Abstract
This retrospective study aimed to evaluate the clinical outcomes of circumferential minimally invasive surgery (CMIS) using lateral lumbar interbody fusion (LLIF) and percutaneous pedicle screw (PPS) in adult spinal deformity (ASD) patients, and to clarify the conditions for achieving postoperative pelvic incidence-lumbar lordosis (PI-LL) < 10°. Demographics and other parameters of ASD patients who underwent CMIS and who were divided into groups G (achieved postoperative PI-LL < 10°) and P (PI-LL ≥ 10°) were compared. Of the 145 included ASD patients who underwent CMIS, the average fused level, bleeding volume, operative time, and number of intervertebral discs that underwent LLIF were 10.3 ± 0.5 segments, 723 ± 375 mL, 366 ± 70 min, and 4.0 segments, respectively. The rod material was titanium alloy in all the cases. The PI-LL significantly improved from 37.3 ± 17.9° to 1.2 ± 12.2° postoperatively. Pre- and postoperative PI, postoperative LL, preoperative PI-LL, PI-LL after LLIF, and postoperative PI-LL were significantly larger in group P. PI-LL after LLIF was identified as a significant risk factor of postoperative PI-LL < 10° by logistic regression, and the cut-off value on receiver operating characteristic curve analysis was 20°. Sufficient correction was achieved by CMIS. If PI-LL after LLIF was ≤20°, it was corrected to the ideal alignment by the PPS procedure.
Keywords: adult spinal deformity, lateral lumbar interbody fusion, percutaneous pedicle screw, circumferential minimally invasive surgery, lumbosacral fusion
1. Introduction
Sagittal and coronal imbalance due to adult spinal deformity (ASD) causes pain and dysfunction in daily life [1,2]. Correction surgery for ASD results in significant improvement in health-related quality of life (HRQOL) [3,4,5,6]. Schwab et al. reported that the Oswestry Disability Index (ODI) was significantly lower in patients with sagittal alignment achieving postoperative pelvic tilt [PT] < 20°, sagittal vertical axis [SVA] < 100 mm, and postoperative pelvic incidence-lumbar lordosis (PI-LL) < 10° [7]. However, high complication and re-surgery rates have been reported to be associated with conventional open surgery [8,9,10,11]. Some elderly patients may not be able to undergo corrective surgery due to the invasiveness and incidence of complications of this conventional surgery. Introduction of a minimally invasive technique would therefore allow more patients with spinal deformities to undergo corrective surgery. In 2006, Ozgur et al. reported a novel approach called extreme lateral interbody fusion (XLIF®: NuVasive, Inc., San Diego, CA, USA) that enabled minimally invasive intervertebral release and interbody fusion [12]. In addition, circumferential minimally invasive surgery (CMIS) in ASD with percutaneous pedicle screw (PPS) is also gaining popularity [13,14]. However, there have been some reports on the insufficient corrective capability of CMIS for ASD [14,15]. On the other hand, there have also been few reports of clear indications for CMIS in patients with ASD. Schwab et al. reported differences in the Oswestry Disability Index between patients with SVA < 50 mm and SVA > 50 mm or between patients with PI-LL < 9° and PI-LL > 9° [7]. However, we only focused on PI-LL because only LL can be adjusted directly by surgery. Since posterior column osteotomy is not performed in CMIS, insufficient correction rather than overcorrection might occur [14,15]. Therefore, in the formula of PI − 10 < LL < PI + 10, we considered that the minimum target value, PI-LL < 10°, is an essential condition for target alignment. In this study, we evaluated the clinical outcomes of CMIS using lateral lumbar interbody fusion (LLIF) and PPS in ASD and clarified the conditions for achieving optimal postoperative alignment with postoperative PI-LL < 10°.
2. Materials and Methods
2.1. Patient Selection
Four surgeons with experience in minimally invasive spine surgery participated in the study. The inclusion criteria were age > 45 years, SVA > 50 mm, PT > 20°, pelvic incidence (PI)-lumbar lordosis (LL) > 10°, fused middle or lower thoracic to ilium, a minimum of two years of follow-up, and availability of standing full-length lateral and anteroposterior (AP) radiographs of the spine at preoperative baseline and at the final follow-up. Patients with ASD who underwent CMIS combined with LLIF and posterior spinopelvic fixation with a PPS system at our institution between January 2017 and November 2019 with a minimum follow-up period of 24 months were included. Patients who underwent surgeries from the upper thoracic to the ilium and had incomplete or inadequate radiographs for complete analysis were excluded. In addition, patients with multi-level iatrogenic kyphosis and those with multi-level posterior bone fusion that did not meet the CMIS indication criteria at our institution were excluded (Table 1). The outcomes examined were the average number of fixed vertebral bodies, the average number of intervertebral levels that underwent LLIF, the number of patients who underwent lateral access corpectomy (LAC), rod diameter and rod material, upper instrumented vertebra (UIV), intraoperative blood loss and operative time, various pre- and postoperative spinopelvic parameters, and complications. Patients were further divided into group G (those who had postoperative PI-LL < 10°) and group P (those who did not meet the postoperative PI-LL < 10° criterion). Spinopelvic parameters were compared between the two groups, and predictors of postoperative PI-LL < 10° were investigated by logistic regression analysis.
Table 1.
Indications for CMIS in ASD.
| Spinal Disease | Indication | |
|---|---|---|
| De novo kyphoscoliosis | GI | |
| Spinal deformity with OVF | GI (with corpectomy) |
|
| Degenerative kyphosis | UI | |
| Adult scoliosis | without bone union | UI |
| anterior bone union | UI | |
| anterior and posterior bone union | UNI (with mini-open Ponte) |
|
| Iatrogenic kyphosis | one level | UI |
| Multi-level | NI | |
Cases with posterior elemental bone fusion and multi-level iatrogenic kyphosis are not indicated for CMIS. CMIS: circumferential minimally invasive surgery; ASD: adult spinal deformity; OVF: osteoporotic vertebral fractures. GI: Good indication; UI: Usually an indication, but sometimes not; UNI: Usually not an indication, but can be an indication for mini-open Ponte osteotomy; NI: Not an indication.
2.2. Surgical Method
LLIF was first performed from L1/2 (in some cases T11/12 or T12/L1) to L4/5, and one week later, posterior fixation with PPS and mini-open transforaminal lumbar interbody fusion (TLIF) were performed at L5/S1. Fixation range was from the middle or lower thoracic spine to the pelvis in all cases. We used percutaneous instrumentation and polyaxial screws at all vertebral levels in all patients and the persuader system for percutaneous instrumentation in the upper instrumented vertebra (UIV) to the pelvis. Lordotic titanium (10°) cages were used in all cases. The cage was filled with autologous iliac bone and a hydroxyapatite/collagen composite (Refit®; HOYA Technosurgical Co., Tokyo, Japan).
