Abstract
In advanced heart failure (AHF) clinical evaluation fails to detect subclinical HF deterioration in outpatient settings. The aim of the study was to determine whether the strategy of intensive outpatient echocardiographic monitoring, followed by treatment modification, reduces mortality and re-hospitalizations at 12 months. Methods: 214 patients with ejection fraction < 30% and >1 hospitalization during the last year underwent clinical evaluation and echocardiography at discharge and were divided into intensive (IMG; N = 143) or standard monitoring group (SMG; N = 71). In IMG, volemic status and left ventricular filling pressure were assessed 14, 30, 90, 180 and 365 days after discharge. HF treatment, particularly diuretic therapy, was temporarily intensified when HF deterioration signs and E/e’ > 15 were detected. In SMG, standard outpatient monitoring without obligatory echocardiography at outpatient visits was performed. Results: We observed lower hospitalization (absolute risk reduction [ARR]-0.343, CI-95%: 0.287–0.434, p < 0.05; number needed to treat [NNT]-2.91) and mortality (ARR-0.159, CI 95%: 0.127–0.224, p < 0.05; NNT-6.29) in IMG at 12 months. One-year survival was 88.8% in IMG and 71.8% in SMG (p < 0.05). Conclusion: In AHF, outpatient monitoring of volemic status and intracardiac filling pressures to individualize treatment may potentially reduce hospitalizations and mortality at 12 months follow-up. Echocardiography-guided outpatient therapy is feasible and clinically beneficial, providing evidence for the larger application of this approach.
Keywords: advanced heart failure, outpatient monitoring, Tissue Doppler echocardiography, left ventricular filling pressure, mortality, rehospitalizations
1. Introduction
Advanced heart failure (AHF) is a clinical syndrome characterized by persistent heart failure (HF) symptoms and progressive left ventricular (LV) dysfunction, despite guideline-based medical therapy [1,2,3,4]. Patients with advanced progressive HF have frequent hospital readmissions due to pulmonary or systemic congestion, high mortality and poor quality of life [2,3]. In severe HF, recurrent hospitalizations increase the risk of adverse events, therefore, the concept of preventing hospital readmissions in this patient population has gained considerable importance over the last few years [5].
Worsening of HF signs and symptoms due to increasing fluid retention and congestion precede hospitalizations for acute HF decompensation. In ambulatory patients, progressive dyspnoea, weight gain, peripheral oedema and crackles on lung auscultation are suggestive of HF deterioration and are important predictors of the upcoming HF decompensation and hospitalization [6]. However, clinical evaluation and lung auscultation may fail to detect subclinical HF decompensation [7,8,9]. On the other hand, intracardiac hemodynamic changes and an increase in LV filling pressure precede the manifestation of HF symptoms and can be detected by echocardiography [10,11,12]. Increased ventricular filling pressure results in high atrial pressure with subclinical HF deterioration [13,14]. Therefore, the possibility to assess filling pressure in ambulatory patients with AHF provides accurate diagnosis of subclinical decompensation, expanding the already established role of echocardiography in heart failure. Early identification of the vulnerable period is important for the timely adjustment of a therapeutic regimen to prevent HF decompensation-related rehospitalizations in AHF patients.
The purpose of this study was to support our hypothesis that the strategy of outpatient echocardiography-guided treatment improves the efficacy of care and outcomes of patients with AHF at 12 months follow-up.
2. Materials and Methods
2.1. Study Design and Population
The prospective study was conducted among the patients corresponding to AHF definition of the European Society of Cardiology (ESC) [2] between January 2017 and February 2018. Eligibility criteria for intensive outpatient echo-guided monitoring included: (1) age between 18 and 90 years, (2) symptomatic patients with NYHA III–IV functional class, despite the optimal guideline-based therapy, (3) more than 1 hospitalization for HF decompensation within the past 12 months, (4) LV ejection fraction (EF) < 30% documented by transthoracic echocardiography, (5) left atrial (LA) volume > 35 mL/m2, and (6) E/e’ > 15 determined by Tissue Doppler (TD) before hospital discharge. Exclusion criteria were: (1) acute myocardial infarction or unstable angina in the past 3 months, (2) severe valvular disease, (3) inability to follow the study protocol, and (4) dialysis.
Before being discharged from hospital, patients were divided in two parallel groups in a ratio 2:1: intensive monitoring group (IMG) with mandatory outpatient echocardiography and standard monitoring group (SMG) with detailed physical examination only.
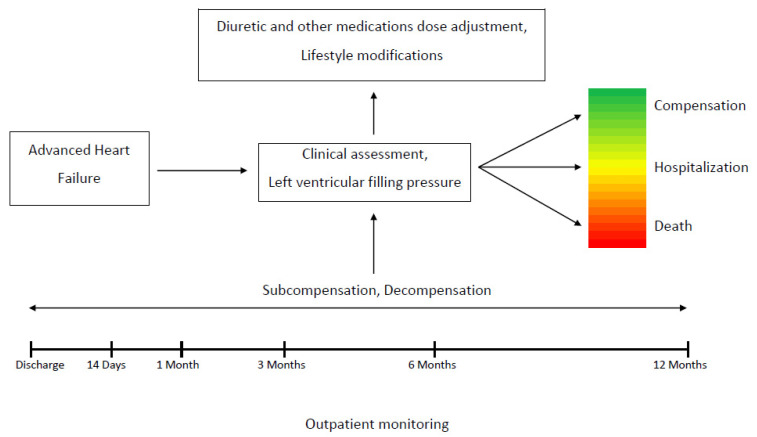
Outpatient evaluation and echocardiography with filling pressure assessment were provided by experienced HF specialists and trained echocardiography physicians according to ESC HFA and ASE guidelines [15,16]. A workflow chart of the present study is presented in Figure 1.
