Abstract
Background
Young adult cancer survivors experience frailty and decreased muscle mass at rates equivalent to much older non-cancer populations indicating accelerated aging. While frailty and low muscle mass can be identified in survivors, their implications for health-related quality of life are not well understood.
Methods
Using cross-sectional analysis of young adult cancer survivors, we assessed frailty with the Fried frailty phenotype and skeletal muscle mass in relation to functional and quality of life outcomes measured by the Medical Outcomes Survey Short-Form 36 (SF-36). Z-tests compared survivors to U.S. population means, and multivariable linear regression models estimated mean SF-36 scores by frailty and muscle mass adjusting for comorbidities, sex, and time from treatment.
Results
Sixty survivors (median age 21 years, range 18–29) participated in the study. Twenty-five (42%) had low muscle mass and 25 were either frail or prefrail. Compared to U.S. population means, survivors reported worse health and functional impairments across SF-36 domains that were more common among survivors with (pre)frailty or low muscle mass. In multivariable linear modeling, (pre)frail survivors (v. non-frail) exhibited lower mean scores for general health (−9.1; p=0.05), physical function (−14.9; p<0.01), and overall physical health (−5.6; p=0.02) independent of comorbid conditions.
Conclusions
Measures of frailty and skeletal muscle mass identify subgroups of young adult cancer survivors with significantly impaired health, functional status, and quality of life independent of medical comorbidities. Identifying survivors with frailty or low muscle mass may provide opportunities for interventions to prevent functional and health declines or to reverse this process.
Keywords: frailty, muscle mass, health-related quality of life, adolescents, young adults, cancer survivorship
Lay Summary:
Young adult cancer survivors age more quickly than peers without cancer which is evidenced by a syndrome of decreased resilience known as frailty. We examined the relationship between frailty (and one of its common components, decreased muscle mass) and quality of life among young adult cancer survivors. Measuring decreased muscle mass and frailty identifies young survivors with poor quality of life including worse general health, fatigue, physical function, and overall physical health as compared to non-frail survivors. Interventions to address components of frailty (low muscle mass, weakness) may improve function and quality of life among young adult cancer survivors.
Precis:
Measuring decreased muscle mass and frailty identifies young adult cancer survivors with poor quality of life including worse general health, fatigue, physical function, and overall physical health as compared to non-frail survivors. Interventions to address components of frailty (low muscle mass, weakness) may improve function and quality of life among young adult cancer survivors.
BACKGROUND
Survival for childhood, adolescent, and young adult cancers has improved for decades with currently more than an estimated 700,000 such survivors in the United States [1]. However, due to cancer and its treatment, the majority of these survivors will develop chronic treatment-related morbidities much earlier in life [2–4]. Many will also experience physiological changes such as loss of skeletal muscle mass [5–7] and deficits in physical and cognitive function that are typically only seen in much older individuals [8–12]. In combination, these impairments reflect a phenomenon of accelerated aging that negatively impacts functional status and quality of life in these younger survivors with decades of life expectancy.
Frailty, a state of decreased physiological reserve associated with increased risk for morbidity and early mortality among older individuals [13, 14], is emerging as a way to characterize accelerated aging among young adult survivors [8, 15–18]. In a study of long-term survivors of childhood cancers (N=1922, on average, 34 years at enrollment and 25 years from diagnosis), the proportion of frail survivors (8%) surpassed that of individuals 65 years and older without a cancer history (7%) [13]. In a five-year prospective follow up study of childhood cancer survivors (N=1509, on average, 30 years at enrollment and 22 years from diagnosis), the prevalence of frailty increased from 6.2% to 13.6% at five years. Additionally, baseline frailty was associated with increased mortality (OR 3.52; 95% CI: 1.95–6.32) [15]. We have reported a similar prevalence of frailty (16%) among survivors of adolescent and young adult cancers (N=184, on average, 39 years at enrollment and 6 years from diagnosis) [19]. Among young adult cancer survivors, measures of physiological age such as frailty likely better estimate the risk for future disability and morbidity as compared to chronological age [4].
While measures of frailty and body composition may characterize the risk for future disability and mortality among young adult cancer survivors [15, 17], these measures may also identify risk for functional and health-related quality of life impairments in this population. Clear evidence demonstrates that among older individuals, both frailty and sarcopenia (a syndrome of decreased muscle mass and impaired muscle function [20]) have been associated with impaired quality of life across physical, emotional, and social domains [21–26]. While self-reported limitations in physical function or disability among adult survivors of childhood cancers have been associated with poor health-related quality of life [27, 28], studies examining health-related quality of life among frail or sarcopenic young adult survivors are lacking. Understanding the association between frailty and quality of life is important for young adult survivors, as addressing components of frailty (low muscle mass and weakness) may not only prevent new-onset chronic morbidities and early mortality but may also improve functional status and quality of life [16]. We assessed frailty and muscle mass in a cross-sectional cohort of young adult cancer survivors in relation to functional and quality of life measures using the Medical Outcomes Study Short Form 36 (SF-36). We hypothesized that both frailty and low muscle mass would be associated with impaired physical function and quality of life for young adult survivors.
METHODS
Study Population and Design
All study activities were reviewed and approved by the UNC Institutional Review Board.
Data for the current study were drawn from a cross-sectional examination of frailty and cellular senescence, the methods for which have been previously described [18]. Survivors of childhood, adolescent, or young adult cancers ages 18–29 years at study enrollment attending a routine survivorship appointment at either the University of North Carolina (UNC) Children’s Oncology or the UNC Adolescent and Young Adult Survivors’ Clinics from January 2018 to August 2019 were identified for study participation. Eligible survivors (1) had received any alkylator, anthracycline, and/or an anthracenedione as part of their cancer treatment regimen and (2) were able to read and understand English. After obtaining informed consent, patients completed measures of wellness and physical function (Medical Outcomes Survey Short Form 36 [SF-36] [29] and the National Health and Nutrition Examination Survey [NHANES] Physical Activity Questionnaire [30]) and evaluations for clinical frailty. Cancer, treatment, and sociodemographic factors were abstracted from the medical record. The intensity of treatment was standardized using a validated measure, the Intensity of Treatment Rating (ITR) Scale, version 3 [31]. Regimens were classified from lowest (1) to highest (4) intensity. Two oncologists blinded to outcomes independently assigned intensity scores with 100% agreement.
