Abstract
Lichen planus is chronic inflammatory mucocutaneous disease. Involvement of nails (nail lichen planus: NLP) could be the only manifestation or it could be associated with the other typical skin and mucous localizations. Typical NLP alterations are linear nail bed dyschromia, longitudinal ridging, splitting, onycholysis, and subungual hyperkeratosis. Pterygium could be observed in advanced stages. Treatment of NLP is challenging. Limited clinical data have suggested that both oral and topical retinoids could be beneficial. Recently, a nail lacquer containing urea (20%), keratinase from Bacillus licheniformis, and hydroxipinacolone retinoate (U-KR lacquer) has been available. This product has shown good efficacy in the treatment of onychodystrophy characterized by onychogryphosis. We have evaluated, in a case series pilot study, the efficacy of this lacquer in subjects with moderate NLP. The product was applied once daily on the affected nails. Ten subjects (6 men and 4 women, mean age 38 years) after their written informed consent, with clinical NLP (2 subjects with histological confirmation) affecting foot or hand nails (mean number of nails involved: 4; range from 1 to 10), were treated for 12 consecutive weeks with U-KR, one application per day. The main endpoint was the evolution of a NLP severity score (NLPSS) evaluating 7 nail signs: grade of onycholysis, longitudinal ridging, splitting, grade of subungual hyperkeratosis, nail bed thickening, dyschromia, and nail pitting. For each item, a 4-grade score (from 0: no sign to 3: severe) was used (range of NLPSS from 0 to 21). At baseline, the NLPSS was 20.8 ± 3. After 12 weeks, the NLPSS showed a significant reduction to 4 ± 8.8, representing an 81% reduction in comparison with baseline value (p = 0.0001), with an absolute difference between means of −16.86 ± 2,586 (95% CI of the difference: from −22.49 to −11.22) The product was very well tolerated. This 10-case pilot study suggests that a nail lacquer with 3 components (urea, keratinase, and a retinoid molecule) could be useful in subjects with NLP. Future controlled trials are warranted to better define the therapeutic potential of this product in NLP treatment.
Keywords: Nail lichen planus, Topical retinoid, Urea, Case report
Introduction
Nail lichen planus (NLP) is characterized by profound nail appearance modification with thinning of the nail plate, linear nail bed dyschromia, longitudinal ridging, onycholysis, and subungual hyperkeratosis [1]. In advanced stages, pterygium is a typical sign of NLP [2]. If not adequately treated, NLP could evolve to permanent nail loss [3]. Treatment of NLP is difficult, and no gold standard treatments are available [4]. Topical very potent corticosteroids, such as clobetasol, or topical tacrolimus [5], could induce limited improvement with a high recurrence rate after discontinuation [6]. Intralesional high potency corticosteroids such as triamcinolone could be effective, at least partially; pain and subungual haematoma are very common side effects limiting this therapeutic approach [7]. Recently a nail lacquer containing urea 20% and keratinase and hydroxipinacolone retinoate 0.1% (U-KR lacquer) has been available. This product has shown good efficacy in the treatment of onychodystrophy characterized by onychogryphosis [8]. Urea is a hydrating and keratolytic substance [9], keratinase is a proteolytic enzyme that digests keratin [10] and hydroxypinacolone retinoate is a cosmetic grade ester of retinoic acid [11]. It is unique in that it processes innate retinoic acid activity, binding directly with retinoid receptors without the need for metabolic breakdown to more biologically active forms [12]. It has been demonstrated to be more stable and cause less skin irritation than retinol [13]. It is commonly used in anti-ageing and anti-acne products [14, 15]. Retinoid molecules have anti-inflammatory and cell proliferation regulation activity [16]. In addition, retinoids possess immune-modulating effects down-regulating inflammatory cytokines production [17]. The oral retinoid alitretinoin 30 mg per day and acitretin have shown to be a promising treatment for NLP [18, 19]. However, the use of systemic retinoids could be associated with relevant side effects and a high risk of foetal malformation in case of pregnancy [20]. Topical application of retinoids could be an interesting therapeutic alternative in subjects with NLP [21]. We have evaluated, in a pilot study, the efficacy of this urea, keratinase, and retinoid lacquer in subjects with moderate NLP.
