Abstract
Background
Increased fluconazole and echinocandin resistance in Candida glabrata requires prompt detection in routine settings. A phenotypic test based on the EUCAST E.DEF 7.3.2 protocol was developed for the detection of fluconazole- and anidulafungin-resistant isolates utilizing the colorimetric dye XTT.
Methods
Thirty-one clinical C. glabrata isolates, 11 anidulafungin resistant and 14 fluconazole resistant, were tested. After optimization studies, 0.5–2.5 × 105 cfu/mL of each isolate in RPMI 1640 + 2% d-glucose medium containing 100 mg/L XTT + 0.78 μΜ menadione and 0.06 mg/L anidulafungin (S breakpoint) or 16 mg/L fluconazole (I breakpoint) in 96-well flat-bottom microtitration plates were incubated at 37°C for 18 h; we also included drug-free wells. XTT absorbance was measured at 450 nm every 15 min. Differences between the drug-free and the drug-treated wells were assessed using Student’s t-test at different timepoints. ROC curves were used in order to identify the best timepoint and cut-off.
Results
The XTT absorbance differences between fluconazole-containing and drug-free wells were significantly lower for the resistant isolates compared with susceptible increased exposure isolates (0.08 ± 0.05 versus 0.25 ± 0.06, respectively, P = 0.005) at 7.5 h, with a difference of <0.157 corresponding to 100% sensitivity and 94% specificity for detection of resistance. The XTT absorbance differences between anidulafungin-containing and drug-free wells were significantly lower for the resistant isolates compared with susceptible isolates (0.08 ± 0.07 versus 0.200 ± 0.03, respectively, P < 0.001) at 5 h, with a difference of <0.145 corresponding to 91% sensitivity and 100% specificity, irrespective of underlying mutations.
Conclusions
A simple, cheap and fast phenotypic test was developed for detection of fluconazole- and anidulafungin-resistant C. glabrata isolates.
Introduction
Invasive Candida infections are estimated to occur globally in more than 250 000 patients per year1 and they are associated with high morbidity and mortality in immunocompromised patients, particularly in the ICU. Although Candida albicans is still the most frequent Candida species, Candida glabrata is the second to third most common Candida species, causing invasive candidiasis in the USA, Australia and Northern Europe.1,2 This is a challenge because C. glabrata, with its propensity to acquire azole3,4 and echinocandin resistance and up to 15% MDR phenotype, is difficult to treat.2,5,6
Given that the mortality of invasive candidiasis doubles with every day of delayed effective antifungal therapy, efforts have been made with regard to fast and accurate detection of resistance.7 The reference methods for antifungal susceptibility testing developed by CLSI8 and EUCAST9 require at least 24 h of incubation. Alternative tests have been proposed, based on molecular techniques,10,11 flow cytometry12,13 and MALDI-TOF MS,14,15 but they have limited application in routine laboratories because of multifactorial mechanisms of azole resistance,16 high complexity, lack of standardization and interpretative guidelines, increased cost and requirement for special equipment and skilled personnel.
Colorimetric methods have been used for antifungal susceptibility testing, since they generate clear-cut endpoints, based on detectable colour changes. XTT is a yellow, water-soluble tetrazolium salt that is rapidly reduced by mitochondrial dehydrogenases of metabolically active yeast cells.17,18 XTT-based methods have a wide use in antifungal susceptibility19,20 and biofilm21 studies. In the present study, we developed a phenotypic test based on the EUCAST E.DEF 7.3.2 protocol for the detection of fluconazole- and anidulafungin-resistant isolates utilizing the colorimetric dye XTT.
Materials and methods
Isolates
A total of 31 clinical C. glabrata isolates were used for both antifungal agents (Table 1), 11 anidulafungin resistant (R) (MICs 0.125–4 mg/L) and 20 anidulafungin susceptible (S) (MICs ≤0.008–0.06 mg/L) and 14 fluconazole resistant (R) (MICs >16 mg/L) and 17 fluconazole susceptible increased exposure (I) (MICs ≤16 mg/L). Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains. All MICs were determined in triplicate using EUCAST E.DEF 7.3.2.9 Isolates were stored in 10% glycerol aqueous solution and were revived by subculturing onto Sabouraud glucose agar plates, 18–24 h prior.
