Abstract
Abstract
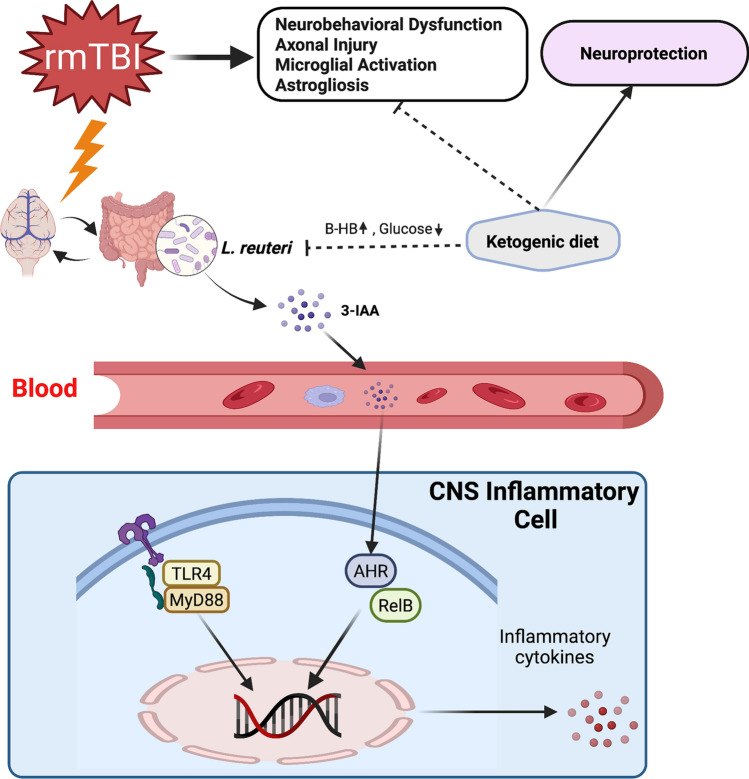
Repetitive mild traumatic brain injury (rmTBI) is associated with a range of neural changes which is characterized by axonal injury and neuroinflammation. Ketogenic diet (KD) is regarded as a potential therapy for facilitating recovery after moderate-severe traumatic brain injury (TBI). However, its effect on rmTBI has not been fully studied. In this study, we evaluated the anti-neuroinflammation effects of KD after rmTBI in adolescent mice and explored the potential mechanisms. Experimentally, specific pathogen-free (SPF) adolescent male C57BL/6 mice received a sham surgery or repetitive mild controlled cortical impacts consecutively for 7 days. The uninjured mice received the standard diet, and the mice with rmTBI were fed either the standard diet or KD for 7 days. One week later, all mice were subjected to behavioral tests and experimental analysis. Results suggest that KD significantly increased blood beta-hydroxybutyrate (β-HB) levels and improved neurological function. KD also reduced white matter damage, microgliosis, and astrogliosis induced by rmTBI. Aryl hydrocarbon receptor (AHR) signaling pathway, which was mediated by indole-3-acetic acid (3-IAA) from Lactobacillus reuteri (L. reuteri) in gut and activated in microglia and astrocytes after rmTBI, was inhibited by KD. The expression level of the toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88) in inflammatory cells, which mediates the NF-κB pathway, was also attenuated by KD. Taken together, our results indicated that KD can promote recovery following rmTBI in adolescent mice. KD may modulate neuroinflammation by altering L. reuteri in gut and its metabolites. The inhibition of indole/AHR pathway and the downregulation of TLR4/MyD88 may play a role in the beneficial effect of KD against neuroinflammation in rmTBI mice.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10571-022-01226-3.
Keywords: Microglia, Astrogliosis, Aryl hydrocarbon receptor, Toll-like receptor, β-Hydroxybutyrate
Introduction
Repetitive mild traumatic brain injury (rmTBI), also known as brain concussion, is a growing medical and economic problem worldwide, accounting for 70–90% of all traumatic brain injury cases. rmTBI might increase the long-term risk for cognitive impairment and dementia, stroke, Parkinson disease, and epilepsy, and is associated with increased long-term mortality rates (Maas et al. 2017). Emerging evidence suggests that rmTBI is associated with histopathological changes such as astrogliosis, microglial activation, axonal injury, and phosphorylated tau immunoreactivity (Yu et al. 2018; Verboon et al. 2021). However, the exact effects of rmTBI on brain functions and the underlying pathological mechanisms remain elusive.
