ABSTRACT
The Gram-negative pathogen Pasteurella multocida is the causative agent of many important animal diseases. While a number of P. multocida virulence factors have been identified, very little is known about how gene expression and protein production is regulated in this organism. One mechanism by which bacteria regulate transcript abundance and protein production is riboregulation, which involves the interaction of a small RNA (sRNA) with a target mRNA to alter transcript stability and/or translational efficiency. This interaction often requires stabilization by an RNA-binding protein such as ProQ or Hfq. In Escherichia coli and a small number of other species, ProQ has been shown to play a critical role in stabilizing sRNA-mRNA interactions and preferentially binds to the 3′ stem-loop regions of the mRNA transcripts, characteristic of intrinsic transcriptional terminators. The aim of this study was to determine the role of ProQ in regulating P. multocida transcript abundance and identify the RNA targets to which it binds. We assessed differentially expressed transcripts in a proQ mutant and identified sites of direct ProQ-RNA interaction using in vivo UV-cross-linking and analysis of cDNA (CRAC). These analyses demonstrated that ProQ binds to, and stabilizes, ProQ-dependent sRNAs and transfer RNAs in P. multocida via adenosine-enriched, highly structured sequences. The binding of ProQ to two RNA molecules was characterized, and these analyses showed that ProQ bound within the coding sequence of the transcript PmVP161_1121, encoding an uncharacterized protein, and within the 3′ region of the putative sRNA Prrc13.
IMPORTANCE Regulation in P. multocida involving the RNA-binding protein Hfq is required for hyaluronic acid capsule production and virulence. This study further expands our understanding of riboregulation by examining the role of a second RNA-binding protein, ProQ, in transcript regulation and abundance in P. multocida.
KEYWORDS: ProQ, sRNA, Pasteurella multocida, RNA-binding proteins
INTRODUCTION
Bacteria often utilize small RNA (sRNA) molecules as regulators of protein production (1). This type of regulation generally involves an sRNA molecule binding to an mRNA target to modulate either transcript abundance or translational efficiency (2). The efficient binding between the sRNA and mRNA target to form a duplex often requires the presence of an RNA-binding protein, as base pairing between the RNA species can involve as few as seven consecutive nucleotides of complementarity (2). The best characterized of these RNA-binding proteins is Hfq. However, several other RNA-binding proteins have been identified that play essential roles in modulating the RNA regulatory network in different bacterial species (3). The RNA-binding proteins ProQ and CspA are involved in posttranscriptional regulation, with each having both unique and shared subsets of sRNAs and/or mRNA targets (3). ProQ was originally identified in Escherichia coli as an osmoregulatory protein that controlled the production of a proline pump called ProP, but later studies involving E. coli and Salmonella enterica demonstrated that ProQ acts as a global RNA-binding protein (4, 5). Structural analysis of E. coli ProQ has identified three distinct domains, a large N-terminal FinO-like domain, a central linker region, and then a C-terminal Tudor domain (5, 6). In Tudor domain-containing eucaryotic proteins and bacterial ProQ proteins, this domain has been shown to bind to ribonuclear proteins, allowing them to bind RNA (7–9). However, experiments studying the interaction of E. coli ProQ with several RNAs have demonstrated that the concave face of the FinO-like domain in ProQ also facilitates binding to the RNA (10). The FinO-like domain is highly conserved among ProQ proteins and shares structural and functional characteristics with the FinO RNA chaperone that is on the E. coli IncF plasmid (10).
Recent high-throughput analyses of other species, using methods such as gradient profiling by sequencing (Grad-seq) and cross-linking immunoprecipitation sequencing (CLIP-seq), have identified RNAs that bind to ProQ and other RNA-binding proteins (4, 11). The S. enterica ProQ was shown to bind to 467 RNA transcripts. Of those, 18% were predicted sRNA molecules, indicating that ProQ interacts with a specialized subset of sRNA molecules to help them form a stable duplex with their mRNA targets (4). Modeling of each of the identified S. enterica ProQ-binding sRNAs indicated all were highly structured, unlike the sRNAs that interacted with Hfq (4). While Hfq is known to bind to a repeated ARN (adenosine, purine, any nucleotide) sequence motif on the distal face and uridine (U)-rich motifs on the lateral and proximal surfaces, no such sequence-specific binding motifs have been identified in ProQ targets (11). However, it is now understood that ProQ binding is dependent on the secondary structure of the RNA, though it is likely other factors are also involved (11). Experiments examining ProQ binding partners have shown ProQ binds to stem-loop regions at the 3′ region of RNA transcripts, often correlating with predicted intrinsic terminators (12). The minimum requirements for intrinsic terminators bound by ProQ include a stem-loop region consisting of at least two base pairs, preceded by an adenosine-rich region and followed by a poly(U) tail consisting of a minimum of three consecutive uridines (12). Experiments investigating ProQ binding to this type of intrinsic terminator at the 3′ region of cspE RNA in S. enterica provided evidence that ProQ protected the transcript from the degradative action of RNase II (11).
A small number of specific ProQ-sRNA interactions have been characterized, including the interaction that involves the E. coli sRNA MalM. MalM can bind to both Hfq and ProQ, but further experiments involving electrophoretic mobility shift assays (EMSA) showed that MalM preferentially bound to ProQ (5). In addition, the S. enterica ProQ was shown to stabilize the RaiZ sRNA, allowing binding to its mRNA target, hupA. This action blocked the ribosome from binding and therefore reduced HupA production (4). Despite HupA being a histone-like protein that acts in transcriptional regulation, no phenotype was identified when the interaction was interrupted (13). ProQ is involved in regulation of virulence in S. enterica (14), as ProQ binds to the 3′-untranslated region (UTR)-derived sRNA STnc540, and the complex binds to a magnesium transporter mRNA that is essential for virulence (14).
While there have been whole-transcriptome studies examining the role of ProQ in enteric species, such as E. coli and S. enterica, and in Neisseria meningitidis, to date there have been no such studies looking at the role of this RNA-binding protein in the Pasteurellaceae family of Gram-negative bacteria. Pasteurella multocida causes a wide range of diseases in a number of important production animals, including hemorrhagic septicemia in cattle and fowl cholera in poultry (15). Strains within this species can be classified into five capsule serogroups, A, B, D, E, and F, based on the composition of the extracellular capsule and into eight lipopolysaccharide (LPS) genotypes, L1 through to L8, based on the gene content within the LPS outer core assembly locus (15). While the roles of capsule, LPS, and other virulence factors such as filamentous hemagglutinin in disease outcome have been well characterized in fowl cholera isolates (16–18), our understanding of how the production of these and other cellular components are regulated is incomplete.
Currently, it is known that both the global transcriptional regulator Fis and the RNA-binding protein/chaperone Hfq are important for the positive regulation of serogroup A hyaluronic acid (HA) capsule production in P. multocida (19, 20). Capsule production was significantly reduced in a P. multocida fis mutant compared to the parent strain and, to a lesser extent, in a P. multocida hfq mutant. In addition, expression of hfq was shown to be reduced in the absence of Fis, indicating that Fis probably has a regulatory effect on Hfq but also acts independently to regulate capsule production (20).
Bioinformatic analyses of the available P. multocida genomes has identified that a ProQ homolog is in all genomes. Identifying the cohort of RNA species that bind to ProQ is a crucial first step in unravelling the regulatory networks in P. multocida and other bacterial pathogens within the Pasteurellaceae family. To this end we have used a number of techniques to comprehensively identify RNAs that interact with ProQ in the highly virulent fowl cholera isolate P. multocida strain VP161 (21).
RESULTS
ProQ production and genomic context in P. multocida.
A ProQ homolog was identified within P. multocida strain VP161 using the Basic Local Alignment Search Tool (BLAST). Analysis of previously published proteomics data of P. multocida strain VP161 grown in heart infusion (HI) medium confirmed that ProQ was produced at mid-exponential growth phase (optical density at 600 nm [OD600], 0.6) (22). The P. multocida proQ is the first of five closely associated genes on the same strand; immediately adjacent to proQ is prc, followed by PmVP161_0957, PmVP161_0956, and gloC (Fig. 1A). There is a G-C-rich stem-loop (5′-TTCCCCCTTTCTTTTTAGGGGGAA-3′) in the intergenic region between PmVP161_0957 and PmVP161_0956 and a predicted Rho-independent terminator (5′-GCCCCTGTTATCAGGGGCTTTT-3′) 16 bp downstream of gloC. In E. coli and S. enterica, proQ is also colocated with prc (but is not associated with homologues of the other three P. multocida genes), and the E. coli prc is transcribed from a promoter located within proQ, 507 bp upstream of the 3′ end of proQ (23). To determine if a similar organization occurred in P. multocida, the 5′ rapid amplification of cDNA ends (5′RACE) technique was used to identify the transcriptional start site of prc. A transcript was detected that started 383 nt upstream of the prc ATG translational start and within proQ (nt 342), indicating that prc is transcribed separately from proQ from a promoter within proQ.
FIG 1.
Genomic location of proQ and growth of the wild-type P. multocida VP161, proQ mutant, and complemented strains in different growth media. (A) Schematic representation of the location of proQ in the genome and the positions of the TargeTron intron (orange box), prc transcriptional start site, and stem-loop sequences. (B and C) Growth curves of WT[EV] (circles), proQ[EV] (squares), and proQ[proQ] (triangles) over 24 h with shaking at 37°C when incubated in heart infusion (HI) broth (B) or HI with 300 mM NaCl (C). (D) Growth curves of WT[EV] (squares), proQ[EV] (circles), and proQ[proQ] (triangles) over 24 h, with shaking at 37°C, when grown in peptone water containing 1% glucose, either untreated (0 μM) (black) or iron-depleted using pretreatment with dipyridyl (150 μM) (gray). Data shown are means ± standard deviations (SD) (n = 3).
ProQ is not involved in the growth or osmotic tolerance of P. multocida.
