Abstract
Background.
Genetically determined hypoparathyroidism can lead to life-threatening episodes of hypocalcemia and, more rarely, to end-stage kidney disease at a young age. Parathyroid allotransplantation is the only curative treatment, and in patients already receiving immunosuppression for kidney transplantation, there may be little additional risk involved. We report the first such case in a child.
Methods.
An 11-y-old girl, known to have hypoparathyroidism secondary to an activating pathogenic variant in the calcium-sensing receptor, developed end-stage kidney disease and was started on intermittent hemodialysis. Since the age of 2.5 y, she had been receiving treatment with exogenous synthetic parathyroid hormone (PTH). In June 2019, at the age of 11.8 y, she received a living-donor kidney and simultaneous parathyroid gland transplant from her father. The kidney was implanted into the right iliac fossa, followed by implantation of the parathyroid gland into the exposed rectus muscle.
Results.
The kidney graft showed immediate function while the intrinsic serum PTH level remained low at 3 ng/L. Exogenous PTH infusion was reduced on day 6 posttransplantation to stimulate PTH production by the new gland, which resulted in improving intrinsic PTH concentrations of 28 ng/L by day 9. Twelve months after transplantation, PTH levels remain in normal range and the kidney graft function is stable with a serum creatinine of 110 μmol/L.
Conclusions.
Simultaneous living donation and transplantation of a kidney and a parathyroid gland into a child is safe and feasible and has the potential to cure primary hypoparathyroidism as well as kidney failure.
INTRODUCTION
Hypoparathyroidism is characterized by inadequately low concentrations of parathyroid hormone (PTH), resulting in decreased calcium reabsorption, decreased bone turnover, and decreased conversion of 25-hydroxyvitamin D to 1,25 hydroxyvitamin D. Consequently, affected patients present with hypocalcemia and hyperphosphatemia.1 Congenital hypoparathyroidism is rare but can occur secondary to genetic disorders, like autosomal dominant hypocalcemia (ADH) type 1 or type 2, which are caused by pathogenic variations in the gene encoding for the Ca2+-sensing receptor (CASR), resulting in abnormal activation of its downstream signaling.1,2 Hypoparathyroidism in children can also occur secondary to autoimmune diseases, and in some cases, the cause of hypoparathyroidism remains undiscovered.3
Hypoparathyroidism can lead to life-threatening episodes of acute hypocalcemia, which can present with convulsions, arrhythmias, cardiac failure, or laryngeal spasm. Chronic hypocalcemia can additionally cause development of cataracts, basal ganglia calcification, as well as characteristic skin changes (eg, dry skin, coarse hair, brittle nails). Conventional medical management with calcitriol can lead to development of nephrocalcinosis, which, if progressive and severe, can result in end-stage kidney disease (ESKD) at a young age.1,4 Dialysis-dependent kidney failure may also result despite no discernible change in severity of nephrocalcinosis and, as such, the exact mechanism of ESKD in patients with hypoparathyroidism remains unknown. Symptomatic treatment requires oral and, in life-threatening situations, rapid intravenous administration of calcium as well as supplementation with 1,25 hydroxyvitamin D analogs. Currently, there is no established long-term physiological treatment to replace nonfunctioning, or abnormally functioning, parathyroid tissue, and furthermore, complications secondary to hypoparathyroidism have been shown to severely impair the patient’s quality of life.4
Autotransplantation of fragments of the parathyroid gland following 4-gland (total) parathyroidectomies, and without the need for revascularization, has been demonstrated to be a feasible way to prevent and treat patients with hypoparathyroidism.5 In the context of hypoparathyroidism and kidney failure, cases of allotransplantation of parathyroid glands from deceased and from living donors have been reported,4 with only a hand full of cases including simultaneous or sequential kidney transplantation, either from one living donor or from different deceased donors,4 as shown in Table 1. To our knowledge, there has been no previous report of a simultaneous kidney and parathyroid gland transplant from a living donor to a child. We report such a case here.
TABLE 1.
