Abstract
IMPORTANCE
Despite bearing a disproportionate burden of heart failure (HF), Black and Hispanic individuals have been poorly represented in HF clinical trials. Underrepresentation in clinical trials limits the generalizability of the findings to these populations and may even introduce uncertainties and hesitancy when translating trial data to the care of people from underrepresented groups. The Heart Failure Collaboratory, a consortium of stakeholders convened to enhance HF therapeutic development, has been dedicated to improving recruitment strategies for patients from diverse and historically underrepresented groups.
OBSERVATIONS
Despite federal policies from the US Food and Drug Administration and National Institutes of Health aimed at improving trial representation, gaps in trial enrollment proportionate to the racial and ethnic composition of the HF population have persisted. Increasing trial globalization with limited US enrollment is a major driver of these patterns. Additional barriers to representative enrollment include inequities in care access, logistical issues in participation, restrictive enrollment criteria, and English language requirements.
CONCLUSIONS AND RELEVANCE
Strategies for improving diverse trial enrollment include methodical study design and site selection, diversification of research leadership and staff, broadening of eligibility criteria, community and patient engagement, and broad stakeholder commitment. In contemporary HF trials, diverse trial enrollment is not only feasible but can be efficiently achieved to improve the generalizability and translation of trial knowledge to clinical practice.
Some racial and ethnic populations, particularly Black and Hispanic individuals, have a greater burden of heart failure (HF), are less likely to receive optimal disease-modifying therapies, and are more likely to die from HF.1–5 Despite this excess risk, these same populations are often the least studied in HF clinical trials.6–10 The importance of addressing the systemic underenrollment of some racial and ethnic groups has been previously highlighted11 and remains an important directive of the US Food and Drug Administration (FDA) Safety and Innovation Act as well as the Inclusion Policy of the National Institutes of Health (NIH).12
The Heart Failure Collaboratory, a consortium of stakeholders convened to enhance therapeutic development and delivery to patients with HF,13 has been dedicated to improving recruitment strategies for diverse patient populations. This article is the direct result of virtual and in-person discussions among Collaboratory stakeholders with the goal of proposing strategies to improve enrollment in HF clinical trials of underrepresented racial and ethnic groups. We focus on the US perspective in the HF clinical trial land scape (with specific attention to enrollment of Black and Hispanic participants) and highlight US federal policies and initiatives to promote improved racial and ethnic representation. We recognize the need as well for improving enrollment of other populations of interest, including women, older adults, and people in rural areas of the United States14–17; however, this call to action focuses on building sustainable pathways to improve future diverse participation with respect to race and ethnicity.
Barriers in Underrepresented HF Trial Enrollment
Many racial and ethnic populations continue to experience socioeconomic deprivation, chronic discrimination, and stress that worsen health outcomes across the lifespan.18–21 The ability and willingness of patients with HF to actively participate in clinical trials is intricately tied to social determinants of health, such as access to health care, education, income equality, and food security as well as cultural and community values.22 This underrepresentation likely results from limited access both to clinical care and the current clinical research enterprise, particularly because HF often requires subspecialty care. Patients are often required to be maximally treated to enter into clinical trials of HF therapeutics; therefore, the routine clinical undertreatment of racial and ethnic minority patients and decreased specialist referral may limit participation in clinical research.
When considering HF clinical eligibility, thresholds for natriuretic peptides, which are known to be constitutively lower in Black individuals, may unintentionally promote underenrollment. Under-represented participants within clinical trials often face significant barriers to trial completion, including transportation access, inflexible work hours, and financial challenges.23–25 Previously identified barriers include mistrust, competing demands, socioeconomic factors, and lack of access to research information.24,26 Perceived benefits and risks of clinical trial research is also a potential barrier to clinical trial participation in Black communities.27 At their core, all of these barriers are, in some part, related to systemic racism and bias, as highlighted in a recent American Heart Association Presidential Advisory.28 Diversity in clinical trials will continue to suffer until there are fundamental changes in the clinical research enterprise, including how patients are identified and recruited.
For Hispanic patients, screening and enrollment may be additionally complicated by differences in primary language and cultural factors. This may be made more difficult by underrepresentation of Hispanic investigators. Some trials may specifically exclude non–English speaking patients, and even in cases where consent documents are translated to other languages, content may not be culturally sensitive. In addition, the presence of translators may distort the relationship between the study team and the patient, adding distance and an element of depersonalization.
From a US perspective, globalization of HF clinical trials29 has resulted in a lower proportion of US patient representation, thereby further limiting diversity and limiting applicability and relevance to management of the US population.11 The proportions of Black and other racial and ethnic groups enrolled in a selection of pivotal contemporary HF clinical trials for medical and device therapies are depicted in Figure 1 and Figure 2, respectively. While not intended to be a systematic examination of HF trials, these selected trials, many of which supported regulatory approval of study therapy, were global trials with limited Black participant enrollment. BEST and HF-ACTION, 2 trials with high proportions of Black participants, were both primarily based in North America. In 1 FDA report of cardiovascular drug trials from 2015 to 2016, only 1.3% of participants outside the United States were Black compared with 15% of participants in the United States.11,12 In 3 recent global trials of HF, enrollment of Black adults was 4% to 7%, driven by limited North American enrollment.30–32 Unfortunately, most trials do not report the numbers of Alaska Native, American Indian, Hispanic, Native Hawaiian, or Other Pacific Islander patients despite a high burden of HF in these populations.
Figure 1.
