Graphical abstract
Keywords: Syringe pump, 3D printing, Electron resonance imaging (ERI), Simulation of physiological processes, Arduino
Abstract
Syringe pumps are routinely used in biomedical imaging laboratories for delivering contrast agents and either infusing or injecting a precise amount of liquids. Commercial syringe pumps that are developed by specialized companies are expensive and only have standard functions, which often do not meet the requirements of individual experiments. In this paper, we demonstrate an open–source single syringe pump with the possibility of adapting to the needs of a researcher. The device that was designed, is controlled by an Arduino Leonardo, along with the stepper motor driver. For sending commands and receiving the current plunger position, a C# software was developed with serial communication via USB. Additionally, the 3D models were made in a universal way, which allows for the use of any syringe size. An example of the application of the syringe pump for biomedical applications was demonstrated using electron resonance imaging (ERI). The single syringe pump tests were demonstrated by simulating the filling of a particular volume inside the resonator. This example reflects the clearance process after an intravascular (I.V) drug administration in the murine model. The experiments were performed on an ERI TM 600 tomograph. The results confirmed that the designed syringe pump allowed for controlling the infusion speed and injected volume. Moreover, we present a user-friendly and open-source graphical interface that is a low-cost alternative for commercial devices.
Specifications table
| Hardware name | Single syringe pump |
|---|---|
| Subject area | Educational Tools and Open Source Alternatives to Existing Infrastructure |
| Hardware type |
|
| Open Source License | GPLv3 |
| Cost of Hardware | $ 112.98 |
| Source File Repository | https://doi.org/10.17632/rw6gz7ymwv.1 |
Hardware in context
Electron paramagnetic resonance (EPR) spectroscopy is a well-known technique for its application in physics and chemistry [1]. As this technique can be performed in 3D and 4D, it is becoming more popular in biomedical and in-vivo studies [2]. The possibility to perform the imaging in two-dimensions and three-dimensions enables the visualization of the spatial distribution of paramagnetic substances, namely spin probes, that are distributed in small laboratory animals, including mice [3], [4], rats [5], and rabbits [6]. ERI is based on the EPR phenomenon and it allows for the mapping of tissue microenvironment parameters such as the level of oxygen (pO2) [7], [8], pH [9], [10], inorganic phosphate [11] and redox state [12].
The development of the rapid scan ERI makes it possible to acquire images with a very high temporal resolution, in the order of a few seconds [13], [14]. The precision and the accuracy of the injected spin probe during the ERI is an important factor for in-vivo studies. Manual injections fail to provide a constant rate of injection, which can lead to an increase in vascular pressure. This can cause animal discomfort or even damage the blood vessels. The use of an insufficient amount of spin probe will cause a reduction in the signal-to-noise (S/N) quality. On the other hand, using an excess volume may be dangerous to animals. A lot of infusion pumps are available on the market, however their prices can reach up to thousands of dollars [15]. They may have a limited functionality and may not be programmable. Unlike these pumps, our solution has reduced production costs and allows the reprogramming of the devices by adding individual functionalities. There are many sources available that shows how one could create a device for laboratory applications, such as a programmable dual syringe pump [16], however this approach requires the use of a touch panel for the control the device, which increases production costs. Therefore, a low-cost infusion pump with different functions and specifications can be a valuable tool for a precise and controllable method of administration of liquid substances into the animal body.
In this work, we demonstrate a low-cost and laboratory-built single-channel syringe pump that is used in a very specific case, namely to simulate the clearance process in mice, which corresponds to the filling of the bladder. This phenomenon is common in ERI and usually causes a distortion of the images obtained from regions close to the bladder [17]. The pump that was designed can be used as a delivery tool of various types of spin probes into the body of the mice. In addition, it functions as a washing device, as in the case of the flushing of the bladder during an in-vivo ERI imaging. An additional feature of our device is the “BOLUS mode“, which is an injection of a discrete amount (volume) of the solution with the chosen flow rate. The main difference between the ‘constant flow rate mode’ and the ‘bolus mode’ is that for the latter, after the injection of a specific volume of solution, the pump stops. This option is extremely important in the case of administering specific doses of drugs or contrast agents in in-vivo studies.
