Abstract
The incidence of malnutrition in the first two years of life has been directly linked with inappropriate complementary feeding practices along with high infectious disease levels. This study was therefore aimed to assess the complementary feeding pattern among mothers of children aged zero to two years in selected health centres in Ado Ekiti, the capital of Ekiti State, Nigeria. The study was cross-sectional in design. One hundred and thirty-five mothers were selected from two health centres within Ado-Ekiti for this study. A semi-structured interviewer-administered questionnaire was used to collect information from the mothers. The questionnaire included questions that assessed the mothers’ socio-demographic characteristics and complementary feeding pattern. Most (62.5%) infants were introduced to complementary foods at 3–5 months old and water (43.3%) at 3 months of age. The main food item given to the infants on commencement of complementary feeding was formula food (45.9%) followed by fermented cereal gruel (37%). The timing of introduction for different food items revealed that in contrast to the use of fermented cereal gruel (23.8%), fewer children were introduced to iron-rich foods (15.1%) and fruits (11%) at 6 months to a year old. Mother’s knowledge of ideal age for the introduction of complementary feeding ( 20.547; p < 0.001) associated significantly with the age of introduction of complementary feeding. More than three-fifth (62.5%) of the respondents had commenced complementary feeding to their infants between 3 and 5 months while an excess of two-fifth (43.3%) of the respondents started giving their children water to drink at 3 months of age. Nurses and nutritionists in primary health care centers should take the lead role in educating mothers about the need for exclusive breastfeeding for the first 6 months of life and appropriate complementary feeding for ages 6–24 months.
Subject terms: Medical research, Outcomes research
Introduction
It has been established that malnutrition is one of the leading causes of death for many of the world's infants, accounting for over a third of the world's under-five deaths1–3. The incidence of malnutrition in the first two years of life has been directly linked with inadequate maternal breastfeeding and complementary feeding practices, along with high infectious disease levels2,4.
Complementary feeding is described as the process that starts when breast milk alone is no longer sufficient to meet infants' nutritional requirements, and thus, along with breast milk, other foods and liquids are required5. This period is from six months of age to two years and it is the most critical growth period for the child, as nutrition deficiencies may result to chronic long-term health problems such as rickets, iron deficiency anemia, stroke, cancer, coronary heart disease among others6.
The food given to children during their weaning period is very crucial as an inadequate complementary diet will significantly inhibit the child's optimum growth, health and cognitive development in the future7,8. Consequently, a complementary food should be healthy, adequate and should start timely. However, complementary feeding has been recognized to be one of the most often compromised and wrongly practiced in a child’s developmental stage. Early initiation and improper weaning practices are common practices in cultures around the world9,10. While some mothers give their children other diet apart from breast milk right from birth, others delay additional diet until more than nine months, with either case resulting into over or under-nutrition11. When complementary feeds are introduced too early (before the age of six months), it may lead to feeding difficulties, increased risk of infection and allergies, early termination of breastfeeding, obesity in children and adults and also affect the feeding behavior of children in the long term12,13.
Furthermore, it has been observed that family pressure, cultural beliefs and the environment are some of the factors responsible for inappropriate complementary feeding practices among the Yorubas of South-western Nigeria14,15. Other determinants have been attributed to the fact that some mothers lack knowledge on the appropriate diet to give to the growing child and also when to introduce the complementary feeds16.
Appropriate feeding practices include the timely introduction of solid and semi-solid foods from six months of age and the enhancement of the quantity and variety of foods consumed by children while maintaining breastfeeding17. The World Health Organization (WHO) implemented a number of new metrics in 2008 to serve as the benchmark for evaluating the quality of practices of infant and young child feeding (IYCF)18. Based on these metrics, many children in low and middle income countries often do not meet adequate complementary feeding requirements19.
Hence, the main objective of this study is to assess the complementary feeding patterns among mothers of children aged 0–2 years in selected primary health centres in Ado Ekiti, Ekiti State, Nigeria. The specific objectives are to:
Determine the age of introduction of complementary feeding to children in the study.
Identify the timing of introduction of different food items to the children in the study
Assess the complementary feeding practices of the mothers in the study setting
Investigate the factors associated with the age of introduction of complementary feeding in the study.