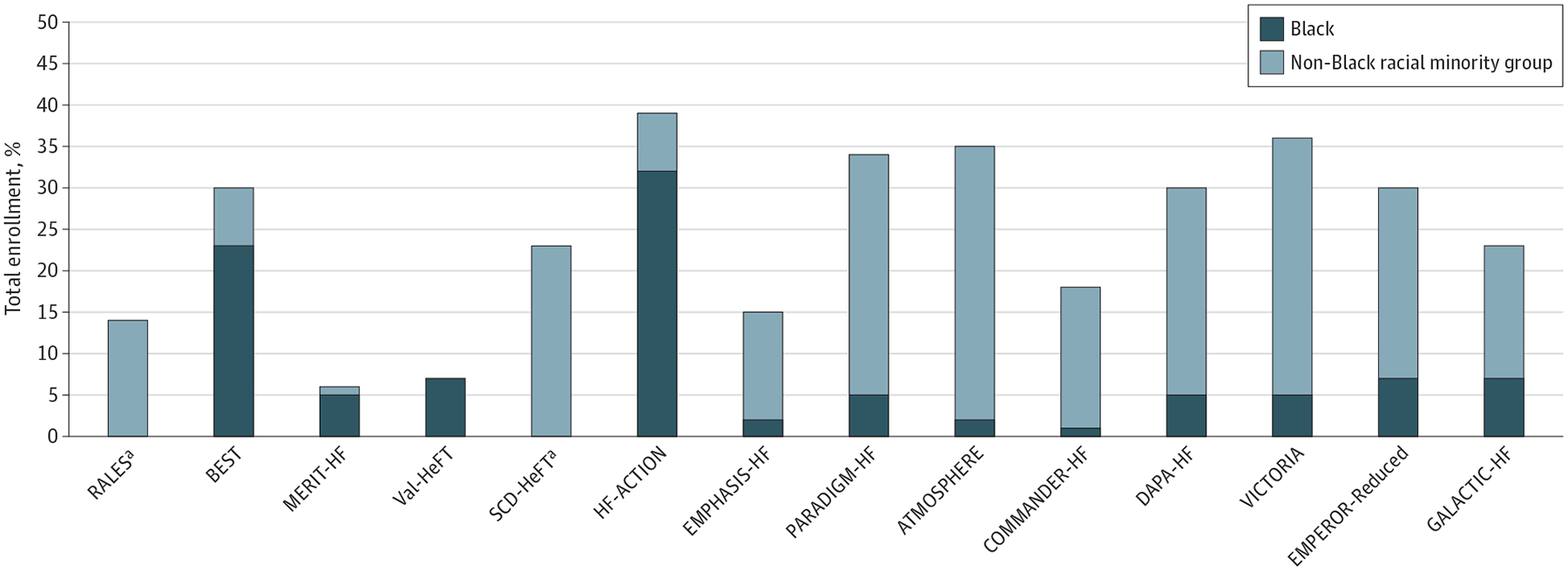
Enrollment of Racial and Ethnic Minority Groups in Select Heart Failure (HF) Medical Therapy Trials
Proportion of racial and ethnic minority groups in select clinical trials is displayed as a percentage of total participants. When reported, the proportion of Black participants is shown. This analysis was not intended to be a systematic review of all trials meeting specific criteria, but rather a sampling of pivotal trials, many of which supported regulatory approval of the studied therapy.
a Further breakdown of racial and ethnic groups was not provided in clinical trial publications.
Figure 2.
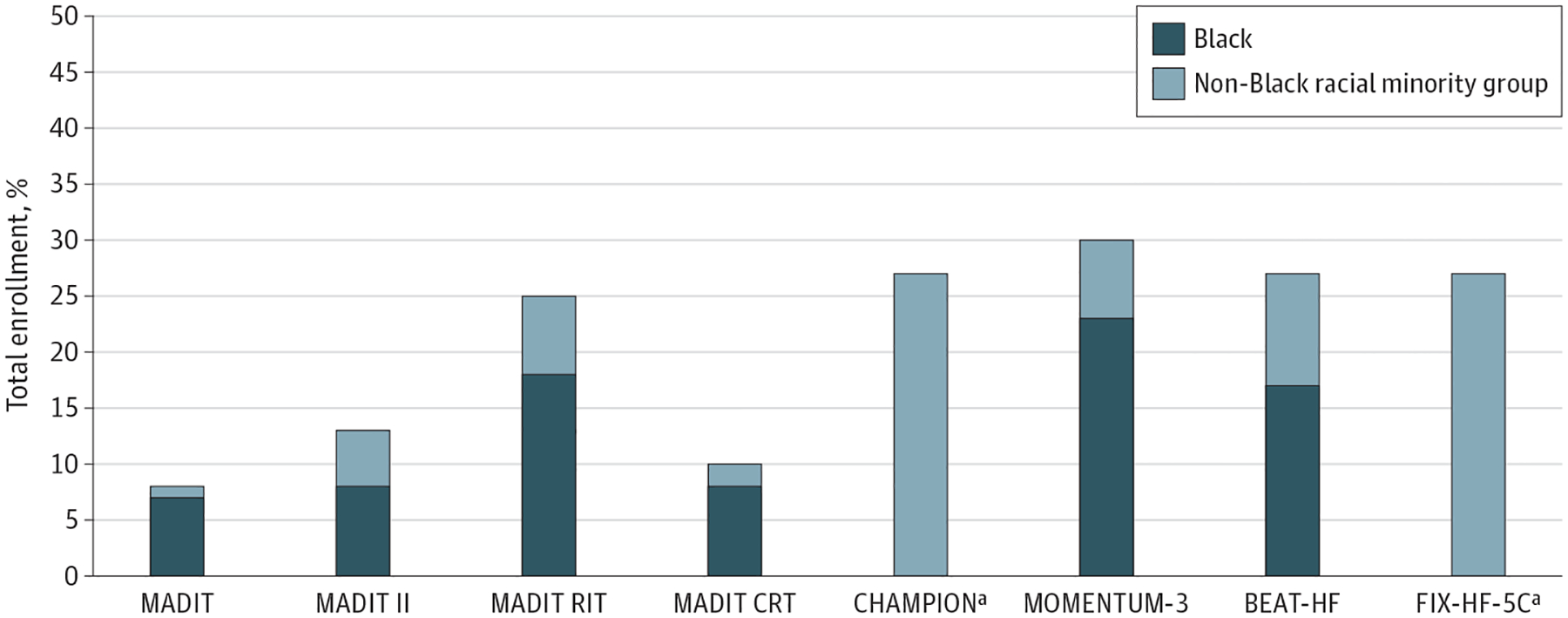
Enrollment of Racial and Ethnic Minority Groups in Key Heart Failure (HF) Device Trials
Proportion of racial and ethnic minority groups in select clinical trials is displayed as a percentage of total enrolled participants. When reported, the proportion of Black participants is shown. This analysis was not intended to be a systematic review of all trials meeting specific criteria, but rather a sampling of pivotal trials, many of which supported regulatory approval of the studied device.
a Further breakdown of racial and ethnic groups was not provided in clinical trial publications.
Continued globalization of HF clinical trials has introduced new patterns of enrollment with recent reports suggesting an increase in the enrollment of certain racial and ethnic participant groups in HF clinical trials. Of 118 HF trials reviewed between 2001 and 2016, data reporting by race and ethnicity was available in less than half (47%). However, of the HF trials that reported race and ethnicity, enrollment of racial and ethnic minority participants increased over time from 13% to 30%. Notably, much of the increase was driven not by increasing enrollment of Black or Hispanic patients but increased enrollment of Asian patients,6 despite a much lower prevalence of HF overall in Asian populations (eFigures 1 and 2 in the Supplement). These patterns largely reflect greater site selection in countries in Asia among global HF trials.
Defining Target Enrollment of Participants of Various Racial and Ethnic Backgrounds
Requiring trial leadership and sponsors to individually power racial and ethnic subgroups to separately assess safety and efficacy may lead to unreasonably large studies, which may be infeasible to conduct. Inherently, subgroups may be underpowered for these statistical determinations even when they are well represented. However, encouraging adequate enrollment is still worthwhile to inform the generalizability of findings and to ascertain potential safety and efficacy signals that could be explored further in postmarketing evaluation or subsequent trials.