The main electronic element used is the Arduino, which is a popular open-source electronics platform associated with easy-to-use hardware and software [18]. The Arduino is found in many applications in academic projects including a high-precision scanning electrochemical microscope [19], a wireless spectrophotometer [20] and a micro syringe autosampler [21], to name few. In addition, many pump designs have been developed that also use Arduino as device control. Some, such as an intelligent infusion flow controlled syringe infusion pump [22], OMIS: The Open Millifluidic Inquiry System for small scale chemical synthesis and analysis [23] and syringe pump created using 3D printing technology and Arduino platform [24] use wireless connection, so in addition to being a precision device, they are also mobile, which allows convenient use anywhere. Rasberry Pi can also be used instead of Arduino, as shown in the project [25]. The advantage of this controller is that it can be programed in Python. In this paper, we have used the Arduino Leonardo board. The communication between the device and the computer is performed via the USB. The device is able to control a single stepper motor with a microcontroller, therefore it is only possible to pump and suck liquids for one specific syringe size at a time. Nevertheless, the pump was designed to allow one to quickly change syringe holders of one syringe size to another.
Hardware description
The base of the pump movement mechanism is an aluminum rail with plastic elements attached to it (Fig. 1). The plastic elements were made using 3D printing technology. The precise injection and suction of liquids are controlled by a NEMA 17 stepper motor. The motor is connected to the Polulu A4988 driver, which is controlled by the Arduino Leonardo board.
Fig. 1.
Three-dimensional visualization of a syringe pump.
The homing procedure, which includes the calibration of the initial syringe position is achieved by using limit switches. The software was developed to simplify the syringe pump as a plug-and-play system.
The device that was designed was tested during an ERI to simulate the filling of the bladder inside a resonator dedicated to in-vivo imaging. Other applications may require different parameters, e.g. lysate generation [16] or microfluidic experiments [26], and can be easily realized by reprogramming the device in the Arduino IDE, or adding different flow rates and syringe volumes in C# code. Additionally, the 3D-printed elements of the pump are universally designed, so with simple modifications it is possible to adjust the device to any size or type of syringe.
Printable elements
All 3D-printed elements are described below. Links to the STL files are included in table 1.
-
•
The push element (Fig. 2A) pushes or pulls the plunger of the syringe.
-
•
The motor holder and the switch holder (Fig. 2B) allows the mounting of the NEMA 17 stepper motor on to the aluminum rail and a limit switch.
-
•
The front stand (Fig. 2C) provides a base for the entire device.
-
•
The rear stand (Fig. 2D) provides a base for the entire device and it contains a place to mount the Arduino Leonardo board.
-
•
The top of the rear stand (Fig. 2E) closes the rear stand and contains a hole for the passing of cables between the motor and the Arduino board.
-
•
The syringe stabilizer (Fig. 2F) holds the syringe in place and prevents vibrations and slippage during the operation of the device.
-
•
The syringe holder 1 (Fig. 2G) is an element necessary for the correct installation of
-
•
a specific syringe size in the pump
-
•
The syringe holder 2 (Fig. 2H) is an element necessary for the correct installation of
-
•
a specific syringe size in the pump.
Table 1.
Designed 3D-printed parts.
| Design file name | File type | Open source license | Location of the file (STL Files) |
|---|---|---|---|
| Push element | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Motor holder and the switch holder | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Front stand | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Rear stand | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Top of the rear stand | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Syringe stabilizer | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Syringe holder 1 | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
| Syringe holder 2 | STL | GPLv3 | https://doi.org/10.17632/rw6gz7ymwv.1 |
Fig. 2.
All three-dimensional models of the designed device. A) Push element. B) Motor holder and the limit switch holder. C) Front stand. D) Rear stand. E) Top of the rear stand. F) Syringe Stabilizer. G) Syringe Holder 1. H) Syringe Holder 2.
Estimated materials costs
Table 2.
Required items for purchase.
