Abstract
Subclinical alterations in left ventricular structure and function are detectable in adolescents with hypertension or obesity. However, data on early echocardiographic abnormalities in seemingly healthy children are lacking. Sex differences in cardiac structure and function have been previously reported, but sex-specific reference values are not available. Specifically, the potential interaction of sex and overweight has not been addressed at all. Anthropometric data, blood pressure and exercise tests were obtained in 356 healthy children. Echocardiographic parameters comprised peak early (E) and late (A) mitral inflow Doppler velocities, E/A ratio, tissue Doppler peak velocities of early (e′) and late diastolic (a′) excursion of mitral/septal annulus and isovolumetric relaxation time (IVRT). Left ventricular mass index (LVMI) and LVMI z-score were calculated. Interaction terms between BMI and sex and stratification by sex were used for analysis. We provide values for echocardiographic parameters for children of two age groups separated by BMI. Overweight/obese children had a significant higher LVMI, lower E/A ratio, higher E/e′ ratios and a longer IVRT. For a given BMI in the upper ranges we demonstrated a higher LVMI in girls than in boys, the IVRT extended significantly more in girls than in boys with increasing BMI. There are sex differences in structural and functional echocardiographic parameters in children and adolescents. Our data not only confirms the importance of overweight and obesity, but demonstrates important interactions between sex and overweight. The greater susceptibility of overweight girls toward echocardiographic changes associated with potential long-term functional impairment needs further exploration and follow-up.
Trial registration number DRKS00012371; Date 18.08.2017.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00246-022-02876-2.
Keywords: Children, Obesity, Echocardiography, Sex differences, Reference values
Introduction
With the recognition of obesity as an important determinant of cardiac damage and remodeling, the early diagnosis of cardiac alterations in children is becoming more important especially in conjunction with the rise of childhood obesity. Early changes in left ventricular structure and function are already detected in adolescents with cardiovascular risk factors, like hypertension or adiposity [1]. Elevated LVMI [2], as well as indices of a lower ventricular compliance in overweight and obese children compared to their lean counterparts were described [3, 4]. Excessive obesity was linked to diastolic dysfunction in children, independent of comorbidities [5].
While the existing data suggest that these changes start very early in life, there is a lack of substantial data on early changes of echocardiographic parameters in children with obesity. The effect of sex has not been addressed to date; sex-specific reference values are not available. In light of different sex-specific phenotypes of cardiac disease in adulthood [6] differences between the sexes in childhood concerning cardiac function and structure should be taken into account. A recent paper investigated children with chronic kidney disease as a high risk group for cardiovascular comorbidities and demonstrated a higher susceptibility for girls toward higher LVMI especially when they were overweight [7]. While, in general, female sex is believed to be cardioprotective numerous studies have shown that in presence of special risk factors women become more susceptible. The situation in childhood, especially prior to puberty as well as a sex-specific influence of BMI on echocardiographic parameters has not been addressed at all.
The aim of our study was to describe structural and functional echocardiographic parameters in children and adolescents of two age groups separated by sex and BMI. Based on our data, we were able to characterize influencing factors associated with the measured parameters and can show that sex-specific differences occur in presence of the important CV risk factor overweight and obesity.
Methods
Study Design and Cohort
Three primary and two secondary schools were randomly chosen. All pupils from the 2nd grade, or 5th grade, respectively, were invited to participate in this cross-sectional prospective study from April to July 2017. Exclusion criteria included structural or congenital heart disease. All parents and children gave written informed consent before participation. The study was approved by the Institutional Review Board (No. 7290) and performed according to the Declaration of Helsinki.
Anthropometric, Blood Pressure and Physical Fitness Assessment
Height, weight and waist circumference were measured, waist-circumference z-score was calculated according to Sharma et al. [8]. Body surface area (BSA) was calculated according to Du Bois [9]. Body mass index z-score (BMIz) was calculated using WHO reference growth standards [10]. We separated our study group according to children’s BMIz in a group with a BMIz < 1.04 and in an overweight/obese group with a BMIz ≥ 1.04. Age was classified into a group of 7–9 and 10–13 year olds, enabling the comparison of echocardiographic parameters to the published reference values from Eidem et al. [11]. Blood pressure (BP) measurement was performed in accordance with current guidelines [12, 13]. In short, BP was measured in a seated position after 5 min of rest, three times on both arms, with at least a 2-min interval between measurements. We used the oscillometric Dinamap device (Dinamap v150; Fa. GE Medical Systems, Chicago, Illinois, USA). The mean was calculated from the second and third measurements, and normalized for sex, age, and height, expressed as z-score [12, 13]. Physical fitness was assessed by graded exercise tests on an ergometer bicycle (sitting position) according to a modified Godfrey protocol [14]. The workload was increased stepwise by 15 W at 1-min intervals. All subjects were encouraged to exercise until exhaustion (breathlessness and leg muscle pain and/or a heart rate ≥ 85% of maximum (calculated 220—age in years) [15].
Echocardiographic Measurements
Transthoracic echocardiography was performed by two uniformly trained investigators. Both were specialized on pediatric echocardiography and followed a standardized protocol using a PHILIPS CX 50 ultrasound machine (Philips Medical Systems, Bothell, USA) equipped with a 5 MHz transducer. Inter- and intra-observer variability was within the range formerly reported by Colan et al. [16]. A segmental analysis was performed to assure segmental anatomy and to exclude a congenital heart defect in accordance with the Guidelines of the American Society of Echocardiography [17].
Left and right ventricular end-diastolic wall thickness and end-diastolic dimensions were obtained from the parasternal short axis view at the level of the papillary muscles using M-mode. LVMI was defined as LV mass/ht2.16 as proposed by Chinali et al. [18]. LVM z-score (LVMz) according to height was calculated as described by Foster et al. [19], LVMIz-for-lean body mass (LBM) was calculated as proposed by Foster et al. [20], both methods of normalization were used for further analysis. Left ventricular end-diastolic dimension z-score (LVEDdz) was calculated according to Lopez et al. [21]. Ejection fraction was measured by the biplane modified Simpson method. For diastolic function pulsed Doppler measurements of mitral and tricuspid inflow (E-, A-wave) were performed in the apical four-chamber view, with the sample volume positioned between the tips of the mitral leaflets within ± 15° of the central volume stream, the E/A ratio was calculated. The time intervals of isovolumetric relaxation time (IVRT) and isovolumetric contraction time (IVCT) were measured from the apical five chamber view using pulse-wave (PW) Doppler positioned to record left ventricular inflow and outflow tract simultaneously. To determine the IVRT, the time interval from the ending of the aortic flow to the beginning of the mitral inflow was measured. For the determination of IVCT, the time interval from the ending of the atrioventricular valve inflow to the beginning of the ventricular outflow was measured. For the further assessment of diastolic function the following PW Tissue Doppler parameters were obtained: peak velocities of early (e′) and late diastolic (a′) excursion of the lateral and septal mitral annulus as well as of the tricuspid annulus. IVRT, obtained by tissue Doppler, was measured from the end of the S-wave to beginning of the following e-wave. Pulmonary venous inflow (PV) was measured in an apical four-chamber view, with the PW sample volume placed as far as possible in the right upper pulmonary vein. The recordings comprised peak systolic velocity (S-wave) as well as peak diastolic velocity (D-wave). Five consecutive cardiac cycles were recorded from every approach. All parameters were measured five times, the median value was taken for further analysis. All measurements were performed by a single investigator. For any given structure, measurements were made only if excellent and unambiguous views were available. Thus, not all structures were measured in all patients.
