Highlights
-
•
Botulinum toxin treatment benefit for CD can wane before typical reinjection cycle.
-
•
Shorter injection cycles of incobotulinumtoxinA are effective for treating CD.
-
•
Shorter injection intervals have no unexpected AEs or loss of treatment effect.
Keywords: Cervical dystonia, Movement disorders, Botulinum toxin, BoNT, IncobotulinumtoxinA
Abstract
Introduction
Some patients with cervical dystonia (CD) receiving long-term botulinum neurotoxin (BoNT) therapy report early waning of treatment benefit before the typical 12-week reinjection interval.
Methods
This phase 4, open-label, randomized, noninferiority study (CD Flex; NCT01486264) compared 2 incobotulinumtoxinA injection schedules (Short Flex: 8 ± 2 weeks; Long Flex: 14 ± 2 weeks) in CD patients. Previous BoNT-responsive subjects who reported acceptable clinical benefit lasting < 10 weeks were recruited. Efficacy and safety were evaluated after 8 injection cycles. The primary endpoint was change in Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) severity subscale 4 weeks after the eighth injection. Secondary endpoints included TWSTRS total and subscale scores. Immunogenicity was assessed in a subset of patients.
Results
Two hundred eighty-two CD patients were randomized and treated (Short Flex, N = 142; Long Flex, N = 140), and 207 completed the study. Significant improvements in TWSTRS severity from study baseline to 4 weeks after cycle 8 were observed in both the Short Flex (4.1 points; P < 0.0001) and Long Flex (2.4 points; P = 0.002) groups; Short Flex was noninferior to Long Flex (LS mean difference = 1.4 points; 95% CI = [−2.9, 0.1] < Δ = 2.0). Key secondary endpoints favored Short Flex intervals. Adverse events (AEs) were comparable between groups. There was no secondary loss of treatment effect.
Conclusion
Injection cycles < 10 weeks for incobotulinumtoxinA are effective (and noninferior to longer intervals) for treating CD patients with early waning of clinical benefit. Shorter injection intervals did not increase AEs or lead to loss of treatment effect.
1. Introduction
Cervical dystonia (CD) is the most common focal dystonia and can present with various abnormal head positions (eg, torticollis, laterocollis, anterocollis, retrocollis). Individuals with CD commonly experience involuntary neck movements, pain, decreased range of motion, tremor, low self-esteem, and impairment of daily activities [1], [2], [3]. Unlike other types of focal dystonia, 75% of individuals with CD frequently report neck pain [2], which is significantly associated with altered employment, reduced productivity, and a need to seek disability benefits [3].
Botulinum neurotoxin (BoNT) therapy is an approved first-line treatment option for CD that blocks acetylcholine release at the neuromuscular junction to lessen muscular contractions [1], [4]. The current BoNT treatment paradigm aims to reduce the magnitude and recurrence of symptoms, and CD patients are typically retreated with 12-week intervals between injections [5]. This injection interval was based primarily on the findings from a retrospective study of individuals who had received an older formulation of onabotulinumtoxinA during the 1980s and early 1990s [5]. This early study reported that more frequent injections at shorter intervals (<3 months) using higher doses of BoNT were associated with resistance and may have reflected antigenicity of associated protein in the older formulation. However, the emergence of new BoNT formulations purified to remove accessory proteins to treat CD and reports of individual variations in the waning of therapeutic benefit highlight the need for evaluating shorter injection intervals to further improve patient outcomes [6], [7]. While many patients with CD benefit from standard injection intervals of BoNT and experience a typical maximum peak response, others have reported a decline in treatment effect at a mean of ∼ 9.5 weeks after injection with several different formulations and a preference for injection intervals ≤ 10 weeks to alleviate symptoms [6]. This early waning of response is not related to secondary treatment failure but is representative of typical patient-to-patient variation in response to BoNT treatment.
IncobotulinumtoxinA, a purified, accessory protein–free BoNT formulation that was shown to be noninferior to onabotulinumtoxinA for adults with CD or blepharospasm, has demonstrated efficacy and safety in two phase 3 clinical trials of patients with CD and is approved by the US Food and Drug Administration (FDA) for treatment of CD in adults [7], [8], [9], [10], [11]. Long-term safety and efficacy of incobotulinumtoxinA for treatment of CD have also been established [12]. To evaluate whether injection intervals shorter than the standard 12 weeks could benefit patients with early waning of effect, we conducted a phase 4 noninferiority study investigating the safety and efficacy of 2 incobotulinumtoxinA injection schedules (Short Flex: 8 ± 2 weeks; Long Flex: 14 ± 2 weeks) in patients with CD who were responsive to BoNT treatment but reported a waning of treatment effect by 10 weeks after their most recent injection. Specifically, this study aimed to determine whether patients receive continuous benefit from shorter injection intervals and whether there were any associated safety concerns with more frequent dosing as compared with a longer dosing interval.
