Abstract
Stress-related mood disorders like anxiety and depression are more prevalent in women than men and are often associated with hypothalamic-pituitary-adrenal (HPA) axis dysregulation. Androgen actions through androgen receptors (ARs) decrease HPA axis responses and stress-associated behaviors. Corticotropin releasing factor (CRF) and its binding to CRF receptor 1 (CRFR1) is also critical for regulation of the HPA axis, anxiety, and depression. We first determined CRFR1/AR co-localization patterns in male and female CRFR1-GFP mice. High co-localization was found within the paraventricular nucleus (PVN), dorsolateral and anteroventral subdivisions of the bed nucleus of the stria terminalis (BSTdl and BSTav), medial preoptic area (MPOA), and posterodorsal medial amygdala (MePD). We next determined whether the non-aromatizable androgen dihydrotestosterone (DHT) regulates CRFR1 expression and stress-induced activation of CRFR1-expressing cells. In the PVN, CRFR1-GFP cell number decreased following gonadectomy (GDX), but DHT treatment reversed this effect. GDX-DHT treated mice also had a decreased CRFR1-GFP cell number within the BSTdl compared to gonad intact and GDX-untreated groups. Following restraint stress GDX-blank mice showed fewer c-Fos/CRFR1 co-localized neurons in the MePD compared to gonad intact and GDX-DHT groups indicating decreased stress-induced activation of CRFR1 neurons following GDX. Higher plasma corticosterone (CORT) was found in GDX males compared to GDX-DHT and sham males following restraint stress, with a negative correlation between PVN CRFR1+ neurons and corticosterone levels 30- and 90-minutes following restraint. Together these findings show androgens can directly alter CRFR1 levels in the brain which may have implications for sex differences in regulation of the HPA axis and stress-related behaviors.
Keywords: androgen, androgen receptor, hypothalamic-pituitary-adrenal axis, corticotropin releasing factor, sex difference, stress
INTRODUCTION
Activation and adaptation of the hypothalamic-pituitary-adrenal (HPA) axis is necessary to promote the survival of organisms when their internal homeostasis is challenged by a perceived threat. The production of neuropeptides corticotropin releasing factor (CRF) and arginine vasopressin (AVP) following activation by a stressor ultimately results in the release of glucocorticoids (primarily cortisol in humans, or corticosterone in rodents) (Vale et al., 1981; Herman et al., 2020). Acting through a negative feedback loop, cortisol feeds back to the hypothalamus to turn off the stress response, while constant HPA axis activation results in dysregulation and an inability to effectively shut down the stress response (Herman et al., 2016; 2020). With continued high levels of cortisol secretion, stress-related diseases such as anxiety and depression are more likely to develop (Keller et al., 2017; Karin et al., 2020; Frimodt-Møller et al., 2019). Sex differences in HPA axis activation and adaptation of the HPA axis to various stressors are prominent in a variety of species including rodents and humans and may contribute to the development of anxiety and mood disorders (Zuloaga et al., 2020; Oyola and Handa, 2017). Women are at twice the risk of being diagnosed with stress-related disorders such as anxiety, depression, and PTSD when compared to men (Kessler et al., 1994; Gater et al., 1998; Weismann et al., 1996).
Androgen actions through the androgen receptor (AR) have been suggested to contribute to sex differences in anxiety and mood disorders due to their inhibitory actions on HPA axis activation. Male mice and rats with the testicular feminization mutation (Tfm), which renders AR non-functional, show increased corticosterone axis responses to stress (Zuloaga et al., 2008a; 2008b; 2011). Supporting these findings, suppression of the HPA axis is found in rodents treated with the non-aromatizable androgen dihydrotestosterone (DHT), which cannot be converted to estradiol (Handa et al., 1994). Furthermore, Tfm mice and rats show elevated anxiety-like behavior (Zuloaga et al., 2008a; 2008b; 2011; Hamson et al., 2014) while male AR knockout mice show increased despair-like behaviors following chronic stress exposure compared to wild type mice (Hung et al., 2019). A similar role of AR has also been reported in humans in which treatment with the AR antagonist flutamide is associated with increased depressive symptoms (Cherrier et al., 2009; Lee et al., 2015) and individuals with complete androgen insensitivity syndrome, resulting from dysfunctional AR, show high rates of depression (~36% incidence; Fliegner et al., 2014).
Although there is substantial evidence indicating signaling through AR regulates the HPA axis and stress-related behaviors, the cell phenotypes and brain regions upon which androgens act to produce these effects is unclear. One potential mechanism is through androgens binding AR within CRF or CRF receptor (CRFR1/CRFR2) expressing neurons. Androgens have been shown to regulate distinct populations of CRF neurons in the paraventricular hypothalamus (PVN) and dorsal bed nucleus of the stria terminalis (BSTdl; Uchida et al., 2019). Furthermore, sex differences in CRFR1 have been reported in several areas of the brain and some CRFR1 populations, including within the PVN, are regulated by androgens. Specifically, previous reports demonstrated that adult male mice have higher levels of CRFR1 in the PVN and gonadectomy (GDX) in males decreases CRFR1 to levels similar to females (Rosinger at al., 2019b; Heck and Handa, 2019). The current study aimed to investigate whether the non-aromatizable androgen DHT regulates CRFR1 levels in the PVN as well as other regions that contain abundant AR and are known to regulate behavioral and neuroendocrine stress responses (BSTdl and ventral BST (BSTav), posterodorsal medial amygdala (MePD), and MPOA). Co-localization of CRFR1 neurons with AR was assessed in the same regions to gain a better understanding of the mechanisms through which androgens might regulate the HPA axis and stress-related behaviors.
EXPERIMENTAL PROCEDURES
Experimental Design
Experiment 1: Determine co-localization patterns of CRFR1 and AR in the male and female mouse brain.
Male and female CRFR1-GFP mice were compared within the PVN, MPOA, BSTav, BSTdl, and MePD for co-expression patterns of CRFR1 and AR.
Experiment 2: Determine whether the non-aromatizable androgen DHT regulates expression of CRFR1 and stress-induced activation of CRFR1-expressing cells.
DHT-treated male CRFR1-GFP mice were compared to gonadectomized untreated and sham mice to determine the role of DHT in regulation of CRFR1 in the PVN, MPOA, BSTav, BSTdl, and MePD.
Animals
Experiments were conducted utilizing a CRFR1 reporter mouse line (bacterial artificial chromosome identified by green fluorescent protein, BAC CRFR1-GFP; Justice et al., 2008) verified by comparing distributions of CRFR1-GFP to in situ CRFR1 mRNA expression (Van Pett et al., 2000; Justice et al., 2008). Our lab and others have utilized this mouse line to determine CRFR1 co-localization patterns and changes in CRFR1 as GFP levels reflect current expression (Rosinger et al., 2019a; 2019b; 2020; De Guzman et al., 2021; Ramot et al., 2017). RT-PCR nucleotide sequences used for genotyping were as follows: CCT ACG GCG TGC AGT GCT TCA GC forward and CGG CGA GCT GCA CGC TGC GTC CTC reverse EGFP350 primers. Maintenance of mice for both experiments included a 12/12 L/D cycle (lights on at 0700), with food and water access ad libitum. All procedures were approved by the University at Albany Institutional Animal Care and Use Committee (IACUC) and met or exceeded the National Institutes of Health guidelines.
Gonadectomy (GDX) and Hormone Treatment
For experiment 2, 60–75-day old male CRFR1-GFP mice were randomly assigned to a treatment group (sham surgery + blank, GDX + blank, GDX + DHT; N = 21, 7 per treatment). Gonadectomies were performed as described in our previously published protocols (Jacobskind et al., 2017; Rosinger et al., 2019a). Carprofen served as the analgesic and was injected prior to surgery and once daily for 2 days after surgery. The sham surgery procedure included anesthetization and incisions in the scrotum. Subcutaneous implants of empty capsules or DHT (10 mm of crystalline DHT (5α-ANDROSTAN-17β-OL-3-ONE) Steraloids Inc). Silastic capsules (1.02 mm id/2.16 mm od) were implanted at the nape of the neck. These DHT capsules are previously described and designed to deliver physiologically relevant concentrations of DHT (Ramzan et al., 2020; Coome et al., 2017; Singh et al., 1995). Brains were collected at 10 days following surgery.
Restraint Stress and Blood Collection
To assess whether CRFR1 cells become activated during psychogenic stress for experiment 2, restraint stress was utilized on 70–85-day-old male mice. Restraint and blood collection occurred between 9 AM-12 PM. Mice were restrained in a well-ventilated plastic tube (L: 6–4/5”, W: 3–9/10”, H: 2–3/5”) for 30 minutes where mice were able to freely move their limbs but not turn around. Tail blood was collected within 3 minutes of placement in the restraint tube to determine basal corticosterone levels using previously described methods (De Guzman et al., 2021). The restraint tube was placed in a plastic cage with bedding. After 30 minutes of restraint stress, tail blood was again collected after which mice were removed from the tube and placed back inside their home cage undisturbed for another 60 minutes (90 minutes after restraint onset). At this timepoint mice underwent rapid decapitation with trunk blood samples and brains collected. Blood was assayed for corticosterone using radioimmunoassay (MP Biomedical) as previously described (Rosinger et al., 2020; Jacobskind et al., 2017; 2018; 2019). The intra-assay coefficient of variance was less than 4%.
Brain Collection and Tissue Sectioning
Brain collection for all sacrificed adult mice consisted of cervical dislocation followed by rapid decapitation. For all mice, brains were extracted from the skull and placed into 4% paraformaldehyde at 4° C. Seminal vesicles and bulbocavernosus/levator ani (BC/LA) muscles were also dissected and weighed to be used as a bioassay for approximating androgen levels and assessing hormone treatment efficacy (Table 1) (Cooke et al., 1999; Rand and Breedlove, 1992). After 24 hours, brains were transferred to a 30% sucrose solution containing sodium azide for preservation. All mouse brains were sectioned at 40 μm on a cryostat (Microm HM505E, MICROM international GmbH, Walldorf, Germany) in the coronal plane into three series. Sectioned brain tissue was stored in cryopreservative solution at 4° C until immunohistochemistry was conducted.
Table 1.
