Abstract
Purpose:
Patient safety errors can arise due to similarity in packaging of medications. We aimed to describe the clinical features of patients presenting with accidental application of joint pain liniments and gum lotion in the eye due to confusion arising from similarity in packaging.
Methods:
This was a retrospective case series with eight consecutive patients presenting from December 2020 to August 2021 with history of accidental application of joint pain liniments or gum lotion in the eye instead of eye drops. All patients underwent visual acuity assessment and slit-lamp examination with fluorescein staining of the cornea to look for corneal involvement and was reassessed till complete resolution.
Results:
Of the eight patients, three were males and five were females. Seven had accidentally applied joint pain liniment, while one had applied gum lotion into the eye. Five of them had corneal involvement ranging from punctate erosions to near-total epithelial defects. Two patients needed referral to a tertiary center and hospital admission. Treatment duration ranged from 2 days to 1 month. Two patients were lost to follow-up
Conclusion:
This study highlights patient safety errors arising from confusion of medication due to similar labeling and packaging of different drugs. While there was no permanent morbidity, such confusions lead to needless discomfort and waste of time, money, and effort for the patient as well as the health-care system.
Keywords: Look Alike-Sound Alike (LASA), medication error, patient safety
Patient safety is one of the most important aspects of health care. Giving the right treatment to the right patient is a key part of patient safety. Unsafe medication practices and medication errors are a leading cause of injury and avoidable harm in health-care systems across the world.[1]
The term “Look Alike-Sound Alike” (LASA) is used to describe a confusion of medication due to the similar labeling and packaging of different drugs, or similar labeling and packaging of the same drug containing different strengths. Confusing medications can cause up to 30% of patient safety errors.[2] In ophthalmology practice, all topical medications come in bottles and there is a high possibility of patients interchanging medications, especially among those who are visually challenged. Though unfortunate and should be prevented at all costs, this confusion can happen and is at least understandable. The series of cases mentioned below, however, are quite peculiar in that the patient confused a joint pain liniment or gum lotion for eye drops due to similar packaging.
Methods
This was a retrospective case series conducted after obtaining Institutional Ethics Committee clearance with adherence to tenets of the Declaration of Helsinki. Patients who presented from December 2020 to August 2021, with history of accidentally applying non-ocular medications such as joint pain liniments or gum lotion to the eyes, were included. All patients had undergone visual acuity assessment and slit-lamp examination with fluorescein staining of the cornea at each visit. Electronic medical records of the patients were accessed to obtain data.
Results
There were three male and five female patients with age ranging from 31 to 63 years. Seven patients gave history of application of joint pain liniment into the eye, while one had applied gum lotion. All patients presented with pain and irritation, among which five who applied the liniment had corneal involvement. Treatment duration till complete healing ranged from 2 days to 1 month, with two patients requiring referral to a tertiary center and hospital admission. All patients had improvement in symptoms and visual acuity after treatment, ranging from 6/12 to 6/6. Two patients were lost to follow-up.
Case 1
A 61-year-old male presented on 12/15/2020, postoperative Day 19 following small incision cataract surgery (SICS) in the left eye (OS), with sudden-onset pain, irritation, and burning sensation following application of liniment Fenlong MR, SKN Organics (diclofenac, capsaicin, and menthol) [Fig. 1] after confusing the bottle with gatifloxacin 0.2% and dexamethasone 0.1% eye drops (Gatilox DM, Sun Pharmaceutical Industries Ltd). Vision at presentation was 6/24. Anterior segment examination revealed a large 8 × 5 mm epithelial defect with surrounding punctate epithelial erosions. He was given immediate saline wash and the eye was patched. The following day review examination showed the epithelial defect to have reduced in size to 7 × 5 mm, and he was asked to continue topical antibiotics and review after 2 days. He was referred to a higher center in view of development of corneal infiltrate with thinning, where he was admitted for 4 days and given oral doxycycline 100 mg BD for 1 week, along with topical antibiotics, cycloplegics, and lubricants. The infiltrate and thinning resolved completely by 1 month, and vision in OS improved to 6/6 with spectacle correction.
Figure 1.