2. Methods
2.1. Study design and subjects
This multicenter, open-label, randomized, noninferiority, phase 4 clinical study (NCT01486264) was conducted at 43 study sites in the United States. Approximately 250 subjects were planned for enrollment; those who signed informed consent and met all inclusion criteria were eligible. This study was conducted in accordance with the Declaration of Helsinki under the approval of institutional ethics committees. Subjects were aged ≥ 18 to < 81 years with documented clinical diagnosis of isolated CD who maintained a stable dose of other medications (if needed) used for dystonia treatment (eg, anticholinergics, baclofen, benzodiazepines). Subjects had at least 3 previous BoNT injections of onabotulinumtoxinA, abobotulinumtoxinA, incobotulinumtoxinA, or rimabotulinumtoxinB within 52 weeks before enrollment; the most recent injection must have shown subject-reported clinical improvement and < 10 weeks of treatment effect. Additionally, subjects must have had at least 2 successful injections (defined as having reported clinical improvement or atrophy of injected muscles) with BoNT prior to the most recent injection at least 12 weeks before enrollment. Key exclusion criteria were concomitant treatment with BoNT for any other indication or diagnosis of significant neuromuscular disease (eg, myasthenia gravis, Lambert-Eaton syndrome, or amyotrophic lateral sclerosis, among others).
After completing screening and enrollment assessments at visit 1 (day 1), eligible subjects were enrolled into the study and randomly assigned to Short Flex or Long Flex incobotulinumtoxinA dosing schedules in a 1:1 ratio. Randomization was performed using interactive voice response system procedures. The initial incobotulinumtoxinA study dose was comparable to a subject’s most recent onabotulinumtoxinA or incobotulinumtoxinA injection (within ± 10%). For subjects enrolled with recent injections of abobotulinumtoxinA or rimabotulinumtoxinB, the initial incobotulinumtoxinA study doses were one-third of the most recent abobotulinumtoxinA dose (within ± 10%) and one-fortieth of the most recent rimabotulinumtoxinB dose (within ± 10%).The first dose of incobotulinumtoxinA was administered at visit 1 (day 1), and subjects were scheduled for 8 subsequent injection visits at 8-week intervals (±2 weeks) for Short Flex and 14-week (±2 weeks) intervals for Long Flex. Injection intervals did not exceed 10 weeks for Short Flex or 16 weeks for Long Flex. A weekly self-assessment (Offset Questionnaire) was completed by subjects following each injection throughout the study after the first 28 days of enrollment and used to assess dosing adjustments. Subjects recorded diary responses to the question, “On most days last week, have you noticed that your CD symptoms are better, worse, or the same as the week prior?” Subjects in the Short Flex group who recorded “worse” responses were eligible to schedule their next injection as early as 6 weeks from the previous injection, while Long Flex subjects remained at 14 weeks (±2 weeks) even if a waning effect was reported earlier.
2.2. Outcomes
The primary efficacy endpoint was change in Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) severity subscale score from baseline to 4 weeks after the eighth injection as determined by trained, blinded, nontreating site personnel. Key secondary endpoints included TWSTRS total score (with the TWSTRS severity subscale conducted by the blinded and unblinded raters) as well as TWSTRS severity, TWSTRS disability, and TWSTRS pain subscale scores (unblinded raters) from baseline to 4 weeks after the eighth injection.
Other secondary outcomes included subject satisfaction and subject-reported global response (9-point Likert scale) evaluated 4 weeks after the eighth injection compared with baseline. Subject satisfaction was scored using a 10-point scale in response to the question, “How satisfied are you at the moment with your current therapy?” Additionally, results of the Cervical Dystonia Impact Profile–58 (CDIP-58), an assessment for measuring the health effect of CD and interventions, were evaluated at baseline and 4 weeks after the eighth injection. Physician-assessed global response (9-point Likert scale) was evaluated at a control visit 4 weeks after the eighth injection compared with another control visit 4 weeks after the first injection. Clinical global impression of severity was evaluated from baseline first injection to the eighth injection. Clinical global impression of severity was scored using a 7-point scale in response to the question “Considering your total clinical experience with this particular population, how ill is the patient at this time?”.
Safety and tolerability were monitored throughout the study and assessed by frequency and severity of adverse events (AEs), as well as by measuring vital signs (including blood pressure, heart rate and respiratory rate). To assess potential resistance due to immunogenicity, a unilateral brow injection (UBI) was performed 4 weeks after the first injection for all subjects and at the withdrawal visit for subjects who discontinued because of lack of treatment effect. Subjects who were nonresponsive to the initial UBI were discontinued from the study. Additional information on immunogenicity determination can be found in the Supplementary Methods.