Body, seminal vesicle, and bulbocavernosus/levator ani (BC/LA) muscle weights.
| Treatment | Body Weight (g) | Seminal Vesicles (g) | BC/LA Muscles (g) |
|---|---|---|---|
| Sham | 28.63 ± 0.42 | 0.39 ± 0.03 | 0.09503 ± 0.0041 |
| GDX-Blank | 27.98 ± 0.59 | 0.13 ± 0.02* | 0.07986 ± 0.0024* |
| GDX-DHT | 28.77 ± 0.58 | 0.52 ± 0.03*# | 0.11218 ± 0.0044*# |
Data reported as mean grams ± the standard error of the mean (SEM).
Indicates p≤0.01 compared to sham,
indicates p≤0.01 compared to GDX-Blank.
Chromogen Immunohistochemistry
To visualize CRFR1-GFP co-localization with AR, sectioned tissue was rinsed in phosphate-buffered saline (PBS; pH 7.6) before being incubated in 1% hydrogen peroxide with 0.3% Triton-X in PBS (PBS-TX) for 10 minutes. Following this initial rinse, tissue was rinsed in PBS and placed in 4% normal goat serum (NGS) with PBS-TX for a 60-minute incubation. Tissue was then transferred into primary GFP antisera (chicken, GFP; Abcam; (RRID: AB300798; 1:10,000)) for incubation overnight. The following day tissue was rinsed in PBS before a 60-minute incubation in biotinylated goat anti-chicken antisera with PBS-TX (Vector Laboratories; 1:500). After several rinses in PBS, sectioned tissue was placed in avidin-biotin complex (ABC Elite kit, Vector Laboratories; 1:1000) before being washed in tris-buffered saline (TBS). Tissue was then incubated in the chromogen diaminobenzidine (DAB). The following day tissue underwent several PBS rinses before antigen retrieval was performed by placing free-floating sections stored in floating Netwell inserts (Corning, USA) into a 98°C citrate buffer solution for 15 minutes. Tissue was rinsed in PBS before incubating in a 4% NGS solution for 60 minutes. Following incubation, the tissue was placed into AR primary antibody overnight (rabbit; Abcam; RRID: AB11156085; 1:500). The following day, tissue was placed in biotinylated goat anti-rabbit antisera with PBS-TX (Vector Laboratories; 1:500). After rinsing in PBS, tissue was placed into ABC. Tissue was rinsed in sodium acetate buffer before being placed into Nickel/DAB solution. Following the Nickel/DAB solution, tissue was rinsed twice in sodium acetate buffer before being rinsed twice in PBS and mounted onto gel-coated slides. When dry, tissue was defatted and cleared in ethanol and xylene solutions and coverslipped using permount. This procedure results in brown CRFR1 label and black AR nuclear label. In this instance, fluorescent immunohistochemistry was not feasible due to the need for antigen retrieval for the AR antibody, which eliminates the GFP signal in this tissue. CRFR1-GFP label validation consisted of using wild type brain tissue sections as a negative control. No signal was found in these wild type sections. Labeling patterns of AR were consistent with previous reports showing extensive labeling in hypothalamic, BST, preotic regions, and hippocampal CA1 (Lu et al. 1998; Chen et al., 2016; Allen Institute ISH database).
Fluorescent Immunohistochemistry
Dual-label fluorescent immunohistochemistry was conducted for experiment 2 to determine co-expression of CRFR1-GFP+ cells with c-Fos, a neural activation marker. Throughout the execution of the IHC, tissue was covered with aluminum foil to avoid signal degradation of endogenous GFP. Tissue was rinsed in phosphate-buffered saline (PBS; pH 7.6) before a 60 min incubation in 4% normal donkey serum (4% NDS) with 0.3% Triton-X in PBS. Following incubation, tissue was placed directly into primary antisera for c-Fos (Santa Cruz; rabbit; RRID: AB2106783; lot no. 12514; 1:250) for overnight incubation at room temperature. The following morning, tissue went through rinses in PBS before being placed into secondary antisera (Jackson Labs; donkey anti-rabbit 594; cat no. 711585152; 1:250) mixed in 4% NDS and PBS-TX for 2.5 hours. After secondary antisera, tissue was rinsed and transferred into the second primary antisera GFP (Abcam; chicken; RRID: AB300798; lot no. GR3190550–18; 1:2000) for incubation in 4% NDS and PBS-TX overnight at room temperature. On day three, tissue was rinsed in PBS before being transferred into the second secondary antisera (Jackson Labs; donkey anti-chicken 488; cat no. 703545155; 1:1500) for 2.5 hours. After undergoing a final rinse in PBS, tissue was mounted and, when dry, coverslipped with DAPI-containing Santa Cruz hard set mounting media. No primary and no secondary controls were utilized to assess the specificity of the tissue labeling. The validation tests of our controls revealed no tissue labeling. Both primary antibodies c-Fos and GFP used for this IHC have been previously validated in our lab (Rosinger et al., 2017; 2019a; 2019b; 2020). Importantly, we have demonstrated that rodents not exposed to a stressor or drug before sacrifice showed very little c-Fos labeling in the extended amygdala, preoptic area, and hypothalamic regions (Jacobskind et al., 2018; Zuloaga et al., 2011; Zuloaga et al., 2014).
Microscopic Analysis
A Nikon 80i microscope equipped with a digital camera was utilized to collect images of CRFR1-GFP as well as co-localization patterns. Images were captured using a 40X (CRFR1/AR) or 20X (fluorescent imaging) objective lens. 40x magnification was used for CRFR1/AR labeled tissue because the antigen retrieval procedure used for this tissue caused shrinkage making it difficult to identify co-labeled cells at 20x, which is our typical magnification for this type of analysis (De Guzman et al., 2021; Rosinger et al., 2020). The Allen Institute mouse brain reference atlas (https://mouse.brain-map.org/static/atlas; Lein et al., 2007) was used to identify brain regions of interest (ROIs) within which CRFR1-GFP and co-labeled neurons were quantified. These regions included the PVN (triangle ROI; plates 62–63), BSTdl (oval ROI; plates 52–53), BSTav (oval ROI; plates 52–53), MePD (rectangle ROI; plate 72–73), and MPOA (oval ROI; plates 53–54). See Figure 1 for approximate location of quantification and shape of ROIs. ImageJ software was used for the quantification of cells, with cells being quantified bilaterally within two atlas-matched sections for each brain section to estimate various cell densities within the selected regions as previously described (De Guzman et al., 2021; Rosinger et al., 2019a; 2019b; 2020). Dual-labeled CRFR1-GFP/AR cells were quantified using ImageJ and were identified as black nuclear AR level present within brown CRFR1-GFP neurons. Co-labeling of c-Fos with CRFR1-GFP cells were evaluated by the presence of red nuclear label (c-Fos) within green neurons (CRFR1-GFP). All cell counts for the study are reported as cells per section. In a previous publication we previously reported CRFR1/AR co-localization in the PVN using the same tissue (Rosinger et al., 2019b). In the present study we increased animal number, re-analyzed data, and replicated previous results. These data are included in the current study as they are important for relative comparison to CRFR1/AR co-localization patterns within other brain regions.
Figure 1. Anatomical locations within which CRFR1-GFP+ and co-labeled cells were quantified.
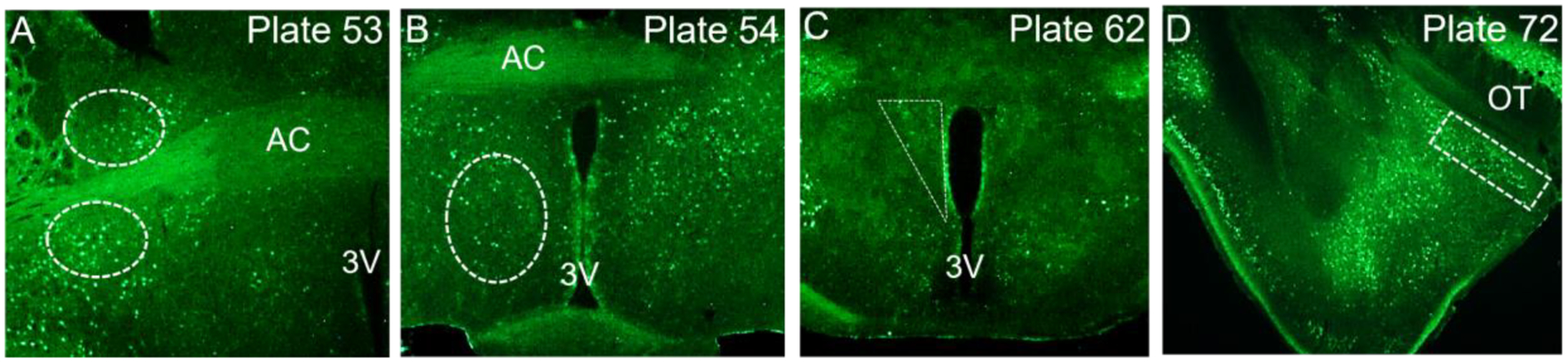
Plate numbers correspond to regions of interest with locations determined by the Allen Mouse Brain Reference Atlas (https://mouse.brain-map.org/static/atlas). Approximate ROI shapes for the BSTdl (A), BSTav (A), MPOA (B), PVN (C) and MePD (D). AC; anterior commissure, OT; optic tract, 3V; third ventricle.
Statistical Analyses
Data were analyzed using student’s t-tests (experiment 1) or one-way and two-way ANOVA (experiment 2). Significant ANOVAs were further analyzed using Tukey’s or Bonferroni post hoc tests. Pearson’s correlations were also performed to assess the association between CRFR1-GFP and CRFR1/c-Fos co-labeled cells with peak stress and recovery corticosterone responses. Parametric tests were used for analysis as sampled distributions for analyses met criteria for homogeneity of variance and normal distribution, which were assessed using F tests and Shapiro-Wilk tests. Analysis was performed using GraphPad Prism v.9. Data are reported as mean ± standard error of the mean (SEM) with significance level set at p ≤ 0.05.
RESULTS
Sex differences in CRFR1-GFP/AR co-localization (Experiment 1)
We replicated previous published data from our lab (Rosinger et al., 2019b), demonstrating high co-localization of AR in CRFR1-GFP neurons within the PVN but no significant sex differences in CRFR1-GFP/AR co-localization (Figure 2A) or percentage of CRFR1-GFP-ir cells co-localizing with AR (Figure 2B). We also reported a greater number of PVN AR+ cells in male mice when compared to females (t(8)=2.877; p ≤ 0.05) as well as a greater number of CRFR1-GFP labeled cells in the male PVN (t(8)=2.254; p ≤ 0.05) (Rosinger et al., 2019b; Figure 2A–D; Figure 3A, F). Along with this re-analysis of the PVN, the present study assessed CRFR1-GFP/AR co-localization patterns in various other stress regulating brain regions.