To determine the role of ProQ within P. multocida, an insertional proQ mutant (AL2973) (Table 1) was constructed in the P. multocida strain VP161 using TargeTron mutagenesis. To ensure transcription of prc was uninterrupted, an intron site at proQ nucleotide (nt) 165 was chosen that was 198 nt upstream of the prc transcriptional start site (Fig. 1A). To generate a complementation vector, the proQ gene was then cloned into the P. multocida vector pBA1100S (pAL1449) (Table 1). Following this, the wild-type (WT) strain was transformed with empty vector pPBA1100S to generate the WT[EV] strain (AL3356) (Table 1), the proQ mutant was transformed with empty vector to generate the proQ[EV] strain (AL3358) (Table 1), and the proQ mutant was transformed with the proQ complementation plasmid pAL1449 to generate the proQ[proQ] strain (AL3357) (Table 1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| P. multocida | ||
| VP161 | Serotype A:1 virulent strain, avian isolate | 21 |
| AL2973 | VP161 proQ TargeTron mutant; Kanr | This study |
| AL3067 | VP161 proQ-hyaD TargeTron double mutant; Kanr | This study |
| AL3068 | AL3067 containing pAL1339; Specr | This study |
| AL3069 | AL3067 containing pAL1332; Specr | This study |
| AL3356 | WT[EV], VP161 containing vector pPBA1100S; Specr | This study |
| AL3357 | proQ[proQ], AL2973 proQ mutant provided with an intact copy of proQ on pAL1449; Specr | This study |
| AL3358 | proQ[EV], AL2973 proQ mutant with vector pPBA1100S; Specr | This study |
| E. coli | ||
| AL1995 | DH5α containing pAL953; Kanr, Specr | 38 |
| AL2227 | DH5α containing pAL1069; Kanr, Specr | 20 |
| AL2708 | DH5α containing pREXY; Specr | This study |
| AL2970 | DH5α containing pAL1291; Kanr, Specr | This study |
| AL3058 | DH5α containing pAL1333; Specr | This study |
| AL3064 | DH5α containing pAL1337; Specr | This study |
| AL3065 | DH5α containing pAL1338; Specr | This study |
| DH5α | deoR endA1 gryA96 hsdR17(rk− mk+) recA1 relA1 supE44 thi-1 (lacZYA-argFV169) ϕ80lacZΔM15, F− | Bethesda Research Laboratory |
| Plasmid | ||
| pAL953 | P. multocida TargeTron vector (Specr) containing group II intron with aph3 (Kanr) | 38 |
| pAL1069 | pAL953 with TargeTron group II intron retargeted to hyaD | 20 |
| pAL1291 | pAL953 with TargeTron group II intron retargeted to proQ | This study |
| pAL1332 | pREXY containing the intact copy of proQ, cloned using primers BAP8088 and BAP8089 | This study |
| pAL1333 | pREXY containing the proQ gene without the stop codon, cloned using primers BAP8088 and BAP8090 | This study |
| pAL1337 | pAL1069 with aph3 (Kanr) removed from group II intron, for generation of markerless hyaD TargeTron mutant | This study |
| pAL1338 | His-TEV-tag cloned in-frame with, and downstream of, proQ into pAL1333 using EcoRI site | This study |
| pAL1339 | 3×FLAG-tag cloned in-frame with and downstream of the His-TEV-tagged proQ into pAL1338 using EcoRI site | This study |
| pAL1449 | Wild-type P. multocida VP161 proQ and promoter region cloned into BamHI and SalI sites of pPBA1100S using primers BAP8573 and BAP8574 | This study |
| pPBA1100S | P. multocida/E. coli shuttle plasmid used to express recombinant proteins or sRNAs under the control of their native promoters; Specr | 22 |
| pREXY | P. multocida/E. coli expression/shuttle vector for expression of recombinant proteins or sRNAs under the control of the P. multocida constitutive Ptpi promoter; Specr | 22 |
In E. coli, ProQ has previously been shown to be involved in the production of ProP, an osmoregulatory protein pump, leading to changes in osmoregulation (24). Therefore, the growth of the proQ[EV] mutant strain was compared to the growth of the WT[EV] strain and the proQ[proQ] strain in rich heart infusion (HI) broth and in HI containing 300 mM NaCl to induce osmotic stress (Fig. 1B and C). Iron is an essential micronutrient that is sequestered in host tissues, and P. multocida requires expression of numerous iron uptake proteins during infection (25). To determine if ProQ played a role in iron uptake, the above-described strains were grown in peptone water with 1% glucose, with or without pretreatment with 150 μM dipyridyl, to deplete media of iron (Fig. 1D). For all conditions, the growth rates of the three strains were indistinguishable, although the proQ[proQ] strain showed a marginally increased lag time compared to both empty vector-containing strains. In summary, ProQ is not required for P. multocida growth in vitro in high-nutrient media, iron-depleted media, or when under osmotic stress.
P. multocida ProQ regulates the expression of sRNAs and tRNAs.
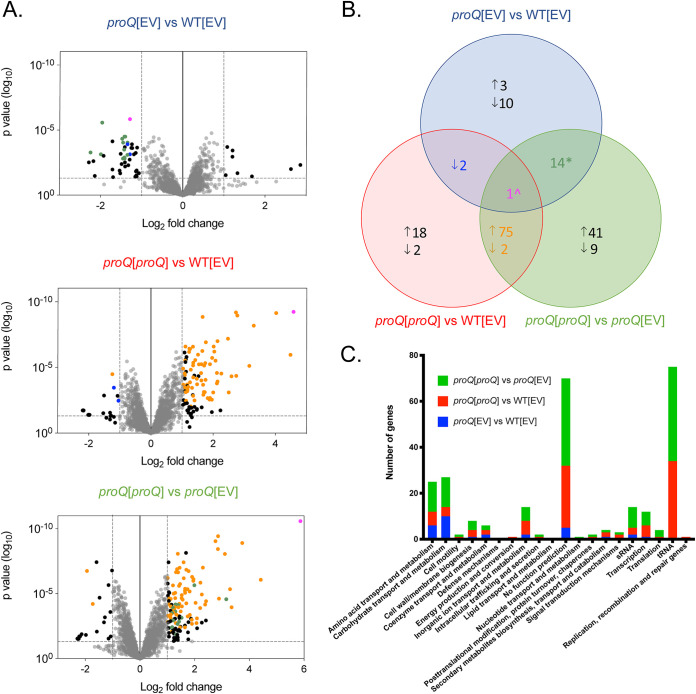
To identify the transcripts that are directly or indirectly affected by the presence of ProQ in P. multocida, comparative transcriptomic analysis was undertaken. Relative transcript abundance was measured in the WT[EV] (AL3356), proQ[EV] (AL3358), and proQ[proQ] (AL3357) strains. These strains were grown in biological triplicate until mid-exponential growth phase (OD600 of 0.6). Strand-specific RNA-sequencing (RNA-seq) libraries were sequenced, and genes were determined to be differentially expressed if they showed a ≥2-fold change in expression (≥1 log2), with a false discovery rate (FDR) of <0.05. Using the transcriptomic data comparisons proQ[EV] versus WT[EV], proQ[proQ] versus WT[EV], and proQ[proQ] versus proQ[EV], a total of 177 differentially expressed genes were identified following the perturbation of proQ expression (Fig. 2A and B; see also Table S1 in the supplemental material).
FIG 2.
Transcriptomic analysis following perturbation of proQ expression in P. multocida strain VP161. (A) Volcano plots showing the log2 fold change in gene expression and P value for each transcript. Each plot shows those transcripts that were significantly differentially expressed in a single comparison (black dots), all three comparisons (pink dot), proQ[EV] versus WT[EV] and proQ[proQ] versus WT[EV] (blue dots), proQ[EV] versus WT[EV] and proQ[proQ] versus proQ[EV] (green dots), and proQ[proQ] versus WT[EV] and proQ[proQ] versus proQ[EV] (orange dots). (B) Venn diagram showing the number of differentially expressed genes in each of the strains and those common to one or more strains. Genes with increased expression are represented by the up arrow and those with decreased expression by the down arrow. Blue-shaded circle labeled proQ[EV] versus WT[EV] shows differential expression of genes in the proQ mutant containing empty vector (AL3358) compared to expression in wild-type VP161 containing empty vector (AL3356). Red-shaded circle labeled proQ[proQ] versus WT[EV] represents the number of differentially expressed genes in the complemented proQ mutant (AL3357) compared to expression in the wild-type strain containing empty vector (AL3356). Green-shaded circle represents the number of differentially expressed genes in proQ[proQ] strain AL3357 compared to expression in the proQ[EV] strain AL3358. *, fourteen genes showed decreased expression in the proQ mutant but increased expression when proQ was expressed on a plasmid; ^, one gene displayed decreased expression in the proQ mutant and increased expression in the complemented proQ mutant. (C) Differentially expressed genes identified by RNA-seq transcriptomic analysis (see Table S1 in the supplemental material), grouped according to cellular function. The number of differentially expressed genes belonging to each function is shown relative to the following analysis groups: proQ[EV] versus WT[EV] (AL3358 versus AL3356, blue bars), proQ[proQ] versus WT[EV] (AL3357 versus AL3356, red bars), and proQ[proQ] versus proQ[EV] (AL3357 versus AL3358, green bars).
There were 30 differentially expressed genes in the proQ mutant compared to expression in the WT, 3 with increased expression and 27 with decreased expression (Fig. 2A and B and Table S1). Of those, 15 had significantly reversed expression of more than 2-fold in the complemented proQ mutant compared to expression in the proQ mutant (Fig. 2A and B and Table S1). In addition, another 7 of the 30 differentially expressed genes in the proQ mutant showed statistically significantly (FDR, <0.05) reversed expression in the proQ complemented strain but at levels between 1.5-fold and 2-fold. Together these data confirm that the plasmid copy of proQ was functional and ProQ acts to stabilize transcripts. Comparison of the RNA transcripts generated from the complemented proQ mutant with those of the WT strain identified 100 differentially expressed genes in the complemented mutant: 94 with increased expression and only six with decreased expression. A comparison of transcripts produced by the complemented proQ mutant with the proQ mutant revealed 142 transcripts differentially expressed when proQ was provided in trans, 131 with increased expression, and 11 with decreased expression (Fig. 2A and B and Table S1). Of these, 78 showed the same pattern of differential expression as transcripts identified when the complemented proQ mutant was compared to the WT, showing good correlation between data sets. Overall, the transcriptomic data indicate that proQ stabilizes transcripts and that providing the proQ mutant with the plasmid-encoded proQ may have resulted in an overabundance of ProQ in the complemented strain (Fig. 2A and B and Table S1).
Using the complete set of differentially expressed transcripts (Table S1), the cellular pathways that each differentially expressed gene was predicted to be involved in were identified using a GO analysis (Fig. 2C). The only significantly enriched group was the carbohydrate metabolism and transport group (19 genes, P = 0.027) (Table 2). In a recent transcriptomic analysis of the P. multocida fowl cholera strains X73 and VP161 (26), 52 putative sRNAs were identified (designated P. multocida regulatory RNA candidate [Prrc], Prrc01 to Prrc52). In our analysis, 9 of the 52 putative sRNAs were differentially expressed when ProQ abundance was altered, namely, Prrc08, Prrc11, Prrc12, Prrc13, Prrc25, Prrc31, Prrc32, Prrc46, and Prrc49 (P = 0.043) (Table 2). In addition, transfer RNAs (tRNAs) were significantly overrepresented in the list of differentially expressed transcripts (42 tRNAs, P < 0.00001) (Table 2). In summary, these data show that changes in ProQ abundance in P. multocida result in the differential expression of a variety of transcripts, particularly those representing tRNAs and sRNAs and those encoding proteins involved in carbohydrate metabolism and transport.
TABLE 2.