2.3. Statistical methods
2.3.1. Analysis sets
The statistical analysis for this study utilized 3 subject analysis sets. The safety evaluation set (SES) included all subjects who were randomly assigned and received at least 1 dose of incobotulinumtoxinA. All randomized subjects who received at least 1 injection and for whom a blinded-rater TWSTRS severity value was available at the baseline injection visit and the control visit 4 weeks after the eighth injection were included in the full analysis set (FAS). The per protocol set (PPS) was inclusive of all subjects from the FAS who were responsive to the UBI at the 4-week control visit and had no major protocol deviations. The primary efficacy analysis was based on observed values for the PPS. Sensitivity analyses of the primary endpoint and evaluation of all secondary endpoints were performed on observed values for the FAS. The SES was used for all analyses of safety endpoints. Details on sample size determination are described in the Supplementary Methods.
2.3.2. Efficacy and safety analyses
Statistical tests were 1-sided with α = 0.025 for the primary endpoint and 2-sided with α = 0.05 for all secondary endpoints. Confidence intervals (CIs) and descriptive P values were given, when appropriate. More details on the composition and statistics of the efficacy and safety analyses are described in the Supplementary Methods.
3. Results
3.1. Subject disposition and baseline characteristics
A total of 282 subjects with CD were randomized, treated, and included in the SES analysis (Short Flex, n = 142; Long Flex, n = 140; Supplemental Fig. 1). One subject randomized to the Long Flex group discontinued the study before receiving study treatment. A total of 207 subjects completed the study (Short Flex, n = 113; Long Flex, n = 94; Supplemental Fig. 1); 198 subjects were included in the FAS (Short Flex, n = 108, 76.1%; Long Flex, n = 90, 64.3%), and 180 subjects were included in the PPS (Short Flex, n = 101, 71.1%; Long Flex, n = 79, 56.4%).
Baseline demographic characteristics were similar between the Short Flex and Long Flex groups (Table 1). Overall, most subjects were female (n = 204, 72.3%). The mean age (SD [range]) of subjects was 57.1 years (11.0 [24–81 years]), and 73% (n = 207) of subjects were aged 18 to 64 years (Table 1). Medical history of pretreatment of CD with botulinum toxin was recorded prior to first injection for onabotulinumtoxinA (n = 265), abobotulinumtoxinA (n = 31), incobotulinumtoxinA (n = 21), and rimabotulinumtoxinB (n = 19) and was similar between groups (Table 1).
Table 1.
Subject baseline demographics (SES).
| Short Flex (n = 142) | Long Flex (n = 140) | Total (N = 282) | |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 36 (25.4) | 42 (30.0) | 78 (27.7) |
| Female | 106 (74.6) | 98 (70.0) | 204 (72.3) |
| Age, mean (SD) [range], y | 57.4 (10.6) [24–79] | 56.7 (11.3) [31–81] | 57.1 (11.0) [24–81] |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 6 (4.2) | 6 (4.3) | 12 (4.3) |
| Not Hispanic or Latino | 136 (95.8) | 134 (95.7) | 270 (95.7) |
| Race, n (%) | |||
| White | 130 (91.5) | 128 (91.4) | 258 (91.5) |
| Black or African | 7 (4.9) | 7 (5.0) | 14 (5.0) |
| Other | 5 (3.5) | 5 (3.6) | 10 (3.5) |
| Height, mean (SD), cm | 166.8 (9.5) | 167.4 (9.7)a | 167.1 (9.6)b |
| BMI, mean (SD), kg/m2 | 27.9 (6.1) | 28.1 (5.4)a | 28.0 (5.8)b |
| Cumulative lifetime dose of BoNT pretreatment | |||
| OnabotulinumtoxinA | |||
| n | 130 | 135 | 265 |
| Units, mean (SD) | 4573 (5386) | 3979 (3867) | 4270 (4675) |
| AbobotulinumtoxinA | |||
| n | 18 | 13 | 31 |
| Units, mean (SD) | 2703 (3465) | 3065 (2665) | 2855 (3111) |
| IncobotulinumtoxinA | |||
| n | 14 | 7 | 21 |
| Units, mean (SD) | 906 (974) | 624 (1035) | 812 (978) |
| RimabotulinumtoxinB | |||
| n | 12 | 7 | 19 |
| Units, mean (SD) | 105,552 (176,036) | 40,750 (37,431) |
81,678 (142,955) |
BMI, body mass index; BoNT, botulinum neurotoxin; SD, standard deviation; SES, safety evaluation set. an = 139. bn = 281.
3.2. IncobotulinumtoxinA dosing and injection intervals
The overall dosing of incobotulinumtoxinA over the eight injection cycles was similar in the Short Flex and Long Flex groups (mean [SD], 272 [112.6] U vs 268 [101.2] U, respectively). As expected, the overall treatment duration was shorter for the Short Flex group (mean [SD], 409.8 [135.9] days) compared with the Long Flex group (mean [SD], 568.1 [221.5] days), as were the injection intervals (mean [SD] Short Flex, 54 [12.7] days; Long Flex, 86 [14.3] days).
