Abstract
Aim
Videofluoroscopy swallow studies (VFSS) are gold standard to diagnose aspiration in children but require resources and radiation compared with clinical feeding evaluation (CFE). We evaluated their added value for diagnosis, feeding management and clinical status.
Methods
A retrospective single‐centre cross‐sectional study of children aged 0–18 years, with respiratory morbidity, referred for VFSS at a tertiary pediatric hospital.
Results
A total of 113 children, median age (range) 2.2 years (0.1–17.9), underwent VFSS. Diagnosis included chronic pulmonary aspiration (CPA), 87 (77%); neurological, 73 (64%); gastrointestinal, 73 (64%) and congenital heart disease, 42 (37%), not mutually exclusive. Forty‐six (41%) aspirated, 9 (8%) only overtly and 37 (33%) including silent aspirations. Those with CPA or cerebral palsy were more likely to have VFSS aspiration, OR 3.2 and 9.8 respectively.
Feeding recommendations after VFSS differed significantly from those based on prior CFE, p < 0.001: The rate of exclusively orally fed children rose from 65% to 79%, p = 0.006; exclusively enterally fed children from 10% to 14%; p = 0.005. During the year after VFSS, there were significantly less antibiotic courses, total and respiratory admissions.
Conclusion
In this population with high prevalence of clinically suspected CPA, VFSS altered feeding management compared with CFE and may have contributed to subsequent clinical improvement.
Keywords: chronic pulmonary aspiration, overt aspiration, silent aspiration, videofluoroscopic swallow study
Abbreviations
- CFE
clinical feeding evaluation
- CHD
congenital heart disease
- OA
overt aspiration
- OPA
oropharyngeal aspiration
- SA
silent aspiration
- SLT
speech and language therapist
- VFSS
videofluoroscopic swallow study
Key Notes.
Children at risk of chronic aspiration require tailor made feeding management to protect their pulmonary health.
In our study, videofluoroscopic swallow study (VFSS)‐ informed decision making enabled some children to switch from enteral to oral feeds, whilst requiring others to stop oral feeds.
Clinical outcomes improved over time, suggesting VFSS might add valuable insights over and above prior clinical assessment.
1. INTRODUCTION
Swallowing involves seamless co‐ordination of voluntary and involuntary neuromuscular activities propagating liquid and food boluses from the mouth through the pharynx and into the oesophagus. Neurologic, 1 , 2 developmental and structural 3 , 4 disorders are associated with swallowing dysfunction resulting in morbidity and some mortality due to aspiration of foreign material into the lung. Clinical feeding evaluations (CFE) by occupational (OT) or speech and language therapists (SLT) are important for the diagnosis of swallowing disorders, swallowing training and referral for instrumental assessment. 5 The therapist inspects the face and oropharynx for anatomic abnormalities and offers different textures, whilst closely observing the swallowing process, auscultating for respiratory sounds and noting voice quality, cough and respiratory distress. When symptoms are unambiguous and improve following intervention, children are often not referred for further evaluation.
Videofluoroscopy swallow studies (VFSS), considered the gold standard for swallowing assessment, are best performed following CFE by the collaboration between the OT or SLT and the paediatric radiologist. 6 Using a variety of radiolabelled textures, information is obtained about anatomy and function, including oro‐pharyngeal transit time, pharyngeal motility and pooling of material in the vallecula and pyriform sinuses. Although VFSS is resource intensive and involves radiation, it provides added value in cases with higher morbidity, or where silent aspiration (SA) without a corresponding protective cough reflex is suspected. 5 , 6 Literature about the relative contribution of CFE and VFSS in dysphagia management in children is scarce, and evidence of the accuracy of CFE in detecting aspirations in children is lacking. 7 Recent studies suggest that CFE may not adequately predict aspiration risk in children, 8 compared with VFSS. 9 Chronic pulmonary aspiration (CPA) can lead to chemical pneumonitis due to the irritant effect of toxic substances such as refluxed gastric contents or bacterial pneumonia. Its diagnosis is of tantamount importance in children, since left untreated it can lead to irreversible lung damage, for example bronchiectasis. This is highly prevalent in children with CPA and can develop rapidly in young children. 10 When identified early, interventions aimed at preventing further airway damage may minimise morbidity and mortality in patients with CPA.