Central to the issue of defining these targets in clinical trials is the anchor by which the population prevalence is characterized. Participants of a particular racial and ethnic background may be appropriately represented, underrepresented, or even overrepresented depending on the specific population considered. The participation-to-prevalence ratio is often used as an aggregate measure of representation in a trial relative to representation in the condition under study. In HF, however, guideline-supported drugs and devices have often been studied in global, heterogeneous participant groups, making correction challenging for regional, national, or subnational prevalence estimates. In countries or regions in which regulatory approval will be sought, trials should be encouraged to target relative enrollment in that country consistent with the racial and ethnic breakdown of the local HF population. Specific targets for absolute patient enrollment may be required by certain regulatory authorities.
Federal Efforts to Increase Diversity in Clinical Trials
The NIH and FDA have been committed to supporting diverse trial enrollment through a series of federal efforts and policies. The NIH’s attention to representative inclusion in clinical studies has evolved since the 1986 Guidance on Inclusion of Women in Clinical Trials. In response to the 1993 Revitalization Act,33 NIH Inclusion Policy required not only representative inclusion of women and minorities but also required that phase 3 clinical trials subject to FDA regulation be designed to include valid statistical analysis stratified by sex, gender, race, and ethnicity.34
Also, in 2017, the NIH initiated requirement that NIH-supported phase 3 clinical trials collect and submit data stratified by sex, gender, race, and ethnicity to the ClinicalTrials.gov registry. All grant applications undergo peer review to ensure target populations reflect the distribution by age, gender, race and ethnicity of the population affected by the disease being studied. Applications that are noncompliant are flagged and cannot be funded unless corrected. Since the Revitalization Act, every Institute must regularly generate Advisory Council Reports certifying compliance with the NIH Policy on Inclusion Guidelines. The NIH has additionally developed outreach strategies and promoted best practices to achieve diversity through providing numerous guidance documents and staff assistance and conducting multiple workshops.
The NIH has established the UNITE initiative to address structural racism within the scientific community. UNITE is a multidisciplinary effort composed of 5 committees with interlinked but related goals of promoting excellence in diversity, equity, and inclusion. These committees seek to understand stakeholder experiences, promote research on health disparities, improve NIH culture, ensure transparency and clear communication, and identify changes in practice, policy, and structures.35
These policies and efforts appear to be effective in improving the standard of enrollment of diverse populations and reporting practices in NIH-funded trials. In a systematic review of contemporary HF trials, the proportion of racial and ethnic minority participants was higher in NIH-funded trials compared with trials supported via other funding mechanisms.6 Furthermore, there may be longitudinal improvements in reporting the breakdown of race and ethnicity of enrolled participants, in part related to requirements for structured fields in clinical trial registries. For instance, an earlier analysis of federally funded clinical trials from 2004 to 2009 found that 21% failed to report sample sizes of racial and ethnic groups and 64% did not report outcomes by race or ethnicity.36 A more recent analysis of trials on ClinicalTrials.gov found that in a random sample, 96% of customized categories included race information and 53% included ethnicity information. In the accompanying publications, 62% reported race and ethnicity information. Among those who did not, 90% still reported race and ethnicity data on ClinicalTrials.gov.37
Notably the NIH policies affect only federally funded research. Therefore, FDA policies and guidance are critically important as well to encourage appropriate representation for private industry and non–federally funded clinical studies and trials. In November 2020, the FDA updated its guidance document on enhancing the diversity of clinical trial populations.38 The document discusses broadening eligibility criteria and avoiding unnecessary exclusions such that a study population better reflects the population most likely to use a particular drug or device.
Importantly, FDA guidance documents are initiatives and recommendations (eTable in the Supplement), not enforceable mandates. In these guidance documents, the FDA acknowledges that it does not establish legally enforceable responsibilities.38 Therefore, major challenges remain in ensuring that these initiatives are translated into practice.
Strategies for Improving Enrollment of Diverse Populations in Clinical Trials
The challenges of clinical trial research that limit recruitment and enrollment of underrepresented patients have been well described in cardiology and HF, drawing attention to the need for sustainable solutions.24,39–41 Enrollment strategies that improve underrepresented participation in HF trials will likely require preemptive and ongoing tactics for successful outcomes (Figure 3).
Figure 3.
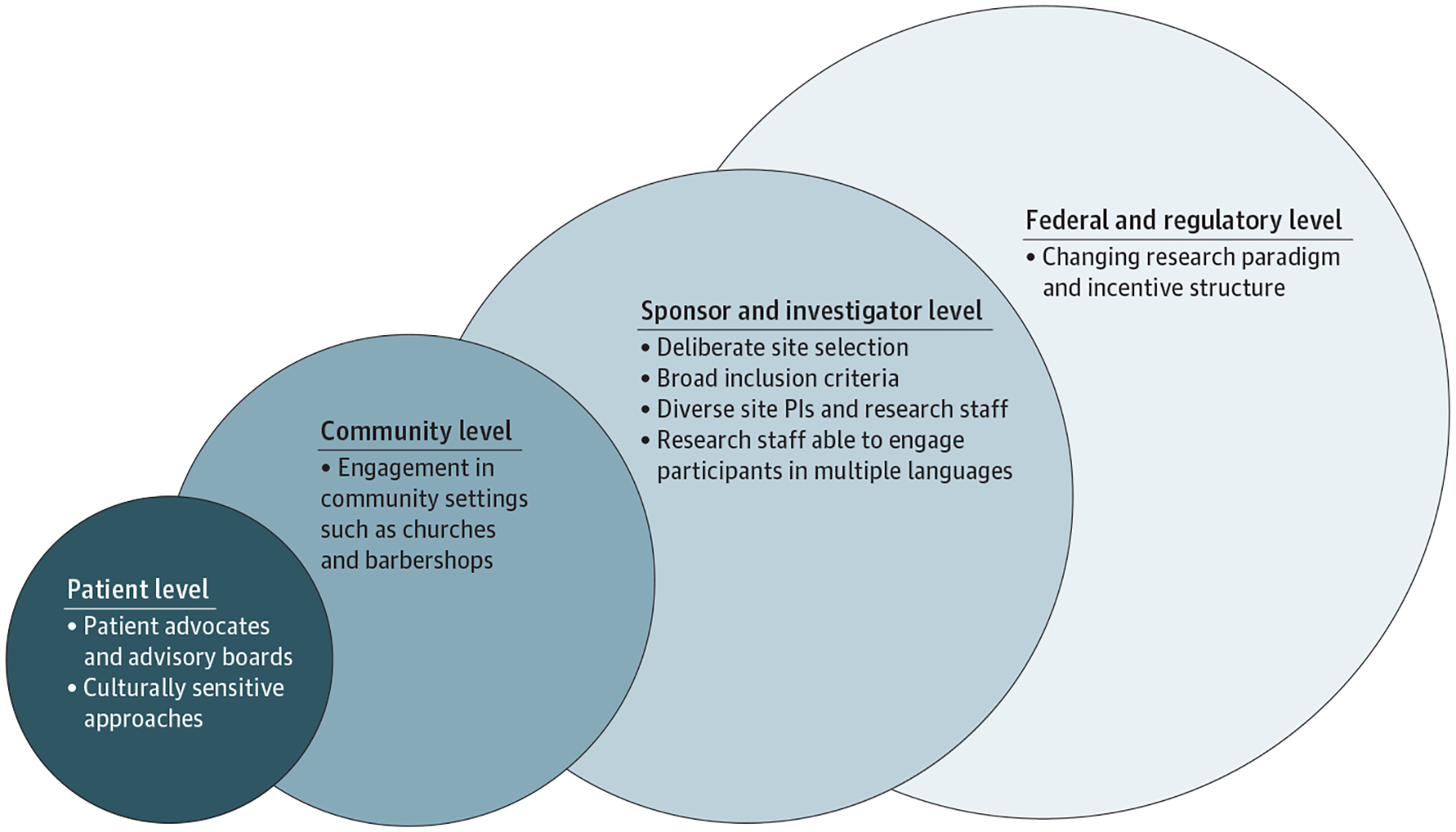
A Roadmap for Increasing Enrollment of Underrepresented Populations in Heart Failure Clinical Trials
Multilevel strategies and solutions are offered to target the breadth of stakeholders involved in heart failure clinical trials, including patients, supporting communities, sponsors, principal investigators (PIs) and research staff, and regulatory bodies.
