Highlights
-
•
Widely targeted metabolome analysis was first performed on two taste wampees.
-
•
Taste related indicators and differential metabolites of wampees were described.
-
•
Key taste metabolites were identified, potential causes were explained.
-
•
This work helps to understand the formation of wampee flavors.
Keywords: Wampee, Sweet taste, Sweet–sour taste, Cultivar, Metabolomics
Abstract
Due to the lack of comprehensive evaluation of all metabolites in wampee, the metabolic reasons for taste differences are unclear. Here, two local varieties YF1 (sweet taste) and YF2 (sweet–sour taste), were selected for quality analysis, followed by ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) based widely targeted metabolomic analysis. YF1 and YF2 were clearly separated by principal component analysis (PCA) and cluster analysis, and 449 metabolites were different between the cultivars, including 29 carbohydrates and 29 organic acids. Among them, d-galactose, d-mannose, and d-fructose 6-phosphate contributed mainly to the sweet taste of the YF1 wampee. l-citramalic acid, 2-hydroxyglutaric acid, and 3-methylmalic acid were the dominant organic acids in YF2 wampee, and therefore, contributed primarily to the sweet–sour taste. The differential metabolites were significantly enriched in the “ascorbate and aldarate metabolism” and “C5-branched dibasic acid metabolism” pathways. Ascorbate played a crucial role in the regulation of sugars and organic acids through those pathways. In addition, high-performance liquid chromatography (HPLC) based quantitative verification exhibited the same specific cultivar variations.
1. Introduction
Wampee [Clausena lansium(Lour.) Skeels] is a tropical fruit of the family Rutaceae. It is usually widely planted in southern China, including Guangxi, Guangdong, and Hainan provinces, and occasionally in India and the United States(Fan et al., 2018, Lim, 2012). The fruit tastes sweet or acidic, such as grapes, and pulp can be used to make jelly, fruit drinks, and desserts (Prasad et al., 2009). Wampee is becoming popular not only because of its unique sweet and sour tastes but also because of its antioxidant (Zhu et al., 2020, Zeng et al., 2020), antifungal (He et al., 2019), and anti-obesity (Huang et al., 2017) effects. Many wampee cultivars are classified according to taste, such as sweet or sweet–sour. Generally, consumers prefer sweet wampee better, while sweet–sour wampee is widely used to prepare beverage and fermented food.
Metabolites contribute significantly to the flavor and taste of fruits. The composition and concentration of primary metabolites, especially carbohydrates and organic acids, are closely related to the sweetness and sourness of fruit (Chen et al., 2009, Guo et al., 2015, Ma et al., 2015, Zhu et al., 2020). Many scientists have focused on the bioactive components in the leaves and peels of wampee because both are traditional herbal remedies for gastrointestinal disorders, asthma, and coughings in China (Li, Wu & Li, 1996). However, as far as we know, the profiling and comparative analysis of the taste components in different varieties is very limited. Recently, wampee fruit was reported to contain different phenolics, flavonoids, and phytochemical compounds between sweet and sweet–sour varieties (Chang et al., 2018). Key taste compounds and metabolic causes between sweet and sweet–sour wampees are still unknown.
Widely targeted metabolome analysis based on ultra-performance liquid chromatography-tandem mass spectrometry (UPLC–MS/MS) is fast, accurate and reliable, and has been successfully used to detect numerous plant metabolites (Chen et al., 2013, Zou et al., 2020). To discover all metabolite contributions to taste, UPLC–MS/MS based metabolomics analysis was performed to identify and quantify all metabolites in the two wampee cultivars. Then, multivariate statistical analysis was used to compare the characteristic metabolites, especially carbohydrates and organic acids. The possible molecular mechanism was also explained by the Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation and pathway analysis. Our findings provide new molecular evidence and metabolic causes related to taste among wampee varieties.