The pathophysiology of rmTBI is characterized by complex changes in cerebral energy metabolism. Normally, the brain uses glucose as the primary energy source; however, immediately after a traumatic injury, the metabolism in the brain switches to increased uptake and utilization of glucose relative to the rate of oxygen utilization. This altered metabolic state, called hyperglycosis, is characterized by uncoupling of glycolysis and oxidative phosphorylation and accumulation of lactic acid. This is followed by a prolonged period of cerebral glucose hypometabolism (Blanco et al. 2016), during which ketone bodies (KB) are used as the main alternative fuel in the brain (Prins et al. 2004; Morris 2005; Thau-Zuchman et al. 2021). Cerebral ketone metabolism is an important compensatory metabolic pathway that bypasses the early glucose metabolic derangements after TBI.
Ketosis, or elevated plasma concentrations of KB, can be induced by a high-fat and low-carbohydrate ketogenic diet (KD) that induces a switch from glucose metabolism to fatty acid metabolism in the body (Prins et al. 2005; Mychasiuk and Rho 2017; Salberg et al. 2019). The classic KD is an isocaloric, high-fat, very low-carbohydrate, and normal-protein diet initially designed to treat patients with refractory epilepsy, particularly children (Koppel and Swerdlow 2018; Ding et al. 2021). Due to the positive effects of KD on refractory epilepsy, its use has been extended to treat a wide variety of diseases, such as Parkinson disease, Alzheimer disease, stroke, and moderate-severe TBI (Rho and Stafstrom 2012; McDonald and Cervenka 2018). Furthermore, KD can improve neurological function and increase the expression of monocarboxylate transporter 1 (MCT1) in the rat after TBI, in an age-dependent manner (Appelberg et al. 2009). Studies have shown that KB may mediate these benefits of KD. However, the exact mechanisms of beneficial effects of KD on TBI are not clear.
Gut microbiota (GM) plays an important role in the host physiology by impacting several metabolic and signaling pathways and neurological functions. The modulation of some of these pathways could be involved in the neuroprotection mediated by KD. It has been shown that KD alters the composition of GM in mice (Olson et al. 2018), and ketosis in humans is associated with the change in GM composition (Klein et al. 2016). Several studies have revealed that the GM can metabolize amino acid tryptophan into indole and its derivatives, that can act as aryl hydrocarbon receptor (AHR) ligands (Agus et al. 2018).
AHR is a ligand-dependent transcription factor that regulates a diverse spectrum of cellular functions by regulating gene expression in a ligand- and cell-type-specific manner and has roles in regulating immunity, stem cell maintenance, and cellular differentiation (Chen et al. 2019). The research on dietary tryptophan derived AHR ligands has identified novel interactions between the gut microbiome and central nervous system (CNS) inflammation.
L. reuteri is found as a gut symbiont in a number of mammalian species and has been described to participate in the transformation of dietary tryptophan into AHR agonists (Zelante et al. 2013). Interestingly, the abundance of L. reuteri is substantially altered in response to the KD (Olson et al. 2018). L. reuteri can use tryptophan as an energy source, producing the AHR agonist indole-3-acetic acid (3-IAA) as a metabolic product (Zelante et al. 2013). The microbial metabolites of tryptophan cross the blood–brain barrier and play a role in the inflammatory processes of the CNS by affecting AHR-driven mechanisms in microglia (Rothhammer et al. 2016). The latest research has also revealed that microglial AHR exerts both pro-inflammatory and anti-inflammatory effects in lipopolysaccharide-activated primary cultures of microglia, depending on the availability of exogenous AHR ligands (Lee et al. 2015). Therefore, the indole/AHR signaling pathway may play an important bridging role between the GM and neuroinflammation induced by rmTBI.