Strategies to improve clinical trial enrollment of underrepresented populations are centered on 3 themes: (1) research study design and site selection; (2) patient, institution, and community engagement; and (3) changing the research paradigm (Table). Addressing these overarching aims is likely to provide a greater understanding of the factors required for the successful participation of underrepresented populations in HF trials. Although the goals of a particular enrollment strategy may be useful in recruiting underrepresented populations into HF trials, some investigators and study sponsors may be concerned about the potential additional costs and time likely necessary to implement these efforts. However, successfully increasing different populations enrolled in clinical trials may ultimately result in faster trial enrollment and lower trial costs. Concrete strategies for enrolling underrepresented populations may be cost-effective and helpful to site investigators and sponsors. Indeed, based on sampling of contemporary trials, proportions of Black participants and women in HF trials were correlated with higher enrollment rates,6 suggesting that their enrollment would not introduce trial hurdles or inefficiencies.
Table.
Strategies to Increase Participation From Underrepresented Populations in Heart Failure Trials
| Theme | Recruitment and enrollment strategies |
|---|---|
| Research study design and site selection | Minimal inclusion and exclusion criteria |
| Capping enrollment for overrepresented groups | |
| Flexibility in participation hours (for patients with work hour limitations) | |
| Bilingual or multilingual staff and study materials | |
| Alleviating barriers to trial participation (ie, transportation, consolidate research visit with medical clinic visits, follow up by phone or at home when possible) | |
| Intentional hiring of investigators and research staff of Alaska Native, American Indian, Black, Hispanic, Native Hawaiian, and other Pacific Islander backgrounds | |
| Global site selection initiatives with increased sites in North America, Africa, and South America | |
| Patient, institution, and community engagement | Partnership with community entities (ie, churches, barbershops, community centers) |
| Research staff participation in health fairs, speaking engagements | |
| Visibility of research team and efforts (marketing and advertising) | |
| Education for clinic staff and professionals | |
| Pretrial evaluations of implementation barriers (ie, clinic flow, feasibility, evaluation of potential recruitment population) | |
| Financial support for staff and clinician time | |
| Study follow-up results provided to clinic and staff | |
| Access to the medical therapy for all clinical trial participants after study (if beneficial) | |
| Changing the research paradigm | Incentives from government agencies |
| Deliberate targets by sponsors | |
| Efforts supported by professional societies and guideline statements |
Research Study Design and Site Selection
Potential strategies to improve enrollment starts with deliberate study design. For example, broad inclusion criteria should be considered to avoid disproportionate exclusion of underrepresented groups. In addition, enrollment of participants from overrepresented groups should intentionally be capped to ensure adequate enrollment of traditionally underrepresented patients. Given racial variation in levels of natriuretic peptides, trials should consider differential thresholds for study entry, accounting for race and other factors.
COVID-19 has advanced the virtual capacities of clinical trials that may extend the reach and accessibility of clinical research practices. Therefore, changes to trial procedures that reduce the burden on participants, such as the use of telemedicine or mobile nurses and phlebotomists who can travel to participants’ homes, may help enroll these populations.38 Recruiters and trial staff must also be aware of social determinants of health that may influence access to internet or even mobile cellular services. Additional recruitment events could be held during evening and weekend hours. Participants should be informed about which costs incurred by their participation in the trial will be reimbursed.
Trial resources and documents should be provided in multiple languages and at an appropriate literacy level and cultural sensitivity.38 Whenever possible, multilingual research staff should be engaged to encourage participation and retention of patients who may have limited comprehension of English. Because language has been identified as an important issue limiting Hispanic patient enrollment, involvement of Spanish-speaking research staff on investigative teams may lessen these barriers. It is important when collecting patient-reported outcomes that language-specific wording be used. For instance, validated HF-specific instruments such as the Kansas City Cardiomyopathy Questionnaire in a patient’s native language should be used when possible.42,43
Deliberate site selection can play an important role in enriching enrollment of particular patient groups.44 This may involve economic incentives to support centers in their enrollment efforts as well as engagement with investigators in other countries to target greater balance in sex, gender, race, and ethnicity in global trials.11 We should learn from past successes: the African-American Heart Failure Trial (A-HeFT) was able to enroll 1100 Black patients with advanced HF exclusively from US sites.45,46 Similarly, the PLATINUM Diversity study was able to enroll 1501 women and patients who identified as American Indian/Alaskan Native, Black, or Hispanic at 52 US sites in less than 10 months.11,44 High enrollment of under-represented populations did not influence study performance metrics.47 Collating cross-trial site experiences and lists of effective sites and investigators may allow for more successful site selection in subsequent trials. Site location may be particularly important in recruiting study participants from these populations.47 Models need to be developed that predict the patient demographics of a proposed study based on study design, geographic location, and other factors.