Figure 2. Co-localization of CRFR1-GFP and AR in regions of the male and female mouse brain.
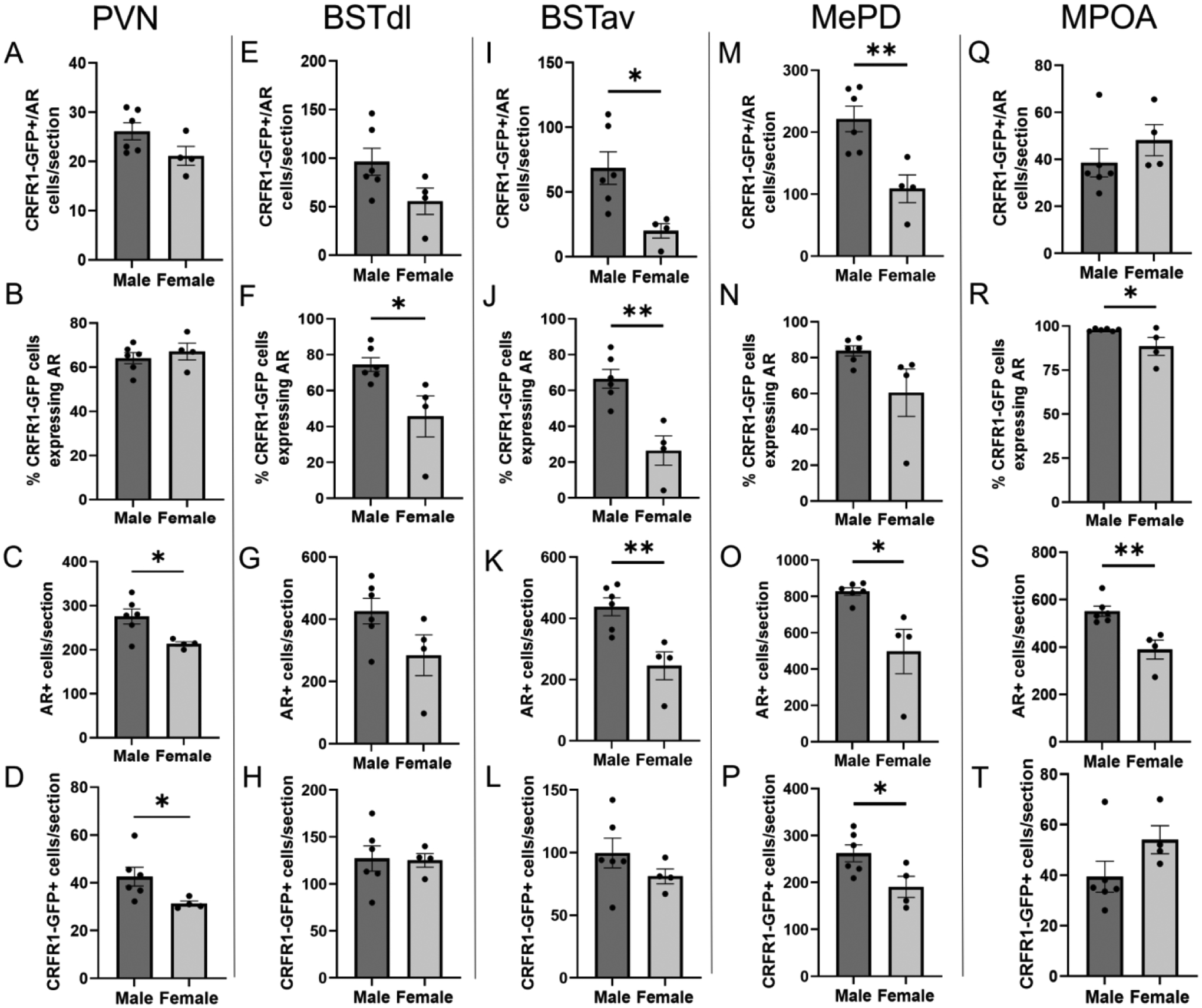
Quantification of CRFR1-GFP/AR co-labeled neurons, the percentage of CRFR1-GFP neurons co-expressing AR, AR+, and CRFR1-GFP+ neurons are shown in the PVN (A-D), BSTdl (E-H), BSTav (I-L), MePD (M-P), and MPOA (Q-T). * Indicates statistical significance p≤0.05, ** p≤0.01 compared to other groups. (PVN data were published in part in a previous publication (Rosinger et al., 2019b), although the data presented reflect an increased N and re-analysis.)
Figure 3. Images of CRFR1-GFP/AR co-labeling.
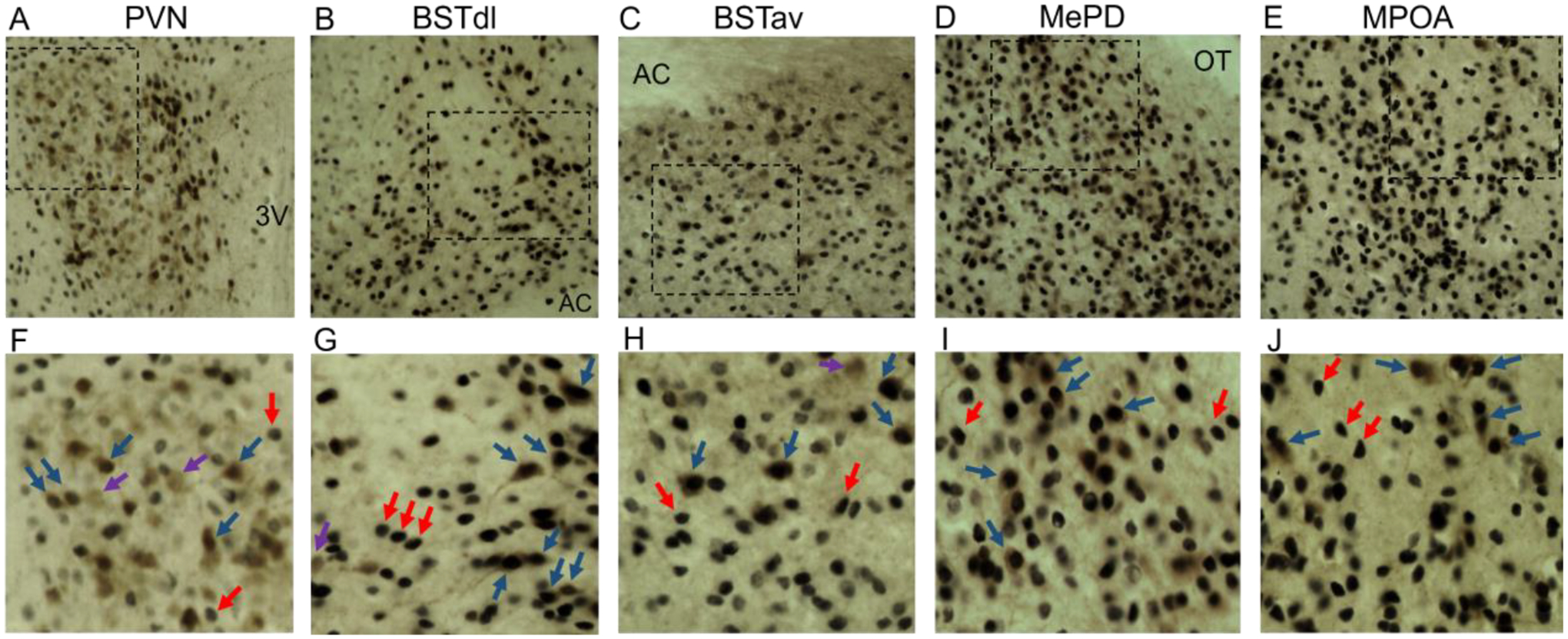
Representative images of CRFR1-GFP (brown), AR (black nuclear), and co-localized cells (brown with black nuclear label) are shown from the (A) PVN, (B) BSTdl, (C) BSTav, (D) MePD, and (E) MPOA of a male mouse. High magnification images of each region are shown directly below in F-J. Purple arrows indicate CRFR1-GFP only, red indicate AR only, and blue indicate examples of co-expressing neurons. 3V, 3rd ventricle; AC, anterior commissure; OT, optic tract.
Analysis of dual-labeled CRFR1-GFP+/AR cells showed high co-localization within the BSTdl, with males demonstrating higher co-localization than females, though this difference didn’t reach the significance threshold (t(8)=1.986; p =0.0823). The percentage of CRFR1-GFP+ neurons expressing AR was greater in the male BSTdl when compared to females (t(8)=2.812; p ≤ 0.05), where males demonstrated ~76% co-localization compared to ~46% of CRFR1-GFP+ cells expressing AR in females. No significant sex differences were found in the number of CRFR1-GFP+ cells though males showed a trend toward a greater number of BSTdl AR+ neurons when compared to females (t(8)=1.943; p =0.0879); Figure 2E–H; Figure 3B, G). High co-localization of AR in CRFR1-GFP neurons was also observed within the BSTav, with male mice demonstrating higher CRFR1-GFP/AR co-localization (t(8)=2.982; p ≤ 0.05) and a higher percentage of CRFR1-GFP+ neurons expressing AR (t(8)=4.357; p ≤ 0.01) with nearly 71% of CRFR1-GFP+ neurons expressing AR compared to 26% in females. Males also show a greater number of AR+ cells compared to females (t(8)=3.741; p ≤ 0.01). However, no significant difference was found between sexes with respect to CRFR1-GFP+ labeled neurons (Figure 2I–L; Figure 3C, H).