Methods
Study location
The study was conducted in selected basic health centres in Ado-Ekiti, the capital of Ekiti State, Nigeria. Ekiti state is situated entirely within the tropics, in the South-west region of Nigeria. Ado-Ekiti has developed basic infrastructure such as primary schools, secondary schools, private hospitals, three tertiary hospitals and few functional primary health centers. The basic health centers selected for this study; Comprehensive Health Center Oke-Iyinmi (CHCO) and Oke-Oniyo Health Centre Ekute (OHCE) were selected because they are part of the most functional primary health centeres in the State.They are located at the centre of the State capital making them easily accessible to both urban and rural residents. The health centers, are mostly patronised by the indigenous people of Ekiti State, who are predominantly Yorubas, farmers and Civil servants. The health centers render breastfeeding support and baby friendly programmes to mothers usually during both antenatal and post-natal clinics. During the clinics, mothers are educated about infant feeding policy such as importance of breast feeding, usage and risk attached to feeding utensils among others.
Study population
Inclusion criteria: Mothers of children aged two years and below attending the two selected primary health care centers in Ado Local Government Area, Ekiti State were eligible to participate.
Exclusion criteria: Mothers with infants that were supplemented earlier due to special conditions such as illness and so on, and non-consenting mothers were excluded from the study.
Study design
The study was cross-sectional in design.
Sample size
Sample size was determined using Taro Yamane formular20:
where n is the minimum sample size required, N is the total population of registered women at both health centers = 178 and is the sampling error = 0.05. Inputting these values in the above formula, yielded a sample size of 123. In order to compensate for non-response or poorly filled questionnaire, an additional 10% was added to make the sample size 135.
Sampling procedure
Proportionate stratified sampling technique was adopted to determine the number of respondents from the two health centres (OHCE and CHCO) while random sampling was employed in the selection of participants from each sub-populations. At the time of this study, the proportion of mothers in OHCE (44) to CHCO (133) was 1:3. Hence, the number of respondents recruited from each stratum is calculated as:
Number of respondents selected from OHCE = 1/4 × 135 = 33.8 = 34 participants
Number of respondents selected from CHCO = 3/4 × 135 = 101.3 = 101 participants
A total of 135 participants were recruited for the study based on calculated sample size from the two primary health care centres.
Data collection instrument
A standardized pretested semi-structured adapted questionnaire was employed for data collection. The adapted questionnaire were from previous studies conducted by11,21.
Validity and reliability of instrument
This study ensured the use of external and content validity. The researchers and two other experts in the field of study closely examined the items in the questionnaire to ensure that they could accurately measure the intended variables. Test–retest method was used to assess the reliability of the instrument. Internal consistency of items showed an intra-class correlation coefficient of 0.75. The questionnaire was administered to 135 respondents at the baby wellness clinic, in the selected primary health care centers in Ado-Ekiti.
Data management and analysis
The data collected for the study were first of all checked for errors, cleaned and then analyzed using the Statistical Package for Social Sciences (SPSS) version 23.0 for Windows, (IBM Corp., Armonk, N.Y., USA)22. Descriptive analysis of socio-demographic characteristics of respondents, age of introduction of complementary food items etc. were presented in frequencies and percentages using tables. The Chi-square test was used to test for significance of association between the independent variables and age of introduction of complementary feeding. P-values < 0.05 were considered statistically significant.
Ethical consideration
Ethical approval for the study was obtained from the Ethics and Research Committee of Afe Babalola University, Ado-Ekiti. Approval for the study was also obtained from Local Authority of the Primary Health Care board, Ekiti State. Written informed consent was obtained from individual participants before commencement of data collection. In addition, respondents were informed of their right to voluntarily participate or withdraw from the study at any stage without adverse consequences. Confidentiality was also observed as the questionnaire bore no name of respondent or any identifying information. All methods were performed in accordance with the relevant guidelines and regulations.
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Ethics and Research Committee of Afe Babalola University, Ado-Ekiti.
Results
A total of 135 mothers were interviewed. Their ages ranged from 17 to 46 years with a mean age of 26.3 ± 4.64 years. Majority (76.3%) of them were aged 20–29 years old. Most of them were married (96.3%), Christians (80%) and of the Yoruba tribe (82.22%). A larger percentage (65.2%) of the participants were self-employed while minority (3.7%) were students. 48.15% of the mothers had secondary education while 8 (5.93%) respondents had no formal education. A higher proportion (35.6%) of the mothers had up to two children while very few (2.1%) had five to eight children. The ages of their children ranged from 1 to 24 months with a mean age of 7.2 4.39 months and a median age of 6 months. Nearly half (49.6%) of the study children were in the age range of 6–12 months. (Table 1).
Table 1.