3.3. Primary efficacy outcome: TWSTRS severity score
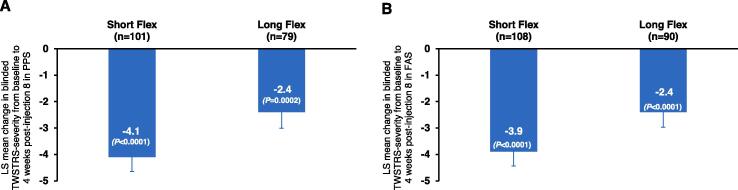
Significant improvements in mean blinded TWSTRS severity score from baseline to 4 weeks after the eighth injection were observed in both the Short Flex (mean change from baseline [SD], −4.1 [5.5] points; P < 0.0001) and Long Flex (mean change from baseline [SD], −2.4 [5.4] points; P = 0.0002) groups (Fig. 1A) in the PPS. Noninferiority of Short Flex compared with Long Flex was shown in an ANCOVA model adjusted for the baseline TWSTRS severity score (95% CI = [−2.9, 0.1] > Δ = 2.0), while there was a numerical trend toward favoring the Short Flex interval (least squares [LS] mean difference [standard error; SE], −1.4 [0.8] points; P = 0.0693) suggesting that both incobotulinumtoxinA injection intervals were effective in managing the clinical symptoms of CD. Similar results from the sensitivity analyses in the FAS with the same baseline adjusted ANCOVA support these results (LS mean difference [SE], −1.3 [0.7] points; P < 0.0001; Fig. 1B).
Fig. 1.
(A) Primary efficacy outcome of change in blinded-rater assessment of TWSTRS severity subscale score from baseline to 4 weeks after the eighth injection in the Short Flex and Long Flex groups reported for all subjects in PPS. PPS included subjects from FAS who were responsive to the UBI at the 4-week control visit with no major protocol deviations. (B) Sensitivity analysis of change in blinded-rater assessment of TWSTRS severity subscale score from baseline to 4 weeks after the eighth injection in the Short Flex and Long Flex groups reported for all subjects in FAS. FAS subset included randomized subjects who received at least 1 injection and for whom a blinded-rater TWSTRS severity value was available at the baseline injection visit and the control visit 4 weeks after the eighth injection. Error bars represent standard error. FAS, full analysis set; LS, least squares; PPS, per protocol set; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale; UBI, unilateral brow injection.
3.4. Key secondary efficacy outcomes: TWSTRS total score and subscales
Unblinded TWSTRS disability and pain subscale scores significantly improved from baseline in both the Short Flex (mean [SE] change in disability subscale, −1.9 [0.4] points, P < 0.0001; mean [SE] change in pain subscale, −2.7 [0.4] points, P < 0.0001) and Long Flex groups (mean [SE] change in disability subscale, −2.1 [0.6] points, P = 0.0008; mean [SE] change in pain subscale, −3.1 [0.5] points, P < 0.0001); however, neither showed clinically relevant differences between Short Flex and Long Flex injection intervals from baseline to 4 weeks after the eighth injection (Table 2). Total TWSTRS scores (blinded and unblinded raters) followed the same trend, with significant improvements from baseline within both the Short Flex (blinded-rater mean [SE] change, −8.5 [1.0] points, P < 0.0001; unblinded-rater mean [SE] change, −9.4 [1.1] points, P < 0.0001); and Long Flex (blinded-rater mean [SE] change, −7.6 [1.2] points, P < 0.0001; unblinded-rater mean [SE] change, −10.3 [1.2] points, P < 0.0001) treatment groups, but there was no significant difference between the two groups (Table 2).
Table 2.
LS Mean Difference Change in TWSTRS Total and Subscale Scores From Baseline to 4 Weeks After Eighth Injection in Short Flex Versus Long Flex Groups (FAS)a.
| Short Flex, Mean (SE) | Long Flex, Mean (SE) | LS Mean difference (SE) (Short Flex − Long Flex) | 95% CI |
Treatment comparison P value |
|
|---|---|---|---|---|---|
| TWSTRS severity (blinded) | −3.9 (0.54) | −2.4 (0.57) | −1.3 (0.72) | (−2.7, 0.2) | 0.0831 |
| TWSTRS total (blinded) | −8.5 (0.99) | −7.6 (1.16) | 0.0 (1.46) | (−2.9, 2.9) | 0.9879 |
| TWSTRS severity (unblinded) | −4.8 (0.60) | −5.1 (0.52) | 0.6 (0.77) | (−0.9, 2.1) | 0.4580 |
| TWSTRS disability (unblinded) | −1.9 (0.43) | −2.1 (0.61) | 0.7 (0.68) | (−0.6, 2.1) | 0.2819 |
| TWSTRS pain (unblinded) | −2.7 (0.41) | −3.1 (0.52) | 0.8 (0.61) | (−0.4, 2.0) | 0.1725 |
| TWSTRS total (unblinded) | −9.4 (1.07) | −10.3 (1.15) | 1.9 (1.52) | (−1.1, 4.9) | 0.2237 |
CI, confidence interval; FAS, full analysis set; LS, least squares; SE, standard error; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale. aFAS includes randomized subjects who received at least 1 injection and for whom a blinded-rater TWSTRS severity value was available at the baseline injection visit and the control visit 4 weeks after the eighth injection.