Strategic site selection is intricately tied to strategic selection of principal investigators. Improving enrollment in clinical trials involves expanding the diversity of both patients and investigator groups. In an analysis of HF trials as well as publications cited in the US and European HF guidelines, HF trials with a female first or senior author were associated with significantly higher proportions of enrolled female participants.48 Therefore, having formalized programming to train investigators of underrepresented groups to become site principal investigators, steering committee members, and lead clinical investigators may have the secondary effect of increasing enrollment of these patient populations. In addition, increased diversity of research coordinators and investigator research teams may help strengthen patient engagement in the research process and enterprise.11 Patients may be more inclined to enroll or follow up when they interact with a health care professional of their same cultural background.38
For studies that are unable to recruit diverse populations, there should be a commitment to phase 4 or postapproval studies with enhanced recruitment of racial and ethnic minority patients to address safety and implementation concerns.11 This approach ensures that these traditionally underrepresented populations are adequately studied at some point during the life cycle of therapeutic development. For example, the Cardio MEMS Post-Approval Study specifically intended to increase representation of populations who were underrepresented in the original pivotal trial, including women, Black participants, patients with preserved ejection fraction, and those with cardiac implantable electronic devices.49 In addition, although the enrollment of Black patients in PARADIGM-HF was low by proportion,50 the subsequent PIONEER-HF trial enrolled 36% Black participants (it is worth noting that the absolute number of Black patients in PARADIGM-HF was greater than those in PIONEER-HF).51 There is recent evidence to suggest that high levels of enrollment of diverse populations in cardiovascular clinical research can be accomplished without compromising enrollment rates or other study performance metrics.47
There are a variety of mechanisms, either through voluntary registries or big clinical data studies, to explore outcomes in diverse populations outside advanced-phase trials. As an example, he FDA established the Sentinel System in 2009, which allows for national surveillance of postmarket medical safety of drugs, biologics, and medical devices.52
Patient, Institution, and Community Engagement
Patient involvement is crucial in the successful recruitment of underrepresented populations.53–56 Several studies suggest using patient advisory boards or advocates before initiating a trial to assess likelihood of success.57,58 Online education-to-action programs have been shown to improve knowledge and intent to participate in biomedical research among Black women.59 Health coaching from nonresearch staff may also help underrepresented populations involved in clinical research. Past participants of other clinical trials may serve as highly informative resources to patients who are new to clinical research. One review of 20 health research studies found minimal differences in the willingness of Black and Hispanic patients to participate in health research compared with White patients, highlighting the importance of ensuring access for all groups.60 Therefore, strategies that promote the patient’s confidence to participate in research are essential to increasing underrepresented patient enrollment.
Trial results may benefit all patients participating in research efforts. Often, the study therapy results are not communicated with participating patients or physicians at study completion. Once an intervention receives an indication for therapy, the financial challenges to obtain the treatment may significantly affect underrepresented populations. Therefore, introducing more effective ways to provide participating patients or clinics with medical treatment may increase the willingness of patients to participate in future studies.
Other important patient factors to consider in clinical trial research are the cultural beliefs and community values of the focus population.61–64 Several underrepresented populations have significant ties to cultural, value-based, or religious beliefs that affect their willingness to participate in clinical trial research. Therefore, strategies to improve enrollment in HF trials should consider both culturally and value-based competencies for the population of interest. Studies suggest that strategies involving churches within the Black community have successfully facilitated the introduction of clinical research efforts.65–68 Considering faith and cultural beliefs in underrepresented populations may be crucial in assisting trial investigators in deploying recruitment and engagement strategies.
It is equally essential to consider establishing healthy and informative communication within communities of underrepresented populations. Several studies have shown varying benefits of a community-based approach concerning clinical trials.23,64,69–72 Because of the heterogeneity of communities and populations, it is likely that strategies to improve under represented participation in clinical trials will need specific individualization to every community. However, individual community-based tactics have been very successful in achieving the desired outcomes. For instance, Victor and colleagues73 evaluated the effectiveness of pharmacist intervention in Black men with uncontrolled hypertension in the Barbershop Hypertension Study. The trial assigned barbershops to a pharmacist-led intervention where barbers encouraged meetings in barbershops with pharmacists who prescribed drug therapy under a collaborative practice agreement with the participants’ doctors. The retention rate in the intervention group was 95%, providing clear evidence that community-based processes can significantly help with recruitment and retention of underrepresented populations. However, the community sites used for Hispanic, Asian, and other populations are likely to be different than those used for Black individuals and may be influenced by cultural and religious factors. Further investigations of this community-based trial design may be similarly feasible in HF, potentially facilitating more expedient and diverse enrollment.
Community clinic engagement has been recognized as an effective means of increasing underrepresented patient involvement in clinical research.57,72,74 Clinical trials involving hospital systems might not engage nonaffiliated community professionals. Frequently, physicians in the community who are not affiliated with the hospital systems are unaware of clinical trial efforts. Although the strategy to involve community clinics and physicians may be important, these efforts present challenges. Community practices unfamiliar with clinical trials research may feel overwhelmed by the complexity of the research process. Therefore, clinical trial investigators should provide information to community professionals perhaps through participation in health fairs or informative discussions from the research staff in the community. Other services, such as meeting with clinic leadership to identify benefits of the study for the clinic, address concerns, and establish protocols to minimize the effect on clinic function, are crucial to the successful involvement of community clinics. Incentives to assist in clinic coverage of staff time and support may also be beneficial. The goal of this multipronged approach is to increase the research “IQ” and support of underrepresented communities through targeted public awareness campaigns that emphasize the importance and virtues of participation in clinical trials.
Changing the Research Paradigm
To improve inequities in HF clinical trials, we need to fundamentally reconsider the way we perform research and the regulatory environment. In addition to the aforementioned strategies, incentives at the sponsor, funder, and federal levels must be better aligned to promote diverse participant enrollment as well as a diverse research enterprise.
Despite federal policies and guidance documents, enrollment targets are not mandated by the FDA. Yet defining recruitment targets has been associated with higher enrollment of Black participants.75 Provision of “carrots” such as streamlined regulatory review processes or extended patent life could be considered to stimulate greater adherence to these policies. For example, the Office of Pediatric Therapeutics76 and the corresponding Pediatric Research Equity Act (PREA)77 offer such incentives to promote adequate study of pediatric populations. The Pediatric Research Equity Act requires new applications to contain a pediatric assessment unless the applicant has obtained a waiver or deferral. Under certain circumstances, by completing these processes, manufacturers receive an additional 6 months of exclusivity. Streamlining the regulatory review process and expediting the time frame for regulatory consideration may be other incentives to enrolling more participants from racial and ethnic minority populations.