2. Materials and methods
2.1. Chemicals and reagents
A 0.1 mol/L NaOH standard solution was purchased from Shenzhen Bolinda Technology Co., Ltd. (Shenzhen, China). Chromatographic grade d-galactose, d-mannose, and d-fructose 6-phosphate were purchased from the National Institute of Metrology (Beijing, China). Chromatographic grade l-citramalic acid, 2-hydroxyglutaric acid, and 3-methylmalic acid were purchased from Dr.Ehrenstorfer GmbH (Augsburg, Germany).
2.2. Materials and sampling
Two wampee cultivars with 90% maturity (102 ± 5 days after flowering; 2.5 ± 2 mm transverse diameter; 14.5%-16.5 % total soluble solids; yellow peel) were selected in this study (Fig. 1). YF1 is a sweet cultivar that originated from Guangdong Province, while YF2 is an ancient local sweet–sour cultivar in Hainan Province and both are widely planted in South China. Wampee fruits were collected from five-year-old trees grown at the Yongfa Research Base of Hainan Academy of Agricultural Sciences (19.7622 N, 110.2117E).
Fig. 1.

Mature fruits of YF1 and YF2 wampees.
Six to eight mature fruits were collected from three trees, peeled, juiced, and mixed to form a biological sample. Each variety has three biological samples, with at least 18 fruits. Then, samples were immediately dried by a freeze-dryer (Scientz-100F, Ningbo, China) and crushed by a mixer mill (MM400, Shanghai, China) with a zirconia bead at 30 Hz for 1.5 min. One hundred milligrams of lyophilized powder was dissolved in 1.2 mL of 70% methanol solution. After vortexing for 30 s every 30 min six times, the sample was stored at 4 °C overnight for extraction. After centrifugation at 12000 rpm for 10 min, the extracts were treated with a nylon syringe filter (0.22 μm, ANPEL, Shanghai, China) before UPLC–MS/MS analysis.
2.3. Quality analysis
The wampee pulp was separated from the peel, and juiced to determine pH, total soluble solids (TSS), titratable acid (TA), and soluble sugar content. A pH meter (PH838, SMART SENSOR, Dongguan, China) and a Brix refractometer (FNV-55, Henan Suijing Environmental Protection Technology Co., Ltd., Luoyang, China) were used to determine pH and TSS, respectively. TA content was measured by 0.1 mol/L NaOH. The soluble sugar content was determined by the sulfuric acid-anthrone colorimetric method at a wavelength of 620 nm. All quality analyses of the samples were repeated three times.
2.4. UPLC–MS/MS based widely targeted metabolome analysis
UPLC–MS/MS analysis was performed according to Deng et al. (2021) with some modifications. Briefly, UPLC (Nexera X2, Shimadzu) was equipped with a column (SB-C18, 2.1 mm × 100 mm, Agilent) and coupled to a 6500 quadrupole-linear ion trap (QTRAP) mass spectrometer. First, 2.0 μL filtered sample was loaded and maintained with a column at 40 °C. The flow rate of the mobile phase was 0.35 mL/min, which consisted of solvent A (ultrapure water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The mobile phase in the column was programmed to start with solvent of 95% A and 5% B. Second, 5% B was linearly increased to 95% within nine minutes and kept at 95% for 1 min. After that, 95% B decreased to 5% within 0.1 min. Finally, a 3 min re-equilibration period was employed.
2.5. KEGG annotation and pathway enrichment analysis of metabolites
Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp) is a database resource for understanding molecular-level information on the genome and chemicals in organisms (Kanehisa et al., 2011). All metabolites were annotated by the KEGG database, followed by enrichment and topological analysis of the pathways where differential metabolites were present. Key pathways were further screened based on the number of differential metabolites.
