Abstract
Haemophilia B is a rare X-linked genetic deficiency of coagulation factor IX (FIX) that, if untreated, can cause recurrent and disabling bleeding, potentially leading to severe arthropathy and/or life-threatening haemorrhage. Recent decades have brought significant improvements in haemophilia B management, including the advent of recombinant FIX and extended half-life FIX. This therapeutic landscape continues to evolve with several non-factor replacement therapies and gene therapies under investigation. Given the rarity of haemophilia B, the evidence base and clinical experience on which to establish clinical guidelines are relatively sparse and are further challenged by features that are distinct from haemophilia A, precluding extrapolation of existing haemophilia A guidelines. Due to the paucity of formal haemophilia B-specific clinical guidance, an international Author Group was convened to develop a clinical practice framework. The group comprised 15 haematology specialists from Europe, Australia, Japan, Latin America and North America, covering adult and paediatric haematology, laboratory medicine and biomedical science. A hybrid approach combining a systematic review of haemophilia B literature with discussion of clinical experience utilized a modified Delphi format to develop a comprehensive set of clinical recommendations. This approach resulted in 29 recommendations for the clinical management of haemophilia B across five topics, including product treatment choice, therapeutic agent laboratory monitoring, pharmacokinetics considerations, inhibitor management and preparing for gene therapy. It is anticipated that this clinical practice framework will complement existing guidelines in the management of people with haemophilia B in routine clinical practice and could be adapted and applied across different regions and countries.
Keywords: consensus, Delphi, guidance, haemophilia B, management, recommendations
Introduction
Haemophilia B is a rare X-linked genetic deficiency of coagulation factor IX (FIX) resulting from mutations in the F9 gene, with a prevalence at birth of 5 cases per 100,000 males. 1 The severity of the disease (mild, moderate or severe) has traditionally been classified according to plasma FIX levels.1,2 If left untreated, severe and some moderate haemophilia B may cause disabling and recurrent bleeding, leading to severe arthropathy. 3 The condition is associated with short- and long-term effects on physical functioning and significantly impaired quality of life (QoL), 4 as well as direct and indirect treatment costs.5–7
Haemophilia B is treated using replacement, intravenous FIX concentrates to elevate plasma FIX levels. Exogenous FIX replacement therapy, including recombinant factor IX (rFIX) and plasma-derived factor IX (pdFIX), can be administered as prophylaxis or on-demand, in addition to perioperative settings. As prophylaxis has the potential to prevent bleeding and provides superior benefits to on-demand therapy, it is endorsed as the standard of care for severe haemophilia B and non-severe haemophilia B with a severe phenotype. 1 However, prophylaxis requires frequent venous access and frequent FIX administrations, which could result in increased costs compared with on-demand treatment.8,9
Based on the plasma half-life of pdFIX and standard half-life (SHL) rFIX, these replacement concentrates usually require an infusion frequency of at least twice a week to maintain plasma FIX levels above the traditional prophylaxis trough level (>1 IU/dl). This confers significant treatment burden that may negatively impact adherence and clinical outcomes.10,11 By modifying the pharmacokinetic (PK) properties of rFIX, the plasma half-life and therefore prophylactic dosing interval can be extended. A potentially important consideration in directing treatment based solely on the plasma levels of administered FIX is the difference in distribution in the intra- and extravascular components for FIX [compared with factor VIII (FVIII)] and between different FIX therapeutic agents in people with haemophilia B (PwHB).12,13
Despite the advantages offered with the use of prophylaxis and the development of extended half-life (EHL) therapies for haemophilia B, it is recognized that patients may still experience subclinical bleeds and joint damage even with prophylactic regimens, 14 and according to most reports, 1.5–10% of patients develop inhibitors against their FIX replacement therapy.15,16 The management of haemophilia B continues to evolve, with several non-factor replacement therapies under investigation, as well as gene therapy (GT). GT is the ultimate FIX replacement therapy for treatment of people with severe haemophilia B. By adding a functional version of the defective gene in situ, GT aims to deliver endogenous expression of functional FIX and ameliorate the disease phenotype. 17 Encouraging but still limited preliminary safety and efficacy evidence from haemophilia B GT trials shows great promise for this potentially one-time treatment option.17,18
Owing to its rarer status, the evidence base and clinical experience of haemophilia B is relatively sparse, and as a result, formal clinical guidance focused specifically on the condition is lacking. An international group of authors was convened in 2020 to develop a set of consensus recommendations for the management of PwHB using a modified Delphi format. The author group utilized a hybrid approach combining a systematic literature review with discussion of clinical experience to inform the development of the recommendations. These recommendations provide a clinical practice framework for the management of PwHB in routine clinical practice based on the published evidence and clinical experience, in conjunction with published guidelines. It is hoped that these recommendations will complement existing haemophilia guidelines and could be adapted and applied across different regions and countries.
Methodology
Haemophilia B modified Delphi steering committee
The international author group was convened in July 2020, comprising 15 specialists in the field of haematology with expertise in treating children and adults with haemophilia B, molecular biology and biomedical science. Together the authors had a global representation, including 12 countries: Australia, Europe (the United Kingdom, Belgium, France, Germany, Spain and Sweden), Japan, Latin America (Brazil and Colombia) and North America (Canada and the United States). Based on gaps in currently available guidance for the management of haemophilia B, the author group identified five key topics on which to develop clinical practice recommendations:
Topic 1: Factor product choice, switching and clinical indications
Topic 2: Specific therapeutic agent laboratory monitoring considerations
Topic 3: PK considerations – modelling, predictions and dose optimization
Topic 4: Inhibitor management and preparing for novel agents
Topic 5: Preparing for gene therapy
A sixth topic on life stage and global treatment goals was considered initially but returned many non-specific publications during the systematic literature search. Given the broad literature base identified, it was agreed that this topic would not be evaluated further in the recommendations at this time.
Development of evidence-based recommendations
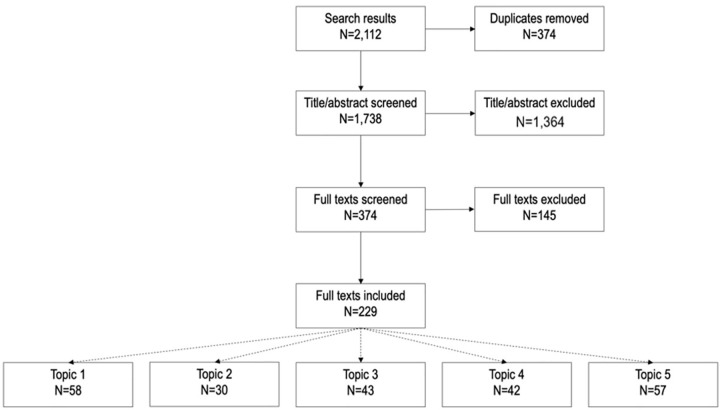
Each topic was assigned either two or three author group members, forming a ‘Topic Group’ to lead the initial development of the consensus recommendations. An extensive literature search (Figure 1 and Supplementary Information 1) was undertaken and a narrative synthesis report, supported by data extraction tables, was used to summarize the evidence base for each topic. In total, 1738 articles were screened and 229 were selected and included in the data analysis (Figure 1). Topic Groups considered the evidence base before discussing and developing a draft set of recommendations for their topic, which was then shared with the full author group to consider alongside the narrative reports of the available published evidence.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow for data selection for development of topic-specific literature reports.
Publications could be included in more than one topic report.
Voting process
The complete author group evaluated the recommendations in two voting rounds. The first round of voting was conducted via an anonymous online survey in which the author group was permitted to select which recommendations would be taken forward and could propose changes to the wording of the recommendations. The second round of voting was conducted during a live meeting and utilized a revised set of recommendations reflecting any proposed changes. The author group discussed and further refined the wording of recommendations before an anonymous vote was taken to determine the level of agreement.
A Delphi approach was used to reach consensus on the proposed recommendations. Individual authors assigned each recommendation a score between 1 (lowest) and 9 (highest), and scores were collated into one of three ranges: 1–3, 4–6 and 7–9. The percentage of individuals scoring within the 7–9 range indicated the level of agreement. Consensus was reached when ⩾75% of individuals had assigned a score of 7–9 for a given recommendation.
Results
A total of 33 recommendations were developed and evaluated in the first round of online voting. All 33 recommendations were retained for further consideration. During the second round of live voting, consensus was reached on 29 recommendations: five for topic 1, five for topic 2, two for topic 3, eight for topic 4 and nine for topic 5 while four recommendations were eliminated (Figure 2 and Supplementary Information 2).
Figure 2.
Flow chart of the modified Delphi process used to reach consensus for the haemophilia B clinical framework.
The two rounds of voting and number of recommendations that were voted on are depicted centrally, with the summary numbers of outcomes introduced or eliminated; the right side of the figure reports the decisional rules and the numbers of outcomes agreed upon by round, and indicates into which topics each recommendation fell.
Recommendations for the management of PwHB in routine clinical practice
Discussion pertaining to the recommended use of approved treatments assumes that users will understand/abide by local licensing regulations and follow guidance on dosing, administration and laboratory requirements.
rFIX and EHL-FIX have significantly improved haemophilia B management in the last 30 years. To avoid joint disease – including minor damage and microbleeds – primary prophylaxis with FIX replacement therapy is recommended as first-line treatment in PwHB with severe disease, 1 and this should be initiated early to prevent overt bleeds and progression to haemophilic arthropathy. Authors highlighted that the decision to initiate prophylaxis is usually driven by FIX assay levels but were also mindful of the limitations of laboratory measurement accuracy at the lowest levels of detection around 1 IU/dl. 19 Physicians should also be aware of the limitations of defining haemophilia B severity based solely on baseline FIX level or bleed phenotype in isolation. For example, a single trauma-related bleed may not accurately reflect a severe phenotype, and similarly, PwHB with the same baseline FIX level can present with varying severity and frequency of bleeding (Table 1). 20
Table 1.