The support of cardiovascular societal organizations is essential to changing the research paradigm, through increasing under-represented researchers and trial participants. The Faculty InstitutionalRecruitmentforSustainableTransformationinitiativethrough the NIH aspires to enhance diversity and inclusion among biomedical faculty.78 For example, the American Heart Association supports career development awards that strongly encourage the participation of underrepresented researchers. The American Heart Association also supports Strategically Focused Research Network grants specifically related to promoting diversity in cardiovascular clinical trials that encourage institutional partnership with majority non-White higher learner institutions, including historically Black colleges and universities and institutions that serve Alaska Native, Pacific Islander, Hispanic, and Native American populations. The Association of Black Cardiologists Clinical Research Network (ABCCRN) provides investigators with a network to identify and vet clinical trial opportunities, match investigators to trials of interest, and provide infrastructure support for research endeavors. In addition, the ABC CRN has also partnered with the Society of Clinical Research Sites to increase investigator diversity in clinical trials. The ABC CRN provides these services regardless of membership to the organization and supports inclusion of all underrepresented trial researchers.79 Opportunities of this nature can significantly affect and support diverse leadership and participation in clinical trials.
Industry sponsorship of programs to increase clinical trial diversity can also play a significant role in addressing underrepresented trial leadership and recruitment. These sponsor-led efforts have both economic and ethical potential, which may facilitate regulatory review and therapeutic advances. Thus, leadership from industry sponsors and professional societies can provide advances toward the career development of underrepresented clinical trial researchers and improve the chances of the increasing diversity among clinical trial participants. Therefore, multifaceted commitment and engagement is essential to improving diversity in clinical trial research.
Conclusions
Important and persistent racial health inequities in HF care and outcomes exist. These inequities are also evidenced by persistent underenrollment of US racial and ethnic minority groups in drug and device trials in HF, especially in the context of global clinical trials. Although federal policies from the US FDA and NIH have promoted improved trial representation over time, gaps have persisted in trial enrollment proportionate to the racial and ethnic composition of the HF population. Increasing trial globalization with limited US enrollment is a major driver to these patterns. Additional barriers to representative enrollment include inequities in care access, logistical issues in participation, restrictive enrollment criteria, and English language requirements.
We suggest being deliberate in trial design and processes to effect change. These strategies include methodical study design and site selection, diversification of research leadership and staff, careful review of eligibility criteria, community and patient engagement, and broad stakeholder commitment. In contemporary HF trials, diverse trial enrollment is not only feasible but can be efficiently achieved to improve the generalizability and translation of trial knowledge to clinical practice.
Supplementary Material
Conflict of Interest Disclosures:
Dr Adamson is an employee of Abbott. Dr Batchelor has received consulting fees from Boston Scientific, Medtronic, V-Wave, Abbott, and Idorsia and has received research support from Abbott and Boston Scientific. Dr George is a current employee for Regeneron, was a former employee of AstraZeneca at the time of this work, and holds stock in AstraZeneca. Dr Ibrahim has received honoraria from Novartis and Roche. Dr Jessup is employed by the American Heart Association. Dr Kitzman has received consulting and/or research support from Bayer, Boehringer Ingelheim, Merck, AstraZeneca, Abbvie, Novartis, Corvia, Novo Nordisk, Rivus, Keyto, Pfizer, Duke Clinical Research Institute, and National Institutes of Health and holds stock in Gilead. Dr Psotka is an employee of the Food and Drug Administration and has received personal fees from Amgen, Cytokinetics, and Windtree. Dr Senatore is an employee of the Food and Drug Administration. Dr Stein is an employee of and shareholder in Boston Scientific. Dr Teerlink has received research support from and is a consultant for Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, EBR Systems, Medtronic, Merck, Novartis, scPharma, and Windtree Therapeutics. Dr Yancy reported spousal salary support, Abbott Labs. Dr Lindenfeld has received consulting and/or research support and/or personal fees from Abbott, Alleviant, AstraZeneca, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards Lifesciences, Impulse Dynamics, Sensible Medical, V-Wave, Vifor, and Volumetrix. Dr O’Connor reported serving as a consultant for Bayer, Merck, and Abiomed. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, and Sanofi and speaker engagements with Novartis and Roche Diagnostics and participates on clinical endpoint committees for studies sponsored by Bayer, Galmed, Novartis, Occlutech, and Impulse Dynamics. No other disclosures were reported.
Footnotes
Publisher's Disclaimer: Disclaimer: Dr Yancy is a deputy editor of JAMA Cardiology. The views expressed in this article are those of the authors and do not necessarily represent the views of the Food and Drug Administration; National Heart, Lung, and Blood Institute; National Institutes of Health; or Department of Health and Human Services.
REFERENCES
- 1.Carnethon MR, Pu J, Howard G, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136 (21):e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 2.Breathett K, Liu WG, Allen LA, et al. African Americans are less likely to receive care by a cardiologist during an intensive care unit admission for heart failure. JACC Heart Fail. 2018;6(5):413–420. doi: 10.1016/j.jchf.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberly LA, Richterman A, Beckett AG, et al. Identification of racial inequities in access to specialized inpatient heart failure care at an academic medical center. Circ Heart Fail. 2019;12 (11):e006214. doi: 10.1161/CIRCHEARTFAILURE.119.006214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 5.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10(7):10. doi: 10.1161/CIRCOUTCOMES.116.003552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3(10):1011–1019. doi: 10.1001/jamacardio.2018.2559 [DOI] [PubMed] [Google Scholar]
- 7.Mohiuddin SM, Hilleman DE. Gender and racial bias in clinical pharmacology trials. Ann Pharmacother. 1993;27(7–8):972–973. doi: 10.1177/106002809302700728 [DOI] [PubMed] [Google Scholar]
- 8.Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? impediments to participation. Ethn Health. 1997;2 (1–2):31–45. doi: 10.1080/13557858.1997.9961813 [DOI] [PubMed] [Google Scholar]
- 9.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112 (2):228–242. doi: 10.1002/cncr.23157 [DOI] [PubMed] [Google Scholar]
- 10.Joseph G, Dohan D. Diversity of participants in clinical trials in an academic medical center: the role of the ‘good study patient?’ Cancer. 2009;115(3): 608–615. doi: 10.1002/cncr.24028 [DOI] [PubMed] [Google Scholar]