Microglial activation is one of the pathological changes occurring after rmTBI, and the mechanism of the regulatory effect of KD on microglial activation remains unknown. Among the variety of receptors involved in the signaling leading to microglial activation, an important contributor is toll-like receptor 4 (TLR4), which is mainly expressed on microglia. It has been reported that microglial pro-inflammatory function is activated by myeloid differentiation primary response 88 (MyD88)-dependent TLR4 signaling pathway after moderate-severe TBI (Zhang et al. 2018). Therefore, we hypothesized that KD would inhibit neuroinflammatory responses after rmTBI by modulating the MyD88-dependent TLR4 signaling pathway.
In this study, we evaluated the therapeutic potential of KD on rmTBI in adolescent mice and explored the underlying mechanisms of its anti-neuroinflammation effects.
Materials and Methods
Animals and Experimental Design
All procedures involving animals in this study were approved by the Animal Care and Experimental Committee of the School of Medicine of Shanghai Jiao Tong University (permit number: RJ2021-0203). A total of 90 specific pathogen-free (SPF), 3–4-week-old adolescent male C57BL/6 mice (10–12 g) were randomly divided into three groups: sham group fed with the standard diet (SCG), rmTBI group fed with the standard diet (TCG), and rmTBI group fed with KD (TKG). Mice were housed in individual cages in a temperature and humidity-controlled animal facility with a 12-h light/dark cycle. Mice were kept in the animal facility for at least 7 days before surgery, and they were given free access to food and water during this period.
The sample size was not predetermined by a statistical method, but our sample sizes are similar to those generally used in the field (Thau-Zuchman et al. 2021). Furthermore, sample sizes for mouse experiments were sufficient for normality, variance homogeneity and statistical analyses. Randomization and blinding (the experimenter being blind to treatment group) were undertaken in all animal experiments. Experimenter blinding was sufficient to control for selection bias.
Repetitive Mild Traumatic Brain Injury Mouse Model
Mice were subjected to deep anesthesia with 2% isoflurane (RWD Life Science Co., Ltd, R510-22, Shenzhen, China) and were placed in a stereotaxic frame in the prone position. After shaving the head, an incision was made along the midline of the scalp, and a self-made concave metal disc was adhered to the head. Controlled cortical impact (CCI) injury was induced using a PinPoint™ PCI3000 Precision Control Impactor™ (Hatteras Instruments, Cary, North Carolina, USA) with the following settings: a 2.5 mm impactor tip with a speed of 1.0 m/s, a depth of 1.5 mm, and a dwell time of 100 ms. SCG mice received the same anesthetic and surgical procedures without impaction. After the injury, the scalp was closed with sutures. Repetitive injuries were induced for a total of 7 times within a 24-h interval. Mice were allowed to recover on a warm carpet (37 °C) until fully awake and active and then returned to their cages.
Diet Interventions and Food Intake Studies
Mice in each group were fed with either the standard diet (Xie Tong Biotechnology Co., Ltd, XTKDCON, Jiangsu, China) or KD (Xie Tong Biotechnology Co., Ltd, XTKD01, Jiangsu, China). TKG mice were not preconditioned with KD and were put on a KD only after the CCI. The standard diet contained 10% protein, 80% carbohydrates, and 10% fat as (% kcal), and KD contained 10% protein and 90% fat (% kcal) as macronutrients. The caloric value of the standard diet and KD were 3.8 kcal/g and 6.7 kcal/g, respectively. In both the standard diet and KD, fat was derived from soybean oil and cocoa butter. Micronutrient content, fiber, and preservatives were matched on a per calorie basis. During experiments, mice had free access to the diets, which were placed in the food well of the cage-top wire lid. The composition of the control diet and KD are shown in Supplementary Tables 3 and 4.
Mice were placed in individual chambers with free access to pre-weighed diets and water. Food uptake was monitored by food weights. Measured values of food intake were normalized to body weight.
Beam Walking
The goal of beam walking task was for the mouse to remain upright while traversing an elevated tapered beam (100 cm long, suspended horizontally 1 m above ground) from one end to the other to reach a safe, dark box. Animals were trained to walk on the beam before surgery to reinforce the goal of the task, and three trials were conducted per mouse, with one-minute breaks between the trials. Performance on the beam was quantified by measuring the time taken by the mouse to walk across the beam and the number of hind-leg foot slips that occurred during the task. The beam-walk balance test was performed on day 7 post-injury.