Table 1.
Median MICs and classification of each strain for anidulafungin and fluconazole
| C. glabrata isolates | FKS mutation | Anidulafungin | Fluconazole | ||
|---|---|---|---|---|---|
| MIC (mg/L) | classification | MIC (mg/L) | classification | ||
| SSI-6133 | FKS2 D663E | 0.25 | R | 32 | R |
| SSI-2250 | FKS2 S663F | 2 | R | 8 | I |
| SSI-4857 | FKS2 L712-STOP/F659-DEL | 4 | R | >32 | R |
| SSI-5324 | FKS2 F659-DEL | 2 | R | 32 | R |
| SSI-3203 | FKS1 S629P | 2 | R | >32 | R |
| SSI-4843 | FKS1 L630Q + FKS2 S663F | 1 | R | 2 | I |
| SSI-5128 | FKS2 S663P | 2 | R | >32 | R |
| SSI-7033 | FKS2 l662W | 0.5 | R | >32 | R |
| SSI-2696a | WT | 0.125 | R | >32 | R |
| AUH-379 | FKS2 S663F | 1 | R | 8 | I |
| SSI-3775 | FKS2 F659S | 2 | R | 16 | I |
| SSI-7968 | WT | 0.016 | S | >32 | R |
| SSI-7966 | WT | 0.016 | S | 4 | I |
| SSI-7967 | WT | 0.016 | S | 4 | I |
| SSI-7969 | WT | 0.016 | S | 8 | I |
| SSI-5502 | WT | 0.03 | S | 8 | I |
| SSI-4914 | FKS2 F659L | 0.06 | S | 2 | I |
| SSI-64.37 | WT | 0.016 | S | 4 | I |
| SSI-6244 | WT | 0.03 | S | >32 | R |
| SSI-6371 | WT | 0.03 | S | 32 | R |
| SSI-62.64 | WT | 0.03 | S | >32 | R |
| SSI-61.31 | WT | 0.03 | S | >32 | R |
| SSI-61.10 | WT | 0.03 | S | 16 | I |
| SSI-60.02 | WT | 0.016 | S | 16 | I |
| SSI-7965 | WT | 0.016 | S | 16 | I |
| SSI-4778 | WT | 0.03 | S | >32 | R |
| SSI-2717 | WT | 0.03 | S | >32 | R |
| AUH-249 | WT | 0.03 | S | 16 | I |
| AUH-312 | WT | 0.016 | S | 16 | I |
| AUH-1670 | WT | 0.016 | S | 16 | I |
| AUH-1740 | WT | 0.03 | S | 16 | I |
S, susceptible; I, susceptible increased exposure; R, resistant; SSI, Statens Serum Institute; AUH, Attikon University Hospital.
This C. glabrata isolate was cultured from a faeces sample from a patient with oesophagitis due to an MDR C. albicans. The C. albicans harboured an S645P alteration, whereas the C. glabrata had WT FKS genes but MICs spanning 0.06–0.125 mg/L.
Compounds and medium
Pure powders of anidulafungin (Pfizer) and fluconazole (Sigma) were dissolved in DMSO to a concentration of 5 and 6.4 mg/mL, respectively. Stocks solutions were stored at −70°C until use. XTT (Sigma) was dissolved at a final concentration of 100–400 mg/L in water supplemented with menadione (Sigma) at a final concentration of 0.78–25 μM from a stock solution of 58 000 μM in absolute ethanol. RPMI 1640 medium (with l-glutamine; without bicarbonate) buffered to pH 7.0 with 0.165 M MOPS (Applichem and Sigma) with 2% d-glucose was used throughout the study.
XTT susceptibility testing
For the XTT assay, 0.5–2.5 × 105 cfu/mL of each isolate in RPMI 1640 + 2% d-glucose medium containing different concentrations of XTT/menadione and the antifungal drug were added to 96-well flat-bottom microtitration plates (200 μL/well). In order to find the optimal concentrations of XTT and menadione that differentiate resistant and susceptible isolates, 100–400 mg/L XTT with 0.78–25 μM menadione were tested together with anidulafungin and fluconazole concentrations close to the EUCAST breakpoints (0.12 and 0.06 mg/L anidulafungin and 32 and 16 mg/L fluconazole). Strains SSI-3203 (resistant to both antifungal drugs), SSI-7696 (susceptible to anidulafungin) and SSI-5502 (susceptible increased exposure to fluconazole) were used in optimization studies. Furthermore, addition of drugs 0, 2 and 4 h after inoculation was also assessed. Drug-free wells were also included. The plates were incubated at 37°C. The metabolic activity in each well was kinetically assessed by measuring the absorbance at 450 nm every 15 min for more than 18 h in a microplate reader (Tecan Infinite F200). Curves linked to metabolic activity were generated for each isolate and each condition.