2.3. Radiological Evaluation
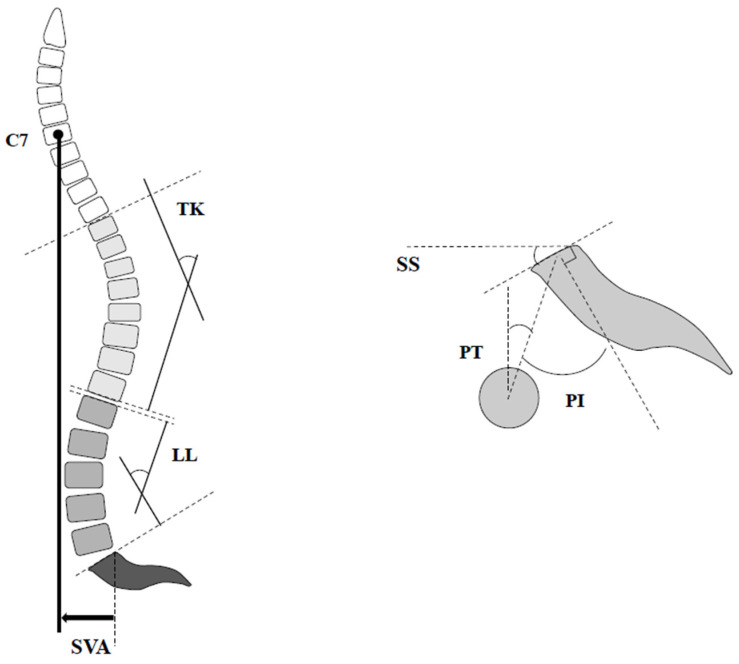
Standing full-length lateral radiograph of the spine was recorded at the preoperative baseline and final follow-up. The following spinopelvic parameters were investigated using the current standard method: PI, LL, PT, thoracic kyphosis (TK), and SVA (Figure 1). Proximal junctional kyphosis (PJK) is defined as the postoperative proximal junctional angle (PJA) between the caudal endplate of the UIV and the cephalad endplate of the UIV+ 2 ≥ 20° and at least 20° greater than the preoperative measurements [16]. Coronal imbalance was defined as ≥30 mm between the central sacral line and the mid-C7 vertebral body.
Figure 1.
Illustration of various spinopelvic parameters. LL: lumbar lordosis; TK: thoracic kyphosis; PI: pelvic incidence; PT: pelvic tilt; SS: sacral slope; SVA: sagittal vertical axis.
2.4. Statistical Analysis
Radiographic and clinical parameters were analyzed using the Mann–Whitney U test or Wilcoxon signed-rank test for continuous data and the Chi-square test for categorical data. Furthermore, logistic regression analysis and receiver operating characteristic (ROC) analysis were performed based on the results of the univariate analysis.
Statistical significance was set at a p value < 0.05. All analyses were performed using JMP software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Demographics
Based on the inclusion criteria, 156 patients were identified, and 11 patients were excluded (two underwent surgeries from the upper thoracic vertebrae to the ilium and nine did not have sufficient radiological data), leaving 145 patients available for analysis. Demographic data are shown in Table 2. The mean surgical age was 73.3 years (48–83 years), and the mean follow-up period was 40.7 months (30–54 months). Female patients accounted for 80.7% of all cases. Average blood loss in the first and second surgery was 104 ± 139.3 mL and 498.2 ± 305.7 mL, respectively. The average operative time in the first and second surgery was 109.6 ± 37.5 min and 233.3 ± 50.9 min, respectively. In comparison, the amount of blood loss and operative time were significantly less in anterior surgery than that in posterior surgery (p < 0.001). The average number of intervertebral levels that underwent LLIF was 4.0 ± 0.5 (3–6). We also performed LLIF at T11/12 or T12/L1 in patients with disc wedging or kyphotic deformity at the thoracolumbar junction. UIV was at T9/T10 in most cases, but in some patients with adult idiopathic scoliosis, the UIV was at T7. There were eight patients who underwent LAC, and the vertebrae that underwent LAC were T12, L1, and L2 in two cases and L3 and L4 in one case. The rod material used was titanium alloy (TA) in all cases, and the diameters and number of rods used were 5.5 mm and 2 rods in 54 cases, 6.0 mm and 2 rods in 48 cases, and 5.5 mm and multi-rods in 43 cases, respectively. Visual analog scale (VAS) back, VAS leg, and ODI were significantly improved after surgery. Final VAS back and ODI were significantly higher in group P. There were no significant differences in the other items (Table 2).
Table 2.
Demographic data.
| Parameter | Whole Group (n = 145) |
Group G (n = 110) |
Group P (n = 35) |
|
|---|---|---|---|---|
| Age (years) | 73.3 ± 6.5 (48–83) | 73.3 ± 6.9 | 73.0 ± 7.7 | |
| Rate of women (%) | 88 | 80.8 | 78.1 | |
| Period of follow-up (months) | 40.7 ± 6.2 (30–54) | 40.7 ± 6.3 | 40.6 ± 6.2 | |
| Rod diameter/number in construct | 5.5 mm/2 rods | 54 (61%) | 40 | 14 |
| 6 mm/2 rods | 48 (30%) | 33 | 15 | |
| 5.5 mm/3 rods | 43 (1%) | 33 | 10 | |
| Number of levels fused | 10.3 ± 0.5 (10–13) | 10.3 ± 0.5 | 10.4 ± 05 | |
| Number of LLIF | 4.0 ± 0.5 (3–6) | 4.0 ± 03 | 4.1 ± 05 | |
| Number of patients with corpectomy(case) |
8 | 7 | 1 | |
| UIV (case) | T7 | 3 (1%) | 3 | 0 |
| T9 | 48 (29%) | 34 | 14 | |
| T10 | 94 (70%) | 72 | 22 | |
| Operative time (min) |
Anterior (first surgery) |
109.6 ± 37.5 (59–273) |
112.4 ± 40.2 | 100.8 ± 26.5 |
| Posterior (second surgery) |
233.3 ± 50.9 ** (171–290) |
233.2 ± 52.1 ** | 233.0 ± 47.7 ** | |
| Blood loss (mL) |
Anterior (first surgery) |
104.3 ± 139.3 (0–970) |
117.7 ± 152.4 | 63.0 ± 75.1 |
| Posterior (second surgery) |
498.2 ± 305.7 ** (79–1530) |
488.6 ± 290.9 ** | 528.1 ± 350.9 ** | |
| VAS back | Before surgery | 6.6 ± 1.4 | 6.4 ± 1.3 | 7.2 ± 1.2 |
| Final | 2.8 ± 0.8 * | 2.6 ± 0.7 *# | 3.3 ± 0.9 * | |
| VAS leg | Before surgery | 5.4 ± 2.2 | 5.5 ± 2.1 | 5.0 ± 2.6 |
| Final | 1.7 ± 1.0 * | 1.7 ± 1.1 * | 1.7 ± 1.1 * | |
| ODI | Before surgery | 37.9 ± 6.2 | 37.3 ± 6.2 | 40.1 ± 5.1 |
| Final | 22.1 ± 5.6 * | 20.8 ± 5.8 *# | 25.9 ± 2.6 * | |
Values are presented as mean ± standard deviation. Wilcoxon signed-rank test. * Statistically significant. UIV: upper instrumented vertebra; LLIF: lateral lumbar interbody fusion. VAS: visual analog scale; ODI: Oswestry Disability Index. * p < 0.001 compared with before surgery, ** p < 0.001 compared with first surgery, # p < 0.001 compared with group P.
