Abstract
Objective
To evaluate the clinical outcome of a hydrogel-based autologous chondrocyte implantation (ACI) for large articular cartilage defects in the knee joint.
Design
Prospective, multicenter, single-arm, phase III clinical trial. ACI was performed in 100 patients with focal full-thickness cartilage defects ranging from 4 to 12 cm2 in size. The primary outcome measure was the responder rate at 2 years using the Knee Injury and Osteoarthritis Outcome Score (KOOS).
Results
Two years after ACI treatment, 93% of patients were KOOS responders having improved by ≥10 points compared with their pre-operative level. The primary endpoint of the study was met and demonstrated that the KOOS response rate is markedly greater than 40% with a lower 95% CI (confidence interval) of 86.1, more than twice the pre-specified no-effect level. KOOS improvement (least squares mean) was 42.0 ± 1.8 points (95% CI between 38.4 and 45.7). Mean changes from baseline were significant in the overall KOOS and in all 5 KOOS subscores from Month 3 (first measurement) to Month 24 (inclusive) (P < 0.0001). The mean MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) score after 24 months reached 80.0 points (95% CI: 70.0-90.0 points) and 92.1 points in lesions ≤ 5 cm2.
Conclusions
Overall, hydrogel-based ACI proved to be a valuable treatment option for patients with large cartilage defects in the knee as demonstrated by early, statistically significant, and clinically meaningful improvement up to 2 years follow-up. Parallel to the clinical improvements, MRI analyses suggested increasing maturation, re-organization, and integration of the repair tissue.
Trial Registration
NCT03319797; EudraCT No.: 2016-002817-22.
Keywords: cartilage repair, large defects, autologous chondrocyte implantation, knee, hydrogel
Introduction
Articular cartilage damage is often associated with significant discomfort such as pain, swelling, and functional impairment. 1 From clinical studies with observation periods of up to 30 years, it is now known that the natural course and the further prognosis of knee cartilage lesions are determined by different aspects. In particular, associated risk factors for the development of premature osteoarthritis (OA) include the size, depth, and localization of the defect, as well as possible additional injuries to knee protective structures, such as ligaments, the meniscus, or other comorbidities like axial or patellar malalignment.2-5
These pre-arthritic deformities have consequences that can lead to abnormal stress and altered biomechanical loading of the affected joint that may induce secondary inflammatory processes associated with the expression of pro-inflammatory cytokines, matrix-degrading metalloproteinases, recruitment and activation of inflammatory cell types, adverse angio- and osteogenesis, and the ingrowth of new sensory nerves and blood vessels into the joint.5,6 The escalation of a pathological joint environment not only favors the emergence of pain and degenerative changes but also inhibits cartilage regeneration. 7 Against this background, there is broad consensus that in case of persistent clinical complaints due to focal cartilage damage, surgical defect therapy and the correction of relevant comorbidities, for example, knee malalignment, should be performed as early as possible. 4
For the biological reconstruction of localized full-thickness cartilage defects, different methods, such as bone marrow stimulating techniques, osteochondral transfer or allografts, particulated or minced cartilage procedures and autologous chondrocyte implantation (ACI), are available. In this context, in addition to long-term observations, several studies, some at the highest level of evidence, have documented that second- and third-generation ACI methods provide the lowest failure and revision rates and the best long-term results for both chondral and osteochondral lesions of the knee, in particular, in defects larger than 2 to 4 cm2 in size.8-13
Furthermore, there is increasing evidence that improved quality of the regenerated tissue (i.e., cartilage with hyaline instead of fibrous characteristics), integration, and surface properties in the absence of vascularization or ossification is beneficial for durable long-term results after cartilage reconstructive surgery.4,8,14 Last, compared with arthrotomy-based, more open surgical approaches, the arthroscopic application of ACI is associated with lower complication rates. 15
For this reason, we have developed a biocompatible and in situ cross-linkable albumin-hyaluronan-based hydrogel as a carrier material for matrix-assisted autologous chondrocyte implantation (M-ACI) procedures. It can be applied either arthroscopically or by a minimally invasive procedure even in difficult defect locations. 16 The cross-linked hydrogel has a water content greater than 95% and does not support cell adhesion for most cell types. However, chondrocytes are anchorage-independent and exhibit high viability as well as a spherical cell morphology in the gel, which promotes and stabilizes their chondrogenic phenotype,17,18 favorable for repair tissue quality and graft survival. 19 Due to the mentioned physical properties, it has barrier function for inflammatory and endothelial cell invasion, resulting in anti-inflammatory, anti-angiogenic, and thus also anti-osteogenic effects.17,18 When applied to a cartilage lesion, the low viscosity cell-seeded biomaterial can flow into the smallest and most irregular-sized defect niches prior to its solidification.
Using this hydrogel as carrier for culture-expanded chondrocytes that were characterized before product release with respect to their potency, identity, and purity according to the European Medicines Agency (EMA) guideline on human cell–based medicinal products (EMEA/CHMP/410869/2006), we have performed a prospective, international, multicenter phase III study to treat 100 patients with large chondral or osteochondral defects of the knee. Post-operative follow-up time is up to 5 years and the results presented here are an analysis of the 2-year time point.
Methods
Study Design and Participants
This prospective, single-arm phase III clinical trial was conducted in full compliance with the principles laid down in the Declaration of Helsinki, the Guideline E6 for Good Clinical Practice of the International Conference on Harmonization (ICH GCP), and relevant local laws and regulations. After approval by the local ethics committees, federal authorities, and study registration (ClinicalTrials.gov identifier: NCT03319797; EudraCT No.: 2016-002817-22), patients who consented in writing to participate in the trial were enrolled and treated between October 2017 and February 2019 for focal symptomatic cartilage defects of the knee at 6 Czech, 5 Hungarian, 3 Lithuanian, 2 German, and 1 Swiss centers.
Males and females aged 18 to 65 years (or ≥14-year-old minors with closed epiphyseal growth plates) with focal cartilage defects of the femoral condyle, trochlea, patella, or tibial plateau of the knee (defect grade of III or IV according to the International Cartilage Regeneration & Joint Preservation Society [ICRS] classification) were eligible for enrollment. The defect size was to range between 4 and 12 cm2, 2 defects as well as prior failed cartilage repair of the index lesion were allowed. Detailed inclusion and exclusion criteria are presented in Table 1 .
Table 1.