Statistical analysis
After subtraction of background absorbance, the differences in XTT absorbance between drug-free and drug-containing wells (deltaΧΤΤ-ABS) were calculated for each isolate and the differences between the resistant and the susceptible isolates were assessed with a non-parametric Mann–Whitney test at different timepoints for each antifungal drug. ROC curve analysis was used in order to determine the earliest timepoint with the highest area under the ROC (AUCROC). Specificity and sensitivity in detecting resistant isolates were calculated at different timepoints and deltaXTT-ABS. From each timepoint the deltaXTT-ABS was chosen based on the highest likelihood ratio [sensitivity/(100−specificity)]. For deltaXTT-ABS with an undefined likelihood ratio (when specificity is 100%), the deltaXTT-ABS with the largest sensitivity was chosen. All analyses were performed using GraphPad Prism 5.03.
Results
Metabolic curves
XTT absorbance data for up to 18 h of incubation were generated using a spectrophotometer. All isolates converted XTT into its orange product, reaching an absorbance of >1.8 for the drug-free control (Figure 1). Metabolic curves were similar across the isolates in the absence of drug, with a linear increase in XTT absorbance up to 6 h, reaching an absorbance of 0.4, a sigmoid increase up to 12 h, reaching an absorbance of 1.6, and a slower increase up to 18 h, reaching a plateau at an absorbance of >2.0. In the presence of antifungal drugs, the metabolic curves of the resistant isolates were similar to the drug-free metabolic curves (Figure 1), whereas the metabolic curves of the susceptible isolates deviated over time. Thus, the metabolic curves of the susceptible isolates were almost flat in the presence of anidulafungin and increased more slowly compared with the drug-free metabolic curve in the presence of fluconazole.
Figure 1.
Metabolic curves generated with 100 mg/L XTT/0.78 μΜ menadione for an anidulafungin- and fluconazole-resistant C. glabrata isolate (SSI-3203) (left-hand panels) and an anidulafungin-susceptible and fluconazole-susceptible increased exposure C. glabrata isolate (SSI-7965) (right-hand panels). An increase around 4 h was observed for all of the curves, probably because of multiplication of Candida cells detected by the spectrophotometer. ANI, anidulafungin; FLC, fluconazole; S, susceptible; I, susceptible increased exposure; R, resistant. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Optimization studies
The metabolic curves for anidulafungin-susceptible and anidulafungin-resistant isolates revealed no significant differences between the different XTT and menadione concentrations (data not shown). Metabolic curves for fluconazole-susceptible increased exposure and fluconazole-resistant isolates with drug added at 0, 2 and 4 h after inoculation showed that lower concentrations of both XTT and menadione with drug added at 0 h resulted in larger differences between drug-containing and drug-free metabolic curves for the susceptible isolate and smaller differences for the resistant isolate (data not shown). Thus, further experiments were conducted with 100 mg/L XTT + 0.78 μM menadione with 0.06 mg/L anidulafungin and 16 mg/L fluconazole.