Clinical Frailty Evaluation
Frailty was assessed using a modified Fried phenotype [13] adapted by Ness [17] and previously used by our study team [18]. The Fried phenotype includes five clinically derived factors: weakness, slowness, decreased skeletal muscle mass, exhaustion, and low energy expenditure. Individuals with three or more factors were classified as frail, those with two factors as pre-frail, and those with ≤ 1 factor were non-frail. Weakness was measured with a hand-held hydraulic dynamometer (model J00105, Lafayette Instrument, Lafayette, IN) using the dominant hand while in a seated position with forearm flexed at the elbow to ninety degrees [13]. The average of two measures was used as the final output. Weakness was classified using standard population cutoffs based on body mass index calculated using height and weight obtained during the clinic visit [13]. Slowness was assessed by a 15-foot walk at the survivor’s usual pace on a hard, non-inclined surface. Women shorter than 159 cm and men shorter than 173 cm in height were considered slow with a time > 7 seconds. Women 159 cm or taller and men 173 cm or taller were considered slow with a time > 6 seconds [13]. No accommodations were made for individuals with health concerns affecting gait such as neuropathy, ataxia, or prosthesis. Skeletal muscle mass was measured using a bioelectrical impedance body composition analyzer [RJL Systems, Quantum IV, Clinton Township, MI) as described by the manufacturer with close attention to the participant’s hydration status. Resistance was measured at an operating frequency of 50kHz at 800 μA. Skeletal muscle mass was calculated from bioelectrical impedance values using the equation derived by Janssen and colleagues who used an RJL systems analyzer. [32, 33] Skeletal muscle index (SMI) was determined by dividing skeletal muscle mass by patient weight. Low muscle mass was defined as an SMI < 0.37 for men and < 0.28 for women as previously determined among a population of young adults [33]. Exhaustion was measured using the vitality subscale on the SF-36 [29]. A score ≤ 40 defined exhaustion, which correlates to one standard deviation below the population mean and the lowest 15.9% of the population. Leisure time energy expenditure was assessed using the NHANES physical activity questionnaire [30]. Low energy expenditure was classified as <383 kcal/week for men and <270 kcal/week for women as previously defined [13, 17].
Patient-Reported Quality of Life
The SF-36 is a well-validated and reliable self-reported 36-item questionnaire that assesses quality of life and impact of health status across eight domains: general health, physical function, limitations in function due to physical or emotional concerns, social function, mental health, vitality, and bodily pain [29, 34]. Two additional summary scores (physical health and mental health) can be generated using the eight subscales. Items assess function in the four weeks prior to survey completion. Subscales and summary scores were calculated and missing data were imputed as described by the SF-36 manual [34]. For scales in which the respondent answered at least 50% of the items, missing items were estimated by averaging the completed items in that scale for that respondent [34].
Statistical Analysis
One-sample z-tests were performed to compare survivor outcomes both to the general United States population (ages ≥ 18 years) and to a young adult subgroup (ages 24–35 years) [34]. Two-sample z-tests were performed to compare the SF-36 scores between subsets of patients within the cohort (low versus normal muscle mass; prefrail and frail versus non-frail). Multivariable generalized linear models were used to calculate mean SF-36 scores according to participants with low muscle mass (v. not) or (pre)frailty (v. non-frail) while adjusting for time off treatment (continuous in years), sex, and presence of one or more comorbidities (categorical as yes/no). P-values were calculated from Wald Chi-Square tests, with the threshold for statistical significance set at <0.05. All analyses were conducted using SAS software v9.4 (Cary, NC).
RESULTS
Sixty young adult cancer survivors participated, with a median age of 21 years at enrollment (range 18–29; median age at diagnosis 13.5, range 1–28) (Table 1). Participants were survivors of childhood, adolescent, and young adult cancers and were on average 6 years from the end of treatment (median 5.75 years, range 3 months-25 years). Participants were mostly female (n=37, 62%) and diagnosed with hematological malignancies (leukemia [40%], lymphoma [22%]). Classification by muscle mass status was completed in 59/60 (98%) survivors; one survivor had undergone bilateral hip replacements preventing BIA assessment. Fifty-six of the 60 survivors (93%) had complete frailty data – two survivors had incomplete survey data, one did not complete grip strength or timed walk, and the fourth was unable to complete the BIA.
Table 1:
Demographic, cancer, and treatment factors among 60 young adult cancer survivors, overall and according to skeletal muscle mass (SMM) and frailty status.