Main Inclusion and Exclusion Criteria.
| Inclusion criteria |
| • Men/women ≥ 18 and ≤ 65 years old or pediatric patients (14-17 years old) with closed epiphyseal growth plate |
| • One or 2 focal, full-thickness cartilage defect(s) of the knee (ICRS grade III or IV) |
| • Defect size ≥ 4 and ≤ 12 cm2 |
| • Defect localization: Femoral condyle, trochlea, patella, or tibial plateau |
| • Intact, well-contained chondral structure surrounding the defect and intact articulating joint surface opposite to the defect(s) to be treated (≤ grade I ICRS) |
| • Stable knee joint or sufficiently reconstructed ligaments and no patella malalignment or sufficiently corrected patella malalignment |
| • Baseline score of < 65/100 in the overall KOOS |
| Exclusion criteria |
| • ICRS grade II cartilage defects in the target knee |
| • Prior biologic reconstructive procedures (e.g., microfracture, mosaicplasty, chondrocyte transplantation) in the target knee at a location different from the defect location to be treated in the trial. Prior biologic reconstructive procedures on the index lesion were accepted (i.e., the prior method has failed), and these procedures were performed ≥ 24 months prior to screening. |
| • Body mass index >35 kg/m2 |
| • More than 50% resection per meniscus in the target knee |
| • Subchondral bone defects more than 2 mm deep unless adjuvant defect filling performed prior to treatment |
| • Drugs or therapy (shortened): Immunosuppressants, systemic or intra-articular steroids, and/or steroid use within 30 days prior to screening |
| • Diseases/conditions (shortened): Metabolic arthropathies, autoimmune disease, immune suppression, history of or current relevant infections, chronic inflammatory arthritis and/or infectious arthritis, uncontrolled diabetes, systemic connective tissue disease, history of borreliosis, history of cancer, osteoporosis, primary hyperparathyroidism or hyperthyroidism without satisfactory treatment, chronic renal failure or patients with prior pathological fractures, any degenerative muscular or neurological condition that would interfere with evaluation of outcome measures |
| • Pregnancy |
| • Uncorrected malalignment (valgus- or varus-deformity) in the target knee |
| • Degenerative joint disease in the target knee as determined by Kellgren and Lawrence grade >2 |
| • Joint space narrowing > 1/3 in the target knee when compared with the other knee or < 3 mm joint space |
| • Arthrofibrosis in the target knee |
| • Diffuse chondromalacia (grade 1 according to Outerbridge allowed) |
ICRS = International Cartilage Regeneration & Joint Preservation Society; KOOS = Knee Injury and Osteoarthritis Outcome Score.
All potential study patients were evaluated preoperatively by MRI of the affected knee joint to assess both the lesion and the surrounding healthy cartilage. Final eligibility was then determined during diagnostic arthroscopic surgery. Of the 132 patients screened, 102 patients were assessed as eligible to participate and were included in the study. Of these, 100 patients were treated with M-ACI, and 2 patients discontinued participation prior to implantation, but after harvest of the cartilage biopsy.
Surgical Technique
In this study, the NOVOCART® Inject plus product (TETEC—Tissue Engineering Technologies AG, Reutlingen, Germany), a 2-component injection system, was investigated: The first component consists of in vitro culture-expanded and characterized autologous articular chondrocytes (2-8 Mio. cells per mL) suspended in a solution containing modified human albumin (maleimido-albumin, MAHSA), isotonic sodium hyaluronate, human serum, and cell culture medium. The second component consists of an α,ω-bisthio-polyethylene glycol cross-linker. By simultaneous injection of the 2 components via a dual-chamber syringe application system, in situ formation of the hydrogel is achieved by cross-linking of the MAHSA moieties.
In the first step, osteochondral biopsies from eligible patients are harvested during arthroscopic surgery from a non-weightbearing area of the knee joint using a single-use trephine (Aesculap, Tuttlingen, Germany). A total of 3 osteochondral cylinders (diameter of 4 mm, about 7 mm deep) per patient are harvested and sent to the manufacturing facility (TETEC—Tissue Engineering Technologies AG, Reutlingen, Germany). Implant production takes 24 ± 5 days starting from tissue processing.
In the second step, M-ACI is performed either arthroscopically or through a mini-arthrotomy approach. NOVOCART® Inject plus is applied to the prepared, dry defect area via the dual-chamber syringe until the defect is completely filled to a height that matches the surrounding native cartilage. The resulting bioresorbable hydrogel anchors the seeded cells within the defect without the need for additional fixation. (In situ) solidification through cross-linking of the hydrogel occurs within 1 to 3 minutes. The leg/knee joint is held stationary in position during this time. After solidification of the transplant, the joint was moved within its physiological range of motion to check joint mobility and graft stability, followed by wound closure.
After surgery, all patients followed a defined rehabilitation protocol based on Hirschmüller et al. 20
Main Assessment Criteria
Primary outcome assessment was performed using the Knee Injury and Osteoarthritis Outcome Score (KOOS), which is a validated and widely accepted patient-reported instrument for assessment of treatment outcome in patients with cartilage defects of the knee. 21 The KOOS ranges from 0 indicating extreme symptoms to 100 indicating no symptoms. An improvement of 8 to 10 points in the KOOS represents the minimal clinically important difference, 22 that is, the smallest change score needed for the effect to be considered clinically relevant. 23 Secondary assessments to KOOS included the subjective International Knee Documentation Committee (IKDC) score,24,25 IKDC objective grading (knee examination), the EQ-5D-5L (standardized measure of quality of life),25-27 and patient satisfaction. All patients were assessed preoperatively (baseline) and then at 3, 6, 12, 18, and 24 months after treatment.
The assessment of repair tissue properties was performed 12 and 24 months after treatment in a subset of 25 patients by the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score,28,29 and T2-mapping.30-36 No baseline status was documented, as both the MOCART score and the T2-mapping characteristics mainly refer to the cartilage repair tissue and thus do not allow relevant pre-operative assessment. The MOCART sum score quantifies 10 items of graft maturation and lesion healing by semi-quantitative, morphological categorization, while the T2 relaxation time-derived variables provide information on the ultrastructural composition of the repair tissue that reflects the integrity and vitality of cartilage and cartilage regenerated tissue. The MOCART sum score ranges from 0 (worst possible outcome) to 100 (normal joint), while decreasing values for T2 relaxation time (up to a certain extent) and smaller T2 standard deviations indicate a higher structural organization of the regenerated tissue.
Moreover, the T2 relaxation time measured in the repair tissue can be set in relation to the T2 relaxation time measured in the surrounding healthy cartilage tissue, thereby resulting in a “global” T2 ratio (if only the full-thickness tissue areas are measured) and in a “zonal” T2 ratio (if, in addition, the differences in T2 relaxation times in the superficial and deep zones of the cartilage areas are considered). Ideal global and zonal T2 ratios are “1” (indicating no difference between regenerated tissue and normal tissue), and the ratio range of 0.8 to 1.2 is regarded as “normal” (and was therefore employed for the analyses of the T2 ratios).
Safety was assessed based primarily on adverse events.
Statistical Analysis
All statistical analyses were performed using the software package SAS, Version 9.4 or higher.
Primary efficacy analysis
The primary study outcome was the overall KOOS responder rate (R) defined as the proportion of patients with a ≥10-points improvement from baseline at Month 24. The no-effect level was set to 40%, thereby resulting in the confirmatory study hypothesis:
Patients with missing 24-month assessment or patients classified as treatment failures were handled as non-responders irrespective of the reason for drop out or their actual KOOS response, respectively.
The H0 hypothesis was tested using a 1-side exact binomial test at a significance level of 0.025. Sufficient treatment efficacy was to be concluded, if the lower bound of the 95% confidence interval (CI) for the KOOS responder rate according to Clopper-Pearson was >40%.
Sensitivity analyses were performed taking into account concomitant analgesic medication and “complete cases” (only non-missing KOOS scores collected at the respective visit were analyzed).
Secondary efficacy endpoints
All other efficacy data were analyzed exploratively.