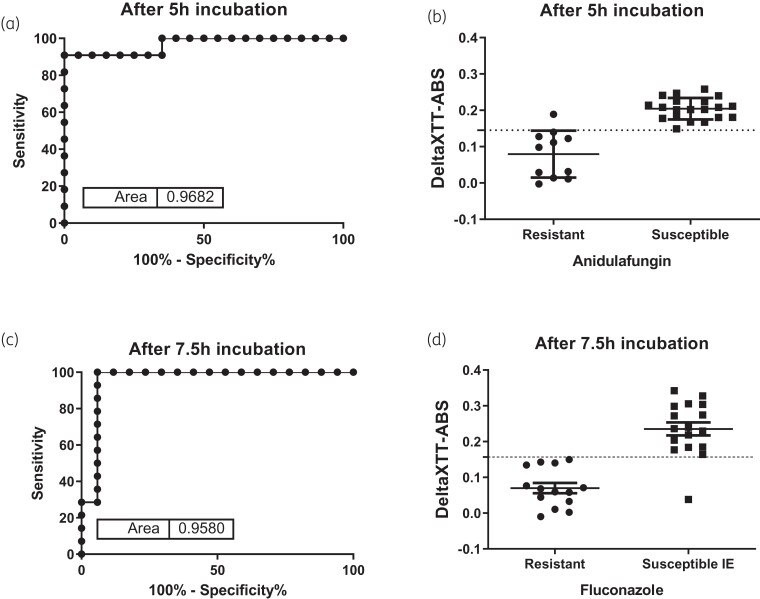
ROC curve analysis
The AUCROC and the sensitivities/specificities at different timepoints are shown in Table 2. Timepoints <5 h for anidulafungin and <6.5 h for fluconazole resulted in specificities and sensitivities <65% (Table 2). The earliest timepoint with the highest sensitivity and specificity was 5 h for anidulafungin (AUCROC = 0.968) and 7.5 h for fluconazole (AUCROC = 0.958). For anidulafungin, the deltaXTT-ABS at 5 h of all resistant isolates, apart from one (strain SSI-7033), were lower than the deltaXTT-ABS of the susceptible isolates (mean ± SD 0.08 ± 0.07 versus 0.200 ± 0.03, respectively, P < 0.001) (Figure 2), with the highest likelihood ratio in ROC curve analysis found for a deltaXTT-ABS of <0.145 resulting in a sensitivity of 90.91% and a specificity of 100% (Table 2). For fluconazole, the deltaXTT-ABS at 7.5 h of resistant isolates were lower than the deltaXTT-ABS of all susceptible increased exposure isolates except one (strain SSI-3775) (mean ± SD 0.08 ± 0.05 versus 0.25 ± 0.06, respectively, P = 0.005) (Figure 2), with the highest likelihood ratio in ROC curve analysis found for a deltaXTT-ABS of <0.157, resulting in a sensitivity of 100% and a specificity of 94.12% (Table 2).
Table 2.
Sensitivities and specificities of XTT method in separating resistant from susceptible/susceptible increased exposure strains at different timepoints
| Drug, timepoint (h) | AUCROC | Cut-off difference | Sensitivity (%) | Specificity (%) | Likelihood ratio |
|---|---|---|---|---|---|
| Anidulafungin | |||||
| 4 | 0.845 | <0.092 | 63.64 | 95 | 12.73 |
| 4.25 | 0.896 | <0.114 | 81.82 | 95 | 16.38 |
| 4.5 | 0.864 | <0.101 | 63.64 | 100 | ND |
| 4.75 | 0.923 | <0.114 | 63.64 | 100 | ND |
| 5a | 0.968 | <0.145 | 90.91 | 100 | ND |
| 5.25 | 0.977 | <0.166 | 90.91 | 100 | ND |
| 5.5b | 0.986 | <0.184 | 90.91 | 100 | ND |
| 5.75 | 0.986 | <0.200 | 90.91 | 100 | ND |
| 6 | 0.986 | <0.216 | 90.91 | 100 | ND |
| Fluconazole | |||||
| 6.5 | 0.878 | <0.112 | 100 | 64.71 | 2.83 |
| 6.75 | 0.899 | <0.127 | 100 | 64.71 | 2.83 |
| 7 | 0.932 | <0.143 | 100 | 76.47 | 4.25 |
| 7.25 | 0.95 | <0.150 | 100 | 88.24 | 8.5 |
| 7.5 | 0.958 | <0.157 | 100 | 94.12 | 17 |
| 7.75 | 0.962 | <0.171 | 100 | 94.12 | 17 |
| 8 | 0.958 | <0.187 | 100 | 94.12 | 17 |
ND, not determined. Likelihood ratios [sensitivity/(100%–specificity)] cannot be calculated because specificity is 100%.
The earliest timepoint with highest sensitivity and specificity is shown in bold.
A 100% sensitivity in distinguishing resistant strains from susceptible strains can be obtained using a deltaXTT-ABS <0.195. However, specificity at this deltaXTT-ABS was only 65% (data not shown).