Statistical Analysis
Our endpoints comprised different echocardiographic parameters (LVMI, LVMIz, mitral E/A ratio, mitral annular E/e′, septal annular E/e′, septal annular IVRT, tricuspid E/A ratio). Data are given as mean ± standard deviations (SD) or absolute and relative frequencies. Echocardiographic parameters were compared between age, sex and BMI categories. Differences in echocardiographic parameters between boys and girls were assessed using t test. Potential covariates were selected for each outcome variable based on prior knowledge. Backward linear regression modeling with a p value of 0.2 or less as selection criteria were performed. Linear regression models for LVMI, LVMz, LVMIz, septal annular IVRT and PV flow D-wave velocity including interaction term between BMI and sex as well as stratification by sex were performed to investigate the varying extent of risk factors on diastolic function and LVMI in boys and girls. A p-value of 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (Statistical Analysis Software, Cary, North Carolina, USA).
Results
Clinical Characteristics of the Study Population
Of the 356 children initially examined, 351 (187 boys; 53%) were included in further analysis. Four children were excluded due to preexisting heart conditions. Two-hundred-two were 2nd grade (8.21 ± 0.52 years of age) and 149 were 5th grade pupils (11.41 ± 0.55 years of age). Both sexes were equally represented in both groups. Anthropometrics, demographical characteristics and clinical details are shown in Table 1. One-hundred-two children (29%) had a BMIz ≥ 1.04 and 55 (16%) had a BMIz ≥ 1.64. The prevalence of overweight children was similar between 2nd and 5th graders (27% vs. 32%; p = 0.2814), while the prevalence of obesity tended to be lower in 2nd grade children (13% vs. 19%; p = 0.0987). The mean BMIz was elevated in both age groups (2nd grade: 0.28; 5th grade: 0.45). The mean waist-circumference z-score increased with age (0.69 vs. 0.91).
Table 1.
Characteristics of the study population
| Variables | Total | Second grade | Fifth grade | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MW | SD | N | MW | SD | N | MW | SD | N | ||
| Age (years) | 9.58 | 1.67 | 351 | 8.21 | 0.52 | 202 | 11.41 | 0.55 | 149 | < 0.0001 |
| Female sex | 47% (164/351) | 47% (95/202) | 46% (69/149) | 0.8481 | ||||||
| Weight (kg) | 36.83 | 12.34 | 351 | 30.21 | 6.87 | 202 | 45.80 | 12.45 | 149 | < 0.0001 |
| Height (cm) | 139.82 | 11.55 | 351 | 132.04 | 6.45 | 202 | 150.36 | 8.03 | 149 | < 0.0001 |
| BMI (kg/m2) | 18.40 | 3.85 | 351 | 17.19 | 2.97 | 202 | 20.04 | 4.29 | 149 | < 0.0001 |
| BMI z-score | 0.36 | 1.12 | 351 | 0.28 | 1.09 | 202 | 0.45 | 1.15 | 149 | 0.1583 |
| Overweight (BMI z-score ≥ 1.04) | 29% (102/351) | 27% (54/202) | 32% (48/149) | 0.2814 | ||||||
| Obese (BMI z-score ≥ 1.64) | 16% (55/351) | 13% (26/202) | 19% (29/149) | 0.0987 | ||||||
| Waist circumference (cm) | 70.35 | 11.91 | 351 | 65.28 | 8.56 | 202 | 77.21 | 12.40 | 149 | < 0.0001 |
| Waist circumference z-score | 0.78 | 0.84 | 351 | 0.69 | 0.84 | 202 | 0.91 | 0.83 | 149 | 0.0136 |
| Systolic BP (mmHg) | 104.04 | 8.21 | 351 | 102.07 | 7.67 | 202 | 106.71 | 8.20 | 149 | < 0.0001 |
| Systolic BP z-score | 0.30 | 0.74 | 351 | 0.35 | 0.71 | 202 | 0.24 | 0.77 | 149 | 0.1653 |
| Systolic BP z-score ≥ 1.282 | 35 (10%) | 19 (9%) | 16 (11%) | 0.6805 | ||||||
| Systolic BP z-score ≥ 1.64 | 12 (3%) | 7 (3%) | 5 (3%) | 0.9554 | ||||||
| Diastolic BP (mmHg) | 60.80 | 5.64 | 351 | 59.60 | 5.35 | 202 | 62.42 | 5.62 | 149 | < .0001 |
| Diastolic BP z-score | 0.05 | 0.49 | 351 | 0.06 | 0.47 | 202 | 0.04 | 0.52 | 149 | 0.7223 |
| Diastolic BP z-score ≥ 1.282 | 5 (1%) | 2 (1%) | 3 (2%) | 0.4239 | ||||||
| Diastolic BP z-score ≥ 1.64 | 1 (0%) | 1 (1%) | 0 (0%) | 0.9555 | ||||||
| Physical fitness (W/kg) | 3.07 | 0.68 | 337 | 3.09 | 0.69 | 196 | 3.04 | 0.66 | 141 | 0.5149 |
| Cholesterol (mg/dl) | 169.38 | 28.31 | 274 | 167.90 | 27.58 | 157 | 171.36 | 29.26 | 117 | 0.3177 |
| HDL (mg/dl) | 57.41 | 12.18 | 273 | 57.87 | 12.14 | 156 | 56.81 | 12.25 | 117 | 0.4804 |
| LDL (mg/dl) | 97.11 | 24.03 | 273 | 94.33 | 21.06 | 156 | 100.80 | 27.15 | 117 | 0.0335 |
| Triglycerides (mg/dl) | 81.48 | 44.93 | 274 | 75.05 | 41.16 | 157 | 90.10 | 48.39 | 117 | 0.0059 |
| Biparental migration | 47% (165/351) | 46% (93/202) | 48% (72/149) | 0.6254 | ||||||
BMI body mass index, BP blood pressure, HDL high density lipoprotein, LDL low density lipoprotein
Echocardiographic Findings of the Study Group
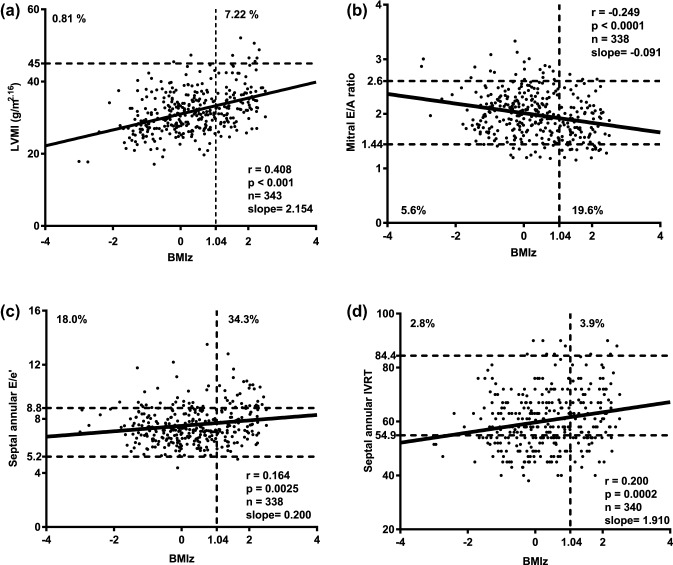
We present our data according to children’s BMIz (< 1.04 and ≥ 1.04) and in comparison to the current reference values published by Eidem et al. [11] (Table 2; Fig. 1). Statistically significant differences between children and adolescents with a BMIz ≥ 1.04 compared to those with BMIz below 1.04 were found for several structural and functional parameters. Left ventricular end-diastolic diameter z-score (LVEDdz) and LVMI [13] were significantly higher in overweight and obese children (p = < 0.0001 and < 0.0001). Furthermore, several diastolic parameters were out of the normal range. While we cannot compare our values with those of the reference studies statistically [11], one can appreciate that for most values the exclusion of overweight and obese children resulted in more favorable values regarding function and morphology. According to the published reference data from Eidem et al., the number of out of range values are presented in Table 2. We only report those cases, in which the measurement pointed toward a potential unfavorable abnormality concerning diastolic function. The comparison of girls and boys revealed significant differences for the mitral and tricuspid E/A ratio as well as for the systolic PV flow (Supplementary Table S1).