Topic 1 Consensus recommendations.
| Topic 1: Factor product choice, switching and clinical indications | |
|---|---|
| 1 | Prophylaxis with FIX should be considered in all people with severe haemophilia B (including those classified as non-severe according to their basal FIX levels but with a severe bleeding phenotype); in these PwHB, prophylaxis should be initiated as early as possible (i.e. prior to the onset of joint bleeding), and thereafter, treatment should not be interrupted |
| 2 | Both SHL-FIX and EHL-rFIX are effective treatment options for prophylaxis in PwHB |
| 3 | Either SHL-FIX or EHL-FIX products can be used to offer adequate haemostatic cover for bleeds, surgery and invasive procedures; when using EHLs, laboratory requirements for product-specific monitoring should be considered |
| 4 | When choosing a product or considering switching to alternative products, venous access, adherence, bleeding phenotype, lifestyle, patient preference and PK should be considered in the context of local licensing and approval status |
| 5 | Dose and frequency of prophylactic FIX treatment should be adapted to the clinical phenotype (e.g. bleed rates) and lifestyle considerations, and not based exclusively on plasma trough levels |
EHL-rFIX, extended half-life–recombinant factor IX; FIX, factor IX; PK, pharmacokinetic; PwHB, people with haemophilia B; SHL, standard half-life.
Table 2 shows that the approved EHL-rFIX and SHL agents, including both pdFIX and rFIX, are effective treatments for PwHB. Currently, no head-to-head clinical trials in PwHB have directly compared SHL-FIX and EHL-FIX using clinically relevant endpoints such as annualized bleeding rate (ABR), annualized joint bleed rates and progression of arthropathy. However, several indirect comparisons have been carried out and indicate favourable efficacy and reduced factor consumption for EHL products compared with SHL products.21–23 A number of factors differ between randomized controlled trials, real world and other study types, including participants and adherence to treatment; therefore comparison of data across these data sources should be interpreted with caution.
Table 2.
Clinical data for approved FIX replacement therapies for the treatment of haemophilia B..
| Phase I/II data for approved FIX replacement therapies for the treatment of haemophilia B | ||||
|---|---|---|---|---|
| Reference or trial NCT | Product/dose(s) | Study characteristics | PK findings | Safety |
| SHL-rFIX | ||||
| Roth et al.
24
N = 56 previously treated PwHB |
Nonacog alfa (BeneFIX®, Pfizer) A baseline PK evaluation was performed on first infusion with rFIX using a single 50-IU/kg intravenous dose |
A 20-centre international prospective, controlled, multicentre study evaluating PK, efficacy, safety and immunogenicity in previously treated PwHB | Mean incremental rFIX recovery was 0.75 IU/dl per IU/kg, 30% lower than expected for pdFIX, although the mean half-life was similar; PK parameters were stable over time 31 participants (57%) increased their dose and 23 (43%) had no change |
• Inhibitor development in 1/56 (low titre, transient) • Thromboembolic events: 1/56 • Allergic reactions: 4/56 • Serious TEAE: 0/56 • TEAE (any): 9/56 (55 events) |
| Windyga et al.
25
NCT01174446 N = 73 for PK analysis of whom N = 14 received on-demand treatment during the prophylaxis/treatment phase |
Nonacog gamma (Rixubis®, Shire) Primary outcome = AUC (0–72 h); equivalence with nonacog alfa (75 ± 5 IU/kg single dose) |
Randomized, crossover phase I/III, prospective, controlled (PK), multicentre study evaluating PK, efficacy, safety and immunogenicity in previously treated patients with severe or moderately severe haemophilia B | PK equivalence (n = 28) between rFIX (nonacog gamma) and a licensed rFIX was confirmed in terms of the ratio of geometric mean AUC (0–72 h) per dose; twice-weekly prophylaxis [mean duration 6.2 (±0.7) months, 1.8 (±0.1) infusions per week, 49.5 (±4.8) IU kg(–1) per infusion] was effective in preventing bleeding episodes, with a significantly lower (79%, p < 0.001) annualized bleed rate (4.2) compared with an on-demand treatment in a historical control group (20.0); 24 of 56 participants on prophylaxis (43%) did not bleed throughout the study observation period | • Inhibitor development: 0/73 • Thromboembolic events: 0/73 • Allergic reactions: 0/73 • Serious TEAE: 0/73 • TEAE (any): 37/73 (90 events) |
| Collins et al.
26
NCT00768287 PK (original crossover): N = 32 previously treated PwHB Repeat PK: N = 14 previously treated PwHB Safety: N = 76 previously treated PwHB |
Trenonacog alfa (IXinity®, Emergent BioSolutions)
a
An initial PK crossover comparison with nonacog alfa was performed on first infusion with rFIX using 75 IU/kg intravenous dose and patients could continue into an open-label study; PwHB on prophylaxis received a of 50–75 IU/kg dose twice weekly and patients receiving on-demand treatment received a 50–100 IU/kg dose depending on the severity of the bleeding episode |
Randomized, multicentre crossover comparison PK arm of a phase II/III study | The lower bound 90% CI for ratio of AUC0-∝ for IB1001/nonacog alfa was 0.89 demonstrating that the primary PK endpoint criterion of >0.80 was met; other PK parameters between the two rFIX products were comparable (Table S1); the repeat PK analysis demonstrated the stability of initial PK parameters during long-term exposure (median: 5.8 months; minimum, maximum: 3.1, 18 months) to IB1001 (Table S1) | • FIX inhibitor development in 0/74 • Thromboembolic events: 0/74 • Allergic reactions: 0/74 • Serious TEAE: 4/74 • TEAE (any): 60/74 (215 events) |
| EHL-rFIX | ||||
| Shapiro et al.
27
NCT00716716 N = 14 previously treated PwHB |
Eftrenonacog alfa (Alprolix® Sobi/Sanofi) 1 PwHB each received 1, 5, 12.5 or 25 IU/kg, and 5 participants each received 50 or 100 IU/kg |
Open-label, dose-escalation trial in previously treated PwHB | Dose proportional increases in activity and Ag exposure were observed; with baseline subtraction, mean activity terminal t1/2 and mean residence time was 56.7 and 71.8 h, respectively; the incremental recovery was 0.93 IU/dl per IU/kg, like plasma-derived FIX | • Inhibitor development in 0/14 • Thromboembolic events: 0/14 • Allergic reactions: 0/14 • Serious TEAE: 2/14 • TEAE (any): 7/14 (16 events) |
| Negrier et al.
28
NCT00956345 N = 16 previously treated PwHB |
Nonacog beta pegol (Refixia®/Rebinyn®, Novo Nordisk) Each PwHB received one dose of their previous FIX product followed by one dose of N9-GP at the same dose level (25, 50 or 100 U/kg) |
A multicentre, multinational, open-label, dose-escalation trial evaluating safety and PK of ascending intravenous doses of nonacog beta pegol in PwHB | The half-life was 93 h, which was five times higher than the patient’s previous product; the incremental recovery of N9-GP was 94% and 20% higher compared with recombinant and plasma-derived products, respectively; these results indicate that N9-GP has the potential to reduce dosing frequency while providing effective treatment of bleeding episodes with a single dose | • Inhibitor development in 0/16 • Thromboembolic events: 0/16 • Allergic reactions: 1/16, transient hypersensitivity • Serious TEAE: 1/16 • TEAE (any): 6/16 (11 events) |
| Santagostino et al.
29
NCT01233440 N = 25 previously treated PwHB |
Albutrepenonacog alfa (Idelvion®, CSL Behring) 25, 50 and 75 IU/kg |
An open-label, multicentre, dose-escalation safety and PK study of albutrepenonacog in PwHB | After 25 or 50 IU/kg albutrepenonacog alfa administration, the baseline-corrected mean FIX activity remained elevated at day 7 (7.4 and 13.4 IU/dl, respectively) and day 14 (2.5 and 5.5 IU/dl, respectively); the incremental recovery was higher than both recombinant and plasma-derived FIX (1.4 versus 0.95 and 1.1 IU/dl per IU/kg, respectively); in the 50-IU/kg cohort (13 participants), the mean half-life was 92 h | • Inhibitor development in 0/25 • Thromboembolic events: 0/13 • Allergic reactions: 0/25 • Serious TEAE: 0/25 • TEAE (any): 13/25 (22 events) |
| Phase II/III and comparative data for approved FIX replacement therapies for the treatment of haemophilia B | |||
|---|---|---|---|
| Reference or NCT number | Product/dose | Safety | Efficacy |
| SHL-rFIX | |||
| Roth et al.
24
N = 56 previously treated PwHB |
Nonacog alfa (BeneFIX®, Pfizer) A baseline PK evaluation was performed on first infusion with rFIX using a single 50 IU/kg intravenous dose |
• Inhibitor development in 1/56 (low titre, transient) • Thromboembolic events: 1/56 • Allergic reactions: 4/56 • Serious TEAE: 0/56 • TEAE (any): 9/56 (55 events) |
Treatment for haemorrhages • On-demand: 55/56 (2758 rFIX infusions for 1796 haemorrhages) The majority of haemorrhages (90.9%) had an ‘excellent’ or ‘good’ response to rFIX treatment |
| Collins et al.