Y-Maze Test
Spatial working memory was measured by the Y-maze test (Kraeuter et al. 2019). A Y-maze is a horizontal maze with three arms (50 cm in length and 10 cm in width) and walls (20 cm in height). The arms are symmetrically inclined to each other at 120°. Animals were set free for spontaneous movement throughout the Y-maze by placing them at the center of the maze. The mice typically like to explore a new arm of the maze, rather than returning to the one previously explored. An alternation sequence is the one when a mouse enters all three arms in a sequence without entering a single-arm twice in a row. A wrong alternation is when a mouse enters an arm two times in a row (Dowling and Allen 2018). The following equation was used to determine the percentage of the wrong alternations:
Tissue Preparation for Light Microscopy
At 7d after rmTBI, mice were subjected to deep anesthesia with 2% isoflurane and perfused transcardially with 4% paraformaldehyde (Beyotime Biotechnology Co., Ltd, P-0099, Shanghai, China). The brains were removed, further fixed at 4 °C overnight in 4% paraformaldehyde, and then immersed in 30% sucrose/phosphate-buffered saline (PBS) (Beyotime Biotechnology Co., Ltd, C0221A, Shanghai, China) at 4 °C overnight. Specimens were mounted in the optimal cutting temperature compound (OCT) (Sakura Finetek Co., Ltd, 4538, Japan). Serial sections were obtained using a cryostat and stained with toluidine blue for 30 min; two to three drops of glacial acetic acid were then added. Once the nucleus and the granulation were clearly visible, the specimens were mounted in Permount or Histoclad. Sections were cut off a microtome and adhered to glass slides with polylysine (all purchased from Beyotime Biotechnology Co., Ltd, C0105M, Shanghai, China). Images of the injured cortex and ipsilateral hippocampus were captured at × 100 using a light microscope (Nikon Labophot; Nikon USA, Melville, NY). There were three mice in each of the three groups.
Beta-hydroxybutyrate (β-HB), Glucose, Total cholesterol, Triglycerides, Total bilirubin, Biliverdin and Hemin Analysis in Plasma
Blood samples were collected (n = 10/group) through intracardiac sampling, plasma was isolated by centrifugation (12,000×g for 5 min), and samples were prepared with EDTA anticoagulant tube. A ketone and glucose monitoring system (FreeStyle Optium Neo, Abbott, NJ, USA) was used to measure blood β-HB and glucose levels according to the manufacturer’s instructions. Total cholesterol (TCs) levels were determined using a cholesterol assay kit (10,007,640, Cayman Chemical Company Ltd., USA). Plasma triglycerides (TGs) were determined with the Triglycerides Assay Kit (TR0100; Sigma, St. Louis, MO, USA). Serum levels of total bilirubin, biliverdin, and hemin were determined with commercial ELISA kits by following the manufacturer’s instructions. Bilirubin (#B4126), biliverdin (#30,891), hemin (#MAK036), and brain β-HB (#MAK272) kits were purchased from Sigma-Aldrich Co., Ltd. (MO, USA).
Silver Staining
Formalin-fixed OCT-embedded Sects. (40-µm-thick) were subjected to silver stain analysis to determine axonal damage. Silver staining was performed by using the FD NeuroSilver Kit II (FD NeuroTechnologies, PK301, Ellicott City, MD) according to the manufacturer's instructions but with some modifications. Densitometric analysis of silver staining was performed on 3 sections per mouse for corpus callosum. Images of the corpus callosum were captured at × 200 by using a light microscope (Nikon Labophot; Nikon USA, Melville, NY) and were converted to grayscale with background subtraction. ImageJ software (NIH, Bethesda, MD) was used to quantify the staining on these digitized images (Winston et al. 2016). Silver staining was expressed in arbitrary units, ranging from 0 (minimum) to 255 (maximum) (Shitaka et al. 2011). There were six mice in each of the three groups.