The change in the overall KOOS from baseline to the 24-month visit was considered a key secondary endpoint and was analyzed by a linear mixed-effect model for repeated measurements (MMRM) using all the longitudinal observations of the overall KOOS score after implantation up to and including Month 24 (except observations obtained after surgical intervention in patients classified as treatment failures). The model included the effects of “country,” “visit,” and “baseline score.” The treatment effect at each post-baseline timepoint was estimated using least square (LS) means for changes from baseline, as well as associated 95% CIs and P values. The analysis was repeated taking into account the concomitant analgesic medication as described for the primary endpoint.
Other continuous secondary efficacy endpoints were analyzed using the MMRM model as described for the key secondary endpoint. Ordinal secondary endpoints were analyzed using ordinal logistic regression or rank-based methods.
Two-sided statistical tests were performed on a level of significance of α = 0.05 and corresponding 2-sided 95% CIs were calculated.
All efficacy analyses were done on the intent-to-treat (ITT) population (i.e., patients who have received M-ACI treatment) and repeated for the per protocol population.
Logistic models were applied to explore the influence of a panel of preselected factors/covariates on the primary efficacy endpoint (KOOS responder rate at Month 24). Potential associations with the key secondary endpoint (KOOS mean change from baseline at Month 24) were explored by adding the respective categorical or continuous effects to the MMRM for the key secondary efficacy analysis.
The association of semi-quantitative MOCART scores and quantitative T2-mapping analyses with the primary efficacy endpoint and the key secondary endpoint were explored using logistic or linear models.
Results
Patient Population
Patient demographic and baseline characteristics are summarized in Table 2 . The ITT analysis population comprised 100 patients (37 women, 63 men) with a mean age of 39.8 ± 11.5 years (range: 15-62 years). The mean body mass index was 27.0 kg/m2, while at least 50% of the study patients were overweight (BMI ≥25) and at least 25% obese (BMI ≥30), respectively. A total of 52 patients (52.0%) had undergone at least 1 knee operation prior to study entry, including 8 patients (8.0%) with prior failed cartilage repair (2 patients thereof had failed twice). The mean time elapsed between the occurrence of the first knee symptoms and study inclusion among study patients was 22.7 ± 26.8 months (range: 0.5-126.6 months).
Table 2.
Patient Demographic and Baseline Characteristics.
| All Patients (N = 100) | |
|---|---|
| Sex, n (%) | |
| Male | 63 (63.0) |
| Female | 37 (37.0) |
| Age (years), mean ± SD (range) | 39.8 ± 11.5 (15-62) |
| Body mass index (kg/m2), mean ± SD (range) | 27.0 ± 4.1 (20.2-34.4) |
| Smoking status, n (%) | |
| Yes | 25 (25.0) |
| No | 75 (75.0) |
| Patients with at least 1 prior surgery, a n (%) | 52 (52.0) |
| Meniscus removal | 27 (27.0) |
| Joint debridement | 16 (16.0) |
| Ligament operation | 15 (15.0) |
| Chondroplasty | 7 (7.0) |
| Time since first symptoms (months), mean ± SD | 22.7 ± 26.8 (0.5-126.6) |
| Concomitant surgeries, b n (%) | 22 (22.0) |
| Ligament operation | 11 (11.0) |
| Osteotomy | 5 (5.0) |
| Meniscus removal | 3 (3.0) |
| Tenoplasty | 3 (3.0) |
| Number of defects per patient, n (%) | |
| One defect | 70 (70) |
| Two defects | 30 (30) |
| Defect location, n defects (%) | |
| Femoral condyle | 80 (61.5) |
| Patellofemoral | 45 (34.6) |
| Tibial plateau | 5 (3.8) |
| ICRS grade, n defects (%) | |
| 3 | 93 (71.5) |
| 4 | 37 (28.5) |
| Lesion etiology, n defects (%) | |
| Traumatic | 78 (60.0) |
| OCD | 6 (4.6) |
| Focal degenerative | 46 (35.4) |
| Defect size (cm2), mean ± SD (range) | |
| All lesions | 4.8 ± 1.9 (1.0-10.4) |
| Larger lesion c | 5.4 ± 1.6 (3.0-10.4) |
| Total d | 6.3 ± 2.1 (4.0-12.5) |
| Operative access, n (%) | |
| Arthroscopy | 46 (46.0) |
| Mini-arthrotomy | 49 (49.0) |
| Open knee surgery | 5 (5.0) |
| Duration of implantation (minutes), mean ± SD (range) | |
| All patients | 46.2 ± 26.2 (10-161) |
| Patients without concomitant surgery | 39.6 ± 19.8 (10-161) |
n = number of patients; n defects = number of defects; ICRS = International Cartilage Regeneration & Joint Preservation Society; OCD = osteochondritis dissecans.
Only surgeries performed in more than 3 patients are given.
Performed concomitantly to tissue harvest or product implantation. Only surgeries performed in more than 1 patient are given.
Lesions were classified into larger and smaller lesions, that is, in patients with 2 lesions, the classification was based on the size of the respective lesions, while in patients with 1 lesion only, this lesion was classified as the larger lesion.
All lesions per patient added to 1 single value.
Seventy patients had only 1 cartilage defect (ICRS grade III or IV), while 30 patients had 2 defects, that is, a total of 130 defects were treated in this study. Most lesions were of traumatic origin (60.0%), while 35.4% were focal degenerative lesions and 4.6% (6 lesions) were caused by osteochondritis dissecans (OCD). Most defects were located at the femoral condyle (61.5%; 11.5% lateral, and 50.0% medial), while 34.6% of the lesions were located patellofemoral and 3.8% tibial.
The mean defect size of the 130 lesions post-debridement was 4.8 ± 1.9 cm2 (range: 1.0-10.4 cm2), while the mean total defect size among the 100 study patients (i.e., all lesions per patient added to 1 single value) was 6.3 ± 2.1 cm2 (range: 4.0-12.5 cm2). In addition, lesions were classified into larger and smaller lesions, that is, in patients with 2 lesions, classification was based on the size of the respective lesions, while in patients with 1 lesion only, this lesion was classified as the larger lesion. The defect size of the 100 larger lesions was 5.4 ± 1.6 cm2 (range: 3.0-10.4 cm2).
In 22 patients (22.0%), other surgical procedures were performed concomitantly to tissue harvest or product implantation, most of them ligament repair operations (11 patients). For the implantation procedure, 49% of patients underwent mini-arthrotomy, 46% had arthroscopy, and 5 patients underwent open knee surgery. The mean duration of the ACI procedure was 46.2 ± 26.2 minutes (median: 40.0 minutes) in the overall population and 39.6 ± 19.8 minutes (median: 35.0 minutes) in patients without concomitant surgeries at implantation.
KOOS Results
Response Rate (Primary Outcome)
Twenty-four months after treatment with NOVOCART® Inject plus, 93% of patients were KOOS responders, that is, with scores improved by ≥10 points compared with their pre-operative level. The study met its primary efficacy endpoint as the 95% CI was between 86.1% and 97.1% and thus the lower 95% CI limit was significantly higher than the pre-specified level of 40% (P < 0.0001). The sensitivity analyses (based on adjustment for increased pain medication given within 7 days prior to the assessment time point and complete cases, respectively), showed results that were almost identical to the main analysis and thus supported its validity.