A 100% sensitivity in distinguishing resistant strains from susceptible strains can be obtained using a deltaXTT-ABS <0.225. However, specificity at this deltaXTT-ABS was 85% (data not shown).
Figure 2.
ROC curves and deltaXTT-ABS for resistant and susceptible C. glabrata isolates for anidulafungin (a and b) and fluconazole (c and d). Horizontal broken lines correspond to optimal deltaXTT-ABS of 0.145 and 0.157 for detecting resistance to anidulafungin and fluconazole, respectively. IE, increased exposure.
Discussion
A EUCAST E.DEF 7.3.2-based broth microdilution colorimetric assay was developed in the present study, enabling the detection of anidulafungin- and fluconazole-resistant C. glabrata isolates within 5 and 7.5 h of incubation with high sensitivity (91% and 100%) and specificity (100% and 94%), respectively. Since only one concentration and a drug-free control are required for detection of resistance, this assay could be incorporated in the 96-well EUCAST standard microplate and generate faster preliminary results while waiting for the final result after 24 h. Thus, detection of resistance is feasible within the 8 h working day, with a high sensitivity and specificity (>91%). Sensitivity was relatively lower for anidulafungin, due to one false-resistant isolate (delayed metabolic activity of the growth control), and specificity was relatively lower for fluconazole, due to one false-susceptible isolate (delayed metabolic activity in the drug-containing well). Prolonged incubation correctly classified these two isolates.
There is an increasing need to develop accurate and fast methods for detection of resistance. Over recent years molecular techniques have been used to detect resistance. These are PCR-based techniques that detect specific mutations in target genes that confer resistance to azoles and echinocandins. Echinocandin resistance is usually associated with specific mutations in the ‘hot-spot’ regions of FKS1 and FKS2 (for C. glabrata only) genes.22 Therefore, a PCR assay that detects most if not all mutations that are dominant could be designed based on local epidemiology. However, no such assays are commercially available yet and translation into level of resistance is dependent on the codon, the specific alteration and the species and thus expertise is required. For the azoles, the situation is more complex, as resistance is associated with multiple mechanisms, including mutations in ERG11 and ERG3 genes, overexpression of ERG genes, MDR or Candida drug resistance (CDR) efflux pumps, mutations in transcription factors or a combination of these mechanisms.11,23,24 Furthermore, they are difficult to use in the daily routine, they are expensive if multiple genes are targeted, skilled personnel are required and, perhaps most importantly, these molecular tests may detect resistance but not susceptibility unless the hot spots are sequenced in contrast to the rapid colorimetric testing presented here.
Flow cytometry has also been used to detect resistance to fluconazole within 1–2 h using serial drug dilutions and FUN-1 probes.25,26 Microfluidic cell-chip technology was used for testing susceptibility of Candida spp. to amphotericin B and fluconazole by differentiating live and dead cells.27 Although these approaches are very promising, they were applied to a small set of isolates not characterized molecularly and required serial dilutions of drugs, special equipment and expertise. MALDI-TOF MS has been used for antifungal susceptibility testing, with a sensitivity and specificity of 94% and 80% in detecting caspofungin-resistant C. glabrata isolates (CLSI MIC 0.5 to >8 mg/L) within 6 h using serial dilutions and caspofungin-resistant C. albicans isolates (CLSI MIC 1–4 mg/L) within 3 h with 1.6% very major errors.14,15 An imaging technique using porous aluminum oxide (PAO) was used to detect, within 1.5 h, microscopic changes in Candida isolates in an RPMI agar plate containing specific concentrations of antifungal drug, with high levels of agreement with EUCAST (>80%).28 However, both methods require rather expensive equipment, a separate set-up and certain expertise.