In the MePD, analysis of dual-labeled CRFR1-GFP+/AR co-expressing cells revealed high co-localization of AR in CRFR1-GFP neurons, with significantly higher co-localization in male mice compared to females (t(8)=3.616; p ≤ 0.01) as well as a higher percentage of CRFR1-GFP+ neurons expressing AR (86%) compared to females (61%), though this difference did not reach statistical significance (t(8)=2.119; p =0.0669). Male mice also showed a greater number of AR+ cells (t(8)=3.318; p ≤ 0.01) and a greater number of CRFR1-GFP+ labeled neurons when compared to females (t(8)=2.485; p ≤ 0.05; Figure 2M–P; Figure 3D, I). High co-localization of AR in CRFR1-GFP neurons was again observed within the MPOA, however no sex differences were found in the number of CRFR1-GFP+/AR cells, though males demonstrated a higher percentage of CRFR1-GFP cells expressing AR (t(8)=2.345; p ≤ 0.05). Male mice also had a greater number of AR+ cells compared to females (t(8)=3.895; p ≤ 0.01). No sex differences in MPOA CRFR1-GFP+ cells were found (Figure 2Q–T; Figure 3E, J).
Effects of gonadectomy and DHT treatment on body weight, seminal vesicles, BC/LA, and stress-induced corticosterone levels (Experiment 2)
Significant effects of treatment were found for seminal vesicle weights (F(2,18)=50.28; p≤ 0.0001) and BC/LA weights (F(2,17)=17.53; p≤ 0.0001) but not body weight (Table 1). Specifically, seminal vesicles were decreased in GDX-Blank mice relative to sham and GDX-DHT mice (p≤ 0.001). Similar decreases in BC/LA weights were found in GDX-Blank compared to sham (p≤ 0.01) and GDX-DHT groups (p≤ 0.001). Furthermore, seminal vesicle and BC/LA weights were also elevated in GDX-DHT relative to sham mice (p≤ 0.01). For corticosterone levels, two-way ANOVA (repeated measures) revealed a significant main effect of time (F(2,39) = 77.21; p ≤ 0.001), treatment (F(2,39) = 77.21; p ≤ 0.001), and an interaction of time and treatment (F(2,39) = 7.25; p ≤ 0.01; Figure 4). Post hoc analyses indicate no differences in baseline corticosterone, but GDX-Blank mice had elevated restraint stress-induced corticosterone levels at 30 minutes (p≤ 0.001) and 90 minutes (p≤ 0.05) compared to sham and GDX-DHT mice. GDX-DHT mice also showed decreased corticosterone relative to sham mice at 30 minutes after restraint onset (p≤ 0.001).
Figure 4. Androgen regulation of corticosterone levels at baseline (0), and 30- and 90-minutes following restraint stress onset.
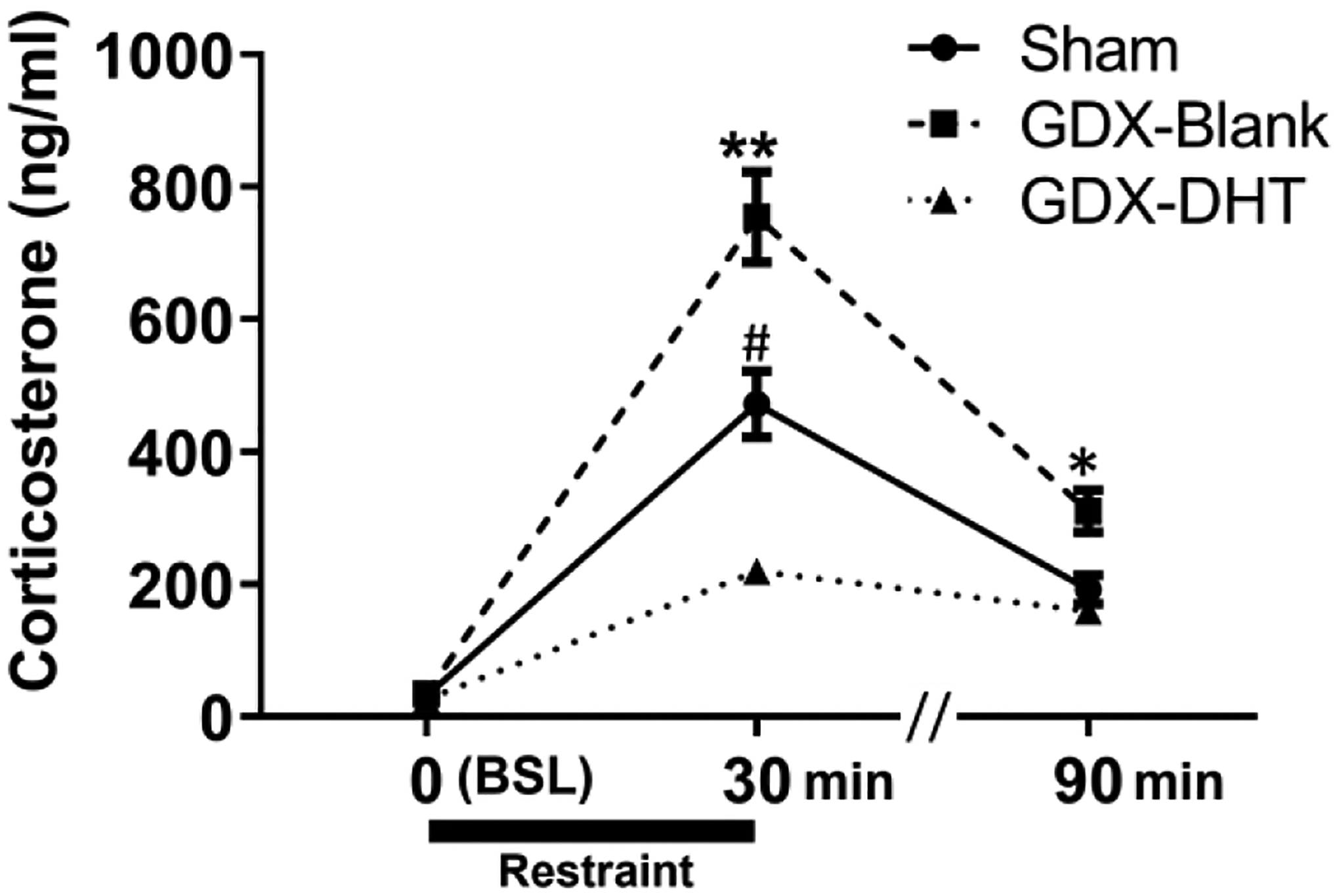
No differences were found at baseline (BSL), but GDX-Blank mice showed significantly elevated levels of corticosterone at 30 and 90 minutes after the onset of restraint stress. * Indicates p≤0.05, ** p≤0.001 for GDX-Blank compared to sham and GDX-DHT groups at the same timepoint. # Indicates p≤0.001 for sham compared to GDX-DHT mice at the 30-minute timepoint.
Androgen effects on CRFR1-GFP cell number and CRFR1-GFP cell activation (c-Fos co-expression) following a 30-minute restraint stress (Experiment 2)
In the PVN, a 1-way ANOVA revealed a significant effect of treatment for the number of CRFR1-GFP neurons (F(2,18)=8.851; p ≤ 0.01; Figure 5A–D). Specifically, we found a decreased CRFR1-GFP cell number following GDX relative to sham mice (p ≤ 0.01), while treatment of gonadectomized mice with DHT reversed this effect (p≤ 0.01). 30-minute restraint stress resulted in no significant difference in c-Fos or c-Fos/CRFR1 co-localized neurons in the PVN of GDX males compared to both sham and DHT treated mice (Table 2; Figure 6D–F).
Figure 5. Androgen regulation of CRFR1-GFP levels in the PVN, BSTdl, BSTav, MePD, and MPOA.
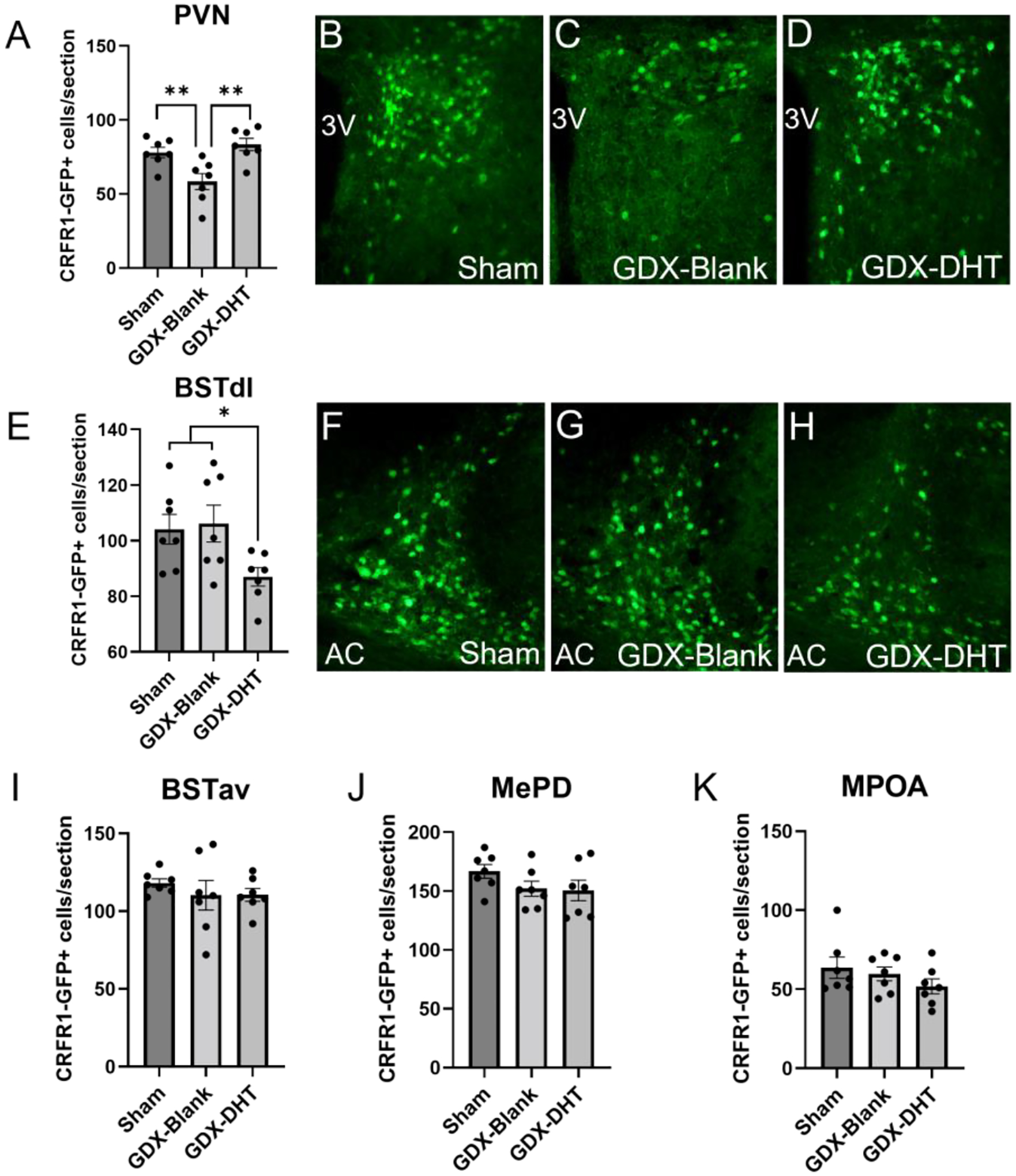
(A-D) In the PVN, GDX-Blank mice showed reduced CRFR1-GFP cell number compared to sham and GDX-DHT groups. (E-H) In the BSTdl, GDX-DHT mice showed a reduced number of CRFR1-GFP neurons relative to sham and GDX-Blank groups. No significant differences were found in the (I) BSTav, (J) MePD, or (K) MPOA. * Indicates p≤0.05, ** p≤0.01 compared to other groups. AC, anterior commissure.