Socio-demographic characteristics of respondents.
| Variable | Frequency (n = 135) | Percent (%) |
|---|---|---|
| Age of mother | ||
| less than 20 | 5 | 3.7 |
| 20–29 | 103 | 76.3 |
| 30–39 | 25 | 18.5 |
| 40 and above | 2 | 1.5 |
| Mean age ± SD | 26.3 ± 4.64 | |
| Marital status | ||
| Single | 5 | 3.7 |
| Married | 130 | 96.3 |
| Religion | ||
| Christianity | 108 | 80.0 |
| Islam | 24 | 17.8 |
| Traditional | 1 | 0.7 |
| Others | 2 | 1.5 |
| Ethnicity | ||
| Yoruba | 111 | 82.22 |
| Hausa | 8 | 5.93 |
| Igbo | 5 | 3.70 |
| Others | 11 | 8.15 |
| Occupation | ||
| Self-employed | 88 | 65.2 |
| Unemployed | 12 | 8.9 |
| Civil servant | 30 | 22.2 |
| Student | 5 | 3.7 |
| Level of Education | ||
| No formal | 8 | 5.93 |
| Primary | 3 | 2.22 |
| Secondary | 65 | 48.15 |
| Tertiary | 59 | 43.70 |
| Number of Children | ||
| 1–2 | 91 | 67.5 |
| 3–4 | 41 | 30.4 |
| 5–8 | 3 | 2.1 |
| Child’s age (months) | ||
| < 6 | 53 | 39.3 |
| 6–12 | 67 | 49.6 |
| 13–19 | 14 | 10.4 |
| 20–24 | 1 | 0.7 |
Majority (88.9%) of the mothers had introduced their infants to water and other foods. Most (62.5%) infants were introduced to food at 3–5 months old and water (43.3%) at 3 months old. Fewer (2.5%) infants were introduced to food at one month and water at birth. (Table 2).
Table 2.
Age at introduction of complementary feeding.
| Variables | Frequency (n = 120) | Percent (%) |
|---|---|---|
| Child's age when introduced to other foods | ||
| At day one | 5 | 4.2 |
| At one month | 3 | 2.5 |
| Three-five months | 75 | 62.5 |
| Six months and above | 37 | 30.8 |
| Child's age when introduced to water | ||
| At birth | 3 | 2.5 |
| At one month | 10 | 8.3 |
| Three months | 52 | 43.3 |
| Between four and five months | 23 | 19.2 |
| At six months | 32 | 26.7 |
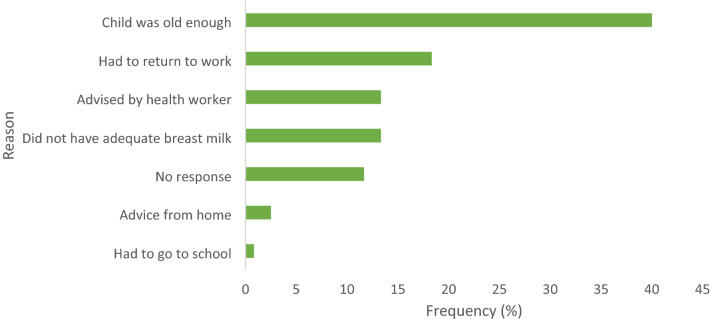
As shown in Fig. 1, two-fifth (40%) of the study population gave complementary food at a particular age because they felt the child was old enough. The second main reason cited by about 18.33% of the mothers was that they had to return to work after their maternity leave.
Figure 1.
Reason for introduction of complementary food at a particular age.
Table 3 displays the timing of introduction of some complementary food items by 110 valid respondents. The food items commonly introduced before three months of age were formular food (40%) and skimmed milk (25%). At three to five months, cereals (29.3%) and formular food (26.3%) were prominent. Cereals (23.8%) and animal products such as fish, meat and eggs (15.1%) were more frequently used at six months to a year old. Fruits (29.1%) and vegetables (28.2) were more often introduced to children above a year old.
Table 3.