| Cohort Characteristic | All Survivors (n=60) | Low SMM (n=25) | Normal SMM (n=34) | (Pre)Frail (n=25) | Non-Frail (n=31) |
|---|---|---|---|---|---|
| Median Age at evaluation, year (Range) | 21 (18–29) | 20.5 (18–29) | 21 (18–29) | 21 (18–29) | 21 (18–29) |
| Female | 37 (62%) | 19 (73%) | 17 (52%) | 20 (80%) | 14 (45%) |
| Cancer Type | |||||
| Acute Leukemia | 24 (40%) | 10 (38%) | 13 (39%) | 10 (40%) | 11 (35%) |
| Lymphoma | 13 (22%) | 5 (19%) | 8 (24%) | 5 (20%) | 7 (23%) |
| Sarcoma | 12 (20%) | 6 (23%) | 6 (18%) | 6 (24%) | 6 (19%) |
| Central Nervous System | 5 (7%) | 4 (15%) | 1 (3%) | 3 (12%) | 2 (6%) |
| Embryonal | 4 (7%) | 1 (4%) | 3 (9%) | 1 (4%) | 3 (10%) |
| Germ Cell Tumor | 2 (3%) | 0 (0%) | 2 (6%) | 1 (4%) | 1 (3%) |
| Treatment Intensity | |||||
| ITRS-1/2 | 15 (25%) | 3 (12%) | 12 (36%) | 3 (12%) | 10 (32%) |
| ITRS-3 | 38 (63%) | 19 (73%) | 18 (55%) | 17 (68%) | 19 (61%) |
| ITRS-4 | 7 (12%) | 4 (15%) | 3 (9%) | 5 (20%) | 2 (7%) |
| Mean Total Anthracycline Dose (mg/m2 Dox)* | 213 (n=50) | 214 (n=20) | 215 (n=29) | 230 (n=19) | 215 (n=27) |
| Comorbidities (≥1) | 24 (40%) | 12 (46%) | 10 (30%) | 11 (44%) | 9 (29%) |
| Median time from treatment end, year (range) | 5.75 (0.25–25) | 4.65 (0.5–25) | 6.25 (0.25–21.5) | 4.00 (0.3–25) | 7.00 (0.25–21.5) |
Calculated for 50 survivors who received anthracycline (doxorubicin equivalents); SMM: skeletal muscle mass, ITRS: intensity of treatment rating scale
Of the 59 survivors completing BIA assessment, 25 (42%) were classified as having low muscle mass. Low muscle mass was more common among female participants, those with sarcoma or CNS cancers, and those who received more intensive therapy (ITR3–4) (Table 1). Of 56 evaluable survivors, 25 (45%) were either frail (16%) or pre-frail (29%); in subsequent analyses, these groups were combined as (pre)frail. As with survivors with low muscle mass, (pre)frailty was more common among females, survivors diagnosed with sarcoma or CNS cancers, and those treated with higher intensity regimens (ITR3–4). Low muscle mass and low energy expenditure were the most common frailty criteria with nearly half of all survivors exhibiting these traits (Table 2).
Table 2:
Criteria for frailty status and frailty classification by biological sex