Table 2.
Echocardiographic Parameters compared to normal values from Eidem et al. [11]
| Variables | 7–9 Years | 10–13 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI z-score < 1.04 | Out of rangeA | BMI z-score ≥ 1.04 | Out of rangeA | Eidem | BMI z-score < 1.04 | Out of rangeA | BMI z-score ≥ 1.04 | Out of rangeA | Eidem | |
| N = 143 | N = 53 | N = 55 | N = 101 | N = 46 | N = 55 | |||||
| Weight (kg)*# | 26.9 ± 3.6 (17.3–38.7) | 39.2 ± 5.4 (28.1–53.7) | 33.8 ± 14.9 | 39.2 ± 7.1 (24.1–58.6) | 59.4 ± 10.4 (40.6–80.4) | 47.2 ± 16.3 | ||||
| BSA (m2)*# | 0.99 ± 0.09 (0.73–1.26) | 1.21 ± 0.1 (0.99–1.49) | 1.07 ± 0.27 | 1.27 ± 0.14 (0.93–1.66) | 1.58 ± 0.17 (1.17–1.9) | 1.37 ± 0.29 | ||||
| Age (years)* | 8.2 ± 0.5 (7.1–9.8) | 8.3 ± 0.5 (7.6–9.3) | 7.91 ± 1.12 | 11.4 ± 0.6 (10.2–13.5) | 11.4 ± 0.5 (10.6–12.4) | 11.99 ± 1.11 | ||||
| Male | 78 (53%) | 29 (54%) | 49% | 57 (56%) | 23 (48%) | 69% | ||||
| Heart rate (bpm) | 78 ± 10 (56–105) | 79 ± 11 (50–102) | 80 ± 11 | 76 ± 11 (52–116) | 79 ± 11 (59–109) | 75 ± 12 | ||||
| Left ventricle | N = 142 | N = 41 | N = 55 | N = 88 | N = 32 | N = 55 | ||||
| Mitral E velocity (cm/s) | 102.8 ± 11.2 (76.2–133) | 3 (2%) | 104.3 ± 14.3 (75.6–139) | 2 (4%) | 94.4 ± 14.8 | 98 ± 13.8 (69–145) | 9 (9%) | 98.5 ± 16.1 (73.8–142) | 5 (11%) | 94.5 ± 16.0 |
| Mitral A velocity (cm/s)*# | 49.9 ± 9.3 (32.7–86.4) | 14 (10%) | 56.8 ± 11.9 (34.8–87) | 16 (32%) | 49.4 ± 12.5 | 51.5 ± 10.1 (32.4–78.6) | 16 (16%) | 57.4 ± 9.1 (43.8–81.3) | 11 (24%) | 49.5 ± 13.8 |
| Mitral E/A ratio *# | 2.1 ± 0.4 (1.2–3.3) | 7 (5%) | 1.9 ± 0.4 (1.2–2.7) | 10 (20%) | 2.0 ± 0.51 | 2.0 ± 0.4 (1.2–3.1) | 7 (7%) | 1.7 ± 0.3 (1.2–2.6) | 10 (22%) | 2.02 ± 0.58 |
| IVRT (PW)* | 52 ± 8.2 (36–76) | 55.4 ± 6.8 (40–67) | 57.3 ± 8.4 (40–76) | 56.2 ± 7.2 (40–74) | ||||||
| IVCT (PW)* | 68.3 ± 12.4 (44–108) | 64.1 ± 7.7 (49–84) | 70.3 ± 10.1 (49–103) | 69.8 ± 12.5 (49–105) | ||||||
| Tissue Doppler imaging | N = 125 | N = 49 | N = 55 | N = 97 | N = 41 | N = 55 | ||||
| Mitral annular e′-wave velocity* | 20.3 ± 2.7 (12.9–27.4) | 2 (1%) | 19.3 ± 3 (13.3–27.1) | 1 (2%) | 17.2 ± 3.7 | 19.7 ± 2.8 (12.3–27.8) | 10 (10%) | 19 ± 3.1 (11.9–28.4) | 11 (25%) | 19.6 ± 3.4 |
| Mitral annular a′-wave velocity* | 6.5 ± 1.5 (4.1–13.4) | 12 (9%) | 7.5 ± 1.7 (4.2–12.1) | 11 (21%) | 6.7 ± 1.9 | 6.4 ± 1.5 (3.5–12.4) | 8 (8%) | 6.4 ± 1.5 (3–9.5) | 7 (16%) | 6.4 ± 1.8 |
| Mitral annular E/e′* | 5.1 ± 0.8 (3.4–7.2) | 0 (0%) | 5.5 ± 1 (3.2–7.5) | 0 (0%) | 5.8 ± 1.9 | 5 ± 0.9 (3.4–10.5) | 8 (8%) | 5.3 ± 1.2 (3.4–9.3) | 7 (16%) | 4.9 ± 1.3 |
| Mitral annular IVRT | 52.6 ± 8 (38–76.5) | 1 (1%) | 53.1 ± 7.2 (38–74) | 0 (0%) | 62.9 ± 11.9 | 57.5 ± 9.9 (38–85) | 2 (2%) | 55.4 ± 9.5 (40–72) | 0 (0%) | 62.6 ± 12.4 |
| Septal annular e′-wave velocity*# | 14.4 ± 1.8 (8.5–18.5) | 10 (7%) | 13.6 ± 1.7 (11–18.1) | 3 (6%) | 13.4 ± 1.9 | 13.3 ± 1.9 (8–18.3) | 24 (24%) | 12.1 ± 1.8 (7.4–17.3) | 16 (36%) | 14.5 ± 2.6 |
| Septal annular a′-wave velocity*# | 6.3 ± 1.2 (4.2–11.4) | 35 (24%) | 6.8 ± 1 (4.7–9.3) | 18 (35%) | 5.9 ± 1.3 | 5.8 ± 1.1 (3.6–10.1) | 3 (3%) | 6.3 ± 1 (4.3–8.9) | 1 (2%) | 6.1 ± 2.3 |
| Septal annular E/e′*# | 7.3 ± 1.1 (5.2–12.2) | 13 (9%) | 7.8 ± 1.3 (5.6–11.7) | 12 (23%) | 7.2 ± 1.6 | 7.5 ± 1.4 (4.4–13.5) | 32 (32%) | 8.3 ± 1.7 (5–12.8) | 23 (51%) | 6.6 ± 1.4 |
| Septal annular IVRT*# | 55.7 ± 8.7 (38–76) | 0 (0%) | 59.0 ± 8.9 (40–76) | 0 (0%) | 65.6 ± 10.7 | 64.2 ± 10.5 (45–90) | 7 (7%) | 68 ± 11.1 (45–90) | 4 (9%) | 72.5 ± 12.3 |
| Right ventricle | N = 134 | N = 46 | N = 55 | N = 95 | N = 44 | N = 55 | ||||
| Tricuspid E velocity (cm/s) | 59.4 ± 7.7 (41.3–76.8) | 7 (5%) | 61 ± 8.7 (43.8–90) | 1 (2%) | 60.5 ± 13.9 | 61.1 ± 9.5 (39.4–90) | 4 (4%) | 59.8 ± 10.9 (36–100.5) | 4 (9%) | 59.6 ± 11.4 |
| Tricuspid A velocity (cm/s) | 38.4 ± 8 (21.4–67.2) | 0 (0%) | 40.4 ± 9 (24.5–60.6) | 0 (0%) | 42.4 ± 10.8 | 40 ± 9.4 (20.3–70) | 0 (0%) | 43.3 ± 9.1 (30–68) | 0 (0%) | 39.2 ± 11.3 |
| Tricuspid E/A ratio# | 1.61 ± 0.34 (0.88–2.67) | 5 (4%) | 1.57 ± 0.34 (1.07–2.83) | 1 (2%) | 1.49 ± 0.40 | 1.59 ± 0.36 (0.85–2.57) | 5 (5%) | 1.40 ± 0.26 (0.62–2.20) | 6 (14%) | 1.61 ± 0.47 |
| Tissue Doppler imaging | N = 131 | N = 44 | N = 55 | N = 92 | N = 37 | N = 55 | ||||
| Tricuspid annular e′-wave velocity* | 16.2 ± 2.3 (10.6–23.3) | 13 (9%) | 14.8 ± 2.5 (10.5–20) | 17 (34%) | 16.5 ± 3.0 | 14.3 ± 3.2 (6–23.9) | 40 (41%) | 13.8 ± 2.5 (9–21) | 14 (38%) | 16.5 ± 3.1 |
| Tricuspid annular a′-wave velocity | 8.8 ± 2.2 (4.6–15.6) | 0 (0%) | 9.1 ± 2.3 (5.1–17.1) | 0 (0%) | 9.8 ± 2.7 | 8.6 ± 2.6 (4.4–17.5) | 0 (0%) | 9.2 ± 2.1 (6–16) | 0 (0%) | 10.3 ± 3.4 |
| Tricuspid annular E/e′* | 3.7 ± 0.7 (2.5–5.9) | 0 (0%) | 4.2 ± 0.7 (3–5.4) | 0 (0%) | 3.6 ± 0.8 | 4.5 ± 1.5 (2.8–13) | 0 (0%) | 4.4 ± 1 (2.6–7.3) | 0 (0%) | 3.5 ± 1.4 |