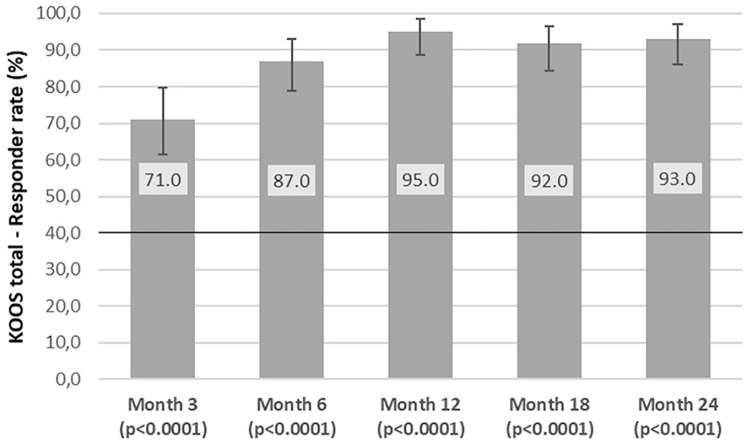
The analysis over time ( Fig. 1 , Table 3 ) showed that the KOOS responder rate had exceeded the threshold of 40% at the time of the first measurement as early as 3 months with 71% responders and a 95% CI between 61.1% and 79.6% (P < 0.0001).
Figure 1.
KOOS responder rates over time through Month 24. Vertical error bars indicate the exact 95% confidence intervals according to Clopper and Pearson. P values are derived from the 1-sided exact binomial test of hypotheses H0: Rate ≤ 40% versus H1: Rate >40%. KOOS = Knee Injury and Osteoarthritis Outcome Score.
Table 3.
Overall KOOS Score Changes From Baseline and Responder Rates Over Time.
| All Patients (N = 100) | ||||
|---|---|---|---|---|
| Baseline | 3 Months | 12 Months | 24 Months | |
| KOOS Score ± SD | 39.8 ± 14.3 | 66.3 ± 15.8 | 78.8 ± 15.0 | 82.4 ± 16.4 |
| LS mean change (SE) | — | 26.2 (1.7) | 38.8 (1.6) | 42.0 (1.8) |
| 95% CI | [22.8-29.6] | [35.6-42.0] | [38.4-45.7] | |
| P value a | <0.0001 | <0.0001 | <0.0001 | |
| Response rate (%) | — | 71.0 | 95.0 | 93.0 |
| 95% CI b | [61.1-79.6] | [88.7-98.4] | [86.1-97.1] | |
| P value c | <0.0001 | <0.0001 | <0.0001 | |
KOOS = Knee Injury and Osteoarthritis Outcome Score; LS = least square; CI = confidence interval; MMRM = mixed-effect model for repeated measurements.
From linear MMRM with “country” and “visit” as fixed factors, and “baseline KOOS score” as covariate. All patients with post-baseline data are included.
Clopper-Pearson (exact) binomial confidence interval.
From 1-sided exact binomial test of hypotheses H0: Rate ≤40% versus H1: Rate >40%.
Changes from baseline
Absolute values for the overall KOOS and changes from baseline are outlined in Table 3 . The mean overall KOOS increased from 39.8 points preoperatively to 82.4 points at the follow-up examination 24 months after treatment. The corresponding mean improvement (LS means) was 42.0 ± 1.8 points (95% CI: 38.4-45.7). The mean changes from baseline were significant in the overall KOOS and in all 5 KOOS subscores at all timepoints measured from Month 3 to Month 24 (P < 0.0001). The greatest improvements at Month 24 (i.e., an increase of more than 40 points) were found in the sports/recreation (LS mean increase: 54.6 ± 2.5; 95% CI: 49.7-59.5) and quality of life (LS mean increase: 47.3 ± 2.6; 95% CI: 42.1-52.4) subscores.
In the covariate analyses covering a broad range of demographic and medical conditions as well as product characteristics (e.g., age, sex, defect size, symptom duration, defect etiology and location, cell density, number of population doublings), the only covariate with a likely impact on overall KOOS changes from baseline was “prior failed cartilage repair” where patients without prior failure achieved higher changes from baseline (mean increase: 43.4 vs. 32.8 points).
Nevertheless, patients with prior failed cartilage repair clearly benefited from treatment with 6 out of 8 patients (75%), including 2 patients who had failed microfracture first and then mosaicplasty, achieving a KOOS and IKDC response ( Fig. 2 ).
Figure 2.
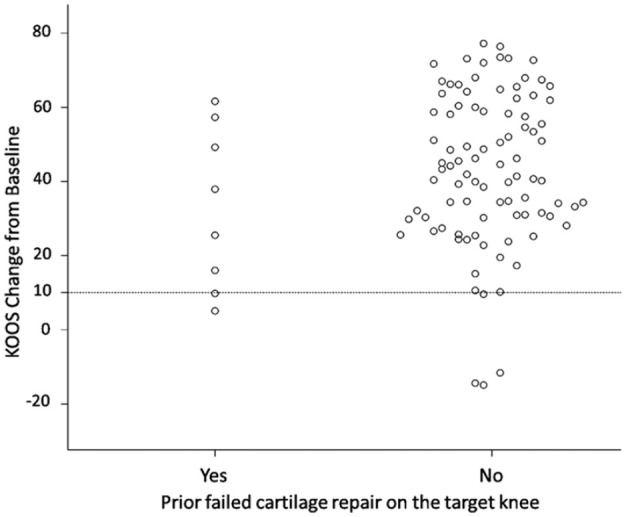
Scatter plot with linear regression model for overall KOOS change from baseline at Month 24 by prior failed cartilage repair. KOOS = Knee Injury and Osteoarthritis Outcome Score.
Other Clinical Efficacy Results
The mean changes from baseline in the IKDC subjective score, which ranges from 0 (worst result) to 100 (best result), were highly significant at all time points (i.e., P < 0.0001; including Month 3) and increased in their extent through Month 24 (LS mean increase from baseline: 41.6 ± 1.9 points; 95% CI: 37.9-45.4). At Month 24, the IKDC subjective responder rates I (improvement of >20.5 points) and II (improvement of ≥11.5 points) were 84.0% (95% CI: 75.3-90.6) and 89.0% (95% CI: 81.2-94.4), respectively.
The analysis of the IKDC objective knee examination at Month 24 showed a nominally significant shift toward the more favorable assessment categories compared with the baseline status. The proportion of patients with a “normal” knee status increased from 52.0% at baseline to 91.4% at Month 24 (Wilcoxon signed-rank test for the change from baseline: P < 0.0001).
Consistent with the improvements in the clinical scores, the EQ-5D-5L health-related quality of life measures (EQ-5D-5L index and visual analogue scale) increased significantly compared with pre-operative assessments from (including) Month 3 through Month 24 (P < 0.0001 for both parameters at all timepoints).
In summary, all secondary endpoints showed results consistent with the primary endpoint, that is, early and significant improvement from (and including) Month 3 and sustained or even increasing improvement up to Month 24.
MRI Results
The main MRI outcomes after 12 and 24 months are summarized in Table 4 .
Table 4.