| Total (n=56) | Female (n=35) | Male (n=21) | ||||||
|---|---|---|---|---|---|---|---|---|
| Frailty Criteria | N | % | N | % | N | % | ||
| Low Muscle Mass | 25 | 45 | 18 | 51 | 7 | 33 | ||
| Exhaustion | 16 | 29 | 14 | 40 | 2 | 10 | ||
| Slowness | 2 | 4 | 2 | 6 | 0 | 0 | ||
| Weakness | 8 | 14 | 5 | 14 | 3 | 14 | ||
| Low Energy Expenditure | 27 | 48 | 19 | 54 | 8 | 38 | ||
| Non-Frail (≤ 1 criterion) | 31 | 55 | 15 | 43 | 16 | 76 | ||
| Pre-Frail (2 criteria) | 16 | 29 | 12 | 34 | 4 | 19 | ||
| Frail (≥ 3 criteria) | 9 | 16 | 8 | 23 | 1 | 5 | ||
Survivors had a statistically significant lower general health score by the SF-36 compared to the general adult standard population (z-score: −3.3, p<0.001). Except for bodily pain, survivors also reported lower scores across all other SF-36 domains and composite scores as compared to the older general U.S. population; however, these deficits were not statistically significant (Figure 1A).
Figure 1:
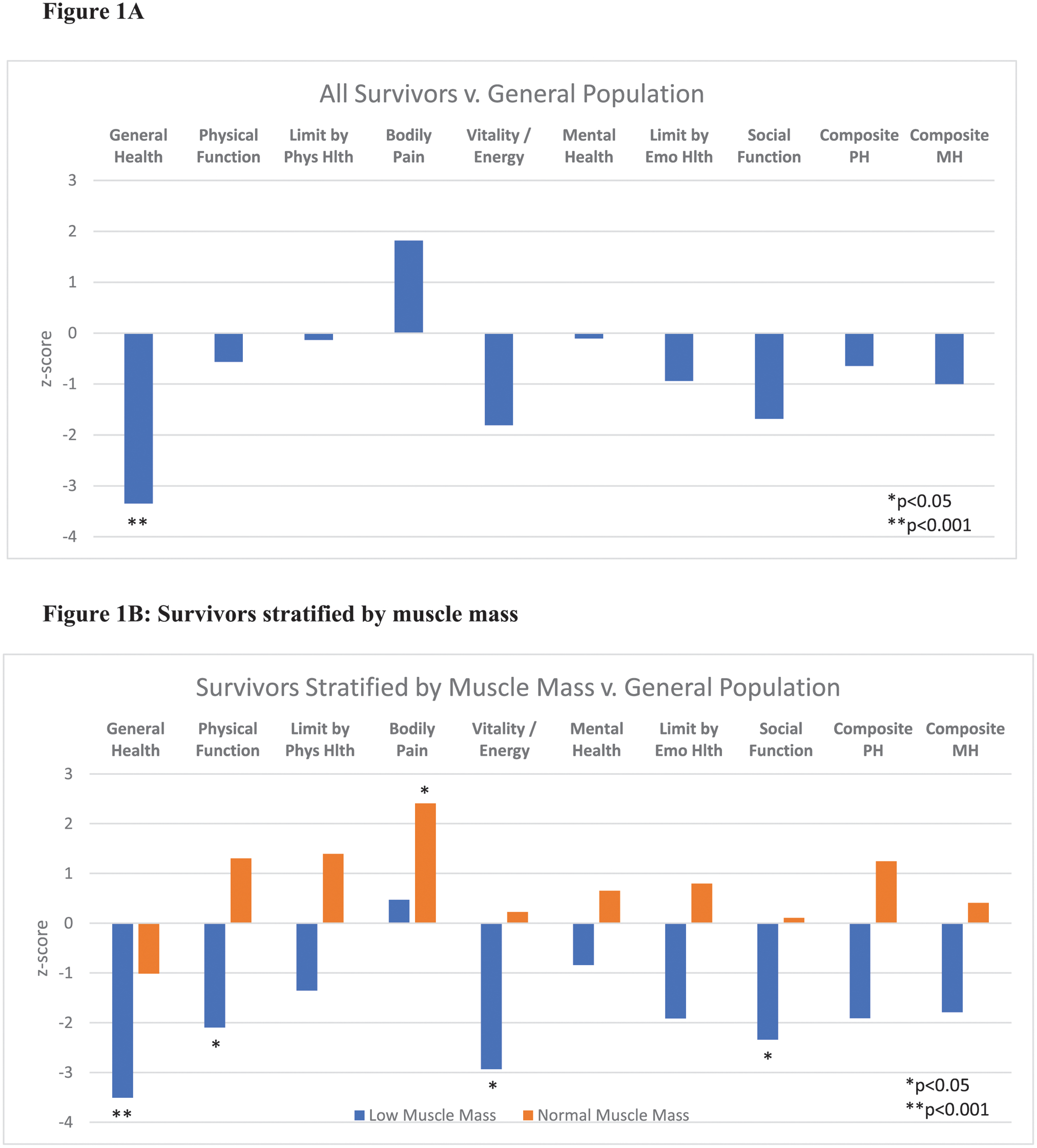
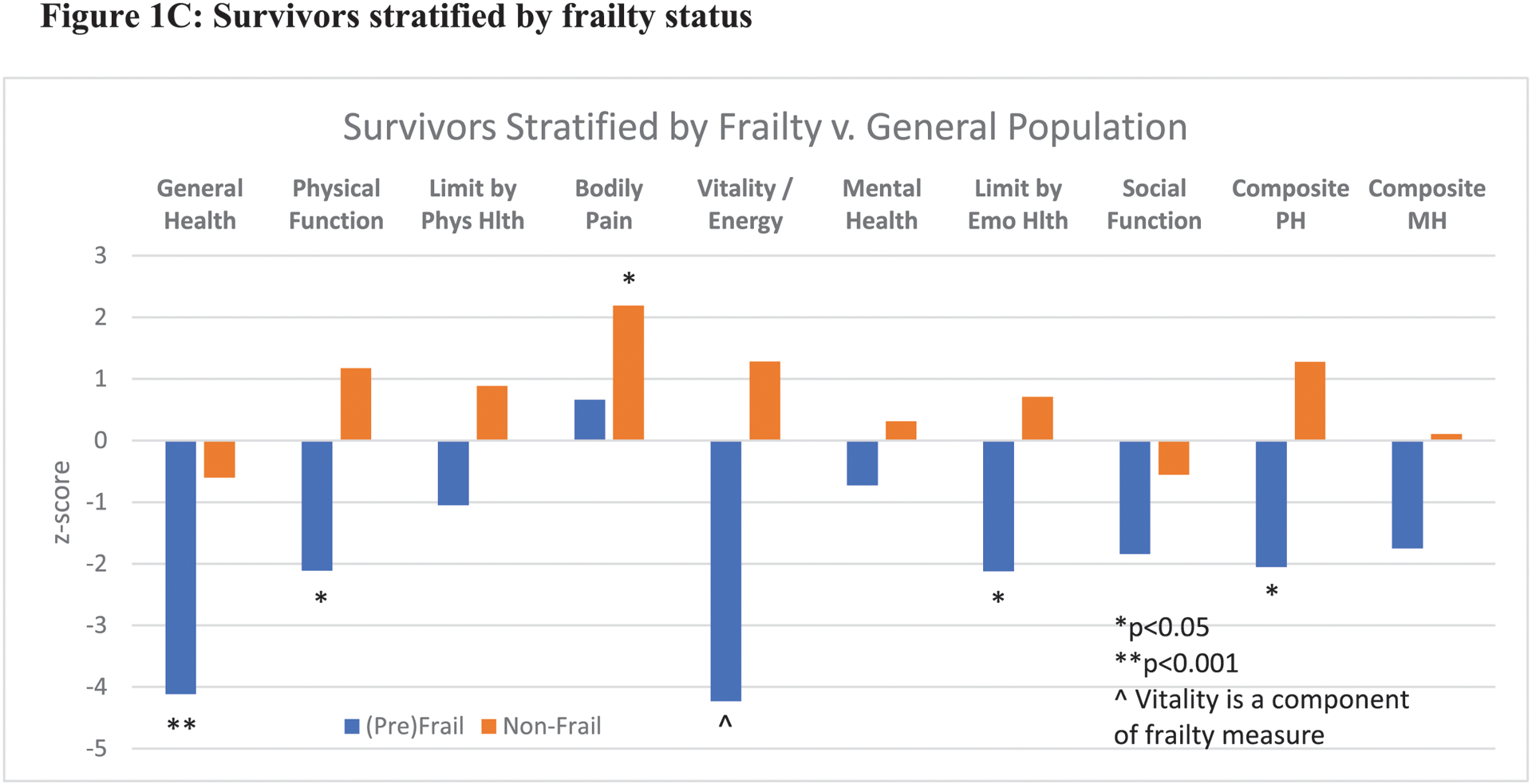
Reported quality of life among AYA survivors as compared to the general U.S. population by SF-36 domains. 1A: Comparison of full study cohort of survivors versus general population means. 1B and 1C: Survivors stratified by muscle mass and frailty status. Phys Hlth: physical health, Emo Hlth: emotional health, PH: physical health, MH: mental health
In analyses stratified by muscle mass and frailty status, several statistically significant deficits were observed among survivors with low muscle mass or (pre)frailty when compared to the general U.S. population (Figures 1B and 1C). Survivors with low muscle mass exhibited impairments in general health (z-score: −3.5, p<0.001), vitality (z-score: −2.9, p<0.01), and social function (z-score: −2.3, p<0.05). Survivors with normal muscle mass demonstrated no statistically significant deficits, and surprisingly, reported less bodily pain than the general US population (z-score: 2.4, p<0.05). Prefrail and frail survivors also exhibited statistically significant deficits in general health (z-score: −4.1, P<0.001), physical health (z-score: −2.1, p<0.05), functional limitations due to emotional health (z-score: −2.1, p<0.05), and composite physical health score (z-score: −2.1, p<0.05).
