents, which represents a younger age range than the other cohorts in this study. It is important to note that there may be important differences between ICS response in adults and adolescents that should be studied in more detail. Despite this, our findings were consistent across these age ranges, which may point more to the overall generalizability of these findings. Third, metabolomic profiling was performed in a different laboratory for CAMP and did not have the broader range of steroid metabolites. We recognize that the variation in the breadth of metabolomic measurements varies by laboratory. Despite these differences, we were able to refine and further validate the robustness of our findings over the four populations we utilized in this study. Finally, while it is important to realize the potential of large EMR databases, it is equally important to recognize that this information is derived from an overrepresentation of individuals with illness and may bias the data or result in confounding by indication. Acknowledging this limitation, we excluded individuals with common relevant comorbid conditions, such as COPD.
This study utilized four independent cohorts with over 14,000 individuals to demonstrate the translational utility of metabolomics for asthma in a precision medicine framework. With a sequential set of scientific inquiries, we utilized global metabolomics to identify substantial reductions in endogenous steroid metabolites associated with prevalent asthma cases compared with controls. With additional clinical data extracted from EMR, as well as extracted cortisol measures, we further demonstrated that this reduction was strongly associated with ICS use among asthma cases and more substantially than previously recognized, with significant effects even at low ICS doses. With these findings, a clinical framework of recommendations specific to asthma cases with ICS use may begin. The utilization of metabolomic data in this study, demonstrates the central role it may play in establishing an initial framework for precision medicine that may be further informed by other omic data types, in addition to enhancing biologic understanding both on the side of clinicians and researchers.
METHODS
Overview of cohorts and study populations
Discovery Study Population: EPIC-Norfolk
EPIC-Norfolk60 is a subset of the EPIC60 multi-center longitudinal cohort study. It includes 25,000 men and women of predominantly European descent. At baseline (between 1993 and 1998), participants provided blood samples and completed a health and lifestyle questionnaire. Plasma samples were stored in the gas phase of liquid nitrogen at −175 degrees Celsius for long-term storage. Asthma status was ascertained based on a combination of self-report of previous diagnosis (“Has the doctor ever told you that you have any of the following: Asthma”?), and any additional cases ascertained at a baseline health check performed by a registered nurse.
Plasma metabolomic data generated on a subset of 10,754 participants were used as the discovery population in this analysis (Table 1). Of these, 661 (6%) individuals had asthma (as previously defined), while the remainder were considered non-asthmatic controls. Individuals with a self-reported previous doctor’s diagnosis of bronchitis, and/or emphysema at baseline were excluded. Participants were largely unfasted and provided consent to participate in the study.
Replication Study Population: MGBB-Asthma
The MGBB (https://biobank.partners.org) is a collection of DNA, serum, and plasma samples from 81,502 fully consented subjects linked to the Research Patient Data Registry (RPDR), a data warehouse that gathers data from multiple electronic medical record (EMR) systems and stores it in a SQL Server database. Researchers may query the RPDR using an online query tool; RPDR currently contains data on 4.6M patients, with 227M encounters, and approximately 900M distinct, coded clinical facts stored in the database dating back to 1986 including demographic data, diagnoses (e.g., ICD-9/ICD-10 codes), procedures (e.g., Current Procedural Terminology (CPT) codes), pharmacy data (e.g. RxNorm), inpatient and outpatient encounter information, provider information, laboratory data, imaging, and pathology data. We applied a validated phenotyping algorithm61 for asthma diagnosis and identified 287 individuals with asthma (positive predictive value > 85%) and 323 controls (negative predictive value > 99%) to generate the MGBB-Asthma population (total N = 610 subjects, Table 1). Completed questionnaires including the demographic details on the biospecimen collection are collected from the individuals who enroll in the Biobank. Non-fasting plasma samples for the MGBB-Asthma cohort were collected between October 2010 and March 2017 and were stored immediately (within 4 hours) in an −80 degrees freezer. Controls were randomly selected from the pool of individuals without asthma with available plasma samples.