The present study aimed to determine the influence of VFSS on feeding management, compared with prior decisions based on CFE and to evaluate clinical status 1 year after VFSS‐directed feeding intervention, as compared to 1 year before.
2. METHODS
2.1. Setting and study population
This was a retrospective single‐centre cross‐sectional study of children who underwent VFSS at a tertiary pediatric hospital (SCMCI). Children had been referred from community settings, ambulatory clinics of SCMCI or following in‐patient evaluation at SCMCI. Included were all children who successfully completed a VFSS between the ages of 0 and 18 years over a period of 7 years. Excluded were children who failed the VFSS technically or due to lack of co‐operation. The study was approved by the local Institutional Review Board, number 0516–17‐RMC.
Medical records were reviewed and coded for chiefly affected organ systems using a modified version of Burklow et al 11 which includes structural abnormalities, neurological conditions, behavioural issues, cardiorespiratory problems and metabolic dysfunction. We did not include behavioural and metabolic categories but added gastrointestinal and genetic categories. Affected organ systems were not mutually exclusive. We further grouped patients according to specific medical diagnoses, again not mutually exclusive, including Down's syndrome, cerebral palsy, developmental delay, tracheo‐esophageal fistula, congenital heart disease, preterm birth (prior to 37 completed weeks), bronchopulmonary dysplasia and clinically suspected chronic pulmonary aspiration.
2.2. Clinical feeding evaluation
When a clinician suspects pulmonary aspiration, our hospital protocol prescribes referral to the OT in their ambulatory clinic at SCMCI for the purpose of feeding assessment. This usually includes a number of sessions and close work with the family to accurately identify and treat the various aspects of dysphagia. Those children for whom sufficient information can be gathered clinically are treated without further investigations. When doubt as to the existence or nature of the dysphagia remains, children are discussed between clinician and OT and referred on to VFSS.
2.3. Videofluoroscopy swallow study
The VFSS was performed with collaboration between an OT and a radiologist in the fluoroscopy suite, using barium to label a variety of food stuffs: thin liquid, thick liquid, purees and solids as appropriate for the age and skill of the child. The OT participating in the VFSS was not blinded to the prior CFE outcome as this information was important for performing the VFSS optimally. Children were scanned using a Siemens Axiom Iconos R200 Fluoroscopy system at a frame rate of 15 per second. Barium sulfate for suspension 98% w/p for oral use (E‐Z‐EM Canada) was diluted with food liquids and solids according to the child's capabilities. Radiation emission in MSv units was recorded, as an approximation due to variations in screening time and body surface area exposed.
Despite the difficulty to imitate the natural feeding environment in the child's home, it was attempted to create a similar milieu, by having the child fed by their caregiver and positioned on a purpose‐built adaptable chair, adjusted to mimic the usual position (Figure 1). Since nasogastric tubes impair swallowing, 12 they were removed prior to the examination. Pulsed serial radiographs of the oropharynx and oesophagus were taken from a lateral view. The distal oesophagus and stomach were included for assessment of reflux. To reduce radiation exposure, when the history suggested aspiration developing with fatigue, multiple swallows were initially performed without fluoroscopy using a safe substance, with a later repetition of fluoroscopic swallow using the highest risk substance.
FIGURE 1.

Purpose‐built adaptable chair, adjustable in a way that mimics the child's usual feeding position
2.4. Coding of clinical feeding evaluation and videofluoroscopy
An independent OT retrospectively reviewed the prose CFE and VFSS reports and scored them using an eight‐point penetration‐aspiration scale for each fluid/ food consistency trialled, which had been validated and previously used in children. 13 , 14 The score was simplified to facilitate comparison between CFE and VFSS 6 (Table 1). For this purpose, penetration was regarded as ‘no aspiration’. Aspiration events were classified as ‘overt aspiration’ or ‘silent aspiration’. A clinical diagnosis of suspected silent aspiration (SA) referred to clues by history and subtle signs such as a wet/ phlegmy vocal quality, lack of speech, a decrease in alertness, drooling, difficulty controlling secretions and an absent gag reflex. 15 Radiologically, SA was scored for detection of material below the level of the true vocal folds without cough or other laryngeal response within 20 s. 16
TABLE 1.