Overview of MRI Outcomes (MOCART Score and T2-Mapping) at Months 12 and 24.
| MRI Variable | All Patients (n = 25) | ||
|---|---|---|---|
| Month 12 | Month 24 | ||
| MOCART sum score | n patients / n lesions | 25 / 30 | 24 / 28 |
| Mean ± SD [95% CI] | 70.0 ± 22.4 [61.6-78.4] | 80.0 ± 25.7 [70.0-90.0] | |
| Median (range) | 75.0 (10-100) | 90.0 (10-100) | |
| T2 Global ratio | n patients / n lesions | 21 / 25 | 20 / 23 |
| Mean ± SD [95% CI] | 1.09 ± 0.23 [1.00-1.19] | 0.98 ± 0.24 [0.87-1.08] | |
| Median (range) | 1.03 (0.77-1.64) | 0.940 (0.69-1.92) | |
| <0.8, n (%) | 2 (8.0) | 4 (17.4) | |
| 0.8-1.2, n (%) | 15 (60.0) | 17 (73.9) | |
| >1.2, n (%) | 8 (32.0) | 2 (8.7) | |
| T2 Zonal ratio | n patients / n lesions | 21 / 25 | 20 / 23 |
| Mean ± SD [95% CI] | 1.10 ± 0.22 [1.01-1.2] | 1.01 ± 0.20 [0.92-1.10] | |
| Median (range) | 1.11 (0.66-1.54) | 0.99 (0.64-1.52) | |
| <0.8, n (%) | 2 (8.0) | 2 (8.7) | |
| 0.8-1.2, n (%) | 15 (60.0) | 18 (78.3) | |
| >1.2, n (%) | 8 (32.0) | 3 (13.0) | |
Percentages and numbers of observations are based on the number of lesions.
MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue; CI = confidence interval.
Among all 25 patients (with 30 lesions), the mean MOCART sum score was 70.0 points (median: 75.0) at Month 12 and increased to 80.0 points (median: 90.0; 95% CI: 70.0-90.0) at Month 24. In the 7 patients with defect sizes of 5 cm2 or less (median split), the MOCART sum score reached 92.1 points at 2 years.
The proportion of lesions within the “ideal range” of 0.8 to 1.2 increased from 60.0% to 73.9% for the T2 global ratio and from 60% to 78.3% for the T2 zonal ratio at Months 12 and 24, respectively. Overall, these MRI-based assessments suggested that the observed clinical improvements through Month 24 were accompanied by progressing graft maturation and cartilage re-organization. In the analyses of potential correlations between clinical and imaging outcomes, there were no significant associations between the MOCART score or the T2-mapping parameters with KOOS outcomes.
Safety Results
Adverse events were assessed in the safety population (i.e., patients with cartilage biopsies taken) comprising 102 patients. The most common treatment-related adverse events occurring in more than 1 patient were arthralgia (18 patients, 17.6%), joint effusion (18 patients, 17.6%), joint swelling (10 patients, 9.8%), joint crepitation (5 patients, 4.9%), and muscle atrophy (2 patients, 2.0%). None of the related adverse events were severe and no patient discontinued the study due to an adverse event; 8 patients (7.8%) experienced moderate and 36 patients (35.3%) experienced mild related adverse events. Most of the related adverse events occurred within the first year post treatment, while late events were reported in 3 patients only (1 patient each with arthralgia, joint crepitation, and transplant failure).
Related serious adverse events were seen in 2 patients: one patient with transplant failure due to complete graft delamination and another patient with lateral patellar compression syndrome which was most probably caused by overtightened sutures of the knee joint capsule during transplantation surgery.
Unplanned subsequent surgical interventions (SSIs) were performed in 7 patients (6.9%). The most common adverse event leading to an unplanned SSI was meniscus injury in 4 patients (3.9%); the other events were reported in 1 patient each (arthrofibrosis, chondropathy, knee deformity, tibia fracture, lateral patellar compression syndrome, and transplant failure). Only 2 of these adverse events were considered treatment-related, the above-mentioned transplant failure (related to the product and the surgical procedure) and lateral patellar compression syndrome (related to surgery) which both had recovered after corrective surgery. Overall, the observed pattern of adverse events was fully consistent with the study population and the treatment method used.
Discussion
Cartilage defects (chondral and osteochondral) are a main risk factor for developing OA in both the knee and hip, which, by definition, is irreversible.37-40 The presence of large full-thickness defects (≥2 cm2) increases the risk of total knee arthroplasty,2,3 and it has been suggested that this may be delayed by ACI treatment. 41 The study reported here demonstrated that 2 years after M-ACI treatment of large cartilage lesions (≥ 4 cm2) with NOVOCART® Inject plus, 93% of patients were KOOS responders, that is, improved by ≥10 points compared with their pre-operative level. Thus, the study met its primary endpoint and demonstrated that the KOOS response rate after treatment with NOVOCART® Inject plus is markedly greater than 40% with a lower 95% CI of 86.1, which is more than twice the pre-specified no-effect level. The favorable outcome in terms of KOOS response rate was also consistently reflected by all secondary variables measured.
While the KOOS response criteria are usually based on the minimal clinically important difference (MCID), that is, 8 to 10 points improvement (www.koos.nu), a substantial clinical benefit (SCB) is defined as the clinical value that the patient considers as substantial improvement. However, according to Ogura et al., a considerable improvement is required to achieve SCB rather than MCID after ACI, and only half of the patients reached SCB. 42 A post hoc analysis using the same SCB criteria as reported by Ogura et al. has shown that in the NOVOCART® Inject plus study, 84% of patients achieved SCB of 30 points improvement in the KOOS sports/recreation subscore and 73% of patients achieved SCB of 37.5 points improvement in the KOOS QoL subscore (mean changes from baseline in SCB responders was 62.9 ± 17.9 for KOOS sports/recreation and 59.2 ± 16.7 for KOOS QoL). These KOOS subscores have shown to be most predictive of patient satisfaction in this setting.43,44 Together with the high KOOS responder rates, the changes from baseline in the overall KOOS, and the high corresponding lower CI limits, the observed percentages of patients who reached SCB underlines the clinical relevance of the improvements achieved after treatment with NOVOCART® Inject plus.
The efficacy results of the present study also compare favorably with other ACI studies in patients with similarly large defect sizes. In a phase II study, where patients with a mean defect size of 5.6 cm2 were treated with Spherox® (co.don AG, Germany), the mean absolute KOOS after 2 years was 73.8 points and the mean IKDC score 70.3 points, which corresponded to changes from baseline of 16.6 and 18.7 points, respectively. 45 In comparison, for NOVOCART® Inject plus, absolute values of 82.4 (LS mean change from baseline: 42.0 points) were achieved in the KOOS and 75.8 (LS mean change from baseline: 41.6 points) in the IKDC score. Some differences exist between these studies in terms of patient age (34 years vs. 39.8 years) and defect characteristics (single vs. multiple defects) that disfavor NOVOCART® Inject plus. Furthermore, baseline KOOS was (per inclusion criteria) lower in the NOVOCART® Inject plus study (39.8 vs. 57.0 points) favoring more room for improvement compared with Spherox® but this does not diminish the higher absolute values achieved in these scores. In a phase III study using matrix-associated ACI that employed a collagen type I/III membrane (MACI®; Genzyme) conducted by Saris et al., defect sizes of cartilage lesions in the knee were also similar to those observed in the NOVOCART® Inject plus trial (index lesion size: 4.9 cm2, total defect size: 5.8 cm2). Two years after treatment with MACI®, the absolute IKDC score had improved from 32.9 to 65.7 score points (NOVOCART® Inject plus: improvement from 34.4 to 75.8 points). 46 Although direct comparison of data from different studies is not entirely unproblematic, it nevertheless shows that the KOOS and IKDC results in the NOVOCART® Inject plus trial are well within the upper range reported previously for ACI treatment in large cartilage defects of the knee.