Summary of previously performed similar cases in the adult population
| Author | Date | Donor(s) | Recipient | Transplant modality | IS |
|---|---|---|---|---|---|
| Groth et al7 | 1973 | Two different deceased donors | 46 y old at time of kidney transplant, parathyroid transplant 52 d later (BG A into O) | Sequential, 2 deceased donors | Yes |
| Wells et al5 | 1975 | One parental living donor | Kidney transplant, parathyroid transplant later on | Sequential, same donor | Yes |
| Duarte et al8 | 1985 | Three different deceased donors | 21 y old at time of kidney transplant, first parathyroid allotransplant 4 y later, further parathyroid allograft later for graft failure | Sequential, 3 deceased donors | Yes |
| Alfrey et al9 | 1992 | One deceased donor (kidney graft), 1 living unrelated donor (parathyroid gland) | 36 y old at time of kidney transplant, parathyroid transplant 2 mo later | Sequential, 2 different donors | Yes |
| Torregrosa et al10 | 2005 | Kidney graft?One living unrelated donor (parathyroid gland) | Not disclosed in abstract | Sequential, 2 different donors | Yes |
| Chapelle et al11 | 2009 | One deceased donor (first kidney graft)One living-related donor (second kidney graft)One deceased donor (third kidney graft + parathyroid gland) | Previous kidney transplant at age 5 and age 9, simultaneous kidney and parathyroid transplant at the age of 32 | Simultaneous, 1 deceased donor | Yes |
| Flechner et al6 | 2010 | One living-related donor sister (first kidney graft)One deceased donor (second kidney graft)One deceased donor (parathyroid gland) | Previous living-donor kidney transplant at the age of 29, second kidney transplant at the age of 36Parathyroid transplant 2 mo after second kidney transplant | Sequential, 3 deceased donors | Yes |
| Giulianotti et al12 | 2014 | One living-related donor mother (first kidney graft)One living-related donor sister (second kidney graft and parathyroid gland) | Previous living-related kidney transplant at the age of 14—graft failed 2 y laterSecond kidney transplant at the age of 17Parathyroid transplant later age >18 y | Sequential, 1 living-related donor | Yes |
| Garcia-Roca et al4 | 2016 | One living-related donor sister (kidney + parathyroid gland) | 23 y old at the time of simultaneous kidney and parathyroid transplant | Simultaneous, 1 living-related donor | Yes |
Shows the summary of all previously described cases of parathyroid and kidney allotransplantation, performed sequentially or simultaneously4,5-12 within the adult population.
IS, immunosuppression.
MATERIALS AND METHODS
We describe the clinical features, management, and outcome of a caucasian girl who presented to her local hospital with seizures secondary to severe hypocalcemia on day 6 of life. Investigations showed a corrected serum calcium level of 1.48 mmol/L (normal concentration: 2.19–2.69 mmol/L), a magnesium level of 0.65 mmol/L (normal concentration: 0.65–1.05 mmol/L), and a PTH level of <0.5 pmol/L (normal concentration: 0.95–5.7 pmol/L).
Hypoparathyroidism
Molecular analysis detected a pathogenic variant in exon 7 of CASR, (c.2528C>A) that has been reported previously in a number of patients with ADH type 1.13
The girl was initially managed with oral 1-alphahydroxycholecalciferol 400 μg daily, which maintained her serum calcium between 1.7 and 1.8 mmol/L (2.24–2.69 mmol/L). At the age of 1.5 y, she was started on recombinant PTH 5 μg twice daily subcutaneously, which was later increased to higher doses and 3 times daily. She was also treated with magnesium, bendroflumethiazide, and cholecalciferol. At 2.5 y and following multiple episodes of symptomatic hypocalcemia, she was commenced on continuous subcutaneous PTH via a small, computerized pump. This has been described previously as a suitable treatment option for patients with ADH.14 The exogenous PTH, teriparatide (1–34) (Forteo), produced in Escherichia coli using recombinant DNA technology, is identical to the 1–34 N-terminal amino acid sequence of endogenous human PTH. Its actions are therefore similar to the known physiological actions of PTH and include stimulation of bone turnover by direct effects on osteoblasts, indirectly increasing the intestinal calcium absorption and increasing the tubular reabsorption of calcium and excretion of phosphate by the kidney. In the course of the following year, chlorothiazide and phosphate supplements were added to her medical management.