Eight‐point penetration‐aspiration scale, designed for videofluoroscopy swallow studies and adapted for clinical assessment
| Category | Score | Description (radiological) | Description (clinical) |
|---|---|---|---|
| No penetration or aspiration | 1 | Contrast does not enter the airway | No aspiration |
| Penetration | 2 | Contrast enters the airway, remains above vocal folds, no residue | |
| 3 | Contrast remains above vocal folds, visible residue remains | ||
| 4 | Contrast contacts vocal folds, no residue | ||
| 5 | Contrast contacts vocal folds, visible residue remains | ||
| Aspiration | 6 | Contrast passes glottis, no sub‐glottic residue visible | Overt aspiration |
| 7 | Contrast passes glottis, visible sub‐glottic residue despite patient's response | ||
| 8 | Contrast passes glottis, visible sub‐glottic residue, absent patient response | Suspected silent aspiration |
For both CFE and VFSS, children were classified as displaying ‘no’, ‘overt’ or ‘silent’ aspiration based on the most pathological behaviour encountered, ‘silent aspiration’ being the most severe form. Thus, silent aspiration with any texture overrode overt aspiration as the classification, even when the latter occurred with another texture.
2.5. Feeding interventions
Following CFE, feeding interventions consisted of any combination of texture adaptation, interface adaptation, oro‐motor stimulation, positioning or a recommendation to remain on enteral feeds only. When more than one CFE was carried out, the assessment closest in time to the VFSS was used for comparison.
The mode of feeding recommended was noted following the CFE (pre‐VFSS) and again post—VFSS. It was categorised as oral, mixed oral with nasogastric tube (NGT) or percutaneous endoscopic gastrostomy (PEG) supplements, or enteral (NGT or PEG) only. In defining changes in management, we simplified the scoring to ‘oral’ for exclusively orally fed children and ‘enteral’ for mixed and purely enterally fed children.
2.6. Clinical status
Medical records from the 12 months preceding and following the VFSS were reviewed to determine the number, nature and length of hospital admissions, primary care and emergency department visits and number of antibiotic courses prescribed.
2.7. Statistics
Data were analysed using SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Demographic factors, organ systems and medical diagnoses were summarised with descriptive statistics. For comparison of difference between two sub‐groups, independent samples t‐test was used when normal distribution was assumed; otherwise, a Mann–Whitney test was used. The findings of the CFE were compared with VFSS data using Fisher's exact test or chi‐square test. Multivariate analysis for medical diagnoses was conducted using a logistic regression model. The McNemar chi‐square test was used to assess the change in feeding route following VFSS.
3. RESULTS
3.1. Study population
Of 128 children referred for VFSS, 113 performed it successfully (Figure 2). Median age was 2.2 years (range 0.1–17.9). Forty‐six of 113 (41%) had oropharyngeal aspiration (9 [8%] overt and 37 [33%] silent), whilst 67 (59%) showed no evidence of aspiration.
FIGURE 2.

Flow diagram of examined patient population including results of clinical feeding evaluation and video‐fluoroscopic swallow studies
At the time of VFSS, 39 (35%) children were fed enterally: 8 gastrostomy, 2 gastro‐jejunal or naso‐jejunal, 1 nasogastric and 28 mixed oral and enteral. The remaining children were fed orally. No child had a tracheostomy.
Organ systems mainly affected and medical diagnoses are shown in Table 2. Most children, 98 out of 113 (87%) had cardiopulmonary involvement, with chronic pulmonary aspiration in 87 (77%) and congenital heart disease in 42 (37%). The latter was associated with at least one other diagnosis in 39 out of 42 (93%) cases. Frequent gastro‐intestinal, 73 (64%) and neurological involvement, 68 (60%) indicate a high prevalence of complex developmental disorders, such as genetic syndromes and cerebral palsy, often resulting in dysphagia. The structural group included children with cleft palate, laryngeal cleft and tracheo‐esophageal fistula.
TABLE 2.