26
NCT00768287 N = 76 previously treated PwHB |
Trenonacog alfa (Ixinity®, Emergent BioSolutions)
a
An initial PK crossover comparison with nonacog alfa was performed on first infusion with rFIX using 75 IU/kg intravenous dose and patients could continue into an open-label study; PwHB on prophylaxis received a 50–75 IU/kg dose twice weekly and patients receiving on-demand treatment received a 50–100 IU/kg dose depending on the severity of the bleeding episode |
• Inhibitor development in 0/76 • Thromboembolic events: 0/76 • Allergic reactions: 0/76 • Serious TEAE: 0/76 • TEAE (any): 57/76 (444 events) of which 15 events in 7/57 participants were considered to be related to treatment |
• ABR on-demand, median (IQR): 16.10 (6.60–71.8) • ABR prophylaxis: median (IQR): 3.55 (0.00–3.46) • 19% of PwHB had zero bleeds in the placebo arm, compared with none in the on-demand arm |
| Windyga et al.25,30
N = 73 for PK analysis of whom N = 14 received on-demand treatment during the prophylaxis/treatment phase |
Nonacog gamma (Rixubis®, Shire) 75 ± 5 IU/kg single or repeat dose |
• Inhibitor development in 0/14 • Thromboembolic events: 0/14 • Allergic reactions: 0/14 • Serious TEAE: 0/14 • TEAE (any): 6/14 (14 events, unrelated to treatment) |
Haemostatic efficacy • Haemostatic FIX levels were achieved peri- and postoperatively (14/14) • Haemostasis was ‘excellent’ intraoperatively in all patients and postoperatively in those without a drain, and ‘excellent’ or ‘good’ at the time of drain removal and day of discharge in those with a drain employed • Following the initial dose, the mean FIX activity level rose from 6.55% to 107.58% for major surgeries and from 3.60% to 81.4% for minor surgeries Actual versus predicted blood loss matched predicted intraoperative blood loss but was equal to or higher than (but less than 150%) the maximum predicted postoperative blood loss reflecting the severity of procedure and FIX requirements |
| EHL-rFIX | |||
| Collins et al.
31
(n = 74) NCT01333111 Phase III, multinational, randomized (prophylaxis groups only), single-blind study |
Nonacog beta pegol (Refixia®/Rebinyn®, Novo Nordisk) 10 IU/kg (n = 30) or 40 IU/kg (n = 29) once weekly |
• FIX inhibitor development in 0/74 • Thromboembolic events: 0/74 • Allergic reactions: 0/74 • Serious TEAE: 4/74 • TEAE (any): 60/74 (215 events) |
|
| • ABR on-demand, median (IQR): 15.58 (9.56–26.47) • ABR prophylaxis (10 IU/kg): median (IQR): 2.93 (0.99–6.02) • ABR prophylaxis (40 IU/kg) median (IQR): 1.04 (0.00–4.00) • 7% of PwHB did not need treatment for bleeding in the on-demand arm • 17% of PwHB did not need treatment for bleeding in the prophylaxis (10 IU/kg) arm • 45% of PwHB did not need treatment for bleeding in the prophylaxis (40 IU/kg) arm |
Powell et al.
21
(n = 119) NCT01027364 Phase III, non-randomized, open-label study |
Eftrenonacog alfa (Alprolix®, Sobi/Sanofi) Group 1: weekly prophylaxis with 50 IU/kg to start, with the dose adjusted as needed; Group 2: interval adjusted prophylaxis with 100 IU/kg at intervals of 10 days to start, with the interval adjusted as needed; Group 3: on-demand treatment of 20–100 IU/kg, with the dose adjusted according to bleeding severity; Group 4: as perioperative care |
• FIX inhibitor development in 0/119 • Thromboembolic events: 0/119 • Allergic reactions: 0/119 • Serious TEAE: 13/119 (one possibly related to eftrenonacog alfa treatment) • TEAE (any): 88/119 |
| • ABR on-demand, mean (±95% CI): 18.67 (14.01–24.89) • ABR prophylaxis (Group 1): mean (±95% CI): 3.12 (2.46–3.95) • ABR prophylaxis (Group 2): mean ± 95% CI: 2.40 (1.67–3.47) • 23% of group 1 had 0 bleeds during the study period • 42.3% of group 2 had 0 bleeds during the study period |
Santagostino et al.
32
(n = 63, 23 on-demand and 40 prophylaxis) Prospective, non-randomized, multinational, open-label phase III study |
Albutrepenonacog alfa (Idelvion®, CSL Behring) 40 patients completed at least 26 weeks on 7-day prophylaxis with a median dose of 40 IU/kg, then 28 (70%) patients switched to the 14-day prophylaxis regimen with 75 IU/kg (21 patients) or to the 10-day regimen with 75 IU/kg (7 patients) |
• FIX inhibitor development in 0/63 • Thromboembolic events: 0/63 • Allergic reactions: 0/63 • Serious TEAE: 2/63 • TEAE (any): 54/63 (347 events) |
| • ABR on-demand, estimated (±95% CI): 13.62 (11.001, 16.868) • ABR prophylaxis, estimated (±95% CI): 0.55 (0.233, 1.322) • 45% of PwHB did not need treatment for bleeding in the prophylaxis group |
|||
ABR, annualized bleeding rate; AUC, area under the curve; CI, confidence interval; EHL, extended half-life; FDA, US Food and Drug Administration; FIX, factor IX; IQR, interquartile range; pdFIX, plasma-derived FIX; NCT, National Clinical Trial; PK, pharmacokinetic; PwHB, people with haemophilia B; rFIX, recombinant FIX; SHL, standard half-life; TEAE, treatment emergent adverse event.
Only licensed for use in the United States (FDA).
Owing to the range of different modified and unmodified FIX therapies, and their variable impact on extravascular distributions, authors stressed that target plasma trough levels may be an oversimplified treatment goal for prophylactic FIX products. 12 Therefore, clinicians should have a thorough understanding of the characteristics of different treatment options as indicated in the product prescribing information (PI)/summary of product characteristics (SmPC), potentially using additional endpoints such as ABR and/or QoL. It would be ideal to evaluate EHL treatments in head-to-head studies, comparing EHL with both SHL and other EHL agents, and reporting both clinical and patient-focused outcomes.
Treatment choice should be a shared decision between the PwHB and physician/nurse prescriber after multidisciplinary discussion, considering the preference of the PwHB (including convenience) and the impact on their QoL. In this respect, PwHB should be informed on differences between different FIX replacement products and how these may affect clinical and patient-relevant outcomes. Several studies that evaluated disease burden, patient perspectives, patient values and resource utilization have revealed that reduced treatment/administrative burden associated with EHL was important to patients and carers,33–35 offering an opportunity to improve adherence.34,35 While treatment choices may impact adherence to treatment for PwHB, adherence is a multifactorial construct that is determined by a number of other features (e.g. socio-economic, patient-related, condition-related, health care system, treatment-related aspects), and factors that still remain to be elucidated. 36
One stage assays (OSA) may use numerous different activated partial thromboplastin time (aPTT) reagents, which can differ in activator type, phospholipid composition and potency estimates between manufacturers, in turn leading to variability in the observed FIX activity. 37 Chromogenic substrate assays (CSA) have fewer reagent choices and demonstrate less variability in the measurement of FIX activity. Kihlberg et al. 38 found that although a third of people with non-severe haemophilia B showed a twofold or greater difference between the results of the OSA and CSA, with CSA presenting the higher value, this difference was not observed in people with severe haemophilia B. Another study showed a discrepancy between OSA and CSA in 17% of patients, this time with all discrepancies demonstrating at least twofold higher FIX activity levels with OSA compared with the CSA, and misclassification of disease severity in 90% of these cases. 39 GT such as FIX-Padua GT also demonstrates variability in the results between CSA and OSA, with CSA returning lower FIX activity than when measured by OSA. 40 Although the use of CSA has received regulatory approval in some regions, in the United States they are designated for ‘Research Use Only’ by the US Food and Drug Administration (FDA), 41 which poses a challenge in laboratories that require accreditation using FDA-approved methods. Another reason for the hesitant adoption of CSA is the lack of familiarity and expertise in their use, as well as perceived higher costs compared with OSA.37,42 However, computer-based cost analysis demonstrated that efficient use of reagents could render the cost comparable between OSA and CSA for both single and batch samples. 42 Therefore, more expansive regulatory approval and a drive to improve education and training in CSA use may enable broader implementation (Table 3).
Table 3.
Topic 2 Consensus recommendations.
| Topic 2: Specific therapeutic agent laboratory monitoring considerations | |
|---|---|
| 1 | Laboratories should be aware that there may be discrepancies between CSA and OSA for diagnostic testing in non-severe haemophilia B |
| 2 | For FIX therapy monitoring, laboratories should participate in proficiency testing for that particular product (e.g. using EQA) and use assays that have been validated in either field studies or locally |
| 3 | CSA provide higher levels of precision and accuracy in the assessment of FIX activity, whereas there may be variability with different OSA assays; however, the CSA may not be suitable for routine monitoring of recombinant FIX-albumin fusion protein (albutrepenonacog alfa) |
| 4 | Clinicians should be aware that insufficient evidence exists for thrombin generation assay or other global assays to guide routine clinical management of PwHB |
| 5 | Laboratories and clinicians should be aware that current FIX-GT demonstrates a consistently lower FIX activity when measured by CSA than by OSA; the choice of which assay should be used to aid clinical decision making is unclear |
CSA, chromogenic substrate assays; EQA, external quality assessment; FIX, factor IX; GT, gene therapy; OSA, one-stage assays; PwHB, people with haemophilia B.
In addition, the laboratory assessment of circulating plasma FIX will not assay any additional extravascular-collagen bound reservoir of FIX, meaning that results may not fully reflect the haemostatic potential of an individual receiving FIX replacement (i.e. measurable plasma FIX and unmeasurable collagen bound FIX). The extravascular distribution of EHL-FIX products is not yet fully understood and appears to be product-dependant, for example, eftrenonacog alfa (Alprolix®, Sanofi/Sobi) may have a greater sequestration in the extravascular space and capacity to bind to Col IV compared with nonacog beta pegol (Refixia®/Rebinyn®, Novo Nordisk)13,43,44 and albutrepenonacog alfa (Idelvion®, CSL Behring).13,44,45 Therefore, it may be inaccurate to assume that the measurable FIX haemostatic activity of some products using circulating plasma levels are directly comparable markers of total haemostatic potential.