- 11.Ortega RF, Yancy CW, Mehran R, Batchelor W. Overcoming lack of diversity in cardiovascular clinical trials: a new challenge and strategies for success. Circulation. 2019;140(21):1690–1692. doi: 10.1161/CIRCULATIONAHA.119.041728 [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. 2015–2016 Global participation in clinical trials report. Published July 2017. https://www.fda.gov/media/106725/download
- 13.O’Connor CM, Psotka MA, Fiuzat M, et al. Improving heart failure therapeutics development in the United States: the Heart Failure Collaboratory. J Am Coll Cardiol. 2018;71(4):443–453. doi: 10.1016/j.jacc.2017.11.048 [DOI] [PubMed] [Google Scholar]
- 14.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15):1682–1688. doi: 10.1001/archinte.162.15.1682 [DOI] [PubMed] [Google Scholar]
- 15.Whitelaw S, Sullivan K, Eliya Y, et al. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur J Heart Fail. 2021;23(1):15–24. doi: 10.1002/ejhf.2034 [DOI] [PubMed] [Google Scholar]
- 16.Whitelaw S, Thabane L, Mamas MA, et al. Characteristics of heart failure trials associated with under-representation of women as lead authors. J Am Coll Cardiol. 2020;76(17):1919–1930. doi: 10.1016/j.jacc.2020.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michos ED, Reddy TK, Gulati M, et al. Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am J Prev Cardiol. 2021;8:100250. doi: 10.1016/j.ajpc.2021.100250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert MA, Slopen N, Williams DR. Cumulative psychological stress and cardiovascular disease risk: a focused review with consideration of black-white disparities. Curr Cardiovasc Risk Rep. 2013;7:318–325. doi: 10.1007/s12170-013-0338-5 [DOI] [Google Scholar]
- 19.McDonald JA, Terry MB, Tehranifar P. Racial and gender discrimination, early life factors, and chronic physical health conditions in midlife. Womens Health Issues. 2014;24(1):e53–e59. doi: 10.1016/j.whi.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135(4):531–554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellers S, Cherepanav D, Hanmer J, Fryback DG, Palta M. Interpersonal discrimination and health-related quality of life among black and white men and women in the United States. Qual Life Res. 2013;22(6):1307–1312. doi: 10.1007/s11136-012-0258-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White-Williams C, Rossi LP, Bittner VA, et al. ; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Epidemiology and Prevention. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141(22):e841–e863. doi: 10.1161/CIR.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 23.Davis TC, Arnold CL, Mills G, Miele L. A qualitative study exploring barriers and facilitators of enrolling underrepresented populations in clinical trials and biobanking. Front Cell Dev Biol. 2019;7:74. doi: 10.3389/fcell.2019.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol. 2019;44(5):148–172. doi: 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Cartmell KB, Bonilha HS, Simpson KN, Ford ME, Bryant DC, Alberg AJ. Patient barriers to cancer clinical trial participation and navigator activities to assist. Adv Cancer Res. 2020;146:139–166. doi: 10.1016/bs.acr.2020.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–e31. doi: 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Forefront. July 2, 2020. doi: 10.1377/forefront.20200630.939347 [DOI] [Google Scholar]
- 28.Churchwell K, Elkind MSV, Benjamin RM, et al. ; American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142 (24):e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 29.Vaduganathan M, Samman Tahhan A, Greene SJ, Okafor M, Kumar S, Butler J. Globalization of heart failure clinical trials: a systematic review of 305 trials conducted over 16 years. Eur J Heart Fail. 2018;20(6):1068–1071. doi: 10.1002/ejhf.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381 (21):1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 31.Packer M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383(15):1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 32.Teerlink JR, Diaz R, Felker GM, et al. ; GALACTIC-HF Investigators. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384(2):105–116. doi: 10.1056/NEJMoa2025797 [DOI] [PubMed] [Google Scholar]
- 33.Faden R, Federman D, eds. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. Volume I. National Academies Press; 1994. [PubMed] [Google Scholar]
- 34.National Institutes of Health. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Published March 18, 1994. https://grants.nih.gov/grants/guide/notice-files/not94-100.html
- 35.National Institutes of Health. UNITE initiative. Accessed February 18, 2021. https://www.nih.gov/ending-structural-racism/unite
- 36.Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt). 2011;20(3): 315–320. doi: 10.1089/jwh.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fain KM, Nelson JT, Tse T, Williams RJ. Race and ethnicity reporting for clinical trials in ClinicalTrials.gov and publications. Contemp Clin Trials. 2021;101:106237. doi: 10.1016/j.cct.2020.106237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration. Enhancing the diversity of clinical trial populations: eligibility criteria, enrollment practices, and trial designs. Published November 2020. https://www.fda.gov/media/127712/download
- 39.Zhu JW, Le N, Wei S, et al. Global representation of heart failure clinical trial leaders, collaborators, and enrolled participants: a bibliometric review 2000–20. Eur Heart J Qual Care Clin Outcomes. Published online August 24, 2021. doi: 10.1093/ehjqcco/qcab058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MS, Shahid I, Siddiqi TJ, et al. Ten-year trends in enrollment of women and minorities in pivotal trials supporting recent US Food and Drug Administration approval of novel cardiometabolic drugs. J Am Heart Assoc. 2020;9(11):e015594. doi: 10.1161/JAHA.119.015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michos ED, Van Spall HGC. Increasing representation and diversity in cardiovascular clinical trial populations. Nat Rev Cardiol. 2021;18 (8):537–538. doi: 10.1038/s41569-021-00583-8 [DOI] [PubMed] [Google Scholar]
- 42.Comín-Colet J, Garin O, Lupón J, et al. ; VALIC-KC study group. Validation of the Spanish version of the Kansas City Cardiomyopathy Questionnaire. Rev Esp Cardiol. 2011;64(1):51–58. doi: 10.1016/j.recesp.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 43.Watanabe-Fujinuma E, Origasa H, Bamber L, et al. Psychometric properties of the Japanese version of the Kansas City Cardiomyopathy Questionnaire in Japanese patients with chronic heart failure. Health Qual Life Outcomes. 2020;18 (1):236. doi: 10.1186/s12955-020-01483-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batchelor W, Kandzari DE, Davis S, et al. Outcomes in women and minorities compared with white men 1 year after everolimus-eluting stent implantation: insights and results from the PLATINUM Diversity and PROMUS Element Plus Post-Approval Study pooled analysis. JAMA Cardiol. 2017;2(12):1303–1313. doi: 10.1001/jamacardio.2017.3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor AL, Ziesche S, Yancy C, et al. ; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351 (20):2049–2057. doi: 10.1056/NEJMoa042934 [DOI] [PubMed] [Google Scholar]
- 46.Taylor AL. The African American Heart Failure Trial: a clinical trial update. Am J Cardiol. 2005;96 (7B):44–48. doi: 10.1016/j.amjcard.2005.07.033 [DOI] [PubMed] [Google Scholar]