Aspiration status according to videofluoroscopy swallow study per patient, according to organ systems and medical diagnoses
| Category | Total | No aspiration | Overt aspiration | Silent aspiration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 113 | % a | n = 67 | % b | % c | n = 9 | % b | % c | n = 37 | % b | % c | |
| Demographics | |||||||||||
| Age ≤1 | 22 | 20% | 11 | 16% | 50% | 1 | 11% | 5% | 10 | 27% | 45% |
| Age >1 | 91 | 80% | 56 | 84% | 61% | 8 | 89% | 9% | 27 | 73% | 30% |
| Male | 67 | 61% | 43 | 64% | 63% | 6 | 67% | 10% | 18 | 49% | 27% |
| Female | 46 | 39% | 24 | 36% | 53% | 3 | 33% | 6% | 19 | 51% | 41% |
| Organ system | |||||||||||
| Neurology | 68 | 68% | 32 | 48% | 47% | 5 | 56% | 7% | 31 | 84% | 46% |
| GI | 73 | 64% | 41 | 61% | 56% | 7 | 78% | 9% | 25 | 68% | 35% |
| Cardiopulmonary | 98 | 87% | 55 | 82% | 56% | 7 | 78% | 7% | 36 | 97% | 37% |
| Genetic | 36 | 32% | 19 | 28% | 53% | 1 | 11% | 3% | 16 | 43% | 44% |
| Structural | 18 | 16% | 15 | 22% | 83% | 0 | 0% | 0% | 3 | 8% | 17% |
| Medical diagnoses | |||||||||||
| Down's Syndrome | 14 | 12% | 7 | 10% | 50% | 1 | 11% | 7% | 6 | 16% | 43% |
| CP | 21 | 19% | 6 | 9% | 29% | 4 | 44% | 19% | 11 | 30% | 52% |
| Dev delay | 37 | 32% | 18 | 26% | 49% | 5 | 56% | 13% | 14 | 37% | 38% |
| Congenital heart ds | 42 | 37% | 24 | 15% | 57% | 3 | 33% | 7% | 15 | 41% | 36% |
| Preterm <37 weeks | 16 | 14% | 10 | 69% | 63% | 1 | 11% | 6% | 5 | 14% | 31% |
| Aspiration lung ds | 87 | 78% | 46 | 3% | 53% | 6 | 67% | 7% | 35 | 95% | 40% |
| BPD | 3 | 3% | 2 | 67% | 0 | 0% | 0% | 1 | 3% | 33% | |
Legend: a indicates percentages of categories for the entire group, not mutually exclusive. b indicates the percentage of categories (demographics/organ systems/medical diagnoses) represented in each of the three aspiration modes (no aspiration, overt aspiration and silent aspiration); for example out of 67 children that did not aspirate, 32 (48%) had neurological abnormalities, 41 (61%) GI abnormalities, etc. c indicates percentage of aspiration status relative to category; for example 32 out of 68 (47%) of all children with neurological problems did not aspirate, 5 (7%) and 31 (46%) showed overt and silent aspirations respectively.
Abbreviations: BPD, broncho‐pulmonary dysplasia; CP, cerebral palsy; Dev delay, developmental delay; GI, gastro‐intestinal.
Percentage of total group.
Percentage of category (demographics/organ systems/medical diagnoses) relative to aspiration status.
Percentage of aspiration status (no aspiration, overt aspiration and silent aspiration) relative to category.
3.2. Predictive value of clinical feeding evaluation
A flow diagram including the results of CFE and VFSS is shown in Figure 2. A normal VFSS was predicted by prior normal CFE in 51 out of 67 (76%) cases. Abnormal VFSS included an element of silent aspiration in 37 out of 46 (80%) children with aspiration. Although 31/37 (84%), had a prior abnormal CFE, 18 of these 31 (58%) had been classified as overt aspirators, possibly due to overt aspiration with some food textures whilst silent aspirations were missed. Of greatest concern, a total of 10 patients had been deemed free of aspiration according to CFE and later found to have abnormal VFSS (4 overt and 6 silent).
3.3. Aspiration status per videofluoroscopy swallow study
The aspiration status per VFSS, according to demographics, organ system involvement and medical diagnoses is shown in Table 2. Throughout, overt aspirations were the least frequent finding, compared with no aspiration and silent aspiration. Only 1/22 infants under 1 year, showed overt aspiration alone on VFSS, whilst 10 (45%) had silent aspirations. According to CFE for these 10, only three had suspected silent aspiration, five had overt aspirations alone and two had no aspirations.
The silent aspiration group had a high representation of neurological and genetic abnormalities, and particularly of clinically suspected aspiration pneumonias. Structural abnormalities were associated with the lowest rates of aspiration.
Figure 3 shows types of aspiration per texture trailed, as identified by VFSS. Among 113 children, 89 were evaluated with thin liquid, 70 with thick liquid, 60 with thin puree, 67 with thick puree and 47 with solids. For all consistencies, ‘no aspiration’ was the most common, and ‘overt aspiration’ the least common result. Of note, 25/70 (36%) of subjects had silent aspiration with thick liquid. The fewest aspirations were noted with solids, with all 5/47 (11%) showing silent aspirations.