2.6. Quantitative verification of key taste metabolites by HPLC
The sugars and organic acids in the sample were extracted by the following methods: a 2 g sample was added to 10 mL deionized water, vortexed for 10 min, and ultrasonically extracted for ten minutes. After being filtered through an aqueous membrane (0.45 μm) and diluted five times, the sugars were analyzed by HPLC (Agilent 1260, USA) equipped with a column (Angela Innoval NH2 5 μm, 4.6 × 250 mm) according to GB5009.8–2016 (National Food Safety Standard for the Determination of Fructose, Glucose, Sucrose, Maltose and Lactose in Foods, CN). The organic acids were analyzed by UPLC (Waters ACQUITY UPLC H-CLASS, USA) equipped with a column (Waters Luna Omega Polar C18, 1.6 μm, 2.1 × 100 mm) according to GB5009.157–2016 (National Food Safety Standard for the Determination of Organic Acids in Foods, CN). All the analyses were repeated four times.
2.7. Statistical analysis
SPSS software(version 22.0, SPSS Inc.) was used to analyze the least significant difference(LSD) at the 5% and 1% levels. OriginPro software(2019b, OriginLab Inc.) was used for image processing.
3. Results and discussion
3.1. Quality analysis of wampee
YF1 (sweet taste) and YF2 (sweet–sour taste) are two distinct wampee cultivars widely cultivated in China. As shown in Table 1, there was no noticeable difference in total soluble solids (TSS) or soluble sugars between YF1 and YF2 wampee juice. However, pH, titratable acid (TA), and sugar-acid ratio showed significant differences. The TA of YF2 wampee was 2.51%, which was 5.34 times higher than that of YF1, resulting in a significantly lower pH and sugar-acid ratio of YF2 wampee. These findings indicated that the various compositions and concentrations of acids in the two cultivars were the main reason for the difference in sugar-acid ratio.
Table 1.
The basic qualities of fruit juice of YF1 and YF2 wampees.
| Cultivars | pH | TSS(%) | Soluble sugar(%) | TA(%) | Sugar-acid ratio |
|---|---|---|---|---|---|
| YF1 | 5.12 ± 0.09a | 16.46 ± 0.66a | 12.34 ± 1.81a | 0.47 ± 0.09b | 26.32 ± 1.48a |
| YF2 | 3.57 ± 0.05b | 14.86 ± 1.77a | 9.93 ± 0.59a | 2.51 ± 0.23a | 3.95 ± 0.25b |
Note: Different letter on the number meant significant difference between YF1 and YF2 wampees(p < 0.05)
Generally, sugars are closely related to the sweetness of the fruit. Organic acids greatly contribute to the sour taste, and their composition has a substantial impact on either sweet or sour taste (Chen et al., 2009, Ma et al., 2019, Minas et al., 2013). Soluble sugars in most mature fruits mainly include fructose, glucose and sucrose, while the composition of organic acids varies greatly among varieties. For instance, citric acid is the main organic acid in citrus fruits (Scherer et al., 2012). In contrast, malic acid is the dominant organic acid in loquat (Chen, Liu & Chen, 2009), apple (Ma et al., 2015). To the best of our knowledge, the comprehensive and comparative analyses of sugars and acids in different wampee cultivars are very limited. Thus, widely targeted metabolome analysis was performed for qualitative and quantitative detection of taste metabolites, especially sugars and acids.
3.2. Widely targeted metabolome analysis of wampee
With the development of UPLC–MS/MS, widely targeted metabolite analysis has been widely used for large-scale identification and profiling of metabolites in several fruits (Oikawa et al., 2015, Wang et al., 2016, Zhang et al., 2020). In total, 1012 metabolites were identified, including carbohydrates, organic acids, amino acids, and other potential taste contributors. We carried out principal component analysis (PCA) on the 1012 metabolites. As shown in Fig. 2A, the contributions of PC1 and PC2 were 50.91% and 14.35%, respectively. The total contribution of the two principal components was 65.26%, which contained the major information of all 1012 metabolites. Three samples of YF1 gathered closely and obviously separated from YF2 and mixed samples(quality control), indicating that there were significant differences among varieties. In other words, YF1, YF2 and mixed samples were clearly separated by PCA. After applying a log10 transformation to the peak areas of each metabolite, hierarchical cluster analysis was performed. This result indicated that YF1 and YF2 wampees were two distinct groups (Fig. 2B). Thus, PCA and hierarchical cluster analysis both demonstrated that YF1 and YF2 had distinct metabolite profiles.