Our patient continued to show persistent low endogenous PTH concentrations pretransplant as measured per routine PTH assays. Adjustments to dosage were based on symptoms and calcium and phosphate serum concentrations. Despite constant adjustments of her PTH dose and the high intake of calcium supplements, her serum calcium and phosphate concentrations remained difficult to manage and she continued to suffer with frequent symptomatic episodes of hypocalcemia. Furthermore, at the age of 10.7 y, she had to undergo bilateral hip pinning because of severe metabolic bone disease and slipped upper femoral epiphyses bilaterally.
Renal Involvement and Progression
Our young patient maintained normal renal function until nearly 9 y of age, before showing steady deterioration and developing ESKD at the age of 11.3 y. She commenced in-center hemodialysis via a central venous catheter while her father was evaluated as a potential kidney donor. An ultrasound study performed to investigate the cause of her deteriorating kidney function showed 2 normal-sized kidneys, but with increased echogenicity and loss of corticomedullary differentiation. In addition, there were bilateral multiple cortical cysts observed. There was no evidence of nephrolithiasis or nephrocalcinosis and no evidence of urinary tract obstruction. The cause of her ESKD remained uncertain.
Transplantation
The donor, her 43-y-old father, was blood group compatible and well matched (HLA mismatch of 0-1-1). As neither, simultaneous parathyroid and kidney donation or transplantation had ever been performed in the United Kingdom previously, relevant regulatory approvals were sought before proceeding. The donor and recipient cases were discussed nationally and approved by the Human Tissue Authority and National Health Service Blood and Transplant. Locally, permissions were granted by our Trust Risk and Assurance Committees at both the adult donor Hospital (Guy’s & St Thomas’s Foundation Hospitals NHS Trust, United Kingdom) and at the pediatric recipient Hospital (Evelina London Children’s Hospital). Before surgery, genetic testing confirmed that the donor did not carry the CASR, c.2528C>A mutation on exon 7 of CASR. Additionally, to our standard donor workup, an ultrasound of the donor’s neck was performed to rule out any thyroid or parathyroid pathologies.
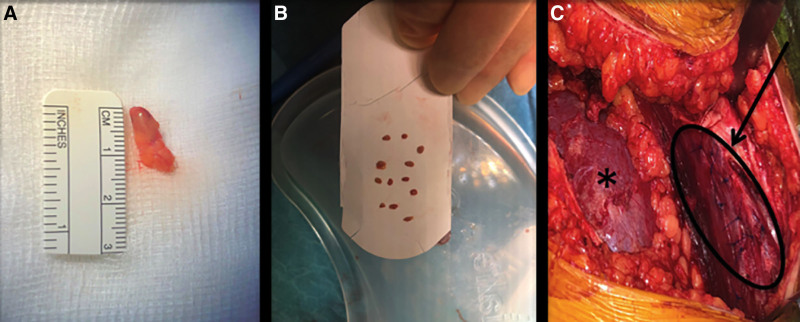
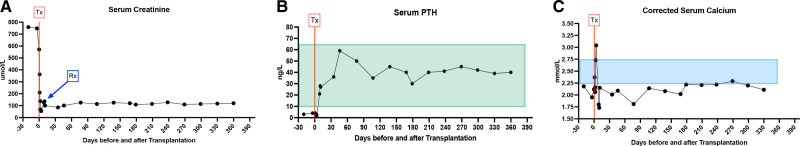
At the age of 11.8 y, in June 2019, the patient received a kidney and a simultaneous parathyroid gland from her 43-y-old father. The donor procedure, a retroperitoneal, left hand-assisted nephrectomy, followed by a right upper gland parathyroidectomy via a Kocher incision, was performed without any complications. A sample of the parathyroid gland was sent off for histological evaluation (frozen section) immediately following harvesting to confirm the tissue to be parathyroid tissue. The kidney was implanted into the recipient’s right iliac fossa via a rectus sparing incision. The parathyroid gland (Figure 1A) was divided into twelve 1.5-mm pieces and transplanted into the anterior aspect of the exposed rectus muscle. This was performed by spreading the muscle fibers to form little pockets for the pieces of tissue (Figure 1B). The implantation sites were then marked with single 4-0 Prolene stitches (Figure 1C, arrow) and the whole area of implantation was marked with 4 liga-clips in case a later resection became necessary. The cold ischemia times were 5 h for the kidney graft and 7 h for the parathyroid gland. For immunosuppression, the Transplant WIthout STeroid protocol was applied, which consisted of basiliximab on days 0 and 4 for induction, tacrolimus, mycophenolate mofetil, and prednisolone, with early steroid withdrawal. The planned milestones for tacrolimus levels were 8 to 12 ng/L in the first 6 mo and 5 to 10 ng/L >6 mo after transplantation. The kidney graft showed primary function with a serum creatinine of 55 µmol/L on postoperative day 3 (Figure 2A). The donor was discharged from the hospital on day 3 following the operation. His serum calcium and PTH levels remained within the normal range perioperatively. He was clinically assessed 2 wk postoperatively and has remained well since.