Figure 1.
Workflow chart of the present study.
A total of 249 patients admitted with HF decompensation were recruited between January 2017 and February 2018 in two University affiliated hospitals in Yerevan, Armenia. The data obtained from 214 patients, who completed the 12 months follow-up period, were used for statistical analysis. The study was approved by the local Ethics Committee and complied with Declaration of Helsinki principles. Informed consent was obtained from all participants before enrolment.
2.2. Clinical Data and Study Protocol
Comorbidities and clinical data of each patient were obtained from patient examination and hospital medical records. Congestion was assessed by presence of crackles on lung auscultation and peripheral oedema. The glomerular filtration rate (GFR) was calculated based on the creatinine levels at discharge using the Cockcroft–Gault equation.
Patients, who met the requirements after the discharge were assigned to intensive or standard monitoring groups and were followed-up for 12 months.
Intensive monitoring included five consecutive hospital visits 14, 30, 90, 180 and 365 days after the discharge. At discharge and during the visits, patients underwent a physical examination, body mass assessment, heart rate, GFR, and electrocardiography (ECG). Echocardiographic evaluation of the LV filling pressure and LA volume were part of intensive monitoring strategy at each outpatient visit. Based on the clinical evaluation of volemic state and echocardiographic data, patients with signs of worsening congestion and high LV filling pressure (E/e’ > 15) were managed by intensification of diuretic treatment (administration of double doses of oral loop diuretics or additional intravenous low dose 20–40 mg furosemide). Additional modifications of HF therapy included switching from renin-angiotensin-aldosterone system (RAAS) inhibitor to sacubitril/valsartan, change of prescribed doses of beta-blockers, and mineralocorticoid receptor antagonists (MRA). Other evidence-based pharmacologic HF therapies were administered per regular protocol during the outpatient period. Patients were hospitalized if treatment modification was not effective in the prevention of HF decompensation.
In SMG, standard outpatient monitoring was performed with the same terms and frequency of hospital visits, but without obligatory echocardiographic study. Outpatient follow-up visits included only physical assessment of congestion and ECG. Decision of treatment modification was based on physical examination only.
2.3. Echocardiographic Evaluation
An experienced cardiologist with everyday practice in echocardiography conducted transthoracic follow-up echocardiographic examinations. The echocardiographist was blinded to the patients’ treatment group. Basic and advanced echocardiographic assessments were performed using a commercially available system (General Electronic [GE] Vivid E9; 2017). All dimension and volume measurements were performed in accordance with accepted guideline recommendations [16,17]. The main treatment targets in the intensive treatment group were E/e’ < 15 and resolution of clinical congestion for the prevention of rehospitalizations. After 5–7 days of short-term loop diuretic treatment intensification, patients returned to previous treatment doses if E/e’ ratio was <15 and signs of congestion were absent.
LA volume was measured from 2-chamber and 4-chamber apical views with the area-length biplane method. Early diastolic velocity of transmitral flow (E) was measured in apical 4-chamber view using the Doppler technique. Septal early diastolic mitral annular velocity (e’) was measured using TD. In patients with atrial fibrillation, E and e’ waves were measured in five consecutive cycles and their average value was used to calculate E/e’ [16].
Investigators were unblinded to group assignment. However, echocardiographic assessment was carried out blindly. Medical history, clinical examinations and treatment changes were recorded at baseline and at each visit during the 12-month follow-up.
2.4. Study Endpoints
All patients were followed-up for 12 months. The primary endpoints were hospitalization for HF decompensation and mortality from cardiac causes. HF hospitalizations were defined as hospital admissions for worsening signs and symptoms of HF with more than 24 h hospital stay. Cardiovascular death included death from acute myocardial infarction, HF, cardiovascular procedures, and sudden cardiac death.
2.5. Statistical Analysis
Statistical analysis was performed using the IBM_22.0.0 SPSS statistical package (IBM, Armonk, NY, USA). Continuous variables with normal distribution were expressed as mean, standard deviation (SD), standard error (SE) and categorical variables presented as numbers and percentages. All p values were from 2-tailed tests, and results were deemed statistically significant at p < 0.05. For the evaluation of effectiveness of new approach (intensive ambulatory monitoring), absolute risk reduction (ARR) and number needed to treat (NNT) were used.
Survival assessment was estimated by the Kaplan–Meier method with adjustment for baseline differences in covariates and was compared using the long rank test.
Due to the non-randomized nature of the study, the propensity score (PS) was performed in order to minimize selection bias between groups. However, both echocardiographic examination and study endpoint assessments were carried out blindly. PS estimates the likelihood of receiving standard or intensive monitoring based on the clinical characteristics of each patient. The covariates included in the logistic regression analysis for PS calculation were age, gender, NYHA class, LA volume index, E/e’ ratio, beta-blockers, digoxin, and angiotensin receptor antagonist/neprilysin inhibitor at baseline, etc.
3. Results
3.1. Baseline Characteristics
Patients baseline characteristics are presented in Table 1.
Table 1.