| Designator | Component | Number | Cost per unit (USD) | Total cost (USD) | Source of materials | Material type |
|---|---|---|---|---|---|---|
| Aluminum rail 10x30x250 | WS-10–30 | 1 | $11.04 | $11.04 | IGUS | Aluminium |
| Threaded brass rod M8 180 mm | 468–7128 | 1 | $24.57 | $24.57 | RSOnline | Brass |
| Hexagonal brass nut M8 | 483–2542 | 6 | $0.34 | $2.04 | RSonline | Brass |
| Nylon screw M8 | 280–492 | 4 | $0.11 | $0.44 | RSOnline | Nylon |
| Screw M3 | 281–013 | 18 | $0.17 | $3.06 | RSOnline | Steel |
| Hex nut M3 | 122–4400 | 14 | $0.02 | $0.28 | RSOnline | Steel |
| Screw M4 | 281–057 | 6 | $0.39 | $2.34 | RSOnline | Steel |
| Hex nut M4 | 189–579 | 14 | $0.06 | $0.84 | RSOnline | Steel |
| Open linear ball bearing M10 | LM-10-UU-OP | 2 | $3.21 | $6.42 | Ebay.com | Steel |
| Shaft coupler 6 × 8 mm D25mm × L30mm | 995,799 | 1 | $8.15 | $8.15 | Banggood | Steel |
| Limit switch NO/NC 2A/125VAC | 1,275,144 | 1 | $1.35 | $1.35 | Banggood | Other |
| Stepper motor: NEMA 17 42HB34F08AB − 1,70A | 42HB34F08AB | 1 | $11.99 | $11.99 | Aliexpress | Other |
| Proto Shield TSX00083 | 2,917,574 | 1 | $10.50 | $10.50 | Farnell | Other |
| Polulu A4988 - RepRap 35 V/2A | 1182 | 1 | $5.95 | $5.95 | Pololu | Other |
| Arduino Leonardo - A000057 | 761–7324 | 1 | $24.01 | $24.01 | RSOnline | Other |
Build instructions
Most of the elements for the pump were printed on a 3D printer using PLA filament. Other items such as screws, nuts and the rest of the mechanical parts were purchased from
a typical store selling building material. Electronic parts like the Arduino or stepper motor driver can be ordered from popular shops like Botland or AVT (see Table 2).
Helpful tools for assembling the syringe pump:
-
•
Socket wrenches (M3, M4, M8)
-
•
Cross screwdriver
-
•
Soldering iron
The combination of electronic parts
The connection of all electronic components in the project is described below. First, the Proto Shield was placed on the Arduino Leonardo. The limit switches and the Polulu A4988 were then connected to the Proto Shield. The NEMA 17 stepper motor was connected directly to the Polulu driver. The pins of the Arduino board were connected to the motor driver accordingly: Pin 2 - Enable, Pin 3,4 - MS1, MS2, Pin 5,6 - STEP, DIR, Pin 13 - limit switch. The RESET and SLEEP inputs were short-circuited (Fig. 4, Fig. 5).
Fig. 4.

Schematic connection of all electronic components (1, 2, 3, 4).
Fig. 5.
Hardware circuit of syringe pump.
Preparation and assembly of mechanical elements
First, it is necessary to print out all the STL files available on the given links in Table 1. Our recommendation is to use PLA filament and print with a dense infill of 40%. After printing, the excess filament from the holes of each element should be removed. The next stage is to assemble the entire device according to the following steps:
Place the open linear ball bearings - one on each side - on the aluminum rail.
Attach the push element (Fig. 2A) to the open linear ball bearings with ten M3 screws and M3 nuts.
In front of the pushing piece, attach the motor holder and the switch holder (Fig. 2B) to the aluminum rail (drill two holes in the aluminum rail and motor holder and attach with two M4 screws and three M4 nuts – Fig. 3). Epoxy resin is then used to attach the limit switch.
Behind the pushing element (Fig. 2A), attach the syringe stabilizer (Fig. 2F) to the aluminum rail, in the same method as for the motor holder and the limit switch holder.
Attach the rear stand (Fig. 2D) to the M4 nuts and motor holder (Fig. 2B) with epoxy resin.
Attach the front stand (Fig. 2C) to the syringe stabilizer (Fig. 2G) with epoxy resin.
Mount the NEMA 17 stepper motor to the motor holder (Fig. 2B) with four M3 screws.
In the central hole of the push element (Fig. 2A) insert two M8 nuts through which the M8 threaded rod will pass.
Install the M8 threaded rod and connect it to the stepper motor with the shaft coupler (6x8mm).
Two syringe holders 2 (Fig. 2H) should be attached to the push element (Fig. 2A) with two M8 nylon screws and a M8 nut. Another two syringe holders 2 (Fig. 2H) and two of syringe holders 1 (Fig. 2G) should be attached to the syringe stabilizer (Fig. 2G) with two M8 nylon screws and two M8 nuts. Syringe holders 1 should be near to syringe stabilizer. Syringe holders 2 have a recess to hide the screw tip.