Fig. 2.
Differential fruit chemotype between YF1 and YF2. (A) PCA analysis of metabolites identified from YF1, YF2 and mix sample. Equal weight of YF1 and YF2 flesh samples were mixed for use as quality control. Each group had three individual samples. For example, YF1-1, YF1-2, YF1-3 were three YF1 wampee samples. (B) Hierarchical cluster analysis of metabolites from YF1 and YF2. The color from green (low) to red (high) indicates the level of each metabolite. The Z score represents the deviation from the mean by standard deviation units.
3.3. Identification and classification of differential metabolites
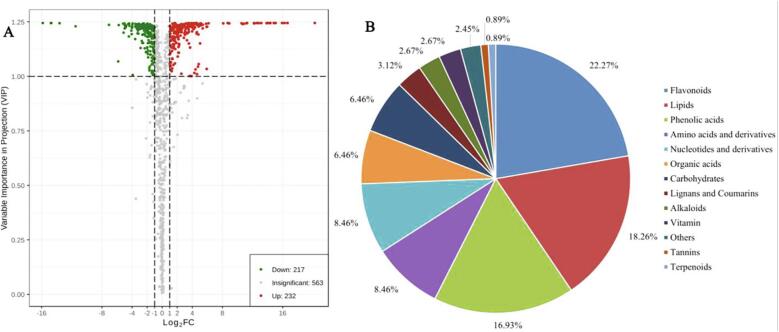
To identify differential metabolites between the two cultivars, we selected metabolites based on fold change ≥ 2 (upregulated) or ≤ 0.5 (downregulated) in YF2 compared with YF1. After that, a variable importance in projection value (VIP ≥ 1) from the orthogonal projections to latent structures-discriminatory analysis (OPLS-DA) model was used to screen these metabolites again. As shown in Fig. 3A, 449 differential metabolites between the two wampee cultivars were identified, 217 of which were downregulated, and 232 metabolites were upregulated in YF2 compared with YF1. The 449 differential metabolites can be categorized into 13 different classes (Fig. 3B), and the majority were flavonoids, lipids, phenolic acids, amino acids and derivatives, nucleotides and derivatives, organic acids, and carbohydrates. Previous studies also demonstrated that wampees contain high levels flavonoids, phenolic acids and carbazole alkaloids in different cultivars, showing high antioxidant and anticancer activities (Sun et al., 2020, Zhu et al., 2020). Many taste metabolites, including organic acids and carbohydrates, were also identified in the two cultivars.
Fig. 3.
Differential metabolites between YF1 and YF2. (A)Volcano plot of the 1012 metabolites identified. (B) Pie chart of the biochemical categories of the 449 differential metabolites.
3.4. Carbohydrates and organic acids
We focused on carbohydrates and organic acids, which may be the main contributors to taste. As shown in Table S1, 29 carbohydrates differentially accumulated in YF1 and YF2 wampees, 21 of which showed a significant difference (p < 0.05). Except for d-arabinono-1,4-lactone, 2-dehydro-3-deoxy-l-arabinonate, and d-glucurono-6,3-lactone, 26 other carbohydrates downregulated in the YF2 wampee compared with the YF1 wampee, which could explain the much sourer taste of YF2 wampee. In particular, the abundances of d-galactose, d-mannose, and d-fructose 6-phosphate, as the major sugars in YF1, were significantly greater than YF2, which contributed primarily to the sweeter taste of the YF1 wampee. Numerous studies have shown that fructose is the main sugar in fruits, such as citrus (Zhou et al., 2018), apple (Jakopic et al., 2012), and mango (Liu et al., 2013). Galactose and mannose were also reported as important components of water-soluble polysaccharides in wolfberry and cherry, and varied from maturity levels and cultivars (Fan et al., 2010).