Patients baseline characteristics in intensive and standard monitoring groups.
| Variable | Intensive Monitoring Group (n = 143) | Standard Monitoring Group (n = 71) | p Value |
|---|---|---|---|
| Age (years) | 66.6 ± 10.1 | 64.6 ± 10.1 | 0.186 |
| Women (n, %) | 30 (21) | 16 (23) | 0.794 |
| Body mass (kg) | 84.6 ± 14.2 | 80.5 ± 13.3 | 0.043 |
| Coronary artery disease (n, %) | 100 (70) | 64 (90) | 0.001 |
| Diabetes mellitus (n, %) | 37 (26) | 18 (25) | 0.934 |
| CKD (n, %) | 51 (36) | 16 (23) | 0.051 |
| Heart rate (beats per minute) | 84.8 ± 15.0 | 79.2 ± 17.4 | 0.016 |
| Sinus rhythm (n, %) | 93 (65) | 54 (76) | 0.102 |
| Atrial fibrillation (n, %) | 48 (34) | 17 (24) | 0.149 |
| Pacemaker (n, %) | 2 (1) | 0 (0) | 0.317 |
| ICD (n, %) | 3 (2) | 2 (3) | 0.743 |
| CRT (n, %) | 4 (3) | 0 (0) | 0.155 |
| Systolic blood pressure (mmHg) | 117.2 ± 17.9 | 119.7 ± 17.4 | 0.326 |
| Diastolic blood pressure (mmHg) | 71.2 ± 10.5 | 75.3 ± 11.9 | <0.001 |
| Creatinine (mmol/L) | 103.9 ± 38.3 | 130 ± 39.4 | <0.001 |
| Potassium (mmol/L) | 4.6 ± 0.5 | 4.2 ± 0.6 | 0.027 |
| NYHA class | |||
| III | 83 (58%) | 49 (69%) | 0.120 |
| IV | 60 (42%) | 22 (31%) | 0.120 |
| Echocardiographic parameters | |||
| LV ejection fraction (%) | 20.1 ± 5.2 | 22.5 ± 4.0 | 0.001 |
| LA volume index (mL/m2) | 51.7 ± 19.1 | 46 ± 10.4 | 0.020 |
| E/e’ ratio | 24.1 ± 6.9 | 15.8 ± 2.2 | <0.001 |
The data are expressed as mean ± SD (standard deviation). CKD—chronic kidney disease; ICD—implantable cardioverter-defibrillator; CRT—cardiac resynchronization therapy; NYHA—New-York Heart Association classification; LV—left ventricle; LA—left atrium.
The mean age of all study subjects was 65.9 ± 10 years, 78.5% of the patients were men. A total of 100 patients in IMG (70%) and 64 in SMG (90%) had a history of coronary artery disease.
Patients in IMG had more severe clinical risk markers compared to SMG. They were also likely to have more severe disease (42% of patients in IMG had NYHA class IV vs. 31% of patients in SMG).
Patients in IMG had higher mean E/e’ ratio (24.1 ± 6.9), higher mean LA volume index (51.7 ± 19.1), and lower mean EF (20.1 ± 5.2) at baseline. Baseline medical treatment of the patients is presented in Table 2.
Table 2.
Medical treatment characteristics at baseline.
| Medical Treatment | Intensive Monitoring Group (n = 143) | Standard Monitoring Group (n = 71) | p Value |
|---|---|---|---|
| Beta-blocker (n, %) | 131 (91.6) | 62 (87.3) | 0.321 |
| ACEi/ARB (n, %) | 123 (86) | 63 (88.7) | 0.014 |
| MRA (n, %) | 136 (95.1) | 62 (87.3) | 0.042 |
| Furosemide, oral (n, %) | 138 (96.5) | 66 (93) | 0.247 |
| Digoxin (n, %) | 36 (25.2) | 12 (16.9) | 0.172 |
| ARNI (n, %) | 16 (11.2) | 1 (1.4) | 0.013 |
| Inotropes (in-hospital) (n, %) | 81 (57) | 24 (33.4) | 0.002 |
| Vasodilators (n, %) | 14 (9.7) | 32 (45) | <0.0001 |
ACEi—Angiotensin-converting enzyme inhibitor; ARB—Angiotensin receptor blocker; MRA—Mineralocorticoid receptor antagonist; ARNI—Angiotensin receptor antagonist/neprilysin inhibitor.
In the IMG group 62 (46.6%), patients underwent an increase in doses and switch to i.v. furosemide at 3 months, 54 (42.2%) at 6 months and 35 (27.8%) at 12 months, whereas in SMG only 4 (7.0%) patients received i.v. furosemide treatment at 3 months and 3 (5.9%) patients at 6 months (Table 3). In SMG, no patients were prescribed i.v. furosemide at 12 months. Despite more frequent switch to i.v. furosemide in IMG, the mean i.v. furosemide doses in both groups at follow-up visits did not differ significantly and were even higher at some time periods in SMG (Table 3).
Table 3.