Attach the Arduino Leonardo to the rear stand (Fig. 2D) with four M3 screws and M3 nuts.
Route all cables through the opening of the top of the rear stand element (Fig. 2E), connecting all electronics according to the diagram (Fig. 3). Finally, the top of the rear stand (Fig. 2E) should be attached to the rear stand (Fig. 2D) with two M4 screw and M4 nuts.
Fig. 3.

The method for attaching the motor holder with the limit switch holder.
The syringe pump, after assembly, is shown in Fig. 6.
Fig. 6.
The assembled syringe pump, after 3D printing.
Software implementation
The software consists of two parts. The first part is developed in the Arduino IDE.
The second part is a high-level software created in Visual Studio. Both parts should be downloaded from: https://doi.org/10.17632/rw6gz7ymwv.1 (Software). The software allows one to communicate with the syringe pump device using a computer. The serial communication properties are set as follows: 115,200 baud rate, none parity, one bit stop and eight data bits.
The sending of commands from the computer to the Arduino board is realized with an ask-and-answer polling scheme see Table 3 and Table 4. In order to install the first script correctly, the Arduino IDE program must be downloaded and installed. Inside this software, it is important to also install the following three libraries: TimerOne and AccelStepper. The downloaded script should be opened using the Arduino IDE software and the Arduino Leonardo board should be connected to the computer via a micro USB cable. The final step is to select a specific board in the program, the appropriate port and then upload the entire script to the controller. The script that is written in C# can be downloaded and compiled in the Visual Studio IDE software - the ability to make changes to the software. After a successful compilation, the executable file should appear in the project directory. The repository also includes the executable version of the program, which is ready to download and run the software - no ability to make changes to the software.
Table 3.
Communication Protocol between PC and Arduino – Commands sent to Arduino.
| Command | Description |
|---|---|
| 10 | Starting injection |
| 20 | Moving forward (1 step) |
| 22 | Moving backward (1 step) |
| 30 | Calibration |
| 80 | Set Timer Period – Delay between steps |
| 32 | Set start position |
| 54 | Stop moving |
| 12 | BOLUS MODE stop position |
| 40 | Starting injection in BOLUS MODE |
| 14 | Position where the syringe is pumped |
| 16 | Changing stepper resolution |
| 35 | Sending actual position |
| 88 | Get device response |
Table 4.
Communication Protocol between PC and Arduino – Commands sent to PC.
| Command | Description |
|---|---|
| <connected> | Device response |
| 1: | Current stepper position |
| 12: | Homing Done |
| 14: | Syringe on start position |
| 16: | BOLUS injection done |
| 18: | Maximum position reached |
Communication Protocol
Operation Instructions
The user interface of the software used for controlling the syringe pump is shown in
Fig. 7A. The inputs that are enabled for use by the user in the functions and methods have been adapted to simulate the physiological processes in EPR imaging. There are limitations present in order to ensure a proper simulation and to protect the tested phantom. A user manual of syringe pump that was designed is described below.
Fig. 7A.
Software for controlling the designed syringe pump.
Operation instructions of the software
Connect the syringe pump device to the power supply and the computer. Run the “Syringe pump.exe” file and note that in the “Connection” panel, the device status should change to “connected”.
After starting the program, the zero position should be set by pressing the “Home” button.
Select or add a new syringe with a specific volume by sliding down the Syringe list, and then confirm with the “Set Syringe” button. After selecting “Add new syringe” from the list, a window will appear allowing you to define your own syringe (Fig. 7B). Enter the name of the syringe, the scale length of the syringe in mm and the volume of the syringe. After completing the Home process, use the Step + and Step- buttons to reach the start of the filled syringe plunger in the pump. The Speed function allows you to change the resolution of the motor operation. After performing this operation, press the “Set current as start position” button and then the “Ok” button. After this operation, the syringe is saved in the program. It allows you to define any type of syringe ranging from 1 to 20 ml. The value of the selected volume is displayed under the “Current syringe” text.
Input an injection flow rate and confirm the selection by pressing the “Set speed” button. The value of the selected injection speed is displayed under the “Current speed” text.
Press the “Start Position” button to bring the push element to the start position for the specified syringe volume.
Install the selected syringe in a holder.
Press the “Start” button to begin pumping. The “Run console” panel displays the elapsed time, the percentage of the process, the amount of liquid injected and the animation of the injecting syringe according to the percentage of the process.