| PV S-wave* | 52.6 ± 8.1 (32.4–74.4) | 0 (0%) | 57.1 ± 8.9 (41.3–85.5) | 0 (0%) | 50.7 ± 11.3 | 49.8 ± 8.7 (32–79.4) | 0 (0%) | 51.4 ± 7.6 (35–72.5) | 0 (0%) | 49.0 ± 11.1 |
| PV D-wave | 67.7 ± 8.6 (34.8–87) | 1 (1%) | 66.6 ± 8.7 (42.8–81.6) | 0 (0%) | 53.3 ± 11.4 | 66.8 ± 9 (47.4–99) | 0 (0%) | 66.1 ± 5.9 (53.7–77.6) | 0 (0%) | 58.4 ± 12.1 |
| EF Simpson biplane* | 68 ± 2.9 (57.3–75.6) | 66.6 ± 3.2 (57–73.2) | 68.2 ± 3.5 (56.8–78) | 69 ± 3.2 (62.8–76.5) | ||||||
| M-mode | N = 134 | N = 47 | N = 95 | N = 40 | ||||||
| IVSd*# | 0.58 ± 0.07 (0.43–0.77) | 0.64 ± 0.08 (0.51–0.88) | 0.65 ± 0.09 (0.48–0.9) | 0.72 ± 0.09 (0.57–0.9) | ||||||
| LVEDd*# | 3.86 ± 0.27 (3.11–4.74) | 3.98 ± 0.28 (3.51–4.71) | 4.14 ± 0.36 (3.38–5.08) | 4.37 ± 0.39 (3.7–5.67) | ||||||
| LVEDdz*# | 0.01 ± 0.75 (− 2.31 to 2.19) | − 0.76 ± 0.68 (− 2.03 to 1.12) | − 0.48 ± 0.77 (− 2.14 to 1.78) | − 1.03 ± 0.91 (− 3.06 to 1.86) | ||||||
| LVPWd*# | 0.58 ± 0.07 (0.4–0.79) | 0.63 ± 0.08 (0.44–0.81) | 0.62 ± 0.09 (0.4–0.9) | 0.7 ± 0.1 (0.56–1) | ||||||
| LVMI (g/m2.16)*# | 30.9 ± 5.2 (17.1–47.3) | 34 ± 5.9 (24.7–52.1) | 30 ± 5.7 (17.8–45.5) | 35.9 ± 6.1 (25.8–50.6) | ||||||
| LVMI (g/m2.7)*# | 27 ± 4.6 (14.7–40.9) | 29 ± 4.9 (21.2–43.3) | 24.4 ± 4.4 (14.7–37.1) | 28.7 ± 4.7 (20–40.2) | ||||||
| LVMz*# | − 1.48 ± 0.95 (− 5.03 to 0.9) | − 0.98 ± 0.89 (− 2.7 to 1.27) | − 1.95 ± 1.15 (− 5.11 to 0.53) | − 0.95 ± 0.9 (− 3.18 to 0.84) | ||||||
BSA body surface area, E peak early mitral inflow Doppler velocities, A peak late mitral inflow Doppler velocities, e′ early diastolic annular myocardial velocity, a′ late diastolic annular myocardial velocity, IVRT isovolumetric relaxation time, IVCT isovolumetric contraction time, PV S-wave pulmonary venous flow velocity systolic, PV D-wave pulmonary venous flow velocity diastolic, EF ejection fraction, IVSd interventricular septal thickness end-diastolic, LVEDd left ventricular end-diastolic dimension, LVEDdz left ventricular end-diastolic dimension z-score, LVPWd left ventricular posterior wall dimension end-diastolic, LVMI left ventricular mass indexed for height2.16, LVMz left ventricular mass z-score adjusted for height
*Significant differences in the group of 7–9 year olds, #significant differences in the group of 10–13 year olds
ANumber of patients in which the measurement pointed toward a potential unfavorable abnormality (± 1 SD compared to Eidem et al.) concerning diastolic function
Fig. 1.
Echocardiographic parameters in conjunction with increasing BMIz. a Higher BMIz was associated with higher left ventricular mass/height 2.16. 7.22% of the overweight and obese children, but only 0.81% of those with BMIz < 1.04 had left ventricular hypertrophy (p < 0.001). The dotted line delineates 45 g/m2.16 as the upper normal limit for LVMI. b Using the current reference values from Eidem et al., 19.6% of the overweight or obese, but only 5.6% of the children with a BMIz < 1.04 had a mitral valve E/A ratio below the lower limit. c Using the current reference values from Eidem et al., 34.3% of the overweight or obese, but only 18% of the children with a BMIz < 1.04 had a septal annular E/e′ ratio above the upper limit. d Using the current reference values from Eidem et al., 3.9% of the overweight or obese, but only 2.8% of the children with a BMIz < 1.04 had an isovolumetric relaxation time (IVRT) above the upper limit. BMIz body mass index z-score, E peak early diastolic inflow Doppler velocity, e early diastolic annular myocardial velocity, IVRT isovolumetric relaxation time, LVMI left ventricular mass index, TDI tissue Doppler imaging
In the following, we will highlight particular morphological and functional aspects of the cohort.
Left Ventricular Mass Index (LVMI)
As indicated above, higher BMI z-scores were associated with increases in LVMI (p < 0.001, Fig. 2). To explore which other factors contributed to a higher LVMI, we performed a multivariable linear regression analysis (Table 3). In addition to BMI, we found that sex, age and physical fitness were independent predictors of a higher LVMI. Importantly, we could not find an association between LVMI and systolic or diastolic BP in our cohort of healthy school children.
Fig. 2.
Left ventricular mass index (LVMI) and left ventricular mass index z-score (LVMIz) stratified according to children’s BMI z-score (BMIz). a Non-overweight children (BMIz < 1.04) had a significant lower LVMI than children overweight/obese (BMIz ≥ 1.04). b Children with BMIz < 1.04 had a significant lower LVMIz than children with a BMIz ≥ 1.04. BMI body mass index, BMIz body mass index z-score, LVMI left ventricular mass index, LVMIz left ventricular mass index z-score
Table 3.