Metabolomic Profiling for EPIC-Norfolk and MGBB-Asthma
In both studies, metabolomic profiling was conducted by Metabolon Inc. (Durham, NC, USA). The profiling methods have been described in detail62. In short, four non-targeted Liquid Chromatography Couple Mass Spectroscopy (LCMS) platforms were performed enabling the broadest coverage of the metabolome: 1) Amines and polar metabolites that ionize in the positive ion mode; 2) Central metabolites and polar metabolites that ionize in the negative ion mode; 3) Polar and non-polar lipids; 4) Free fatty acids, bile acids, and metabolites of intermediate polarity. All reagents and columns for this project were purchased in bulk from a single lot and all instruments were calibrated for mass resolution and mass accuracy daily. Coefficients of variation were measured in blinded QC samples randomly distributed among study samples, and batch variation is controlled for. Metabolites were identified by their mass-to-charge ratio (m/z), retention time (rt), and through a comparison to a library of purified known standards. Peaks were quantified using the area-under-the-(ROC) curve. Metabolite measures were median normalized across run days (with medians set to 1).
In EPIC-Norfolk, measurements were made in citrate plasma samples taken at baseline, for two sets of samples, each consisting of approximately 6,000 quasi-randomly selected individuals. Individuals with high levels of metabolite missingness were excluded. Metabolites present in at least 30 asthma cases in both measurement sets were included in the analyses. Metabolite measures were natural log transformed, windsorized at 5 SD and standardized (μ = 0, SD = 1). Analyses were performed within each of the two metabolite measurements sets individually, and results were meta-analyzed to pool the associations from the two measurement sets, using a fixed effects inverse variance weighted meta-analysis. In the MGBB-Asthma, missing metabolite values were imputed by replacement with half the minimum value for each metabolite in all samples. Metabolites that are of unknown identity (or with format X-nnnnn) can be quantified and tracked, and therefore Metabolon is confident that they represent biologically relevant molecules and not analytical artefacts. Metabolites with an interquartile range of zero were excluded from further analysis (n=129) with 904 metabolites remaining for the analysis (Supplementary Table 5).
Randomized controlled trial (RCT) of ICS: CAMP
CAMP is a double blind RCT that randomized 1,041 children with mild-to-moderate asthma aged 5 to 12 years to low dose inhaled steroid budesonide (200 mcg), nedocromil (8 mg) or matching placebos, treatment twice daily during 4.3 years of the trial63-65. Samples for the CAMP clinical trial were obtained from the clinical sites at the randomization visit between December 1993 and September 1995 and again at the end of the clinical trial between December 1997 and December 1999 and were stored in an −80-degree freezer. Cortisone and cortisol were both among the metabolites measured via global untargeted metabolomic profiling at the Broad Institute using 560 serum samples (Table 1) collected from CAMP individuals at baseline and four years later at the end of the trial. The quality control information is detailed elsewhere66. The RCT design of CAMP was used to further validate the cause-effect relationship between long-term ICS use and changes in cortisone and cortisol levels.
ICS effect and clinical cortisol measurements: EMR-Cortisol
We queried the RPDR in MGBB to identify individuals who obtained clinical cortisol measurements from cortisol testing (most often as a part of adrenal insufficiency testing, specifically the adrenocorticotropic hormone (ACTH) stimulation test). Given the diurnal variation in cortisol levels, we obtained and extracted detailed information on specifications of the cortisol measurements, including the time and date of the blood draw. For individuals with multiple cortisol measurements on different dates, we recorded the minimum, maximum, mean, and median cortisol values, only considering the initial measurement from any single visit. To avoid confounding by other medications and to avoid misclassification due to controls that may have taken ICS medications for reasons other than asthma, we used a stringent measure of ICS treatment: subjects having a count of greater than or equal to at least 10 prescriptions of their most common ICS medication were categorized as “ICS use”, while subjects with less than 10 prescription counts of their most common ICS medication were categorized as “no ICS use”. We then stratified these groups based on asthma affection status and ICS treatment, resulting in the following four categories: 1) no asthma/no ICS use; 2) no asthma/ICS use; 3) asthma/no ICS use; 4) asthma/ICS use. Subjects with COPD were excluded from this analysis. In total, we identified 2,235 individuals that were included in the EMR-Cortisol cohort (Table 1). Twenty-three of these subjects overlapped with MGBB-asthma.