FIGURE 3.
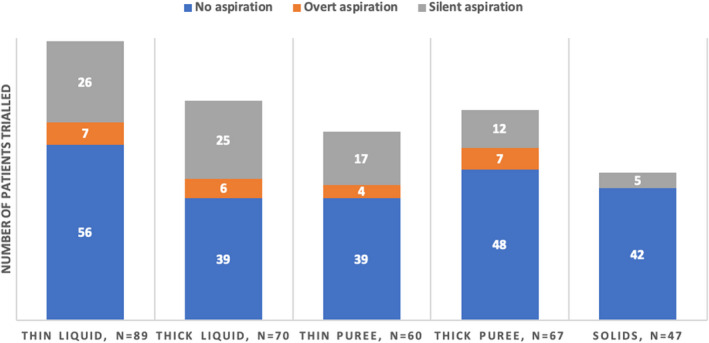
Aspiration status—no aspirations, overt or silent aspirations—according to food texture trialled, as identified on videofluoroscopy swallow study. N = 113
A logistic regression model, relating medical diagnoses to the presence of aspiration (combined overt and silent), according to VFSS is shown in Table 3. It shows that children with chronic pulmonary aspiration and cerebral palsy were more prone to VFSS aspiration, with an OR of 3.2 [95% CI = 1.4–12.4] and 9.8 [95% CI = 2.2–42.8] respectively. These observations hold true following adjustment for age.
TABLE 3.
Logistic regression model describing the relationship between medical diagnoses and the presence of aspiration (combined overt and silent), according to VFSS
| Medical diagnoses | OR | 95% CI | p value | AOR | 95% CI | p value |
|---|---|---|---|---|---|---|
| CP | 9.8* | 2.2–42.8 | 0.002 | 10.21* | 2.27–45.95 | 0.002 |
| Aspiration lung disease | 3.2* | 1.4–12.4 | 0.009 | 3.3* | 1.82–12.7 | 0.008 |
| Developmental delay | 1.8 | 0.6–4.9 | 0.3 | 1.64 | 0.58–4.65 | 0.351 |
| Congenital heart disease | 1.7 | 0.5–5.7 | 0.4 | 1.61 | 0.47–5.53 | 0.444 |
| Preterm <37 weeks | 0.2 | 0.04–1.5 | 0.1 | 0.24 | 0.04–1.41 | 0.115 |
| Down's Syndrome | 0.8 | 0.2–3.9 | 0.7 | 0.77 | 0.15–3.88 | 0.755 |
| BPD | 0.6 | 0.02–14.6 | 0.7 | 0.59 | 0.23–14.9 | 0.741 |
Abbreviations: AOR, age adjusted odds ratio; BPD, broncho‐pulmonary dysplasia; CI, confidence interval; CP, cerebral palsy; OR, odds ratio.
Significant at p < 0.05.
3.4. Feeding interventions
Following 101 out of 113 CFEs, and prior to the VFSS, the OT suggested changes in the way the child was fed, most frequently texture adaptation 89/113 (79%) but also interface adaptation, oro‐motor stimulation and positioning. Five children were instructed to stop oral feeds following CFE. Of these five, four had previously been exclusively orally fed and one on mixed feeds. The recommendation was sustained in two of those five children following VFSS, whist it was found safe to resume oral feeding in the remaining three children. Following VFSS, 12 children were asked to stop oral feeding, only two having received that recommendation following CFE. Six had previously been exclusively orally fed and six mixed fed. Taking all feeding route recommendations post VFSS together, they differed significantly from those based on the preceding CFE, p < 0.001. The rate of exclusively orally fed children rose from 65% to 79%; p = 0.006 following VFSS, whilst the rate of exclusively enterally fed children also increased, from 10% to 14%; p = 0.005.
3.5. Clinical status 1 year pre vs. 1 year post VFSS
In the year following VFSS, there were significantly less total and respiratory related hospital admissions, and less antibiotic courses were administered, see Table 4. Although the number of intensive care admissions also decreased, and community visits increased, neither of these reached statistical significance.
TABLE 4.