The method described in the present study is cheap, with minimal hands-on time, requires no particular equipment and expertise and can be combined with the reference broth microdilution method in the same set-up. The only requirement is a microplate reader that can perform continuous measurements, which is present to most laboratories, due to its wide application. The method is very sensitive (100%) and specific (94%) in detecting fluconazole-resistant C. glabrata isolates within 7.5 h at a deltaXTT-ABS <0.157. Detection of echinocandin-resistant isolates can be achieved even earlier, after 5 h, with a sensitivity of 91% and a specificity of 100% at a deltaXTT-ABS <0.145. The method can detect resistance irrespective of the specific FKS mutation, providing that the mutation confers an MIC elevation. A higher sensitivity (100%) in detecting echinocandin resistance could be achieved by using a deltaXTT-ABS <0.195, although specificity was reduced (65%). The false results were due to the fact that one fluconazole-susceptible increased exposure isolate had a deltaXTT-ABS <0.157 (0.038) after 7.5 h, due to delayed metabolic activity of the growth control, and one anidulafungin-resistant isolate had a deltaXTT-ABS >0.145 (0.189) after 5 h, due to delayed metabolic activity in the drug-containing well (Figure 3). The false resistance for fluconazole could be overcome by introducing a cut-off of 0.324 for the drug-free control at 5.5 h in order to detect isolates with slow metabolic activity, whereas the false susceptibility for anidulafungin could be overcome by further incubation. More information on pathogen susceptibility and reduction of false results could be obtained by testing more than one concentration.
Figure 3.
Examples of metabolic curves that showed false resistance to fluconazole, due to delayed metabolic activity of the drug-free growth control (a), or false susceptibility to anidulafungin, due to delayed metabolic activity in the drug-containing well (b). ANI, anidulafungin; FLC, fluconazole; GC, growth control; I, susceptible increased exposure; R, resistant. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
XTT has been used previously for early susceptibility testing of Aspergillus and Mucorales spp. within 6–8 h of incubation.29,30 For yeast, XTT was used to detect resistance, but almost 24 h was needed.31 In that study, the CLSI protocol was used with a lower inoculum size and RPMI with lower d-glucose. Unfortunately, XTT and menadione concentrations were not mentioned in order to conclude whether concentrations were sufficient to detect yeast metabolism, as XTT/menadione concentrations are important parameters for assessing metabolic activity. In the present study, XTT/menadione concentrations were optimized and in combination with the richer RPMI medium supplemented with 2% d-glucose and the higher inoculum size (0.5–2.5 × 105 cfu/mL) of the EUCAST method we were able to detect metabolism early during incubation and distinguish resistant from susceptible isolates within one working day. The new method could be used for different Candida species and antifungal drugs after proper optimization of in vitro conditions.
In conclusion, the present paper describes a simple, cheap and fast phenotypic test that can detect resistance of C. glabrata to two classes of antifungal drugs. This opens the field for application to other drugs and species and possibly even directly from positive blood cultures, as long as a high fungal inoculum can be obtained free from other metabolically active cells. Fast phenotypic tests that correlate with reference methodologies of antifungal susceptibility testing can be useful tools in clinical laboratories for detecting antifungal resistance and guiding early appropriate antifungal therapy.
Contributor Information
Panagiota-Christina Georgiou, Clinical Microbiology Laboratory, Attikon University Hospital, Athens, Greece.
Maiken Cavling Arendrup, Unit of Mycology, Statens Serum Institute, Copenhagen, Denmark; Department of Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Joseph Meletiadis, Clinical Microbiology Laboratory, Attikon University Hospital, Athens, Greece; Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Rotterdam, The Netherlands.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
References
- 1. Pappas PG, Lionakis MS, Arendrup MCet al. Invasive candidiasis. Nat Rev Dis Primers 2018; 4: 18026. [DOI] [PubMed] [Google Scholar]
- 2. Healey KR, Perlin DS. Fungal resistance to echinocandins and the MDR phenomenon in Candida glabrata. J Fungi 2018; 4: 105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 2015; 58: 2–13. [DOI] [PubMed] [Google Scholar]
- 4. Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 2019; 25: 792–8. [DOI] [PubMed] [Google Scholar]
- 5. Arendrup MC, Patterson TF. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 2017; 216Suppl 3: S445–51. [DOI] [PubMed] [Google Scholar]
- 6. Colombo AL, Júnior JNDA, Guinea J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis 2017; 30: 528–38. [DOI] [PubMed] [Google Scholar]
- 7. Posteraro B, Sanguinetti M. The future of fungal susceptibility testing. Future Microbiol 2014; 9: 947–67. [DOI] [PubMed] [Google Scholar]
- 8. CLSI . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Fourth Edition: M27. 2017.