Carbohydrate transport or metabolism genes, putative sRNA transcripts, and tRNA transcripts that showed differential expression when proQ was inactivated or expressed in trans in P. multocida strain VP161b
| PMVP161 locus tag | Gene name | Predicted product/protein domain | Log2 fold changea (FDR) |
General functional group | ||
|---|---|---|---|---|---|---|
| proQ[EV] vs WT[EV] | proQ[proQ] vs WT[EV] | proQ[proQ] vs proQ[EV] | ||||
| PmVP161_1790 | PmVP161_1790 | TRAP dicarboxylate transporter, DctP subunit subfamily, putative | −0.13 (0.6108) | 0.88 (0.0002) | 1 (0) | Carbohydrate transport and metabolism genes |
| PmVP161_1812 | lsrA | ABC transporter domain protein | −0.57 (0.3276) | 0.52 (0.179) | 1.09 (0.0098) | Carbohydrate transport and metabolism genes |
| PmVP161_0883 | nagE | PTS permease for N-acetylglucosamine and glucose | −0.58 (0.1516) | 0.58 (0.0595) | 1.16 (0.0012) | Carbohydrate transport and metabolism genes |
| PmVP161_1947 | siaP | TRAP dicarboxylate transporter-DctP subunit subfamily, putative | −0.62 (0.2352) | 0.5 (0.231) | 1.12 (0.009) | Carbohydrate transport and metabolism genes |
| PmVP161_0129 | mglB | Galactose ABC transporter, periplasmic-binding protein | −1.12 (0.147) | 0.18 (0.7709) | 1.3 (0.023) | Carbohydrate transport and metabolism genes |
| PmVP161_0127 | mglC | Beta-methylgalactoside transporter | −1.11 (0.0161) | −0.31 (0.3274) | 0.8 (0.0176) | Carbohydrate transport and metabolism genes |
| PmVP161_0128 | mglA_1 | Galactose/methyl galactoside transporter ATP-binding protein | −1.23 (0.0127) | −0.48 (0.1375) | 0.75 (0.0289) | Carbohydrate transport and metabolism genes |
| PmVP161_1584 | glpT | Glycerol-3-phosphate transporter | −1.23 (0.0161) | −0.33 (0.3798) | 0.9 (0.0151) | Carbohydrate transport and metabolism genes |
| PmVP161_1511 | rbsA_2 | Ribose transport ATP-binding protein RbsA | −1.92 (0.0324) | −0.99 (0.1439) | 0.93 (0.102) | Carbohydrate transport and metabolism genes |
| PmVP161_1505 | fbaA_2 | Fructose-bisphosphate aldolase (E.C. 4.1.2.13) | −1.34 (0.0115) | −0.17 (0.6517) | 1.17 (0.0033) | Carbohydrate transport and metabolism genes |
| PmVP161_1504 | ydjH | Pfkb family carbohydrate kinase family | −1.4 (0.0098) | −0.19 (0.5988) | 1.21 (0.0017) | Carbohydrate transport and metabolism genes |
| PmVP161_1503 | xylB | d-Xylulose kinase | −1.42 (0.0098) | −0.11 (0.7729) | 1.3 (0.0012) | Carbohydrate transport and metabolism genes |
| PmVP161_1797 | yhjE | Shikimate transporter and similar proteins of the major facilitator superfamily; cd17369 | −1.42 (0.0186) | −0.21 (0.6442) | 1.21 (0.0082) | Carbohydrate transport and metabolism genes |
| PmVP161_1509 | rbsB_2 | Periplasmic binding proteins and sugar binding domain of LacI family | −1.42 (0.0411) | −0.24 (0.7106) | 1.18 (0.0196) | Carbohydrate transport and metabolism genes |
| PmVP161_0954 | dctM_1 | TRAP dicarboxylate transporter-DctM subunit | −1.99 (0.0302) | −0.66 (0.2937) | 1.33 (0.0466) | Carbohydrate transport and metabolism genes |
| PmVP161_1075 | rbsD | High-affinity ribose transport protein | −0.7 (0.3061) | −1.51 (0.0129) | −0.81 (0.126) | Carbohydrate transport and metabolism genes |
| PmVP161_1281 | srlB | Glucitol/sorbitol-specific PTS system component IIA | 0.38 (0.4048) | 1.02 (0.0045) | 0.64 (0.027) | Carbohydrate transport and metabolism genes |
| PmVP161_1482 | gntT | Gluconate permease | −0.09 (0.9033) | 1.04 (0.0035) | 1.12 (0.0015) | Carbohydrate transport and metabolism genes |
| PmVP161_1885 | uhpT | Hexose phosphate transport protein | −0.21 (0.7783) | 1.32 (0.0052) | 1.54 (0.0015) | Carbohydrate transport and metabolism genes |
| PmVP161_2247 | Prrc49 | Putative sRNA | −0.34 (0.78) | 1.3 (0.0559) | 1.65 (0.0156) | Putative sRNA |
| PmVP161_2211 | Prrc12 | Putative sRNA | −0.48 (0.0313) | 0.52 (0.0066) | 1 (0.0001) | Putative sRNA |
| PmVP161_2223 | Prrc25 | Putative sRNA | −0.81 (0.5907) | 1.29 (0.1345) | 2.09 (0.0222) | Putative sRNA |
| PmVP161_2244 | Prrc46 | Putative sRNA | −0.94 (0.4984) | 1.48 (0.0724) | 2.41 (0.0082) | Putative sRNA |
| PmVP161_2210 | Prrc11 | Putative sRNA | −1.35 (0.0313) | −0.27 (0.5701) | 1.07 (0.0212) | Putative sRNA |
| PmVP161_2212 | Prrc13 | Putative sRNA | −2.24 (0.0256) | 0.92 (0.1921) | 3.16 (0.0006) | Putative sRNA |
| PmVP161_2229 | Prrc31 | Putative sRNA | 0.07 (0.9407) | 1.2 (0.0046) | 1.13 (0.0041) | Putative sRNA |
| PmVP161_2207 | Prrc08 | Putative sRNA | 0.23 (0.8305) | 2.27 (0.001) | 2.04 (0.001) | Putative sRNA |
| PmVP161_2230 | Prrc32 | Putative sRNA | 0.25 (0.8745) | 1.9 (0.0175) | 1.65 (0.02) | Putative sRNA |
| PmVP161_1476 | PmVP161_1476 | tRNA Thr | −0.07 (0.9701) | 1.4 (0.0507) | 1.47 (0.0308) | tRNA |
| PmVP161_1864 | PmVP161_1864 | tRNA Phe | −0.16 (0.9354) | 1.21 (0.1012) | 1.37 (0.0491) | tRNA |
| PmVP161_1924 | PmVP161_1924 | tRNA Glu | −0.17 (0.9101) | 1.22 (0.0695) | 1.39 (0.0303) | tRNA |
| PmVP161_0447 | PmVP161_0447 | tRNA Arg | −0.25 (0.8449) | 1.24 (0.0534) | 1.48 (0.0184) | tRNA |
| PmVP161_0451 | PmVP161_0451 | tRNA Glu | −0.26 (0.8397) | 1.17 (0.0773) | 1.43 (0.027) | tRNA |
| PmVP161_1302 | PmVP161_1302 | tRNA Arg | −0.47 (0.4891) | 0.79 (0.0723) | 1.26 (0.007) | tRNA |
| PmVP161_0861 | PmVP161_0861 | tRNA Leu | −0.63 (0.5383) | 1.24 (0.052) | 1.87 (0.0066) | tRNA |
| PmVP161_0279 | PmVP161_0279 | tRNA Leu | −0.63 (0.6656) | 1.63 (0.0533) | 2.26 (0.0111) | tRNA |
| PmVP161_1453 | PmVP161_1453 | tRNA Sec | 0.35 (0.6801) | 1.16 (0.0325) | 0.81 (0.0794) | tRNA |
| PmVP161_0673 | PmVP161_0673 | tRNA Leu | −0.02 (0.9906) | 1.89 (0.0209) | 1.9 (0.0127) | tRNA |
| PmVP161_0209 | PmVP161_0209 | tRNA Lys | −0.03 (0.9714) | 1.63 (0.0003) | 1.65 (0.0002) | tRNA |
| PmVP161_0208 | PmVP161_0208 | tRNA Lys | −0.04 (0.9558) | 1.43 (0.0002) | 1.47 (0.0001) | tRNA |
| PmVP161_0105 | PmVP161_0105 | tRNA Ser | −0.04 (0.9714) | 1.7 (0.0085) | 1.75 (0.0051) | tRNA |
| PmVP161_1478 | PmVP161_1478 | tRNA Gly | −0.09 (0.9576) | 1.73 (0.0085) | 1.82 (0.0044) | tRNA |
| PmVP161_0173 | PmVP161_0173 | tRNA Cys | −0.11 (0.9598) | 1.96 (0.0214) | 2.07 (0.0115) | tRNA |
| PmVP161_0106 | PmVP161_0106 | tRNA Arg | −0.12 (0.7379) | 2.74 (0) | 2.86 (0) | tRNA |
| PmVP161_0383 | PmVP161_0383 | tRNA Ser | −0.13 (0.943) | 3.16 (0.0004) | 3.3 (0.0002) | tRNA |
| PmVP161_0211 | PmVP161_0211 | tRNA Lys | −0.14 (0.9101) | 2.12 (0.001) | 2.26 (0.0004) | tRNA |
| PmVP161_0174 | PmVP161_0174 | tRNA Gly | −0.14 (0.941) | 2.75 (0.0013) | 2.89 (0.0006) | tRNA |
| PmVP161_1703 | PmVP161_1703 | tRNA Glu | −0.19 (0.8952) | 1.28 (0.0476) | 1.47 (0.02) | tRNA |
| PmVP161_1127 | PmVP161_1127 | tRNA Asp | −0.21 (0.7868) | 1.49 (0.0035) | 1.7 (0.0011) | tRNA |
| PmVP161_0045 | PmVP161_0045 | tRNA Ala | −0.26 (0.6404) | 1.02 (0.0083) | 1.28 (0.0016) | tRNA |
| PmVP161_0909 | PmVP161_0909 | tRNA Gly | −0.26 (0.6423) | 1.26 (0.0027) | 1.52 (0.0006) | tRNA |
| PmVP161_0642 | PmVP161_0642 | tRNA Val | −0.26 (0.8665) | 1.83 (0.0175) | 2.09 (0.0064) | tRNA |
| PmVP161_0672 | PmVP161_0672 | tRNA Gln | −0.28 (0.8078) | 2.15 (0.0025) | 2.42 (0.0008) | tRNA |
| PmVP161_1929 | PmVP161_1929 | tRNA Trp | −0.85 (0.5964) | 2.5 (0.0087) | 3.35 (0.0016) | tRNA |
| PmVP161_0041 | PmVP161_0041 | tRNA Val | 0.02 (0.973) | 2.78 (0) | 2.76 (0) | tRNA |
| PmVP161_1477 | PmVP161_1477 | tRNA Tyr | 0.04 (0.9701) | 1.36 (0.0132) | 1.31 (0.0116) | tRNA |
| PmVP161_1928 | PmVP161_1928 | tRNA Asp | 0.05 (0.9593) | 1.62 (0.001) | 1.56 (0.0007) | tRNA |
| PmVP161_0210 | PmVP161_0210 | tRNA Lys | 0.06 (0.949) | 1.71 (0.0004) | 1.65 (0.0003) | tRNA |
| PmVP161_0180 | PmVP161_0180 | tRNA Ser | 0.07 (0.9714) | 4.49 (0.0001) | 4.42 (0.0001) | tRNA |
| PmVP161_0172 | PmVP161_0172 | tRNA Leu | 0.08 (0.9232) | 2.04 (0.0001) | 1.96 (0.0001) | tRNA |
| PmVP161_1128 | PmVP161_1128 | tRNA Asp | 0.08 (0.9367) | 1.9 (0.0003) | 1.82 (0.0003) | tRNA |
| PmVP161_1865 | PmVP161_1865 | tRNA Asn | 0.11 (0.9551) | 2 (0.0088) | 1.9 (0.0075) | tRNA |
| PmVP161_0040 | PmVP161_0040 | tRNA Val | 0.18 (0.7747) | 2.48 (0) | 2.3 (0) | tRNA |
| PmVP161_0043 | PmVP161_0043 | tRNA Val | 0.2 (0.7686) | 3.31 (0) | 3.11 (0) | tRNA |
| PmVP161_0252 | PmVP161_0252 | tRNA Met | 0.21 (0.8934) | 2.59 (0.0014) | 2.38 (0.0012) | tRNA |
| PmVP161_0107 | PmVP161_0107 | tRNA Arg | 0.25 (0.6908) | 1.48 (0.0018) | 1.22 (0.003) | tRNA |
| PmVP161_0671 | PmVP161_0671 | tRNA Gln | 0.25 (0.8207) | 1.4 (0.0236) | 1.15 (0.0338) | tRNA |
| PmVP161_0212 | PmVP161_0212 | tRNA Lys | 0.27 (0.7513) | 1.76 (0.002) | 1.49 (0.0027) | tRNA |
| PmVP161_0042 | PmVP161_0042 | tRNA Val | 0.29 (0.601) | 4.03 (0) | 3.74 (0) | tRNA |
| PmVP161_0674 | PmVP161_0674 | tRNA Met | 0.3 (0.7824) | 2.12 (0.0031) | 1.83 (0.0042) | tRNA |
Transcript expression ratio is shown as a log2 value with the corresponding false discovery rate (FDR) shown in parentheses. Shaded boxes indicate a statistically significant difference in expression.
The following comparisons were performed: P. multocida proQ mutant containing empty vector, AL3358, compared to wild-type parent VP161 containing empty vector, AL3356 (proQ[EV] versus WT[EV]); complemented proQ mutant, AL3357, compared to wild-type parent VP161 containing empty vector (proQ[proQ] versus WT[EV]); and complemented proQ mutant compared to proQ mutant containing empty vector (proQ[proQ] versus proQ[EV]). Data are a subset of the full list of differentially expressed genes provided in Table S1.
The RNA-binding proteins Hfq and ProQ are both expressed by P. multocida and have been shown in other species to have overlapping regulons (27). To determine if the P. multocida RNA chaperones have overlapping RNA targets, the transcriptomic profile of the hfq mutant (20) was compared to the complete list of proQ differentially expressed transcripts. A list of 34 transcripts was found to be differentially expressed in both data sets (Table S2) and included 12 tRNAs and two sRNAs (Prrc11 and Prrc08). Of the 34 transcripts, only two showed decreased expression in both data sets, while three showed increased expression in both data sets. The remaining 29 transcripts showed increased expression in the hfq mutant strain and decreased expression in the proQ mutant strain. Therefore, similar to what has been seen in other species (27), P. multocida ProQ and Hfq have a subset of their RNA targets in common but predominantly display opposing effects on transcript stability.