Clinical outcomes 1 year pre vs. 1 year post VFSS, per patient (n = 113). Values displayed as mean (SD)
| Outcome | Year pre VFSS | Year post VFSS | p‐value |
|---|---|---|---|
| Total no of admissions | 3.42 (3.42) | 2.33 (2.59) | <0.001 |
| No of urgent admissions | 2.05 (2.1) | 1.12 (1.05) | <0.001 |
| Total days of admission | 20.92 (13.66) | 9.78 (7.54) | <0.001 |
| Respiratory admissions | 1.54 (0.87) | 0.97 (0.66) | 0.04 |
| Intensive care admissions | 0.28 (0.18) | 0.19 (0.09) | N/S |
| Total community visits | 14.64 (9.65) | 16.49 (10.46) | N/S |
| Respiratory community visits | 2.44 (2.27) | 2.3 (2.02) | N/S |
| Emergency room visits | 3.69 (3.24) | 3.12 (2.86) | N/S |
| Antibiotic courses | 2.88 (2.56) | 1.8 (0.89) | <0.001 |
4. DISCUSSION
In this retrospective cross‐sectional study examining children with suspected aspiration and a high prevalence of respiratory morbidity, we found that feeding management recommendations based on VFSS differed considerably from those based on prior CFE. Our study is one of few that describes clinical outcomes following VFSS guided interventions. 3 , 4 , 8 , 9 , 11 Although there may be some spontaneous improvement over time, the significant decrease in hospitalisations and requirement for antibiotics within 1 year of feeding intervention is noteworthy.
Our findings of missed aspirations by CFE as well as ‘incorrect’ feeding decisions compared with the gold standard, suggest that VFSS should be considered for cases of significant pulmonary morbidity and suspected aspiration. The OT‐suspected silent aspirations on CFE based on indirect clues such as a wet voice, 15 , 17 but missed over 20% of children with abnormal VFSS. Conversely, over 20% of those with normal VFSS were considered to have aspiration based on CFE. Nevertheless, as discrepancies may in part be due to the intermittent nature of aspiration including during evaluation, collaboration between the managing pulmonologist and the OT who reviews the child one or more times, remains essential in addition to VFSS. 18
‘No aspiration’ was the most frequent finding on VFSS with all consistencies trialled. In this regard, VFSS provides important affirmation of safety for oral feeding, when this is in doubt. The paucity of overt aspiration alone detected by VFSS may reflect management of these cases following CFE alone, as previously recommended 19 without children having been referred to VFSS and thus not part of our study.
Up to 90% of children with neurological and maturational disorders, for example familial dysautonomia or Down's syndrome have silent aspiration on VFSS. 20 In our population, too, these groups showed far more silent than overt aspirations.
Of infants under 1 year, a critical time in terms of oro‐motor skill acquisition, 21 , 22 almost half showed silent aspirations, whereas overt aspirations were rarely observed. Most had neurological and genetic abnormalities, as well as clinical aspiration pneumonias. As expected, structural defects of the airway did not give rise to silent aspirations, as these children cough upon exposure to foreign material in the airway. 6
The contribution of laryngeal penetration, remaining above the vocal cords, 23 to chronic pulmonary aspiration is debated. In a recent study, it appeared significant as subsequent thickening of feeds was associated with decreased symptoms and hospitalisation. 24 Although we did not count penetration as aspiration in this study, scores of 4 or 5 on the penetration‐aspiration scale, representing penetration, might well have been associated with a cough response or change in voice, possibly leading to CFE diagnosis of overt or silent aspiration.
There are few reports on the outcome following VFSS‐guided feeding intervention. We have shown that children improved clinically across a number of domains in the year following such integrated feeding management. This is in keeping with a large recent retrospective cohort study in young infants showing that thickening feeds after observing silent aspirations on VFSS reduced the risk of acute respiratory infection. 24 , 25
Broader considerations including parental concerns and choices should be part of management decisions. 26 Feeding represents a key channel of communication which families are often reluctant to forsake, even knowing their child might be at risk of aspiration. Clinicians must be sensitive to this complex interaction. Our study was not designed to take these issues into consideration, but the first step is certainly an accurate diagnosis of the extent and nature of aspiration.