Laboratory assays must be internally and externally validated, and the level of variation estimated. An external quality assessment (EQA) programme provides information on test accuracy and long-term reagent-analyser performance. Participation in such programmes improves laboratory performance and diagnosis. In terms of best practice, laboratories should be encouraged to participate in EQA, including those intended to monitor post-infusion levels, programmes, to follow relevant guidelines,46,47 and should carefully consider the most appropriate reference standard. 48 To further optimize methodology, EQA themselves may benefit from harmonizing their practices. 49
The choice of aPTT reagent can impact the accuracy of measured FIX activity and this may differ between EHL agent and assay type, and these challenges are also relevant with FIX GT;50,51 therefore, laboratories should use an assay and an aPTT reagent that has been validated for a specific FIX product. 52 The World Federation of Hemophilia (WFH) and the United Kingdom Haemophilia Centre Doctors’ Organisation (UKHCDO) has provided guidance on laboratory measurement of most FIX therapies.1,53 In summary, when measuring pdFIX, there are no OSA or CSA that are unsuitable; for nonacoag alfa (BeneFIX®, Pfizer) and nonacog gamma (Rixubis®), there are no OSA that are unsuitable; for eftrenonacog alfa (Alprolix® Sobi/Sanofi), OSA using Actin, Actin FS, Actin FSL, Pathromtin SL, Cephascreen or SynthASil and all CSA are suitable, but OSA using CK Prest and other kaolin-based reagents should be avoided; for albutrepenonacog alfa (Idelvion®, CSL Behring), OSA using Pathromtin SL and SynthASil are suitable, but OSA using CK Prest, SynthAFax or Actin FS and all CSA should be avoided; and finally, for nonacog beta pegol (Refixia®/Rebinyn®, Novo Nordisk), OSA using SynthAFax, DG Synth or Cephascreen and all CSA are suitable, but OSA using APTT-SP, Actin, Actin FS, Actin FSL, SynthASil and Pathromtin SL should be avoided. 53 Trenacog alfa (Xinity®, Emergent BioSolutions) which is only approved in the United States (FDA) details the following laboratory monitoring in the PI, ‘FIX activity measurements in the clinical laboratory may be affected by the type of aPTT reagent or laboratory standard used’, 54 so local validation of assays should be performed prior to monitoring this product.
Assay discrepancies associated with the International Standard (IS) for plasma or in-house plasma standards traceable to the plasma IS are rare53,55 In addition to the PI/SmPC guidance for laboratory assessment of FIX products, the FDA requires the use of pre-specified plasma reference standards for approval.
The authors emphasized that certain PK data for PwHB have been extrapolated from haemophilia A patient data, which may not take into consideration factors and evidence appropriate to haemophilia B. For example, the clinical significance of targeting a 1 IU/dl trough level is evident for individuals with FVIII deficiency but less so for those with FIX deficiency. 56
PK analysis may help inform the dosing regimen for PwHB with a full case history, including details on joint status, phenotype and product; however, establishing meaningful PK measurements for dosing or product comparisons is challenging for FIX replacement therapies (Table 4). This is due to inherent differences in extravascular distribution between products, patients and over time (Table 5). In terms of individualized haemophilia therapy, PK data can be used to guide prophylaxis, for example, to assess initial post-infusion levels to ascertain the FIX dosing required to achieve a peak for a specific product, and if suboptimal, the dosing can be increased. However, these measures only consider circulating plasma levels, so the extravascular levels and potential haemostatic effects thereof must also be considered. In this regard, findings from preclinical studies evaluating SHL-rFIX and eftrenonacog alfa (Alprolix®, Sobi/Sanofi) suggest that extensive reservoirs of extravascular FIX are at least as relevant for determining haemostasis as the circulating, measurable FIX. 57 The cross-reacting material (CRM) status may also impact PK measurements, for example, in CRM positive mice; native dysfunctional FIX antigen may occupy extravascular binding sites, interfering with the competitive binding of infused rFIX 58 impacting the in vivo recovery, and therefore prophylactic haemostasis. Therefore, while data suggest that extravascular distribution contributes to haemostasis, 57 the recovery and volume of distribution vary depending on which FIX agent is used, so direct comparisons of different FIX agents cannot be made.
Table 4.
Topic 3 Consensus recommendations.
| Topic 3: PK considerations – modelling, predictions and dose optimization | |
|---|---|
| 1 | We recommend that clinicians review product-specific characteristics, as well as patient phenotype and joint status, to determine whether PK analysis may guide individualized prophylaxis dosing |
| 2 | Population PK analysis should be considered, acknowledging the different extravascular distribution and optimal sampling times of each specific FIX product |
FIX, factor IX; PK, pharmacokinetic.
Table 5.
Pharmacokinetic parameter estimates for approved treatments of haemophilia B.
| Treatment (reference) | Recovery (IU d/l)/(IU/kg) | Half-life (h) | Mean residence time (h) in adults | Elimination clearance (ml/h/kg) in adults | Volume of steady-state distribution (ml/kg) in adults |
|---|---|---|---|---|---|
| pdFIX | |||||
| pdFIX (Replenine-VF®, Bio Products Laboratory) 59 | 1.16 | 19.0 | 24.9 | 4.52 | 122.1 |
| pdFIX (Haemonine®, Biotest) 60 | 1.5 ± 0.5 | 28.5 ± 12.1 | 33 | 200 ml/h | N.R. |
| SHL-rFIX | |||||
| Nonacog alfa (BeneFIX®, Pfizer) 61 | 0.73 ± 0.20 | 22.4 ± 5.3 | N.R. | 8.0 ± 0.6 | 225 ± 59 |
| Trenacog alfa (Ixinity®, Emergent BioSolutions) 54 | 0.98 ± 0.21 | Mean ± SD: 24 h (±7) | Mean ± SD: 30 (±6) | Mean ± SD: 5.6 (±1.3) | Mean ± SD: 193 (±62) |
| Nonacog gamma (Rixubis®, Shire) 62 | 0.87 ± 0.22 | Mean ± SD: 26.70 ± 9.55 Median (range): 24.58 (15.83–52.34) |
Mean ± SD: 30.82 ± 7.26 Median (range): 28.93 (22.25–47.78) |
Mean ± SD: 6.4 ± 1.3 Median (range): 6.2 (4.3–9.1) |
Mean ± SD: 202 ± 77 Median (range): 172 (110–394) |
| EHL-rFIX | |||||
| Eftrenonecog alfa (Aprolix®, Sobi/Sanofi) 63 (patient ⩾19 years old) | 0.92 (95% CI: 0.77–1.10) | 77.60 (95% CI: 70.05–85.95) | 95.82 (95% CI: 88.44–106.21) | 3.17 (95% CI: 2.85–3.51) | 303.4 (95% CI: 275.1–334.6) |
| Albutrepenonacog alfa (Idelvion®, CSL Behring) 64 | 1.18 (0.86–1.86) | 95.3 h (51.5–135.7) | N.R. | 0.875 (0.748–1.294) | N.R. |
| Nonacog beta pegol (Refixia®/Rebinyn®, Novo Nordisk) 65 | 1.9 | 115 | 158 | 0.4 | 66 |
CI, confidence interval; EHL, extended half-life; N.R. not reported; pdFIX, plasma-derived factor IX; rFIX, recombinant FIX; SD, standard deviation; SHL, standard half-life.
Despite the potential influence of extravascular distribution of different FIX concentrates, at a product level, the author group recommended that a full PK profile should be expected to comprise the evaluation of multiple blood samples, for example, at least eight samplings over 72 h for SHL, with additional sampling up to 2 weeks for EHL treatments, 1 with the last sample taken when basal levels are reached. It was highlighted that for some rFIX products in the literature, the PK-sampling period utilized was too short to fully account for full reversion to baseline and thus may give erroneous predictions of half-life incomparable with other studies and/or products. 13 At a patient-management level, population PK is possible using a predictive algorithm derived from a large data repository for a given FIX product, for instance, Web-Accessible Population Pharmacokinetic Service–Haemophilia (WAPPS–Hemo). 66 Consequently, a PK profile can be characterized for an FIX product using more acceptable sparse sampling to guide clinical management. The International Society on Thrombosis and Hemostasis (ISTH) guidelines (2017) provide further guidance on how to interpret and apply individual population PK assessments in clinical practice. 67 The author group highlighted the importance of considering the area under the curve and volume of distribution when considering concentrate characteristics and treatment options, although neither parameter is currently widely translated to guide individual clinical management.
Anaphylaxis or severe allergic reactions are serious complications of inhibitor development in PwHB, which may also be associated with the development of nephrotic syndrome in a small proportion of patients.68,69 Inhibitor development and the risk of an allergic reaction appear to be independent of the brand of FIX replacement therapy.69,70 Higher FIX dosing may be associated with inhibitor development and increased risk of adverse events; 71 however, this has also been observed in some PwHB receiving low FIX doses. However, currently there are no data identifying any FIX concentrate as immunologically superior to others in reducing inhibitor risk (Table 6). 70
Table 6.
Topic 4 Consensus recommendations.
| Topic 4: Inhibitor management and preparing for novel agents | |
|---|---|
| 1 | In people with severe haemophilia B, the causative F9 genetic defect should be determined as soon as possible after diagnosis to identify those at increased risk of inhibitor development and/or severe allergic reaction |
| 2 | Inhibitor screening should be routinely performed in all people with severe haemophilia B and scrutiny intensified if developing allergic reactions towards FIX and/or in those patients with inadequate response to FIX replacement therapy |
| 3 | FIX infusion and close clinical observation for allergic reaction should occur in the hospital setting during the first 20 EDs in people with severe haemophilia B |
| 4 | Recombinant activated factor VII should be the first choice for bleeding control and/or surgical cover in people with severe haemophilia B and high-responding inhibitors, as well as in those who have developed allergic reactions; aPCC is an option, but the content of FIX and associated risk of anamnesis and/or worsening of allergic reaction(s) needs to be considered |
| 5 | ITI to eradicate persistent inhibitors should be considered in people with severe haemophilia B; however, the relative benefits and risks need to be taken into account; ITI should only be initiated in a haemophilia treatment centre with an experienced team |
| 6 | Patients should be closely monitored during ITI for the development of nephrotic syndrome and/or severe allergic reactions |
| 7 | For those patients who have an allergic reaction, desensitization should be considered; importantly, further serious allergic reaction(s) should be anticipated in these patients, and subsequent infusions should occur in the hospital setting with appropriate resuscitation expertise and equipment |
| 8 | For FIX inhibitor eradication, ITI protocols with a combination of FIX and immunosuppressive agents may be considered as a first-line treatment |
aPCC, activated prothrombin complex concentrate; EDs, exposure days; FIX, factor IX; ITI, immune tolerance induction.