In Vivo Longitudinal Magnetic Resonance Imaging
In vivo MRI studies were performed on a 7-Telsa Bruker Biospec 70/20 USR spectrometer (Bruker BioSpin Corporation, Billerica, Massachusetts, USA) 7 days after the induction of rmTBI. Animals were placed onto a cradle with a stereotaxic head holder. Anesthesia was induced with 2% isoflurane via N2O/O2 (1:1) gas through a nose cone. Body temperature was maintained at 37.0 °C using warm air, monitored, and controlled via a rectal temperature probe (CWE model TC-1000, Ardmore, Pennsylvania, USA). Conventional MR scanning sequence included T2WI and diffusion tensor imaging (DTI).
A whole brain anatomical T2-weighted scan using a Rapid Acquisition with Refocused Echoes (RARE) sequence was performed with the following parameters: TR = 5500 ms, TE = 37 ms, RARE factor = 8, FOV = 2.5 × 2.5 cm, in plane resolution = 109 × 109 μm, 600 μm slice thickness, 45 slices, and 12 min acquisition time. The diffusion images were acquired with a spin-echo and echo-planar imaging (EPI) sequence between the olfactory bulb and the cerebellum.
The diffusion tensor images were obtained for fractional anisotropy (FA) and mean diffusivity (MD) using a weighted linear least squares method (Winston et al. 2016). Finally, a region-of-interest (ROI) analysis was performed in the corpus callosum using the RadiAnt DICOM Viewer.
Immunohistochemical Staining
Formalin-fixed OCT-embedded frozen sections (40 µm thick) were permeabilized with 0.3% Triton X-100 (Beyotime Biotechnology Co., Ltd, ST797, Shanghai, China) in PBS, followed by blocking for 4 h in 0.3% Triton X-100 with 10% donkey serum (Beyotime Biotechnology Co., Ltd, A7039, Shanghai, China). The sections were incubated in the primary antibody diluted in 0.3% Triton X-100 with 10% donkey serum for 48 h at 4 °C. After primary antibody incubation, the sections were washed in PBS and incubated in the secondary antibody diluted in 0.3% Triton X-100 with 10% donkey serum for 4 h at room temperature (RT). Sections were washed three times in PBS and incubated in Hoechst 33,342 (1:2,000; Thermo Fisher) for 15 min at RT for nuclear counterstaining. The sections were then mounted on microscope slides, sealed with clear nail polish, and stored at 4 °C for preservation. The negative control sections were incubated with only secondary antibodies. There were six mice in each of the three groups. The antibody information is listed in Supplementary Tables 1 and 2.
In our study, omission of the primary antibody and incubation of the sections only with secondary antibodies was used to confirm the specificity of the different secondary antibodies.
Immunofluorescence Microscopy Analysis
In order to assess microglial infiltration and astrogliosis, at least 6 selected microscope fields in hippocampus were observed in each group (× 200 magnifications; Zeiss LSM880; Zeiss, Germany), and microglia or astrocytes present in these fields were statistically analyzed. Morphology of microglia and astrocytes were quantified using ImageJ software (NIH, Bethesda, MD) program as described previously (Sun et al. 2012). Furthermore, to observe the cellular localization and expression of AHR and RelB in microglia, randomly selected microscope fields were observed in each group (× 400 magnifications; Zeiss LSM880; Zeiss, Germany). The negative control sections were observed in the same conditions. There were six mice in each of the three groups.
Western Blot Analysis
The hippocampus was harvested on day 7 after induction of rmTBI. The frozen brain samples were mechanically lysed in 20 mM tris (hydroxymethyl) aminomethane (Tris, pH 7.6), containing 0.2% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1% deoxycholate, 1 mM phenylmethylsulphonyl fluoride, and 0.11 IU/mL aprotinin (all purchased from Sigma–Aldrich, Inc., St. Louis, MO, USA). The lysates were centrifuged at 12,000 × g for 15 min at 4 °C and the supernatants were collected. The protein concentrations in the supernatants were estimated by the Bradford method.