Statistical Analysis
Overview of Analytic Approach
An illustration of our analytic strategy is presented in Fig. 1. Briefly, we utilized a discovery and replication approach to identify metabolites (predictor variable) associated with prevalent asthma (outcome variable), using the EPIC-Norfolk (discovery) and biobank-derived Mass General Brigham Biobank-Asthma (MGBB-Asthma) (replication) cohorts. As these findings implicated steroid-associated metabolites, we then assessed the impact of ICS use on the replicated asthma-associated metabolites within MGBB-Asthma cohort and further evaluated the impact of ICS use using cortisol and cortisone measures from the four-year ICS RCT, CAMP63,65. Using cortisol as a biomarker for adrenal suppression from ICS use, we queried the MGBB EMR to identify individuals with cortisol measures to create the EMR-Cortisol cohort and assessed the impact of ICS use on these cortisol measures. Analyses in the EPIC-Norfolk cohort were performed in Stata 14.2. All other analyses were performed in R version 4.0.367. The p-values presented are two-sided and have been adjusted for multiple comparisons. The EPIC-Norfolk study was approved by the Norwich Local Ethics Committee (REC Ref. 7898CN01). The research work for Biobank cohorts was approved by the IRB of Mass General Brigham (# 2015P000983, #2014P001109). For CAMP data, all study procedures were approved by the Institutional Review Board of the Brigham and Women’s Hospital (Protocol#: 1999-P-001549/29, the Partners Human Research Committee (PHRC), # 2002P000331, ClinicalTrials.gov Identifier: NCT00000575). All study participants provided written consent at enrollment. Consent from the guardian and assent was obtained from all children participating in the CAMP trial.
Discovery and Replication for Metabolite-Asthma Associations
Multivariable logistic regression models were employed in EPIC-Norfolk to assess the association between log-transformed metabolite levels with asthma affection status where the number of participants varied for each metabolite. Models were adjusted for age, sex, BMI and smoking status (current, former and never). We did not adjust for ethnicity, as the EPIC-Norfolk population is mostly White (99.7%). To correct for multiple testing, we applied a stringent Bonferroni significance threshold (0.05/number of statistical tests in EPIC-Norfolk). In MGBB-Asthma, multivariable logistic regression models adjusted for age, sex, race, BMI and smoking status were used to replicate the associations between the Bonferroni significant metabolites and asthma case status. An association was considered “replicated” if: 1) The effect estimate (Odds Ratio) is in the same direction as the initial association finding and 2) The False Discovery Rate (FDR)68 is <5%.
Steroid Metabolites by ICS treatment in MGBB-Asthma
Using the MGBB, we obtained information on asthma medication use (Supplementary Table 6. We created a binary measure of inhaled corticosteroid (ICS) use as the outcome using information on the total number of ICS prescriptions. To identify the optimal binary threshold, we considered several cutoffs for ICS prescription count: >0, ≥4, ≥6 and ≥10. Importantly, our findings were robust to all four prescription cutoff selections. A count of four prescriptions was selected as the threshold to define a binary cut-off for ICS. Specifically, subjects with at least four ICS prescriptions were categorized as using ICS while subjects with less than four ICS prescription counts were categorized as not treated with ICS. The following medications were utilized to create the prescription count: Beclomethasone, Dipropionate, Budesonide, Ciclesonide, Inhaled dexamethasone, Flunisolide, Fluticasone, Fluticasone/Salmeterol, Mometasone and Triamcinolone. An ordinal measure of asthma severity was created using EMR indications of mild intermittent, mild persistent, moderate persistent, and severe persistent asthma. A binary indicator of OCS use was defined by at least one prescription of the following oral medications in the past year: Dexamethasone, Methylprednisolone, Prednisolone, Prednisone. Any healthy controls with intake of ICS and individuals with COPD were excluded from the analysis.