- 9. Arendrup MC, Meletiadis J, Mouton JWet al. EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.2. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. 2020; 1–21. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf. [DOI] [PubMed]
- 10. Fluit AC, Visser MR, Schmitz FJ. Molecular detection of antimicrobial resistance. Clin Microbiol Rev 2001; 14: 836–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perlin DS. Antifungal drug resistance: do molecular methods provide a way forward? Curr Opin Infect Dis 2009; 22: 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vale-Silva LA, Buchta V. Antifungal susceptibility testing by flow cytometry: Is it the future? Mycoses 2006; 49: 261–73. [DOI] [PubMed] [Google Scholar]
- 13. Rudensky B, Broidie E, Yinnon AMet al. Rapid flow-cytometric susceptibility testing of Candida species. J Antimicrob Chemother 2005; 55: 106–9. [DOI] [PubMed] [Google Scholar]
- 14. Vatanshenassan M, Boekhout T, Lass-Flörl Cet al. Proof of concept for MBT ASTRA, a rapid matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based method to detect caspofungin resistance in Candida albicans and Candida glabrata. J Clin Microbiol 2018; 56: e00420-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vella A, De Carolis E, Vaccaro Let al. Rapid antifungal susceptibility testing by matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis. J Clin Microbiol 2013; 51: 2964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morio F, Jensen RH, Le Pape Pet al. Molecular basis of antifungal drug resistance in yeasts. Int J Antimicrob Agents 2017; 50: 599–606. [DOI] [PubMed] [Google Scholar]
- 17. Paull KD, Shoemaker RH, Boyd MRet al. The synthesis of XTT: a new tetrazolium reagent that is bioreducible to a water-soluble formazan. J Heterocycl Chem 1988; 25: 911–4. [Google Scholar]
- 18. Hawser SP, Norris H, Jessup CJet al. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5- [(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for. J Clin Microbiol 1998; 36: 1450–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meletiadis J, Mouton JW, Meis JFet al. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis {2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide}, for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 2001; 39: 4256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tellier R, Krajden M, Grigoriew GAet al. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother 1992; 36: 1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pierce CG, Uppuluri P, Tristan ARet al. A simple and reproducible 96 well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 2008; 3: 1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perlin DS. Echinocandin resistance in Candida. Clin Infect Dis 2015; 61Suppl 6: S612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 2008; 46: 120–8. [DOI] [PubMed] [Google Scholar]
- 24. Consortium OPATHY, Gabaldon T. Recent trends in molecular diagnostics of yeast infections: from PCR to NGS. FEMS Microbiol Rev 2019; 43: 517–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pina-Vaz C, Sansonetty F, Rodrigues AGet al. Susceptibility to fluconazole of Candida clinical isolates determined by FUN-1 staining with flow cytometry and epifluorescence microscopy. J Med Microbiol 2001; 50: 375–82. [DOI] [PubMed] [Google Scholar]
- 26. Wenisch C, Moore CB, Krause Ret al. Antifungal susceptibility testing of fluconazole by flow cytometry correlates with clinical outcome. J Clin Microbiol 2001; 39: 2458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bouquet O, Kocsis B, Kilár Fet al. Amphotericin B and fluconazole susceptibility of Candida species determined by cell-chip technology. Mycoses 2012; 55: e90–6. [DOI] [PubMed] [Google Scholar]
- 28. Ingham CJ, Boonstra S, Levels Set al. Rapid susceptibility testing and microcolony analysis of Candida spp. cultured and imaged on porous aluminum oxide. PLoS One 2012; 7: e33818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antachopoulos C, Meletiadis J, Sein Tet al. Use of high inoculum for early metabolic signalling and rapid susceptibility testing of Aspergillus species. J Antimicrob Chemother 2007; 59: 230–7. [DOI] [PubMed] [Google Scholar]
- 30. Antachopoulos C, Meletiadis J, Roilides Eet al. Rapid susceptibility testing of medically important zygomycetes by XTT assay. J Clin Microbiol 2006; 44: 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kretschmar M, Nichterlein T, Kuntz Pet al. Rapid detection of susceptibility to fluconazole in Candida species by a bioluminescence assay of intracellular ATP. Diagn Microbiol Infect Dis 1996; 25: 117–21. [DOI] [PubMed] [Google Scholar]