3.2. Radiographic Parameters
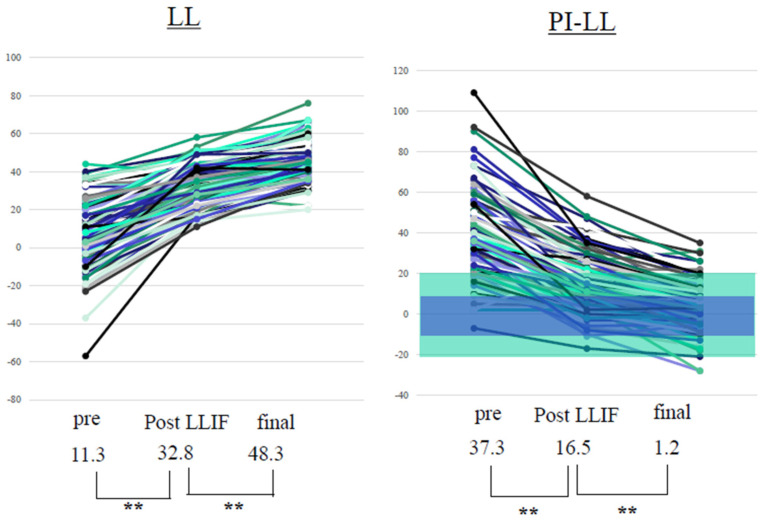
PI-LL significantly improved from 37.3 ± 17.9° to 1.2 ± 12.2° after surgery. The other parameters also improved significantly after surgery (Table 3). In LL, an average of 15.5 ± 10.1° (4–42°) was obtained with PPS alone. After surgery, 60% of all cases had PI + 10 > LL > PI − 10, 70% had PI + 15 > LL > PI − 15, and 90% had PI + 20 > LL > PI − 20 (Figure 2). There was a low correlation between preoperative and postoperative LL and between preoperative PI-LL and the LL change using PPS. Meanwhile, a high correlation was observed between PI-LL after LLIF and postoperative PI-LL, and between preoperative PI-LL and total LL change. A moderate correlation was also observed between preoperative and postoperative PI-LL and between PI-LL after LLIF and LL change with PPS (Figure 3).
Table 3.
Spinopelvic parameters.
| Parameter | Pre-Op | Post-Op | Final |
p Value (Pre-Op vs. Final) |
|---|---|---|---|---|
| PI | 47.3 ± 10.5 | 48.0 ± 11.1 | 47.9 ± 10.8 | 0.542 |
| PI-LL | 37.3 ± 17.9 | 1.2 ± 12.2 | 2.7 ± 12.1 | <0.001 * |
| LL | 11.3 ± 15.9 | 48.3 ± 10.3 | 46.5 ± 10.8 | <0.001 * |
| PT | 31.8 ± 11.2 | 17.5 ± 9.8 | 18.5 ± 9.2 | <0.001 * |
| TK | 19.4 ± 16.3 | 39.2 ± 10.8 | 42.3 ± 11.6 | <0.001 * |
| SVA | 83.3 ± 50.1 | 15.7 ± 35.0 | 38.0 ± 36.5 | <0.001 * |
Values are presented as mean ± standard deviation. Wilcoxon signed-rank test. * Statistically significant. There was significant improvement in all parameters, except PI, after surgery. LL: lumbar lordosis; PI: pelvic incidence; TK: thoracic kyphosis; PT: pelvic tilt; SVA: sagittal vertical axis.
Figure 2.
Preoperative and postoperative LL and PI-LL. Values are presented as mean ± standard deviation. Wilcoxon signed-rank test. ** Statistically significant. PPS improved LL by 15.5° (4–42°). After CMIS, 60% of all patients met PI + 10° ≥ LL ≤ PI − 10° (blue range) and 90% met PI + 20 > LL > PI − 20 (green range). LL: lumbar lordosis; PI: pelvic incidence; PPS: percutaneous pedicle screw; LLIF: lateral lumbar interbody fusion. ** p < 0.001.
Figure 3.
Correlation between various parameters. There was a low correlation between preoperative and postoperative LL (a) and between preoperative PI-LL and LL change using PPS (e). A high correlation was observed between PI-LL after LLIF and postoperative PI-LL (c) and between preoperative PI-LL and total LL change (d). A moderate correlation was observed between preoperative and postoperative PI-LL (b) and between PI-LL after LLIF and LL change with PPS (f). These findings indicate that greater correction is achieved in cases of greater deformity. LL: lumbar lordosis; ΔLL: change in LL; PI: pelvic incidence; LLIF: lateral lumbar interbody fusion; PPS: percutaneous pedicle screw.
Comparing various spinopelvic parameters between the two groups, the pre- and postoperative PI, PI-LL after LLIF, pre- and postoperative PI-LL, and pre- and postoperative PT were significantly larger in group P (pre- and postoperative PI, p ≤ 0.001; pre- and postoperative PI-LL and PI-LL after LLIF, p ≤ 0.001; pre- and postoperative PT, p ≤ 0.001) than in group G. Postoperative LL was significantly smaller in group P (postoperative LL, p ≤ 0.001) than in group G (Table 4). Based on the results of univariate analysis, the four preoperative items (preoperative PI, preoperative PT, preoperative PI-LL, and PI-LL after LLIF) were independent variables, and postoperative PI-LL > 10° was the dependent variable. Logistic regression analysis revealed that PI-LL after LLIF and preoperative PI were risk factors for postoperative PI-LL > 10° (Table 5). ROC curve analysis of postoperative PI-LL > 10° using two items revealed that the cut-off values for each item were as follows: preoperative PI of 56° (area under the curve [AUC] 0.78) and PI-LL after LLIF of 20° (AUC 0.84) (Table 6).
Table 4.