FIGURE 1.
Intraoperative photographs of parathyroid transplantation into the exposed rectus muscle. The donated upper right quadrant parathyroid gland immediately after donation is shown in (A) with some fat tissue still attached (parathyroid gland marked by black circle); the same gland after division into 12 pieces of approximately 1.5 mm in size before transplantation into the recipient is shown in (B); and the transplanted kidney in its position within the right iliac fossa (*) with the parathyroid transplant within the rectus muscle (arrow) in (C).
FIGURE 2.
The patient’s serum creatinine levels are shown in micromole per liter in (A), from the time of Tx (red line), with follow-up values (days after transplant shown on x-axis). The episode of rejection is marked with an arrow in blue. PTH serum levels in nanograms per liter for the same time period are shown in (B), with the time of Tx (red line) and the normal range for PTH levels (10–65 ng/L) highlighted in light green. C, The corrected serum calcium levels in millimoles per liter for the same period of follow-up in our patient are shown, with the time of transplantation highlighted (Tx, red line) and the normal range (2.25–2.75 mmol/L) being marked in light blue. PTH, parathyroid hormone; Tx, transplantation.
RESULTS
Recovery of Parathyroid Gland and Renal Function
At the time of transplantation, our patient (body weight 47 kg) was receiving 66 μg of teriparatide per day, 10 drops of cholecalciferol once daily (2400 IU), sevelamer 800 mg 3 times a day, calcium acetate 475 mg 3 times a day, and calcium acetate 475 mg with snacks when symptomatic.
In the immediate period following transplantation, the function of the parathyroid transplant could be easily monitored by our standard PTH assay (Roche Elecsys PTH method), which is a sandwich assay using 2 monoclonal antibodies: a biotinylated monoclonal antibody against the N-terminal fragment of PTH (1–37) and a ruthenium-labeled monoclonal antibody against the C-terminal fragment (38–84), and therefore exclusively detects the whole PTH molecule. In contrast to that, synthetic PTH (teriparatide) is limited to the 1–34 part of the PTH molecule and therefore not detected by this assay. The intrinsic PTH concentration, at the time of transplantation and immediately posttransplantation, was 3 ng/L (normal range, 10–65 ng/L) (Figure 2B). Perioperatively, the patient was commenced on intravenous calcium supplementation, but on day 3, calcium infusions were stopped because of hypercalcemia (Figure 2C). The rate of synthetic PTH infusion via the pump was titrated to maintain the corrected serum calcium above 1.8 mmol/L but below normal range (2.25–2.75 mmol/L) with an aim to stimulate the endogenous PTH production by the transplanted gland. The rate of the PTH pump was first reduced on day 6 after transplantation and titrated almost daily for the next month. As a result, the endogenous serum PTH concentration rose to 21 ng/L day 8 and 28 ng/L on day 9 following transplantation. Thereafter, her PTH levels remained within physiological range for the first time in her life, as shown in Figure 2B. At first, she was however still pump dependent, and the endogenous PTH level was inappropriately normal for her low serum calcium levels (Figure 2C).