To quantify the relative reduction in steroid metabolite levels based on ICS use, MGBB-Asthma subjects were stratified by their asthma affection status. Asthma cases were further stratified by ICS treatment. This resulted in four sub-group comparisons: 1) asthma cases versus controls; 2) asthma cases with ICS treatment versus controls; 3) asthma cases not treated with ICS versus controls; 4) asthma cases with ICS treatment versus asthma cases not treated with ICS. Multivariable logistic regression models using pairwise comparisons adjusted for age, gender, race, BMI and smoking status were utilized to compare metabolite levels between groups. Analyses comparing asthma cases with ICS and without ICS treatment were additionally adjusted for asthma severity and oral corticosteroid use in the past year.
Evaluation of the ICS dose on cortisol in MGBB-Asthma
We extracted the identified individuals from MGBB-Asthma prescribed Fluticasone propionate, over the 5 years prior to plasma collection and extracted the median fluticasone dosage level. Dose was considered both as a continuous measure and in groups defined using the GINA 20211 guidelines of “no ICS use”, “low ICS dose” (44-200 mcg) and “moderate to high ICS dose” (>200 mcg). This resulted in 82 individuals that were categorized in the “low dose” group (44-200 mcg), and 20 individuals were categorized in the “moderate to high dose” group (>200 mcg). There were 81 individuals that were categorized in the “No ICS” group.
Linear models were utilized with dose as the predictor variable, and adjusted for age, sex, race, smoking status, BMI and the frequency of the medication use while cortisol was the response variable. Dosage was measured using the actual dosage values, and as both an ordinal and categorical variable using the GINA 2021 classifications described above. A trend test was used to evaluate the significance of the ordinal dose variable on cortisol while individual estimates were generated when comparing low ICS dose use and moderate to high ICS dose to the reference group with no ICS use.
ICS effect on cortisone and cortisol using the CAMP RCT
We utilized multivariable linear regression models and individuals from CAMP to further assess the relationship between cortisol and cortisone levels in children randomized to low dose ICS (budesonide) verses those randomized to nedocromil or placebo. Considering both baseline and the end of the four-year clinical trial, the models were adjusted for age, gender, race, BMI, and an interaction variable between age and randomized ICS-use for the end of the trial model, as puberty directly influences steroid levels. We further performed a rigorous assessment of the impact of potential confounders on the ICS and steroid metabolite associations observed in CAMP, including measures of FEV1, cumulative hospitalizations, emergency room visits, total eosinophils, and total Immunoglobulin E (IgE). Because CAMP is an RCT that specifically randomized individuals to low-dose ICS budesonide (200mcg), the findings provide a direct estimate of low dose ICS use on cortisone and cortisol.
Quantification of Cortisol and adrenal insufficiency within the EMR
We investigated minimum cortisol levels (mcg/dL) recorded in EMR-Cortisol subjects throughout a 24-hour period, stratified as above on asthma status and ICS use. To account for differences in sample availability across the 24-hour period, subjects were binned into three time categories based on sample collection time: 4:00am-12:00pm, 12:00pm-6:00pm and 6:00pm-4:00am. The mean cortisol levels were subjected to smoothing interpolation using loess curve regression fitting. Tukey’s HSD69 test was used to identify significant differences between the asthma/ICS subgroups. Pairwise comparisons between the subgroups were also performed using generalized linear models, adjusted for collection time, age, gender and race.
We further queried the EMR for asthma cases that were tested for adrenal insufficiency within the last five years. Information was extracted on the presence or absence of four primary adrenal insufficiency symptoms70,71 including fatigue, weight loss, hyperpigmentation and anemia using ICD10 codes. Presence of ICS use/treatment was defined as four or more ICS prescriptions and absence of ICS was defined as no ICS prescriptions. To account for potential confounding by asthma severity, we restricted this analysis to “mild” asthma cases (n=755), as identified by a physician’s report via the EMR. Logistic regression models were applied to this set of mild asthma cases, with ICS use as the primary exposure of interest and each adrenal insufficiency symptom as the outcome, while adjusting for age, sex, race, BMI, and smoking (former and current).