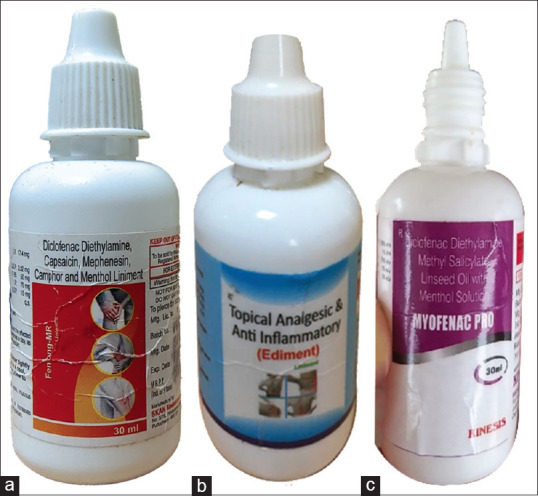
Packaging of joint pain liniments: (a) Fenlong MR (b)Ediment and (c) Myofenac Pro
Case 2
A 59-year-old female presented on 12/19/2020, 29 days after cataract surgery, with burning sensation in right eye (OD) following accidental application of Fenlong MR liniment instead of gatifloxacin 0.3% and prednisolone 1% eye drops (Gate-P, Ajanta Pharma). Visual acuity at presentation was 6/9. Anterior segment examination revealed mild conjunctival congestion, occasional cells in the anterior chamber, and posterior chamber intraocular lens. Saline wash was given, and the patient was asked to continue Gate-P eye drops 4 times a day for 1 week along with lubricants. During subsequent follow-up after 1 week, her vision was 6/9 with no active lesion and she was asked to taper steroids.
Case 3
A 63-year-old female patient presented on 12/25/2020 with pain in OS following accidental application of Fenlong MR liniment instead of Carboxymethylcellulose (CMC) 0.5% eye drops (Tear Drops, Sun Pharma) the previous night. Vision at presentation was 6/60. Anterior segment examination revealed lid edema, diffuse conjunctival congestion, and a large 6 mm × 6 mm epithelial defect. Saline wash was performed. Patient was started on CMC 0.5% eye drops, ciprofloxacin eye ointment, moxifloxacin 0.5% eye drops, and homatropine 2% eye drops. She was seen the next day and referred to a higher center in view of increasing size of epithelial defect, where the eye was patched and bandaged for 3 days and she was started on Oral Doxycycline 100 mg twice daily. After 3 days, the epithelium had healed, vision improved to 6/18, and the patient was asked to continue lubricants and antibiotics eye drops. By 01/20/2021, her vision had improved to 6/9 in OS and she was advised to continue only lubricant eye drops.
Case 4
A 60 year old male presented on 01/09/2021 with complaints of redness and irritation following accidental application of Fenlong MR. He confused the liniment with an over-the-counter eye-drops bought for a stick injury sustained 3 days prior. Vision at presentation was 6/24. Anterior segment examination showed diffuse conjunctival congestion and diffuse punctate epithelial erosions on the cornea. Saline wash was done and the patient was started on lubricants, antibiotic eye drops and ointment. He was lost to follow-up.
Case 5
A 31-year-old male, an information technology (IT) professional, presented on 01/14/21 with irritation, pain, and watering in OD following accidental application of Myofenac Pro (Kinesis Pharmaceuticals Pvt Ltd) rubefacient oil [Fig. 1] after confusing it with olopatadine 0.1% (Winolap, Sun Pharmaceuticals) eye drops prescribed the day before. Vision at presentation was 6/6. Anterior segment showed mild congestion. Saline wash was given and he was advised lubricant eye drops. Symptoms resolved after 2 days. Patient did not have any persisting sequelae or complications.
Case 6
A 57-year-old female presented on 01/29/2021 with redness and pain in OS following accidental application of Ediment liniment (Acumen Pharmaceuticals) [Fig. 1]. Vision at presentation was 6/12. Anterior segment examination revealed near-total epithelial defect with diffuse congestion and lid edema [Fig. 2]. She was started on topical antibiotics, cycloplegics, and lubricants. On subsequent follow-up the next day, the patient was found to have a healing epithelial defect and she was asked to continue the same medication along with application of bandage contact lens (BCL). BCL was removed after 2 days as the epithelial defect had healed to 3 × 2 mm. Patient was lost to follow-up.
Figure 2.