Standardized models for the endpoints of different echocardiographic parameters
| LVMI | LVMz | ||||||
|---|---|---|---|---|---|---|---|
| β | SD | p | β | SD | p | ||
| Intercept | 32.466 | 0.8696 | 0.0007 | Intercept | − 1.3483 | 0.1527 | 0.0126 |
| Girls | − 21.0763 | 5.1787 | < 0.0001 | Girls | − 4.2042 | 0.9497 | < 0.0001 |
| Boys | 0 | Boys | 0 | ||||
| Age | − 19.8192 | 5.6944 | 0.0006 | Age | − 5.9264 | 1.0495 | < 0.0001 |
| BMI | 60.0535 | 7.1486 | < 0.0001 | BMI | 10.9623 | 1.367 | < 0.0001 |
| Physical fitness | 20.8346 | 6.9082 | 0.0028 | Physical fitness | 3.6986 | 1.2615 | 0.0036 |
| Systolic BP right arm | − 1.4964 | 1.0137 | 0.1409 | ||||
| Mitral E/A ratio | Mitral annular E/e′ | ||||||
|---|---|---|---|---|---|---|---|
| β | SD | p | β | SD | p | ||
| Intercept | 2.0144 | 0.04536 | 0.0005 | Intercept | 5.1167 | 0.105 | 0.0004 |
| Girls | − 0.8167 | 0.3862 | 0.0352 | Girls | − 1.1741 | 0.9041 | 0.195 |
| Boys | 0 | Boys | 0 | ||||
| Age | − 0.6634 | 0.4343 | 0.1276 | Age | − 2.0024 | 1.0048 | 0.0471 |
| BMI | − 1.4953 | 0.4231 | 0.0005 | BMI | 3.199 | 0.9763 | 0.0012 |
| Diastolic BP right arm | − 1.0966 | 0.4029 | 0.0069 | ||||
| Septal annular E/e′ | Septal annular IVRT | ||||||
|---|---|---|---|---|---|---|---|
| β | SD | p | β | SD | p | ||
| Intercept | 7.6456 | 0.1711 | 0.0005 | Intercept | 60.5364 | 2.338 | 0.0015 |
| Girls | − 1.6997 | 1.3082 | 0.1948 | Girls | 6.0434 | 9.2896 | 0.5158 |
| Boys | 0 | Boys | 0 | ||||
| Age | 0.786 | 1.4675 | 0.5926 | Age | 34.0376 | 10.5477 | 0.0014 |
| BMI | 5.4841 | 1.4157 | 0.0001 | BMI | 36.3556 | 9.9999 | 0.0003 |
| Diastolic BP right arm | 29.1607 | 9.5348 | 0.0024 | ||||
| Heart rate | − 46.9614 | 9.343 | < .0001 | ||||
| Tricuspid E/A ratio | PV D-wave | ||||||
|---|---|---|---|---|---|---|---|
| β | SD | p | β | SD | p | ||
| Intercept | 1.6628 | 0.09019 | 0.0029 | Intercept | 67.0637 | 0.4690 | < 0.0001 |
| Girls | − 0.5104 | 0.3263 | 0.1188 | Girls | − 18.5681 | 8.8170 | 0.0360 |
| Boys | 0 | Boys | 0 | ||||
| Age | − 0.2558 | 0.377 | 0.4979 | Age | 1.6547 | 9.3987 | 0.8604 |
| BMI | − 0.9516 | 0.355 | 0.0077 | BMI | − 20.6708 | 11.9846 | 0.0855 |
| Physical fitness | − 12.3673 | 11.5314 | 0.2843 | ||||
LVMI left ventricular mass indexed for height2.16, LVMz left ventricular mass z-score adjusted for height, E peak early mitral inflow Doppler velocities, A peak late mitral inflow Doppler velocities, e′ early diastolic annular myocardial velocity, IVRT isovolumetric relaxation time, PV D-wave pulmonary venous flow velocity diastolic
We further explored sex differences and found higher LVMI in boys than in girls for children with a BMIz < 1.04 (p < 0.0001), but this difference was no longer observed in overweight and obese children (p = 0.096, Fig. 3). We performed a sex stratified analysis to investigate how changes in BMI affect LVMI in each sex (Table 4). Our results support the observation that girls and boys with higher BMI values have comparable absolute LVMI values. We show that, e.g., at a BMI value of 30 kg/m2 girls are expected to have an estimated LVMI of 37.9 g/m2 whereas boys will have 39.5 g/m2. This convergence at higher BMI values is the result of a greater increase in LVMI for a given BMI change. Per 1 kg/m2 BMI increase LVMI increases by 0.5272 g/m2.16 in boys, whereas by 0.7336 g/m2.16 in girls.
Fig. 3.
Left ventricular mass demonstrated for boys and girls separated according to children’s BMIz. a Significant difference in LVMI between boys and girls with a BMIz < 1.04. b The difference of LVMI between boys and girls was no longer found in children with a BMIz ≥ 1.04. c There is a sex-specific difference between the slopes for LVMI with increasing BMIz. BMIz body mass index z-score, LVMI left ventricular mass index
Table 4.
Interaction terms between sex and BMI as well as stratification by sex
| LVMI | LVMz | ||||||
|---|---|---|---|---|---|---|---|
| β | SD | p | β | SD | p | ||
| Intercept | 27.7298 | 2.4435 | 0.0077 | Intercept | − 1.1683 | 0.4406 | 0.1177 |
| Age | − 0.544 | 0.1867 | 0.0038 | Age | − 0.1854 | 0.03379 | < 0.0001 |
| Girls | − 6.3765 | 2.7264 | 0.0199 | Girls | − 1.2689 | 0.4941 | 0.0107 |
| Boys | 0 | Boys | 0 | ||||
| BMI | 0.6236 | 0.1076 | < 0.0001 | BMI | 0.1014 | 0.0195 | < 0.0001 |
| BMI * Girls | 0.1794 | 0.1456 | 0.2187 | BMI * Girls | 0.03799 | 0.02638 | 0.1508 |
| BMI * Boys | 0 | BMI * Boys | 0 | ||||
| Intercept | 24.5598 | 2.3497 | 0.009 | Intercept | − 2.3085 | 0.4236 | 0.0321 |
| BMI * Boys | 0.5272 | 0.1109 | < 0.0001 | BMI * Boys | 0.07035 | 0.01923 | 0.0003 |
| Intercept | 16.8644 | 1.9312 | 0.0129 | Intercept | − 3.8709 | 0.4164 | 0.0114 |
| BMI * Girls | 0.7336 | 0.09871 | < 0.0001 | BMI * Girls | 0.1173 | 0.02042 | < 0.0001 |
| Septal annular IVRT | PV D-wave | ||||||
|---|---|---|---|---|---|---|---|
| β | SD | p | β | SD | p | ||
| Intercept | 41.8147 | 4.8547 | 0.0132 | Intercept | 66.6673 | 3.6558 | 0.003 |
| Age | 1.5155 | 0.3518 | < 0.0001 | Age | − 0.04501 | 0.2947 | 0.8787 |
| Girls | − 10.6783 | 5.0561 | 0.0354 | Girls | 8.1222 | 4.4779 | 0.0706 |
| Boys | 0 | Boys | 0 | ||||
| BMI | 0.2529 | 0.1997 | 0.2064 | BMI | 0.09589 | 0.1739 | 0.5817 |
| BMI * Girls | 0.5525 | 0.2698 | 0.0414 | BMI * Girls | − 0.54 | 0.2378 | 0.0238 |
| BMI * Boys | 0 | BMI * Boys | 0 | ||||
| Intercept | 52.5448 | 5.2389 | 0.0098 | Intercept | 66.3696 | 3.0802 | 0.0021 |
| BMI * Boys | 0.4507 | 0.2031 | 0.0278 | BMI * Boys | 0.08843 | 0.1662 | 0.5953 |
| Intercept | 40.884 | 4.3985 | 0.0114 | Intercept | 74.5022 | 3.2425 | 0.0019 |
| BMI * Girls | 1.0609 | 0.1989 | < 0.0001 | BMI * Girls | − 0.4517 | 0.1697 | 0.0086 |
LVMI left ventricular mass indexed for height2.16, LVMz left ventricular mass z-score adjusted for height, IVRT isovolumetric relaxation time, PV D-wave pulmonary venous flow velocity diastolic
We also calculated LVMIz [20], expressing LV mass relative to LBM, meant as sensitivity analysis. Similar to our calculations based on LVMI by Chinali et al., we only saw very few children with LV hypertrophy. Using LBM normalized values we did not expect to see an effect of BMI. However, we confirmed our previous observation that higher BMI values were associated with smaller increases in LVM in boys (boys: β = − 0.033, p = 0.045; girls: β = − 0.005, p = 0.72) (Supplementary Table S2).