When compared to a young adult standard U.S. population (ages 24–35 years), even greater deficits in health-related quality of life were observed among survivors, most significantly in general health (z-score −6.0, p<0.001), physical function (z-score −4.5, p<0.001), and composite physical health score (z-score −4.7, p<0.001) (Figure 2A). In analyses stratified by low muscle mass and frailty status, the deficits among survivors as compared to the young adult standard population were overwhelmingly reported among the survivors with low muscle mass or (pre)frailty (Figures 2B and 2C). Survivors with low muscle mass or (pre)frailty exhibited statistically significant deficits in physical, emotional, and social functioning.
Figure 2:
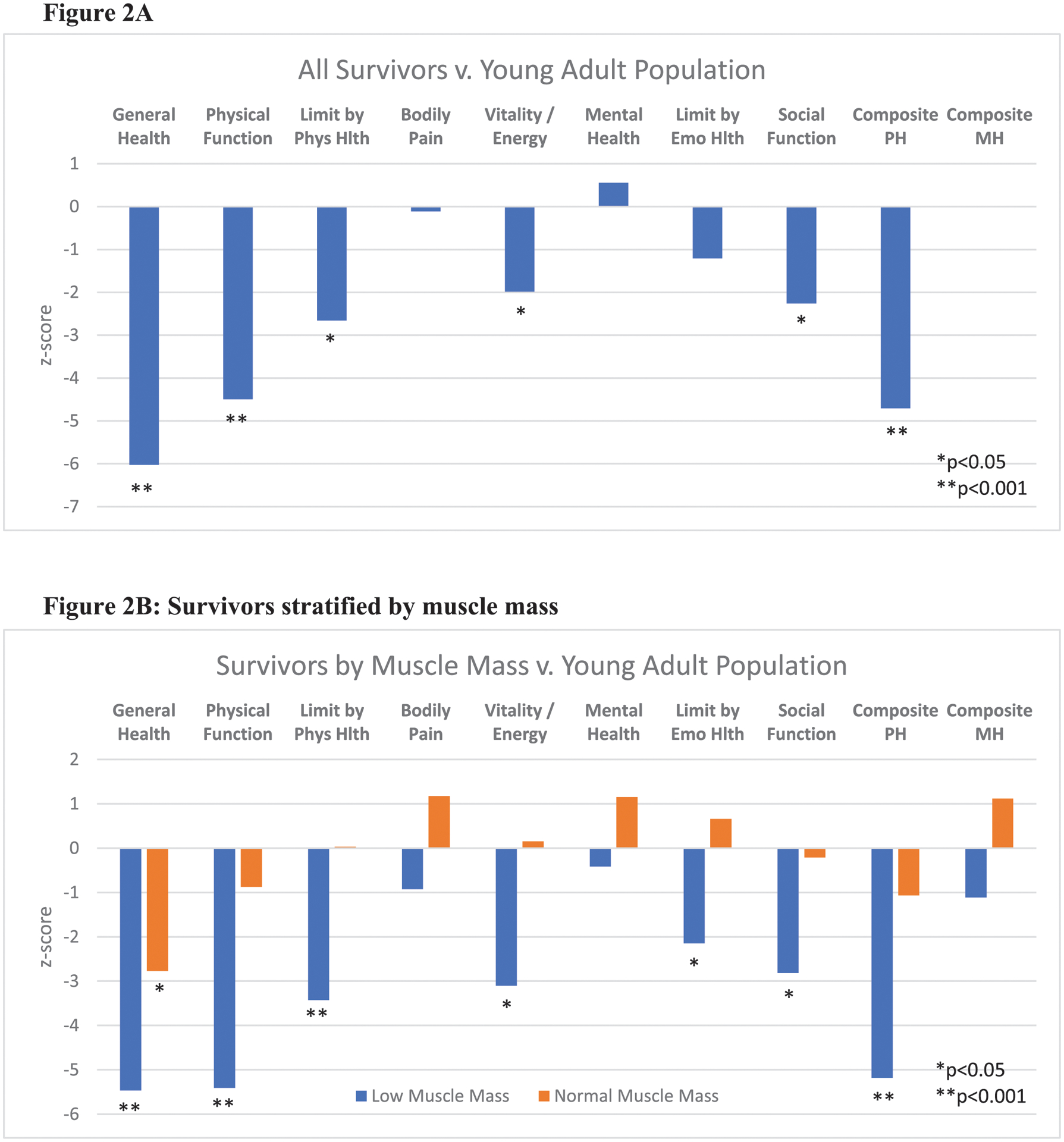
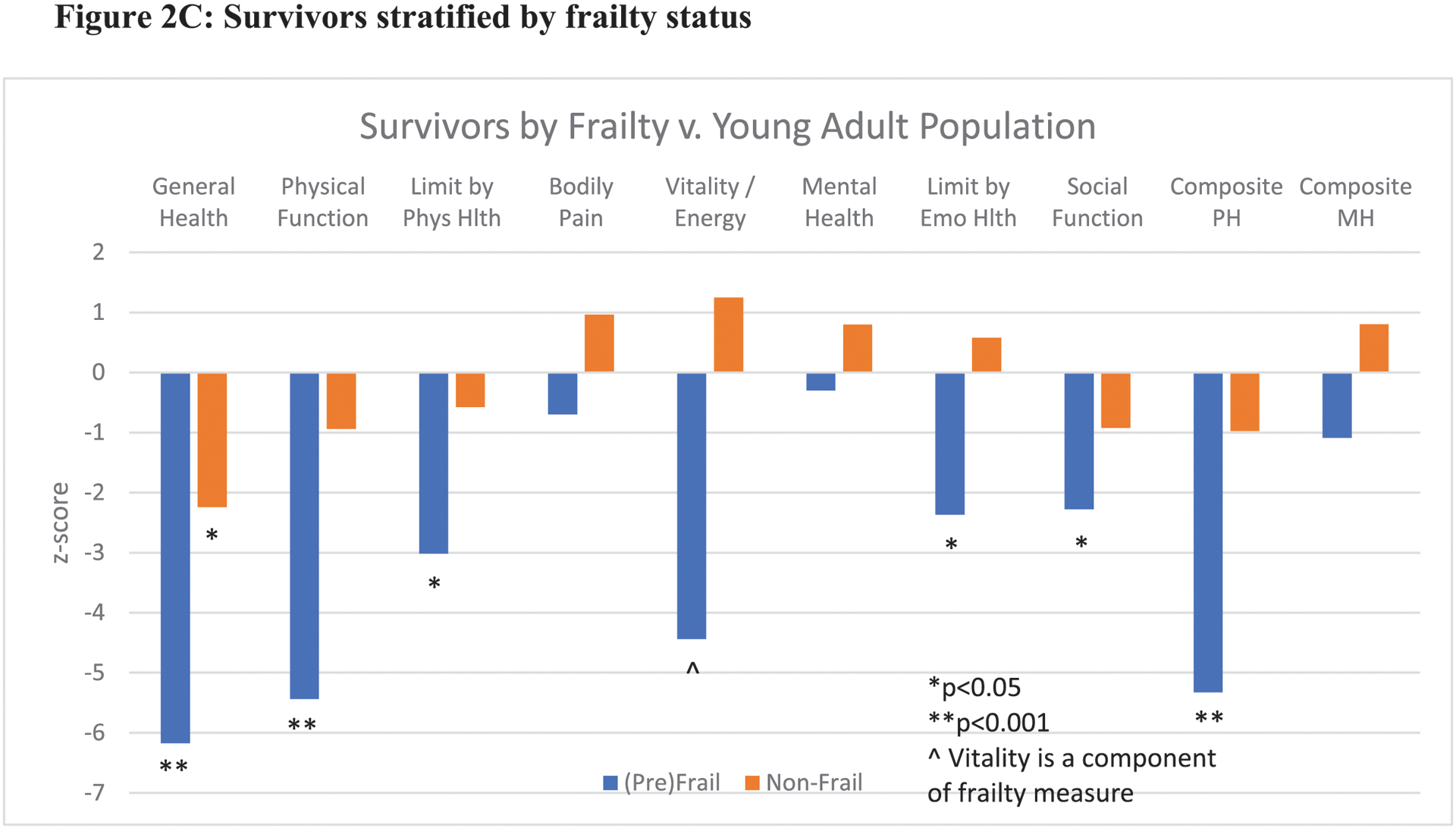
Reported quality of life among AYA survivors as compared to a young adult (24–35yrs) U.S. population by SF-36 domains. 2A: Comparison of full study cohort of survivors versus general population means. 2B and 2C: Survivors stratified by muscle mass and frailty status. Phys Hlth: physical health, Emo Hlth: emotional health, PH: physical health, MH: mental health
Using multivariable linear regression, mean SF-36 scores were lower across all domains for survivors with low muscle mass (v. normal muscle mass) and (pre)frailty (v. non-frail) (Table 3). Compared to survivors with normal muscle mass, survivors with low muscle mass exhibited poorer physical function (mean difference in score −13.2, p=0.01), greater limitations due to physical (−15.3, p=0.05) and emotional health (−14.4, p=0.06), more fatigue (−10.6, p=0.03), and lower overall physical health (−5.1, p=0.02). Compared to non-frail survivors, (pre)frail survivors had greater impairment of general health (−9.1, p=0.05), physical function (−14.9, p<0.01), and overall physical health (−5.6, p=0.02).