Diuretic doses at baseline and follow-up visit.
| Furosemide, Oral | at Discharge | 3 Months | 6 Months | 12 Months | |
| IMG | Study group (n) | 142 | 133 | 128 | 126 |
| Patient number (n, %) | 141 (99.3%) | 126 (94.7%) | 123 (96.1%) | 122 (96.8%) | |
| Mean dose (mg) | 44.96 ± 16.76 | 59.52 ± 32.64 | 63.74 ± 35.61 | 65.74 ± 39.29 | |
| SMG | Control Group (n) | 71 | 57 | 51 | 51 |
| Patient number (n, %) | 66 (93%) | 55 (96.5%) | 36 (70.6%) | 13 (25.5%) | |
| Mean dose (mg) | 64.55 ± 23.48 | 58.91 ± 30.65 | 64.44 ± 34.84 | 61.54 ± 35.08 | |
| Furosemide, i.v. | at discharge | 3 months | 6 months | 12 months | |
| IMG | Patient number (n, %) | 28 (19.7%) | 62 (46.6%) | 54 (42.2%) | 35 (27.8%) |
| Mean dose (mg) | 25.71 ± 10.69 | 36.45 ± 21.89 | 43.70 ± 25.50 | 62.29 ± 36.23 | |
| SMG | Patient number (n, %) | 25 (35.2%) | 4 (7.0%) | 3 (5.9%) | 0 |
| Mean dose (mg) | 64.8 ± 20.23 | 65.00 ± 25.17 | 86.67 ± 30.55 | 0 | |
| Torasemide, Oral | at discharge | 3 months | 6 months | 12 months | |
| IMG | Patient number (n, %) | 61 (43.0%) | 81 (60.9%) | 83 (64.8%) | 82 (65.1%) |
| Mean dose (mg) | 8.28 ± 3.28 | 10.43 ± 4.89 | 10.18 ± 4.58 | 10.06 ± 4.87 | |
| SMG | Patient number (n, %) | 2 (2.8%) | 3 (5.3%) | 2 (3.9%) | 2 (3.9%) |
| Mean dose (mg) | 10 | 10 | 10 | 10 | |
The data are expressed as mean ± SD (standard deviation). IMG—intensive monitoring group; SMG—standard monitoring group.
3.2. Outcomes
We observed a total of 93 hospitalizations for HF decompensation over the 12 months follow-up, including 43 (30%) in the IMG, and 50 (70%) in the SMG. Eight (6%) patients in IMG and 14 (19.7%) patients SMG had more than one hospitalization for HF decompensation within the study period.
Hospitalizations for other reasons, such as stroke, cancer and implantable cardioverter-defibrillator (ICD) implantation occurred in three patients in SMG.
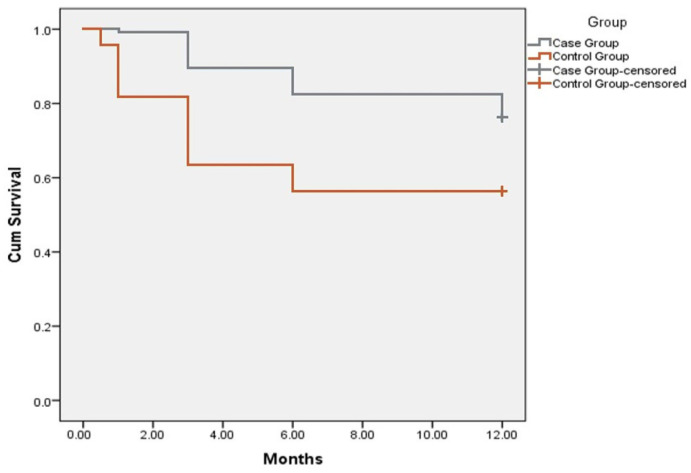
We observed a statistically significant (p < 0.05) reduction in hospitalization rates for HF decompensation in IMG. Compared to the SMG, significantly lower hospitalization rates were observed in IMG at 1, 3 and 6 months after discharge (Figure 2).
Figure 2.
Kaplan–Meier curve showing hospitalization rates in intensive monitoring group compared to standard monitoring (control) group during 12 months follow-up period. Y axis represents Cum Survival, X axis—follow-up period represented by months.
A total of 20 (28.2%) deaths were observed in SMG, including two cases of deaths from non-cardiac causes. In IMG, 16 deaths (11.2%) occurred during the follow-up, among them 13 individuals died from cardiovascular causes and three patients from cancer. The causes of cardiovascular deaths were acute myocardial infarction in three patients, sudden cardiac death in two, and HF decompensation in eight patients. Compared to SMG, we observed a significant decrease in mortality rates in IMG at 3, 6 and 12 months of follow-up with a significantly lower mortality rates at 12 months after the discharge (p < 0.05).
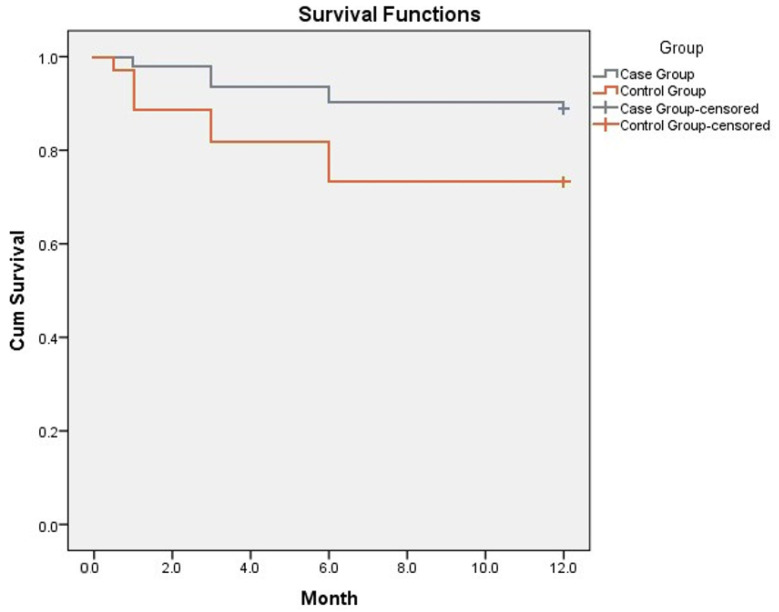
Kaplan–Meier curve for 1-year survival showed an improved survival in IMG. Mean survival in the intensive monitoring group was 11.2 ± 0.22 months (CI 10.75–11.61) and in the standard monitoring group 9.61 ± 0.49 months (CI 10.75–11.61) (Figure 3). The data suggested that 1-year survival was 88.8% in the IMG and 71.8% in the control group (p < 0.05).