Cases Report Presentation
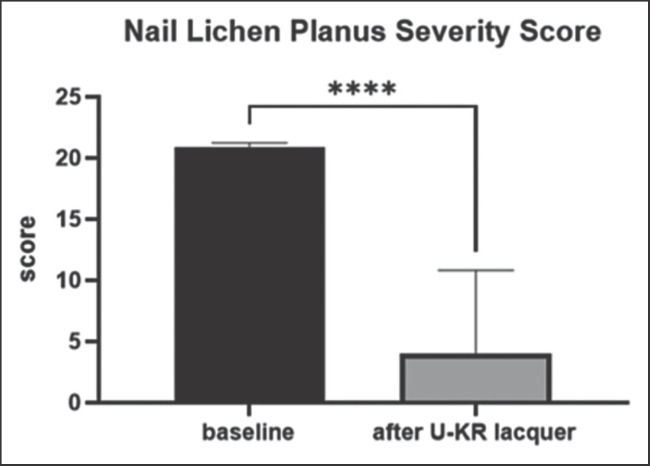
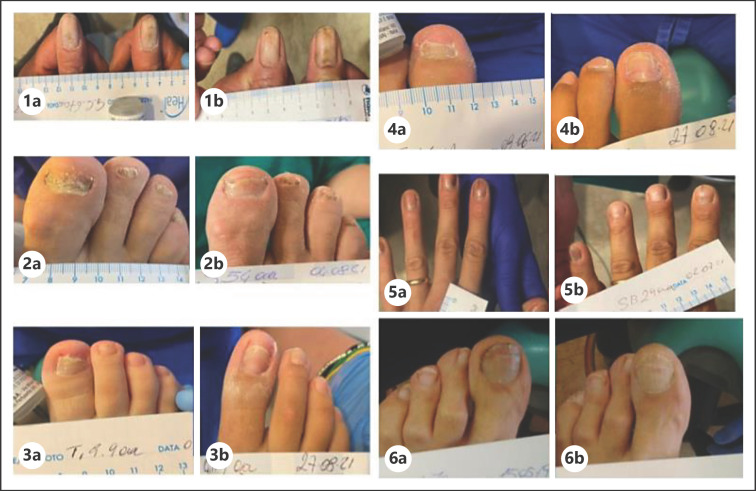
Ten subjects (6 men and 4 women, mean age 38 years) after their written informed consent, with clinical NLP (2 subjects with histological confirmation) affecting the foot or hand nails (mean number of nails involved: 4; range from 1 to 10), were treated for 12 consecutive weeks with EU-KR, one application per day. The presence of pterygium was an exclusion criterion. The main endpoint was the evolution of an NLP severity score (NLPSS) of the most affected nail, evaluating 7 nail signs: (1) grade of onycholysis, (2) longitudinal ridging, (3) splitting, (4) grade of subungual hyperkeratosis, (5) nail bed thickening, (6) dyschromia, and (7) nail pitting. For each item, a 4-grade score (from 0: no sign to 3: severe) was used (range of NLPSS from 0 to 21). At baseline, the NLPSS was 20.8 ± 3. After 12 weeks, the NLPSS showed a significant reduction (p < 0.0001; paired T Test) to 4 ± 8.8, representing an 81% reduction in comparison with baseline value, with an absolute difference between means of −16.86 ± 2,586 (95% CI of the difference: from −22.49 to −11.22) (Fig. 1). Figure 2 reports colour pictures of 6 subjects with NLP before and after treatment with the U-KR lacquer.
Fig. 1.
NLPSS (mean ± SD) at baseline and after 12-week treatment with U-KR lacquer. ***p < 0.0001: paired T Test.
Fig. 2.
Colour pictures of 6 (1, 2, 3, 4, 5, and 6) subjects before (a) and after (b) treatment with U-KR lacquer.