The “Stop” button stops the pumping at a specific stage. The process can be resumed from the current position by pressing the “Start” button. To start the pumping process from the beginning, press the “Start Position” button, and then the “Start” button.
A manually controlled injection and suction of liquids is achieved using the “Step +” and “Step -” buttons.
To change the resolution of the stepper motor movement, the “Move speed” panel is used. The radio buttons allows the setting of the resolution: 1x, 2x and 4x.
In addition, the “Bolus mode” button activates the “Bolus” panel. It allows the injection of any volume of liquid at any pumping rate in a controlled manner.
Fig. 7B.
Software for controlling the designed syringe pump – Add new syringe.
Detailed documentation for the software can be found in the repository in README file: https://doi.org/10.17632/rw6gz7ymwv.1 (Software).
Validation and Characterization
Characterization of the designed syringe pump
The accuracy of the syringe pump was estimated based on the value of the pitch of the metric thread for the M8 rod, amounting to 1.25 mm during each motor revolution. Therefore, it was possible to precisely determine the places where the pushing element would move to, and determine its current position. The zero-point position was defined by the limit switch.
A single step of a NEMA 17 stepper motor equates to a rotation of 1.8°, therefore a full revolution takes 200 steps. Each step of the NEMA 17 motor resulted in the movement of the plunger by 0.006 mm. By dividing the above measured values by this distance of 0.006 mm, the resulting value obtained was the number of motor steps needed for moving the pushing element to reach the measured value.
The flow rates should function properly. Because of that, there should be a delay between every motor step. For this purpose, the “Delay” conversion factor was determined as follows:
The numerator of the formula defines the total time [μs] of the pumping process, while the denominator is the number of motor steps required to inject a given volume of liquid that corresponds to the length of the syringe given in [mm]. The characteristics of the device that was designed are presented in Table 5.
Table 5.
Characterization of the designed syringe pump.
| Single Syringe Pump | |
|---|---|
| Flow rate range | 1–20 ml: 0.1 – 500 ml/h |
| Type | Single-channel |
| BOLUS | YES |
| Price | $ 112.98 |
Validation of the flow rate of designed syringe pump by ERI (The bladder filling simulation) and scale
The syringe pump was tested using the ERI TM 600 tomograph. The flow rate and the accuracy of the pump were tested by imaging the incrementing volume of the spin probe inside the resonator. This situation is analogous to the filling of the bladder after the intravenous injection of the spin probe into to the body of the mouse. During in-vivo EPRI, the bladder of the mouse accumulates the spin probe due to the clearance process, which results in intensifying the EPR signal from bladder region, and causes distortions of the obtained images, which may lead to misinterpretations. With time, the bladder signal becomes more intense and covers the image area. This problem and its solutions, which use an infusion pump, were demonstrated elsewhere [17]. The development of measurement techniques and analysis algorithms requires a device that can simulate the processes taking place in a living organism. One of the major problems may be the gradual detuning of the measuring system due to the volume of the liquid increasing in the resonator. Such a process was simulated using a pump that was designed and manufactured for the purposes of this study. With the use of the pump, a spin probe that dissolved in water was infused from one syringe to another (or another container). They were connected with each other by a PE tube. The first syringe was mounted in the pump and the second (designed to simulate
a gradual filling of the bladder) was placed inside the ERI resonator.
To conduct the experiment, a 5 ml syringe with a 5 ml/h flow rate was selected. CW 3D imaging was performed. The single point imaging (SPI) protocol was applied for the acquisition of data in a k-space domain with a size of 21x21x21 (which corresponds to 9 261 scans) (Fig. 8).
Fig. 8.

Simplified process of creating a 3D image using the Single Point Imaging method.
3D ERI imaging - Before the start of imaging for each measured volumes of liquid, the tomograph resonator was tuned. With the use of the tomograph control software, the size of the encoded k-space and maximum gradient was defined as follow: k-space size 21x21x21, maximum gradient 2 G, scan amplitude 10 G, scan time 5 ms and 100 ms. The data obtained was loaded into a reconstruction software. Next, the tau parameter was selected – a specific point from each scan in k-space. A 3D discrete Fourier transform (3D FFT) was applied in order to reconstruct the images from the k – spaces that were obtained. This reconstruction procedure provides the images of a specific volume of liquid that was injected.