Table 2.
Total c-Fos counts quantified in various brain regions of restrained male mice.
| Treatment | |||
|---|---|---|---|
| Region | Sham | GDX-Blank | GDX-DHT |
| PVN | 223.8 ± 10.9 | 232.1 ± 5.8 | 242.8 ± 7.8 |
| BSTdl | 39.4 ± 2.7 | 40.0 ± 3.9 | 42.1 ± 4.7 |
| BSTav | 118.2 ± 2.6 | 110.0 ± 9.5 | 110.2 ± 4.1 |
| MePD | 58.4 ± 5.0 | 42.3 ± 5.8 | 52.2 ± 6.0 |
| MPOA | 35.7 ± 5.2 | 38.5 ± 5.6 | 24.9 ± 2.5* |
Means ± SEMs are shown as cells per section.
Indicates p≤0.05 compared to the GDX-blank group.
Figure 6. CRFR1-GFP/c-Fos co-localization in male mice following restraint stress.
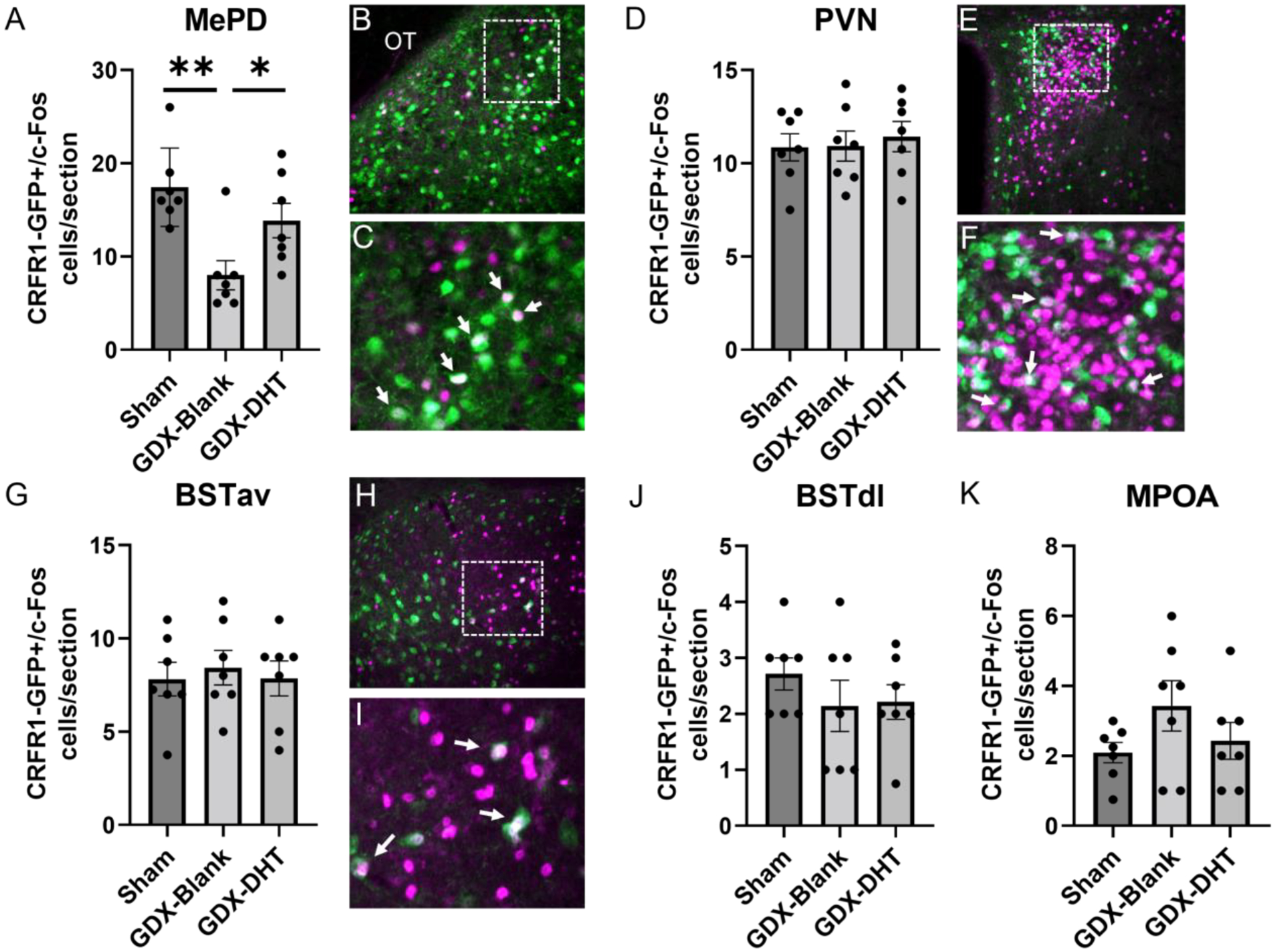
(A-C) In the MePD, GDX-Blank mice showed fewer CRFR1-GFP/c-Fos co-localized cells compared to sham and GDX-DHT groups. No significant differences were found in the PVN (D-F), BSTav (G-I), BSTdl (J), or MPOA (K). CRFR1-GFP, green; c-Fos, magenta. * Indicates p≤0.05, ** p≤0.01 compared to other groups. OT, optic tract. Arrows indicate examples of co-labeled neurons.
In the BSTdl a 1-way ANOVA revealed a significant effect of treatment (F(2,18)=3.970; p≤ 0.05) in which CRFR1-GFP neurons were decreased in GDX-DHT mice compared to sham and GDX-blank groups (p≤ 0.01; Figure 5E–H). There were no significant differences in c-Fos/CRFR1-GFP co-localized neurons in the BSTdl (Figure 6J). Furthermore, no significant differences in CRFR1-GFP cell number (Figure 5I) or c-Fos/CRFR1-GFP co-localized neurons (Figure 6G–I) were found in the BSTav. Within the MePD, no significant differences in CRFR1-GFP cell number following GDX or DHT treatment were found (Figure 5J). However, a 1-way ANOVA revealed a main effect of treatment on the number of c-Fos/CRFR1-GFP co-localized neurons (F(2,18)=8.098; p≤ 0.01; Figure 6A–C). Specifically, fewer c-Fos/CRFR1-GFP co-labeled cells were found in restrained GDX-blank compared to both sham and DHT treated groups in the MePD (p≤ 0.05). In the MPOA no significant effect of treatment was found for the number of CRFR1-GFP (Figure 5K) or co-localized c-Fos/CRFR1-GFP neurons (Figure 6K). Interestingly, DHT treatment resulted in a decrease in c-Fos+ neurons in the MPOA when compared to GDX-blank mice (p ≤ 0.05; Table 2).
Correlations between CRFR1-GFP and CRFR1-GFP/c-Fos cells with peak stress (30 min) and recovery (90 min) corticosterone responses (Experiment 2)
Pearson’s correlations were primarily performed to test the hypothesis that changes in CRFR1-GFP neurons in the PVN would be correlated with corticosterone responses to stress at 30 and 90 minutes after stress initiation. This was based on previous findings that indicate PVN CRFR1 neurons are part of a local negative feedback circuit that decreases release of CRF and in turn corticosterone release (Jiang et al., 2018; 2019). Correlations were also performed in other CRFR1 populations with largely unknown roles in HPA axis regulation to determine whether there is an association with corticosterone release. In the PVN, a negative correlation was found between the number of PVN CRFR1+ neurons and corticosterone levels at 30 minutes (r = −0.6022, p≤ 0.01; Figure 7A,B) and 90 minutes (r = −0.7996, p≤ 0.001; Figure 7A,C) after restraint onset, indicating high PVN CRFR1 is associated with lower corticosterone. No significant correlations were found between CRFR1-GFP cell number and 30- or 90-minute corticosterone levels for other brain regions (Figure 7A). We next performed correlational analyses between CRFR1-GFP/c-Fos co-localized cells and 30- or 90-minute stress-induced corticosterone levels. A significant negative correlation was found between MePD CRFR1-GFP/c-Fos cells and 30-minute corticosterone levels (r = −0.4922, p≤ 0.05; Figure 7A, D) indicating higher levels of dual-labeled neurons were associated with lower levels of corticosterone. No other correlations between CRFR1-GFP/c-Fos and corticosterone within other brain regions reached statistical significance.
Figure 7. Correlations between corticosterone levels at 30- and 90-minutes after restraint onset and CRFR1-GFP and CRFR1/c-Fos levels in various brain regions.