Inhibitor screening requires assays such as the Nijmegen–Bethesda assay 72 that have been locally validated by laboratories that take part in an appropriate EQA programme, and it is vital for any comprehensive haemophilia treatment programme to be able to detect emerging inhibitory activity as early as possible to direct appropriate medical treatment and consider eradication of inhibitors. Screening should be at least every third exposure day (ED) during the first 20 EDs, and more frequently if any concerns about allergic reaction or poor response. During this first 20 ED period for patients with severe haemophilia B, infusions should be undertaken in a hospital setting, with appropriate resuscitation facilities for clinical observation of allergic reactions and inhibitor emergence. Once through this early period of treatment exposure, screening should then be performed every 3–6 months until 150 EDs and annually thereafter.1,73 Because the risk of inhibitor development is believed to be highest during the first 50–75 EDs, 16 the number of EDs for previously untreated patients initiating treatment should be tracked by the care team to ensure timely inhibitor screening practice. Logistically, this can be more challenging to monitor in those receiving on-demand versus prophylactic treatment and for exposures administered at care facilities distant to the coordinating centre. In summary, proactive monitoring of all early treatment exposures in a previously untreated PwHB should be prioritized to detect and appropriately manage emerging inhibitors and their complications.
Several features are associated with increased risk of inhibitor development and severe allergic reactions (e.g. large deletions or nonsense mutations in the F9 gene) 16 and resultant disease severity, with this complication occurring almost exclusively in the severe form of haemophilia B (inhibitor rates are 4.9%, 0.5% and 0.1% in severe, moderate and mild haemophilia B, respectively). 74 Inhibitor screening is warranted in non-severe haemophilia B on clinical suspicion or after an allergic reaction. Following an unselected, previously untreated persons with severe haemophilia B cohort (n = 154), The Pediatric Network for haemophilia management (Ped Net) group describes a cumulative inhibitor incidence of 9.3% at 75 EDs, higher than the historically quoted <5%, with allergic reactions in 28.6% of those developing an inhibitor. 16 Inhibitor development is also more commonly associated with Black race and younger age. 74 Awareness of the F9 genotype in an individual with severe or moderate haemophilia B confirms the need for clinical scrutiny, particularly for early exposures in PwHB deemed ‘high-risk’ (e.g. those with a large deletion); however, the recommended precautions for early exposures should not be de-escalated for those with severe or moderate HB who are perceived to be genotypically ‘lower risk’.
Two therapeutic options are available for PwHB with inhibitors experiencing bleeds, trauma or requiring surgical intervention: activated Prothrombin Complex Concentrate (aPCC) or activated recombinant factor VII (rFVIIa). aPCC and rFVIIa75,76 bypass functional FIX activity and have considerably improved the management of acute bleeds and QoL in patients with chronic inhibitors and demonstrated efficacy and acceptable safety in PwHB undergoing surgery and in acute care.77–79 It should be noted that in the most recent WFH guidance it is recommended that PwHB who have high-responding inhibitors, or in those with low-responding inhibitors who develop allergic reactions or anaphylaxis, rFVIIa should be the first choice for controlling bleeding because aPCC contains FIX and may stimulate an anamnestic response, including further anaphylaxis. 1 As with treatment of inhibitors in people with haemophilia A (PwHA), immune tolerance induction (ITI) is used in PwHB developing high titre anti-FIX inhibitors, although there are some differences centred around reducing adverse reaction risks, which are more common in PwHB than PwHA and associated with its low success rate. 80 PwHB who are candidates for ITI and have an accompanying allergic phenotype require desensitization with gradually titrated doses of FIX. 81 Desensitization protocols are typically performed in a hospital setting and may involve immune modulation, for example, plasmapheresis, steroids or rituximab before, during or after the escalating dose.81–84 There are conflicting views as to when ITI should be initiated in PwHB after inhibitor detection. 1 Nephrotic syndrome may impede the success of ITI treatment in PwHB, further contributing to the low success rate (30–35%) of this treatment; 85 therefore, patients undergoing ITI should regularly undergo proteinuria screening, for example, with every inhibitor titre check. The risk of inhibitor development may influence the choice of first-line haemophilia treatment in the future, particularly in patients at high risk of inhibitor development. Compared with haemophilia A, the incidence of haemophilia B is small; therefore, large studies, pooled data or real-world data are required to better inform strategies for prevention and eradication of FIX inhibitors; for example, when to initiate ITI in PwHB after inhibitor detection or which (if any) FIX concentrates are less immunogenic and reduce inhibitor risk.
Recently, there has been increasing interest in ‘non-factor’ treatments that enhance coagulation irrespective of inhibitor development, such as the FVIII-mimetic treatment emicizumab, approved for the treatment of PwHA,86,87 or agents that ‘rebalance’ haemostasis, such as investigational agents fitusiran (Sanofi), marstacimab (Pfizer) and concizumab (Novo Nordisk),86,88,89 which may be utilized in haemophilia A or B regardless of inhibitor status, and consequently may change treatment decisions regarding the approach to inhibitor management and/or the need to restore tolerance to FIX in these patients, particularly if complicated with severe anaphylaxis and/or ITI failure. However, at the time of writing, these agents remain investigational in late-stage clinical trials.
By reducing or eradicating the need for exogenous FIX for prolonged periods, GT using gene addition offers the potential to lessen disease burden for PwHB with a single-dose GT treatment.90,91 Research efforts to date have focused on using a recombinant liver-directed adeno-associated virus (AAV) vector with a functional F9 gene cassette, targeted at the liver to enable endogenous expression of FIX, replacing the otherwise missing or mutant FIX.90,91 In recent GT trials in severe or moderately severe (<2 IU/dl FIX) PwHB, zero bleeds were reported in 39/54 patients 26 weeks after a single dose of etranacogene dezaparvovec (UniQure) in the pivotal HOPE-B trial 92 and in 12/15 patients ⩾1 year after a single dose of fidanacogene elaparvovec (Pfizer) in a phase II trial. 93 These data are promising; however, research is ongoing and long-term safety data in PwHB are needed. Furthermore, the question as to whether GT offers a lifelong functional cure depends on the duration of effect, which in turn may be influenced by liver turnover diluting non-integrated GT episomes or low level, non-targeted integration into the host genome (Table 7).
Table 7.
Topic 5 Consensus recommendations.
| Topic 5: Preparing for GT | |
|---|---|
| 1 | Based on current AAV haemophilia B GT trial data, this therapy should be considered as a future treatment option in adults with severe haemophilia B |
| 2 | As part of the informed consent process, patients should be made aware of the unpredictability of achieved FIX level and duration of expression |
| 3 | With liver-directed AAV GT for haemophilia B, patients should be aware that pre-existing liver pathology may be an exclusion criterion; for those proceeding to GT, patients should be counselled about other potential sources of hepatotoxicity that may interfere with FIX expression (e.g. medication use, alcohol) |
| 4 | Clinicians should be aware that a rise in transaminase levels during the acute phase of GT may indicate an immune response that can potentially threaten the expression of FIX; close monitoring of transaminase levels is needed to ensure that timely immunosuppression can be implemented |
| 5 | Clinicians should consider that the specific geographic pattern of AAV seropositivity may help direct which GT is chosen |
| 6 | When establishing a programme for haemophilia B GT, it is important to set up a network of care directed by experienced haemophilia treaters to include comprehensive education programmes for patients, haemophilia centre staff, extended multidisciplinary team and allied services |
| 7 | Patients and HCPs should be well informed of the potential need for either prophylactic or interventional immune suppression following GT administration, including duration and potential side effect profiles |
| 8 | Patients and HCPs should be aware of the need for long-term safety and efficacy follow-up, including assessment of liver health and levels of FIX expression, coordinated by the haemophilia centre |
| 9 | Centres and stakeholders, including regulators, payers and patients, should recognize the importance of participating in a post-authorization registry to gather real-world data on safety and efficacy of haemophilia B GT |
AAV, adeno-associated virus; FIX, factor IX; GT, gene therapy; HCP, healthcare provider.
The main toxicity of concern in the early period after GT dosing in clinical trials is the elevation in liver transaminases after vector infusion, which may be attributable to an anti-capsid T-cell immune response in some 90 but not all cases. 94 When used prophylactically or before the peak in aspartate transaminase/alanine transaminase, timely intervention with corticosteroids has been found to control such episodes; 94 however, in some patients, transgene expression may be partially or completely lost. 18 The importance of pre-existing AAV seropositivity is yet unclear; in some cases, the success of GT may be hindered by pre-existing humoral capsid immunity. However, in the HOPE-B trial, despite 23/54 patients having neutralizing antibodies (nAbs) to AAV5, no correlation of nAbs and expressed FIX activity was observed in titres up to 678, and only one of these patients (nAb titre of 3212.3) was unresponsive to GT. 92 This may not be a generalizable phenomenon to other AAV serotypes and would require further serotype-specific data. Because seroprevalence to different AAV serotypes varies geographically, being able to select from multiple GT products that use various AAV vector serotypes may potentially avoid this complication.95,96 Decreased factor expression or elevated transaminases, both with and without cellular immune responses justify the use of immunosuppression strategies to protect transduced hepatocytes.91,94,97–100 These immune suppression protocols may differ between platforms and are likely to evolve over time (Table 7).