Spinopelvic parameters (group P vs. group G).
| Variable | Group P (n = 35) |
Group G (n = 110) |
p Value | |
|---|---|---|---|---|
| PI | Pre-PI | 57.1 ± 10.6 | 44.8 ± 10.4 | <0.001 * |
| Post-PI | 58.2 ± 10.7 | 46.5 ± 10.7 | <0.001 * | |
| LL | Pre-LL | 10.4 ± 13.8 | 11.5 ± 16.7 | 0.590 |
| LL after LLIF | 28.5 ± 10.4 | 33.3 ± 11.1 | 0.065 | |
| Post-LL | 41.4 ± 10.0 | 50.4 ± 9.5 | <0.001 * | |
| ΔLL | ΔLL (with LLIF) | 21.1 ± 11.2 | 21.7 ± 12.9 | 0.776 |
| ΔLL (with PPS) | 10.0 ± 8.8 | 17.1 ± 10.0 | 0.109 | |
| Total ΔLL | 31.1 ± 15.1 | 38.8 ± 16.7 | 0.210 | |
| PI-LL | Pre-PI-LL | 44.6 ± 17.1 | 35.0 ± 17.7 | <0.001 * |
| PI-LL after LLIF | 26.7 ± 7.7 | 13.1 ± 12.0 | <0.001 * | |
| Post-PI-LL | 16.7 ± 5.2 | −3.8 ± 8.8 | <0.001 * | |
| PT | Pre-PT | 37.6 ± 11.5 | 29.9 ± 10.6 | <0.001 * |
| Post-PT | 26.3 ± 7.6 | 14.8 ± 8.5 | <0.001 * | |
| TK | Pre-TK | 18.9 ± 15.2 | 19.6 ± 16.9 | 0.305 |
| Post-TK | 36.4 ± 8.8 | 37.5 ± 11.4 | 0.625 | |
Values are presented as mean ± standard deviation. Mann–Whitney U test. * Statistically significant. LLIF: lateral lumbar interbody fusion; LL: lumbar lordosis; PI: pelvic incidence; TK: thoracic kyphosis; PT: pelvic tilt.
Table 5.
Postoperative PI-LL > 10° risk factors (logistic regression analysis).
| Odd Ratio | 95% CI | p Value | |
|---|---|---|---|
| Pre-op PI | 0.93 | 0.87–0.99 | 0.032 * |
| PI-LL after LLIF | 0.87 | 0.79–0.95 | 0.001 * |
| Pre-op PT | 0.98 | 0.90–1.06 | 0.618 |
| Pre-op PI-LL | 1.04 | 0.99–1.10 | 0.068 |
* Statistically significant. LLIF: lateral lumbar interbody fusion; LL: lumbar lordosis; PI: pelvic incidence; PT: pelvic tilt; CI: confidence interval.
Table 6.
ROC curve analysis.
| Cut-Off Value | Sensitivity | Specificity | AUC | |
|---|---|---|---|---|
| PI-LL after LLIF | 20° | 0.95 | 0.65 | 0.846 |
| PI | 56° | 0.75 | 0.83 | 0.781 |
On performing an ROC analysis of postoperative PI-LL > 10° using two items, the cut-off values for each item were as follows: PI-LL after LLIF of 20° (AUC 0.84) and PI of 56° (AUC 0.78). ROC: receiver operating characteristic; LLIF: lateral lumbar interbody fusion; LL: lumbar lordosis; PI: pelvic incidence; AUC: area under the curve.
3.3. Clinical Outcomes
VAS back, VAS leg, and ODI were significantly improved after surgery (p ≤ 0.001). The ODI improved from a preoperative average of 37.9 ± 6.2 to a final average of 22.1 ± 5.6 (p ≤ 0.001).
3.4. Complications
The rate of PJK was 13% (re-surgery, 8%), and there was no statistically significant difference in rod diameter and number. Rod fracture (RF) was observed in 20% of patients, and it occurred significantly more frequently when using two 5.5 mm rods than with two 6.0 mm rods and multiple 5.5 mm rods (5.5 mm vs. 6.0 mm, p = 0.008 and 5.5 mm vs. multi, p = 0.001). The rate of neurological deficits was 6% (three cases recovered within 3 months and two cases remained paralyzed). Transient thigh symptoms due to LLIF were observed in 39% of patients, but all patients recovered within 3 months. The proportions of patients with infection, breakage of SAI screw, and coronal imbalance after surgery were 1%, 4%, and 13%, respectively (Table 7).
Table 7.
Complications.
| n = 145 | ≤30 Days | 30 Days 3 Years |
After 3 Years | Total | p Value | ||
|---|---|---|---|---|---|---|---|
| PJK | 5.5 mm, 2 rods (n = 54) (case) | 3 | 4 | 0 | 7 (13%) | n.s. | |
| 6 mm, 2 rods (n = 48) (case) | 2 | 6 | 0 | 8 (17%) | |||
| 5.5 mm, multi-rods (n = 43) (case) | 1 | 3 | 0 | 4 (9%) | |||
| Total (case) | 6 | 13 | 0 | 19 (13 %) | |||
| Revision (case) | 3 | 9 | 0 | 12 (8%) | |||
| Rod fracture | Rod diameter, number |
5.5 mm, 2 rods (n = 54) (case) |
0 | 18 | 2 | 20 (37%) |
5.5 mm vs. 6 mm: 0.008 * 5.5 mm vs. multi: 0.001 * |
| 6 mm, 2 rods (n = 48) (case) |
0 | 7 | 0 | 7 (15%) |
|||
| 5.5 mm, multi-rods (n = 43) (case) |
0 | 4 | 0 | 4 (9%) |
|||
| Total (case) | 0 | 29 | 2 | 31 (21%) | |||
| Reasons | Nonunion (case) | 0 | 19 | 0 | 19 (13%) | ||
| ALL rupture (case) | 0 | 9 | 0 | 9 (6%) | |||
| After union (case) | 0 | 1 | 2 | 3 (2%) | |||
| Revision (case) | 0 | 28 | 0 | 28 (19%) | |||
| Neurological deficit (case) | 5 (transient 3, permanent 2) |
0 | 0 | 5 (3%) | |||
| Thigh symptom (case) | 56 (transient) |
0 | 0 | 56 (39%) | |||
| Infection (case) | 1 | 1 | 0 | 2 (1%) | |||
| Breakage of SAI (case) | 0 | 6 (revision 3) |
0 | 6 (4%) | |||
| Coronal imbalance (case) | 16 | 2 | 1 | 19 (13%) | |||
Chi-square test. * Statistically significant. PJK: proximal junctional kyphosis; ALL: anterior longitudinal ligament; SAI: S2 alar iliac screw; n.s.: not significant.
4. Discussion
Improvements in various spinopelvic parameters and global alignment in ASD correlate with improvements in clinical outcomes and HRQOL [17]. Previous reports showed that traditional open ASD correction surgery results in a high percentage of complications and long-term hospitalization [17,18]. However, it has been reported that the CMIS in ASD is less invasive with reduced incidence of complications [14,19]. In addition, there are some reports on the usefulness of hybrid surgery with open posterior and Ponte osteotomy after LLIF [14,19]. However, lifting the intervertebral space with an LLIF cage also opens the facet joints and provides indirect decompressions of the spinal canal and intervertebral foramen. Subsequently, by applying rods, lordosis can be obtained in the process of closing the open facet joint. Therefore, even if it is a PPS procedure without Ponte osteotomy, sufficient correction is possible without causing nerve root impingement (Figure 4). Compared to the procedure of dissecting the posterior back muscles, exposing the lamina, and performing a Ponte osteotomy, it is obvious that correction surgery using LLIF and PPS in ASD is less invasive [14,19].
Figure 4.