3.5. Additional secondary outcomes: Patient experience and physician assessments
Multiple patient and physician assessments favored the Short Flex injection interval. Subject satisfaction scored from 1 (completely satisfied) to 10 (completely unsatisfied) was significantly improved compared with baseline over 8 injections in the Short Flex group (mean [SE] change, −1.2 [0.4] points; P = 0.0007), but not in the Long Flex group (mean [SE] change, −0.6 [0.4] points; P = 0.1322; Supplemental Fig. 2A). A significant improvement was also observed in the Short Flex group for physician-assessed clinical global impression of severity at 4 weeks after the eighth injection (mean [SE] change, −0.5 [0.1] points; P < 0.0001). CDIP-58 total score was significantly improved in both the Short Flex and Long Flex groups (Short Flex mean [SE] within-treatment difference, −14.4 [2.0], P < 0.0001; Long Flex mean [SE] within-treatment difference, −12.4 [2.0], P < 0.0001) but showed a slight trend in favoring the Short Flex group (mean [SE] between-treatment change, −2.0 [2.8], P = 0.4809; Supplemental Fig. 2B). More details on secondary outcomes are described in the Supplementary Results.
3.6. Safety outcomes and immunogenicity
Overall, 210 subjects (74.5%) reported at least one treatment-emergent AE (TEAE); 9 subjects (3.2%) had a TEAE leading to discontinuation and 2 subjects had a fatal TEAE unrelated to incobotulinumtoxinA. The number of subjects with at least one TEAE was similar between the Short Flex (n = 111, 78.2%) and Long Flex groups (n = 99, 70.7%). Among all subjects, the most common TEAEs were dysphagia (25.2%), headache (10.3%), and muscle weakness (7.8%; Table 3). Serious TEAEs were reported in 26 subjects (9.2%); however, none were related to incobotulinumtoxinA.
Table 3.
Treatment-Emergent Adverse Events and nAb Assessments (SES).
| Short Flex (n = 142) | Long Flex (n = 140) | Total (N = 282) | |
|---|---|---|---|
| TEAEs, n (%)a | |||
| Gastrointestinal disorders | |||
| Dysphagia | 36 (25.4) | 35 (25.0) | 71 (25.2) |
| Nausea | 6 (4.2) | 9 (6.4) | 15 (5.3) |
| Infections and infestations | |||
| Sinusitis | 6 (4.2) | 7 (5.0) | 13 (4.6) |
| Upper respiratory tract infection | 9 (6.3) | 4 (2.9) | 13 (4.6) |
| Nasopharyngitis | 8 (5.6) | 4 (2.9) | 12 (4.3) |
| Musculoskeletal and connective tissue disorders | |||
| Muscle weakness | 10 (7.0) | 12 (8.6) | 22 (7.8) |
| Neck pain | 9 (6.3) | 9 (6.4) | 18 (6.4) |
| Back pain | 5 (3.5) | 10 (7.1) | 15 (5.3) |
| Musculoskeletal pain | 3 (2.1) | 9 (6.4) | 12 (4.3) |
| Arthralgia | 3 (2.1) | 7 (5.0) | 10 (3.5) |
| Nervous system disorders | |||
| Headache | 12 (8.5) | 17 (12.1) | 29 (10.3) |
| Dizziness | 5 (3.5) | 7 (5.0) | 12 (4.3) |
| Injury, poisoning, procedural complication | |||
| Fall | 8 (5.6) | 7 (5.0) | 15 (5.3) |
| Serious TEAEs, n (%)b | 15 (10.6) | 11 (7.9) | 26 (9.2) |
| TEAEs leading to discontinuation, n (%) | 6 (4.2) | 3 (2.1) | 9 (3.2) |
| TEAEs leading to death, n (%)c | 1 (0.7) | 1 (0.7) | 2 (0.7) |
| Positive nAb assessments, n (%)d | |||
| Baseline | |||
| HDA+ | 4 (2.8) | 3 (2.1) | 7 (2.5) |
| MPA+ | 3 (2.1) | 2 (1.4) | 5 (1.8) |
| End of study | |||
| HDA+ | 4 (2.8) | 2 (1.4) | 6 (2.1) |
| MPA+ | 3 (2.1) | 2 (1.4) | 5 (1.8) |
BoNT, botulinum neurotoxin; FIA, fluorescent immunoassay; HDA, hemidiaphragm assay; MPA, mouse protection assay; nAb, neutralizing antibody; SES, safety evaluation set; TEAE, treatment-emergent adverse event. aIncidence of TEAEs reported by ≥ 5% of subjects in either treatment group. bNo serious TEAEs were related to incobotulinumtoxinA. cTEAEs leading to death were not related to the study treatment; events included severe myocardial ischemia and completed suicide. dBinding antibodies to BoNT were identified using FIA as an initial screen, with positive results followed up with more sensitive assessments for nAbs (initial screening data not shown for clarity). HDA and MPA are sensitive confirmatory analyses for presence of nAbs. HDA is ∼ 25 times more sensitive than MPA.