Test preparation - Two identical 5 ml syringes were connected to each other by a PE tube. The trityl Oxo61was used as a spin probe. The filled syringe was mounted in the pump and the other was placed in the center of the resonator. The last step in preparing the pump was to turn on the power and then connect it to the computer via a USB cable. The syringe size (5 ml), and the flow rate (5 ml/h) were selected in the control software (Fig. 9).
Fig. 9.
The connection of the syringes and their arrangement in the pump and the resonator of the tomograph.
Ten 3D images were made for volumes between 0.2 and 2 ml. In order to compare the quality of the images that were obtained, measurements with two acquisition times: 5 ms and 100 ms were performed (Fig. 10). The analysis of the images showed a proportional filling of the second syringe as a function of time. This indicated the correct operation of the infusion pump and the process of the simulation of the bladder filling. Changing the acquisition time from
Fig. 10.
Obtained 3D images for a syringe with a volume of 5 ml, flow rate 5 ml / h and for two acquisition times: 5 and 100 ms. The tau parameter is the same for each image: 5 ms: tau = 8. 100 ms: tau = 11 (where tau means one selected point from the Fourier transform from a given projection).
5 ms to 100 ms provided a better signal to noise ratio of the acquired scans. The images that were obtained using a longer acquisition time allowed for a better quality and more accurate visualization of the liquid in the syringe.
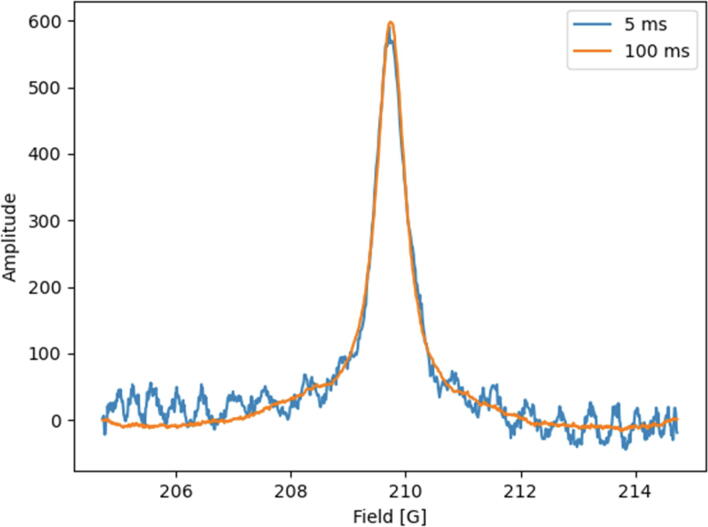
Additionally, spectroscopic analysis was performed. For this purpose, the EPR signal was collected for each tested step without the application of a magnetic field gradient (Fig. 11).
Fig. 11.
Collected signals for spectroscopic analysis - signal quality depends on the volume of liquid in the syringe. The above measurements show an exemplary signal for two data collection times: 5 ms and 100 ms without additional filtering. Noticeable noise reduction as the acquisition time increases. The above acquisition method was developed by Novilet.
The maximum of all measured volumes amplitudes was determined (Fig. 12). The graphs show the amplitude as a function of the volume of the spin probe. An increase in the amplitude of the signal is directly proportional to the syringe volume in the resonator. Also, this information confirms the correct operation of the infusion pump and the simulated process. At the same time, the correct operation of the tuning and compensation system of the resonator was confirmed.
Fig. 12.
Maximum amplitudes of collected EPR signals for each tested volume. The error on the X axis was determined from: and is less than 5% (therefore is insignificant for the measurement) while the Y axis error is 2% of the maximum amplitude value.
The quality factor of the resonator depends on the amount of the lossy substance in the imaging volume. By integration of the absorption signal, the relative concentration of spins in the resonator was obtained (Fig. 13). Similarly, a linear relation between the concentration and the injected volume was noticed which indicates the high accuracy and precision of the device that was designed.
Fig. 13.
Percentage of spins in the resonator for each tested volume. Error bars were determined in accordance with Fig. 12.
Finally, the pump was tested using a scale (RADWAG AS 110.R2). The test was performed with a 5 ml volume syringe and a flow rate of 60 ml/h. A container was placed on the scale to which 0.2 ml of liquid was pumped according to the software.