Figure 3.
Kaplan–Meier curve showing survival rates in intensive and standard monitoring groups. Y axis represents Cum Survival, X axis—follow-up period represented by months.
The absolute risk reduction for rehospitalizations was 0.36, and the number needed to treat (NNT) was 2.78, indicating that a total of 2.78 patients would need intensive monitoring (rather than standard) for one additional patient to avoid rehospitalization over a 12-month period. When corrected with PS, absolute risk reduction remained significant (ARR = 0.343, CI 95%: 0.287–0.434, p < 0.05). PS was not significantly associated with risk reduction (p > 0.05). After correction NNT was 2.91.
The absolute risk reduction for survival was estimated at 0.17, and the NNT was 5.88, indicating that a total of 5.88 patients would need an intensive monitoring (rather than standard) for one additional patient to survive over a 12-month period. When corrected with PS, absolute risk reduction remained significant (ARR = 0.159, CI 95%: 0.127–0.224, p < 0.05). PS was not significantly associated with risk reduction (p > 0.05). After correction, NNT was 6.29.
4. Discussion
Patients with AHF remain at high risk of death and rehospitalizations despite advances in HF therapy. During the last decade, it has become evident that ambulatory monitoring of patients with HF leads to fewer decompensations. Furthermore, a personalized approach remains an important step towards the achievement of a better outcome [18]. The implementation of focused echocardiography in such settings is supported both by previous and current ESC guidelines on Heart Failure and ASE guidelines [15,16,19].
Frequently, haemodynamic deterioration precedes clinical congestion by days or weeks [20]. Therefore, clinical congestion may be seen as the “tip of an iceberg” of the haemodynamic compromise. Moreover, prolonged elevated filling pressure predisposes to organ injury and hypoperfusion, neurohormonal and proinflammatory response. Multiorgan dysfunction, particularly renal and hepatic failure, contributes to poor survival in this patient group [21]. Determination and quantification of haemodynamic congestion are crucial steps in the examination of HF patients. Thus, the failure to recognize subclinical elevation of pulmonary capillary wedge pressure (PCWP) may negatively affect the prognosis of HF patients [22]. An increase in LV filling pressure preceding the onset and aggravation of HF symptoms may be easily detected by echocardiography in patients with subclinical HF decompensation as an early predictor of HF deterioration [10]. Therefore, recognition of an increase in LV filling pressure at outpatient settings may enable early identification of AHF patients in the “vulnerable phase” of subclinical decompensation [23]. This offers a window for a timely escalation of diuretic therapy for the prevention of rehospitalizations.
Echocardiography is a safe, accessible and low-cost examination technique, which can be successfully used for the detection of subclinical HF decompensation. However, echocardiographic monitoring continues to have a limited application in outpatient settings.
Comprehensive echocardiography with TD imaging overcomes the limitations of physical examination and clinical picture-based strategies for the prediction of HF rehospitalizations. Moreover, the latter has been reported to have low sensitivity and efficacy in the early identification of upcoming hospital readmissions [8,9].
Over the last few decades, multiple prospective multicentre studies have investigated remote monitoring approaches based on the assessment of body weight, symptoms, blood pressure, heart rate, and capillary oxygen saturation. However, analyses of these studies show no consistent benefit of non-hemodynamic monitoring in HF patients [24,25,26,27,28]. In AHF patients, cardiac filling pressures rise several weeks before deterioration and hospitalization. Meanwhile, symptoms of clinical congestion occur usually shortly before the hospitalization [29]. This has led to the development of several invasive monitoring devices during the last years.
Invasive guidance of decongestive therapy guided by pulmonary artery pressure was associated with a significant reduction in hospitalizations in outpatient chronic HF patients in the CHAMPION trial [30] However, invasive monitoring has several limitations with regard to availability and safety. Moreover, in the ESCAPE trial, treatment of patients with acute HF, guided by pulmonary artery catheter, was non-beneficial compared with conventional therapy [31]. The US Post Approval Study (PAS) showed a 58% reduction in HF-related hospitalizations in the first year after CardioMEMS implantation compared with 1 year before implantation. Furthermore, a reduction in HF hospitalizations, mortality and all-cause mortality was observed after CardioMEMS implantation. However, patients included in the PAS study were their own historical controls and there has been no randomized comparison to standard care without pulmonary artery monitoring [32,33,34].