Organic acids are crucial factors in fruit flavor (Chen et al., 2009). In total, 29 organic acids were differentially accumulated and 25 exhibited significant differences (p < 0.05). This finding is consistent with the result that TA showed significant differences between YF1 and YF2 wampees (Table 1). Among them, l-citramalic acid, 2-hydroxyglutaric acid, and 3-methylmalic acid accumulated at the highest concentrations significantly in YF2 wampee compared with YF1 wampee. These three massive organic acids were the main reasons for the low sugar/acid ratio of YF2 wampee (Table 1), therefore, contributing mostly to acidic taste. The dominant acids are malic and citric in most fruits, and their final concentrations are influenced by the balance of biosynthesis and degradation of organic acids in mature fruit (Diakou et al., 2000). We speculated that the biosynthesis and degradation of organic acids, especially three major organic acids, in the two cultivar wampees were significantly different. The possible molecular mechanism of carbohydrate and organic acid regulation needs to be further studied.
3.5. KEGG classification and enrichment analysis of differential metabolites
KEGG enrichment was carried out for the 449 differential metabolites to uncover the molecular mechanism related to taste. The KEGG pathway analysis revealed that a significant enrichment occurred in “ascorbate and aldarate metabolism” and “C5-branched dibasic acid metabolism”. As shown in Fig. 4, the Log2YF2/YF1 of l-ascorbate was 20.2, and the peak area of l-ascorbate in YF2 wampee was 1253888-fold higher than that of YF1 wampee. This result indicated that l-ascorbate might play a vital role in the above main pathways.
Fig. 4.
Maps of KEGG pathways involved in key differential metabolites. Note: The pathway maps include “ascorbate and aldarate metabolism” and “C5-branched dibasic acid metabolism”. The colored circles in front of each metabolite indicate log2YF2/YF1 values.
According to maps of KEGG pathways (Fig. 4), on the one hand, the large consumption of sugars as precursor substances, especially d-glucuronate and d-glucarate, caused a considerable accumulation of ascorbate in YF1 wampee. On the other hand, several organic acids, including (S)-citramalate and itaconate, were produced through the “C5-branched dibasic acid metabolism pathway” by ascorbate degradation. Ascorbate is reported as the biosynthetic precursor of l-tartaric and oxalic acids in several plants (Debolt, Melino & Ford, 2007). It is the dominant organic acid in many fruits, such as grape (Keskin et al., 2021), wolfberry (Zhao et al., 2015). Our findings proved that carbohydrates and organic acids associated with ascorbate synthesis and degradation might be vital for wampee taste differences. Flavonoids, lipids, lignans, and coumarins were also identified in our widely targeted metabolite analysis. However, they were not significantly enriched in the KEGG pathways; therefore, they were deemed unlikely to be the main contributors to the taste differences between the two wampees.
3.6. Quantitative verification of key taste metabolites by HPLC
Six key taste metabolites, including three carbohydrates (d-galactose, d-mannose, d-fructose 6-phosphate) and three organic acids (l-citramalic acid, 2-hydroxyglutaric acid, 3-methylmalic acid) were selected for quantitative verification by HPLC. As shown in Fig. 5, all key taste metabolites exhibited extremely significant differences (p < 0.01) between cultivars, which was similar to the metabolomics results. The contents of d-galactose, d-mannose, d-fructose 6-phosphate in YF1 wampee with sweet taste were significantly higher than those in YF2 wampee. In contrast, the accumulation of l-citramalic acid, 2-hydroxyglutaric acid, and 3-methylmalic acid in YF2 wampee with sweet–sour taste was significantly higher than that in YF1 wampee. In particular, the l-citramalic acid contents were 968.0 mg/kg and 8611.3 mg/kg in YF1 and YF2 wampees, respectively, which exhibited the greatest differences between cultivars. Therefore, the notably different accumulation of the three carbohydrates and three acids between YF1 and YF2 was one of the main reasons for the different sugar-acid ratios, leading to the individual sweet or sweet–sour tastes. In general, sour fruits contain higher levels of ascorbate (Liang et al., 2017). As one of the important substrates for ascorbate biosynthesis, sugar content exhibited a close correlation with ascorbate in fruit (Mario et al., 2021, Assoi et al., 2021). This correlation could be one of the main reasons for the low carbohydrates and high ascorbic acid contents found in YF2 wampee. The large amount of l-citramalic acid found in YF2 wampee was another reason for its much more sour taste.