The weight of the liquid was then checked. After that, the process was repeated until 5 ml was pumped (Fig. 14). Analogous to previous experiments, a linear relationship between the mass and the injected volume was also observed which demonstrates the high accuracy and precision of the syringe pump that was designed.
Fig. 14.
The values of the measured mass on the scale depending on each tested volume. The X axis error bars were determined in accordance with Fig. 12, while the Y axis error bars result from the graduation of the device used, which is insignificant as well.
Conclusions and device summary
In this paper we described the design and the use of a low-cost syringe pump. The main application of the constructed tool was the simulation of the filling of the bladder with a spin probe, which is a typical phenomenon during in-vivo ERI imaging. However, many other biological/clinical purposes, e.g. contrast and drug delivery, can be simulated with an infusion pump. The device may be an alternative to the commercially developed pumps due to the cost and functionality. A significant advantage of the project when compared to commercial solutions is the ability to customize the software for individual purposes. It is possible to change the software in accordance with the requirements of the currently conducted experiment or simulation. In general, due to the low cost of production, the syringe pump that was designed can become a useful tool in imaging laboratories, and can be an alternative to expensive commercial devices.
This research was funded (supported) by the Smart Growth Operation Program under the project POIR.01.01.01–00-0025/15 and POIR.01.02.00–00-0077/18
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ohx.2021.e00194.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Eaton G.R., Eaton S.S., Barr D.P., Weber R.T., editors. Quantitative EPR. Springer Vienna; Vienna: 2010. [Google Scholar]
- 2.Berliner J.L. Kluwer Academic Publishers-Plenum Publishers; New York, Boston, Dordrecht, London, Moscow: 2003. In vivo EPR (ESR) Theory and Appliation. [Google Scholar]
- 3.Utsumi H., Muto E., Masuda S., Hamada A. In vivo ESR measurement of free radicals in whole mice. Biochem. Biophys. Res. Commun. 1990;172(3):1342–1348. doi: 10.1016/0006-291X(90)91597-L. [DOI] [PubMed] [Google Scholar]
- 4.Afeworki M., van Dam G.M., Devasahayam N., Murugesan R., Cook J., Coffin D., Larsen J.H., Mitchell J.B., Subramanian S., Krishna M.C. Three-dimensional whole body imaging of spin probes in mice by time-domain radiofrequency electron paramagnetic resonance. Magn. Reson. Med. 2000;43:375–382. doi: 10.1002/(sici)1522-2594(200003)43:3<375::aid-mrm9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Feldman A., Wildman E., Bartolinini G., Piette L.H. In vivo electron spin resonance in rats. Phys. Med. Biol. 1975;20(4):602–612. doi: 10.1088/0031-9155/20/4/007. [DOI] [PubMed] [Google Scholar]
- 6.Epel B., Haney C.R., Hleihel D., Wardrip C., Barth E.D., Halpern H.J. Electron paramagnetic resonance oxygen imaging of a rabbit tumor using localized spin probe delivery. Med. Phys. 2010;37(6Part1):2553–2559. doi: 10.1118/1.3425787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epel B., Redler G., Halpern H.J. How in vivo EPR measures and images oxygen. Adv. Exp. Med. Biol. 2014;812:113–119. doi: 10.1007/978-1-4939-0620-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elas M., Magwood J.M., Butler B., Li C., Wardak R., DeVries R., Barth E.D., Epel B., Rubinstein S., Pelizzari C.A., Weichselbaum R.R., Halpern H.J. EPR Oxygen Images Predict Tumor Control by a 50% Tumor Control Radiation Dose. Cancer Res. 2013;73(17):5328–5335. doi: 10.1158/0008-5472.CAN-13-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koda S., Goodwin J., Khramtsov V.V., Fujii H., Hirata H. Electron Paramagnetic Resonance-Based pH Mapping Using Spectral-Spatial Imaging of Sequentially Scanned Spectra. Anal. Chem. 2012;84(8):3833–3837. doi: 10.1021/ac203415w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobko A.A., Eubank T.D., Driesschaert B., Khramtsov V.V. In Vivo EPR Assessment of pH, pO2, Redox Status, and Concentrations of Phosphate and Glutathione in the Tumor Microenvironment. J. Vis. Exp. 2018 doi: 10.3791/56624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobko A.A., Eubank T.D., Driesschaert B., Dhimitruka I., Evans J., Mohammad R., Tchekneva E.E., Dikov M.M., Khramtsov V.V. Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci. Rep. 2017;7:41233. doi: 10.1038/srep41233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni A.C., Bratasz A., Rivera B., Krishna M.C., Kuppusamy P. Redox Mapping of Biological Samples Using EPR Imaging. Isr. J. Chem. 2008;48(1):27–31. doi: 10.1560/IJC.48.1.27. [DOI] [Google Scholar]
- 13.Baranowski M., Gonet M., Czechowski T., Kucinska M., Plewinski A., Szczepanik P., Murias M. Dynamic Electron Paramagnetic Resonance Imaging: Modern Technique for Biodistribution and Pharmacokinetic Imaging. J. Phys. Chem. C. 2020;124(36):19743–19752. doi: 10.1021/acs.jpcc.0c0570310.1021/acs.jpcc.0c05703.s00110.1021/acs.jpcc.0c05703.s002. [DOI] [Google Scholar]
- 14.Gonet M., Baranowski M., Czechowski T., Kucinska M., Plewinski A., Szczepanik P., Jurga S., Murias M. Multiharmonic electron paramagnetic resonance imaging as an innovative approach for in vivo studies. Free Radical Biol. Med. 2020;152:271–279. doi: 10.1016/j.freeradbiomed.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Guangzhou WIT Medical Technology Co, Syringe Pump WIT-302, (2020). : http://www.witmedical.com/pd.jsp?id=26.
- 16.Garcia V.E., Liu J., DeRisi J.L. Low-Cost Touchscreen Driven Programmable Dual Syringe Pump for Life Science Applications. BioRxiv. 2018 doi: 10.1101/288290. [DOI] [Google Scholar]
- 17.C.R. Haney A.D. Parasca K. Ichikawa B.B. Williams M. Elas C.A. Pelizzari H.J. Halpern Reduction of image artifacts in mice by bladder flushing with a novel double-lumen urethral catheter Mol Imaging. 5 3 2006 7290.2006.00020 10.2310/7290.2006.00020 [PMC free article] [PubMed]
- 18.Arduino - Home, (n.d.). https://www.arduino.cc/ (accessed November 9, 2020).
- 19.Guver A., Fifita N., Milas P., Straker M., Guy M., Green K., Yildirim T., Unlu I., Yigit M.V., Ozturk B. A low-cost and high-precision scanning electrochemical microscope built with open source tools. HardwareX. 2019;6:e00082. doi: 10.1016/j.ohx.2019.e00082. [DOI] [Google Scholar]
- 20.Laganovska K., Zolotarjovs A., Vázquez M., Mc Donnell K., Liepins J., Ben-Yoav H., Karitans V., Smits K. Portable low-cost open-source wireless spectrophotometer for fast and reliable measurements. HardwareX. 2020;7:e00108. doi: 10.1016/j.ohx.2020.e00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalho M.C., Murray R.H. Osmar, the open-source microsyringe autosampler. HardwareX. 2018;3:10–38. doi: 10.1016/j.ohx.2018.01.001. [DOI] [Google Scholar]
- 22.N. Merhi N. Mohamad G. HajjMoussa A. ElSayed S.H. Bamashmos L. Hamawy M. HajjHassan M.A. Ali A. Kassem An Intelligent Infusion Flow Controlled Syringe Infusion Pump 2019 IEEE Cairo, Egypt 48 52 10.1109/ICM48031.2019.9021516
- 23.LeSuer R.J., Osgood K.L., Stelnicki K.E., Mendez J.L. OMIS: The Open Millifluidic Inquiry System for small scale chemical synthesis and analysis. HardwareX. 2018;4:e00038. doi: 10.1016/j.ohx.2018.e00038. [DOI] [Google Scholar]
- 24.Samokhin A.S. Syringe Pump Created using 3D Printing Technology and Arduino Platform. J Anal Chem. 2020;75(3):416–421. doi: 10.1134/S1061934820030156. [DOI] [Google Scholar]
- 25.Wijnen B., Hunt E.J., Anzalone G.C., Pearce J.M., Gilestro G.F. Open-Source Syringe Pump Library. PLoS ONE. 2014;9(9):e107216. doi: 10.1371/journal.pone.0107216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lake J.R., Heyde K.C., Ruder W.C., Wanunu M. Low-cost feedback-controlled syringe pressure pumps for microfluidics applications. PLoS ONE. 2017;12(4):e0175089. doi: 10.1371/journal.pone.0175089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.