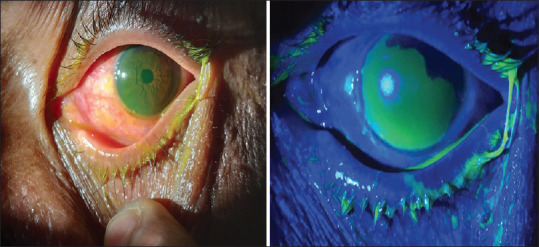
Anterior segment photograph showing fluorescein stained large epithelial defect with conjunctival congestion under diffuse illumination and cobalt blue filter
Case 7
A 47-year-old female patient presented on 05/21/21 with complaints of swelling, redness, and irritation of OD for 3 days following application of Fenlong MR liniment accidentally mistaking it for lubricant eye drops. She had consulted a nearby hospital where she was advised lubricants, topical antibiotics, and cycloplegics. As her symptoms did not subside, she was referred to us for further management. At presentation, her vision in OD was 6/60. Anterior segment examination revealed lid edema, diffuse conjunctival congestion, and chemosis with large 8 × 7 mm central epithelial defect with Descemet’s membrane folds [Fig. 3a and b]. A BCL was applied, and she was asked to continue the same medications and oral nonsteroidal anti-inflammatory agents. She was asked to review after 4 days. On follow-up, the patient was symptomatically better, her vision had improved to 6/36, and the epithelial defect had healed with only few punctate erosions [Fig. 3c and d]. BCL was removed, and the patient was started on loteprednol etabonate 0.5%-tobramycin 0.3% eye drops 4 times/day to reduce inflammation and was asked to review after 1 week.
Figure 3.
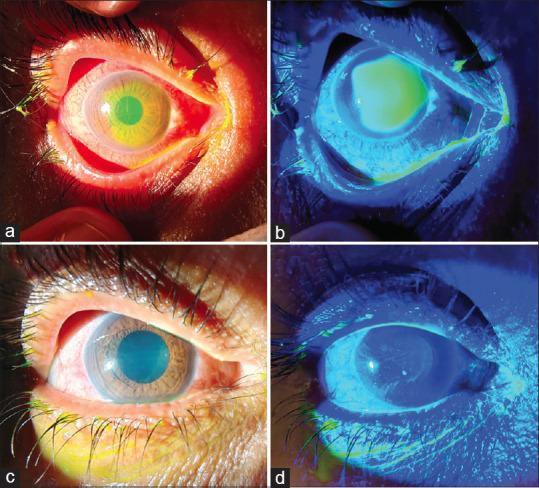
Anterior segment photograph showing fluorescein stained epithelial defect at presentation (a), under cobalt-blue filter (b) and improvement after 3 days of treatment and application of BCL (c and d). BCL = bandage contact lens
Case 8
A senior gynecologist of 62 years of age attended our hospital for routine checkup and she was found to have open-angle glaucoma in her OD. She was given brinzolamide 1%-timolol 0.5% (Azarga, Alcon Laboratories) eye drops, which had a white, opaque eye drop container. The patient was already using topical zinc sulfate oral drops (Zingisol, ICPA Health Products Ltd) for her gum infection, which had been prescribed by her dentist. It was also packed in a white, opaque container [Fig. 4]. The patient had accidentally applied zinc sulfate in her OD and developed severe irritation. She came to the emergency department on 08/13/2021 and her vision in OD was 6/9. The eye was washed thoroughly with sterile water and moxifloxacin ointment was given. The next day morning, her corneal epithelium was found intact and the vision was 6/6 with correction.
Figure 4.
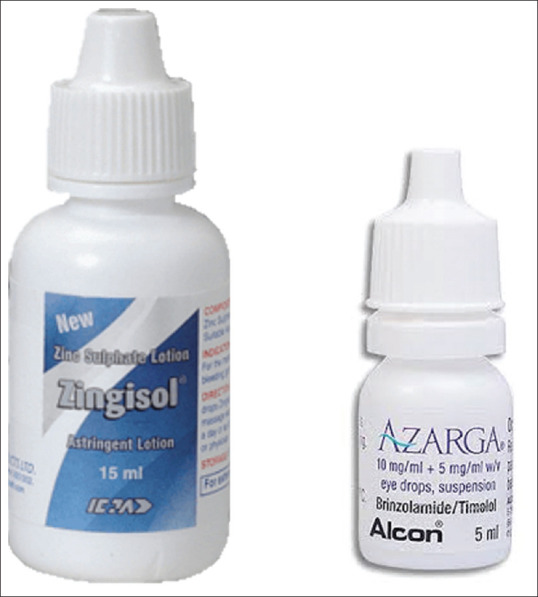
Image showing similarity of packaging of Zingisol gum astringent compared with Azarga eye drops
Discussion
The definition of medication error is “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health-care professional, patient, or consumer.”[3] Packaging of medications is an important aspect in preventing errors. Similarity in packaging is the main reason behind one-third of all cases of confusing medications.[2]
The liniments Fenlong MR contains a combination of diclofenac, capsaicin, mephenesin, camphor, and menthol, while Myofenac Pro and Ediment contain diclofenac, methyl salicylate, and menthol, all of which can cause extreme irritation, pain, and epithelial erosion when applied in the eye. Zingisol is an astringent lotion containing zinc sulfate used to reduce gum bleeding. The bottle also mentions that any contact with eyes, wounds, or broken skin may cause irritation, burning, and stinging sensation. Unfortunately, all these medications come in droppers similar to the ones used in ophthalmology, thus leading to accidental application into the eye [Fig. 5].