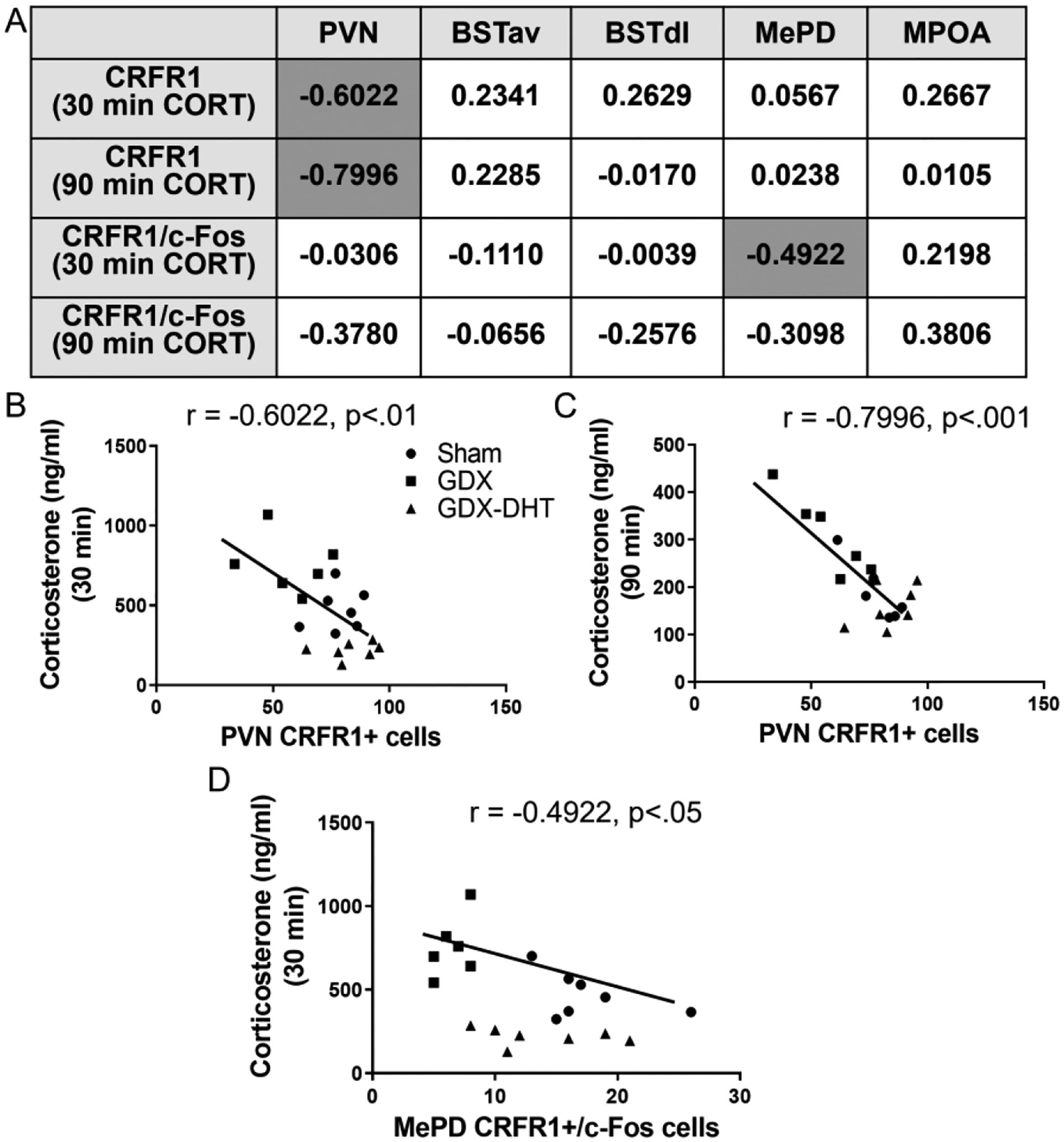
Dark gray cells in the box (A) indicate significant positive or negative correlations (p≤ 0.05). Statistically significant correlations are also graphed in B-D.
DISCUSSION
The first goal of this study was to determine whether specific CRFR1 neuron populations co-localize with AR and if this differed by sex. Analysis of co-localization of CRFR1 and AR revealed generally high co-localization across all brain regions examined. Furthermore, significantly higher numbers of CRFR1-GFP+ cells co-localizing with AR were found within the BSTav and MePD in males when compared to females. Overall, these findings indicate a potential mechanism for ARs to regulate behavioral and neuroendocrine stress responses given the known role of CRFR1 in regulating these functions. In addition, we sought to determine whether CRFR1 levels and stress-induced activation of CRFR1-GFP-ir neurons within the PVN, MPOA, MePD, and BST were regulated by androgens, specifically the non-aromatizable androgen DHT. We replicate previous findings that gonadectomy decreases CRFR1 in the PVN of male mice (Rosinger et al., 2019b; Heck and Handa, 2019) and now demonstrate this decrease in CRFR1 is restored by DHT treatment. Furthermore, the MePD demonstrated a decrease in c-Fos/CRFR1 co-localization following restraint stress in GDX males compared to sham operated and GDX-DHT males.
Co-localization of CRFR1/AR in male and female mice
Analysis of co-localization of CRFR1 and AR revealed high co-labeling of AR in CRFR1 neurons within the BSTdl, BSTav, MPOA, and MePD, adding to our previous report showing high co-expression within the PVN (Rosinger et al., 2019b). Sex differences in the number and/or percentage of CRFR1-GFP neurons that co-express AR was also found in the BSTdl, BSTav, MePD, and MPOA, with males showing higher levels compared to females. The BST is a region known to regulate anxiety-like behaviors where it acts as a convergence site for information from other regions associated with the control of emotional, autonomic, and behavioral responses to stress such as the amygdala and hypothalamus (Sink et al., 2013; Callahan et al., 2013; Lebow and Chen, 2016). Importantly, CRF has been demonstrated to act via CRFR1 within subdivisions of the BST to increase anxiety-like behaviors (Pomrenze et al., 2019; Sahuque et al., 2006). Therefore, presence of AR expressed by CRFR1 neurons suggests a mechanism through which androgens could regulate stress-related behaviors. AR-containing cells of the posterior BST project to the parvocellular PVN and have been suggested to regulate the HPA axis through these projections (Williamson and Viau, 2007; Bingham et al., 2011). It is possible that CRFR1/AR co-expressing neurons are involved in this process although further studies are needed to determine the function of these neurons.
The MePD also showed a sex difference with higher co-localization of AR in CRFR1 cells in males when compared to females. Interestingly, there was also a sex difference in MePD CRFR1-GFP cell number with higher levels in males compared to females. The MePD is known to be activated by stressful stimuli and appears to play a role in male rivalry aggression and social recognition (Davey and Grossmann, 2016; Sandi and Haller, 2015). The MePD is also known to contain high levels of AR when compared to females (Cara et al., 2021; Commins and Yahr, 1985; Simerly et al., 1990), and a recent study has indicated a role for AR in the posterior medial amygdala in controlling conditioned defeat in hamsters (Cooper et al., 2021). Furthermore, testosterone’s reduction of anxiety and fear responses in mice are associated with androgen-induced changes in the MePD (Tong et al., 2019). Thus, the CRFR1/AR co-localized neurons within the MePD may potentially contribute to androgen regulation of a variety of behavioral stress responses including those related to fear, anxiety, and social defeat.
CRFR1 neurons of the MPOA showed nearly 100% co-localization with AR in male mice and 88% co-localization in female mice. The MPOA has been implicated as a key site where androgens bind to ultimately modulate HPA responses to stress. AR expressing neurons of the MPOA project to the PVN and discrete lesions of the MPOA eliminate the ability of androgens to suppress HPA axis reactivity (Williamson and Viau, 2007; 2008). Furthermore, the MPOA has been indicated as an area that shows alterations in neural activation in androgen receptor deficient Tfm compared to wild type rats after exposure to an open field stressor (Zuloaga et al., 2011). Further studies are needed to determine the potential role of this select population of MPOA CRFR1/AR co-expressing neurons in regulating these stress responses. In future studies it will also be important to map co-expression patterns of estrogen receptors (ERα and ERβ) with CRFR1 to better understand mechanisms (cell types/brain regions) through which androgens might regulate neuroendocrine and behavioral stress responses. Conversion of testosterone to 17β-estradiol and subsequent binding to ERα and ERβ modulate HPA axis responses to stress and anxiety-like behaviors (Handa et al., 2009). These effects are receptor dependent, with binding to ERα increasing HPA axis responses to stress and anxiety-like behaviors and binding to ERβ producing opposite effects (Weiser and Handa, 2009; Weiser and Handa, 2009; Lund et al, 2006). Furthermore, DHT can be further converted to 3β-diol which interacts with ERβ to decrease stress responsivity (Lund et al., 2004;2006; Pak et al., 2005). Each of the brain regions assessed in this study contain high levels of ERα and ERβ (Mitra et al., 2003; Merchenthaler at el., 2004; Zuloaga et al., 2014) and are therefore also potential sites for regulating these effects, possibly through modulation of CRF receptors (Zuloaga et al., 2020).
Beyond co-localization with CRFR1, our results revealed that male mice displayed overall greater numbers of AR+ cells in the BSTav, MePD, and MPOA adding to our previous report of greater PVN AR in males compared to females (Rosinger et al., 2019b; also shown in Figure 2C). Overall, these findings reflect potential for enhanced responsivity to androgens in these regions. Within the posterior division of the BST, Lu et al. (1998) reported intermediate AR-ir staining in male mice with very little staining in females. We expand upon these findings by showing a sex difference in another subdivision of the mouse BST (BSTav). Sex differences in BST AR (male>female) are also found in other species including rats and hamsters (McGinnis and Katz, 1996; Roselli, 1991; Wood and Newman, 1999; Herbison, 1995; McAbee and DonCarlos, 1998). Similarly, greater AR in the MePD of males compared to females corresponds with previous reports of sex differences in rats (Roselli et al., 1989). Additionally, we replicate the findings of Lu et al. (1998) who assessed CF-1 mice, by reporting a sex difference where males demonstrate higher levels of AR in the MPOA.
Androgen effects on CRFR1 levels and CRFR1-GFP-ir activation (c-Fos co-expression) in mice subjected to restraint stress
Previous studies have reported greater CRFR1 levels within the male PVN of mice that decrease to levels similar to females following GDX (Rosinger et al., 2019b; Heck et al., 2019). Therefore, a primary aim of the current study was to determine whether PVN CRFR1 levels are maintained in males due to actions of DHT. We further assessed potential changes in CRFR1 resulting from gonadectomy and DHT replacement in other brain regions containing high levels of CRFR1 co-localization with AR. Restraint-induced corticosterone levels were also assessed and replicated the well-established effects of gonadectomy to increase, and DHT to decrease, corticosterone levels (Handa et al., 1994a; 1994b). The more potent suppression of corticosterone in DHT-treated in comparison to gonad intact mice may be due to the capsule producing a supraphysiological amount of DHT which is reflected by modestly greater seminal vesicle and bulbocavernosus/levator ani weights.