Extended Data
Extended Data Fig. 1. Plasma metabolites in EPIC-Norfolk cohort.
A. Manhattan plot of metabolites sorted by their pathways on x-axis and negative log10 of P-value on the y-axis. The cut off horizontal lines on the y-axis highlight the metabolites significantly associated with asthma outcome at a P-value <0.05 and at the Bonferroni threshold (n=35 metabolites, P-value<5.14x10−5). The legend key shape and color show the direction of effect for the metabolites and the main pathway they belong to, respectively. B. Volcano Plot showing the effect size of the metabolites with OR on the x-axis and negative log10 of P-value on the y-axis. The metabolites colored in red are significant at a Bonferroni threshold of P-value<5.14x10−5. Multivariable logistic regression models were used to obtain odds ratio and p-values comparing asthma cases with controls (A, B).
Extended Data Fig. 2. Principal steroid hormone biosynthesis pathways with mineralocorticoid, glucocorticoid and androgen metabolites highlighting the replicated metabolites between EPIC-Norfolk cohort and Mass General Brigham Biobank.
Our annotated metabolites colored in red have been mapped to these pathways with their precursors or intermediates.
Abbreviations: CRH: Corticotropin releasing hormone; ACTH: Adrenocorticotropic hormone
Supplementary Material
Funding/Support and acknowledgements:
Effort from PK, JALS and STW is supported by P01HL132825 from the National Heart, Lung and Blood Institute, National Institutes of Health (NIH/NHLBI), USA. IDS is funded by the Medical Research Council (MC_UU_00006/1 - Etiology and Mechanisms). Effort for RK, DIS, MC, KM, MS and JALS is supported by R01HL123915 from the NIH/NHLBI and W81XWH-17-1-0533 from the U.S.A Department of Defense. Effort for RSK is supported by K01HL146980 from the NIH/NHLBI. Effort for SHC is supported by K01HL153941 from the NIH/NHLBI. Effort for MH and JALS is supported by R01HL141826 from the NIH/NHLBI. Effort for AD is supported by K01HL130629 from the NIH/NHLBI. Effort for AD and JALS is supported by 1R01HL152244 from the NIH/NHLBI. Effort for MM and JALS is supported by R01HL155742 from the NIH/NHLBI. Effort for HK is supported by the Jane and Aatos Erkko Foundation, the Paulo Foundation, and the Pediatric Research Foundation. Effort for KLS is supported by K08HL148178 from the NIH/NHLBI. Effort for MM is supported by R01HL139634 from the NIH/NHLBI. Effort for AW is supported by K23HL151819 from the NIH/NHLBI and Thrasher Research Fund Award (15115). Effort for ACW is supported by 1R01HD085993 from the NICHD. Effort for YV is supported by K23AI130408 from the NIAID. Effort for JALS, STW, EWK is supported by the NIH U01HG008685. Effort for PZ and CEW was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (JP19K21239), the Japanese Environment Research and Technology Development Fund (No. 5-1752), the Gunma University Initiative for Advanced Research (GIAR), the Japan-Sweden Research Cooperative Program between JSPS and STINT (grant no. JPJSBP-1201854), the Swedish Heart Lung Foundation (HLF 20180290, HLF 20200693), and the Swedish Research Council (2016-02798). The EPIC-Norfolk study (https://doi.org/10.22025/2019.10.105.00004) has received funding from the Medical Research Council (MR/N003284/1 MC-UU_12015/1 and MC_UU_00006/1) and Cancer Research UK (C864/A14136). Metabolite measurements in the EPIC-Norfolk study were supported by the MRC Cambridge Initiative in Metabolic Science (MR/L00002/1) and the Innovative Medicines Initiative Joint Undertaking under EMIF grant agreement no. 115372. We are grateful to all the participants who have been part of the project and to the many members of the study teams at the University of Cambridge who have enabled this research. We thank the staff and participants of the EPIC-Norfolk, Mass General Brigham Biobank and CAMP studies for their contributions. The Figure 1 was created with BioRender (https://biorender.com/).