Immunogenicity assessments for neutralizing antibodies were not collected at baseline following the second protocol amendment, thus results were only available for some subjects and limit meaningful comparisons before and after treatment. Additionally, antibody assessments in subjects following reported loss of treatment effect were not consistently performed. Fluorescent immunoassay (FIA) screening was conducted for a total of 196 subjects at baseline and 157 subjects at study termination; hemidiaphragm assay (HDA) or mouse protection assay (MPA) confirmatory analyses were conducted for 39 subjects at baseline and 44 subjects at termination who had positive FIA results. Overall, 11 subjects tested positive for neutralizing antibodies by the HDA or MPA assay after 8 treatment cycles (Short Flex, n = 7; Long Flex, n = 4; Table 3), and there were no notable differences between treatment groups. Of the subjects who discontinued the study for lack of efficacy, none showed a UBI response indicating loss of effect or converted from an antibody-negative test at baseline to a positive test at the end of study via HDA or MPA. Thus, there was no evidence of the development of neutralizing antibodies associated with secondary treatment failure.
4. Discussion
Some patients with CD who are responsive to treatment and receive BoNT injections at standard 12-week treatment intervals report a waning of clinical effect and reemergence of symptoms before reinjection [6]. Previous clinical trials have shown that flexible, repeated injections of incobotulinumtoxinA are safe and effective for treatment of CD [8], [12], [13]. Patients with CD who reported a decline in clinical effect prior to 10 weeks with previous BoNT therapy entered this randomized, phase 4 clinical study of incobotulinumtoxinA, the results of which demonstrated that shorter injection intervals (Short Flex: 8 ± 2 weeks) are noninferior to longer injection intervals (Long Flex: 14 ± 2 weeks) and tend to have numerically more favorable clinical outcomes than the longer interval without compromising safety.
From baseline to 4 weeks after the eighth injection of incobotulinumtoxinA both Short Flex and Long Flex groups showed significant improvements in the primary efficacy variable (blinded TWSTRS severity subscore); the LS mean difference (SE) of Short Flex minus Long Flex score was –1.4 (0.75), which is less than the noninferiority margin, and the negative difference indicates a numerically greater reduction of severity with Short Flex intervals. More frequent injections of incobotulinumtoxinA did not lead to higher rates of nonresponse in patients who previously reported early waning of clinical benefit from BoNT therapy. Importantly, in addition to the primary outcome, the subject satisfaction score was also significantly improved in the Short Flex, but not the Long Flex, treatment group. The subject-reported CDIP-58 subscales (pain/discomfort, sleep, annoyance, mood; Supplemental Fig. 2B) and total scores also demonstrated numerical trends in favor of the Short Flex interval. All of these findings are in line with the previously reported patient preference for shorter injection intervals and further illustrate the advantages of adapting BoNT treatment schedules to meet individual variations in responsiveness and expectations of treatment satisfaction [6].
IncobotulinumtoxinA was generally well tolerated, and there were no new or unexpected safety findings with either treatment regimen. The most frequently reported TEAEs were comparable between the treatment groups and consistent with those previously reported for incobotulinumtoxinA [7], [8], [12]. Given the multiple protocol amendments, one limitation of the study is that the immunogenicity data are too sparse to allow for meaningful conclusions. However, there were no cases of secondary nonresponse associated with the development of new neutralizing antibodies in either incobotulinumtoxinA treatment group among those tested. These findings are congruent with data on the immunogenicity of incobotulinumtoxinA treatment in the current literature and favorable for long-term treatment with incobotulinumtoxinA. These studies suggest that incobotulinumtoxinA treatment does not increase incidence of neutralizing antibodies in CD patients pretreated with other BoNT formulations [14], [15], [16].
Overall, this study demonstrates that CD patients who experience early waning of BoNT effect benefit from shorter injection cycles of incobotulinumtoxinA without increased risk, which enables clinicians to rethink the canonical treatment paradigm of a 12-week reinjection interval. While patients were responsive to both treatment regimens, a significant satisfaction advantage for patients with CD can be realized by shortening the reinjection intervals. Accordingly, these data should encourage physicians to align their injection strategy more closely with patients’ clinical response.
5. Data sharing statement
Merz will share data that relate to the results reported in this article with qualified researchers who provide a valid research question based on a data sharing contract. Proposals should be submitted to data-sharing@merz.com. Data are not available 5 years after publication.