When health care providers, patients, caregivers and advocates are considering a trial of investigational GT, the fundamentals of this treatment should be discussed, including availability of different AAV gene transfer platforms each with nuanced differences in protocol, immunosuppression and inclusion criteria for candidates. Guidance by Sidonio et al. 101 discusses these aspects of GT treatment and provides infographic visual aids that are accessible to patients and may help them make an informed decision. Using an appropriate lexicon, patients should be educated on the variation observed between trials and study participants receiving the same therapy to ensure PwHB can make an informed decision, 102 and understand that trial populations tend to exclude patients with abnormal liver or kidney function, hepatitis or HIV, or current/past history of inhibitors. 103
Owing to the enduring nature of GT, there is a need for long-term follow-up for years after treatment and as such patients will need to remain in close contact with their haemophilia treatment centre. Therefore, when setting up Centres of Excellence for haemophilia B GT, a multidisciplinary team of experts must be able to cater for practical aspects of the patient treatment and management, such as infusion needs, immunosuppression protocols, follow-up and surveillance, while meeting Genetically Modified Organism requirements and including comprehensive risk assessments and documentation. 104
The WFH has established a World Gene Therapy Registry (WFH GTR) to gather global post-authorization surveillance, which is needed to gather sufficient long-term follow-up data to allow robust evaluation of safety and efficacy for GT. This cohesive effort by the American Thrombosis and Hemostasis Network (ATHN), the European Haemophilia Consortium (EHC), the ISTH, the US National Hemophilia Foundation (NHF) and industry GT development partners aims to provide robust, long-term safety and efficacy data, which will be available to health care providers treating patients with GT. 105
Conclusion
In this Delphi consensus process, we have developed a comprehensive set of clinical recommendations to guide the management of PwHB across five key topics and several recommendations on how data collection could be homogenized in future studies.
rFIX and EHL-FIX have significantly improved haemophilia B management in the last 30 years, and the therapeutic landscape shows promise with several non-factor replacement therapies and GTs currently under investigation. Although similarities between haemophilia A and haemophilia B exist, the intrinsic size and structural differences between FVIII and FIX proteins mean that data from haemophilia A cannot simply be extrapolated to haemophilia B. The relative rarity of haemophilia B and resultant paucity of large studies further highlight the need for robust studies investigating haemophilia B management and comparing treatments.
It is hoped that the recommendations for clinical management provided here will complement existing guidelines and support the optimal management of PwHB. Areas requiring future studies are identified to support the generation of robust evidence and it is anticipated that additional research will facilitate the refinement of guidance in those areas lacking a strong evidence base.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207221085202 for International consensus recommendations on the management of people with haemophilia B by Daniel P. Hart, Davide Matino, Jan Astermark, Gerard Dolan, Roseline d’Oiron, Cédric Hermans, Victor Jiménez-Yuste, Adriana Linares, Tadashi Matsushita, Simon McRae, Margareth C. Ozelo, Sean Platton, Darrel Stafford, Robert F. Sidonio and Andreas Tiede in Therapeutic Advances in Hematology
Footnotes
Author contributions: Daniel P. Hart: Conceptualization; Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing.
Davide Matino: Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing.
Jan Astermark: Formal analysis; Methodology; Validation; Writing – review & editing.
Gerard Dolan: Formal analysis; Methodology; Validation; Writing – review & editing.
Roseline d’Oiron: Formal analysis; Methodology; Validation; Writing – review & editing.
Cédric Hermans: Formal analysis; Methodology; Validation; Writing – review & editing.
Victor Jiménez-Yuste: Formal analysis; Methodology; Validation; Writing – review & editing.
Adriana Linares: Formal analysis; Methodology; Validation; Writing – review & editing.
Tadashi Matsushita: Formal analysis; Methodology; Validation; Writing – review & editing.
Simon McRae: Formal analysis; Methodology; Validation; Writing – review & editing.
Margareth C. Ozelo: Formal analysis; Methodology; Validation; Writing – review & editing.
Sean Platton: Formal analysis; Methodology; Validation; Writing – review & editing.
Darrel Stafford: Formal analysis; Methodology; Validation; Writing – review & editing.
Robert F. Sidonio, Jr.: Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing.
Andreas Tiede: Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Daniel P Hart has received personal or institutional honoraria from Bayer, Biotest, BioMarin, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, Spark, Takeda and UniQure; and personal or institutional consulting fees from Bayer, Biotest, BioMarin, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, Spark, Takeda, UniQure. Davide Matino has received honoraria from Novo Nordisk, Octapharma, Pfizer, Sanofi and Sobi; received grant support from Bayer, Octapharma and Pfizer (to McMaster University); and has participated on Advisory Boards for Novonordisk, Pfizer and Sobi. Jan Astermark has received honoraria from Bayer, BioMarin, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Shire/Takeda and Sobi; received grant support from Bayer, CSL Behring, Shire/Takeda and Sobi/Biogen; and has participated on Data Safety Monitoring Boards or Advisory Boards for Bayer, BioMarin, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Shire/Takeda, Sobi and Uniqure. Gerard Dolan has received honoraria from CSL Behring, Novo Nordisk, Pfizer and Sobi; personal consulting fees from CSL Behring, Novo Nordisk, Pfizer and Sobi; support for attending meetings or travel from CSL Behring, Novo Nordisk, Pfizer and Sobi; and has participated on Data Safety Monitoring Boards or Advisory Boards for CSL Behring, Novo Nordisk, Pfizer and Sobi. Roseline d’Oiron has received honoraria from Baxalta/Shire/Takeda, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche and Sobi; support for attending meetings and/or travel from Baxalta/Shire/Takeda, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche and Sobi; and has participated on Data Safety Monitoring Boards or Advisory Boards for Baxalta/Shire/Takeda, Biomarin, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Spark Therapeutics, Sobi and UniQure. Cédric Hermans has received honoraria from Bayer, Biomarin, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Takeda and Uniqure; consulting fees from Bayer, Biomarin, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Takeda and Uniqure; and has participated on a Data Safety Monitoring Board or Advisory Board for CSL Behring. Victor Jiménez-Yuste has received honoraria from CSL Behring, Novo Nordisk, Pfizer and Sobi; and consulting fees from CSL Behring, Novo Nordisk, Pfizer and Sobi. Adriana Linares has declared no conflict of interest. Tadashi Matsushita has received honoraria from Bayer, Chugai, CSL Behring, JB, KMB, Novo Nordisk, Pfizer, Sanofi and Takeda; consulting fees from Bayer; grant and research support from Chugai, JB and KMB; a leadership or fiduciary role from Aichi Pt Union; and has participated on Data Safety Monitoring Boards or Advisory Boards for Bayer, Chugai, Novo Nordisk, Sanofi and Takeda. Simon McRae has received honoraria from Pfizer; and a leadership or fiduciary role from the Australian Bleeding Disorders Registry. Margareth C. Ozelo has received personal honoraria from Bayer, BioMarin, Novo Nordisk, Roche and Takeda; consulting fees from Pfizer; grant and research support from BioMarin, Novo Nordisk, Pfizer, Roche, Sanofi and Takeda; support for attending meetings and/or travel from Novo Nordisk, Roche and Takeda; a leadership or fiduciary role from Novo Nordisk; other financial or non-financial interests from Grifols; and has participated on Data Safety Monitoring Boards or Advisory Boards for Bayer, BioMarin, Novo Nordisk, Roche, Sanofi and Takeda. Sean Platton has received personal honoraria from Pfizer; and has participated on a Data Safety Monitoring Board or Advisory Board for Novo Nordisk. Darrel Stafford has received honoraria from Sobi; and royalties and patents from VKOR. Robert F. Sidonio has received honoraria from BioMarin and Sanofi; consulting fees from Bayer, BioMarin, Genentech, Hema Biologics, Kedtion, Novo Nordisk, Octapharma, Takeda; research grants from Genentech, Octapharma and Takeda; and has participated on a Data Safety Monitoring Board or Advisory Board for Uniqure. Andreas Tiede has received personal or institutional honoraria from Bayer, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi and Takeda; and research grant/support from Bayer, Biotest, Chugai, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi and Takeda.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing was provided by Aisling Koning and Debbie McIntosh of Synergy Medical Communications, London, UK, and was supported by Pfizer. Editorial assistance and support with the submission of this manuscript was provided by Kyle Lambe of Synergy Medical Communications, London, UK, and was supported by Pfizer. The authors have authorized this support and approved the inclusion of all conflicting interests and funding disclosures.
ORCID iD: Daniel P. Hart  https://orcid.org/0000-0001-9084-8598
https://orcid.org/0000-0001-9084-8598
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Daniel P. Hart, The Royal London Hospital Haemophilia Centre, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Whitechapel Road, London E1 2AD, UK.
Davide Matino, Department of Medicine, McMaster University and The Thrombosis and Atherosclerosis Research Institute, Hamilton, ON, Canada.
Jan Astermark, Institution of Translational Medicine and Department of Hematology, Oncology and Radiation Physics, Lund University, Skåne University Hospital, Malmö, Sweden.
Gerard Dolan, Centre for Haemostasis and Thrombosis, St Thomas’ Hospital, London, UK.
Roseline d’Oiron, Centre for Haemophilia and Constitutional Bleeding Disorders, Hôpital Bicêtre AP-HP Université Paris-Saclay, Le Kremlin-Bicêtre, France.
Cédric Hermans, Haemostasis and Thrombosis Unit, Division of Haematology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain (UCLouvain), Brussels, Belgium.
Victor Jiménez-Yuste, Hospital Universitario La Paz, Autónoma University, Madrid, Spain.
Adriana Linares, Grupo de Oncohematología Pediátrica, Universidad Nacional de Colombia, Bogotá, Colombia; Programa de Hemofilia, Clínica Infantil Colsubsidio, Bogotá, Colombia.
Tadashi Matsushita, Department of Transfusion Medicine, Nagoya University Hospital, Nagoya, Japan.
Simon McRae, Launceston General Hospital, Launceston, TAS, Australia.
Margareth C. Ozelo, Hemocentro UNICAMP, University of Campinas, Campinas, Brazil
Sean Platton, The Royal London Hospital Haemophilia Centre, Royal London Hospital, Barts Health NHS Trust, London, UK.