Figure 5.
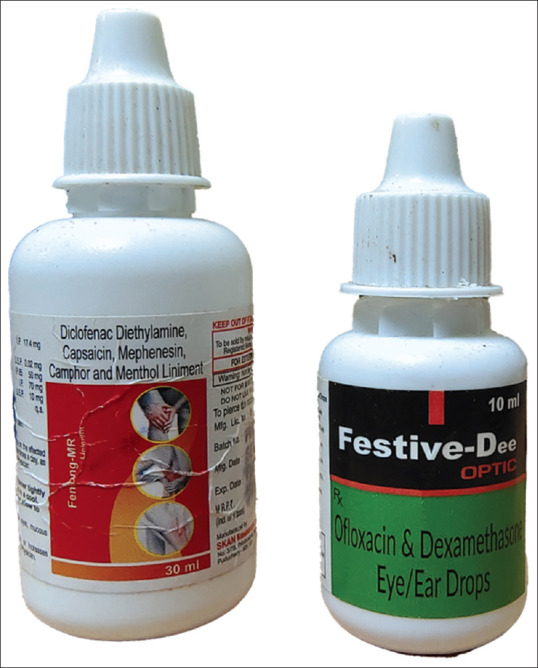
Image showing similarity of packaging of joint pain liniment Fenlong MR and 10 ml Festive Dee eye drops
Fortunately, while there was no permanent morbidity, this easily preventable error in patient safety led to patients losing 2-30 workdays, causing emotional and financial burden to them and their families, especially as five of them were daily wage workers. What was surprising was that the confusion was also seen in a 31-year-old IT professional and even in a senior gynecologist [Table 1].
Table 1.
Characteristics and summary of the eight patients in the series
| Number | Age, years | Gender | Occupation | Agent used | Confused with | Vision at presentation | Corneal involvement | Needing hospital admission/referral to tertiary center | Time till healing of corneal lesion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | Male | Farmer | Fenlong MR | Gatilox DM | 6/24 | Yes | Yes | 1 month |
| 2 | 59 | Female | Homemaker | Fenlong MR | Gate-P | 6/9 | No | No | 1 week |
| 3 | 63 | Female | Farmer | Fenlong MR | Tear drops | 6/60 | Yes | Yes | 1 month |
| 4 | 60 | Male | Manual laborer | Fenlong MR | Unknown | 6/24 | Yes | No | Lost to follow-up |
| 5 | 31 | Male | Software engineer | Myofenac Pro | Winolap | 6/6 | No | No | 2 days |
| 6 | 57 | Female | Farmer | Ediment | Unknown | 6/12 | Yes | No | Lost to follow-up |
| 7 | 47 | Female | Farmer | Fenlong MR | Zivifresh | 6/60 | Yes | No | 4 days |
| 8 | 62 | Female | Gynecologist | Zingisol | Azarga | 6/9 | No | No | 2 days |
In view of the above, a formal complaint was lodged with the Assistant Director of Drugs Control and is in process for the same.
Conclusion
In a developing country like India, especially in rural areas, it is of utmost importance to make sure that such confusions do not arise from similar packaging of medications, as it leads to needless discomfort and waste of time, money, and effort for the patient as well as the health-care system.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Medication without Harm-Global Patient Safety Challenge on Medication Safety. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 2.Schnoor J, Rogalski C, Frontini R, Engelmann N, Heyde CE. Case report of a medication error by look-alike packaging: A classic surrogate marker of an unsafe system. Patient Saf Surg. 2015;9:12. doi: 10.1186/s13037-014-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Coordinating Council for Medication Error Reporting and Prevention. What is a medication error? [Last accessed on 2007 Oct 01]. Available from: www.nccmerp.org/aboutMedErrors.html .