ProQ binds to a variety of RNA species at Rho-independent terminators.
To identify RNA species that interact directly with ProQ, in vivo UV cross-linking and analysis of cDNA (CRAC) was employed (28). To facilitate purification of interacting RNA species, an expression plasmid, pAL1339 (Table 1), was constructed that encoded ProQ translationally fused at the C-terminal end with a dual-affinity purification tag, His6-TEV-3×FLAG (HTF). To allow for safe use of viable P. multocida in the UV cross-linking apparatus, the P. multocida proQ mutant used for the CRAC experiments was attenuated for virulence by inactivating hyaD, a gene essential to produce capsule in this pathogen (Fig. S1). The proQ-hyaD double mutant was provided with the plasmid encoding ProQ-HTF (pAL1339) to generate the strain AL3068 (Table 1). To generate an untagged ProQ control strain, the proQ-hyaD mutant was provided with pAL1132, encoding a wild-type copy of proQ, to generate strain AL3069 (Table 1).
Strains expressing H-TF-tagged and untagged recombinant ProQ (AL3068 and AL3069, respectively) were separately grown to an OD600 of 0.8 in HI medium before treatment with 1,800 mJ of UV-C to covalently cross-link RNA-protein complexes in the cell. RNA species that cross-linked with ProQ were recovered by initial coimmunoprecipitation of the tagged ProQ-HTF under native conditions using M2 anti-FLAG resin and then under stringent denaturing conditions (using 6 M guanidinium HCl) with immobilized metal affinity chromatography (IMAC). UV cross-linked RNAs were recovered by proteinase K digestion of ProQ-RNA complexes and then reverse transcribed and sequenced on an Illumina NextSeq platform. Reads from ProQ cross-linked RNAs were mapped to the P. multocida VP161 genome to identify the regions with increased association with the ProQ-HTF protein.
The cDNA sequencing data generated from the coimmunoprecipitated RNA was analyzed using DeSeq2 (29) and PyCRAC (30). CRAC data generated using the tagged ProQ was compared with data generated from the untagged ProQ to remove nonspecific binding partners. Following the comparison, a total of 67 RNA species showed a significant association with the ProQ-HTF protein (Table 3). Of these, 29 (43%) were protein-coding transcripts (Table 3, Fig. 3A), including 19 ribosomal proteins (13 50S and 6 30S ribosomal subunits) that were highly overrepresented (P < 0.00001). Interestingly, the second most common RNA species binding to ProQ-HTF represented the 3′ untranslated region (UTR) of genes (26 transcripts; 39%) (Fig. 3A). Additionally, five tRNA species showed significant binding to ProQ-HTF, namely, tRNA Met (PmVP161_0674), tRNA Cys (PmVP161_0173), tRNA Ser (PmVP161_0105), tRNA Ala (PmVP161_0045), and tRNA Trp (PmVP161_1929) (Table 3, Fig. 3A). The ProQ-HTF protein was also shown to bind to six intergenic regions predicted to encode sRNA molecules (Table 3, Fig. 3A). Two of these regions had previously been identified as the predicted sRNAs Prrc13 and Prrc37 based on the abundance of intergenic transcripts in WT P. multocida (26), and four were identified here for the first time (Prrc54, Prrc55, Prrc56, and Prrc57) (Table 4). The data generated for each of the RNA species identified using CRAC analysis was then compared with the corresponding transcriptomic data (Table 3). The comparison showed that of the 67 RNA species that bound to ProQ-HTF in CRAC analysis, 10 were also identified in the RNA-seq transcriptomic analysis as having increased levels of transcript in the complemented proQ mutant AL3357 (compared to expression in either the WT strain AL3356 or the proQ mutant AL3358), including five tRNAs, PmVP161_1121, rpmF, rseA, the predicted sRNA Prrc13, and the 3′ UTR of PmVP161_0663 (≥2-fold change in expression; FDR of <0.05) (Table 3 and Table S1). The same comparisons identified only one ProQ-bound RNA species that had reduced transcript levels in the complemented proQ mutant AL3357, namely, tsf, encoding translation elongation factor Ts (Table 3).
TABLE 3.
Transcripts that bound to ProQ, as determined using CRACb
| PmVP161 locus tag | Gene name | Predicted product | RNA type | General function prediction | Differential expression, log2 FCa (FDR) |
||
|---|---|---|---|---|---|---|---|
| proQ[EV] vs WT[EV] | proQ[proQ] vs WT[EV] | proQ[proQ] vs proQ[EV] | |||||
| PmVP161_0045 | tRNA Ala | tRNA Ala | tRNA | Translation | −0.26 (0.64) | 1.02 (0.008) | 1.28 (0.002) |
| PmVP161_0053 | groL | 60-kDa chaperonin | 3′UTR | Posttranslational modification | NA | N/A | NA |
| PmVP161_0105 | tRNA Ser | tRNA Ser | tRNA | Translation | −0.04 (0.97) | 1.71 (0.009) | 1.75 (0.005) |
| PmVP161_0168 | PmVP161_0168 | Putative nicotinate phosphoribosyltransferase | 3′UTR | Coenzyme transport and metabolism | NA | N/A | NA |
| PmVP161_0173 | tRNA Cys | tRNA Cys | tRNA | Translation | −0.11 (0.96) | 1.96 (0.02) | 2.07 (0.01) |
| PmVP161_0192 | PmVP161_0192 | YebC/PmpR family DNA binding transcriptional regulator | CDS | No function prediction | NA | N/A | NA |
| PmVP161_0193 | PmVP161_0193 | Membrane protein | 3′UTR | No function prediction | NA | NA | NA |
| PmVP161_0242 | purA | Adenylosuccinate synthetase | 3′UTR | Nucleotide transport and metabolism | NA | NA | NA |
| PmVP161_0322 | rng | Cytoplasmic axial filament protein | 3′UTR | Translation | NA | NA | NA |
| PmVP161_0327 | rplU | 50S ribosomal protein L21 | CDS | Translation | NA | NA | NA |
| PmVP161_0424 | PmVP161_0424 | Hypothetical | CDS | No function prediction | NA | NA | NA |
| PmVP161_0547 | pcp | Glycine zipper TM2 domain containing protein | 3′UTR | Cell wall/membrane biogenesis | NA | NA | NA |
| PmVP161_0590 | rplT | 50S ribosomal protein L20 | 3′UTR | Translation | NA | NA | NA |
| PmVP161_0615 | mepS | Lipoprotein | CDS | Cell wall/membrane biogenesis | NA | NA | NA |
| PmVP161_0641 | pykA | Pyruvate kinase | 3′UTR | Carbohydrate transport and metabolism | NA | NA | NA |
| PmVP161_0663 | PmVP161_0663 | Hypothetical protein PM0674 | 3′UTR | No function prediction | 0.09 (0.75) | 1.11 (4.37E−05) | 1.02 (5.21E−05) |
| PmVP161_0674 | tRNA Met | tRNA Met | tRNA | Translation | 0.30 (0.78) | 2.12 (0.003) | 1.83 (0.004) |
| PmVP161_0711 | nrdA | Ribonucleotide-diphosphate reductase subunit alpha | 3′UTR | Nucleotide transport and metabolism | NA | NA | NA |
| PmVP161_0787 | ompA | Outer membrane protein OmpA | 3′UTR | Cell wall/membrane biogenesis | NA | NA | NA |
| PmVP161_0800 | ahpC | Antioxidant, AhpC/Tsa family | 3′UTR | Posttranslational modification | NA | NA | NA |
| PmVP161_0805 | ihfB | Integration host factor, beta subunit | CDS | Replication, recombination and repair | NA | NA | NA |
| PmVP161_0901 | aceF | Dihydrolipoamide acetyltransferase | CDS | Energy production and conversion | NA | NA | NA |
| PmVP161_0915 | hflX | GTPase | CDS | No function prediction | NA | NA | NA |
| PmVP161_0936 | accA | Acetyl-coenzyme A carboxylase, carboxyl transferase subunit alpha | 3′UTR | Lipid transport and metabolism | NA | NA | NA |
| PmVP161_1012 | smpB | SsrA-binding protein | CDS | Posttranslational modification | NA | NA | NA |
| PmVP161_1017 | PmVP161_1017 | Conserved hypothetical protein | CDS | No function prediction | NA | NA | NA |
| PmVP161_1098 | mraZ | Transcriptional regulator MraZ | 3′UTR | Cell cycle control, mitosis and meiosis | NA | NA | NA |
| PmVP161_1121 | PmVP161_1121 | Hypothetical | CDS | No function prediction | 0.11 (0.80) | 2.02 (4.38E−05) | 1.91 (0.00004) |
| PmVP161_1222 | PmVP161_1222 | Hypothetical protein PM0016 | 3′UTR | No function prediction | NA | NA | NA |
| PmVP161_1266 | tsf | Translation elongation factor Ts | CDS | Translation | −0.18 (0.81) | −1.07 (0.01) | −0.89 (0.02) |
| PmVP161_1267 | rpsB | 30S ribosomal protein S2 | 3′UTR | Translation | NA | NA | NA |
| PmVP161_1300 | folD | Methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase | 3′UTR | Coenzyme transport and metabolism | NA | NA | NA |
| PmVP161_1322 | rpmF | 50S ribosomal protein L32 | CDS | Translation genes | −0.44 (0.61) | 0.75 (0.18) | 1.19 (0.03) |
| PmVP161_1446 | rseA | Sigma-E factor negative regulatory protein-RNA polymerase sigma factor RpoE | CDS | Translation | −0.41 (0.39) | 1.03 (0.01) | 1.44 (0.001) |
| PmVP161_1480 | tufA_1 | Elongation factor Tu | 3′UTR | Translation | NA | NA | NA |
| PmVP161_1522 | rplQ | 50S ribosomal protein L17 | 3′UTR | Translation | NA | NA | NA |
| PmVP161_1523 | rpoA | DNA-directed RNA polymerase subunit alpha | CDS | Transcription | NA | NA | NA |
| PmVP161_1526 | rpsM | Ribosomal protein S13/S18 | CDS | Translation | NA | NA | NA |
| PmVP161_1531 | rpsE | 30S ribosomal protein S5-50S ribosomal protein L18 | CDS | Translation | NA | NA | NA |
| PmVP161_1536 | rplE | 50S ribosomal protein L5 | CDS | Translation | NA | NA | NA |
| PmVP161_1537 | rplX | 50S ribosomal protein L24-L14 | CDS | Translation | NA | NA | NA |
| PmVP161_1548 | rplP | 50S ribosomal protein L16-30S ribosomal protein S3 | CDS | Translation | NA | NA | NA |
| PmVP161_1551 | rpsS | 30S ribosomal protein S19-50S ribosomal protein L2 | 5′UTR | Translation | NA | NA | NA |
| PmVP161_1553 | rplW | 50S ribosomal protein L23 | CDS | Translation | NA | NA | NA |
| PmVP161_1555 | rplC | 50S ribosomal protein L3-30S ribosomal protein S10 | CDS | Translation | NA | NA | NA |
| PmVP161_1621 | ygaP | Rhodanese-like domain protein | 3′UTR | Inorganic ion transport and metabolism | NA | NA | NA |
| PmVP161_1782 | araD | l-Ribulose-5-phosphate 4-epimerase | 3′UTR | Carbohydrate transport and metabolism | NA | NA | NA |
| PmVP161_1834 | rpsP | 30S ribosomal protein S16-16S rRNA processing protein rim | CDS | Translation | NA | NA | NA |
| PmVP161_1863 | mltC | Membrane-bound lytic murein transglycosylase C | 3′UTR | Cell wall/membrane biogenesis | NA | NA | NA |
| PmVP161_1894 | PmVP161_1894 | FkbP-type peptidyl-prolyl cis-trans isomerase | CDS | Posttranslational modification | NA | NA | NA |
| PmVP161_1905 | rplK | 50S ribosomal protein L11 | CDS | Translation | NA | NA | NA |
| PmVP161_1906 | rplA | 50S ribosomal protein L1 | CDS | Translation | NA | NA | NA |
| PmVP161_1916 | hupA | DNA-binding protein HU-alpha | 3′UTR | Replication, recombination and repair | NA | NA | NA |
| PmVP161_1929 | tRNA Trp | tRNA Trp | tRNA | Translation | −0.85 (0.60) | 2.50 (0.009) | 3.35 (0.002) |
| PmVP161_1963 | cysK | Cystine synthase A | CDS | Amino acid transport and metabolism | NA | NA | NA |
| PmVP161_2001 | PmVP161_2001 | YajQ family cyclic di-GMP-binding protein | 3′UTR | No function prediction | NA | NA | NA |
| PmVP161_2022 | rpsU | 30S ribosomal protein S21 | 3′UTR | Translation | NA | NA | NA |
| PmVP161_2039 | aroK | Shikimate kinase | CDS | Amino acid transport and metabolism | NA | NA | NA |
| PmVP161_2044 | secA | Preprotein translocase, SecA subunit | 3′UTR | Intracellular trafficking and secretion | NA | NA | NA |
| PmVP161_2089 | rpsR | 30S ribosomal protein S18 | CDS | Translation | NA | NA | NA |
| PmVP161_2090 | rplI | 50S ribosomal protein L9 | CDS | Translation | NA | NA | NA |
| PmVP161_2212 | Prrc13 | sRNA | sRNA | sRNA | −2.24 (0.03) | 0.92 (0.19) | 3.16 (0.0006) |
| PmVP161_2235 | Prrc37 | sRNA | sRNA | sRNA | NA | NA | NA |
| PmVP161_2251 | Prrc54 | sRNA | sRNA | sRNA | NA | NA | NA |
| PmVP161_2252 | Prrc55 | sRNA | sRNA | sRNA | NA | NA | NA |
| PmVP161_2253 | Prrc56 | sRNA | sRNA | sRNA | NA | NA | NA |
| PmVP161_2254 | Prrc57 | sRNA | sRNA | sRNA | NA | NA | NA |
Transcript expression ratio is shown as a log2 fold change (FC) value with the corresponding false discovery rate (FDR) shown in brackets. Shaded boxes indicate a statistically significant difference in expression. NA, gene not identified as differentially expressed in comparative transcriptomic analysis (Table S1).