Table 3:
Multivariable model estimating mean differences in SF-36 domain scores among AYA survivors by muscle mass and frailty status.
| Low SMM v. Normal SMM | (Pre)Frail v. Non-Frail | |||
|---|---|---|---|---|
| SF-36 Domain | Estimate | p-value | Estimate | p-value |
| General Health | −7.1 | 0.11 | −9.1 | 0.05 |
| Physical Function | −13.2 | 0.01 | −14.9 | <0.01 |
| Limitations due to Physical Health | −15.3 | 0.05 | −8.4 | 0.33 |
| Bodily Pain | −4.8 | 0.33 | −0.7 | 0.89 |
| Vitality / Energy | −10.6 | 0.03 | * | * |
| Mental Health | −4.5 | 0.28 | −1.1 | 0.81 |
| Limitations due to Emotional Health | −14.4 | 0.06 | −10.1 | 0.24 |
| Social Function | −8.6 | 0.17 | −2.1 | 0.76 |
| Composite Physical Health | −5.1 | 0.02 | −5.6 | 0.02 |
| Composite Mental Health | −2.9 | 0.24 | −1.6 | 0.56 |
Models adjusted for time off therapy, sex, and presence of ≥ 1 comorbidities; SMM: skeletal muscle mass; *Vitality is a component of frailty measure
Vitality is a component of frailty measure
DISCUSSION
Childhood and young adult cancer survivors often experience accelerated aging manifested by early functional impairments, declining physiology, and medical comorbidities usually seen in much older individuals. In our study of young adult cancer survivors, frailty and low muscle mass, clinical indicators of aging, were common. (Pre)Frailty and low muscle mass identify a high-risk sub-group of survivors experiencing significant health, well-being, and functional impairments independent of medical comorbidities.