Fig. 5.
Quantitative verification of key taste metabolites by HPLC. (A) Quantitative verification of carbohydrates. (B) Quantitative verification of organic acids. Note: ** on the column meant extremely significant difference between YF1 and YF2 wampees (p < 0.01).
4. Conclusions
In this study, we performed UPLC–MS/MS based widely targeted metabolome analysis on two typical wampees, YF1 (sweet taste) and YF2 (sweet–sour taste). A total of 449 differential metabolites were identified between the two cultivars, including 29 carbohydrates and 29 organic acids. Among them, d-galactose, d-mannose, and d-fructose 6-phosphate contributed mostly to the sweet taste of the YF1 wampee. l-citramalic acid, 2-hydroxyglutaric acid, 3-methylmalic acid, and quinic acid were the dominant organic acids in YF2 wampee. Furthermore, KEGG pathway enrichment analysis also indicated that “ascorbate and aldarate metabolism” and “C5-branched dibasic acid metabolism” were the main underlying causes of differences in tastes between the cultivars, and ascorbate played a vital role in the regulation of sugars and organic acids. The above results provide important insights into the taste-forming components and mechanism of wampees.
CRediT authorship contribution statement
Qing-chun Yin: Investigation, Methodology, Writing – original draft. Jian-bang Ji: Conceptualization, Supervision. Rong-hu Zhang: Investigation, Methodology, Writing – review & editing. Zhou-wei Duan: Investigation, Writing – review & editing. Hui Xie: Investigation, Writing – review & editing. Zhe Chen: Writing – review & editing. Fu-chu Hu: Writing – review & editing. Hao Deng: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was funded by Scientific and Technical Project of Hainan Province (KYYS-2021-03) and Scientific Research Institute Innovation Project of Hainan Province (jscx202009), China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100261.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Assoi E.K., Bagui O.K., Kouakou B.K., Gbogbo A.Y., Zoueu J.T. Estimating maturity by measuring ph, sugar, dry matter, water and vitamin c content of cashew apple (anacardium occidentale) from remote spectral reflectance data using neural network. Australian Journal of Crop Science. 2021;15(7):1029–1034. doi: 10.21475/ajcs.21.15.07.p3075. [DOI] [Google Scholar]
- Chang X., Ye Y., Pan J., Lin Z., Qiu J., Guo X., Lu Y. Comparative assessment of phytochemical profiles and antioxidant activities in selected five varieties of wampee (Clausena lansium) fruits. International Journal of Food Science & Technology. 2018;53(12):2680–2686. doi: 10.1111/ijfs.13877. [DOI] [Google Scholar]
- Chen F., Liu X., Chen L. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chemistry. 2009;114(2):657–664. doi: 10.1016/j.foodchem.2008.10.003. [DOI] [Google Scholar]
- Chen W., Gong L., Guo Z., Wang W., Zhang H., Liu X.…Luo J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Molecular Plant. 2013;6(6):1769–1780. doi: 10.1093/mp/sst080. [DOI] [PubMed] [Google Scholar]
- Debolt S., Melino V., Ford C.M. Ascorbate as a biosynthetic precursor in plants. Annals of Botany. 2007;99(1):3–8. doi: 10.1093/aob/mcl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Yin Q.C., Lin Y.Q., Feng J.C., Chen Z., Zhang R.H. Analysis on quality differences associated with metabolomics of rambutan during different temperature storage. Food Chemistry: Molecular Sciences. 2021;3 doi: 10.1016/j.fochms.2021.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakou P., Svanella L., Raymond P., Gaudillère J.P., Moing A. Phosphoenolpyruvate carboxylase during grape berry development: Protein level, enzyme activity and regulation. Australian Journal of Plant Physiology. 2000;27:221–229. doi: 10.1071/PP99141. [DOI] [Google Scholar]