The mechanism of correction surgery using LLIF and PPS. Spreading the intervertebral space with insertion of a LLIF cage also opens the facet joints and provides indirect decompression of the intervertebral foramen. Subsequently, by applying a rod, lordosis can be obtained by closing the facet joint. Therefore, even if PPS procedure without Ponte osteotomy was undertaken, sufficient correction is possible without causing nerve root impingement (a-1,a-2,a-3). The contracture facet joints were released and dilated with insertion of LLIF. LLIF procedure can dissociate not only the anterior element, but also the posterior element indirectly (b-1,b-2). LLIF: lateral lumbar interbody fusion; PPS: percutaneous pedicle screw.
4.1. Pros and Cons of Staged Surgery
Staged surgery for adult spinal deformities has been reported. Tahn et al. reported that the incidence of complications between unstaged and staged surgery was not significantly different, but that staged surgery was associated with significant hospital stays and transfusions [20]. On the other hand, Anand et al. reported that staged surgery for ASD can reduce admission to the intensive care unit [21]. There are several reasons why CMIS involves two stages. The first is that the corrective impact of each surgery can be evaluated accurately. The second is to reduce the invasiveness of the surgery. As the duration of surgery increases, the amount of bleeding gradually increases, the blood is diluted and, as a result, hemostatic functions deteriorate, and the amount of bleeding is anticipated to increase. The third reason is that the surgical method can be reconsidered by evaluating the alignment and changes in neurological symptoms after the first surgery. Anand et al. reported that after performing a multi-level LLIF, the alignment was evaluated in a standing position to determine whether to use the PPS procedure or the open procedure in the second surgery. If sufficient correction is achieved in the first surgery, the PPS procedure is indicated, or the fixed range may be reduced in some cases. If sufficient intervertebral release and correction are not achieved after the first surgery, open surgery might be needed. In addition, if indirect decompression by LLIF does not produce sufficient improvements in neurological symptoms, direct decompression must be performed in the second surgery. Two-stage surgery is very useful because such evaluation and reconsideration of the surgical procedure can be performed. The only disadvantage of staged surgery is the extended duration of hospital stay. However, this is not a big problem considering the above-mentioned advantages.
4.2. Correction Force/Indications for CMIS for ASD
Reports of CMIS for ASD are gradually increasing, but there are many reports on its ineffectiveness in cases of severe deformation. Mummaneni et al. reported the minimally invasive spinal deformity surgery (MISDEF) algorithm based on the severity of deformity and spinopelvic parameters [22]. They suggested that patients with mild deformities (PI-LL < 30° and PT < 25°) can undergo CMIS. Furthermore, they indicated MISDEF2 as multi-level MIS in patients with PI-LL < 30° and CMIS with ACR or mini-open PSO in patients with PI-LL ≥ 30° [23]. Haque et al. reported that the amount of postoperative SVA change was larger in conventional open surgery than in CMIS, and postoperative PI-LL was larger in CMIS than in hybrid and conventional open surgery [14]. This suggests that the corrective force in the MIS procedure is weaker than that in the traditional posterior open surgery. Park et al. and Mundis et al. reported that ASD patients who underwent combined severe fixed sagittal imbalance and spinopelvic malalignment were poor candidates for MIS surgery alone due to the high risk of residual postoperative deformity and fixed sagittal imbalance [19,24]. In this study, the following were noted: a low correlation between pre- and postoperative LL and between preoperative PI-LL and LL change with PPS, a high correlation between preoperative PI-LL and total LL change and between post-LLIF PI-LL and postoperative PI-LL, and a moderate correlation between pre- and postoperative PI-LL and between post-LLIF after PI-LL and LL change with PPS. This indicates that higher correction is achieved in cases with severe spinal deformity and that PI-LL after LLIF strongly affects postoperative PI-LL. This is similarly suggested by the results of the multivariate analysis. Furthermore, the preoperative mean PI-LL was 35° and the preoperative mean PT was 31° in this study, indicating that the degree of deformity in patients with ASD was higher compared to those in previous reports on CMIS for ASD [14,15]. This suggests that sufficient anterior intervertebral release by LLIF and the creation of an appropriate rod contour can achieve sufficient correction for severe spinal deformity with posterior fixation with PPS. In this study, logistic regression analysis revealed that PI-LL after LLIF and preoperative PI were risk factors of postoperative PI-LL > 10°, and ROC analysis of postoperative PI-LL > 10° using two items revealed that the cut-off values for each item were as follows: preoperative PI of 56° and PI-LL after LLIF of 20°. This suggested that the tolerance for target LL is greater in patients with high PI, indicating that this is similar to the previous formula [25,26]. Since the flexibility of spinal deformity changes remarkably after LLIF, it is difficult to use preoperative parameters as the indication criteria for CMIS. From the results of ROC analysis, we believe that PI-LL after LLIF ≤ 20° can be used as one of the indication criteria for CMIS.
4.3. Complications
Park et al. reported that the hybrid group demonstrated higher absolute improvement in radiographic parameters at the expense of a higher complication rate compared with the CMIS group. The complication rate (major or minor) in the hybrid group was 55% compared to 33% in the CMIS group [19]. This finding correlates with that of Wang et al., who demonstrated a major complication rate of 40% in patients undergoing hybrid surgery compared to 14% in the CMIS group [27]. Complications specific to the LLIF approach include transient anterior thigh dysesthesias and hip flexor weakness, which occur in 15% to 40% of patients undergoing LLIF [28,29,30]. In this study, the incidence of thigh symptoms was 39% in patients who underwent LLIF, which was almost similar to that reported in previous reports. However, all the patients recovered within 3 months, which was not clinically significant.
4.4. Proximal Junctional Kyphosis
Proximal junctional kyphosis (PJK) and proximal junctional failure (PJF) are common potential postoperative complications [31]. There are various reports on the incidence of PJK after correction surgery for ASD, which ranges from approximately 20–50% [11,31,32]. PJK is considered to be caused by multiple factors, including age, thoracolumbar spine muscle mass, overcorrection, level of UIV, preoperative SVA, fixation to ilium, and terminal rod contour [33,34,35]. Rhee et al. reported that PJK is seen less frequently in anterior spinal fusion than in posterior spinal fusion [36]. Some studies report that the reduction in posterior soft tissue damage using PPS is useful in the prevention of PJK [13,34,37]. In this study, the incidence of PJK was approximately 13% and the re-surgery rate was 8%, which is lower than that in previous reports [32,33,34]. The first reason for the low incidence of PJK is due to the effect of a reduction in the posterior soft tissue damage by using PPS, which is similar to those of past reports [13,34,37]. The second reason is ensuring sufficient kyphosis of terminal rod contour, suitable for postoperative reciprocal change, as in our previous report [35]. Moreover, in this study, the incidence of PJK tended to be higher when a 6 mm rod was used than when a 5.5 mm rod was used. As reported by Cahill and Lange, the use of softer implants is predicted to reduce the load on the junction, reduce adjacent disc degeneration, and reduce the occurrence of PJK [34,38]. A transition rod that has high rigidity at the lumbar level and low rigidity at the thoracic spine may be useful for preventing PJK in the future, and development is desired.