It has been shown by various working groups that failed previous cartilage repair surgeries, such as microfracture, negatively affect clinical outcome when compared with ACI as a first-line treatment.47-50 This was also observed in the NOVOCART® Inject plus study; however, patients who had failed prior cartilage treatment (including 2 patients who had failed cartilage repair twice) still benefited from treatment with NOVOCART® Inject plus as indicated by a mean KOOS change from baseline of 32.8 points and responder rates of 75% in KOOS and IKDC, respectively.
Semi-quantitative and quantitative assessment of the repair tissue by MRI-based MOCART scoring and T2-mapping demonstrated progressive graft maturation and cartilage re-organization from 12 to 24 months. This is consistent with the current literature for the knee joint indicating that complete cartilage graft maturation after ACI requires at least 12 to 24 months.51,52
However, no correlation was observed between MRI data and patient-reported outcome scores which is consistent with numerous systemic review and meta-analysis literature reports.53-55 Follow-up time may be an important parameter in this respect as many studies with a shorter follow-up time of 2 years or less did not find any correlations between MRI parameters and clinical outcome,56-58 whereas such correlations were observed in longer term studies.59-61 Nonetheless, good MRI results at earlier timepoints may be predictive for long-term outcome as demonstrated by McCarthy et al., where MRI parameters assessed at 12 months showed significant correlation with durable longer term outcomes. 14
NOVOCART® Inject can be administered arthroscopically, due to the low-viscosity, injectable nature of the chondrocyte-loaded biomaterial. These properties also result in filling of the smallest defect niches, promoting a homogeneous cell distribution and good ingrowth of regenerated tissue into the defect bed. The adhesive properties of the hydrogel alleviate the need for additional fixation of the implant making it suitable for difficult-to-reach locations, for example, tibia plateau or in the hip as well as avoiding deleterious effects of suture fixation. 62
An extension of the MRI data in our study using gray-level co-occurrence matrix (GLCM) texture analyses of T2 quantitative maps did show that while cartilage tissue adjacent to the repair site is negatively affected by a lesion, treatment with NOVOCART® Inject improved the texture to approximate healthy tissue over time along with maturation of the cartilage implant (Jancova et al. unpublished results). A suitable M-ACI product therefore does not treat cartilage defects in isolation but leads to overall improvement of the joint environment in the sense of OA prevention.
The short cross-linking time of 1 to 3 minutes and the minimally invasive applicability without additional implant fixation also significantly reduce the cut-to-suture time for product implantation. Detrimental structural, biochemical, and metabolic changes of human articular cartilage as well as a significantly increased risk of adverse events (surgical-site infection, sepsis, extended length of hospital stay, and readmission) have been correlated with increased operative times.62,63 As shown in the present study, NOVOCART® Inject implantation requires a mean cut-to-suture time of 39.6 minutes in patients without concomitant surgeries with even shorter times reported elsewhere. 64 No corresponding data on surgery duration have been published for other ACI products. However, for Spherox®, the spheroids alone need about 20 minutes to attach to the subchondral bone plate of the cartilage defect before the wound can be closed (based on the summary of product characteristics).
In about half of the patients in this study, graft implantation was performed by mini-arthrotomy, due most likely to surgeons’ preferences based on similarities of incision lengths and a better view of the surgical site. While the choice of the access procedure (arthroscopic vs. mini-arthrotomy) does not play a major role in terms of the efficacy and safety outcomes, open knee surgeries for ACI are associated with longer hospitalization and a higher complication rate.65,66
The safety profile of NOVOCART® Inject plus observed in the phase III study was consistent with the established safety experience from published studies with other M-ACI products where arthralgia, joint effusion, and joint swelling represented the most commonly reported treatment-related adverse events.45,46,57 Most of these events were attributed to the respective surgical procedure and occurred within the first year post treatment, while late events were rare. Two reoperations, one of which involved the graft and was considered a transplant failure, were performed to address complications in connection with M-ACI treatment. Overall, no safety concerns were identified from the phase III trial, and the safety profile for treatment with NOVOCART® Inject plus is considered favorable.
Limitations
The lack of a control group is certainly a limitation of this study; however, a suitable control group was not available at the time of study initiation. Based on current evidence, an indication for ACI is given for symptomatic cartilage defects starting from defect sizes of more than 2 to 4 cm2, while microfracture and mosaicplasty are still considered a treatment option for smaller defects.4,13 In particular, lesions > 4 cm2 should not be treated with bone marrow stimulating techniques or autologous osteochondral grafting. Therefore, microfracture or mosaicplasty would not have been appropriate comparators for the present trial targeting patients with mid-size to large articular cartilage defects due to ethical concerns as agreed with the European Medicines Agency during scientific advice. The only alternative would have been another ACI product; however, at the time of study initiation, no authorized ACI products were available in Europe that could have served as comparator for NOVOCART® Inject plus in a controlled study and therefore a single-arm design was chosen.
Conclusions
Overall, M-ACI with NOVOCART® Inject plus in the present phase III trial has been shown to be a safe and effective treatment option for patients with large cartilage defects in the knee as demonstrated by clear and consistent results across all investigated efficacy variables at 2 years of follow-up: Statistically significant and clinically meaningful improvements in clinical parameters from the pre-operative status were achieved as early as 3 months after treatment (first time point measured) and were maintained through Month 24. The primary study endpoint was met in a confirmatory manner from Month 3 onward to Month 24. Parallel to the clinical improvements, the MRI analyses were also consistent with an increasing maturation, re-organization, and integration of the repair tissue.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by TETEC—Tissue Engineering Technologies AG.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.T. received payment for central MRI assessment by TETEC AG. C.G., A.Kö., and A.Ki. are employees of TETEC AG. R.S. is an employee of Aesculap Biologics LLC. All other authors received an investigator fee as outlined in the initial clinical trial authorization documents and accepted by the corresponding ethics committees.
Ethical Approval: The trial was approved by the ethics committees responsible for the respective centers and by the local regulatory authorities. The main ethics committee was the Bayerische Landesärztekammer, Germany (No. 17012).