Discussion & Conclusion
Lichen planus is an inflammatory disorder affecting skin, hair, oral mucosa, and nail [22]. The worldwide prevalence is estimated around 1%. NLP could be the only clinical manifestation of the disease. NLP is one of the few nails pathological conditions characterized by a high risk of permanent nail loss; therefore, prompt treatment is mandatory. However, NLP is a destructive inflammatory onychodystrophy that is often difficult to treat. The demonstration of the involvement of nail matrix is very common (up to 80% of cases) when histopathology diagnosis is performed in comparison with the simple clinical evaluation. Histopathology in NLP in general shows hyperkeratosis, acanthosis, elongation of rete ridges, and lymphocytes infiltration [23]. In more detail, in NLP there is irregular acanthosis; the mid-dermal cells appear larger, flatter, and paler than usual. A focal increase of granular layer (causing the Wickham striae on skin and mucosa) is observed with degeneration of basal epidermal cells. Flattened rete ridges of saw-tooth appearance are observed with focal vacuolization of the basal keratinocytes and separation of epidermis from dermis (the so-called Max Joseph spaces). In this regard, retinoid molecules could be able to modulate keratinocytes proliferation and reduce the inflammatory response [24]. Interestingly, lichen planus is believed to be an autoimmune disease characterized by increased type 1 interferon production [25]. Retinoids with an affinity for RXR receptors could reduce the upregulation of IFN. The oral retinoid alitretinoin and acitretin have shown to be efficacious in NLP [26]; however, systemic therapy with retinoids could be problematic to manage. Topical application of retinoids (i.e., tazarotene) could be an interesting alternative. This 10-case pilot study suggests that a nail lacquer with 3 components (urea, keratinase, and a retinoid molecule) could be effective and safe in subjects with moderate NLP in improving the main signs of nail involvement. Some limitations of the present cases report should be taken into account in evaluating these results. First, this is a description of clinical cases not a controlled trial. The diagnosis of NLP was only clinical and not histologically confirmed in most patients (8/10). A non-specific nail appearance improvement of the disease could not be completely ruled out even if this is not the common evolution of NLP. Future controlled trials are warranted to better define the therapeutic potential and the histological modification induced by this product in NLP treatment.
Statement of Ethics
This cases report collection complies with the guidelines for human studies. The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The subjects have given their written informed consent to publish their case including the images used in this article. In relation of the nature of the product used (a cosmetic) and the study design (a collection of cases), ethical approval is not required for this study in accordance with local or national guidelines. Written informed consent was obtained from participants and in one case by legal guardians for publication of the details of their medical case and used images.
Conflict of Interest Statement
L.A. declared no conflicts of interest. M.M. is an employee of Difa Cooper, Cantabria Labs group.
Funding Sources
This cases report collection was partially funded by Cantabria Labs Difa Cooper.
Conflict of Interest Statement
L.A. declared no conflicts of interest. M.M. is an employee of Difa Cooper, Cantabria Labs group.
Author Contributions
L.A. evaluated clinically all the subjects of this case series and performed the evaluation of the severity score and collected the image. M.M. participated in the writing of the final manuscript and data analysis.
Data Availability Statement
Source data are available upon specific request. These data are only available upon request because they are collected from subjects' personal medical recording files.
References
- 1.Tosti A, Peluso AM, Fanti PA, Piraccini BM. Nail lichen planus: clinical and pathologic study of twenty-four patients. J Am Acad Dermatol. 1993;28((5 Pt 1)):724–30. doi: 10.1016/0190-9622(93)70100-8. [DOI] [PubMed] [Google Scholar]
- 2.Zaias N. The nail in lichen planus. Arch Dermatol. 1970;101((3)):264–71. [PubMed] [Google Scholar]
- 3.Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366((8)):723–32. doi: 10.1056/NEJMcp1103641. [DOI] [PubMed] [Google Scholar]