4.5. Rod Fractures
RF is a frequent implant-related complication following ASD surgery with an incidence of 6.8–22% [39,40]. In this study, the incidence of RF was 21%. The incidence of RF was 30% in patients who received the 5.5 mm rod, which is higher than that in previous reports, but the frequency is equal or less than that in those who received the 6 mm and multi-rod. This is because in CMIS, the paraspinal muscles were not detached, the spinal lamina was not exposed, and bone grafting was not performed [15]. In addition, since posterior facet joint resection was not performed, the facet joint worked as a hinge when LL was formed with the ideal rod. As a result, there were cases where the contact between the vertebral body and cage was insufficient. We considered this to be the reason for slow bone fusion and higher incidence of RF in cases using 5.5 mm rod than in conventional open surgery. Moreover, RF occurred more frequently with the usage of two 5.5 mm rods than with two 6.0 mm rods and 5.5 mm multi-rods in this study. Merrill et al. reported the usefulness of multi-rod application as a countermeasure for RF [41]. In this study, the usefulness of 6 mm rods and multi-rods in the prevention of RF was clear. It is possible to increase the bone fusion rate and reduce RF by increasing the rod diameter and the number of rods, increasing the durability of rods, and delaying the time of breakage due to wear, in CMIS procedures that allow time for bone fusion. Izeki et al. reported that spontaneous facet fusion was confirmed in patients with degenerative disease who underwent surgery with LLIF and PPS [42]. He noted that spontaneous fusion may be achieved in patients with facet degeneration. In ASD patients who underwent CMIS, both anterior intervertebral bone fusion by LLIF and spontaneous facet fusion without bone grafting will be achieved over time.
4.6. Infection
Haddad et al. reported that the incidence of deep wound infection was 16% in traditional open surgery [43]. Eastlack et al. and Uribe et al. reported that postoperative infections following ASD is lower in MIS than in hybrids [15,44]. Eastlack et al. reported that the postoperative infection rate in ASD was 1.5% [14], and it was 1% in this study, which is similar. There are two reasons for the low infection rate of CMIS in ASD. The first reason is the reduction in paraspinal muscle detachment due to the use of PPS and the consequent reduction in exposure to bacteria. The second one is the reduction in operative time and blood loss. The operative time was shorter and blood loss was lesser in CMIS than in hybrid surgery with LLIF and posterior open instrumentation [19,45].
We have shown that CMIS using LLIF and PPS is indicated for de novo scoliosis, spinal deformity with vertebral body fracture, and adult idiopathic scoliosis (AS) without interbody fusion or only one level of intervertebral fusion. Contraindications are cases of iatrogenic kyphosis with multi-level vertebral fusion and posterior element bone fusion and cases of AS with multi-vertebral bone fusion (Table 1). However, it is important to reassess the flexibility of the spine after performing LLIF. Since there are many cases in which flexibility is significantly changed due to the release of anterior interbody bridging and posterior facet joints due to LLIF, reassessment of flexibility after LLIF is important.
4.7. Limitations
This study has several limitations. First, the sample size was small. Additional studies with large sample size are required for validating the results. The second limitation is related to the lack of bone mineral density (BMD) evaluation. In this study, the average age of the patients was 73.3 years, and 88% of the total patients were women; consequently, most of them would have an osteoporotic spine. Low vertebral bone density alters the biomechanical impact of implants on vertebral bodies and may increase implant-related complications, such as vertebral fractures and implant failure. Thus, we believe that a large-scale prospective study including the evaluation of BMD will be necessary in future. The third limitation is that the follow-up period differed, depending on the rod diameter and material. Since the patients who received multiple rods in this study had the lowest follow-up period, RF may increase in the future. Thus, further long-term follow-up is important. The fourth limitation is that the target alignment in this study was PI-LL < 10°. There are some reports that the target alignment differs between patients with low PI and those with high PI [25,26]. In addition, the ideal alignment differs depending on the age [26]. Further research on the achievement conditions for target alignment according to age and PI is desired in the future.
5. Conclusions
We reported the clinical results of CMIS in ASD and clarified the conditions for achieving postoperative PI-LL < 10°. Sufficient correction was obtained using CMIS with LIF and PPS. It was suggested that if PI-LL is ≤20° after LLIF, postoperative PI-LL ≤ 10° can be achieved even with correction by PPS.
Author Contributions
M.I. substantially contributed to study conception and design, performed data acquisition, and supervised the study. S.T. analyzed and interpreted the data. T.A., Y.T., M.P., M.A., T.S. contributed approval of the version of the manuscript to be published. Investigation, M.I., Y.T., M.P. and M.A. Supervision, T.A. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study was approved by the institutional review board of the Kansai Medical University Hospital (2020189). The study was also carried out in line with the principles of the Declaration of Helsinki.
Informed Consent Statement
Written informed consent was obtained from the patients prior to study participation and for the publication of this report and any accompanying images.