Survivors with frailty or pre-frailty reported significantly worse general, physical, emotional, and social health than a general U.S. comparator population, which was on average, older than the survivors (mean age: 58 years) [35]. This is suggestive of accelerated aging among these survivors. Additionally, (pre)frail survivors had significantly poorer health than their non-frail survivor peers. These findings suggest that not only is frailty evident among young adult survivors, it is associated with increased risk for adverse functional and health outcomes. As a clinical indicator of accelerated aging, the physical frailty phenotype identifies survivors at high risk for negative outcomes. Interventions to mitigate aspects of frailty such as low muscle mass or weakness may help improve function and quality of life for survivors in addition to reducing the risks for subsequent morbidity and mortality.
Prior studies of long-term survivors of childhood cancers (20–30 years from treatment) observed a significant proportion of survivors to be (pre)frail and who were at increased risk for incident medical morbidities and early mortality [15, 17]. Identifying frail survivors may allow for earlier interventions to prevent further functional and health declines or even to reverse this process [36–38]. Our study adds to these findings demonstrating that (pre)frailty is common among survivors who were older at the time of treatment and who are earlier in survivorship, on average six years post-treatment with a median age of 21 years. Our findings suggest that opportunities to reverse the impact of frailty on young adult survivors may present early in survivorship.
Further, we demonstrate that low muscle mass, a common frailty criterion in our study population, is by itself a risk factor for poor function and health. While loss of skeletal muscle mass is an established feature of aging in humans [39], our findings show that this process can occur much earlier among young adult survivors. Among older adults, low muscle mass and sarcopenia are known to associate with functional impairment, decreased health-related quality of life, and increased mortality [33, 39–41]. Our findings show that low muscle mass, as assessed using BIA, is associated with poor general health, physical function, energy, social well-being, and overall physical health among young adult survivors. Except in the pain subscale, survivors with low muscle mass uniformly reported lower scores across SF-36 subscales and composite scales as compared to an older general U.S. population and had lower scores in all subscales and composite scales when compared to a young adult standard U.S. population. Further, assessment of skeletal muscle mass using BIA, a non-invasive method that could be scaled and incorporated into clinical workflows, identifies young adult survivors at risk for adverse health and functional outcomes. Survivors with low muscle mass may benefit from early interventions such as prescribed physical activity and resistance regimens to prevent further muscle loss or even to reverse this process [37].
While survivors with low muscle mass or (pre)frailty were observed to have the most significant deficits in physical health domains, they also experience impairment in social and emotional health domains. These findings are not unexpected as survivors with impaired physical function and disabilities can experience limitations in carrying out social activities and would be at risk for poor mental health due to these limitations. Depression or social isolation could contribute to decreased physical activity, poor diet, or unhealthy behaviors that subsequently lead to further loss of muscle mass or the development of (pre)frailty. Due to the cross-sectional design of this study, we are unable to determine causality between decreased muscle mass or frailty and deficits in social functioning and emotional health. While further prospective studies are needed to clarify causation within this relationship, it is clear from these results that physical, emotional, and social deficits are closely associated with low muscle mass or frailty among survivors.
Our findings are limited by the cross-sectional design of the study. While there is a clear association, we are unable to determine if frailty and low muscle mass in our study population lead to adverse outcomes such as functional impairment or poor health or if functional difficulties and impaired health are the cause for decreased muscle mass and frailty. Based on prior studies that have demonstrated a higher risk for incident medical morbidities, disability[13–15, 17, 33, 40], and poor health among individuals with baseline frailty or low muscle mass, we hypothesize that this same causal relationship exists for young adult survivors with frailty and low muscle mass early in survivorship. We are pursuing prospective studies with repeated measures of body composition and the frailty phenotype to help answer this question. Additionally, due to the small cohort size we are unable to examine the associations between muscle mass or frailty and health outcomes in subgroups such as by cancer type, specific treatment exposures, or sociodemographic factors that might modify these associations or serve as confounders. We were able to estimate the association between these aging markers and health outcomes while adjusting for the presence of medical comorbidities, biological sex, and time from treatment completion. Further, our study population only included survivors who received cytotoxic chemotherapies limiting our ability to describe the relationship between frailty and functional status among survivors treated solely with other modalities such as surgical, radiation, hormonal, and immune therapies. We anticipate that subsequent studies will include AYAs treated with these modalities.
In summary, we observed (pre)frailty and low skeletal muscle mass to be common among young adult cancer survivors early in survivorship and that these clinical indicators of aging are associated with significant functional and health impairment. Frailty and skeletal muscle mass assessment among survivors may help to risk stratify survivors that would most benefit from early interventions to reverse or mitigate loss of function, disability, and poor health. Future work to prospectively assess these associations and define the best interventions to address low muscle mass and frailty in young adult survivors in order to promote decades of health and well-being is needed.
Acknowledgements:
The authors would like to thank Dr. Anne Kazak and colleagues for granting us permission to use the Intensity of Treatment Rating Scale.
Funding:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490 (ABS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support was also provided by the Hyundai Hope on Wheels Foundation (ABS).