Timing of introduction of complementary food items (n = 110).
| Variable | < 3 months | 3–5 months | 6 months-1 yr | > 1 yr | Never givenb |
|---|---|---|---|---|---|
| Cerealsa | 2(10) | 49(29.3) | 52(23.8) | 1(0.9) | 6(2.4) |
| Vegetables | 1(5) | 5(3) | 29(13.3) | 31(28.2) | 44(17.3) |
| Fish/Meat/Eggs | 0 | 21(12.6) | 33(15.1) | 29(26.4) | 27(10.6) |
| Skimmed milk | 5(25) | 24(14.4) | 25(11.5) | 5(4.5) | 51(20) |
| Formular food | 8(40) | 44(26.3) | 30(13.8) | 3(2.7) | 25(9.8) |
| Cow milk | 4(20) | 18(10.8) | 25(11.5) | 9(8.2) | 54(21.1) |
| Fruits | 0(0) | 6(3.6) | 24(11) | 32(29.1) | 48(18.8) |
aIncluding rice, fermented cereal gruel made from maize, millet or guinea corn (pap).
bComplementary feeding had not yet been introduced to the infant when mother filled the questionnaire.
When the respondents who had introduced other foods were asked questions on the ideal age for the introduction of complementary feeding, a greater proportion (38.3%) of them felt complementary feeding was appropriate for infants aged 3–5 months old followed by 44 (36.7%) respondents who felt it was appropriate for babies aged 6 months and above (Table 4).
Table 4.
Complementary feeding practices of mothers.
| Variables | Frequency (n = 120) | Percent (%) |
|---|---|---|
| Mother’s knowledge of ideal age for introducing other foods | ||
| From day one | 1 | 0.8 |
| At one month | 2 | 1.7 |
| Three-five months | 46 | 38.3 |
| Six months and above | 44 | 36.7 |
| No idea | 27 | 22.5 |
| Still breastfeed with complementary food | ||
| Yes | 103 | 85.8 |
| Thickness of child's food | ||
| Same as other people in the family | 3 | 2.50 |
| Thick enough to stay on a spoon | 70 | 58.33 |
| Watery similar to breast milk | 46 | 38.33 |
| Others | 1 | 0.83 |
| Feeding a sick child | ||
| Feed slowly and patiently | 87 | 72.5 |
| Give favorite foods | 18 | 15.0 |
| Feed the child forcefully | 15 | 12.5 |
| Feeding utensils use | ||
| Feeding bottle | 37 | 30.8 |
| Bowl and spoon | 71 | 59.2 |
| Hand feeding | 7 | 5.8 |
| Others | 5 | 4.2 |
| Wash and sterilize feeding utensils | ||
| Sometimes | 18 | 15.0 |
| Always | 101 | 84.2 |
| Never | 1 | 0.8 |
| Wash hands before feeding child | ||
| Sometimes | 9 | 7.5 |
| Always | 110 | 91.7 |
| Never | 1 | 0.8 |
| Wash hands after changing diaper | ||
| Sometimes | 7 | 5.83 |
| Always | 112 | 93.33 |
| Never | 1 | 0.83 |
Frequency of breast feeding: Of the 120 (88.9%) mothers who had introduced other foods, a majority (85.8%) breastfed their babies simultaneously with complementary feeding. (Table 4).
Thickness of child's food and feeding a sick child: Most of the complementary food given was thick enough to stay on a spoon (58.33%) as the population of the babies comprised mostly of 6–12 month olds (Table 1) followed by watery food texture similar to breast milk (38.33%). While majority (72.5%) of the mothers practiced feeding their babies slowly and patiently in the event of sickness, very few (12.5%) practiced forceful feeding. (Table 4).
Feeding utensils and Hygiene: The most commonly used feeding utensil was bowl and spoon (59.2%) followed by feeding bottle (30.8%). About 5.8% still practiced hand feeding which is a norm in the Yoruba culture of south-western Nigeria. Most of the respondents practiced a relatively good hygiene as 84.2% always wash and sterilize feeding utensils, 91.7% wash hands before feeding their children and nearly 93.33% wash hands immediately after changing a diaper. (Table 4).
Majority (80%) of the children breastfed three times or lesser daily, were aged 6 months or older. Surprisingly, a lower proportion (43.5%) of the children breastfed more than thrice daily, were less than 6 months old. These results were comparable but not significant. (Table 5).
Table 5.
Complementary feeding practices: frequency of breastfeeding child.
| Age of Child | ||||
|---|---|---|---|---|
| Frequency of breastfeeding | < 6 months | ≥ 6 months | p-value | |
| ≤ 3 times | 1(20) | 4(80) | 0.334y | 0.564 |
| > 3 times | 50(43.5) | 65(56.5) | ||
: Chi-square test; y: Yate’s correction.