Our young patient’s serum creatinine level has remained stable since transplantation (Figure 2A), except from an initial rise because of an episode of T cell–mediated rejection (type 1A, Banff Criteria) on postoperative day 10, which was successfully treated with 4 pulses of methylprednisolone. PTH levels remained stable and after discussion with the involved endocrinology team at the time, rejection of the parathyroid tissue was deemed highly unlikely. Some further slight rises in serum creatinine were detected later on and attributed to calcineurin inhibitor toxicity, resulting in tacrolimus dose adjustments. Donor-specific antibodies were not detected. In regards to her virology on follow-up, she remained negative for cytomegaly virus and BK and had mild Ebstein-Barr virus viremia (viral load 834 IU/mL) at 12 mo after her transplantation, which was managed by monitoring and adaptation of her immunosuppression. Our patient’s serum creatinine level steadily increased to a new baseline around 110 μmol/L because of growth and significant weight gain (70 kg, measured 1 y after transplantation). Exogenous PTH via the pump was weaned off and could be stopped completely 22 wk following transplantation. At the time of follow-up, 12 mo after transplantation, she was clinically very well, with a serum creatinine of 109 μmol/L, a PTH level of 32 ng/L, and a serum calcium level of 2.24 mmol/L. After 12 mo, her weight was 60 kg and she continued to take calcium 500 mg twice daily (12.5 mmol per tablet, equivalent to 0.4 mmol/kg/d), and cholecalciferol 2400 units once daily for bone health.
DISCUSSION
We herein report the first case of simultaneous living-related kidney and parathyroid gland donation and transplantation in a child. Our case report shows that obtaining both organs simultaneously is safe and feasible and can result in the cure of both PTH deficiency and kidney failure. In the setting of simultaneous transplantation, there are no additional risks to the recipient in terms of immunosuppression. Also, obtaining both organs from the same donor reduces the exposure to different HLA antigens. Following parathyroid gland transplantation, the resulting PTH secretion is likely to protect the kidney graft from damage related to the management of hypoparathyroidism, including nephrocalcinosis or nephrolithiasis.
Hypoparathyroidism and its complications can have fatal consequences and can significantly deteriorate the quality of life of affected patients. The technique of parathyroid cryopreservation and autotransplantation have been established and previously described in detail, especially in the context of hypoparathyroidism as a potential complication following parathyroid surgery.5,15,16 Parathyroid autografts are typically placed heterotopically into a forearm or sternocleidomastoid muscle. This technique results in a low incidence of permanent hypoparathyroidism following radical parathyroidectomy, and recurrent hypercalcemia can be easily managed by local excision of a portion of the grafted tissue17 if it becomes necessary. However, when parathyroid tissue is transplanted into an arm, there can be differences in PTH levels depending on which side the blood is drawn. Central rectal implantation, like in our case, avoids this issue.
As a consequence of the pathogenic variant detected in our patient’s CASR, the parathyroid gland calcium-sensing receptors were constantly activated and, therefore, would continuously “sense” calcium concentrations in the blood stream to be normal, even when they were low. Therefore, endogenous PTH production would not be stimulated, despite lower than normal serum calcium concentrations, resulting in systemic symptomatic hypocalcemia. Similarly, the CaSRs in the renal tubule (on the blood side of the cell wall) would sense “normal” blood calcium concentrations and therefore downregulate the renal outer medullary potassium channel resulting in a decrease of sodium reabsorption through the renal Na-K-2Cl cotransporter, leading to polydipsia and polyuria. Thus, this genetic defect resulted in our patient presenting with hypocalcemia, hypomagnesemia, hypercalciuria with a potential for nephrocalcinosis, and problems with sodium and water reabsorption.