The proteins (20 μg/lane) were separated on 12% SDS polyacrylamide gels and electro-transferred onto a polyvinylidene difluoride membrane (Bio-Rad Lab, Hercules, CA). The membrane was blocked with 5% skim milk (Beyotime Biotechnology Co., Ltd, P0216, Shanghai, China) (w/v) in Tris-buffered saline with 0.1% Tween-20 (TBST) (Beyotime Biotechnology Co., Ltd, ST825, Shanghai, China) for 1 h at room temperature and incubated with primary antibodies diluted in TBST for 24 h. After the membrane had been washed three times in TBST, 15 min each time, it was incubated with the appropriate horseradish peroxidase-conjugated secondary antibody diluted in TBST for 1 h. The cross-reactive protein bands were visualized by enhanced chemiluminescence Western blot detection reagents (Millipore, Burlington, MA), and the results were quantified by Quantity One Software (Bio Rad, Hercules, CA, USA). The band densities were calculated as ratios of TLR4 and MyD88/β-tubulin in the lanes. There were six mice in each of the three groups. The antibody information is listed in Supplementary Tables 1 and 2.
Enzyme-linked Immunosorbent Assay (ELISA) Analysis of Tumor Necrosis Factor (TNF), Interleukin-1 β (IL-1β), Chemokine (C-X-C motif) ligand 1 (CXCL1), Interferon Regulatory Factor 3 (IRF-3), and 3-IAA
On day 7 after rmTBI induction, mice were subjected to deep anesthesia using 2% isoflurane. The brains were quickly removed by dissection and kept over ice in the physiologic salt solution. The hippocampus specimens were separated, cut into small pieces, dispersed by repeated aspiration into a pipette tip, and suspended in 1 mL of physiologic salt solution with protease inhibitor (Beyotime Biotechnology Co., Ltd, P1006, Shanghai, China) in a test tube. Blood was collected by cardiac puncture, and the plasma was obtained by centrifugation (12,000 × g for 5 min). The colon was washed and flushed with PBS to remove lumenal contents. Tissue samples were sonicated on ice in 10 s intervals at 20 mV in RIPA lysis buffer (Beyotime Biotechnology Co., Ltd, P0013B, Shanghai, China), and the homogenates were centrifuged at 7500 rpm for 20 min. The supernatants were used for measuring the concentrations of cytokines and chemokines with commercial ELISA kits by following the manufacturer’s instructions.
TNF (#JL10484), IL-1β (#JL18442), CCL1 (#JL42145), and IRF3 ELISA (#JL20345) kits were purchased from Jiang Lai Biotechnology Co., Ltd. (Shanghai, China). Quantification of 3-IAA in mouse was performed using an ELISA kit (abx150354, Abbexa Ltd., Sugar Land, TX, USA) according to a previously described method (Constante et al. 2021). There were six mice in each of the three groups.
DNA Extraction from Fecal Samples
Bacterial DNA from frozen fecal samples was extracted using a QIAamp DNA stool mini kit (Qiagen, Hilden, 51,504, Germany) according to the manufacturer’s instructions and stored at − 20 °C until use. Nanodrop One spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used to quantify the DNA concentration, and the quality of the extracted DNA was estimated by the ratio of absorbance at 260 and 280 nm.
Standard Curve for qPCR
The standard curve was constructed by plotting the threshold cycles (Ct) values against the log input extracted DNA from respective dilutions of bacterial suspension of a reference strain of L. reuteri (Jomehzadeh et al. 2020). Briefly, L. reuteri reference strain was grown in 5 mL of Man, Rogosa & Sharpe broth medium (MRS) (Huaikai microbial Co., Ltd, 027312, Guangdong, China) to an OD 600 nm of 0.6 on a Nanodrop One spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and serially diluted to a final concentration range of 101–107 CFU mL−1. A 100μL of each dilution was plated on MRS agar and incubated under the microaerophilic condition for 48 h at 37 °C. Colonies were then enumerated and used for colony-forming unit extrapolation (CFU per milliliter). All experiments were performed at least in triplicate, and the average titer (CFU mL−1) of three replicates was determined. Community DNA from feces was extracted using a QIAamp DNA stool mini kit (Qiagen, Hilden, 51,504, Germany) as described above. By comparing the Ct values acquired to the standard curve, the number of cells of L. reuteri in the fecal samples were determined.