ORCID iD: A. Kirner  https://orcid.org/0000-0001-8687-8284
https://orcid.org/0000-0001-8687-8284
References
- 1. Heir S, Nerhus TK, Rotterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of Knee Injury and Osteoarthritis Outcome Score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231-7. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 2. Sanders TL, Pareek A, Obey MR, Johnson NR, Carey JL, Stuart MJ, et al. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am J Sports Med. 2017;45(8):1799-805. doi: 10.1177/0363546517699846. [DOI] [PubMed] [Google Scholar]
- 3. Everhart JS, Abouljoud MM, Kirven JC, Flanigan DC. Full-thickness cartilage defects are important independent predictive factors for progression to total knee arthroplasty in older adults with minimal to moderate osteoarthritis: data from the osteoarthritis initiative. J Bone Joint Surg Am. 2019;101(1):56-63. doi: 10.2106/JBJS.17.01657. [DOI] [PubMed] [Google Scholar]
- 4. Niemeyer P, Albrecht D, Andereya S, Angele P, Ateschrang A, Aurich M, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23(3):426-35. doi: 10.1016/j.knee.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 5. Khella CM, Asgarian R, Horvath JM, Rolauffs B, Hart ML. An evidence-based systematic review of human knee post-traumatic osteoarthritis (PTOA): timeline of clinical presentation and disease markers, comparison of knee joint PTOA models and early disease implications. Int J Mol Sci. 2021;22(4):1996. doi: 10.3390/ijms22041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390-8. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 7. Yue D, Du L, Zhang B, Wu H, Yang Q, Wang M, et al. Time-dependently appeared microenvironmental changes and mechanism after cartilage or joint damage and the influences on cartilage regeneration. Organogenesis. 2021;17:85-99. doi: 10.1080/15476278.2021.1991199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3786-99. doi: 10.1007/s00167-016-4300-1. [DOI] [PubMed] [Google Scholar]
- 9. Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. 2017;24(3):508-17. doi: 10.1016/j.knee.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 10. Jones KJ, Kelley BV, Arshi A, McAllister DR, Fabricant PD. Comparative effectiveness of cartilage repair with respect to the minimal clinically important difference. Am J Sports Med. 2019;47(13):3284-93. doi: 10.1177/0363546518824552. [DOI] [PubMed] [Google Scholar]
- 11. Zaffagnini S, Boffa A, Andriolo L, Reale D, Busacca M, Di Martino A, et al. Mosaicplasty versus matrix-assisted autologous chondrocyte transplantation for knee cartilage defects: a long-term clinical and imaging evaluation. Appl Sci. 2020;10:4615. [Google Scholar]
- 12. Zamborsky R, Danisovic L. Surgical techniques for knee cartilage repair: an updated large-scale systematic review and network meta-analysis of randomized controlled trials. Arthroscopy. 2020;36(3):845-58. doi: 10.1016/j.arthro.2019.11.096. [DOI] [PubMed] [Google Scholar]
- 13. Biant LC, McNicholas MJ, Sprowson AP, Spalding T. The surgical management of symptomatic articular cartilage defects of the knee: consensus statements from United Kingdom knee surgeons. Knee. 2015;22(5):446-9. doi: 10.1016/j.knee.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 14. McCarthy HS, McCall IW, Williams JM, Mennan C, Dugard MN, Richardson JB, et al. Magnetic resonance imaging parameters at 1 year correlate with clinical outcomes up to 17 years after autologous chondrocyte implantation. Orthop J Sports Med. 2018;6(8). doi: 10.1177/2325967118788280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-91. doi: 10.1016/j.joca.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 16. Bretschneider H, Trattnig S, Landgraeber S, Hartmann A, Günther K-P, Dienst M, et al. Arthroscopic matrix-associated, injectable autologous chondrocyte transplantation of the hip: significant improvement in patient-related outcome and good transplant quality in MRI assessment. Knee Surg Sports Traumatol Arthrosc. 2020;28:1317-24. doi: 10.1007/s00167-019-05466-7. [DOI] [PubMed] [Google Scholar]
- 17. Benz K, Freudigmann C, Müller J, Wurst H, Albrecht D, Badke A, et al. A polyethylene glycol-crosslinked serum albumin/hyaluronan hydrogel for the cultivation of chondrogenic cell types. Adv Eng Mater. 2010;12(9):B535-51. [Google Scholar]
- 18. Scholz B, Kinzelmann C, Benz K, Mollenhauer J, Wurst H, Schlosshauer B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur Cell Mater. 2010;20:24-36; discussion 36-7. [DOI] [PubMed] [Google Scholar]
- 19. Ackermann J, Merkely G, Mestriner AB, Shah N, Gomoll AH. Increased chondrocytic gene expression is associated with improved repair tissue quality and graft survival in patients after autologous chondrocyte implantation. Am J Sports Med. 2019;47(12):2919-26. doi: 10.1177/0363546519868213. [DOI] [PubMed] [Google Scholar]
- 20. Hirschmüller A, Baur H, Braun S, Kreuz PC, Suedkamp NP, Niemeyer P. Rehabilitation after autologous chondrocyte implantation for isolated cartilage defects of the knee. Am J Sports Med. 2011;39:2686-96. doi: 10.1177/0363546511404204. [DOI] [PubMed] [Google Scholar]
- 21. Mithoefer K, Saris DBF, Farr J, Kon E, Zaslav K, Cole BJ, et al. Guidelines for the design and conduct of clinical studies in knee articular cartilage repair: international cartilage repair society recommendations based on current scientific evidence and standards of clinical care. Cartilage. 2011;2(2):100-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roos EM, Lohmander LS. The Knee Injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Vet HC, Ostelo RW, Terwee CB, van der Roer N, Knol DL, Beckerman H, et al. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16(1):131-42. doi: 10.1007/s11136-006-9109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600-13. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 25. Roos EM, Engelhart L, Ranstam J, Anderson AF, Irrgang JJ, Marx RG, et al. ICRS recommendation document: patient-reported outcome instruments for use in patients with articular cartilage defects. Cartilage. 2011;2(2):122-36. doi: 10.1177/1947603510391084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ludwig K, Graf von der Schulenburg J-M, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663-74. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 29. Trattnig S, Millington SA, Szomolanyi P, Marlovits S. MR imaging of osteochondral grafts and autologous chondrocyte implantation. Eur Radiol. 2007;17(1):103-18. doi: 10.1007/s00330-006-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Domayer SE, Welsch GH, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P, et al. MRI monitoring of cartilage repair in the knee: a review. Semin Musculoskelet Radiol. 2008;12(4):302-17. doi: 10.1055/s-0028-1100638. [DOI] [PubMed] [Google Scholar]
- 31. Domayer SE, Welsch GH, Nehrer S, Chiari C, Dorotka R, Szomolanyi P, et al. T2-mapping and dGEMRIC after autologous chondrocyte implantation with a fibrin-based scaffold in the knee: preliminary results. Eur J Radiol. 2010;73(3):636-42. doi: 10.1016/j.ejrad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 32. Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology. 2008;247(1):154-61. doi: 10.1148/radiol.2471070688. [DOI] [PubMed] [Google Scholar]
- 33. Welsch GH, Mamisch TC, Quirbach S, Zak L, Marlovits S, Trattnig S. Evaluation and comparison of cartilage repair tissue of the patella and medial femoral condyle by using morphological MRI and biochemical zonal T2 mapping. Eur Radiol. 2009;19(5):1253-62. doi: 10.1007/s00330-008-1249-6. [DOI] [PubMed] [Google Scholar]