Left Ventricular Diastolic Function
As indicated above overweight and obese children also tended to have more unfavorable echocardiographic diastolic parameters than children with BMIz < 1.04. Early mitral and tricuspid inflow velocities (E) were similar between the groups, but both mitral and tricuspid A-wave velocities were increased, resulting in a decreased E/A ratio. The values for the E/e′ ratio (septal and mitral annular) were higher in overweight and obese children and the IVRT measured at the septal annulus was significant longer. The effect of increasing BMIz is also highlighted in Fig. 1.
Multivariable linear regression analysis was used to further explore contributing factors for the selected diastolic parameters (Table 3). The E/A ratio of the mitral valve was independently associated with sex, BMI and diastolic BP. Girls showed a significant lower E/A ratio compared to boys while age had no effect. Interestingly higher BMI was associated with an increase in the A-wave, while the E-wave essentially was left unchanged. E/e′ ratio (either septal or mitral) were only predicted by BMI without a contribution of sex and age. IVRT was independently associated with age, BMI and diastolic BP, while heart rate was inversely related.
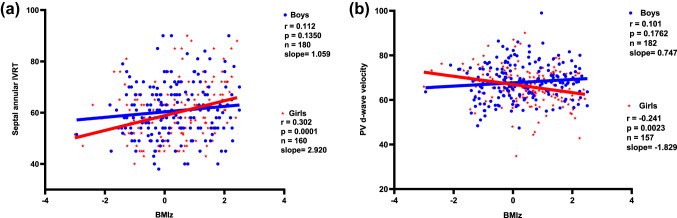
We assumed again an interaction with sex. By creating an interaction term (Table 4; Fig. 4a), we demonstrated a significantly higher increase for IVRT in girls for a given BMI. Sex stratified regression models showed how BMI affect IVRT in boys and girls differently. We found a prolonged IVRT of 0.5525 ms for a given BMI in girls compared to boys. The stratified regression analysis demonstrated that a BMI increase of 1 kg/m2 was associated with an IVRT increase of 0.4507 ms in boys (p = 0.028), while in girls IVRT extended significantly more with 1.0609 ms (p < 0.001).
Fig. 4.
a Sex-specific difference between the slopes for a isovolumetric relaxation time (IVRT) with increasing BMIz and b for diastolic pulmonary venous flow (PV d-wave) with increasing BMIz. BMIz body mass index z-score, IVRT isovolumetric relaxation time, PVd pulmonary venous flow velocity diastolic
Interestingly this phenomenon was also found in the association between reduction of diastolic PV flow and BMI, in which girls showed a reduction of 0.54 m/s with a BMI increase of 1 kg/m2 compared to boys. The sex stratified regression model confirmed a reduced diastolic PV flow with increasing BMI in girls (Fig. 4b; Table 4).
Discussion
This cross-sectional study on echocardiographic changes in a large cohort of apparently healthy German school children significantly extends the available data currently used to assess morphological and functional cardiac aspects. We provide values for many parameters used in daily clinical assessment for two age groups separated by children’s BMI. We show significant structural (LVMI) and functional (A-wave velocities, E/A ratio, pulmonary flow velocities) differences between girls and boys. Our data confirm the importance of overweight and obesity for many of the parameters used in routine work-up of patients. We extend current data by showing a greater effect of overweight and obesity on different echocardiographic parameters in girls.
Sex Differences
Sex differences in LVMI as an important structural parameter have been repeatedly reported [22]. However, information about sex-related differences in diastolic echocardiographic parameters in children is scarce most likely due to the limited number of cases available in earlier reference studies [23, 24]. Values for several echocardiographic parameters measured in girls from our study were found in the respective upper or lower ranges of normality, indicating a real biological difference between the sexes. Apart from those physiological differences, we demonstrate that in girls the same BMI increase resulted in a greater increase in LVMI as well as a greater increase in IVRT and diastolic PV flow compared to boys. These findings suggest that there are sex-related negative effects of being overweight or obese on mass as well as compliance of the left ventricle with increasing BMI. We therefore assume a higher susceptibility toward cardiac changes in presence of increasing BMI as an important cardiovascular risk factor in girls compared to boys. Our findings in these otherwise healthy but overweight children are in accordance with data from a large cohort of children with chronic kidney disease, in whom a stronger association of obesity with LVMIz and LVH was demonstrated among girls compared to boys [7]. A greater vulnerability of girls upon cardiovascular stresses had been demonstrated in older girls after renal transplantation that displayed higher BP values when exposed to an additional risk, i.e., higher trough levels for cyclosporine A [25]. Further support comes from recent adult data. The protective effect of female sex on CV health is lost in presence of additional comorbidities and stresses (such as type 2 diabetes, hypertension, hypercholesterinaemia, sedentary lifestyle, mental stress) [26–28].
While peripubertal and perimenopausal differences point toward a hormonal cause for CVD risk [29], our data suggest that risk factors earlier in life must be taken into account, as most of our study population had not reached puberty yet. A possible explanation for our findings is a significantly higher adipose mass in females than in males for each BMI category, as described by De Simone et al. [30] and for pediatric age by Taylor et al. [31]. Adipose tissue is considered to be a metabolic highly active tissue and a large endocrine organ [32] expressing several hormones, growth factors and cytokines [33, 34]. It has been shown that especially in women adiposity and inflammation pathways are highly relevant in the development of CVD [35]. Lau et al. [36] demonstrated sex differences in circulating biomarkers, with significant higher levels of leptin and ceruloplasmin in women than in men. For leptin levels, there were found associations with diastolic function (E/e′) in adults, after adjusting for age, sex and BMI [37]. Higher ceruloplasmin levels were associated with heart failure and were weakly associated with CVD [38]. Unfortunately we do not have information on leptin, ceruloplasmin or other factors derived from adipose tissue of our cohort. Notably, a correlation of high-sensitive C-reactive protein with echocardiographic parameters was not found.
Impact of Obesity
The importance of obesity for the different echocardiographic parameters has been controversially discussed especially concerning echocardiographic parameters of diastolic function. But in nearly all studies, indices of LV mass were greater in overweight and obese than in normal weight children or adolescents [39–42]. It should be noted that, the prevalence of left ventricular hypertrophy in overweight children varies dependent of the method used for normalization [43]. Children in our study showed a higher LVMI and LVMz with increasing BMI, which is in agreement with previous pediatric studies, describing a correlation between BMI and LVMI [4, 5, 39, 44–47]. In agreement with recent results from larger cohorts of children [41] the influence of BP, often discussed as possible reason for a higher LVMI in overweight children [4], could not be confirmed in our cohort.