As presented in Table 6, there were no significant associations between socio-demographic variables such as respondent’s age (p = 0.719), marital status (p = 0.849), religion (p = 0.272), respondent’s ethnicity (p = 0.544), occupation (p = 0.671), level of education (p = 0.636) number of children (p = 1.0) and child’s age (p = 0.108) and age of introduced complementary feeding. However, mother’s knowledge on ideal age for introduction of complementary feeding was significantly associated (p < 0.001) with age of introduction of complementary feeding. 91.8% of the mothers who felt complementary feeding was appropriate for infants less than 6 months old introduced their infants to other foods at that period.
Table 6.
Association between selected variables and age of introduction of complementary feeding.
| Variable | Age of introduction | |||
|---|---|---|---|---|
| < 6 months | 6 months | p-value | ||
| Age of mother (years) | ||||
| < 20 | 4(80) | 1(20) | 2.329 | 0.507 |
| 20–29 | 66(71.7) | 26(28.3) | ||
| 30–39 | 12(57.1) | 9(42.9) | ||
| 40 and above | 1(50) | 1(50) | ||
| Marital status | ||||
| Single | 4(80) | 1(20) | 0.002y | 0.964 |
| Married | 79(68.7) | 36(31.3) | ||
| Religion | ||||
| Christianity | 67(69.8) | 29(30.2) | 3.214 | 0.36 |
| Islam | 14(66.7) | 7(33.3) | ||
| Traditional | 0(0) | 1(100) | ||
| Others | 2(100.0) | 0(0) | ||
| Ethnicity | ||||
| Yoruba | 68(68.0) | 32(32.0) | 2.141 | 0.544 |
| Hausa | 7(87.5) | 1(12.5) | ||
| Igbo | 2(50.0) | 2(50.0) | ||
| Others | 6(75.0) | 2(25.0) | ||
| Occupation | ||||
| Self-employed | 54(73.0) | 20(27.0) | 2.713 | 0.438 |
| Unemployed | 8(66.7) | 4(33.3) | ||
| Civil servant | 19(65.5) | 10(34.5) | ||
| Student | 2(40.0) | 3(60.0) | ||
| Level of Education | ||||
| No formal | 4(57.1) | 3(42.9) | 3.389 | 0.335 |
| Primary | 2(66.7) | 1(33.3) | ||
| Secondary | 44(77.2) | 13(22.8) | ||
| Tertiary | 33(62.3) | 20(37.7) | ||
| Number of Children | ||||
| 0–3 | 72(69.2) | 32(30.8) | 0.000y | 1.000 |
| 4–8 | 11(68.8) | 5(31.2) | ||
| Child’s age | ||||
| < 6 months | 32(80) | 8(20) | 2.584y | 0.108 |
| ≥ 6 months | 51(69.2) | 29(30.8) | ||
| Mother's knowledge of ideal age | ||||
| < 6 months | 45(91.8) | 4(8.2) | 20.547 | < 0.001* |
| ≥ 6 months | 25(56.8) | 19(43.2) | ||
| Don’t know | 13(48.1) | 14(51.9) | ||
: Chi-square test; y: Yates correction; *: p-value < 0.05.
Discussion
Based on the joint recommendation of the WHO and the United Nations Children’s Fund (UNICEF), the introduction of nutritionally-adequate and safe complementary foods to an infant should be at 6 months7. However, results from this study showed that most infants were introduced to complementary feeding (62.5%) as early as 3–5 months old and water (43.3%) at 3 months old. These findings is in congruence with previous studies where complementary feeding was introduced as early as 3 months old23,24. A number of reasons were given for early introduction of complementary feeding; topmost among them was that child was old enough (40%). This reason has been highlighted in a previous study by Alzaheb (2016)25, but differs from prior studies where mothers felt breastfeeding alone was insufficient for the growth and development of their children1,26.
Furthermore, the timing of introduction for different food items was looked into in this study. The results revealed that in contrast to the use of cereals (23.8%) such as fermented cereal gruel which is an important source of energy, fewer children were introduced to iron-rich foods (15.1%) and fruits (11%) at 6 months to a year old. This is also the norm in several West African countries including Ethiopia where complementary foods are based on thin cereal gruel and are low in foods from meat, eggs or fish, especially among low-income groups due to socioeconomic factors, taboos, and ignorance1,27,28. Likewise, in Guatemala, most family foods used as complementary foods were considerably short in micronutrients such as calcium, iron, zinc, even when adequate amounts of protein, B vitamins and vitamin C were provided29. Interestingly, a wide lag exist between the findings in this study and the practice in a developed country like Netherlands where majority of infants had higher intake of iron-rich foods (51.9%), vegetables (57.4%) and fruits (49.6%) at age six months and above24. Moreover, the findings of this study also differ from correct practice developed by the World Health Organisation for infant and young child feeding (IYCF) which recommends the consumption of at least four food groups; at least one animal-source food, at least one vitamin A-rich fruit and vegetable, legumes and nuts, eggs, in addition to a staple food (grain, root or tuber) in a day for children at 6 to 23 months of age18.