Locus tag/gene name predicted product/type and general function prediction are shown for all transcripts that bound to HTF-tagged ProQ. For comparison between the two experiments conducted in this study, comparative transcriptomic data corresponding to each of the identified transcripts is also provided in the last three columns. Comparisons are proQ mutant containing empty vector, AL3358, compared to wild-type VP161 containing empty vector, AL3356, (proQ[EV] versus WT[EV]); complemented proQ mutant, AL3357, compared to wild-type VP161 containing empty vector (proQ[proQ] versus WT[EV]); complemented proQ mutant compared to proQ mutant containing empty vector (proQ[proQ] versus proQ[EV]).
FIG 3.
Analysis of transcripts that bind directly to ProQ, as determined by CRAC analysis. (A) Distribution of ProQ-bound RNA species: sRNA, small RNA; CDS, protein coding sequence; 3UTR, 3′ untranslated region of CDS; 5UTR, 5′ untranslated region of CDS. (B) Cumulative distribution functions [Fn(x)] for differentially expressed transcripts, defined as ≥1 log2 change in expression with a false discovery rate of <0.05 (log2 FC), in proQ mutant (AL3358) compared to the WT (AL3356) (B) and the proQ mutant (AL3358) compared to the complemented proQ mutant (AL3357) (C). Blue lines indicate the Fn(x) for transcripts that bound to ProQ as determined by CRAC analysis, and red lines indicate the Fn(x) for transcripts that did not bind to ProQ. (D) ProQ binding site frequency as a function of position on the transcripts relative to the beginning of the predicted terminator stem-loop sequence. (E) ProQ binding sequence (top), pairing (middle), and structure (bottom) motifs (S, stem; E, external bulge; B, bulge loop; H, hairpin loop; and I, internal loop) as determined from transcripts bound at the beginning of the terminator stem-loop as identified using GraphProt.
TABLE 4.
ProQ-associated putative sRNAs identified in P. multocida strain VP161 using UV-cross-linking and analysis of cDNA (CRAC)a
| Name | Predicted sRNA sequence (5′ to 3′) | Position on VP161 genome | Size (bp) |
|---|---|---|---|
| Prrc13 | CCUGUUUUGGACGAAAGUAAGUGCAAGAAGACUAUCAUACACACUUGUUUUGUUAACCCUCAUGCAUUUAUUCGUUACAGGCACGGAAGACACGAUGAGCAAGGAUGCCUUUCAGUUAAGGGCAAAGCGAUGGCUUUGCCCUUGUUUGUU | 425368–425517 | 150 |
| Prrc37 | AUUUAAUUUUCGUUCCUUGCUUCCGCAGAUGGGUAGCUGGUCUAACAGUCGGAGGCUGAUUAACCCGUAAGGAGCAGUAAUGCGACACUACGAAAUCGUGUUUAUGGUUCAUCCGGACCAAAGCGAACAAG | 2230876–2231006, overlaps 5′ region (sense strand) of CDS PmVP161_2087 | 131 |
| Prrc54 | UAAACAUACUCACAGGCGGUCACUUGCCGAUAAAAUUUUGCAAAUUUUUCCCACAAUCUGACCGCUUGCCUUUUC | 1224402–1224476 | 75 |
| Prrc55 | CUGCUCGUCCUAUCCUAUUCUUAAGAAAUGCGAAA | 1090579–1090613 | 35 |
| Prrc56 | CCGCACUUUGGGCGUCAAUAUAACUAAAGCACCUUGAGUCAG | 731136–731095 | 42 |
| Prrc57 | UAACAAUUAAUAACAAAAAUGGAGAGUUGAAUUAAUUU | 743859–743822 | 38 |
VP161 genome GenBank assembly accession no. GCA_5011390865.1.
To further validate that ProQ activity resulted in increased transcript abundance, comparative transcript abundance was assessed. Transcripts extracted from the RNA-seq data were grouped into two groups, those identified as binding to ProQ (in CRAC analysis) and those that did not bind to ProQ. Two comparisons were made. The first compared ProQ-bound and unbound transcript abundance between the proQ mutant and the wild-type strain (Fig. 3B), and the second compared ProQ-bound and unbound transcript abundances between the complemented proQ mutant and the proQ mutant (Fig. 3C). These data clearly showed that RNA species that bound to ProQ showed decreased transcript abundance in the proQ mutant and increased abundance in the proQ mutant with a functional copy of proQ provided in trans, supporting the proposition that ProQ stabilizes a subset of transcripts in P. multocida (Fig. 3C).
As mutations occur in the nucleotide sequence where RNA targets have been cross-linked to the ProQ protein during CRAC, the sequences of putative ProQ-RNA binding sites were identified and examined for the presence of conserved sequences or structural motifs. No universal sequence motif could be identified, a finding consistent with ProQ binding site analyses in other bacterial species. As ProQ in other bacterial species has been shown to bind intrinsic terminators, we examined the ProQ-bound transcripts identified through CRAC for association with P. multocida intrinsic terminators that were predicted using RNAMotif (31). ProQ was shown to bind between −6 nt and +6 nt relative to the start of 5′ terminator stem loops (Fig. 3D). Additionally, these stem loops were enriched for adenosine residues compared to those found on other transcripts (Fig. 3E). Collectively, these data indicate that a significant number of P. multocida transcripts are stabilized by ProQ, and this is facilitated by ProQ binding to adenosine-rich sequences at the 5′ end of terminator stem loops.
ProQ stabilizes PmVP161_1121 mRNA.
Comparative transcriptomic analyses, and CRAC analysis using HTF-tagged ProQ, identified a transcript, PmVP161_1121, that strongly associated with ProQ (Table 3). The encoded 78-amino-acid protein is of unknown function, with no known protein domains, and has been separately identified as a bona fide protein by proteomic analyses of the parent strain, P. multocida strain VP161 (22). Homologues of PmVP161_1121 are on a number of the publicly available P. multocida genomes and on genomes representing Haemophilus influenzae, Haemophilus parainfluenzae, Mannhaemia haemolytica, and several Neisseria species. A maximum likelihood phylogenetic tree previously generated using a core genome alignment of 296 P. multocida reference genomes (32) included 22 genomes containing PmVP161_1121. Interestingly, all 22 P. multocida strains encoding homologues of PmVP161_1121 clustered together to form a unique clade, and all belonged to serogroup A capsule group and lipopolysaccharide genotype L1 (32). Differential transcriptomic analysis showed that the PmVP161_1121 transcript was increased approximately 4-fold in the complemented proQ mutant compared to expression in the WT strain and the proQ mutant (log2 fold changes of 2.02 and 1.91, respectively) (Table S1). As PmVP161_1121 was also shown to directly bind HTF-tagged ProQ in the CRAC analysis (Fig. 4A), these data suggest that ProQ is important for the stabilization of the PmVP161_1121 transcript in strain VP161. RNA secondary structure at the ProQ binding site within the PmVP161_1121 transcript was predicted using RNAfold (33) and visualized in VARNA (Fig. 4B) (34) and revealed the ProQ binding site in PmVP161_1121 mRNA is likely to be highly structured and fold into multiple stem loops. To confirm the dysregulation of the target, Northern blotting was used to assess the levels of PmVP161_1121 transcript in the WT P. multocida, the proQ mutant, and the complemented proQ mutant. A PmVP161_1121-specific digoxigenin (DIG)-labeled RNA probe detected two RNA species approximately 230 nt (correlating with the predicted size of the PmVP161_1121 transcript) and 260 nt in length (Fig. 4C). Densitometry was used to assess the relative levels of the 230-nt PmVP161_1121 transcript in each of the strains examined. The amounts of the 230-nt PmVP161_1121 transcript in the WT strain compared to that in the proQ mutant were not statistically different, confirming the data generated by transcriptomics (Fig. 4C, Table 3). This finding suggests that the expression of PmVP161_1121 is intrinsically low in the WT strain VP161 under the conditions tested; therefore, any changes in PmVP161_1121 transcript abundance when ProQ is absent are below the level of detection. However, 3-fold more of the 230-nt PmVP161_1121 transcript was identified in the complemented proQ mutant than in the WT or the proQ mutant (Fig. 4D), confirming data generated by transcriptomic analysis and CRAC analysis showing a clear association between ProQ and PmVP161_1121. Together, the experimental data and modeling prediction indicate ProQ directly binds to the PmVP161_1121 transcript by binding to stem loops within the coding region.
FIG 4.
Analysis of ProQ interaction with mRNA PmVP161_1121. (A) RNA-seq read coverage over the PmVP161_1121 transcript as determined by CRAC analysis of RNA samples isolated from strains expressing either tagged-ProQ (+) or untagged-ProQ (−). CRAC experiments were performed in biological triplicate (R1 to R3). Read counts are shown on a scale of 0 to 3,000 reads for all plots. (B) The secondary structure of the PmVP161_1121 transcript as predicted using RNAfold and indicating the residues interacting with ProQ region (red circles). (C) Northern blot detection (in biological triplicate) measuring the abundance of the PmVP161_1121 transcript (∼230 bp) in RNA isolated from the wild-type P. multocida VP161 containing empty vector (AL3356), proQ mutant containing empty vector (AL3358), and the complemented proQ mutant (AL3357). (D) Densitometry analysis of 230-nt PmVP161_1121 transcript detected in the northern blot using total RNA production and determined by SYBR-stained rRNA for standardization. Data shown are means ± SD; n = 3. **, P < 0.005; ***, P < 0.0005 by Mann-Whitney test.