To date, there are several studies on cardiothoracic ultrasound monitoring aimed to provide decongestion in patients with acute and chronic HF (Table 4). In these studies, different patient groups were assessed by several measurements. In most of the studies, patients in the cardiothoracic-guided treatment arm seemed to be more effectively decongested compared to patients on standard treatment. However, E/e’ parameter and LA re-modelling assessments provide a more integrated approach among different cardiac ultra-sound measurements and are applied in routine clinical use. Thus, our study validated echo-guided parameters and demonstrated the reliable efficacy of echocardiography-guided treatment in ambulatory specific patient groups.
Table 4.
Review of studies on the role of cardiothoracic monitoring on prognosis in acute and chronic heart failure patients.
| Author (Year) | Study Type | Number of Patients | Patient Characteristics | Methodology | Outcomes | Limitations and Pitfalls |
|---|---|---|---|---|---|---|
| Ohman J., Harjola V-P., 2018 [35] | Pilot, prospective | 20 | Acute HF, E/e’ > 15, pulmonary congestion | E/e’, IVC index, LUS | Decrease of all-cause death and acute HF rehospitalisation. Better decongestion of patients | Small pilot study with unequal population of patients in two groups |
| Rivas-Lasarte M., Alvarez-Garcia J., 2019 (LUS-HF) [36] | Randomized trial | 123 | HF, high NT-proBNP, pulmonary congestion | LUS | LUS-guided strategy reduced hospitalisations and mortality at 6-month follow-up | Treatment protocol was not exclusively based on LUS findings |
| Marini C., Fragasso G., 2020 [37] | Multicentre, randomized | 244 | Chronic HF outpatient | LUS | Mid-term reduction of hospitalisations with LUS-guided managements | Mid-term (90 days) follow-up |
| Pang P., Russel F., 2021 (BLUSHED-AHF) [38] | Multicentre, randomized | 130 | Acute HF | LUS | No benefit of LUS-guided strategy compared to usual care at 90 days. No benefit of B-lines < 15 after 6 h decongestion, however faster resolution of congestion after 48 h |
No assessment of long-term rehospitalisation |
| Sisakian H., Shahnazaryan S., 2022 | Prospective | 214 | Advanced chronic HF | E/e, LV filling pressure | Decrease of hospitalisations and mortality in echo-guided group by intensive monitoring at 12-month follow-up | Exclusion of patients with severe valvular disease. Preliminary un-blinded selection |
HF—heart failure; IVC—inferior vena cava; NT-proBNP—N-terminal pro-B-type natriuretic peptide; LV—left ventricle; LUS—lung ultrasound.
The simplicity of echocardiographic application and measurements makes it potentially useful for larger groups of HF patients at relatively lower risk, where invasive telemonitoring may have more impact in sicker patients.
One of the main objectives of our study was to prevent frequent hospitalizations in patients with AHF. The optimization of treatment using non-invasive easy monitoring should be implemented for such patients at outpatient periods.
In our study of patients with advanced HF, NYHA III–IV and LVEF < 30%, intensive outpatient monitoring of LV filling pressures with need-based adjustment of HF treatment was associated with improved short and long-term survival and lower risk of rehospitalizations. The substantial reduction in both hospitalization and mortality were observed predominantly at a 3–6 months period. The data analysis suggested an improvement in 1-year survival with an absolute risk reduction of hospitalizations and mortality in IMG.
In our opinion, the main diagnostic tool of the observed beneficial effects is the assessment of LV filling pressures as early predictive parameters of HF decompensation, which precedes clinical deterioration. Changes in intracardiac hemodynamics and LV filling pressure elevation precede the development of symptoms and signs of congestion, and, finally, lead to HF hospitalization [10]. In our study, LA volume and E/e’ measurements allowed early identification of LV filling elevation and intracardiac hemodynamic worsening and predicted HF decompensation in subclinical patients. Thus, in terminally ill patients, intensive echocardiographic monitoring with repeated measurement of LA volume and E/e’ assessment by TD echocardiography may predict and prevent HF deterioration and, therefore, may lead to the reduction of hospitalizations and improvement of the prognosis.
In IMG, diuretic treatment intensification was guided by repeated blind outpatient echocardiographic assessments of LV filling pressures. Specifically, loop diuretic dose up-titration and/or addition of intravenous furosemide was provided in accordance with E/e’ ratio increase and volemic status. On the other hand, in SMG, the decision of changing diuretic dose was based on the clinical picture and physical examination alone. Thus, repeated echocardiographic measurements of LV filling pressures in patients with AHF may warranty a targeted and timely intensification of diuretic therapy. This fact can explain the difference in outcomes in two arms, particularly a statistically significant reduction of hospitalization and mortality rates in IMG.
Our findings are in line with our initial hypothesis, that echocardiography-guided therapy would improve outcomes and reinforce the importance of echocardiography-detected subclinical congestion in the management of AHF patients. TD examination is a quick and easy to perform technique which is available in most cardiology departments. The results of this study highlight that currently, many patients may be receiving inadequate treatment and may probably be undertreated. Therefore, based on the results of the study, HF specialists are encouraged to use LV filling pressure measurements more frequently for the detection of subclinical HF deterioration and to enable more effective and timely treatment of these patients.
Study Limitations
As all observational studies our study carries the possibility for bias, leading to an over or underestimation of the intensive monitoring approach. The unblinded design of the study may represent a potential limitation. Although two groups of study populations were homogeneous regarding the main baseline characteristics, we provided a propensity score calculation to minimize selection bias.