Complementary feeding practices are directly related to healthy children. In this study, breast-feeding on demand (more than thrice daily) was highly practiced. This practice is also common among the Hausas of North-western Nigeria30,31. The most commonly used feeding utensil was bowl and spoon (59.2%), which is similar to other studies in Nigeria32,33. Bottle feeding (30.8%) and hand feeding (5.8%) were practiced by few respondents. This is in disparity to what was observed in Sudan where 59.2% mothers fed their children with their hands34. Most of the respondents practiced a relatively good hygiene as 84.2% wash and sterilize feeding utensils, 91.7% wash hands before feeding a child and nearly 93.33% wash hands immediately after changing a diaper. This is contrary to previous studies done in Bangladesh, Ethiopia and Tanzania, where majority of the mothers had very poor hygienic practice during complementary feeding35–37.
The importance of educating mothers on appropriate complementary feeding practices has been underscored in older studies conducted within and outside Africa16,26,38–40. The findings of this study further affirm the fact that mother’s knowledge of the ideal age for the introduction of complementary feeding relates with the age of introduction of complementary feeding. 91.8% of mothers who felt complementary feeding was appropriate for infants less than six months old introduced their infants to other foods at that age. This outcome has been substantiated in earlier studies where most mothers were ignorant of the WHO recommended age for complementary feeding initiation33,41,41.
Conclusion
More than three-fifth (62.5%) of the respondents had commenced complementary feeding to their infants between 3–5 months while more than two-fifth (43.3%) started giving their children water to drink at 3 months of age. Mother’s knowledge of ideal age for introduction of complementary feeding was the major factor for early complementary feeding initiation in this study. These findings imply that intervention strategies such as educating mothers about the need for exclusive breastfeeding for the first 6 months of life and complementary feeding from 6 to 24 months, is mandatory particularly at the grassroots to ensure optimal nutrition for children aged two and below.
Acknowledgements
The authors wish to acknowledge the mothers who participated in this study.
Authors Contribution
D.T.E. and A.H. designed the study. D.T.E. and O.A.I. wrote the first draft of the manuscript. A.H. and D.T.E. implemented the research. O.A.I. managed the analyses of the study. A.H., A.J.A., D.T.E. and O.A.I. managed the literature searches. O.A.I. and D.T.E. revised the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated/analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aliyu I, Duru C, Lawal TOM, A. Breastfeeding and weaning practices among Nigerian women. J. Med. Invest. Pract. 2014;9(4):140. doi: 10.4103/9783-1230.157054. [DOI] [Google Scholar]
- 2.Udoh EE, Amodu OK. Complementary feeding practices among mothers and nutritional status of infants in Akpabuyo Area, Cross River State Nigeria. Springerplus. 2016;5(1):2073. doi: 10.1186/s40064-016-3751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akeredolu IA, Osisanya JO, Seriki-Mosadolorun JS, Okorafor U. Mothers’ nutritional knowledge, infant feeding practices and nutritional status of children (0–24 months) in Lagos State, Nigeria. Eur. J. Nutrit. Food Saf. 2014;4(4):364–374. doi: 10.9734/EJNFS/2014/7604. [DOI] [Google Scholar]
- 4.Akpor O, Oluwadare T, Taiwo O, Aladenika B, Akpor O. Feeding and weaning practices among mothers of under-five children in selected primary health care centres in Ado-Ekiti, Ekiti, Nigeria. Potravinars. Slovak J. Food Sci. 2020;14:42–51. doi: 10.5219/1211. [DOI] [Google Scholar]