Role of the Funder/Sponsor:
The external funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Competing Interests: The authors declare no competing interests.
Reporting Summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Code availability
All code for data processing and analyses are available via GitHub at:
https://github.com/CDNMBioinformatics/PartnersBiobank_asthma_metabolomics
https://github.com/CDNMBioinformatics/RPDR
Data availability
The EPIC-Norfolk data can be requested by bona fide researchers for specified scientific purposes via the study website (https://www.mrc-epid.cam.ac.uk/research/studies/epic-norfolk/). Requests for the other datasets can be made by researchers via a data use agreement for specific scientific inquiries. Datasets for the other cohorts is subject to controlled access. These restrictions apply, given the sensitive nature of patient data and the possibility of identifying individuals via the use of electronic medical records in conjunction with omic data. Please contact the corresponding author to create a data use agreement. The corresponding author will respond within 10 days to your request.
References:
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2021. Available at: www.ginasthma.org.
- 2.Masoli M, Fabian D, Holt S & Beasley R The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59, 469–478 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Becker AB & Abrams EM Asthma guidelines: the Global Initiative for Asthma in relation to national guidelines. Curr. Opin. Allergy Clin. Immunol 17, 99–103 (2017). [DOI] [PubMed] [Google Scholar]
- 4.(2018)., C. gov. CDC - Asthma - Data and Surveillance - Asthma Surveillance Data. [Google Scholar]
- 5.Greally M, Jagoe WS & Greally J The genetics of asthma. Ir. Med. J 75, 403–405 (1982). [PubMed] [Google Scholar]
- 6.Dold S, Wjst M, von Mutius E, Reitmeir P & Stiepel E Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch. Dis. Child 67, 1018–1022 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins MA, Hopper JL & Giles GG Regressive logistic modeling of familial aggregation for asthma in 7,394 population-based nuclear families. Genet. Epidemiol 14, 317–332 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Sharma S et al. The genomic origins of asthma. Thorax 69, 481–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louisias M, Ramadan A, Naja AS & Phipatanakul W The Effects of the Environment on Asthma Disease Activity. Immunol Allergy Clin North Am 39, 163–175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinke SN et al. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J 49, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly RS et al. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 151, 262–277 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RS et al. Plasma metabolite profiles in children with current asthma. Clin Exp Allergy 48, 1297–1304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGeachie MJ et al. The metabolomics of asthma control: a promising link between genetics and disease. Immunity, Inflamm. Dis 3, 224–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamko DJ, Sykes BD & Rowe BH The metabolomics of asthma: novel diagnostic potential. Chest 141, 1295–1302 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Bush A Translating Asthma: Dissecting the Role of Metabolomics, Genomics and Personalized Medicine. Indian J. Pediatr 85, 643–650 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Snowden S, Dahlen SE & Wheelock CE Application of metabolomics approaches to the study of respiratory diseases. Bioanalysis 4, 2265–2290 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Checkley W et al. Identifying biomarkers for asthma diagnosis using targeted metabolomics approaches. Respir. Med 121, 59–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pite H, Morais-Almeida M & Rocha SM Metabolomics in asthma: where do we stand? Curr. Opin. Pulm. Med 24, 94–103 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Crompton G A brief history of inhaled asthma therapy over the last fifty years. Prim. Care Respir. J 15, 326–331 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duplantier JE, Nelson RPJ, Morelli AR, Good RA & Kornfeld SJ Hypothalamic-pituitary-adrenal axis suppression associated with the use of inhaled fluticasone propionate. J. Allergy Clin. Immunol 102, 699–700 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Guilbert TW et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med 354, 1985–1997 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Allen DB Effects of inhaled steroids on growth, bone metabolism and adrenal function. Expert Rev Respir Med 1, 65–74 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Lapi F, Kezouh A, Suissa S & Ernst P The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur. Respir. J 42, 79–86 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Gurnell M, Heaney