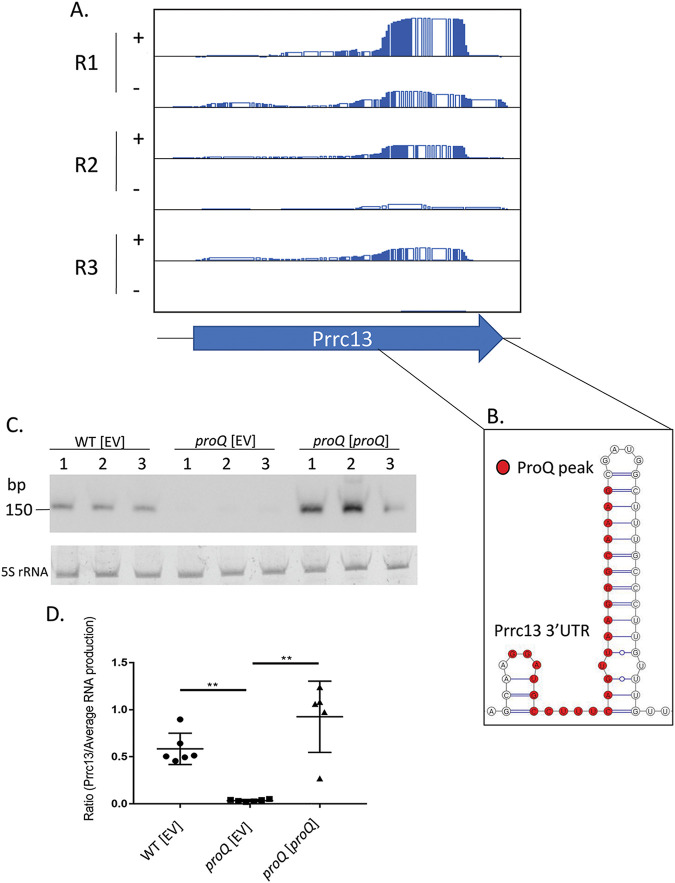
The sRNA Prrc13 requires ProQ for stability.
The putative sRNA Prrc13 in P. multocida strain VP161 was shown in this study to be dysregulated by altering ProQ abundance. Prrc13 was first identified as a putative sRNA in WT strain VP161 (26) and is immediately upstream of PmVP161_0400, encoding a predicted metal-dependent hydrolase belonging to the YdjM superfamily of proteins. Analysis of the CRAC data showed that ProQ directly bound the 3′ region of Prrc13, adjacent to the region containing the predicted intrinsic terminator (Fig. 5A and B). Comparative transcriptomic analysis revealed there was 4.2-fold reduced expression of Prrc13 in the proQ mutant compared to expression in the WT strain (log2 fold change of −2.24) (Table 2) and an 8.9-fold increase in expression in the complemented proQ mutant compared to the proQ mutant (log2 fold change of 3.16) (Table 2). To confirm this dysregulation, a Northern blot and densitometry analysis were performed to compare the levels of Prrc13 in the WT, the proQ mutant, and the complemented proQ mutant. A hybridizing band corresponding to the Prrc13 transcript was present in samples from the WT and the complemented proQ mutant but not the P. multocida proQ mutant (Fig. 5C and D). Together, these data indicate that ProQ stabilizes the Prrc13 transcript through direct interaction involving the 3′ termination region.
FIG 5.
Analysis of ProQ interaction with the predicted sRNA Prrc13. (A) RNA-seq read coverage over the Prrc13 transcript following CRAC analysis of RNA samples isolated from strains expressing either tagged ProQ (+) or untagged ProQ (−). CRAC experiments were performed in biological triplicate (R1 to R3). Read counts are shown on a scale of 0 to 3,000 reads for all plots. (B) The secondary structure of the 3′ region of Prrc13 as predicted using RNAfold. Residues binding to ProQ are indicated (red circles). (C) Northern blot detection of Prrc13 transcript. RNA was isolated from the wild-type P. multocida VP161 containing empty vector (AL3356), the proQ mutant containing empty vector (AL3358), and the complemented proQ mutant (AL3357). (D) Densitometry performed on transcript identified in Northern blot. Prrc13 expression is normalized relative to the average RNA production. Data shown are mean ± SD; n = 5 to 6. **, P < 0.005 by Mann-Whitney test.
DISCUSSION
In this study, comparative transcriptomics and ProQ-specific CRAC analysis were employed to identify the RNAs bound and regulated by ProQ in the P. multocida fowl cholera strain VP161. In concordance with data from S. enterica, E. coli, and N. meningitidis (11, 13, 35), we find that ProQ binds to structured regions of the RNA transcripts in the region of intrinsic terminator stem loops. Unlike other bacterial species, P. multocida does not appear to encode other FinO domain proteins, so it likely relies primarily on ProQ, Hfq, and CsrA for RNA binding and regulation (36). Our study indicates that ProQ is unlikely to play a role during in vitro growth of P. multocida or under conditions of osmotic stress or iron depletion. This contrasts with the P. multocida RNA-binding protein Hfq, which was shown to be involved in the regulation of iron-sequestering proteins (20). In N. meningitidis, deletion of hfq but not proQ reduced the ability of the strain to grow in rich medium. However, inactivation of proQ in the hfq mutant led to a further reduction in growth, suggesting that in N. meningitis there is a cohort of RNA transcripts that could be stabilized via ProQ activity in the absence of Hfq (35).
The transcriptomic analysis indicates that P. multocida ProQ associates with carbohydrate and metabolism gene transcripts and a number of tRNAs and sRNAs. Of the nine sRNAs that were differentially expressed in response to changes in ProQ, only one, Prrc12, is also known to be differentially expressed in an hfq mutant (log2 fold change, 2.02) in P. multocida (26). This indicates that within P. multocida, Hfq and ProQ have limited overlap in the RNA species to which they bind. Five tRNAs bound to ProQ and showed increased expression when ProQ was in abundance in the complemented proQ mutant but decreased expression in the proQ mutant, indicating that ProQ plays an important role in stabilizing these particular tRNAs. Interestingly, a study in S. enterica using a UV cross-linking method similar to CRAC found ProQ bound predominantly to the 3′ UTRs of coding transcripts, and only three tRNA binding partners were identified (11). None of the tRNAs were homologs of those we identified in P. multocida; however, they may have a similar secondary structure.
Immediately downstream of proQ on the same strand on the P. multocida strain VP161 genome is prc, followed by three other genes. We analyzed the region for potential termination sequences and used 5′RACE to identify the prc transcriptional start. The gene prc was expressed from its own promoter within proQ, similar to the arrangement observed in E. coli (23). To circumvent any downstream polar effects from the mutagenesis of proQ, the TargeTron insertion site chosen for inactivation of proQ was located upstream of the prc transcriptional start. Expression of prc in the proQ mutant was reduced but highly increased when the proQ mutant was provided with a functional copy of proQ gene in trans, indicating that the prc transcript is stabilized by ProQ. Indeed, the 5′ UTR of prc was shown to bind ProQ in the CRAC analysis. Therefore, the decrease in prc expression in the mutant is likely due to the lack of ProQ binding rather than effects of insertional mutagenesis.
Our data clearly show that ProQ plays a crucial role in the stabilization of the PmVP161_1121 mRNA and the putative sRNA Prrc13. Strains with homologues of PmVP161_1121 cluster within the same clade on a P. multocida core gene phylogeny tree, and all belong to capsule serogroup A and LPS genotype 1 (32). The majority of the P. multocida A:1 strains in this clade are fowl cholera disease isolates (32), and it would be of interest to explore the role of this protein in the disease process in future studies. A recent Hfq CRAC analysis in P. multocida has shown that the PmVP161_1121 transcript does not show evidence of binding to Hfq (26). However, ProQ was shown to directly bind to the PmVP161_1121 transcript at a predicted central stem-loop region and by doing so stabilized the transcript. Interestingly, two hybridizing transcripts were detected by Northern blotting, one correlating with the predicted size of the PmVP161_1121 transcript and a slightly larger transcript, indicating there is a second, alternative transcription start site upstream.
Prrc13 is a highly expressed, novel sRNA that is encoded between PmVP161_0399, predicted to encode a helix-turn-helix-type transcriptional regulator (COG3423) and similar to H. influenzae Ner, and PmVP161_0400, encoding a predicted metal-dependent hydrolase (COG1988). It is likely that Prrc13 exclusively interacts with ProQ, as recent CRAC analysis examining Hfq-RNA binding partners revealed Prrc13 does not directly interact with Hfq (26). Our data indicate that ProQ directly binds to Prrc13, and many other transcripts, at the beginning of the predicted 3′ terminator stem-loop, a position similar to that commonly observed for ProQ binding in S. enterica, E. coli, and N. meningitidis. ProQ has been shown to bind mRNA and stabilize the transcript by preventing transcript degradation by RNase II (11). Our study indicates that the P. multocida ProQ can also stabilize the Prrc13 sRNA, as the proQ mutant produced very low levels of Prrc13 transcript, but transcript levels were restored when a functional copy of proQ was provided to the mutant in trans.
Although P. multocida is a member of the Gammaproteobacteria, as are the well-studied pathogens E. coli, N. meningitidis, and S. enterica, the genomic GC content is approximately 10% lower. When examining the function of RNA-binding proteins, the GC content of the genome is an important consideration, as transcripts with a relatively high-GC form strong duplexes and are predicted to have a reduced need for further stabilization by RNA-binding proteins. This has been observed in Mycobacterium tuberculosis (genomic GC content of approximately 65%), where the 6C sRNA could interact with target transcripts using C-rich loops without the need of a chaperone protein (37). Our results demonstrate that ProQ is required to stabilize a broad range of structural, regulatory, and messenger RNAs in the P. multocida transcriptome and does so by binding to A-rich regions of highly structured regions of RNA transcripts. We have shown that the P. multocida ProQ is involved primarily in stabilization of a core set of RNAs that are different from those bound by Hfq. ProQ binds to highly structured sites on RNA targets, and no clear binding sequence motif was identified. This work builds a foundation for further characterization of ProQ-RNA interactions and is the first characterization of ProQ RNA binding in the Pasteurellaceae family.
MATERIALS AND METHODS
Bacterial strains, media, plasmids, and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. P. multocida strains were grown in HI (Oxoid) or peptone water (Oxoid) with 10% glucose at 37°C with shaking (200 rpm). E. coli strains were grown in lysogeny broth (LB; Oxoid). For growth on solid media, media were supplemented with 1% agar. Media were supplemented with the appropriate antibiotic as required (50 μg/mL spectinomycin and/or 50 μg/mL kanamycin). For growth curve analysis, OD600 readings were taken every 30 min using a FLUOstar omega microplate reader (BMG Labtech).
DNA manipulations.
Genomic DNA and plasmid DNA were purified using the genomic DNA extraction kit (RBC Bioscience Corp.) and the Macherey-Nagel NucleoSpin plasmid QuickPure kit (Fisher-Scientific), respectively, according to the manufacturer's instructions. Restriction endonucleases (New England Biolabs) and ligase (Roche) were used according to the manufacturer's instructions. PCR amplifications were performed using Taq DNA polymerase (Roche) or Phusion high-fidelity DNA polymerase (Roche). Oligonucleotides (primers) were manufactured by Sigma-Aldrich and are listed in Table 5. For cloning purposes, PCR products were first purified using the NucleoSpin gel and PCR clean-up kit (Macherey-Nagel) according to the manufacturer's instructions. Each sequencing reaction was performed using the BigDye Terminator v3.1 cycle sequencing kit (Roche) using PCR product or plasmid DNA as the template according to the manufacturer’s instructions or using genomic DNA as the template, as previously described (38). Sanger sequencing data were generated by the Micromon Genomics facility, Monash University, and analyzed using VectorNTI (Invitrogen). Nucleotide and amino acid sequence comparisons/alignments were performed using BLAST (NCBI) and Clustal Omega (EBI).
TABLE 5.