- 5.World Health Organization. Infant and young child feeding. 2020. Accessed August 3, 2020 at https://www.who.int/en/news-room/fact-sheets/detail/infant-and-young-child-feeding
- 6.Haimi M, Lerner A. Nutritional deficiencies in the pediatric age group in a multicultural developed country, Israel. World J. Clin. Cases WJCC. 2014;2(5):120. doi: 10.12998/wjcc.v2.i5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oloko M, Ekpo R. Exploring traditional weaning practices in North Western Nigeria; food, knowledge and culture: A step towards safeguarding community food security. Acad. J. Interdiscip. Stud. 2018;7(2):97–97. doi: 10.2478/ajis-2018-0050. [DOI] [Google Scholar]
- 8.National Population Commission (NPC) [Nigeria] and ICF International. 2014. Nigeria Demographic and Health Survey 2013. Abuja, Nigeria, and Rockville, Maryland, USA: NPC and ICF International. Accessed August 3, 2020. https://dhsprogram.com/pubs/pdf/FR293/FR293.pdf
- 9.Salim S, Kalsoom S, Humayun A. Weaning practices and perceptions of mothers residing in urban slums of Lahore, Pakistan: A focus group design. Ann. King Edward Med. Univ. 2016;22(4):1. [Google Scholar]
- 10.Gonah L, Mutambara J. Determinants of weaning practices among mothers of infants aged below 12 months in Masvingo, Zimbabwe. Ann. Glob. Health. 2016;82(5):875–884. doi: 10.1016/j.aogh.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Aina F, Kio J, Olajide TE, Ogunfowokan O, Awoniyi AM, Nwaokocha C. Infant weaning knowledge and practice among mothers attending infant welfare clinic in three primary healthcare centres in Ikenne local government area, Ogun state, Nigeria. IJAR. 2017;3(12):227–230. [Google Scholar]
- 12.World Health Organization. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. World Health Organization; 2009 [PubMed]
- 13.Kavitha S, Nadhiya C, Parimalavalli R. Study of Complementary feeding practices among mothers of infants aged six months to one year. Healthline. 2014;5(2):29–35. [Google Scholar]
- 14.Agunbiade OM, Ogunleye OV. Constraints to exclusive breastfeeding practice among breastfeeding mothers in Southwest Nigeria: Implications for scaling up. Int. Breastfeed. J. 2012;7(1):5. doi: 10.1186/1746-4358-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imonikebe BU. Weaning Practices and Nutritional Status of Infants in Isoko North and South Local Government Areas in Delta State, Nigeria. Afr. Res. Rev. 2009;3(4):1. doi: 10.4314/afrrev.v3i4.47556. [DOI] [Google Scholar]
- 16.Motee A, Ramasawmy D, Pugo-Gunsam P, Jeewon R. An assessment of the breastfeeding practices and infant feeding pattern among mothers in Mauritius. J. Nutrit. Metab. 2013;2013:1. doi: 10.1155/2013/243852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassa T, Meshesha B, Haji Y, Ebrahim J. Appropriate complementary feeding practices and associated factors among mothers of children age 6–23 months in Southern Ethiopia, 2015. BMC Pediatr. 2016;16(1):131. doi: 10.1186/s12887-016-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . Indicators for Assessing Infant and Young Child Feeding Practices: Part1 Definitions. World Health Organization; 2008. [Google Scholar]
- 19.Issaka AI, Agho KE, Page AL, Burns P, Stevens GJ, Dibley MJ. The problem of suboptimal complementary feeding practices in West Africa: what is the way forward? Matern. Child Nutrit. 2015;11:53–60. doi: 10.1111/mcn.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamane T. Statistics, An Introductory: Analysis. 2. Harper and Row; 1967. [Google Scholar]
- 21.Mwita LO. Correlates of complementary feeding practices among caregivers of infants and young children aged 6–24 months at Mbagathi district hospital, Nairobi (Doctoral dissertation, University of Nairobi)
- 22.IBM. SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp. Released 2015.