Quantitative Real-Time PCR
As previously described, Real-time quantitative PCR was performed using L. reuteri specific primers (Jomehzadeh et al. 2020). Total bacterial DNA was applied as a template for qPCR. The qPCR reaction volume of 20 μL contained 2 μL of 50 ng/μL of DNA template, each of forward and reverse primer in a volume of 0.4 μL, 10 μL of SYBR Advantage Premix, and nuclease-free water added to obtain the final volume of 20 μL (all purchased from Beyotime Biotechnology Co., Ltd, D7265, Shanghai, China). Initial denaturation at 95 °C for 30 s were followed by 40 cycles at 95 °C for 10 s, annealing at 60 °C for 30 s and extension at 70 °C for 15 s, and final elongation at 72 °C for 5 min. All experiments were performed in triplicates. There were six mice in each of the three groups. The following L. reuteri specific primer pair was used: Lreu-1: 5′-CAGACAATCTTTGATTGTTTAG-3′; Lreu-4: 5′-GCTTGTTGGTTTGGGCTCTTC-3′.
Statistical Analysis
All data are presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software, San Diego, USA). All data were subjected to one-way ANOVA followed by Tukey’s test. Statistical significance was inferred at P < 0.05. Normality was checked by Shapiro–Wilk test, and homogeneity of variance was checked by Brown-Forsythe test in multiple groups. The results showed that the variance was similar. The results of the tests for normality and variance homogeneity are shown in Supplementary Tables 5 and 6.
Results
A Stable Repetitive Mild Traumatic Brain Injury Mouse Model Was Established
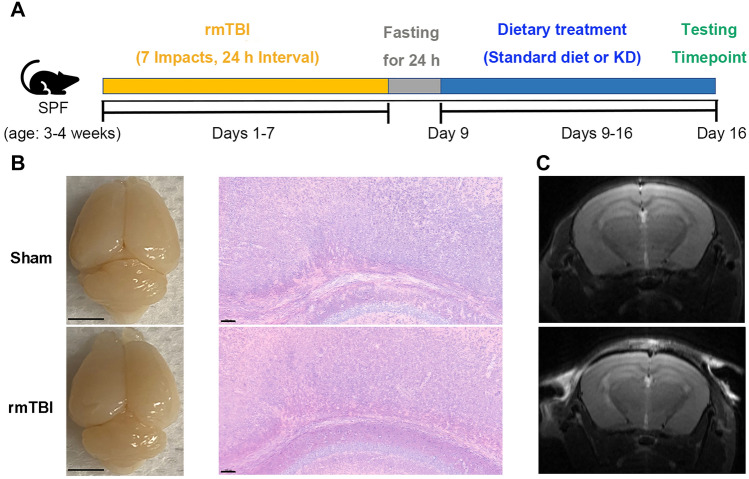
The rmTBI mouse model was induced with the CCI device. All mice were then subjected to dietary interventions. One week later, all mice were subjected to behavioral tests and experimental analysis (Fig. 1A). The hematoxylin & eosin (HE) staining of neurons and T2-weighted imaging (T2WI) were performed in our study. The general view and HE staining of the brains showed that rmTBI did not induce acute brain damage, including contusion and hemorrhage (Fig. 1B). Furthermore, no acute brain damage was observed in the brains of SCG and TCG mice in T2WI, suggesting that the impacts did not induce a moderate or severe brain injury (Fig. 1C).
Fig. 1.