Darrel Stafford, Department of Biology, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Robert F. Sidonio, Jr., Aflac Cancer and Blood Disorders, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA, USA.
Andreas Tiede, Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover Medical School, Hannover, Germany.
References
- 1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26(Suppl. 6): 1–158. [DOI] [PubMed] [Google Scholar]
- 2. White GC, 2nd, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 2001; 85: 560. [PubMed] [Google Scholar]
- 3. Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorb 2011; 1: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carvalhosa AM, Henrard S, Lambert C, et al. Physical and mental quality of life in adult patients with haemophilia in Belgium: the impact of financial issues. Haemophilia 2014; 20: 479–485. [DOI] [PubMed] [Google Scholar]
- 5. O’Hara J, Hughes D, Camp C, et al. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis 2017; 12: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Escobar MA. Health economics in haemophilia: a review from the clinician’s perspective. Haemophilia 2010; 16(Suppl. 3): 29–34. [DOI] [PubMed] [Google Scholar]
- 7. Johnson KA, Zhou ZY. Costs of care in hemophilia and possible implications of health care reform. Hematology Am Soc Hematol Educ Program 2011; 2011: 413–418. [DOI] [PubMed] [Google Scholar]
- 8. Coppola A, Tagliaferri A, Di Capua M, et al. Prophylaxis in children with hemophilia: evidence-based achievements, old and new challenges. Semin Thromb Hemost 2012; 38: 79–94. [DOI] [PubMed] [Google Scholar]
- 9. Nazeef M, Sheehan JP. New developments in the management of moderate-to-severe hemophilia B. J Blood Med 2016; 7: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peyvandi F, Garagiola I, Biguzzi E. Advances in the treatment of bleeding disorders. J Thromb Haemost 2016; 14: 2095–2106. [DOI] [PubMed] [Google Scholar]
- 11. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet 2016; 388: 187–197. [DOI] [PubMed] [Google Scholar]
- 12. Stafford DW. Extravascular FIX and coagulation. Thromb J 2016; 14(Suppl. 1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iorio A, Fischer K, Blanchette V, et al. Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost 2017; 117: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 14. Olivieri M, Kurnik K, Pfluger T, et al. Identification and long-term observation of early joint damage by magnetic resonance imaging in clinically asymptomatic joints in patients with haemophilia A or B despite prophylaxis. Haemophilia 2012; 18: 369–374. [DOI] [PubMed] [Google Scholar]
- 15. DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol 2007; 138: 305–315. [DOI] [PubMed] [Google Scholar]
- 16. Male C, Andersson NG, Rafowicz A, et al. Inhibitor incidence in an unselected cohort of previously untreated patients with severe haemophilia B: a PedNet study. Haematologica 2020; 106: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nathwani AC, Davidoff AM, Tuddenham EGD. Gene therapy for hemophilia. Hematol Oncol Clin North Am 2017; 31: 853–868. [DOI] [PubMed] [Google Scholar]
- 18. George LA. Hemophilia gene therapy comes of age. Blood Adv 2017; 1: 2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peyvandi F, Kenet G, Pekrul I, et al. Laboratory testing in hemophilia: impact of factor and non-factor replacement therapy on coagulation assays. J Thromb Haemost 2020; 18: 1242–1255. [DOI] [PubMed] [Google Scholar]
- 20. Santagostino E, Mancuso ME, Tripodi A, et al. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost 2010; 8: 737–743. [DOI] [PubMed] [Google Scholar]
- 21. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med 2013; 369: 2313–2323. [DOI] [PubMed] [Google Scholar]
- 22. Iorio A, Krishnan S, Myrén KJ, et al. Continuous prophylaxis with recombinant factor IX Fc fusion protein and conventional recombinant factor IX products: comparisons of efficacy and weekly factor consumption. J Med Econ 2017; 20: 337–344. [DOI] [PubMed] [Google Scholar]
- 23. Powell J, Shapiro A, Ragni M, et al. Switching to recombinant factor IX Fc fusion protein prophylaxis results in fewer infusions, decreased factor IX consumption and lower bleeding rates. Br J Haematol 2015; 168: 113–123. [DOI] [PubMed] [Google Scholar]
- 24. Roth DA, Kessler CM, Pasi KJ, et al. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood 2001; 98: 3600–3606. [DOI] [PubMed] [Google Scholar]
- 25. Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicentre phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level </=2%) haemophilia B. Haemophilia 2014; 20: 15–24. [DOI] [PubMed] [Google Scholar]
- 26. Collins PW, Quon DVK, Makris M, et al. Pharmacokinetics, safety and efficacy of a recombinant factor IX product, trenonacog alfa in previously treated haemophilia B patients. Haemophilia 2018; 24: 104–112. [DOI] [PubMed] [Google Scholar]
- 27. Shapiro AD, Ragni MV, Valentino LA, et al. Recombinant factor IX-Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood 2012; 119: 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Negrier C, Knobe K, Tiede A, et al. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood 2011; 118: 2695–2701. [DOI] [PubMed] [Google Scholar]
- 29. Santagostino E, Negrier C, Klamroth R, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood 2012; 120: 2405–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Windyga J, Lissitchkov T, Stasyshyn O, et al. Efficacy and safety of a recombinant factor IX (Bax326) in previously treated patients with severe or moderately severe haemophilia B undergoing surgical or other invasive procedures: a prospective, open-label, uncontrolled, multicentre, phase III study. Haemophilia 2014; 20: 651–658. [DOI] [PubMed] [Google Scholar]
- 31. Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood 2014; 124: 3880–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood 2016; 127: 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun HLYM, Poon M-C, Lee A, et al. Observational study of real-world factor utilization and health outcomes in patients with hemophilia in Canada. Blood 2018; 132: 4813. [Google Scholar]
- 34. Furlan R, Krishnan S, Vietri J. Patient and parent preferences for characteristics of prophylactic treatment in hemophilia. Pat Pref Adher 2015; 9: 1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shapiro A, Chaudhury A, Jain N, et al. Real-world data on the use of rFIXFc in subjects with hemophilia B for up to 3.7 years demonstrates improved bleed control and adherence with reduced treatment burden. Blood 2018; 132: 2493. [Google Scholar]
- 36. Strike K, Chan A, Iorio A, et al. Predictors of treatment adherence in patients with chronic disease using the multidimensional adherence model: unique considerations for patients with haemophilia. The Journal of Haemophilia Practice 2020; 7: 92–101. [Google Scholar]
- 37. Kitchen S, Signer-Romero K, Key NS. Current laboratory practices in the diagnosis and management of haemophilia: a global assessment. Haemophilia 2015; 21: 550–557. [DOI] [PubMed] [Google Scholar]
- 38. Kihlberg K, Strandberg K, Rosen S, et al. Discrepancies between the one-stage clotting assay and the chromogenic assay in haemophilia B. Haemophilia 2017; 23: 620–627. [DOI] [PubMed] [Google Scholar]
- 39. Kloosterman KRHS, van Balen EC, Smit C, et al. Factor IX assay discrepancy in non-severe hemophilia B – a cross-sectional study in the Netherlands. Haeomphilia 2020; 26: 21. [Google Scholar]
- 40. Le Quellec S, Dane AP, Barbon E, et al. Recombinant adeno-associated viral vectors expressing human coagulation FIX-E456H variant in hemophilia B mice. Thromb Haemost 2019; 119: 1956–1967. [DOI] [PubMed] [Google Scholar]
- 41. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one-stage and chromogenic assays of factor VIII activity. J Thromb Haemost 2016; 14: 248–261. [DOI] [PubMed] [Google Scholar]
- 42. Kitchen S, Blakemore J, Friedman KD, et al. A computer-based model to assess costs associated with the use of factor VIII and factor IX one-stage and chromogenic activity assays. J Thromb Haemost 2016; 14: 757–764. [DOI] [PubMed] [Google Scholar]
- 43. Salas J, Van Der Flier A, Hong VP, et al. Extravascular distribution of conventional and Ehl FIX products using in vivo SPECT imaging analysis in hemophilia B mice. Blood 2017; 130: 1061. [Google Scholar]
- 44. Mann DM, Stafford KA, Poon MC, et al. The Function of extravascular coagulation factor IX in haemostasis. Haemophilia 2021; 27: 332–339. [DOI] [PubMed] [Google Scholar]
- 45. Rampotas A, Desborough MJR, Raza-Burton S, et al. A single centre retrospective study of low dose prophylaxis with extended half-life factor IX for severe haemophilia B. Haemophilia 2020; 26: 278–281. [DOI] [PubMed] [Google Scholar]
- 46. Kitchen SMA, Echenagucia M. Diagnosis of hemophilia and other bleeding disorders: a laboratory manual, 2010, http://www1.wfh.org/publication/files/pdf-1283.pdf
- 47. van Moort I, Meijer P, Priem-Visser D, et al. Analytical variation in factor VIII one-stage and chromogenic assays: experiences from the ECAT external quality assessment programme. Haemophilia 2019; 25: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilmot HV, Hogwood J, Gray E. Recombinant factor IX: discrepancies between one-stage clotting and chromogenic assays. Haemophilia 2014; 20: 891–897. [DOI] [PubMed] [Google Scholar]
- 49. Favaloro EJ, Jennings I, Olson J, et al. Towards harmonization of external quality assessment/proficiency testing in hemostasis. Clin Chem Lab Med 2018; 57: 115–126. [DOI] [PubMed] [Google Scholar]
- 50. Bowyer AE, Hillarp A, Ezban M, et al. Measuring factor IX activity of nonacog beta pegol with commercially available one-stage clotting and chromogenic assay kits: a two-center study. J Thromb Haemost 2016; 14: 1428–1435. [DOI] [PubMed] [Google Scholar]
- 51. Bowyer AE, Lowe AE, Tiefenbacher S. Laboratory issues in gene therapy and emicizumab. Haemophilia 2021; 27(Suppl. 3): 142–147. [DOI] [PubMed] [Google Scholar]