LG, Price D & Menzies-Gow A Long-term corticosteroid use, adrenal insufficiency and the need for steroid-sparing treatment in adult severe asthma. J. Intern. Med 290, 240–256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todd GR, Wright D & Ryan M Acute adrenal insufficiency in a patient with asthma after changing from fluticasone propionate to budesonide. J. Allergy Clin. Immunol 103, 956–957 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Drake AJ et al. Symptomatic adrenal insufficiency presenting with hypoglycaemia in children with asthma receiving high dose inhaled fluticasone propionate. BMJ 324, 1081–1082 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd GRG et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur. Respir. J 19, 1207–1209 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Macdessi JS et al. Adrenal crises in children treated with high-dose inhaled corticosteroids for asthma. Med. J. Aust 178, 214–216 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Santiago AH & Ratzan S Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. International journal of pediatric endocrinology 2010, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RW et al. Prevalence of hypothalamic-pituitary-adrenal axis suppression in children treated for asthma with inhaled corticosteroid. Paediatr. Child Health 17, e34–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay CM & Spratt DI Adrenal insufficiency in a woman secondary to standard-dose inhaled fluticasone propionate therapy. Endocrinol. diabetes Metab. case reports 2014, 130080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeley D Inhaled corticosteroids for asthma: guidance is inconsistent. BMJ (Clinical research ed.) 367, l6934 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Meyers DA, Bleecker ER, Holloway JW & Holgate ST Asthma genetics and personalised medicine. Lancet. Respir. Med 2, 405–415 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keskin O et al. Genetic associations of the response to inhaled corticosteroids in asthma: a systematic review. Clin. Transl. Allergy 9, 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Pacheco N, Pino-Yanes M & Flores C Genomic Predictors of Asthma Phenotypes and Treatment Response. Front. Pediatr 7, 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijverberg SJH, Farzan N, Slob EMA, Neerincx AH & Maitland-van der Zee AH Treatment response heterogeneity in asthma: the role of genetic variation. Expert Rev. Respir. Med 12, 55–65 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Cazzola M, Rogliani P, Calzetta L & Matera MG Pharmacogenomic Response of Inhaled Corticosteroids for the Treatment of Asthma: Considerations for Therapy. Pharmgenomics. Pers. Med 13, 261–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueiredo RG, Costa RS, Figueiredo CA & Cruz AA Genetic Determinants of Poor Response to Treatment in Severe Asthma. Int. J. Mol. Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawcutt DB et al. Susceptibility to corticosteroid-induced adrenal suppression: a genome-wide association study. Lancet. Respir. Med 6, 442–450 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frey LJ Data integration strategies for predictive analytics in precision medicine. Per. Med 15, 543–551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donovan BM, Bastarache L, Turi KN, Zutter MM & Hartert TV The current state of omics technologies in the clinical management of asthma and allergic diseases. Ann. allergy, asthma Immunol. Off. Publ. Am. Coll. Allergy, Asthma, Immunol 123, 550–557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbaraly T et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: analysis of two cohort studies. Sci. Rep 8, 8620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakkeheim E, Mowinckel P, Carlsen KH, Burney P& Lødrup Carlsen KC Reduced basal salivary cortisol in children with asthma and allergic rhinitis. Acta Paediatr. 99, 1705–1711 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Shin YS et al. The impact of asthma control on salivary cortisol level in adult asthmatics. Allergy. Asthma Immunol. Res 6, 463–466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorsey MJ, Cohen LE, Phipatanakul W, Denufrio D & Schneider LC Assessment of adrenal suppression in children with asthma treated with inhaled corticosteroids: use of dehydroepiandrosterone sulfate as a screening test. Ann. allergy, asthma Immunol. Off. Publ. Am. Coll. Allergy, Asthma, Immunol 97, 182–186 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Svendsen UG et al. High-dose inhaled steroids in the management of asthma. A comparison of the effects of budesonide and beclomethasone dipropionate on pulmonary function, symptoms, bronchial responsiveness and the adrenal function. Allergy 47, 174–180 (1992). [DOI] [PubMed] [Google Scholar]