- Fan H.P., Mazza G., Liao X.J. Purification, composition and antioxidant activity of polysaccharides from wolfberry, cherry, kiwi and cranberry fruits. Croatian Journal of Food Science & Technology. 2010;2(1):9–17. [Google Scholar]
- Fan Y.J., Chen H.Q., Mei W.L., Kong F.D., Li F.X., Chen P.W.…Dai H.F. Nematicidal amide alkaloids from the seeds of Clausena lansium. Fitoterapla. 2018;128:20–25. doi: 10.1016/j.fitote.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Guo S., Duan J., Qian D., Tang Y., Wu D., Su S.…Zhao Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chemistry. 2015;167:468–474. doi: 10.1016/j.foodchem.2014.07.013. [DOI] [PubMed] [Google Scholar]
- He X., Zhang L., Chen J., Sui J., Yi G., Wu J., Ma Y. Correlation between chemical composition and antifungal activity of Clausena lansium essential oil against Candida spp. Molecules. 2019;24(7):1394. doi: 10.3390/molecules24071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Li D., Xu Y.S., Feng Z.L., Meng F.C., Zhang Q.W.…Lin L.G. Clausoxamine, an alkaloid possessing a 1,3-oxazine-4-one ring from the seeds of clausena lansium and the anti-obesity effect of lansiumamide B. RSC Advances. 2017;7(74):46900–46905. doi: 10.1039/C7RA09793J. [DOI] [Google Scholar]
- Jakopic J., Slatnar A., Stampar F., Veberic R., Simoncic A. Analysis of selected primary metabolites and phenolic profile of ‘Golden Delicious’ apples from four production systems. Fruits. 2012;67(5):377–386. doi: 10.1051/fruits/2012032. [DOI] [Google Scholar]
- Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Research. 2011;40(D1):D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin N., Kunter B., Elik H., Kaya Z., Keskin S. Anom approach for the statistical evaluation of organic acid contents of clones of the grape variety 'kalecik karas'. Mitteilungen Klosterneuburg. 2021;71(2):126–138. [Google Scholar]
- Li S.H., Wu S.L., Li W.S. Amides and coumarin from the leaves of Clausena lansium. Journal of Chinese Pharmaceutical Sciences. 1996;48(5):367–373. [Google Scholar]
- Liang D., Zhu T., Ni Z., Lin L., Tang Y., Wang Z.…Ezura H. Ascorbic acid metabolism during sweet cherry (Prunus avium) fruit development. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T.K. Springer; Berlin: 2012. Clausena lansium., Edible Medicinal and Non-Medicinal Plants; pp. 871–883. [Google Scholar]
- Liu F., Fu S., Bi X., Chen F., Liao X., Hu X., Wu J. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chemistry. 2013;138(1):396–405. doi: 10.1016/j.foodchem.2012.09.111. [DOI] [PubMed] [Google Scholar]
- Ma B.Q., Ding Y.D., Li C.Y., Li M.J., Ma F.W., Yuan Y.Y. Comparative proteomic analysis reveals key proteins linked to the accumulation of soluble sugars and organic acids in the mature fruits of the wild malus species. Plants. 2019;8(11):488. doi: 10.3390/plants8110488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Chen J., Zheng H., Fang T., Ogutu C., Li S.…Wu B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chemistry. 2015;172:86–91. doi: 10.1016/j.foodchem.