- 4.Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39((2)):221–30. doi: 10.1016/j.det.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Ujiie H, Shibaki A, Akiyama M, Shimizu H. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90((2)):218–9. doi: 10.2340/00015555-0814. [DOI] [PubMed] [Google Scholar]
- 6.Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. J Eur Acad Dermatol Venereol. 2012;26((10)):1304–9. doi: 10.1111/j.1468-3083.2011.04288.x. [DOI] [PubMed] [Google Scholar]
- 7.Grover C, Bansal S, Nanda S, Reddy BS. Efficacy of triamcinolone acetonide in various acquired nail dystrophies. J Dermatol. 2005;32((12)):963–8. doi: 10.1111/j.1346-8138.2005.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 8.Adamo L, Milani M. An assessor-blinded trial. 29th EADV 2020 Virtual Congress; 2020. Improvement of onychodystrophy by a nail lacquer containing urea, keratinase and hydroxypinacolone retinoate. Abstract. [Google Scholar]
- 9.Pan M, Heinecke G, Bernardo S, Tsui C, Levitt J. Urea: a comprehensive review of the clinical literature. Dermatol Online J. 2013;19:20392. [PubMed] [Google Scholar]
- 10.Lin X, Lee CG, Casale ES, Shih JC. Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Appl Environ Microbiol. 1992;58((10)):3271–5. doi: 10.1128/aem.58.10.3271-3275.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temova Rakuša Ž, Škufca P, Kristl A, Roškar R. Retinoid stability and degradation kinetics in commercial cosmetic products. J Cosmet Dermatol. 2021;20((7)):2350–8. doi: 10.1111/jocd.13852. [DOI] [PubMed] [Google Scholar]
- 12.Jun SH, Kim H, Lee H, Song JE, Park SG, Kang NG. Synthesis of retinol-loaded lipid nanocarrier via vacuum emulsification to improve topical skin delivery. Polymers. 2021;13((5)):826. doi: 10.3390/polym13050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truchuelo MT, Jiménez N, Jaén P. Assessment of the efficacy and tolerance of a new combination of retinoids and depigmenting agents in the treatment of melasma. J Cosmet Dermatol. 2014;13((4)):261–8. doi: 10.1111/jocd.12110. [DOI] [PubMed] [Google Scholar]
- 14.Cameli N, Mariano M, Abril E, Berardesca E. Clinical evaluation of the efficacy of a new retinoic-complex (Retinsphere®) product for the treatment of skin photoaging. Esper Dermatol. 2012;14:29–34. [Google Scholar]
- 15.Veraldi S, Barbareschi M, Guanziroli E, Bettoli V, Minghetti S, Capitanio, et al. Treatment of mild to moderate acne with a fixed combination of hydroxypinacolone retinoate, retinol glycospheres and papain glycospheres. G Ital Dermatol Venereol. 2015;150((2)):143–7. [PubMed] [Google Scholar]
- 16.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10((9)):1002–13. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 17.Reifen R. Vitamin A as an anti-inflammatory agent. Proc Nutr Soc. 2002;61((3)):397–400. doi: 10.1079/PNS2002172. [DOI] [PubMed] [Google Scholar]
- 18.Dehesa L, Tosti A. Treatment of inflammatory nail disorders. Dermatol Ther. 2012;25((6)):525–34. doi: 10.1111/j.1529-8019.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 19.Alsenaid A, Eder I, Ruzicka T, Braun-Falco M, Wolf R. Successful treatment of nail lichen planus with alitretinoin: report of 2 cases and review of the literature. Dermatology. 2014;229((4)):293–6. doi: 10.1159/000365655. [DOI] [PubMed] [Google Scholar]
- 20.Katugampola RP, Finlay AY. Oral retinoid therapy for disorders of keratinization: single‐centre retrospective 25 years' experience on 23 patients. Br J Dermatol. 2006;154((2)):267–76. doi: 10.1111/j.1365-2133.2005.06906.x. [DOI] [PubMed] [Google Scholar]
- 21.Prevost NM, English JC., 3rd Palliative treatment of fingernail lichen planus. J Drugs Dermatol. 2007;6((2)):202–4. [PubMed] [Google Scholar]
- 22.Katta R. Lichen planus. Am Fam Physician. 2000;61((11)):3319–8. [PubMed] [Google Scholar]
- 23.Magalhães GM, Succi ICB, Sousa MAJ. Base for the histopathological study of nail lesions. An Bras Dermatol. 2003;78((1)):49–61. [Google Scholar]
- 24.Jean J, Soucy J, Pouliot R. Effects of retinoic acid on keratinocyte proliferation and differentiation in a psoriatic skin model. Tissue Eng Part A. 2011;17((13–14)):1859–68. doi: 10.1089/ten.TEA.2010.0463. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel J, Scheler M, Proelss J, Bieber T, Tüting T. Type I interferon-associated cytotoxic inflammation in lichen planus. J Cutan Pathol. 2006;33((10)):672–8. doi: 10.1111/j.1600-0560.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 26.Bubna AK. Alitretinoin in dermatology: an update. Indian J Dermatol. 2015 Sep–Oct;60((5)):520. doi: 10.4103/0019-5154.164426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data are available upon specific request. These data are only available upon request because they are collected from subjects' personal medical recording files.