Participating physicians were free to choose an intensive versus standard outpatient monitoring approach. The baseline differences in several clinical parameters, such as atrial fibrillation, NYHA class IV, E/e’ ratio, and treatment with MRA suggest, that physicians were more likely to provide intensive monitoring and follow-up to high-risk patients with more severe disease. Despite the higher baseline risk of patients in IMG, mortality and hospitalization rates were lower in this group compared to SMG. Besides the expected differences in NYHA class and E/e’ ratio, we observed significantly more cases of chronic kidney disease in IMG. Another limitation of study was exclusion of severe valvular heart disease, which may limit generalization of our results to such patients.
5. Conclusions
Hemodynamic monitoring by echocardiography is a widely available and cost-effective method for the outpatient assessment of patients with AHF. It can be implemented at different stages of care for the identification of vulnerable, high-risk patients, allowing optimal and timely adjustment of therapeutic regimens for the prevention of rehospitalizations. TD examination of LV filling pressures provides risk assessment for patients with subclinical decompensation prior to the clinically apparent HF decompensation. Frequent echocardiographic monitoring of filling pressures in AHF patients may lead to a considerable reduction in recurrent hospitalizations and mortality at a 12-month period.
Author Contributions
Conceptualization, H.S., Y.L. and S.S.; Data curation, H.S. and S.S.; Formal analysis, A.C.; Investigation, S.S., S.P., A.M. (Armine Minasyan), G.M., M.H., A.M. (Ashkhen Maghaqelyan) and S.M.-S.; Methodology, H.S. and S.S.; Project administration, H.S.; Resources, S.P., A.M. (Armine Minasyan), G.M., M.H., A.M. (Ashkhen Maghaqelyan) and S.M.-S.; Software, A.C.; Supervision, H.S.; Validation, H.S., S.S. and Y.L.; Visualization, H.S.; Writing—original draft, H.S., S.S. and Y.L.; Writing—review and editing, H.S. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Yerevan State Medical University (#3/14-15, 20 November 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The access numbers will be provided prior to the publication.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology Founda-tion/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Crespo-Leiro M.G., Metra M., Lund L.H., Milicic D., Costanzo M.R., Filippatos G., Gustafsson F., Tsui S., Barge-Caballero E., De Jonge N., et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018;20:1505–1535. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 3.Metra M., Dinatolo E., Dasseni N. Advanced Heart Failure the New Heart Failure Association Definition of Advanced Heart Failure Advanced Heart Failure. Card. Fail. Rev. 2019;5:5–8. doi: 10.15420/cfr.2018.43.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metra M., Ponikowski P., Dickstein K., McMurray J.J., Gavazzi A., Bergh C.-H., Fraser A.G., Jaarsma T., Pitsis A., Mohacsi P., et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Ziaeian B., Fonarow G.C. The Prevention of Hospital Readmissions in Heart Failure. Prog. Cardiovasc. Dis. 2016;58:379–385. doi: 10.1016/j.pcad.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damy T., Kallvikbacka-Bennett A., Zhang J., Goode K., Buga L., Hobkirk J., Yassin A., Dubois-Randé J.-L., Hittinger L., Cleland J.G., et al. Does the physical examination still have a role in patients with suspected heart failure? Eur. J. Heart Fail. 2011;13:1340–1348. doi: 10.1093/eurjhf/hfr128. [DOI] [PubMed] [Google Scholar]
- 7.Platz E., Lewis E.F., Uno H., Peck J., Pivetta E., Merz A., Hempel D., Wilson C., Frasure S.E., Jhund P., et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur. Heart J. 2016;37:1244–1251. doi: 10.1093/eurheartj/ehv745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampert B.C., Emani S. Remote hemodynamic monitoring for ambulatory left ventricular assist device patients. J. Thorac. Dis. 2015;7:2165–2171. doi: 10.3978/j.issn.2072-1439.2015.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamson P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009;6:287–292. doi: 10.1007/s11897-009-0039-z. [DOI] [PubMed] [Google Scholar]
- 10.Mangi M.A., Rehman H., Rafique M., Illovsky M. Ambulatory Heart Failure Monitoring: A Systemic Review. Cureus. 2017;9:e1174. doi: 10.7759/cureus.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vazir A., Cowie M.R. Assessing Acute Decompensated Heart Failure—Strategies and Tools. Eur. Cardiol. Rev. 2012;8:128. doi: 10.15420/ecr.2012.8.2.128. [DOI] [Google Scholar]
- 12.Vignon P. Cardiovascular failure and weaning. Ann. Transl. Med. 2018;6:354. doi: 10.21037/atm.2018.05.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dokainish H., Nguyen J.S., Bobek J., Goswami R., Lakkis N.M. Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: An echocardiographic and invasive haemodynamic study. Eur. J. Echocardiogr. 2011;12:857–864. doi: 10.1093/ejechocard/jer157. [DOI] [PubMed] [Google Scholar]