Oligonucleotides used in this study
| Name | Sequencea (5′–3′) | Description |
|---|---|---|
| BAP612 | GTAAAACGACGGCCAGT | Vector-specific primer (M13 forward/universal primer) used to sequence PCR products cloned into the multiple cloning site (MCS) of pPBA1100S, pAL99 or pREXY |
| BAP2067 | GGAAGGAACAGTTTCTCTGGATTG | Forward primer used to PCR-amplify region flanking TargeTron insertion site in P. multocida VP161 hyaD |
| BAP2679 | TTGTGTGGAATTGTGAGCGGA | Vector-specific primer used to PCR products cloned into the MCS of pPBA1100S, pAL99 or pREXY, on opposite side of MCS relative to BAP612 |
| BAP7971 | AAAAAAGCTTATAATTATCCTTAGTAAGCAAAACAGTGCGCCCAGATAGGGTG | For proQ mutagenesis, IBS TargeTron primer used in PCR to target intron to proQ |
| BAP7972 | CAGATTGTACAAATGTGGTGATAACAGATAAGTCAAAACACATAACTTACCTTTCTTTGT | For proQ mutagenesis, EBS1d TargeTron primer used in PCR to target intron to proQ |
| BAP7973 | TGAACGCAAGTTTCTAATTTCGATTCTTACTCGATAGAGGAAAGTGTCT | For proQ mutagenesis, EBS2 TargeTron primer used in PCR to target intron to proQ |
| BAP8088 | GCTTTTCCCGGGGTAAATTAATGTAT | Forward primer for PCR amplification of proQ, primer includes the proQ start codon and an XmaI site for cloning into pREXY |
| BAP8089 | ACTTATGAATTCTTATGCAAACAGATGTTCA | Reverse primer for amplification of proQ, primer includes the native proQ stop codon and an EcoRI site for cloning into pREXY |
| BAP8090 | TATTTCGAATTCTGCAAACAGATGTTC | Reverse primer for amplification of proQ, in same position as BAP8089 but native stop codon excluded, contains an EcoRI site for cloning into pREXY |
| BAP8098 | AATTAATGGAGCACCATCACCATCACCATGATTATGATATTCCAACTACTGCTAGCGAGAATTTGTATTTTCAGGGTG | Sense strand encoding His TEV tag, anneals to BAP8099 to produce double-stranded DNA with an EcoRI sticky end at 3′ end and an EcoRI compatible end at 5′ end, used to construct pAL1338 |
| BAP8099 | AATTCACCCTGAAAATACAAATTCTCGCTAGCAGTAGTTGGAATATCATAATCATGGTGATGGTGATGGTGCTCCATT | Antisense strand encoding His-TEV tag with EcoRI compatible ends; used with BAP8098 as described above, used to construct pAL1338 |
| BAP8100 | AATTGGACTACAAAGATGACGACGATAAAGACTACAAAGATGACGACGATAAAGACTACAAAGATGACGACGATAAATGAG | Sense strand encoding 3xFLAG tag, anneals to BAP8101 to produce double-stranded DNA with an EcoRI sticky end at 3′ end and an EcoRI compatible end at 5′ end, cloned into EcoRI site downstream of His TEV tag in pAL1338 to construct pAL1339 |
| BAP8101 | AATTCTCATTTATCGTCGTCATCTTTGTAGTCTTTATCGTCGTCATCTTTGTAGTCTTTATCGTCGTCATCTTTGTAGTCC | Antisense strand encoding 3×FLAG; used to anneal with BAP8100 as described above, used to construct pAL1339 |
| BAP8516 | ATTTTCTTCCGTAGGTTGTGGCGTAA | Reverse primer, anneals to nt 113 to 138 in prc, used for 5′RACE |
| BAP8517 | GGCTGTACTGCCTCGACAAGATTAAAGCTCAA | Reverse primer, anneals to nt 59 to 89 in prc, used for 5′RACE |
| BAP8571 | CGCACTGATTGAAAACAAGG | Forward primer for PCR amplification of PmVP161_1121 region, anneals 26 bp upstream of predicted start codon, for in vitro transcription production of a riboprobe |
| BAP8572 | TAATACGACTCACTATAGGGCATTAAGGGCTTTCCCCAGT | Reverse PCR primer, anneals 179 bp downstream of predicted start codon for PmVP161_1121, for in vitro transcription production of a riboprobe, contains T7 RNA polymerase promoter sequence at 5′ end (underlined) |
| BAP8573 | ATTCAGGGATCCGCCACGTGTAATAAACAGAGCCA | Forward primer for PCR amplification of proQ, including the native promoter region, contains a BamHI site for cloning into pPBA1100S |
| BAP8574 | TAATTGGTCGACTTCCGTAGGTTGTGGCGTAAC | Reverse primer for PCR amplification of proQ, contains a SalI site for cloning into pPBA1100S |
| BAP8610 | TGGACGAAAGTAAGTGCAAGAA | Forward primer for PCR amplification of an 82-bp fragment of the sRNA Prrc13, for in vitro transcription production of a riboprobe |
| BAP8611 | TAATACGACTCACTATAGCTTCCGTGCCTGTAACGAAT | Reverse primer for amplification of an 82-bp fragment representing the putative sRNA Prrc13, contains T7 RNA polymerase promoter sequence at 5′ end (underlined), for in vitro transcription production of a riboprobe |
| EBS universal | CGAAATTAGAAACTTGCGTTCAGTAAAC | TargeTron universal primer used for Sanger sequencing and PCR-based retargeting of TargeTron group II intron |
All restriction sites and restriction site compatible ends are shown in boldface. T7 RNA polymerase promoter sequence is shown underlined.
Construction of P. multocida mutants and expression plasmids.
To inactivate proQ and hyaD in the P. multocida strain VP161, TargeTron insertional mutagenesis (Sigma-Aldrich) was used as previously described (19, 38), with the following modifications. The specificity of the group II intron within the E. coli-P. multocida TargeTron shuttle vector, pAL953 (Table 1) (38), or a vector containing a markerless intron (described below) was targeted to the gene of interest using the splice overlap extension (SOE) PCR method described previously (38). The plasmid containing the retargeted intron (Table 1) was used to transform the appropriate P. multocida strain by electroporation. Mutants containing a TargeTron group II intron insertion in the target gene were identified using an initial PCR with primers flanking the target gene (Table 5) followed by a second PCR using a TargeTron-specific EBS universal primer (Table 5) paired with the appropriate primer flanking the target gene. The correct insertion of the intron into the genome was confirmed using Sanger sequencing with genomic DNA isolated from the mutant as the template and EBS universal primer.
To construct a proQ complementation strain, proQ and its predicted native promoter region was PCR-amplified using P. multocida VP161 genomic DNA with primers BAP8573 and BAP8574 (Table 5). The PCR product was digested with BamHI and SalI and ligated to similarly digested vector pPBA1100S (Table 1). The ligation mix was used to transform E. coli DH5α, and recovered transformants were screened for the correct plasmid using colony PCR with vector-specific primers that flanked the cloning site (BAP2679 and BAP612) (Table 5). Plasmids were extracted from three transformants that were positive by colony PCR, and separate Sanger sequencing reactions were performed using vector-specific primers that flanked the inserted DNA. One plasmid with the correct sequence was designated pAL1449 (Table 1) and was used to transform the P. multocida proQ mutant AL2973, resulting in the complementation strain, AL3357 (Table 1). The empty vector was also used to transform the proQ mutant strain and wild-type VP161, resulting in the strains AL3358 and AL3356 (Table 1), respectively.
RNA extraction and whole-genome transcriptomic analyses using RNA-seq.
RNA extractions were performed as described previously (22) using cultures grown to an OD600 of 0.6 (biological triplicate). Strand-specific RNA sequencing was performed using the SureSelect strand-specific RNA library preparation kit (Agilent) per the manufacturer’s instructions, with RNA isolated from the following strains: P. multocida wild-type VP161 containing empty vector (AL3356), VP161 proQ mutant containing empty vector (AL3358), and the proQ mutant with pAL1449 containing an intact copy of proQ under the control of the native promoter (AL3357) (Table 1). Libraries were sequenced on an Illumina NextSeq at the Micromon Genomics facility, Monash University.
The trimmed fastq files were aligned to the VP161 genome (GenBank accession no. NZ_CP048792.1) and aligned using R subread (39). Reads were quantified with featureCounts (40), producing the raw gene count matrix. The QC for the raw genes counts matrix and various quality control metrics were summarized in a MultiQC report (41). The genes counts matrix was then analyzed with the Degust (42) web tool, including differential expression analysis and several quality plots, such as classical multidimensional scaling (MDS) and MA plots. In this analysis, limma/voom (43) was used for model fitting. Degust (42) largely follows limma/voom workflow, including trimmed mean of M values (TMM) normalization (44) for RNA composition and several quality plots, such as classical multidimensional scaling and MA plots. Differentially expressed genes were defined as those showing a >2-fold (log2 fold change of 1) change in expression with a false discovery rate (FDR) of <0.05.
Northern blotting.
Northern blotting was performed as described previously (45), with the following modifications. PCR products were generated using genomic VP161 DNA as the template with primers BAP8571 and BAP8572, specific for PmVP161_1121, or primers BAP8610 and BAP8611, specific for Prrc13 (Table 5). DIG-labeled RNA probes were synthesized by in vitro transcription from each PCR product using the appropriate primers. Reverse primers BAP8572 and BAP8611 contained a T7 promoter sequence to facilitate in vitro transcription, which was performed per the manufacturer’s instructions, using the DIG northern starter kit version 10 (Roche). Detection of hybridizing fragments was performed as described previously (22).
5′RACE.
The 5′ transcriptional start site of prc was determined using 5′RACE per reference 22, using the primer BAP8516 (reverse primer; anneals to nt 113 to 138 in prc) with the commercially supplied outer primer in a first-round PCR and then the primer BAP8517 (reverse primer; anneals to nt 59 to 89 in prc) with the commercially supplied inner primer in a second-round PCR.
Construction of strains for CRAC.
To conduct CRAC experiments, the P. multocida strains were required to be safe for use in the UV-cross-linking apparatus. To attenuate the proQ mutant, AL2973, a markerless intron was inserted into hyaD, a gene essential for capsule production in this strain. To do this, the previously constructed TargeTron plasmid targeted to the VP161 hyaD gene (pAL1069) (Table 1) (20), was digested with MscI and religated to remove the kanamycin resistance gene present on the intron. The resulting TargeTron plasmid, pAL1337, was used to inactivate hyaD in the proQ mutant (AL2973) (Table 1) as described above. Transformants with an acapsular phenotype were screened for the correct intron insertion into hyaD by colony PCR with BAP2067 (located upstream of the target region) paired with EBS universal primer (Table 1). Three putative hyaD mutants were then Sanger sequenced using genomic DNA as the template with a hyaD-specific primer (Table 5) to confirm the site of intron insertion. Southern blotting was also employed using DIG-labeled probes, one specific for the kanamycin gene (on intron in proQ) to detect specifically the proQ intron insertion and one specific for group II intron sequence to confirm the position of both the introns at the correct sites. One of the hyaD-proQ double mutants confirmed by Sanger sequencing and Southern blotting to have the expected profile (see Fig. S1 in the supplemental material) was designated AL3067 (Table 1) and used for further study.
To express an HTF-tagged ProQ protein for CRAC analysis, a multistep cloning process was employed. The proQ gene was PCR-amplified without the stop codon using Phusion high-fidelity DNA polymerase (NEB) with VP161 genomic DNA and the primers BAP8088 and BAP8090 (Table 1). The PCR product generated was digested with XmaI/EcoRI and ligated to similarly digested pREXY to generate the interim plasmid, pAL1333 (Table 1). To insert the His-TEV tag sequence (including a 3′ stop codon) in-frame at the 3′ end of proQ in pAL1333, 50 μM primers BAP8098 and BAP8099 (Table 1) were combined in annealing buffer (10 mM Tris, pH 7.5 to 8.0, 50 mM NaCl, 1 mM EDTA) and subjected to 95°C for 5 min, followed by a gradual cooling (thermal cycler reduced by 1°C per cycle for 70 cycles, followed by a 12°C hold). The annealed, double-stranded DNA fragment with EcoRI compatible ends (designed to generate an EcoRI site at the 3′ end but not at the 5′ end) was then ligated to EcoRI-digested pAL1333. Plasmid was isolated from three colonies with the correct colony PCR profile (using vector primers BAP2679 and BAP612), and one of the plasmids confirmed to have the correct sequence was designated pAL1338 (Table 1). Next, a double-stranded DNA fragment encoding a 3× FLAG tag (constructed by annealing the primers BAP8100 and BAP8101 using method described above) and with EcoRI-compatible ends was ligated to EcoRI-digested pAL1338. Plasmid was isolated from three colonies with the correct colony PCR profile and sequenced. One of the correct plasmids was designated pAL1339. For generating the control plasmid, encoding untagged ProQ, the proQ forward primer BAP8088 was used with the reverse primer, BAP8089, which included the proQ native stop codon. The PCR product generated was cloned into pREXY in the same manner as that described above for the interim plasmid pAL1333 to generate the plasmid pAL1332 (Table 1). For use in the CRAC experiments, the plasmids pAL1339 (encoding HTF-tagged ProQ) and pAL1332 (control encoding untagged ProQ) were then separately used to transform the P. multocida hyaD-proQ double mutant (AL3067) to generate the strain AL3068 and the control strain AL3069, respectively (Table 1). The expression of HTF-tagged ProQ in AL3068 was confirmed using immunoblotting with an anti-FLAG antibody (data not shown).