- 47.Batchelor WB, Damluji AA, Yong C, et al. Does study subject diversity influence cardiology research site performance? insights from 2 U.S. national coronary stent registries. Am Heart J. 2021; 236:37–48. doi: 10.1016/j.ahj.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reza N, Tahhan AS, Mahmud N, et al. Representation of women authors in international heart failure guidelines and contemporary clinical trials. Circ Heart Fail. 2020;13(8):e006605. doi: 10.1161/CIRCHEARTFAILURE.119.006605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shavelle DM, Desai AS, Abraham WT, et al. ; CardioMEMS Post-Approval Study Investigators. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS Post-Approval Study. Circ Heart Fail. 2020;13(8): e006863. doi: 10.1161/CIRCHEARTFAILURE.119.006863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMurray JJV, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 51.Berardi C, Braunwald E, Morrow DA, et al. ; PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in Black Americans: data from the PIONEER-HF Trial. JACC Heart Fail. 2020;8(10): 859–866. doi: 10.1016/j.jchf.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 52.Cocoros NM, Fuller CC, Adimadhyam S, et al. ; FDA-Sentinel COVID-19 Working Group. A COVID-19-ready public health surveillance system: the FDA’s Sentinel System. Pharmacoepidemiol Drug Saf. 2021;30(7):827–837. doi: 10.1002/pds.5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community. 2004;12(5):382–388. doi: 10.1111/j.1365-2524.2004.00507.x [DOI] [PubMed] [Google Scholar]
- 54.Rucker-Whitaker C, Flynn KJ, Kravitz G, Eaton C, Calvin JE, Powell LH. Understanding African-American participation in a behavioral intervention: results from focus groups. Contemp Clin Trials. 2006;27(3):274–286. doi: 10.1016/j.cct.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 55.Branson RD, Davis K Jr, Butler KL. African Americans’ participation in clinical research: importance, barriers, and solutions. Am J Surg. 2007;193(1):32–39. doi: 10.1016/j.amjsurg.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 56.Fracasso PM, Goodner SA, Creekmore AN, et al. Coaching intervention as a strategy for minority recruitment to cancer clinical trials. J Oncol Pract. 2013;9(6):294–299. doi: 10.1200/JOP.2013.000982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang B, De Vore D, Chirinos C, et al. Strategies for recruitment and retention of underrepresented populations with chronic obstructive pulmonary disease for a clinical trial. BMC Med Res Methodol. 2019;19(1):39. doi: 10.1186/s12874-019-0679-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heller C, Balls-Berry JE, Nery JD, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials. 2014;39 (2):169–182. doi: 10.1016/j.cct.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radecki Breitkopf C, Williams KP, Ridgeway JL, et al. Linking education to action: a program to increase research participation among African American women. J Womens Health (Larchmt). 2018;27(10):1242–1249. doi: 10.1089/jwh.2017.6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3 (2):e19. doi: 10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma GX, Seals B, Tan Y, Wang SY, Lee R, Fang CY. Increasing Asian American participation in clinical trials by addressing community concerns. Clin Trials. 2014;11(3):328–335. doi: 10.1177/1740774514522561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otado J, Kwagyan J, Edwards D, Ukaegbu A, Rockcliffe F, Osafo N. Culturally competent strategies for recruitment and retention of African American populations into clinical trials. Clin Transl Sci. 2015;8(5):460–466. doi: 10.1111/cts.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivers DA, Pal T, Vadaparampil ST, Adams LA, Dash-Pitts L, Quinn GP. A community-academic partnership to explore informational needs of African American women as a primer for cancer clinical trial recruitment. Ethn Health. 2019;24(6): 679–693. doi: 10.1080/13557858.2017.1367762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sankaré IC, Bross R, Brown AF, et al. Strategies to build trust and recruit African American and Latino community residents for health research: a cohort study. Clin Transl Sci. 2015;8(5):412–420. doi: 10.1111/cts.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng J, McLaughlin M, Pariera K, Murphy S. A comparison between Caucasians and African Americans in willingness to participate in cancer clinical trials: the roles of knowledge, distrust, information sources, and religiosity. J Health Commun. 2016;21(6):669–677. doi: 10.1080/10810730.2016.1153760 [DOI] [PubMed] [Google Scholar]
- 66.O’Brien B, Shrestha S, Stanley MA, et al. Positive and negative religious coping as predictors of distress among minority older adults. Int J Geriatr Psychiatry. 2019;34(1):54–59. doi: 10.1002/gps.4983 [DOI] [PubMed] [Google Scholar]
- 67.Rivers D, August EM, Sehovic I, Lee Green B, Quinn GP. A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemp Clin Trials. 2013;35 (2):13–32. doi: 10.1016/j.cct.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 68.Yeary KHK, Moore PC, Gauss CH, et al. Reach and adoption of a randomized weight loss maintenance trial in rural African Americans of faith: the WORD (Wholeness, Oneness, Righteousness, Deliverance). Am J Health Promot. 2019;33(4): 549–557. doi: 10.1177/0890117118805065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diaz T, Navarro JR, Chen EH. An institutional approach to fostering inclusion and addressing racial bias: implications for diversity in academic medicine. Teach Learn Med. 2020;32(1):110–116. doi: 10.1080/10401334.2019.1670665 [DOI] [PubMed] [Google Scholar]
- 70.Skolarus LE, Cowdery J, Dome M, et al. Reach Out churches: a community-based participatory research pilot trial to assess the feasibility of a mobile health technology intervention to reduce blood pressure among African Americans. Health Promot Pract. 2018;19(4):495–505. doi: 10.1177/1524839917710893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson LM, Adeney KL, Shinn C, Safranek S, Buckner-Brown J, Krause LK. Community coalition-driven interventions to reduce health disparities among racial and ethnic minority populations. Cochrane Database Syst Rev. 2015; (6): CD009905. doi: 10.1002/14651858.CD009905.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson DA, Joosten YA, Wilkins CH, Shibao CA. Case study: community engagement and clinical trial success: outreach to African American women. Clin Transl Sci. 2015;8(4):388–390. doi: 10.1111/cts.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in Black barbershops. N Engl J Med. 2018; 378(14):1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta A, Calfas KJ, Marshall SJ, et al. Clinical trial management of participant recruitment, enrollment, engagement, and retention in the SMART study using a Marketing and Information Technology (MARKIT) model. Contemp Clin Trials. 2015;42:185–195. doi: 10.1016/j.cct.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prasanna A, Miller HN, Wu Y, et al. Recruitment of Black adults into cardiovascular disease trials. J Am Heart Assoc. 2021;10(17):e021108. doi: 10.1161/JAHA.121.021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.US Food and Drug Administration. Office of Pediatric Therapeutics. Reviewed October 25, 2021. https://www.fda.gov/about-fda/office-clinical-policy-and-programs/office-pediatric-therapeutics
- 77.US Food and Drug Administration. Pediatric Research Equity Act (Public Law 108–155). Updated November 7, 2019. https://www.fda.gov/drugs/development-resources/pediatric-research-equity-act-prea
- 78.National Institutes of Health. Faculty Institutional Recruitment for Sustainable Transformation (FIRST). Updated July 22, 2021. https://www.nimhd.nih.gov/programs/collab/first/
- 79.Association of Black Cardiologists Clinical Research Network. Accessed December 29, 2020. https://abcardio.org/clinical-research-network/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