Bearing in mind our obligation to keep the radiation dose as low as reasonably achievable (ALARA), 27 VFSS was performed by intermittent screening, which might risk missing penetration and aspiration events. New technologies, such as low‐dose digital pulsed video‐fluoroscopic swallow examinations, might reduce radiation doses even further. 28
Our study has a number of limitations, in particular, its retrospective design and lack of control group. To enable comparison between clinical and radiological assessments, the validated 8‐point penetration‐aspiration score had to be adapted. Although we took care to apply the score consistently and based on evidence, some uncertainly about the clinical categorisation remains. This cannot be completely avoided as it is this uncertainty that often leads to referral to VFSS. We demonstrated clinical improvement within a short period of time following the VFSS, however swallowing dysfunction does tend to improve spontaneously with time and maturation. 22 This might have introduced a bias resulting from ‘regression to the mean’, but availing a control group without the use of VFSS even in a prospective study would clearly be unethical. Our patient group consisted exclusively of children referred to VFSS and not those managed by OT and CFE alone, providing a bias to more severe cases. Conversely, there was insufficient VFSS data on seven children due to poor co‐operation, possibly skewing our cohort towards ‘milder’ cases who could cooperate. A further limitation lies in the fact that children underwent CFE and VFSS on different occasions and each of these represent brief glimpses of a complex reality with intermittent aspirations occurring during both methods of evaluation. This is exemplified by the finding that five patients with overt aspirations detected clinically, displayed only silent aspirations during VFSS.
To conclude, in our selected population of children with a high prevalence of chronic pulmonary aspiration, VFSS resulted in frequent change in feeding route compared with prior CFE alone and may have contributed to the clinical improvement observed. We suggest to consider VFSS as part of an integrative approach to feeding management when aspiration is suspected.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to declare.
AUTHOR CONTRIBUTION
Dr Stafler had primary responsibility for protocol development, patient screening, enrolment, outcome assessment, preliminary data analysis and writing the manuscript. Dr Akel participated in the development of the protocol and was responsible for data collection and tabulation. Drs Mei‐Zahav, Levine, Grozovski, Mrs Eshel and Shimoni participated in the development of the protocol and analytical framework for the study and contributed to the writing of the manuscript. Drs Gendler, Blau and Prais supervised the design and execution of the study, performed the final data analyses and contributed to the writing of the manuscript.
Stafler P, Akel K, Eshel Y, et al. Videofluoroscopy compared with clinical feeding evaluation in children with suspected aspiration. Acta Paediatr. 2022;111:1441–1449. doi: 10.1111/apa.16338
Patrick Stafler and Khaled Akel contributed equally to this manuscript.
Funding information
This study has had no funding.
REFERENCES
- 1. Tutor JD, Gosa MM. Dysphagia and aspiration in children. Pediatr Pulmonol. 2012;47(4):321‐337. [DOI] [PubMed] [Google Scholar]
- 2. Schwarz SM, Corredor J, Fisher‐Medina J, Cohen J, Rabinowitz S. Diagnosis and treatment of feeding disorders in children with developmental disabilities. Pediatrics. 2001;108(3):671‐676. [DOI] [PubMed] [Google Scholar]
- 3. Sheikh S, Allen E, Shell R, et al. Chronic aspiration without gastroesophageal reflux as a cause of chronic respiratory symptoms in neurologically normal infants. Chest. 2001;120(4):1190‐1195. [DOI] [PubMed] [Google Scholar]
- 4. Heuschkel RB, Fletcher K, Hill A, Buonomo C, Bousvaros A, Nurko S. Isolated neonatal swallowing dysfunction: a case series and review of the literature. Dig Dis Sci. 2003;48(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 5. Breton SMaS, editor. Infant and Child Feeding adn Swallowing. American Occupational Therapy Association, Inc.; 2013. [Google Scholar]
- 6. Weir KA, McMahon S, Taylor S, Chang AB. Oropharyngeal aspiration and silent aspiration in children. Chest. 2011;140(3):589‐597. [DOI] [PubMed] [Google Scholar]