- 14.Tang W.W., Shrestha K., Mullens W., Borowski A.G., Martin M.G., Troughton R.W., Klein A.L. Impact of Left Ventricular Remodeling on Diagnostic and Prognostic Value of Tissue Doppler Indices in Chronic Systolic Heart Failure. J. Card. Fail. 2011;17:128–134. doi: 10.1016/j.cardfail.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.-P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. Erratum in 2018, 39, 860. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., Dokainish H., Edvardsen T., Flachskampf F.A., Gillebert T.C., Klein A.L., Lancellotti P., et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:233–271. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez A.F. Relationship Between Early Physician Follow-up and 30-Day Readmission Among Medicare Beneficiaries Hospitalized for Heart Failure. JAMA J. Am. Med. Assoc. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 19.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghiade M., Follath F., Ponikowski P., Barsuk J.H., Blair J.E., Cleland J.G., Dickstein K., Drazner M.H., Fonarow G.C., Jaarsma T., et al. Assessing and grading congestion in acute heart failure: A scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010;12:423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 21.Zymliński R., Sokolski M., Biegus J., Siwołowski P., Nawrocka-Millward S., Sokolska J., Dudkowiak M., Marciniak D., Todd J., Jankowska E.A., et al. Multi-organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur. J. Heart Fail. 2019;21:744–750. doi: 10.1002/ejhf.1378. [DOI] [PubMed] [Google Scholar]
- 22.Lucas C., Johnson W., Hamilton M.A., Fonarow G., Woo M.A., Flavell C.M., Creaser J.A., Stevenson L.W. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am. Heart J. 2000;140:840–847. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 23.Murphy N., Shanks M., Alderman P. Management of Heart Failure with Outpatient Technology. J. Nurse Pract. 2018;15:12–18. doi: 10.1016/j.nurpra.2018.07.004. [DOI] [Google Scholar]
- 24.Angermann C.E., Störk S., Gelbrich G., Faller H., Jahns R., Frantz S., Loeffler M., Ertl G. Competence Network Heart Failure. Mode of Action and Effects of Standardized Collaborative Disease Management on Mortality and Morbidity in Patients with Systolic Heart Failure: The Interdisciplinary Network for Heart Failure (INH) study. Circ. Heart Fail. 2012;5:25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry S.I., Mattera J.A., Curtis J.P., Spertus J.A., Herrin J., Lin Z., Phillips C.O., Hodshon B.V., Cooper L.S., Krumholz H.M. Telemonitoring in Patients with Heart Failure. N. Engl. J. Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleland J.G., Louis A.A., Rigby A.S., Janssens U., Balk A.H. Noninvasive Home Telemonitoring for Patients with Heart Failure at High Risk of Recurrent Admission and Death: The Trans-European Network-Home-Care Management System (TEN-HMS) study. J. Am. Coll. Cardiol. 2005;45:1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Koehler F., Koehler K., Deckwart O., Prescher S., Wegscheider K., Kirwan B.-A., Winkler S., Vettorazzi E., Bruch L., Oeff M., et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–1057. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 28.Koehler F., Winkler S., Schieber M., Sechtem U., Stangl K., Böhm M., Boll H., Baumann G., Honold M., Koehler K., et al. Impact of Remote Telemedical Management on Mortality and Hospitalizations in Ambulatory Patients with Chronic Heart Failure. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 29.Zile M.R., Bennett T.D., Sutton M.S.J., Cho Y.K., Adamson P.B., Aaron M.F., Aranda J.J.M., Abraham W.T., Smart F.W., Stevenson L.W., et al. Transition from Chronic Compensated to Acute Decompensated Heart Failure. Circulation. 2008;118:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 30.Abraham W.T., Stevenson L.W., Bourge R.C., Lindenfeld J.A., Bauman J.G., Adamson P.B., CHAMPION Trial Study Group Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 31.Binanay C., Califf R.M., Hasselblad V., O’Connor C.M., Shah M.R., Sopko G., Stevenson L.W., Francis G.S., Leier C.V., Miller L.W., et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness. JAMA J. Am. Med. Assoc. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 32.Abraham W.T., Adamson P.B., Bourge R.C., Aaron M.F., Costanzo M.R., Stevenson L.W., Strickland W., Neelagaru S., Raval N., Krueger S., et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 33.Vaduganathan M., DeFilippis E.M., Fonarow G.C., Butler J., Mehra M.R. Postmarketing Adverse Events Related to the CardioMEMS HF System. JAMA Cardiol. 2017;2:1277–1279. doi: 10.1001/jamacardio.2017.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai A.S., Bhimaraj A., Bharmi R., Jermyn R., Bhatt K., Shavelle D., Redfield M.M., Hull R., Pelzel J., Davis K., et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice. J. Am. Coll. Cardiol. 2017;69:2357–2365. doi: 10.1016/j.jacc.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Öhman J., Harjola V.-P., Karjalainen P., Lassus J. Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Fail. 2018;5:120–128. doi: 10.1002/ehf2.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivas-Lasarte M., Alvarez-Garcia J., Fernández-Martínez J., Maestro A., López-López L., Solé-González E., Pirla M.J., Mesado N., Mirabet S., Fluvià P., et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study) Eur. J. Heart Fail. 2019;21:1605–1613. doi: 10.1002/ejhf.1604. [DOI] [PubMed] [Google Scholar]
- 37.Marini C., Fragasso G., Italia L., Sisakian H., Tufaro V., Ingallina G., Stella S., Ancona F., LoIacono F., Innelli P., et al. Lung ultrasound-guided therapy reduces acute decompensation events in chronic heart failure. Heart. 2020;106:1934–1939. doi: 10.1136/heartjnl-2019-316429. [DOI] [PubMed] [Google Scholar]
- 38.Pang P.S., Russell F.M., Ehrman R., Ferre R., Gargani L., Levy P.D., Noble V., Lane K.A., Li X., Collins S.P. Lung Ultrasound–Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF) JACC Heart Fail. 2021;9:638–648. doi: 10.1016/j.jchf.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The access numbers will be provided prior to the publication.