Preparation of ProQ CRAC libraries and analysis of binding.
ProQ-specific CRAC was performed using P. multocida strains AL3068 and AL3069 grown to an OD600 of 0.8 in HI media (in biological triplicate) per Sy et al. (46). Identification of RNA molecules binding to ProQ was determined per Sy et al. (46). The UV-CRAC protocol involves a nuclease digestion of protein-bound RNAs and mutations occur in the RNA sequence where the RNA has directly cross-linked to the protein, and this allowed for a broad identification of bound RNAs as well as the exact location of the nucleotides involved in ProQ binding.
Data availability.
All transcriptomics data have been deposited in GEO with the identifier GSE196834.
ACKNOWLEDGMENTS
This work was supported in part by the Australian Research Council discovery project grant number 150103715. E.G. was supported by a Monash University MBio Postgraduate Discovery Scholarship. B.S., J.L.W., and J.J.T. were supported by NHMRC grants (GNT1139313 and GNT1161161).
Footnotes
Supplemental material is available online only.
Contributor Information
John D. Boyce, Email: john.boyce@monash.edu.
Laurie E. Comstock, Duchossois Family Institute
REFERENCES
- 1.Adams PP, Storz G. 2020. Prevalence of small base-pairing RNAs derived from diverse genomic loci. Biochim Biophys Acta Gene Regul Mech 1863:194524. 10.1016/j.bbagrm.2020.194524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891. 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attaiech L, Glover JN, Charpentier X. 2017. RNA chaperones step out of Hfq's shadow. Trends Microbiol 25:247–249. 10.1016/j.tim.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Smirnov A, Forstner KU, Holmqvist E, Otto A, Gunster R, Becher D, Reinhardt R, Vogel J. 2016. Grad-seq guides the discovery of ProQ as a major small RNA-binding protein. Proc Natl Acad Sci USA 113:11591–11596. 10.1073/pnas.1609981113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez G, Hardwick S, Maslen SL, Skehel JM, Holmqvist E, Vogel J, Bateman A, Luisi B, Broadhurst RW. 2017. Structure of the Escherichia coli ProQ RNA chaperone protein. RNA 23:696–711. 10.1261/rna.060343.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glover M, J N, Chaulk SG, Edwards RA, Arthur D, Lu J, Frost LS. 2015. The FinO family of bacterial RNA chaperones. Plasmid 78:79–87. 10.1016/j.plasmid.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Ponting CP. 1997. Tudor domains in proteins that interact with RNA. Trends Biochem Sci 22:51–52. 10.1016/s0968-0004(96)30049-2. [DOI] [PubMed] [Google Scholar]
- 8.Rizvanovic A, Kjellin J, Söderbom F, Holmqvist E. 2021. Saturation mutagenesis charts the functional landscape of Salmonella ProQ and reveals a gene regulatory function of its C-terminal domain. Nucleic Acids Res 49:9992–10006. 10.1093/nar/gkab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Mouali Y, Ponath F, Scharrer V, Wenner N, Hinton JC, Vogel J. 2021. Scanning mutagenesis of RNA-binding protein ProQ reveals a quality control role for the Lon protease. RNA 27:1512–1527. 10.1261/rna.078954.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey S, Gravel CM, Stockert OM, Wang CD, Hegner CL, LeBlanc H, Berry KE. 2020. Genetic identification of the functional surface for RNA binding by Escherichia coli ProQ. Nucleic Acids Res 48:4507–4520. 10.1093/nar/gkaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmqvist E, Li L, Bischler T, Barquist L, Vogel J. 2018. Global maps of ProQ binding in vivo reveal target recognition via RNA structure and stability control at mRNA 3' ends. Mol Cell 70:971–982. 10.1016/j.molcel.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Holmqvist E, Berggren S, Rizvanovic A. 2020. RNA-binding activity and regulatory functions of the emerging sRNA-binding protein ProQ. Biochim Biophys Acta Gene Regul Mech 1863:194596. 10.1016/j.bbagrm.2020.194596. [DOI] [PubMed] [Google Scholar]
- 13.Smirnov A, Wang C, Drewry LL, Vogel J. 2017. Molecular mechanism of mRNA repression in trans by a ProQ-dependent small RNA. EMBO J 36:1029–1045. 10.15252/embj.201696127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westermann AJ, Venturini E, Sellin ME, Förstner KU, Hardt WD, Vogel J. 2019. The major RNA-binding protein ProQ impacts virulence gene expression in Salmonella enterica serovar Typhimurium. mBio 10:e02504-18. 10.1128/mBio.02504-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkie IW, Harper M, Boyce JD, Adler B. 2012. Pasteurella multocida: diseases and pathogenesis. Curr Top Microbiol Immunol 361:1–22. 10.1007/82_2012_216. [DOI] [PubMed] [Google Scholar]
- 16.Chung JY, Wilkie I, Boyce JD, Townsend KM, Frost AJ, Ghoddusi M, Adler B. 2001. Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroup A. Infect Immun 69:2487–2492. 10.1128/IAI.69.4.2487-2492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper M, Cox A, St Michael F, Parnas H, Wilkie I, Blackall PJ, Adler B, Boyce JD. 2007. Decoration of Pasteurella multocida lipopolysaccharide with phosphocholine is important for virulence. J Bacteriol 189:7384–7391. 10.1128/JB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatum FM, Yersin AG, Briggs RE. 2005. Construction and virulence of a Pasteurella multocida fhaB2 mutant in turkeys. Microb Pathog 39:9–17. 10.1016/j.micpath.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Steen JA, Steen JA, Harrison P, Seemann T, Wilkie I, Harper M, Adler B, Boyce JD. 2010. Fis is essential for capsule production in Pasteurella multocida and regulates expression of other important virulence factors. PLoS Pathog 6:e1000750. 10.1371/journal.ppat.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mégroz M, Kleifeld O, Wright A, Powell D, Harrison P, Adler B, Harper M, Boyce JD. 2016. The RNA-binding chaperone Hfq is an important global regulator of gene expression in Pasteurella multocida and plays a crucial role in production of a number of virulence factors, including hyaluronic acid capsule. Infect Immun 84:1361–1370. 10.1128/IAI.00122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkie IW, Grimes SE, O'Boyle D, Frost AJ. 2000. The virulence and protective efficacy for chickens of Pasteurella multocida administered by different routes. Vet Microbiol 72:57–68. 10.1016/S0378-1135(99)00187-X. [DOI] [PubMed] [Google Scholar]
- 22.Gulliver EL, Wright A, Deveson Lucas D, Megroz M, Kleifeld O, Schittenhelm R, Powell D, Seemann T, Bulitta J, Harper M, Boyce JD. 2018. Determination of the small RNA GcvB regulon in the Gram-negative bacterial pathogen Pasteurella multocida and identification of the GcvB seed binding region. RNA 24:704–720. 10.1261/rna.063248.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr CH, Culham DE, Marom D, Wood JM. 2014. Salinity-dependent impacts of ProQ, Prc, and Spr deficiencies on Escherichia coli cell structure. J Bacteriol 196:1286–1296. 10.1128/JB.00827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MN, Kwok SC, Hodges RS, Wood JM. 2007. Structural and functional analysis of ProQ: an osmoregulatory protein of Escherichia coli. Biochemistry 46:3084–3095. 10.1021/bi6023786. [DOI] [PubMed] [Google Scholar]
- 25.Paustian ML, May BJ, Kapur V. 2001. Pasteurella multocida gene expression in response to iron limitation. Infect Immun 69:4109–4115. 10.1128/IAI.69.6.4109-4115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megroz M. 2020. The role of Hfq and sRNAs in regulation of Pasteurella multocida gene expression. PhD thesis. Monash University, Clayton, Australia. 10.26180/5ece1b0d2fc43. [DOI] [Google Scholar]
- 27.Melamed S, Adams PP, Zhang A, Zhang H, Storz G. 2020. RNA-RNA interactomes of ProQ and Hfq reveal overlapping and competing roles. Mol Cell 77:411–425. 10.1016/j.molcel.2019.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tree JJ, Granneman S, McAteer SP, Tollervey D, Gally DL. 2014. Identification of bacteriophage-encoded anti-sRNAs in pathogenic Escherichia coli. Mol Cell 55:199–213. 10.1016/j.molcel.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb S, Hector RD, Kudla G, Granneman S. 2014. PAR-CLIP data indicate that Nrd1-Nab3-dependent transcription termination regulates expression of hundreds of protein coding genes in yeast. Genome Biol 15:R8. 10.1186/gb-2014-15-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macke TJ, Ecker DJ, Gutell RR, Gautheret D, Case DA, Sampath R. 2001. RNAMotif, an RNA secondary structure definition and search algorithm. Nucleic Acids Res 29:4724–4735. 10.1093/nar/29.22.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smallman T. 2021. Investigation of Pasteurella multocida pathogenesis using transposon-directed insertion site sequencing and comparative genomics. PhD thesis. Monash University, Victoria, Australia. [Google Scholar]
- 33.Lorenz R, Bernhart SH, Honer Zu Siederdissen C, Tafer H, Flamm C, Stadler PF, Hofacker IL. 2011. ViennaRNA package 2.0. Algorithms Mol Biol 6:26. 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darty K, Denise A, Ponty Y. 2009. VARNA: interactive drawing and editing of the RNA secondary structure. Bioinformatics 25:1974–1975. 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauriedl S, Gerovac M, Heidrich N, Bischler T, Barquist L, Vogel J, Schoen C. 2020. The minimal meningococcal ProQ protein has an intrinsic capacity for structure-based global RNA recognition. Nat Commun 11:2823. 10.1038/s41467-020-16650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerovac M, El Mouali Y, Kuper J, Kisker C, Barquist L, Vogel J. 2020. Global discovery of bacterial RNA-binding proteins by RNase-sensitive gradient profiles reports a new FinO domain protein. RNA 26:1448–1463. 10.1261/rna.076992.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mai J, Rao C, Watt J, Sun X, Lin C, Zhang L, Liu J. 2019. Mycobacterium tuberculosis 6C sRNA binds multiple mRNA targets via C-rich loops independent of RNA chaperones. Nucleic Acids Res 47:4292–4307. 10.1093/nar/gkz149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper M, St Michael F, John M, Vinogradov E, Steen JA, van Dorsten L, Steen JA, Turni C, Blackall PJ, Adler B, Cox AD, Boyce JD. 2013. Pasteurella multocida Heddleston serovar 3 and 4 strains share a common lipopolysaccharide biosynthesis locus but display both inter- and intrastrain lipopolysaccharide heterogeneity. J Bacteriol 195:4854–4864. 10.1128/JB.00779-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y, Smyth GK, Shi W. 2019. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res 47:e47. 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 41.Ewels P, Magnusson M, Lundin S, Käller M. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048. 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David R, Powell AP, Milton M. 2019. Degust: interactive Rna-Seq analysis. Zenodo 10.5281/zenodo.3258932. [DOI] [Google Scholar]
- 43.Law CW, Chen Y, Shi W, Smyth GK. 2014. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15:R29. 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson MD, Oshlack A. 2010. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11:R25. 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rio DC. 2014. Northern blots for small RNAs and microRNAs. Cold Spring Harb Protoc 2014:793–797. 10.1101/pdb.prot080838. [DOI] [PubMed] [Google Scholar]
- 46.Sy B, Wong J, Granneman S, Tollervey D, Gally D, Tree JJ. 2018. High-resolution, high-throughput analysis of Hfq-binding sites using UV crosslinking and analysis of cDNA (CRAC). Methods Mol Biol 1737:251–272. 10.1007/978-1-4939-7634-8_15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1Table S2Figure S1. Download jb.00592-21-s0001.pdf, PDF file, 0.3 MB (327.9KB, pdf)
Data Availability Statement
All transcriptomics data have been deposited in GEO with the identifier GSE196834.