In adulthood early subclinical changes of diastolic parameters are very sensitive indicators for disturbances of myocardial energy metabolism [48]. It is known that, overweight and obesity lead to energetic abnormalities and insufficient energy supply of the myocardium [49], but the evaluation of diastolic function is still a very complex field of science. Our data on lower LV compliance in case of overweight and obesity resemble data from larger studies [5]. Di Salvo et al. investigated 150 obese children and adolescents and showed a significant higher E/e′ ratio as well as a longer IVRT in these subjects in comparison to a control group [50]. Dhuper et al. presented a higher E/e′ ratio and lower mitral E/A ratio in 213 obese children, while subjects with hypertension were not excluded and the effect of obesity solely could not be rated [46]. While some smaller studies could not show any differences between overweight and normal weight [44], others did but with heterogeneous results for the different parameters describing diastolic function [4, 5, 40]. Especially a difference in E/e′ and E/A ratio could be observed in several studies [4, 39, 46, 51]. A possible explanation for these partly contradicting results may be that in presence of chronic volume overload—as assumed in overweight and obesity—only minimal changes in tissue Doppler velocities can be expected [52]. Studies with rather smaller sample sizes might have had not enough power to show significant differences in all the parameters.
Our findings suggest that changes in cardiac structure start early in life and occur at much lower BMIz level than expected. This implicates an urgent need for an effective obesity prevention in children and adolescents, with a special focus on obesity-related cardiac alterations in girls.
Study Limitations
Potential limitation of the current study is the lack of information about the pubertal status of our study participants. Since sex-specific hormone levels play a significant role for cardiovascular health in adults, a closer investigation of pubertal status would have been favorable. This cross-sectional study design does not provide information about the potential progression of the described cardiac alterations in adulthood or potential reversibility. So the clinical significance of these findings remains unknown and will require longitudinal follow-up over several years to determine their predictive value. LVMI and left ventricular diastolic function were probably also influenced by other factors that could not be examined, e.g. hyperinsulinemia or leptin levels.
Conclusion
There are significant structural and functional sex differences in a number of echocardiographic parameters in children and adolescents. Our data confirm the importance of overweight and obesity for many of the parameters used in routine work-up of patients with changes in myocardial structure and function, indicating an early onset of potentially unfavorable alterations in the myocardium. We demonstrate a higher susceptibility of girls by showing a negative effect of being overweight or obese on different echocardiographic parameters in girls. Further studies are needed to explore how the knowledge on sex-specific risk factors can be implemented in current risk management strategies to maximize benefit especially for girls.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- BMI
Body mass index
- BMIz
Body mass index z-score
- BP
Blood pressure
- BSA
Body surface area
- CVD
Cardiovascular disease
- HDL
High-density lipoprotein cholesterol
- LDL
Low-density lipoprotein cholesterol
- A
Peak late mitral inflow Doppler velocities
- a′
Late diastolic annular myocardial velocity
- E
Peak early mitral inflow Doppler velocities
- e′
Early diastolic annular myocardial velocity
- EF
Ejection fraction
- IVCT
Isovolumetric contraction time
- IVRT
Isovolumetric relaxation time
- IVSd
Interventricular end-diastolic septum thickness
- LVEDd
Left ventricular end-diastolic dimension
- LVEDdz
Left ventricular end-diastolic dimension z-score
- LVM
Left ventricular mass
- LVMz
Left ventricular mass z-score
- LVMI
Left ventricular mass index
- LVMIz
Left ventricular mass index z-score
- LVPWd
Left ventricular end-diastolic posterior wall thickness
- PV D-wave
Diastolic pulmonary venous flow velocity
- PV S-wave
Systolic pulmonary venous flow velocity
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by Braukmann-Wittenberg-Herz-Stiftung.
Data Availability
Data available upon reasonable request to the authors.
Code Availability
Not applicable.
Consent for Publication
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
The study was approved by the Institutional Review Board (No. 7290) and performed according to the Declaration of Helsinki.
Informed Consent
All parents and children gave their written informed consent before participation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jeannine von der Born and Sarah Baberowski have contributed equally to this study.
References
- 1.Tran AH, Flynn JT, Becker RC, Daniels SR, Falkner BE, Ferguson M, Hanevold CD, Hooper SR, Ingelfinger JR, Lande MB, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels JA, Urbina EM. Subclinical systolic and diastolic dysfunction is evident in youth with elevated blood pressure. Hypertension. 2020;75:1551–1556. doi: 10.1161/HYPERTENSIONAHA.119.14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di BP, Capaldo B, Forziato C, Sanguigno E, Di FT, Scilla C, Cavuto L, Saitta F, Sibilio G, Moio N. Central adiposity and left ventricular mass in obese children. Nutr Metab Cardiovasc Dis. 2008;18:613–617. doi: 10.1016/j.numecd.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T, Manlhiot C, Jaeggi ET, Bradley TJ, Mertens L. Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiogr. 2012;25:401–410.e401. doi: 10.1016/j.echo.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, Zimmer M, Mende M, Linke A, Kiess W, Schuler G, Korner A, Erbs S. Childhood obesity: impact on cardiac geometry and function. JACC Cardiovasc Imaging. 2014;7:1198–1205. doi: 10.1016/j.jcmg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–1319. doi: 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 6.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/S0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 7.Brady TM, Roem J, Cox C, Schneider MF, Wilson AC, Furth SL, Warady BA, Mitsnefes M. Adiposity, sex, and cardiovascular disease risk in children with CKD: a longitudinal study of youth enrolled in the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis. 2020;76:166–173. doi: 10.1053/j.ajkd.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78:723–729. doi: 10.1038/pr.2015.160. [DOI] [PubMed] [Google Scholar]
- 9.Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311; discussion 312–303 [PubMed]
- 10.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 11.Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, Ayres NA, Bezold LI, O'Brian Smith E, Pignatelli RH. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–221. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167:653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 13.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, Subcommittee on Screening and Management of High Blood Pressure in Children Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017 doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 14.Klijn PH, van der Net J, Kimpen JL, Helders PJ, van der Ent CK. Longitudinal determinants of peak aerobic performance in children with cystic fibrosis. Chest. 2003;124:2215–2219. doi: 10.1378/chest.124.6.2215. [DOI] [PubMed] [Google Scholar]
- 15.Greiwing A, Hager A, Kreiker C, Kroidl RF, Lehnigk B, Scheid P, Schomaker R, Schwarz S. Kursbuch Spiroergometrie. In: Kroidl RF, Schwarz S, Lehnigk B, editors. Technik und Befundung verständlich gemacht. Stuttgart: Georg Thieme Verlag; 2010. [Google Scholar]
- 16.Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, Chen S, Golding F, Radojewski E, Camitta M, Carboni M, Rychik J, Stylianou M, Tani LY, Selamet Tierney ES, Wang Y, Sleeper LA, Pediatric Heart Network I. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–854.e846. doi: 10.1016/j.echo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J, Task Force of the Pediatric Council of the American Society of Echocardiography, Pediatric Council of the American Society of Echocardiography Guidelines and standards for performance of a pediatric echocardiogram: a Report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–1430. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Chinali M, Emma F, Esposito C, Rinelli G, Franceschini A, Doyon A, Raimondi F, Pongiglione G, Schaefer F, Matteucci MC. Left ventricular mass indexing in infants, children, and adolescents: a simplified approach for the identification of left ventricular hypertrophy in clinical practice. J Pediatr. 2016;170:193–198. doi: 10.1016/j.jpeds.2015.10.085. [DOI] [PubMed] [Google Scholar]