It is interesting to discuss here the possible cause of kidney dysfunction in our patient. It remained difficult to understand why the young girl developed chronic kidney disease that progressed to ESKD and why she developed kidney cysts without marked nephrocalcinosis. Kidney cysts with nephrocalcinosis, particularly in the medulla, and chronic kidney disease have been described in other inherited disorders including kidney tubular disorders although the cause of cyst development often remains poorly understood.18 In contrast, our patient’s kidneys remained of good size and, although echogenic, did not present with typical ultrasonographic features of nephrocalcinosis. In addition, the cysts were cortical rather than medullary in distribution. Other possible causes of kidney dysfunction may include a recognized adverse effect of teriparatide, although its mechanism remains unknown and difficult to prove.19 Considering the radiological findings and the distribution of the cysts in the girl’s kidneys, it is also possible that she had a different kidney disease. However, the absence of any family history of kidney disease or cystic kidney involvement of either parents make this less likely. We have not investigated our patient for other possible causes of primary renal disease.
If we had proceeded with kidney transplantation only, this would have had no impact on the defect involving her parathyroid glands and she would continue to be dependent on exogenous PTH infusion. It is likely that the defect in her native kidneys, and some of the resulting symptoms, may have been alleviated by the presence of the transplanted kidney, which will express wild-type CaSR and not the pathogenic form. Transplanting parathyroid glands without simultaneous kidney transplantation would have led to severe nephrocalcinosis as the kidneys would still express the aberrant CaSR, resulting in hypercalciuria in response to the normal serum calcium levels following transplanted parathyroid glands. For this reason, simultaneous parathyroid (single gland) and kidney transplant from her father was offered with a curative intention for both the hypoparathyroidism and the ESKD.
Although allotransplantation of parathyroid tissue using cryopreservation, tissue culture or other techniques, has been explored and described as a procedure to treat severe (postsurgical) hypocalcemia, its use has been reported only as case reports. Only a few clinical studies are available in the literature and its application in clinical routine is not possible in many centers because of the requirement for highly specialized laboratory and personnel infrastructure.6,20-24 Furthermore, parathyroid tissue expresses major histocompatibility class I and class II antigens, and for ideal long-term allograft function, immunosuppression should be performed. Several strategies have been investigated to avoid immunosuppression in parathyroid allotransplantation, with different success and graft survival rates.22,24-27 Nawrot et al24 used specific antibodies, Hasse et al26 used a microencapsulation technique, and Timm et al27 investigated the application of short-term immunosuppression in a rodent model. Nawrot et al24 reported the largest clinical series using cultured parathyroid tissue with successful implantation of 116 parathyroid transplants into 85 patients without the need for immunosuppression. Alternatively, in cases without immunosuppression, residual parathyroid tissue from the donors was cryopreserved for subsequent retransplantation, in case of rejection episodes.20,28-30 Furthermore, parathyroid allotransplantation has been successfully performed in already immunosuppressed patients, for example, following renal transplantation, but it is rarely indicated.
Results of previous reports including only adult patients and other related cases in the published literature, as summarized previously by Garcia-Roca et al,4 were used to inform the discussions with both parents. In their review of the literature, 18 cases of parathyroid allotransplantation from deceased and living donors were mentioned.4 In 9 of those cases, kidney transplantation was also performed, but only in 2 cases, both transplants were performed simultaneously. The recipients in both cases were adults and in 1 case, both organs were from a deceased donor and in the other case, both organs came from a living-related donor. In all remaining 7 cases, the organs were transplanted sequentially, one with both organs from the same living donor and all the others with organs from different deceased donors. Table 1 summarizes all previous cases of parathyroid and kidney transplantation, simultaneously or sequentially, into 1 recipient.
In summary, our case report demonstrates that simultaneous parathyroid and kidney transplantation in children is safe and feasible and it should be offered to all children with congenital hypoparathyroidism and related kidney failure. Living donation and transplantation of the parathyroid gland alone could be considered as the treatment of choice in children with certain types of hypoparathyroidism, with the option to perform living kidney donation from the same donor, if needed. Parathyroid transplantation can improve the quality of life of affected children dramatically, despite the need for immunosuppression, and is a much cheaper treatment option in the long run. In case of simultaneous parathyroid and kidney transplantation, number of procedures for both the donor and recipient are reduced to a single procedure. Furthermore, in cases with the same donor, exposure to different HLA antigens is additionally minimized. In our case, the functioning parathyroid gland could potentially protect the kidney graft from damage by the underlying disease.