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Mario F., Vitor A.S., Alicia E.V., Dominique A., Noemi R.L., Castillo A.G.…B. The role of GDP-L-galactose phosphorylase in the control of ascorbate biosynthesis. Plant Physiology. 2021;185(4):1574–1594. doi: 10.1093/plphys/kiab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas I.S., Crisosto G.M., Holcroft D., Vasilakakis M., Crisosto C.H. Postharvest handling of plums (Prunus salicina Lindl.) at 10°C to save energy and preserve fruit quality using an innovative application system of 1-MCP. Postharvest Biology and Technology. 2013;76:1–9. doi: 10.1016/j.postharvbio.2012.08.013. [DOI] [Google Scholar]
- Oikawa A., Otsuka T., Nakabayashi R., Jikumaru Y., Isuzugawa K., Murayama H.…Osorio-Algar S. Metabolic profiling of developing pear fruits reveals dynamic variation in primary and secondary metabolites, including plant hormones. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, K. N., Hao, J., Yi, C., Zhang, D., Qiu, S., Jiang, Y., Zhang, M., & Chen, F. (2009). Antioxidant and anticancer activities of wampee (Clausena lansium (Lour.) Skeels) Peel. Journal of Biomedicine and Biotechnology, 2009, 612850. 10.1155/2009/612805. [DOI] [PMC free article] [PubMed]
- Scherer R., Rybka A.C.P., Ballus C.A., Meinhart A.D., Filho J.T., Godoy H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chemistry. 2012;135(1):150–154. doi: 10.1016/j.foodchem.2012.03.111. [DOI] [Google Scholar]
- Sun X.Y., Ma J., Li C.J., Zang Y.D., Zhang D.M. Carbazole alkaloids with bioactivities from the stems of clausena lansium. Phytochemistry Letters. 2020;38:28–32. doi: 10.1016/j.phytol.2020.03.004. [DOI] [Google Scholar]
- Wang S., Tu H., Wan J., Chen W., Liu X., Luo J.…Zhang H. Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chemistry. 2016;199:8–17. doi: 10.1016/j.foodchem.2015.11.113. [DOI] [PubMed] [Google Scholar]
- Zeng J.K., Jiang Z.T., Li W., Zhang L.B., Shao Y.Z. Effects of UV-C irradiation on postharvest quality and antioxidant properties of wampee fruit (Clausena lansium (Lour.) Skeels) during cold storage. Fruits. 2020;75(1):36–43. doi: 10.17660/th2020/75.1.4. [DOI] [Google Scholar]
- Zhang X., Li X., Su M., Du J., Zhou H., Li X., Ye Z. A comparative UPLC-Q-TOF/MS-based metabolomics approach for distinguishing peach (Prunus persica (L.) Batsch) fruit cultivars with varying antioxidant activity. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109531. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li H., Xi W., An W., Niu L., Cao Y.…Yin Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chemistry. 2015;173:718–724. doi: 10.1016/j.foodchem.2014.10.082. [DOI] [PubMed] [Google Scholar]
- Zhou Y., He W., Zheng W., Tan Q., Xie Z., Zheng C., Hu C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chemistry. 2018;259:278–285. doi: 10.1016/j.foodchem.2018.03.102. [DOI] [PubMed] [Google Scholar]
- Zhu, T. T., Zuo, W. J., Yan, J., Wen, P., Pei, Z. S., Lian, H. Y., & Yang, H. C. (2020). Comparative assessment of the antioxidant activities among the extracts of different parts of Clausena lansium (Lour.) Skeels in human gingival fibroblast cells. Evidence-based Complementary and Alternative Medicine, 2020, 3958098. 10.1155/2020/3958098. [DOI] [PMC free article] [PubMed]
- Zou S., Wu J., Shahid M.Q., He Y., Lin S., Liu Z., Yang X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chemistry. 2020;323 doi: 10.1016/j.foodchem.2020.126822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.