6. Funding source
CRediT authorship contribution statement
Cynthia Comella: Investigation, Writing – review & editing. Robert A. Hauser: Investigation, Writing – review & editing. Stuart H. Isaacson: Investigation, Writing – review & editing. Daniel Truong: Investigation, Writing – review & editing. Odinachi Oguh: Investigation, Writing – review & editing. Jennifer Hui: Investigation, Writing – review & editing. Eric S. Molho: Investigation, Writing – review & editing. Matthew Brodsky: Investigation, Writing – review & editing. Erin Furr-Stimming: Investigation, Writing – review & editing. Georg Comes: Validation, Formal analysis, Writing – review & editing. Michael A. Hast: Writing – review & editing. David Charles: Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Disclosures: Cynthia Comella serves on the editorial board of Clinical Neuropharmacology and Sleep Medicine. She has received compensation/honoraria for services as a consultant or an advisory committee member for Acadia Pharmaceuticals, Acorda Therapeutics, Adamas Pharmaceuticals, AEON Biopharma, Allergan, Ipsen Pharmaceuticals, Jazz Pharmaceuticals, Lundbeck, Merz Pharmaceuticals, Neurocrine Biosciences, Revance Therapeutics, and Sunovion Pharmaceuticals. She receives royalties from Wolters Kluwer. Robert Hauser has received consulting fees from AbbVie, Acadia, Acorda, Adamas, Alterity, Amneal, Aptinyx, Britannia, Cerevance, Curium Pharma, Enterin, Inhibikase, Jazz, KeiferRx, Kyowa Kirin, Lundbeck A/S, Merck, Merz, Neurocrine Biosciences, Novus, Pharma Two B, Pharmather, Revance Therapeutics, Roche, Sage Therapeutics, Scion NeuroStim, Sio Gene Therapies, Sunovion, Supernus, Tolmar, US WorldMeds, and Vivifi Biotech and has received speaker fees from AbbVie, Acorda, Adamas, Amneal, Kyowa Kirin, Neurocrine Biosciences, and Sunovion. He holds stock in Axial Biotherapeutics and Inhibikase. His institution, University of South Florida, received research fees from AbbVie, Axovant Sciences, Biogen, Bukwang Pharmaceuticals, Cavion, Centogene, Cerevance, Cerevel Therapeutics, Cynapsus Therapeutics, Enterin, F. Hoffmann-La Roche, Genentech, Global Kinetics Corporation, Impax Specialty Pharma, Intec Pharma, Integrative Research Laboratories Sweden AB, Jazz Pharmaceuticals, MJFF, Neuraly, NeuroDerm, Neurocrine Biosciences, Northwestern University, Pfizer, Pharma Two B, Revance Therapeutics, Sanofi US Services, Sun Pharma Advanced Research Company, Sunovion Pharmaceuticals, and UCB Biopharma SPRL. Stuart Isaacson has received honoraria for CME for, was a consultant for, received research grants from, and/or was a promotional speaker on behalf of Abbvie, Allergan, Ipsen, Merz, Revance, Supernus, and US World Meds. Daniel Truong has received research funding from Abbvie, Acorda, Aeon, Auspex, Biogen, Bukwang, Cerevel, Cynapsus, Daiichi Sankyo Pharma, Eli Lilly, Enterin, Ipsen, Kyowa, Lundbeck, Merz, National Institute of Neurological Disorders and Stroke, Neurocrine, Neuroderm, Parkinson’s Foundation, Prilenia, Revance, and Sunovion. He has received honoraria for consulting and speaker activities from Acorda, Neurocrine, TEVA, an US Worldmed. Odinachi Oguh is a paid speaker and consultant for Sunovion Pharmaceuticals. Jennifer Hui is an advisory board member for Acorda, serves as a consultant for Sunovion, and receives grant support from Roche. Eric Molho is on the speakers bureau for Neurocrine Biosciences; received research funding from Amneal Pharmaceuticals, Biohaven Pharmaceuticals, Cerevel Therapeutics, CHDI/HSG, and Enterin; and received educational grants from AbbVie and Merz Pharmaceuticals. Matthew Brodsky has no conflicts of interest to disclose. Erin Furr-Stimming receives research funding from Cures within Reach, HDSA, Neurocrine, Prilenia, Roche/Genetech, and Uniqure; has consulted for Teva; and is on the speakers bureau for Sunovion; none of these are related to Xeomin or conflict with work being presented. Georg Comes and Michael Hast and are employees of Merz Pharmaceuticals. David Charles received income from Alliance for Patient Access, Merz, Newronika, Revance, and Supernus for consulting services. His institution, Vanderbilt University Medical Center, receives income from grants or contracts with Abbott, AbbVie, Aeon, Boston Scientific, Impax, Intec, Ipsen, Lundbeck, Medtronic, Merz, Novartis, Pharma Two B, and Supernus for research or educational programs that he has led.
Acknowledgments
This study was sponsored by Merz Pharmaceuticals, LLC. Writing and editorial assistance was provided under the direction of the authors by Katie Veleta, PhD, and Chris Lawrence, PhD, ELS, MedThink SciCom, and was funded by Merz Pharmaceuticals, LLC.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2022.100142.