ACKNOWLEDGMENTS
The author (M.D.S.) acknowledges financial support from the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre and Clinical Research Facilities awards to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
J.B., M.I., and L.R. participated in medical management before transplantation. M.D.S., N.W., H.J., M.C., and J.B. participated in medical management after transplantation. N.K., P.C., M.I., M.D.S., J.H., and R.G. participated in planning of procedure. N.K. and P.G. participated in donor kidney procurement and parathyroidectomy. N.V. participated in kidney transplantation. N.K. and N.V. participated in parathyroid transplantation. C.B. participated in photography. N.V. participated in data curation and writing original draft preparation. All authors participated in writing, review, and editing. All authors have read and agreed to the published version of the article.
The authors declare no conflicts of interest.
REFERENCES
- 1.Mannstadt M, Bilezikian JP, Thakker RV, et al. Hypoparathyroidism. Nat Rev Dis Primers. 2017;3:17055. [DOI] [PubMed] [Google Scholar]
- 2.Moon JE, Lee SJ, Park SH, et al. De novo a novel variant of CaSR gene in a neonate with congenital hypoparathyroidism. Ann Pediatr Endocrinol Metab. 2018;23:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linglart A. Congenital hypoparathyroidism. Abstract presented at: 19th European Congress of Endocrinology; May 21, 2017; Lisbon, Portugal. [Google Scholar]
- 4.Garcia-Roca R, Garcia-Aroz S, Tzvetanov IG, et al. Simultaneous living donor kidney and parathyroid allotransplantation: first case report and review of literature. Transplantation. 2016;100:1318–1321. [DOI] [PubMed] [Google Scholar]
- 5.Wells SA, Jr, Gunnells JC, Shelburne JD, et al. Transplantation of the parathyroid glands in man: clinical indications and results. Surgery. 1975;78:34–44. [PubMed] [Google Scholar]
- 6.Flechner SM, Berber E, Askar M, et al. Allotransplantation of cryopreserved parathyroid tissue for severe hypocalcemia in a renal transplant recipient. Am J Transplant. 2010;10:2061–2065. [DOI] [PubMed] [Google Scholar]
- 7.Groth CG, Hammond WS, Iwatsuki S, et al. Survival of a homologous parathyroid implant in an immunosuppressed patient. Lancet. 1973;1:1082–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte B, Mozes MF, John E, et al. Parathyroid allotransplantation in the treatment of complicated idiopathic primary hypoparathyroidism. Surgery. 1985;98:1072–1076. [PubMed] [Google Scholar]
- 9.Alfrey EJ, Perloff LJ, Asplund MW, et al. Normocalcemia thirteen years after successful parathyroid allografting in a recipient of a renal transplant. Surgery. 1992;111:234–236. [PubMed] [Google Scholar]
- 10.Torregrosa NM, Rodríguez JM, Llorente S, et al. Definitive treatment for persistent hypoparathyroidism in a kidney transplant patient: parathyroid allotransplantation. Thyroid. 2005;15:1299–1302. [DOI] [PubMed] [Google Scholar]
- 11.Chapelle T, Meuris K, Roeyen G, et al. Simultaneous kidney-parathyroid allotransplantation from a single donor after 20 years of tetany: a case report. Transplant Proc. 2009;41:599–600. [DOI] [PubMed] [Google Scholar]
- 12.Giulianotti PC, D’Amico G, Tzvetanov I, et al. Living donor parathyroid allotransplantation with robotic transaxillary procurement in a kidney transplant recipient. Transpl Int. 2014;27:e43–e45. [DOI] [PubMed] [Google Scholar]
- 13.Fox A, Gilbert R. Use of teriparatide in a four year old patient with autosomal dominant hypocalcaemia. Arch Dis Child. 2016;101:e2. [DOI] [PubMed] [Google Scholar]