- 23.Moreira LC, Lopes LH, Bauleo ME, Sarno F. Introduction of complementary foods in infants. Einstein (São Paulo) 2019;17(3):1. doi: 10.31744/einstein_journal/2019AO4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Van Grieken A, Van Der Velde LA, Vlasblom E, Beltman M, L’Hoir MP, Boere-Boonekamp MM, Raat H. Factors associated with early introduction of complementary feeding and consumption of non-recommended foods among Dutch infants: The BeeBOFT study. BMC Public Health. 2019;19(1):388. doi: 10.1186/s12889-019-6722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alzaheb RA. Factors associated with the early introduction of complementary feeding in Saudi Arabia. Int. J. Environ. Res. Public Health. 2016;13(7):702. doi: 10.3390/ijerph13070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trivedi BY, Vyas SN, Dave BN, Desai KA. Complementary feeding practices among mothers of Waghodia Taluka of Vadodara: A knowledge, attitude, and practice study. International Journal of Medical Science and Public Health. 2015;4:647–651. doi: 10.5455/ijmsph.2015.13102014133. [DOI] [Google Scholar]
- 27.Onofiok NO, Nnanyelugo DO. Weaning foods in West Africa: Nutritional problems and possible solutions. Food Nutrit. Bull. 1998;19(1):27–33. doi: 10.1177/156482659801900105. [DOI] [Google Scholar]
- 28.Abeshu MA, Lelisa A, Geleta B. Complementary feeding: review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries–lessons from Ethiopia. Front. Nutr. 2016;17(3):41. doi: 10.3389/fnut.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen LH. Adequacy of family foods for complementary feeding. Am. J. Clin. Nutr. 2012;95:785–786. doi: 10.3945/ajcn.112.035675. [DOI] [PubMed] [Google Scholar]
- 30.Umar AS, Oche MO. Breastfeeding and weaning practices in an urban slum, North Western Nigeria. Int. J. Trop. Dis Health. 2013;3(2):114–125. doi: 10.9734/IJTDH/2013/1337. [DOI] [Google Scholar]
- 31.Okafoagu NC, Oche OM, Raji MO, Onankpa B, Raji I. Factors influencing complementary and weaning practices among women in rural communities of Sokoto state, Nigeria. Pan Afr. Med. J. 2017;28(1):1. doi: 10.11604/pamj.2017.28.254.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olatona FA, Odozi MA, Amu EO. Complementary feeding practices among mothers of children under five years of age in Satellite Town, Lagos, Nigeria. Food Publ. Health. 2014;4(3):93–98. [Google Scholar]
- 33.Olatona FA, Adenihun JO, Aderibigbe SA, Adeniyi OF. Complementary feeding knowledge, practices, and dietary diversity among mothers of under-five children in an urban community in Lagos State, Nigeria. Int. J. MCH AIDS. 2017;6(1):46. doi: 10.21106/ijma.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed SG. Infants feeding and weaning practices among mothers in Northern Kordofan state, Sudan. Eur. Sci. J. 2014;10(24):165–181. [Google Scholar]
- 35.Saleh F, Ara F, Hoque MA, Alam MS. Complementary feeding practices among mothers in selected slums of Dhaka city: a descriptive study. J. Health Popul. Nutr. 2014;32(1):89–96. [PMC free article] [PubMed] [Google Scholar]
- 36.Mshida HA, Kassim N, Kimanya ME, Mpolya E. Influence of water, sanitation, and hygiene practices on common infections among under-five children in Longido and Monduli districts of Arusha Tanzania. J. Environ. Public Health. 2017;25:1. doi: 10.1155/2017/9235168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demmelash AA, Melese BD, Admasu FT, Bayih ET, Yitbarek GY. Hygienic practice during complementary feeding and associated factors among mothers of children aged 6–24 months in Bahir Dar Zuria District, Northwest Ethiopia, 2019. J. Environ. Public Health. 2020;20:2020. doi: 10.1155/2020/2075351. [DOI] [Google Scholar]
- 38.Yemane S, Awoke T, Gebreslassie M. Timely initiation of complementary feeding practice and associated factors among mothers of children aged from 6 to 24 months in Axum town, North Ethiopia. Int. J. Nutr. Food Sci. 2014;3(5):438–442. doi: 10.11648/j.ijnfs.20140305.21. [DOI] [Google Scholar]
- 39.Saaka M, Larbi A, Mutaru S, Hoeschle-Zeledon I. Magnitude and factors associated with appropriate complementary feeding among children 6–23 months in northern Ghana. BMC Nutrit. 2016;2(1):2. doi: 10.1186/s40795-015-0037-3. [DOI] [Google Scholar]
- 40.Dhami MV, Ogbo FA, Osuagwu UL, Agho KE. Prevalence and factors associated with complementary feeding practices among children aged 6–23 months in India: a regional analysis. BMC Public Health. 2019;19(1):1034. doi: 10.1186/s12889-019-7360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jemide JO, Ene-Obong HN, Edet EE, Udoh EE. Association of maternal nutrition knowledge and child feeding practices with nutritional status of children in Calabar South Local Government Area, Cross River State, Nigeria. Int. J. Home Sci. 2016;2(1):293–298. [Google Scholar]
- 42.Owais A, Suchdev PS, Schwartz B, Kleinbaum DG, Faruque AS, Das SK, Stein AD. Maternal knowledge and attitudes towards complementary feeding in relation to timing of its initiation in rural Bangladesh. BMC nutrition. 2019;5(1):7. doi: 10.1186/s40795-019-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated/analysed during the current study are available from the corresponding author on reasonable request.