- 34. Welsch GH, Trattnig S, Domayer S, Marlovits S, White LM, Mamisch TC. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis Cartilage. 2009;17(9):1219-27. [DOI] [PubMed] [Google Scholar]
- 35. Welsch GH, Zak L, Mamisch TC, Resinger C, Marlovits S, Trattnig S. Three-dimensional Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score assessed with an isotropic three-dimensional true fast imaging with steady-state precession sequence at 3.0 Tesla. Invest Radiol. 2009;44(9):603-12. doi: 10.1097/RLI.0b013e3181b5333c. [DOI] [PubMed] [Google Scholar]
- 36. Kurkijärvi JE, Mattila L, Ojala RO, Vasara AI, Jurvelin JS, Kiviranta I, et al. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthritis Cartilage. 2007;15(4):372-8. doi: 10.1016/j.joca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 37. Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52(7):2033-9. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 38. Hafezi-Nejad N, Zikria B, Eng J, Carrino JA, Demehri S. Predictive value of semi-quantitative MRI-based scoring systems for future knee replacement: data from the osteoarthritis initiative. Skeletal Radiol. 2015;44(11):1655-62. doi: 10.1007/s00256-015-2217-2. [DOI] [PubMed] [Google Scholar]
- 39. Jungmann PM, Kraus MS, Nardo L, Liebl H, Alizai H, Joseph GB, et al. T(2) relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: longitudinal data from the osteoarthritis initiative. J Magn Reson Imaging. 2013;38(6):1415-24. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dwyer MK, Tumpowsky C, Boone A, Lee J, McCarthy JC. What is the association between articular cartilage damage and subsequent THA 20 years after hip arthroscopy for labral tears? Clin Orthop Relat Res. 2019;477(5):1211-20. doi: 10.1097/CORR.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Windt TS, de Vonk LA, Brittberg M, Saris DB. Treatment and prevention of (early) osteoarthritis using articular cartilage repair-fact or fiction? A systematic review. Cartilage. 2013;4(Suppl 3):5S-12S. doi: 10.1177/1947603513486560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogura T, Ackermann J, Barbieri Mestriner A, Merkely G, Gomoll AH. Minimal clinically important differences and substantial clinical benefit in patient-reported outcome measures after autologous chondrocyte implantation. Cartilage. 2020;11:412-22. doi: 10.1177/1947603518799839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ebert JR, Smith A, Wood DJ, Ackland TR. A comparison of the responsiveness of 4 commonly used patient-reported outcome instruments at 5 years after matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2013;41(12):2791-9. doi: 10.1177/0363546513502314. [DOI] [PubMed] [Google Scholar]
- 44. Faber S, Seiferth N, Angele P, Spahn G, Buhs M, Zinser W, et al. Factors correlating with patients’ satisfaction after undergoing cartilage repair surgery-data from the German Cartilage Registry (KnorpelRegister DGOU). Int Orthop. 2022;46:457-64. doi: 10.1186/s13018-020-01668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niemeyer P, Laute V, Zinser W, John T, Becher C, Diehl P, et al. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1130-43. doi: 10.1007/s00167-019-05786-8. [DOI] [PubMed] [Google Scholar]
- 46. Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42(6):1384-94. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 47. Lamplot JD, Schafer KA, Matava MJ. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sports Med. 2018;6(3). doi: 10.1177/2325967118761871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-8. [DOI] [PubMed] [Google Scholar]
- 49. Schuette HB, Kraeutler MJ, Schrock JB, McCarty EC. Primary autologous chondrocyte implantation of the knee versus autologous chondrocyte implantation after failed marrow stimulation: a systematic review. Am J Sports Med. 2021;49:2536-41. doi: 10.1177/0363546520968284. [DOI] [PubMed] [Google Scholar]
- 50. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325-31. doi: 10.1177/0363546511425651. [DOI] [PubMed] [Google Scholar]
- 51. Marlovits S, Zeller P, Singer P, Resinger C, Vécsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57(1):24-31. [DOI] [PubMed] [Google Scholar]
- 52. Niethammer TR, Safi E, Ficklscherer A, Horng A, Feist M, Feist-Pagenstert I, et al. Graft maturation of autologous chondrocyte implantation: magnetic resonance investigation with T2 mapping. Am J Sports Med. 2014;42(9):2199-204. doi: 10.1177/0363546514538756. [DOI] [PubMed] [Google Scholar]
- 53. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41(6):1426-34. doi: 10.1177/0363546513485931. [DOI] [PubMed] [Google Scholar]
- 54. de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695-702. doi: 10.1177/0363546512473258. [DOI] [PubMed] [Google Scholar]
- 55. Lansdown DA, Wang K, Cotter E, Davey A, Cole BJ. Relationship between quantitative MRI biomarkers and patient-reported outcome measures after cartilage repair surgery: a systematic review. Orthop J Sports Med. 2018;6(4). doi: 10.1177/2325967118765448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Henderson IJP, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-6. doi: 10.1302/0301-620x.85b7.13782. [DOI] [PubMed] [Google Scholar]
- 57. Niemeyer P, Laute V, Zinser W, Becher C, Kolombe T, Fay J, et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7(7). doi: 10.1177/2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ebert JR, Smith A, Fallon M, Wood DJ, Ackland TR. Correlation between clinical and radiological outcomes after matrix-induced autologous chondrocyte implantation in the femoral condyles. Am J Sports Med. 2014;42(8):1857-64. doi: 10.1177/0363546514534942. [DOI] [PubMed] [Google Scholar]
- 59. Kreuz PC, Müller S, von Keudell A, Tischer T, Kaps C, Niemeyer P, et al. Influence of sex on the outcome of autologous chondrocyte implantation in chondral defects of the knee. Am J Sports Med. 2013;41(7):1541-8. doi: 10.1177/0363546513489262. [DOI] [PubMed] [Google Scholar]
- 60. Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668-75. doi: 10.1177/0363546511404675. [DOI] [PubMed] [Google Scholar]
- 61. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23(4):381-7. doi: 10.1016/j.arthro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 62. Shen P, Li X, Xie G, Huangfu X, Zhao J. Time-dependent effects of arthroscopic conditions on human articular cartilage: an in vivo study. Arthroscopy. 2016;32(12):2582-91. doi: 10.1016/j.arthro.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 63. Gowd AK, Liu JN, Bohl DD, Agarwalla A, Cabarcas BC, Manderle BJ, et al. Operative Time as an independent and modifiable risk factor for short-term complications after knee arthroscopy. Arthroscopy. 2019;35(7):2089-98. doi: 10.1016/j.arthro.2019.01.059. [DOI] [PubMed] [Google Scholar]
- 64. Schlumberger M, Schuster P, Bulow HJ, Mayer P, Eichinger M, Richter J. Arthroscopic autologous chondrocyte implantation in the knee with an in situ crosslinking matrix: minimum 4-year clinical results of 15 cases and 1 histological evaluation. Arch Orthop Trauma Surg. 2019;139(11):1607-15. doi: 10.1007/s00402-019-03243-2. [DOI] [PubMed] [Google Scholar]
- 65. Migliorini F, Eschweiler J, Spiezia F, van de Wall BJM, Knobe M, Tingart M, et al. Arthroscopy versus mini-arthrotomy approach for matrix-induced autologous chondrocyte implantation in the knee: a systematic review. J Orthop Traumatol. 2021;22(1):23. doi: 10.1186/s10195-021-00588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Edwards PK, Ebert JR, Janes GC, Wood D, Fallon M, Ackland T. Arthroscopic versus open matrix-induced autologous chondrocyte implantation: results and implications for rehabilitation. J Sport Rehabil. 2014;23(3):203-15. doi: 10.1123/jsr.2013-0042. [DOI] [PubMed] [Google Scholar]