Footnotes
Potential Conflicts of Interest: none
References
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019–2021. Atlanta: American Cancer Society, 2019. [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309(22):2371–81 doi: 10.1001/jama.2013.6296[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355(15):1572–82 doi: 10.1056/NEJMsa060185[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14(1):61–70 doi: 10.1038/nrc3634[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guida JL, Ahles TA, Belsky D, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst 2019;111(12):1245–54 doi: 10.1093/jnci/djz136[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joffe L, Schadler KL, Shen W, Ladas EJ. Body Composition in Pediatric Solid Tumors: State of the Science and Future Directions. J Natl Cancer Inst Monogr 2019;2019(54):144–48 doi: 10.1093/jncimonographs/lgz018[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orgel E, Mueske NM, Sposto R, Gilsanz V, Freyer DR, Mittelman SD. Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy. Leuk Lymphoma 2018;59(1):138–45 doi: 10.3109/10428194.2015.1136741[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness KK, Kirkland JL, Gramatges MM, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol 2018;36(21):2206–15 doi: 10.1200/JCO.2017.76.7467[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenian SH, Gibson CJ, Rockne RC, Ness KK. Premature Aging in Young Cancer Survivors. J Natl Cancer Inst 2019;111(3):226–32 doi: 10.1093/jnci/djy229[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book 2014:e423–30 doi: 10.14694/EdBook_AM.2014.34.e423[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 11.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol 2012;30(29):3618–24 doi: 10.1200/JCO.2012.42.6841[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Brussel M, Takken T, Lucia A, van der Net J, Helders PJ. Is physical fitness decreased in survivors of childhood leukemia? A systematic review. Leukemia 2005;19(1):13–7 doi: 10.1038/sj.leu.2403547[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–56 doi: 10.1093/gerona/56.3.m146[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ 2011;183(8):E487–94 doi: 10.1503/cmaj.101271[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaney A, Howell CR, Krull KR, et al. Progression of Frailty in Survivors of Childhood Cancer: a St. Jude Lifetime Cohort Report. J Natl Cancer Inst 2021. doi: 10.1093/jnci/djab033[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ness KK, Howell CR, Bjornard KL. Frailty and quality of life in adult survivors of childhood cancer. Expert Rev Qual Life Cancer Care 2017;2(2):79–85 doi: 10.1080/23809000.2017.1300507[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 2013;31(36):4496–503 doi: 10.1200/JCO.2013.52.2268[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smitherman AB, Wood WA, Mitin N, et al. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16(INK4a) and frailty. Cancer 2020;126(22):4975–83 doi: 10.1002/cncr.33112[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smitherman AB, Anderson C, Lund JL, Bensen JT, Rosenstein DL, Nichols HB. Frailty and Comorbidities Among Survivors of Adolescent and Young Adult Cancer: A Cross-Sectional Examination of a Hospital-Based Survivorship Cohort. J Adolesc Young Adult Oncol 2018;7(3):374–83 doi: 10.1089/jayao.2017.0103[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393(10191):2636–46 doi: 10.1016/S0140-6736(19)31138-9[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 21.Rizzoli R, Reginster JY, Arnal JF, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013;93(2):101–20 doi: 10.1007/s00223-013-9758-y[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crocker TF, Brown L, Clegg A, et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Qual Life Res 2019;28(8):2041–56 doi: 10.1007/s11136-019-02149-1[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henchoz Y, Bula C, Guessous I, Santos-Eggimann B. Association between Physical Frailty and Quality of Life in a Representative Sample of Community-Dwelling Swiss Older People. J Nutr Health Aging 2017;21(5):585–92 doi: 10.1007/s12603-016-0772-4[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 24.Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health 2016;70(7):716–21 doi: 10.1136/jech-2015-206717[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Aguilar M, Garcia-Lara JM, Aguilar-Navarro S, Navarrete-Reyes AP, Amieva H, Avila-Funes JA. The Phenotype of Frailty and Health-Related Quality of Life. J Frailty Aging 2013;2(1):2–7 doi: 10.14283/jfa.2013.1[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Tsekoura M, Kastrinis A, Katsoulaki M, Billis E, Gliatis J. Sarcopenia and Its Impact on Quality of Life. Adv Exp Med Biol 2017;987:213–18 doi: 10.1007/978-3-319-57379-3_19[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Ness KK, Gurney JG, Zeltzer LK, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil 2008;89(1):128–36 doi: 10.1016/j.apmr.2007.08.123[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan R, Clohisy DR, Neglia JP, et al. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: the Childhood Cancer Survivor Study. Br J Cancer 2004;91(11):1858–65 doi: 10.1038/sj.bjc.6602220[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83 [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). In: U.S. Department of Health and Human Services CfDCaP, ed. Hyattsville, MD, 2009. [Google Scholar]
- 31.Kazak AE, Hocking MC, Ittenbach RF, et al. A revision of the intensity of treatment rating scale: classifying the intensity of pediatric cancer treatment. Pediatr Blood Cancer 2012;59(1):96–9 doi: 10.1002/pbc.23320[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000;89(2):465–71 doi: 10.1152/jappl.2000.89.2.465[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 33.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50(5):889–96 doi: 10.1046/j.1532-5415.2002.50216.x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE Jr. SF-36 Health Survey. The use of psychological testing for treatment planning and outcomes assessment, 2nd ed. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers, 1999:1227–46. [Google Scholar]
- 35.Ware JE, Kosinski M and Keller SD SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: Health Assessment Lab, 1994. [Google Scholar]
- 36.Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci 2015;70(2):216–22 doi: 10.1093/gerona/glu099[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzetti E, Calvani R, Tosato M, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res 2017;29(1):35–42 doi: 10.1007/s40520-016-0705-4[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 38.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 2006;166(8):860–6 doi: 10.1001/archinte.166.8.860[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 39.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 2008;12(7):433–50 doi: 10.1007/BF02982704[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams GR, Rier HN, McDonald A, Shachar SS. Sarcopenia & aging in cancer. J Geriatr Oncol 2019;10(3):374–77 doi: 10.1016/j.jgo.2018.10.009[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams GR, Dunne RF, Giri S, Shachar SS, Caan BJ. Sarcopenia in the Older Adult With Cancer. J Clin Oncol 2021;39(19):2068–78 doi: 10.1200/JCO.21.00102[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]