Contributor Information
Cynthia Comella, Email: Cynthia_Comella@rush.edu.
Robert A. Hauser, Email: rhauser@usf.edu.
Stuart H. Isaacson, Email: isaacson@ParkinsonsCenter.org.
Daniel Truong, Email: dtruong@pmdi.org.
Odinachi Oguh, Email: OGUHO@ccf.org.
Jennifer Hui, Email: Jennifer.Hui@med.usc.edu.
Eric S. Molho, Email: MolhoE@amc.edu.
Matthew Brodsky, Email: brodskym@ohsu.edu.
Erin Furr-Stimming, Email: Erin.E.Furr@uth.tmc.edu.
Georg Comes, Email: Georg.comes@merz.de.
Michael A. Hast, Email: Michael.hast@merz.com.
David Charles, Email: david.charles@vumc.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brashear A. Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics. 2009;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J., Brin M.F., Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov. Disord. 1991;6(2):119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 3.Molho E.S., Agarwal N., Regan K., Higgins D.S., Factor S.A. Effect of cervical dystonia on employment: a retrospective analysis of the ability of treatment to restore premorbid employment status. Mov. Disord. 2009;24:1384–1387. doi: 10.1002/mds.22622. [DOI] [PubMed] [Google Scholar]
- 4.Simpson D.M., Hallett M., Ashman E.J., Comella C.L., Green M.W., Gronseth G.S., et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818–1826. doi: 10.1212/WNL.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene P., Fahn S., Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov. Disord. 1994;9(2):213–217. doi: 10.1002/mds.870090216. [DOI] [PubMed] [Google Scholar]
- 6.Sethi K.D., Rodriguez R., Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J. Med. Econ. 2012;15:419–423. doi: 10.3111/13696998.2011.653726. [DOI] [PubMed] [Google Scholar]
- 7.Benecke R., Jost W.H., Kanovsky P., Ruzicka E., Comes G., Grafe S. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology. 2005;64(11):1949–1951. doi: 10.1212/01.WNL.0000163767.99354.C3. [DOI] [PubMed] [Google Scholar]
- 8.Comella C.L., Jankovic J., Truong D.D., Hanschmann A., Grafe S. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J. Neurol. Sci. 2011;308(1-2):103–109. doi: 10.1016/j.jns.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez H.H., Pappert E.J., Comella C.L., Evidente V.G.H., Truong D.D., Verma A., Jankovic J. Efficacy and safety of incobotulinumtoxinA in subjects previously treated with botulinum toxin versus toxin-naïve subjects with cervical dystonia. Tremor Other Hyperkinet. Mov. 2013;3(0):03. doi: 10.7916/D87P8X43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xeomin [package insert]. Frankfurt am Main, Germany: Merz Pharmaceuticals GmbH; 2020.
- 11.Jankovic J. Clinical efficacy and tolerability of Xeomin in the treatment of blepharospasm. Eur. J. Neurol. 2009;16(suppl 2):14–18. doi: 10.1111/j.1468-1331.2009.02880.x. [DOI] [PubMed] [Google Scholar]
- 12.Dressler D., Paus S., Seitzinger A., Gebhardt B., Kupsch A., Bock U., Ceballos-Baumann A., Dengler R., Ebke M., Hagenah J., Haslinger B., Hecht M., Heide G., Hellwig S., Kupsch A., Paus S., Schiefer J., Storch A., Vieregge P., Vogt T., Volkmann J., Woldag H. Long-term efficacy and safety of incobotulinumtoxinA injections in patients with cervical dystonia. J. Neurol. Neurosurg. Psychiatry. 2013;84(9):1014–1019. doi: 10.1136/jnnp-2012-303608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evidente V.G.H., Fernandez H.H., LeDoux M.S., Brashear A., Grafe S., Hanschmann A., Comella C.L. A randomized, double-blind study of repeated incobotulinumtoxinA (Xeomin®) in cervical dystonia. J. Neural. Transm. (Vienna) 2013;120(12):1699–1707. doi: 10.1007/s00702-013-1048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.H. Hefter, C. Hartmann, U. Kahlen, M. Moll, H. Bigalke, Prospective analysis of neutralising antibody titres in secondary non-responders under continuous treatment with a botulinumtoxin type A preparation free of complexing proteins—a single cohort 4-year follow-up study, BMJ Open 2 (2012) e000646. [DOI] [PMC free article] [PubMed]
- 15.Hefter H., Brauns R., Ürer B., Rosenthal D., Albrecht P. Effective long-term treatment with incobotulinumtoxin (Xeomin®) without neutralizing antibody induction: a monocentric, cross-sectional study. J. Neurol. 2020;267:1340–1347. doi: 10.1007/s00415-019-09681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr W.W., Neal J., Sublett J.W. Immunogenicity of botulinum toxin formulations: potential therapeutic implications. Adv. Ther. 2021;38:5046–5064. doi: 10.1007/s12325-021-01882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.