Within the PVN we replicated previous reports of a decrease in CRFR1 after gonadectomy (Rosinger et al., 2019b; Heck et al., 2019) and further report that PVN CRFR1 levels are restored by DHT. Our correlational analyses also indicate a negative association between PVN CRFR1+ neuron number and corticosterone levels at 30- and 90- minutes following restraint stress. This finding suggests a possible role for androgen regulation of CRFR1 neurons within the PVN in controlling corticosterone release. CRF released within the PVN has been shown to signal to neighboring CRFR1 expressing neurons to regulate HPA axis negative feedback (Jiang et al., 2018). Specifically, intra-PVN CRF release activates CRFR1 neurons which have GABAergic synapses on CRF neurons which function to suppress further CRF release through a local negative feedback circuit (Jiang et al., 2018; 2019). Therefore, the increase in PVN CRFR1 driven by androgens, and resulting elevation in GABAergic input to CRF neurons, may be a mechanism through which androgens suppress stress induced HPA axis activity. The role of PVN CRFR1 in regulating behavioral stress responses is not as well understood, however this might also be a site of action through which androgens regulate anxiety- and depressive-like behaviors (Zuloaga et al., 2008; 2011; 2020). Although effects on PVN CRFR1 levels were found, activation of these cells was unaffected by androgens. It remains possible that these neurons were engaged, and effects might be detected using a marker upstream of c-Fos such as phosphorylated CREB that more broadly detects changes in transcriptional activity. Of note, there was substantial c-Fos in the PVN that was not co-expressed in CRFR1 neurons. It is likely that much of this c-Fos is localized in CRF, and potentially vasopressin, expressing neurons based on previous studies using restraint stress (Cusulin et al., 2013; Walker et al., 2019; Sugimoto et al., 2018). It is also possible that androgens might be selectively mediating activation patterns of CRF and vasopressin neurons although further studies are needed to explore this possibility.
Within the BSTdl, we report decreased levels of CRFR1-GFP in DHT-treated mice relative to other groups. One possibility is that this effect is driven by supraphysiological levels of DHT, which may suppress CRFR1 only at high levels. Another explanation for decreased BSTdl CRFR1 in DHT-treated mice is that other gonadal hormones regulate BSTdl CRFR1 in an opposing manner to DHT. For example, testicular androgens can be converted to estrogens which subsequently bind estrogen receptors. ERα is highly expressed in the mouse BST (Mitra et al., 2003; Merchenthaler et al., 2004), thus it is possible that estrogen binding to ERα might have opposing effects, resulting in elevated CRFR1. Estrogens have previously been reported to drive increases in CRF in the BSTdl (Uchida et al., 2019). Thus, the diminished levels of estrogens in GDX+DHT mice along with elevated levels of DHT, which potently binds AR, might drive the decrease in CRFR1 in the BSTdl. Functionally, this decrease in CRFR1 by DHT has implications for understanding the mechanisms whereby androgens regulate behaviors associated with the BSTdl, including fear and anxiety-like responses. It remains unclear as to why BSTdl CRFR1 neurons are affected by androgens whereas other CRFR1 populations, including within the BSTav are not, particularly since AR co-expression in CRFR1 neurons is only slightly greater in the BSTdl. Interestingly, CRF neurons in the BSTdl are reported to be sexually dimorphic and gonadal hormone responsive whereas CRF neurons in the BSTav are not (Uchida et al., 2019). This suggests there may be greater overall intrinsic sensitivity to gonadal steroid hormones in the BSTdl relative to the BSTav, although the mechanisms of this are unclear.
Unlike the BSTdl, the BSTav showed no effects of androgen manipulation on CRFR1 levels or stress-induced activation of these neurons. Interestingly, a sex difference in activation of BSTav CRFR1 neurons has previously been demonstrated with female mice showing an attenuation in stress-induced activation following exposure to chronic variable stress, while chronic stress did not alter activation of these neurons in male mice (Rosinger et al., 2020). This suggests that BSTav CRFR1 neurons may play a role in sexually dimorphic adaptations to chronic stress, and it is possible that androgens contribute to this effect. Further studies involving chronic variable stress in androgen-treated mice are needed to test this hypothesis.
In the MePD neither gonadectomy or DHT treatment altered CRFR1-GFP levels, however gonadectomized mice showed a decrease in CRFR1-GFP/c-Fos colocalized cells compared to sham and DHT-treated mice. Interestingly, we also report a positive correlation between CRFR1-GFP/c-Fos cells and peak stress (30 min) corticosterone levels. The medial amygdala has previously been implicated as a region that projects to the PVN to regulate neuroendocrine stress responses (Dayas et al., 1999). Our current findings further suggest a role of MePD CRFR1 neurons as a potential site for androgen regulation of neuroendocrine stress responses. The MePD has also been indicated as a region in which androgens act to alter behavioral stress responses associated with fear, anxiety, and social defeat (Cooper et al., 2021; Tong et al., 2019). Furthermore, AR-deficient Tfm rats show decreased c-Fos in the MePD compared to wild type rats following exposure to an open field stressor (Zuloaga et al., 2011), again suggesting the MePD as a potential mediator of androgen/stress interactions. No effects of androgen were found in the MPOA. Interestingly this region also showed alterations in stress-induced c-Fos in Tfm compared to wild type rats (Zuloaga et al., 2011) and is also implicated as a site for androgen regulation of HPA axis responses to stress (Williamson and Viau, 2007; 2008). Although CRFR1 levels and activation of these MPOA neurons were not affected by androgens it remains possible that they contribute to androgen regulation of stress responses. Alternatively, other cell types in the MPOA may be critical to this response.
Overall, these findings indicate that androgens can directly alter levels of CRFR1 in the brain and thus may potentially contribute to sex differences in the regulation of the HPA axis and the stress-related behaviors. We also report high levels of CRFR1 co-localization with AR in various stress responsive brain regions indicating a receptor mechanism through which DHT likely mediates these changes in CRFR1. These co-localization patterns are also sexually dimorphic, suggesting a potentially increased sensitivity in males for androgens to modify CRFR1 neurons and the functions they regulate. Future studies will aim to determine the significance of androgen actions on CRFR1 expressing neurons in mediating effects on behavioral and neuroendocrine stress responses.
ACKNOWLEDGMENTS
We thank James Dias and Barbara Weaver for assistance with and use of their equipment for the radioimmunoassay. We also acknowledge Robert J. Handa who assisted in aspects of experimental design and whose mentorship and groundbreaking research in the field continues to serve as an inspiration. This research was supported by University at Albany Research Initiation Funds (DZ), NIH R15-MH118692 (DZ), and NIH-HD09641101, NIH-HD097331, NIH-DC017149 (PF).
Footnotes
Conflict of Interest
None declared.
REFERENCES
- 1.Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V. Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology. 2011. Jun;36(7):1433–43. doi: 10.1038/npp.2011.27. Epub 2011 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cara AL, Henson EL, Beekly BG, Elias CF. Distribution of androgen receptor mRNA in the prepubertal male and female mouse brain. J Neuroendocrinol. 2021. Dec;33(12):e13063. doi: 10.1111/jne.13063. Epub 2021 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009. Mar;18(3):237–47. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CV, Brummet JL, Jordan CL, Breedlove SM. Down, But Not Out: Partial Elimination of Androgen Receptors in the Male Mouse Brain Does Not Affect Androgenic Regulation of Anxiety or HPA Activity. Endocrinology. 2016. Feb;157(2):764–73. doi: 10.1210/en.2015-1417. Epub 2015 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinard CT, Barnes AK, Adler SG, Cooper MA. Winning agonistic encounters increases testosterone and androgen receptor expression in Syrian hamsters. Horm Behav. 2016. Nov;86:27–35. doi: 10.1016/j.yhbeh.2016.09.002. Epub 2016 Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J Comp Neurol. 1985. Jan 22;231(4):473–89. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- 7.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999. Jun 22;96(13):7538–40. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coome LA, Swift-Gallant A, Ramzan F, Melhuish Beaupre L, Brkic T, Monks DA. Neural androgen receptor overexpression affects cell number in the spinal nucleus of the bulbocavernosus. J Neuroendocrinol. 2017. Sep;29(9). doi: 10.1111/jne.12515. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MA, Clinard CT, Dulka BN, Grizzell JA, Loewen AL, Campbell AV, Adler SG. Gonadal steroid hormone receptors in the medial amygdala contribute to experience-dependent changes in stress vulnerability. Psychoneuroendocrinology. 2021. Jul;129:105249. doi: 10.1016/j.psyneuen.2021.105249. Epub 2021 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev. 2016. Feb;37(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999. Jul;11(7):2312–22. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 12.De Guzman RM, Rosinger ZJ, Parra KE, Jacobskind JS, Justice NJ, Zuloaga DG. Alterations in corticotropin-releasing factor receptor type 1 in the preoptic area and hypothalamus in mice during the postpartum period. Horm Behav. 2021. Sep;135:105044. doi: 10.1016/j.yhbeh.2021.105044. Epub 2021 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001. Oct 15;21(20):8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fliegner M, Krupp K, Brunner F, Rall K, Brucker SY, Briken P, Richter-Appelt H. Sexual life and sexual wellness in individuals with complete androgen insensitivity syndrome (CAIS) and Mayer-Rokitansky-Küster-Hauser Syndrome (MRKHS). J Sex Med. 2014. Mar;11(3):729–42. doi: 10.1111/jsm.12321. Epub 2013 Oct 25. [DOI] [PubMed] [Google Scholar]
- 15.Frimodt-Møller KE, Møllegaard Jepsen JR, Feldt-Rasmussen U, Krogh J. Hippocampal Volume, Cognitive Functions, Depression, Anxiety, and Quality of Life in Patients With Cushing Syndrome. J Clin Endocrinol Metab. 2019. Oct 1;104(10):4563–4577. doi: 10.1210/jc.2019-00749. [DOI] [PubMed] [Google Scholar]
- 16.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998. May;55(5):405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 17.Hamson DK, Jones BA, Csupity AS, Ali FM, Watson NV. Androgen insensitive male rats display increased anxiety-like behavior on the elevated plus maze. Behav Brain Res. 2014. Feb 1;259:158–63. doi: 10.1016/j.bbr.2013.11.021. Epub 2013 Nov 20. [DOI] [PubMed] [Google Scholar]