- 52. Bowyer AE, Shepherd MF, Kitchen S, et al. Measurement of extended half-life recombinant factor IX products in clinical practice. Int J Lab Hematol 2019; 41: e46–e49. [DOI] [PubMed] [Google Scholar]
- 53. Gray E, Kitchen S, Bowyer A, et al. Laboratory measurement of factor replacement therapies in the treatment of congenital haemophilia: a United Kingdom Haemophilia Centre Doctors’ organiSation guideline. Haemophilia 2020; 26: 6–16. [DOI] [PubMed] [Google Scholar]
- 54. Aptevo BioTherapeutics. IXINITY® Highlights of prescribing information 2015, https://www.fda.gov/media/91715/download
- 55. Kitchen S, Gray E, Mertens K. Monitoring of modified factor VIII and IX products. Haemophilia 2014; 20(Suppl. 4): 36–42. [DOI] [PubMed] [Google Scholar]
- 56. Bjorkman S. Pharmacokinetics of plasma-derived and recombinant factor IX – implications for prophylaxis and on-demand therapy. Haemophilia 2013; 19: 808–813. [DOI] [PubMed] [Google Scholar]
- 57. Cooley B, Funkhouser W, Monroe D, et al. Prophylactic efficacy of BeneFIX vs Alprolix in hemophilia B mice. Blood 2016; 128: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cooley B, Broze GJ, Jr, Mann DM, et al. Dysfunctional endogenous FIX impairs prophylaxis in a mouse hemophilia B model. Blood 2019; 133: 2445–2451. [DOI] [PubMed] [Google Scholar]
- 59. EMA. Replenine-VF summary of product characteristics, 2020, https://www.medicines.org.uk/emc/product/5542/smpc#gref
- 60. EMA. Haemonine 500 summary of product characteristics, 2016, https://www.medicines.org.uk/emc/product/580/smpc#gref
- 61. EMA. BeneFix® summary of product characteristics, 2019, https://www.ema.europa.eu/en/documents/product-information/benefix-epar-product-information_en.pdf
- 62. EMA. Rixubis® summary of product characteristics, 2014, https://www.ema.europa.eu/en/documents/product-information/rixubis-epar-product-information_en.pdf
- 63. EMA. Alprolix, INN-eftrenonacog alfa: summary of product characteristics, 2016, https://www.ema.europa.eu/en/documents/product-information/alprolix-epar-product-information_en.pdf
- 64. EMA. Idelvion, INN-albutrepenonacog alfa: summary of product characteristics, 2016, https://www.ema.europa.eu/en/documents/product-information/idelvion-epar-product-information_en.pdf
- 65. EMA. Refixia, INN-nonacog beta pegol: summary of product characteristics, 2017, https://www.ema.europa.eu/en/documents/product-information/refixia-epar-product-information_en.pdf
- 66. WAPPS-Hemo, 2021, https://www.wapps-hemo.org/
- 67. Iorio A, Blanchette V, Blatny J, et al. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost 2017; 15: 2461–2465. [DOI] [PubMed] [Google Scholar]
- 68. Chitlur M, Warrier I, Rajpurkar M, et al. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997-2006). Haemophilia 2009; 15: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 69. Recht M, Pollmann H, Tagliaferri A, et al. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia 2011; 17: 494–499. [DOI] [PubMed] [Google Scholar]
- 70. Fischer K, Lassila R, Peyvandi F, et al. Inhibitor development in haemophilia according to concentrate. Four-year results from the European HAemophilia Safety Surveillance (EUHASS) project. Thromb Haemost 2015; 113: 968–975. [DOI] [PubMed] [Google Scholar]
- 71. Ragni MV, Ojeifo O, Feng J, et al. Risk factors for inhibitor formation in haemophilia: a prevalent case-control study. Haemophilia 2009; 15: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Verbruggen B, Novakova I, Wessels H, et al. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost 1995; 73: 247–251. [PubMed] [Google Scholar]
- 73. Collins PW, Chalmers E, Hart DP, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition). UK Haemophilia Centre Doctors Organization. Br J Haematol 2013; 160: 153–170. [DOI] [PubMed] [Google Scholar]
- 74. Puetz J, Soucie JM, Kempton CL, et al. Prevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection database. Haemophilia 2014; 20: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Negrier C, Lienhart A, Numerof R, et al. SURgical interventions with FEIBA (SURF): international registry of surgery in haemophilia patients with inhibitory antibodies. Haemophilia 2013; 19: e143–50. [DOI] [PubMed] [Google Scholar]
- 76. Negrier C, Voisin S, Baghaei F, et al. Global post-authorization safety surveillance study: real-world data on prophylaxis and on-demand treatment using FEIBA (an activated prothrombin complex concentrate). Blood Coagul Fibrinolysis 2016; 27: 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dimichele D, Negrier C. A retrospective postlicensure survey of FEIBA efficacy and safety. Haemophilia 2006; 12: 352–362. [DOI] [PubMed] [Google Scholar]
- 78. Meeks SL, Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematology Am Soc Hematol Educ Program 2016; 2016: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gringeri A, Leissinger C, Cortesi PA, et al. Health-related quality of life in patients with haemophilia and inhibitors on prophylaxis with anti-inhibitor complex concentrate: results from the Pro-FEIBA study. Haemophilia 2013; 19: 736–743. [DOI] [PubMed] [Google Scholar]
- 80. DiMichele DM, Kroner BL. and North American Immune Tolerance Study Group. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost 2002; 87: 52–57. [PubMed] [Google Scholar]
- 81. Dioun AF, Ewenstein BM, Geha RS, et al. IgE-mediated allergy and desensitization to factor IX in hemophilia B. J Allergy Clin Immunol 1998; 102: 113–117. [DOI] [PubMed] [Google Scholar]
- 82. Barnes C, Brewin T, Ekert H. Induction of immune tolerance and suppression of anaphylaxis in a child with haemophilia B by simple plasmapheresis and antigen exposure: progress report. Haemophilia 2001; 7: 439–440. [DOI] [PubMed] [Google Scholar]
- 83. Shibata M, Shima M, Misu H, et al. Management of haemophilia B inhibitor patients with anaphylactic reactions to FIX concentrates. Haemophilia 2003; 9: 269–271. [DOI] [PubMed] [Google Scholar]
- 84. Alexander S, Hopewell S, Hunter S, et al. Rituximab and desensitization for a patient with severe factor IX deficiency, inhibitors, and history of anaphylaxis. J Pediatr Hematol Oncol 2008; 30: 93–95. [DOI] [PubMed] [Google Scholar]
- 85. Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood 1997; 89: 1115–1116. [PubMed] [Google Scholar]
- 86. Franchini M, Mannucci PM. Non-factor replacement therapy for haemophilia: a current update. Blood Transfus 2018; 16: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. EMA. Hemlibra 150 mg/mL solution for injection summary of product characteristics, 2018, https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf
- 88. Pasi KJ, Lissitchkov T, Mamonov V, et al. Targeting of antithrombin in hemophilia A or B with investigational siRNA therapeutic fitusiran – results of the phase 1 inhibitor cohort. J Thromb Haemost 2021; 19: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shapiro AD, Angchaisuksiri P, Astermark J, et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood 2019; 134: 1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Batty P, Lillicrap D. Advances and challenges for hemophilia gene therapy. Hum Mol Genet 2019; 28: R95–R101. [DOI] [PubMed] [Google Scholar]
- 91. Miesbach W, Meijer K, Coppens M, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018; 131: 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pipe SRM, Key NS, Leebeek FWG, et al. LBA-6 first data from the phase 3 HOPE-B gene therapy trial: efficacy and safety of etranacogene dezaparvovec (AAV5-Padua hFIX variant; AMT-061) in adults with severe or moderate-severe hemophilia B treated irrespective of pre-existing anti-capsid neutralizing antibodies. In: Presented at the 62nd ASH Annual Meeting and Exposition, December 2020. [Google Scholar]
- 93. George LA, Sullivan SK, Rasko JEJ, et al. Efficacy and safety in 15 hemophilia B patients treated with the AAV gene therapy vector fidanacogene elaparvovec and followed for at least 1 year. In: Presented at the 61st ASH Annual Meeting and Exposition, December 2019. [Google Scholar]
- 94. Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014; 371: 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999; 59: 406–411 [DOI] [PubMed] [Google Scholar]
- 96. Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010; 21: 704–712. [DOI] [PubMed] [Google Scholar]
- 97. George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017; 377: 2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med 2017; 377: 2519–2530. [DOI] [PubMed] [Google Scholar]
- 99. Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Monahan PE, Sun J, Gui T, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther 2015; 26: 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sidonio RF, Jr, Pipe SW, Callaghan MU, et al. Discussing investigational AAV gene therapy with hemophilia patients: a guide. Blood Rev 2020: 100759. [DOI] [PubMed] [Google Scholar]
- 102. Hart DP, Branchford BR, Hendry S, et al. Optimizing language for effective communication of gene therapy concepts with hemophilia patients: a qualitative study. Orphanet J Rare Dis 2021; 16: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miesbach W, O’Mahony B, Key NS, et al. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia 2019; 25: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. European Commission. Genetically Modified Organism (GMO) aspects for investigational medicinal products, https://ec.europa.eu/health/human-use/advanced-therapies/gmo_investiganional_en (accessed 18 March 2021).
- 105. Konkle BA, Coffin D, Pierce GF, et al. World federation of hemophilia gene therapy registry. Haemophilia 2020; 26: 563–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207221085202 for International consensus recommendations on the management of people with haemophilia B by Daniel P. Hart, Davide Matino, Jan Astermark, Gerard Dolan, Roseline d’Oiron, Cédric Hermans, Victor Jiménez-Yuste, Adriana Linares, Tadashi Matsushita, Simon McRae, Margareth C. Ozelo, Sean Platton, Darrel Stafford, Robert F. Sidonio and Andreas Tiede in Therapeutic Advances in Hematology