Data Availability Statement
The data used in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
All authors declared that there are no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bess S., Protopsaltis T.S., Lafage V., Lafage R., Ames C.P., Errico T., Smith J.S. Clinical and radiographic evaluation of adult spinal deformity. Clin. Spine Surg. 2016;29:6–16. doi: 10.1097/BSD.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 2.Bess S., Line B., Fu K.M., McCarthy I., Lafage V., Schwab F., Shaffrey C., Ames C., Akbarnia B., Jo H., et al. The health impact of symptomatic adult spinal deformity: Comparison of deformity types to United States population norms and chronic diseases. Spine. 2016;41:224–233. doi: 10.1097/BRS.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acaroglu E., Yavuz A.C., Guler U.O., Yuksel S., Yavuz Y., Domingo-Sabat M., Pellise F., Alanay A., Perez Grueso F.S., Kleinstück F., et al. A decision analysis to identify the ideal treatment for adult spinal deformity: Is surgery better than non-surgical treatment in improving health-related quality of life and decreasing the disease burden? Eur. Spine J. 2016;25:2390–2400. doi: 10.1007/s00586-016-4413-8. [DOI] [PubMed] [Google Scholar]
- 4.Djurasovic M., Glassman S.D. Correlation of radiographic and clinical findings in spinal deformities. Neurosurg. Clin. N. Am. 2007;18:223–227. doi: 10.1016/j.nec.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Gum J.L., Glassman S.D., Douglas L.R., Carreon L.Y. Correlation between cervical spine sagittal alignment and clinical outcome after anterior cervical discectomy and fusion. Am. J. Orthop. 2012;41:E81–E84. [PubMed] [Google Scholar]
- 6.Lenke L.G., Fehlings M.G., Shaffrey C.I., Cheung K.M., Carreon L., Dekutoski M.B., Schwab F.J., Boachie-Adjei O., Kebaish K.M., Ames C.P., et al. Neurologic outcomes of complex adult spinal deformity surgery: Results of the prospective, multicenter Scoli-RISK-1 Study. Spine. 2016;41:204–212. doi: 10.1097/BRS.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 7.Schwab F., Patel A., Ungar B., Farcy J.P., Lafage V. Adult spinal deformity-postoperative standing imbalance: Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine. 2010;35:2224–2231. doi: 10.1097/BRS.0b013e3181ee6bd4. [DOI] [PubMed] [Google Scholar]
- 8.Kothari P., Lee N.J., Leven D.M., Lakomkin N., Shin J.I., Skovrlj B., Steinberger J., Guzman J.Z., Cho S.K. Impact of gender on 30-day complications after adult spinal deformity surgery. Spine. 2016;41:1133–1138. doi: 10.1097/BRS.0000000000001499. [DOI] [PubMed] [Google Scholar]
- 9.Maruo K., Ha Y., Inoue S., Samuel S., Okada E., Hu S.S., Deviren V., Burch S., William S., Ames C.P., et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine. 2013;38:E1469–E1476. doi: 10.1097/BRS.0b013e3182a51d43. [DOI] [PubMed] [Google Scholar]
- 10.Mok J.M., Cloyd J.M., Bradford D.S., Hu S.S., Deviren V., Smith J.A., Tay B., Berven S.H. Reoperation after primary fusion for adult spinal deformity: Rate, reason, and timing. Spine. 2009;34:832–839. doi: 10.1097/BRS.0b013e31819f2080. [DOI] [PubMed] [Google Scholar]
- 11.Pichelmann M.A., Lenke L.G., Bridwell K.H., Good C.R., O’Leary P.T., Sides B.A. Revision rates following primary adult spinal deformity surgery: Six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine. 2010;35:219–226. doi: 10.1097/BRS.0b013e3181c91180. [DOI] [PubMed] [Google Scholar]
- 12.Ozgur B.M., Aryan H.E., Pimenta L., Taylor W.R. Extreme lateral interbody fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Mummaneni P.V., Park P., Fu K.M., Wang M.Y., Nguyen S., Lafage V., Uribe J.S., Ziewacz J., Terran J., Okonkwo D.O., et al. Does minimally invasive percutaneous posterior instrumentation reduce risk of proximal junctional kyphosis in adult spinal deformity surgery? A propensity-matched cohort analysis. Neurosurgery. 2016;78:101–108. doi: 10.1227/NEU.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 14.Haque R.M., Mundis G.M., Jr., Ahmed Y., El Ahmadieh T.Y., Wang M.Y., Mummaneni P.V., Uribe J.S., Okonkwo D.O., Eastlack R.K., Anand N., et al. Comparison of radiographic results after minimally invasive, hybrid, and open surgery for adult spinal deformity: A multicenter study of 184 patients. Neurosurg. Focus. 2014;36:E13. doi: 10.3171/2014.3.FOCUS1424. [DOI] [PubMed] [Google Scholar]
- 15.Eastlack R.K., Srinivas R., Mundis G.M., Nguyen S., Mummaneni P.V., Okonkwo D.O., Kanter A.S., Anand N., Park P., Nunley P., et al. Early and late reoperation rates with various MIS techniques for adult spinal deformity correction. Glob. Spine J. 2019;9:41–47. doi: 10.1177/2192568218761032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheer J.K., Fakurnejad S., Lau D., Daubs M.D., Coe J.D., Paonessa K.J., LaGrone M.O., Amaral R.A., Trobisch P.D., Lee J.-H., et al. Results of the 2014 SRS survey on PJK/PJF: A report on variation of select SRS member practice patterns, treatment indications, and opinions on classification development. Spine. 2015;40:829–840. doi: 10.1097/BRS.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 17.Glassman S.D., Hamill C.L., Bridwell K.H., Schwab F.J., Dimar J.R., Lowe T.G. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine. 2007;32:2764–2770. doi: 10.1097/BRS.0b013e31815a7644. [DOI] [PubMed] [Google Scholar]
- 18.Smith J.S., Klineberg E., Lafage V., Shaffrey C.I., Schwab F., Lafage R., Hostin R., Mundis G.M., Errico T.J., Kim H.J., et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J. Neurosurg. Spine. 2016;25:1–14. doi: 10.3171/2015.11.SPINE151036. [DOI] [PubMed] [Google Scholar]
- 19.Park P., Wang M.Y., Lafage V., Nguyen S., Ziewacz J., Okonkwo D.O., Uribe J.S., Eastlack R.K., Anand N., Haque R., et al. Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J. Neurosurg. Spine. 2015;22:374–380. doi: 10.3171/2014.9.SPINE131004. [DOI] [PubMed] [Google Scholar]
- 20.Than K.D., Park P., Tran S., Mundis G.M., Fu K.M., Uribe J.S., Okonkwo D.O., Nunley P.D., Fessler R.G., Eastlack R.K., et al. Analysis of complications with staged surgery for less invasive treatment of adult spinal deformity. World Neurosurg. 2019;126:e1337–e1342. doi: 10.1016/j.wneu.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 21.Anand N., Kong C., Fessler R.G. A staged protocol for circumferential minimally invasive surgical correction of adult spinal deformity. Neurosurgery. 2017;81:733–739. doi: 10.1093/neuros/nyx353. [DOI] [PubMed] [Google Scholar]
- 22.Mummaneni P.V., Shaffrey C.I., Lenke L.G., Park P., Wang M.Y., La Marca F., Smith J.S., Mundis G.M., Okonkwo D.O., Moal B., et al. The minimally invasive spinal deformity surgery algorithm: A reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg. Focus. 2014;36:E6. doi: 10.3171/2014.3.FOCUS1413. [DOI] [PubMed] [Google Scholar]
- 23.Mummaneni P.V., Park P., Shaffrey C.I., Wang M.Y., Uribe J.S., Fessler R.G., Chou D., Kanter A.S., Okonkwo D.O., Mundis G.M., et al. The MISDEF2 algorithm: An updated algorithm for patient selection in minimally invasive deformity surgery. J. Neurosurg. Spine. 2019;32:221–228. doi: 10.3171/2019.7.SPINE181104. [DOI] [PubMed] [Google Scholar]