- 47.Boe J, Bakke P, Rodolen T, Skovlund E & Gulsvik A High-dose inhaled steroids in asthmatics: moderate efficacy gain and suppression of the hypothalamic-pituitary-adrenal (HPA) axis. Research Council of the Norwegian Thoracic Society. Eur Respir J 7, 2179–2184 (1994). [DOI] [PubMed] [Google Scholar]
- 48.Afilalo M et al. Efficacy of inhaled steroids (beclomethasone dipropionate) for treatment of mild to moderately severe asthma in the emergency department: a randomized clinical trial. Ann. Emerg. Med 33, 304–309 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Chang CC & Tam AY Suppression of adrenal function in children on inhaled steroids. J Paediatr Child Heal. 27, 232–234 (1991). [DOI] [PubMed] [Google Scholar]
- 50.Kannisto S, Korppi M, Remes K & Voutilainen R Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. J Clin Endocrinol Metab 85, 652–657 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Ahmet A, Kim H & Spier S Adrenal suppression: A practical guide to the screening and management of this under-recognized complication of inhaled corticosteroid therapy. Allergy, asthma, Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol 7, 13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aalbers R, Vogelmeier C & Kuna P Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respir. Med 111, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 53.O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, L. P. Asthma progression and mortality: The role of inhaled corticosteroids. Eur. Respir. J (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho MH & Tantisira KG Adrenal insufficiency and ICS: genetics takes a breath. Lancet. Respir. Med 6, 407–408 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Wenzel SE Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med 18, 716–725 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Suissa S, Ernst P, Benayoun S, Baltzan M & Cai B Low-dose inhaled corticosteroids and the prevention of death from asthma. N. Engl. J. Med 343, 332–336 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Wade M et al. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur. J. Endocrinol 162, 109–113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt S et al. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ 323, 253–256 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sannarangappa V & Jalleh R Inhaled corticosteroids and secondary adrenal insufficiency. Open Respir. Med. J 8, 93–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Day N et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br. J. Cancer 80 Suppl 1, 95–103 (1999). [PubMed] [Google Scholar]
- 61.Yu S et al. Toward high-throughput phenotyping: unbiased automated feature extraction and selection from knowledge sources. J. Am. Med. Inform. Assoc 22, 993–1000 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly RS et al. Metabolomic profiling of lung function in Costa-Rican children with asthma. Biochim. Biophys. acta. Mol. basis Dis 1863, 1590–1595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control. Clin. Trials 20, 91–120 (1999). [PubMed] [Google Scholar]
- 64.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med 343, 1054–1063 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Strunk RC et al. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J. Pediatr 154, 682–687 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly RS et al. An Integrative Transcriptomic and Metabolomic Study of Lung Function in Children With Asthma. Chest 154, 335–348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Team, R. C. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. (2020). [Google Scholar]
- 68.Benjamini Y & Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 289–300 (1995). [Google Scholar]
- 69.Haynes W Tukey’s Test. in Encyclopedia of Systems Biology (eds. Dubitzky W, Wolkenhauer O, Cho K-H & Yokota H) 2303–2304 (Springer; New York, 2013). doi: 10.1007/978-1-4419-9863-7_1212 [DOI] [Google Scholar]
- 70.Neary N & Nieman L Adrenal insufficiency: etiology, diagnosis and treatment. Curr. Opin. Endocrinol. Diabetes. Obes 17, 217–223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pazderska A & Pearce SH Adrenal insufficiency - recognition and management. Clin. Med 17, 258–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The EPIC-Norfolk data can be requested by bona fide researchers for specified scientific purposes via the study website (https://www.mrc-epid.cam.ac.uk/research/studies/epic-norfolk/). Requests for the other datasets can be made by researchers via a data use agreement for specific scientific inquiries. Datasets for the other cohorts is subject to controlled access. These restrictions apply, given the sensitive nature of patient data and the possibility of identifying individuals via the use of electronic medical records in conjunction with omic data. Please contact the corresponding author to create a data use agreement. The corresponding author will respond within 10 days to your request.