- 18.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994. Dec;28(4):464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 19.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994. Jan;55(1):117–24. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 20.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21(4):351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019. Jan;44(1):45–58. doi: 10.1038/s41386-018-0167-9. Epub 2018 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbison AE. Sexually dimorphic expression of androgen receptor immunoreactivity by somatostatin neurones in rat hypothalamic periventricular nucleus and bed nucleus of the stria terminalis. J Neuroendocrinol. 1995;7(7):543–553. doi: 10.1111/j.1365-2826.1995.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 23.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016. Mar 15;6(2):603–21. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman JP, Nawreen N, Smail MA, Cotella EM. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress. 2020. Nov;23(6):617–632. doi: 10.1080/10253890.2020.1859475. Epub 2020 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung YY, Huang YL, Chang C, Kang HY. Deficiency in Androgen Receptor Aggravates the Depressive-Like Behaviors in Chronic Mild Stress Model of Depression. Cells. 2019. Sep 2;8(9):1021. doi: 10.3390/cells8091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobskind JS, Rosinger ZJ, Brooks ML, Zuloaga DG. Stress-induced neural activation is altered during early withdrawal from chronic methamphetamine. Behav Brain Res. 2019. Jul 2;366:67–76. doi: 10.1016/j.bbr.2019.03.034. Epub 2019 Mar 19. [DOI] [PubMed] [Google Scholar]
- 27.Jacobskind JS, Rosinger ZJ, Gonzalez T, Zuloaga KL, Zuloaga DG. Chronic Methamphetamine Exposure Attenuates Neural Activation in Hypothalamic-Pituitary-Adrenal Axis-Associated Brain Regions in a Sex-specific Manner. Neuroscience. 2018. Jun 1;380:132–145. doi: 10.1016/j.neuroscience.2018.04.010. Epub 2018 Apr 19. [DOI] [PubMed] [Google Scholar]
- 28.Jacobskind JS, Rosinger ZJ, Zuloaga DG. Hypothalamic-pituitary-adrenal axis responsiveness to methamphetamine is modulated by gonadectomy in males. Brain Res. 2017. Dec 15;1677:74–85. doi: 10.1016/j.brainres.2017.09.020. Epub 2017 Sep 21. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z, Rajamanickam S, Justice NJ. CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol Stress. 2019. Aug 10;11:100192. doi: 10.1016/j.ynstr.2019.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Z, Rajamanickam S, Justice NJ. Local Corticotropin-Releasing Factor Signaling in the Hypothalamic Paraventricular Nucleus. J Neurosci. 2018. Feb 21;38(8):1874–1890. doi: 10.1523/JNEUROSCI.1492-17.2017. Epub 2018 Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008. Dec 1;511(4):479–96. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karin O, Raz M, Tendler A, Bar A, Korem Kohanim Y, Milo T, Alon U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol Syst Biol. 2020. Jul;16(7):e9510. doi: 10.15252/msb.20209510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, Schatzberg AF. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017. Apr;22(4):527–536. doi: 10.1038/mp.2016.120. Epub 2016 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994. Jan;30(1):15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee M, Jim HS, Fishman M, Zachariah B, Heysek R, Biagioli M, Jacobsen PB. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psychooncology. 2015. Apr;24(4):472–7. doi: 10.1002/pon.3608. Epub 2014 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, … Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007. Jan 11;445(7124):168–76. doi: 10.1038/nature05453. Epub 2006 Dec 6. [DOI] [PubMed] [Google Scholar]
- 37.Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998. Apr;139(4):1594–601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- 38.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365(1):43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 40.McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139(4):1738–1745. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- 41.McGinnis MY, Katz SE. Sex differences in cytosolic androgen receptors in gonadectomized male and female rats. J Neuroendocrinol. 1996. Mar;8(3):193–7. doi: 10.1046/j.1365-2826.1996.04494.x. [DOI] [PubMed] [Google Scholar]
- 42.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004. May 24;473(2):270–91. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 43.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003. May;144(5):2055–67. doi: 10.1210/en.2002-221069. Erratum in: Endocrinology. 2003 Jul;144(7):2844. [DOI] [PubMed] [Google Scholar]
- 44.Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017. Sep;20(5):476–494. doi: 10.1080/10253890.2017.1369523. Epub 2017 Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146(1):147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- 46.Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, Morikawa H, Hopf FW, Messing RO. A Corticotropin Releasing Factor Network in the Extended Amygdala for Anxiety. J Neurosci. 2019. Feb 6;39(6):1030–1043. doi: 10.1523/JNEUROSCI.2143-18.2018. Epub 2018 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, Chen A. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat Neurosci. 2017. Mar;20(3):385–388. doi: 10.1038/nn.4491. Epub 2017 Jan 30. [DOI] [PubMed] [Google Scholar]
- 48.Ramzan F, Baumbach J, Monks AD, Zovkic IB. Histone H2A.Z is required for androgen receptor-mediated effects on fear memory. Neurobiol Learn Mem. 2020. Nov;175:107311. doi: 10.1016/j.nlm.2020.107311. Epub 2020 Sep 8. [DOI] [PubMed] [Google Scholar]
- 49.Rand MN, Breedlove SM. Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol. 1992. Feb;23(1):17–30. doi: 10.1002/neu.480230104. [DOI] [PubMed] [Google Scholar]
- 50.Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991. Mar;128(3):1310–6. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- 51.Roselli CE, Handa RJ, Resko JA. Quantitative distribution of nuclear androgen receptors in microdissected areas of the rat brain. Neuroendocrinology. 1989. May;49(5):449–53. doi: 10.1159/000125151. [DOI] [PubMed] [Google Scholar]
- 52.Rosinger ZJ, De Guzman RM, Jacobskind JS, Saglimbeni B, Malone M, Fico D, Justice NJ, Forni PE, Zuloaga DG. Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations. Physiol Behav. 2020. May 15;219:112847. doi: 10.1016/j.physbeh.2020.112847. Epub 2020 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosinger ZJ, Jacobskind JS, Bulanchuk N, Malone M, Fico D, Justice NJ, Zuloaga DG. Characterization and gonadal hormone regulation of a sexually dimorphic corticotropin-releasing factor receptor 1 cell group. J Comp Neurol. 2019a. Apr 15;527(6):1056–1069. doi: 10.1002/cne.24588. Epub 2018 Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosinger ZJ, Jacobskind JS, De Guzman RM, Justice NJ, Zuloaga DG. A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus. Neuroscience. 2019b. Jun 15;409:195–203. doi: 10.1016/j.neuroscience.2019.04.045. Epub 2019 May 2. Erratum in: Neuroscience. 2020 Jan 21;428:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosinger ZJ, Jacobskind JS, Park SG, Justice NJ, Zuloaga DG. Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: A novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience. 2017. Oct 11;361:167–178. doi: 10.1016/j.neuroscience.2017.08.016. Epub 2017 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology (Berl). 2006. May;186(1):122–32. doi: 10.1007/s00213-006-0362-y. Epub 2006 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015. May;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- 58.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990. Apr 1;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 59.Singh J, O’Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995. Dec;136(12):5311–21. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- 60.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981. Oct;78(10):6517–21. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugimoto K, Ohmomo H, Shutoh F, Nogami H, Hisano S. Presentation of noise during acute restraint stress attenuates expression of immediate early genes and arginine vasopressin in the hypothalamic paraventricular nucleus but not corticosterone secretion in rats. Neurosci Res. 2015;96:20–29. doi: 10.1016/j.neures.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Tong WH, Abdulai-Saiku S, Vyas A. Testosterone Reduces Fear and Causes Drastic Hypomethylation of Arginine Vasopressin Promoter in Medial Extended Amygdala of Male Mice. Front Behav Neurosci. 2019. Feb 26;13:33. doi: 10.3389/fnbeh.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uchida K, Otsuka H, Morishita M, Tsukahara S, Sato T, Sakimura K, Itoi K. Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biol Sex Differ. 2019. Jan 28;10(1):6. doi: 10.1186/s13293-019-0221-2. Erratum in: Biol Sex Differ. 2019 Feb 19;10(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000. Dec 11;428(2):191–212. doi: . [DOI] [PubMed] [Google Scholar]
- 65.Walker LC, Cornish LC, Lawrence AJ, Campbell EJ. The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: A validation study. Neuropharmacology. 2019;154:96–106. doi: 10.1016/j.neuropharm.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 66.Wamsteeker Cusulin JI, Füzesi T, Watts AG, Bains JS. Characterization of Corticotropin-Releasing Hormone neurons in the Paraventricular Nucleus of the Hypothalamus of Crh-IRES-Cre Mutant Mice. PLOS ONE. 2013;8(5): e64943. doi: 10.1371/journal.pone.0064943. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150(4):1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lépine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996. Jul 24–31;276(4):293–9. [PubMed] [Google Scholar]
- 70.Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007. Aug 20;503(6):717–40. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- 71.Williamson M, Viau V. Selective contributions of the medial preoptic nucleus to testosterone-dependant regulation of the paraventricular nucleus of the hypothalamus and the HPA axis. Am J Physiol Regul Integr Comp Physiol. 2008. Oct;295(4):R1020–30. doi: 10.1152/ajpregu.90389.2008. Epub 2008 Aug 6. [DOI] [PubMed] [Google Scholar]
- 72.Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol. 1999. Jun 5;39(3):359–70. doi: . [DOI] [PubMed] [Google Scholar]
- 73.Zuloaga DG, Heck AL, De Guzman RM, Handa RJ. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol Sex Differ. 2020. Jul 29;11(1):44. doi: 10.1186/s13293-020-00319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008. Nov;54(5):758–66. doi: 10.1016/j.yhbeh.2008.08.004. Epub 2008 Aug 15. [DOI] [PubMed] [Google Scholar]
- 75.Zuloaga DG, Poort JE, Jordan CL, Breedlove SM. Male rats with the testicular feminization mutation of the androgen receptor display elevated anxiety-related behavior and corticosterone response to mild stress. Horm Behav. 2011. Sep;60(4):380–8. doi: 10.1016/j.yhbeh.2011.07.008. Epub 2011 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008. May;53(5):613–26. doi: 10.1016/j.yhbeh.2008.01.013. Epub 2008 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor β expression in the mouse forebrain: age and sex differences. J Comp Neurol. 2014. Feb 1;522(2):358–71. doi: 10.1002/cne.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]