- 7. Calvo I, Conway A, Henriques F, Walshe M. Diagnostic accuracy of the clinical feeding evaluation in detecting aspiration in children: a systematic review. Dev Med Child Neurol. 2016;58(6):541‐553. [DOI] [PubMed] [Google Scholar]
- 8. Duncan DR, Mitchell PD, Larson K, Rosen RL. Presenting signs and symptoms do not predict aspiration risk in children. J Pediatr. 2018;201:141‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva‐Munhoz Lde F, Buhler KE, Limongi SC. Comparison between clinical and videofluoroscopic evaluation of swallowing in children with suspected dysphagia. Codas. 2015;27(2):186‐192. [DOI] [PubMed] [Google Scholar]
- 10. Piccione JC, McPhail GL, Fenchel MC, Brody AS, Boesch RP. Bronchiectasis in chronic pulmonary aspiration: risk factors and clinical implications. Pediatr Pulmonol. 2012;47(5):447‐452. [DOI] [PubMed] [Google Scholar]
- 11. Burklow KA, Phelps AN, Schultz JR, McConnell K, Rudolph C. Classifying complex pediatric feeding disorders. J Pediatr Gastroenterol Nutr. 1998;27(2):143‐147. [DOI] [PubMed] [Google Scholar]
- 12. Alnassar M, Oudjhane K, Davila J. Nasogastric tubes and videofluoroscopic swallowing studies in children. Pediatr Radiol. 2011;41(3):317‐321. [DOI] [PubMed] [Google Scholar]
- 13. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration‐aspiration scale. Dysphagia. 1996;11(2):93‐98. [DOI] [PubMed] [Google Scholar]
- 14. Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration‐aspiration scale. Dysphagia. 1999;14(4):228‐232. [DOI] [PubMed] [Google Scholar]
- 15. Garon BR, Sierzant T, Ormiston C. Silent aspiration: results of 2,000 video fluoroscopic evaluations. J Neurosci Nurs. 2009;41(4):178‐185; quiz 86‐7. [PubMed] [Google Scholar]
- 16. Arvedson J, Rogers B, Buck G, Smart P, Msall M. Silent aspiration prominent in children with dysphagia. Int J Pediatr Otorhinolaryngol. 1994;28(2–3):173‐181. [DOI] [PubMed] [Google Scholar]
- 17. Rosenfeld M, Emerson J, Williams‐Warren J, et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(3):359‐365. [DOI] [PubMed] [Google Scholar]
- 18. Boesch RP, Daines C, Willging JP, et al. Advances in the diagnosis and management of chronic pulmonary aspiration in children. Eur Respir J. 2006;28(4):847‐861. [DOI] [PubMed] [Google Scholar]
- 19. Suiter DM, Leder SB, Karas DE. The 3‐ounce (90‐cc) water swallow challenge: a screening test for children with suspected oropharyngeal dysphagia. Otolaryngol Head Neck Surg. 2009;140(2):187‐190. [DOI] [PubMed] [Google Scholar]
- 20. Jackson A, Maybee J, Moran MK, Wolter‐Warmerdam K, Hickey F. Clinical characteristics of dysphagia in children with down syndrome. Dysphagia. 2016;31(5):663‐671. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez K, Spittle AJ, Slattery JM, Morgan AT. Oromotor feeding in children born before 30 weeks’ gestation and term‐born peers at 12 months’ corrected age. J Pediatr. 2016;178:113‐118 e1. [DOI] [PubMed] [Google Scholar]
- 22. Lau C. Development of suck and swallow mechanisms in infants. Ann Nutr Metab. 2015;66(Suppl 5):7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brodsky L. Dysphagia with respiratory/pulmonary presentation: assessment and management. Semin Speech Lang. 1997;18(1):13‐22; quiz ‐3. [DOI] [PubMed] [Google Scholar]
- 24. Duncan DR, Larson K, Davidson K, May K, Rahbar R, Rosen RL. Feeding interventions are associated with improved outcomes in children with laryngeal penetration. J Pediatr Gastroenterol Nutr. 2019;68(2):218‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coon ER, Srivastava R, Stoddard GJ, Reilly S, Maloney CG, Bratton SL. Infant videofluoroscopic swallow study testing, swallowing interventions, and future acute respiratory illness. Hosp Pediatr. 2016;6(12):707‐713. [DOI] [PubMed] [Google Scholar]
- 26. Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Dev Disabil Res Rev. 2008;14(2):118‐127. [DOI] [PubMed] [Google Scholar]
- 27. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. 2007;37(2–4):1‐332. [DOI] [PubMed] [Google Scholar]
- 28. Weiss J, Notohamiprodjo M, Neumaier K, et al. Feasibility of low‐dose digital pulsed video‐fluoroscopic swallow exams (VFSE): effects on radiation dose and image quality. Acta Radiol. 2017;58(9):1037‐1044. [DOI] [PubMed] [Google Scholar]