- 24.Mundis G., Uribe J.S., Mummaneni P.V., Anand N., Park P., Okonkwo D.O., Kanter A.S., Fessler R.G., Nguyen S., Akbarnia B.A., et al. 172-A critical analysis of sagittal plane deformity correction with minimally invasive surgery: A 2-year follow-up study of deformity patients categorized by the SRS-Schwab Classification. Neurosurgery. 2015;62:222–223. doi: 10.1227/01.neu.0000467136.50891.a8. [DOI] [Google Scholar]
- 25.Yamato Y., Hasegawa T., Kobayashi S., Yasuda T., Togawa D., Arima H., Oe S., Iida T., Matsumura A., Hosogane N., et al. Calculation of the target lumbar lordosis angle for restoring an optimal pelvic tilt in elderly patients with adult spinal deformity. Spine. 2016;41:E211–E217. doi: 10.1097/BRS.0000000000001209. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa K., Okamoto M., Hatsushikano S., Shimoda H., Ono M., Watanabe K. Normative values of spino-pelvic sagittal alignment, balance, age, and health-related quality of life in a cohort of healthy adult subjects. Eur. Spine J. 2016;25:3675–3686. doi: 10.1007/s00586-016-4702-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang M.Y., Mummaneni P.V., Fu K.M., Anand N., Okonkwo D.O., Kanter A.S., La Marca F., Fessler R., Uribe J., Shaffrey C.I., et al. Less invasive surgery for treating adult spinal deformities: Ceiling effects for deformity correction with 3 different techniques. Neurosurg. Focus. 2014;36:E12. doi: 10.3171/2014.3.FOCUS1423. [DOI] [PubMed] [Google Scholar]
- 28.Moller D.J., Slimack N.P., Acosta F.L., Jr., Koski T.R., Fessler R.G., Liu J.C. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg. Focus. 2011;31:E4. doi: 10.3171/2011.7.FOCUS11137. [DOI] [PubMed] [Google Scholar]
- 29.Tempel Z.J., Gandhoke G.S., Bonfield C.M., Okonkwo D.O., Kanter A.S. Radiographic and clinical outcomes following combined lateral lumbar interbody fusion and posterior segmental stabilization in patients with adult degenerative scoliosis. Neurosurg. Focus. 2014;36:E11. doi: 10.3171/2014.3.FOCUS13368. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers W.B., Gerber E.J., Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine. 2011;36:26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.J., Lenke L.G., Shaffrey C.I., Van Alstyne E.M., Skelly A.C. Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: A systematic review. Spine. 2012;37:S144–S164. doi: 10.1097/BRS.0b013e31826d611b. [DOI] [PubMed] [Google Scholar]
- 32.Yagi M., Akilah K.B., Boachie-Adjei O. Incidence, risk factors and classification of proximal junctional kyphosis: Surgical outcomes review of adult idiopathic scoliosis. Spine. 2011;36:E60–E68. doi: 10.1097/BRS.0b013e3181eeaee2. [DOI] [PubMed] [Google Scholar]
- 33.Bridwell K.H., Lenke L.G., Cho S.K., Pahys J.M., Zebala L.P., Dorward I.G., Cho W., Baldus C., Hill B.W., Kang M.M. Proximal junctional kyphosis in primary adult deformity surgery: Evaluation of 20 degrees as a critical angle. Neurosurgery. 2013;72:899–906. doi: 10.1227/NEU.0b013e31828bacd8. [DOI] [PubMed] [Google Scholar]
- 34.Cahill P.J., Wang W., Asghar J., Booker R., Betz R.R., Ramsey C., Baran G. The use of a transition rod may prevent proximal junctional kyphosis in the thoracic spine after scoliosis surgery: A finite element analysis. Spine. 2012;37:E687–E695. doi: 10.1097/BRS.0b013e318246d4f2. [DOI] [PubMed] [Google Scholar]
- 35.Ishihara M., Taniguchi S., Adachi T., Kushida T., Paku M., Ando M., Saito T., Kotani Y., Tani Y. Rod contour and overcorrection are risk factors of proximal junctional kyphosis after adult spinal deformity correction surgery. Eur. Spine J. 2021;30:1208–1214. doi: 10.1007/s00586-021-06761-z. [DOI] [PubMed] [Google Scholar]
- 36.Rhee J.M., Bridwell K.H., Won D.S., Lenke L.G., Chotigavanichaya C., Hanson D.S. Sagittal plane analysis of adolescent idiopathic scoliosis: The effect of anterior versus posterior instrumentation. Spine. 2002;27:2350–2356. doi: 10.1097/00007632-200211010-00008. [DOI] [PubMed] [Google Scholar]
- 37.Korkmaz M., Akgul T., Sariyilmaz K., Ozkunt O., Dikici F., Yazicioglu O. Effectiveness of posterior structures in the development of proximal junctional kyphosis following posterior instrumentation: A biomechanical study in a sheep spine model. Acta Orthop. Traumatol. Turc. 2019;53:385–389. doi: 10.1016/j.aott.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lange T., Schmoelz W., Gosheger G., Eichinger M., Heinrichs C.H., Boevingloh A.S., Schulte T.L. Is a gradual reduction of stiffness on top of posterior instrumentation possible with a suitable proximal implant? A biomechanical study. Spine J. 2017;17:1148–1155. doi: 10.1016/j.spinee.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Barton C., Noshchenko A., Patel V., Cain C., Kleck C., Burger E. Risk factors for rod fracture after posterior correction of adult spinal deformity with osteotomy: A retrospective case-series. Scoliosis. 2015;10:30. doi: 10.1186/s13013-015-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith J.S., Shaffrey E., Klineberg E., Shaffrey C.I., Lafage V., Schwab F.J., Protopsaltis T., Scheer J.K., Mundis G.M., Fu K.-M.G., et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J. Neurosurg. Spine. 2014;21:994–1003. doi: 10.3171/2014.9.SPINE131176. [DOI] [PubMed] [Google Scholar]
- 41.Merrill R.K., Kim J.S., Leven D.M., Kim J.H., Cho S.K. Multi-rod constructs can prevent rod breakage and pseudarthrosis at the lumbosacral junction in adult spinal deformity. Glob. Spine J. 2017;7:514–520. doi: 10.1177/2192568217699392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izeki M., Fujio K., Ota S., Soga S., Matsuda S.J. Radiological follow-up of the degenerated facet joints after lateral lumbar interbody fusion with percutaneous pedicle screw fixation: Focus on spontaneous facet joint fusion. J. Orthop. Sci. 2021 doi: 10.1016/j.jos.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Haddad S., Núñez-Pereira S., Pigrau C., Rodríguez-Pardo D., Vila-Casademunt A., Alanay A., Acaroglu E.R., Kleinstueck F.S., Obeid I., Perez-Grueso F.J.S., et al. The impact of deep surgical site infection on surgical outcomes after posterior adult spinal deformity surgery: A matched control study. Eur. Spine J. 2018;27:2518–2528. doi: 10.1007/s00586-018-5583-3. [DOI] [PubMed] [Google Scholar]
- 44.Uribe J.S., Deukmedjian A.R., Mummaneni P.V., Fu K.M., Mundis G.M., Okonkwo D.O., Kanter A.S., Eastlack R., Wang M.Y., Anand N., et al. Complications in adult spinal deformity surgery: An analysis of minimally invasive, hybrid, and open surgical techniques. Neurosurg. Focus. 2014;36:E15. doi: 10.3171/2014.3.FOCUS13534. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton D.K., Kanter A.S., Bolinger B.D., Mundis G.M., Nguyen S., Mummaneni P.V., Anand N., Fessler R.G., Passias P.G., Park P., et al. Reoperation rates in minimally invasive, hybrid and open surgical treatment for adult spinal deformity with minimum 2-year follow-up. Eur. Spine J. 2016;25:2605–2611. doi: 10.1007/s00586-016-4443-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available upon reasonable request from the corresponding author.