- 14.Gevers E, Buck J, Ashman N, et al. Continuous subcutaneous PTH infusion in autosomal dominant hypocalcaemia. Abstract presented at: 8th International Conference on Children's Bone Health; June 2017; Würzburg, Germany. [Google Scholar]
- 15.Malmaeus J, Akerström G, Johansson H, et al. Parathyroid autotransplantation. An investigation of parathyroid autograft function. Acta Chir Scand. 1983;149:545–554. [PubMed] [Google Scholar]
- 16.Krausz MM, Ashkenazi I, Alfici R. Parathyroid autotransplantation in adults and children. Harefuah. 2017;156:167–170. [PubMed] [Google Scholar]
- 17.Brunt LM, Sicard GA. Current status of parathyroid autotransplantation. Semin Surg Oncol. 1990;6:115–121. [DOI] [PubMed] [Google Scholar]
- 18.Besouw MTP, Bienias M, Walsh P, et al. Clinical and molecular aspects of distal renal tubular acidosis in children. Pediatr Nephrol. 2017;32:987–996. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Annex I: summary of product characteristics. Available at https://www.ema.europa.eu/en/documents/product-information/forsteo-epar-product-information_en.pdf. Accessed April 19, 2020.
- 20.Aysan E, Yucesan E, Idiz UO, et al. Discharging a patient treated with parathyroid allotransplantation after having been hospitalized for 3.5 years with permanent hypoparathyroidism: a case report. Transplant Proc. 2019;51:3186–3188. [DOI] [PubMed] [Google Scholar]
- 21.Kukreja SC, Johnson PA, Ayala G, et al. Allotransplantation of rat parathyroid glands: effects of organ culture and transplantation into the adrenal gland. Experientia. 1979;35:559–560. [DOI] [PubMed] [Google Scholar]
- 22.Aysan E, Altug B, Ercan C, et al. Parathyroid allotransplant with a new technique: a prospective clinical trial. Exp Clin Transplant. 2016;14:431–435. [DOI] [PubMed] [Google Scholar]
- 23.Barczyński M, Gołkowski F, Nawrot I. Parathyroid transplantation in thyroid surgery. Gland Surg. 2017;6:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nawrot I, Woźniewicz B, Tołłoczko T, et al. Allotransplantation of cultured parathyroid progenitor cells without immunosuppression: clinical results. Transplantation. 2007;83:734–740. [DOI] [PubMed] [Google Scholar]
- 25.Tołłoczko T, Woiniewicz B, Sawicki A, et al. Allotransplantation of cultured human parathyroid cells: present status and perspectives. Transplant Proc. 1997;29:998–1000. [DOI] [PubMed] [Google Scholar]
- 26.Hasse C, Zielke A, Klöck G, et al. First successful xenotransplantation of microencapsulated human parathyroid tissue in experimental hypoparathyroidism: long-term function without immunosuppression. J Microencapsul. 1997;14:617–626. [DOI] [PubMed] [Google Scholar]
- 27.Timm S, Otto C, Begrich D, et al. Short-term immunosuppression after rat parathyroid allotransplantation. Microsurgery. 2003;23:503–507. [DOI] [PubMed] [Google Scholar]
- 28.Cabané P, Gac P, Amat J, et al. Allotransplant of microencapsulated parathyroid tissue in severe postsurgical hypoparathyroidism: a case report. Transplant Proc. 2009;41:3879–3883. [DOI] [PubMed] [Google Scholar]
- 29.Aysan E, Kilic U, Gok O, et al. Parathyroid allotransplant for persistent hypocalcaemia: a new technique involving short-term culture. Exp Clin Transplant. 2016;14:238–241. [DOI] [PubMed] [Google Scholar]
- 30.Kunori T, Tsuchiya T, Itoh J, et al. Improvement of postoperative hypocalcemia by repeated allotransplantation of parathyroid tissue without anti-rejection therapy. Tohoku J Exp Med. 1991;165:33–40. [DOI] [PubMed] [Google Scholar]