- 19.Foster BJ, Mackie AS, Mitsnefes M, Ali H, Mamber S, Colan SD. A novel method of expressing left ventricular mass relative to body size in children. Circulation. 2008;117:2769–2775. doi: 10.1161/CIRCULATIONAHA.107.741157. [DOI] [PubMed] [Google Scholar]
- 20.Foster BJ, Khoury PR, Kimball TR, Mackie AS, Mitsnefes M. New reference centiles for left ventricular mass relative to lean body mass in children. J Am Soc Echocardiogr. 2016;29:441–447.e442. doi: 10.1016/j.echo.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Lopez L, Colan S, Stylianou M, Granger S, Trachtenberg F, Frommelt P, Pearson G, Camarda J, Cnota J, Cohen M, Dragulescu A, Frommelt M, Garuba O, Johnson T, Lai W, Mahgerefteh J, Pignatelli R, Prakash A, Sachdeva R, Soriano B, Soslow J, Spurney C, Srivastava S, Taylor C, Thankavel P, van der Velde M, Minich L, Pediatric Heart Network Investigators Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017 doi: 10.1161/circimaging.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Cantinotti M, Lopez L. Nomograms for blood flow and tissue Doppler velocities to evaluate diastolic function in children: a critical review. J Am Soc Echocardiogr. 2013;26:126–141. doi: 10.1016/j.echo.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Kapuku GK, Davis HC, Shah N, McMillan AM, Harshfield GA. Gender differences in diastolic function among youth. Pediatr Cardiol. 2008;29:102–107. doi: 10.1007/s00246-007-9093-z. [DOI] [PubMed] [Google Scholar]
- 25.Sugianto RI, Schmidt BMW, Memaran N, Duzova A, Topaloglu R, Seeman T, Konig S, Dello Strologo L, Murer L, Ozcakar ZB, Bald M, Shenoy M, Buescher A, Hoyer PF, Pohl M, Billing H, Oh J, Staude H, Pohl M, Genc G, Klaus G, Alparslan C, Grenda R, Rubik J, Krupka K, Tonshoff B, Wuhl E, Melk A. Sex and age as determinants for high blood pressure in pediatric renal transplant recipients: a longitudinal analysis of the CERTAIN Registry. Pediatr Nephrol. 2020;35:415–426. doi: 10.1007/s00467-019-04395-4. [DOI] [PubMed] [Google Scholar]
- 26.Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35:111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sillars A, Ho FK, Pell GP, Gill JMR, Sattar N, Gray S, Celis-Morales C. Sex differences in the association of risk factors for heart failure incidence and mortality. Heart. 2020;106:203–212. doi: 10.1136/heartjnl-2019-314878. [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E. A journey in diabetes: from clinical physiology to novel therapeutics: the 2020 Banting Medal for Scientific Achievement Lecture. Diabetes. 2021;70:338–346. doi: 10.2337/dbi20-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shlyakhto E. Gendered innovations in the study of cardiovascular diseases. Adv Exp Med Biol. 2018;1065:655–675. doi: 10.1007/978-3-319-77932-4_40. [DOI] [PubMed] [Google Scholar]
- 30.De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, Lee ET, Howard BV. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens. 2011;29:1431–1438. doi: 10.1097/HJH.0b013e328347a093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72:490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- 32.Unamuno X, Gomez-Ambrosi J, Rodriguez A, Becerril S, Fruhbeck G, Catalan V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Investig. 2018;48:e12997. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 33.Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15:507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 34.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 35.Motiwala SR, Sarma A, Januzzi JL, O'Donoghue ML. Biomarkers in ACS and heart failure: should men and women be interpreted differently? Clin Chem. 2014;60:35–43. doi: 10.1373/clinchem.2013.202531. [DOI] [PubMed] [Google Scholar]
- 36.Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, Lyass A, Larson MG, Levy D, Ho JE. Sex differences in circulating biomarkers of cardiovascular disease. J Am Coll Cardiol. 2019;74:1543–1553. doi: 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawaguchi T, Nakajima T, Haruyama A, Hasegawa T, Shibasaki I, Nakajima T, Kaneda H, Arikawa T, Obi S, Sakuma M, Ogawa H, Takei Y, Toyoda S, Nakamura F, Abe S, Fukuda H, Inoue T. Association of serum leptin and adiponectin concentrations with echocardiographic parameters and pathophysiological states in patients with cardiovascular disease receiving cardiovascular surgery. PLoS ONE. 2019;14:e0225008. doi: 10.1371/journal.pone.0225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dadu RT, Dodge R, Nambi V, Virani SS, Hoogeveen RC, Smith NL, Chen F, Pankow JS, Guild C, Tang WH, Boerwinkle E, Hazen SL, Ballantyne CM. Ceruloplasmin and heart failure in the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2013;6:936–943. doi: 10.1161/CIRCHEARTFAILURE.113.000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porcar-Almela M, Codoner-Franch P, Tuzon M, Navarro-Solera M, Carrasco-Luna J, Ferrando J. Left ventricular diastolic function and cardiometabolic factors in obese normotensive children. Nutr Metab Cardiovasc Dis. 2015;25:108–115. doi: 10.1016/j.numecd.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, Devereux RB. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol. 2006;47:2267–2273. doi: 10.1016/j.jacc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Kharod AM, Ramlogan SR, Kumar S, Raghuveer T, Drake W, Dai H, Raghuveer G. Childhood obesity increases left-ventricular mass irrespective of blood pressure status. Pediatr Cardiol. 2014;35:353–360. doi: 10.1007/s00246-013-0782-5. [DOI] [PubMed] [Google Scholar]
- 42.Eisenmann JC, Malina RM, Tremblay A, Bouchard C. Adiposity and cardiac dimensions among 9- to 18-year-old youth: the Quebec Family Study. J Hum Hypertens. 2007;21:114–119. doi: 10.1038/sj.jhh.1002116. [DOI] [PubMed] [Google Scholar]
- 43.Mahgerefteh J, Linder J, Silver EJ, Hazin P, Ceresnak S, Hsu D, Lopez L. The prevalence of left ventricular hypertrophy in obese children varies depending on the method utilized to determine left ventricular mass. Pediatr Cardiol. 2016;37:993–1002. doi: 10.1007/s00246-016-1380-0. [DOI] [PubMed] [Google Scholar]
- 44.Di Bonito P, Capaldo B, Forziato C, Sanguigno E, Di Fraia T, Scilla C, Cavuto L, Saitta F, Sibilio G, Moio N. Central adiposity and left ventricular mass in obese children. Nutr Metab Cardiovasc Dis. 2008;18:613–617. doi: 10.1016/j.numecd.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Crowley DI, Khoury PR, Urbina EM, Ippisch HM, Kimball TR. Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr. 2011;158:709–714.e701. doi: 10.1016/j.jpeds.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity (Silver Spring) 2011;19:128–133. doi: 10.1038/oby.2010.134. [DOI] [PubMed] [Google Scholar]
- 47.Rodicio MM, Domenech de Miguel V, Guinda Jimenez M, Cigarran Guldris S, Lopez Franco MM, Estany Gestal A, Couce ML, Leis Trabazo MR. Early cardiac abnormalities in obese children and their relationship with adiposity. Nutrition. 2018;46:83–89. doi: 10.1016/j.nut.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Serizawa T, Vogel WM, Apstein CS, Grossman W. Comparison of acute alterations in left ventricular relaxation and diastolic chamber stiffness induced by hypoxia and ischemia. Role of myocardial oxygen supply–demand imbalance. J Clin Investig. 1981;68:91–102. doi: 10.1172/JCI110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 50.Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, Rea A, Fratta F, Castaldi B, D'Andrea A, Calabro P, Miele T, Coppola F, Russo MG, Caso P, Perrone L, Calabro R. Abnormal myocardial deformation properties in obese, non-hypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J. 2006;27:2689–2695. doi: 10.1093/eurheartj/ehl163. [DOI] [PubMed] [Google Scholar]
- 51.Harada K, Orino T, Takada G. Body mass index can predict left ventricular diastolic filling in asymptomatic obese children. Pediatr Cardiol. 2001;22:273–278. doi: 10.1007/s002460010228. [DOI] [PubMed] [Google Scholar]
- 52.Lai WW, Mertens L, Cohen M, Geva T. Echocardiography in pediatric and congenital heart disease: from fetus to adult. Hoboken: Wiley; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request to the authors.
Not applicable.
Consent for Publication
Not applicable.