A stable repetitive mild traumatic brain injury mouse model was established. A Experimental design for rmTBI model and diet interventions. Mice received a sham surgery or repetitive mild impacts consecutively for 7 days, after which they were fed a standard diet or KD for 7 more days. The subsequent behavioral tests and molecular experiments were performed on day 7 post-injury. B The general view and HE staining of the brains from SCG and TCG mice 7 days after rmTBI. No acute brain damage was observed on the brains of SCG and TCG mice. General view, scale bar = 0.5 cm; HE staining, scale bar = 100 μm. C Representative T2WI of the brains from sham and rmTBI mice. rmTBI did not induce acute macroscopic brain damage, including contusion and hemorrhage
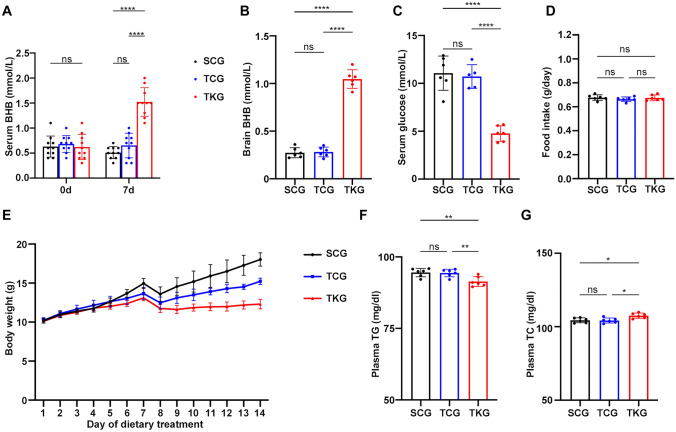
KD Elevated Serum β-HB, Brain β-HB, Plasma TC and Decreased Serum Glucose, Plasma TG After rmTBI
β-HB and glucose levels were measured in serum with ketone and glucose monitoring system. It is well-established that KD is associated with an induction of higher ketone levels and low glucose levels (Olson et al. 2018). Significantly increased serum β-HB level, brain β-HB level and decreased serum glucose level were detected in TKG mice, compared with the SCG and TCG mice (Fig. 2A, B, C). As shown in Fig. 2D, no significant change in food intake were observed. Moreover, we observed that KD-treated mice did not gain as much bodyweight as other groups did (Fig. 2E).
Fig. 2.
KD elevated serum β-HB, brain β-HB, plasma TC and decreased serum glucose, plasma TG after rmTBI. A, B and C Levels of serum β-HB, brain β-HB and glucose in mice fed standard diet or KD for 7 days. Data in the bar graphs represent mean ± SD. Serum β-HB: n = 10; one-way ANOVA; p = 0.3586 SCG vs TCG, ****p < 0.0001 SCG vs TKG, ****p < 0.0001 TCG vs TKG; F2, 27 = 2.272, p = 0.1225; brain β-HB: n = 6; one-way ANOVA; p = 0.9772 SCG vs TCG, ****p < 0.0001 SCG vs TKG, ****p < 0.0001 TCG vs TKG; F2, 15 = 0.9734, p = 0.4004. serum glucose: n = 6; one-way ANOVA; p = 0.7692 SCG vs TCG, ****p < 0.0001 SCG vs TKG, ****p < 0.0001 TCG vs TKG; F2, 15 = 1.908, p = 0.1826. D The amount of food intake in mice of different groups. N = 6; one-way ANOVA; p = 0.5732 SCG vs TCG, p = 0.9910 SCG vs TKG, p = 0.6505 TCG vs TKG; F2, 15 = 0.1667, p = 0.8480. E Line graph showing the dynamic changes in body weight of mice in different groups. n = 10/each group. F, G Levels of TG and TC in mice fed standard diet or KD for 7 days. Plasma TG: n = 6; one-way ANOVA; p = 0.9830 SCG vs TCG, **p = 0.0070 SCG vs TKG, **p = 0.0099 TCG vs TKG; F2, 15 = 0.1073, p = 0.8990; plasma TC: n = 6; one-way ANOVA; p = 0.9893 SCG vs TCG, *p = 0.0152 SCG vs TKG, *p = 0.0115 TCG vs TKG; F2, 15 = 0.04835, p = 0.9529
TG, TC, total bilirubin, biliverdin and hemin levels were measured in plasma with commercially available kits, respectively. Considerably reduced plasma TG level and increased plasma TC level were detected in TKG mice, compared with the SCG and TCG mice (Fig. 2F, G). Significantly increased serum total bilirubin was observed in TCG and TKG mice, compared with the SCG mice. Although serum levels of biliverdin and hemin were rose modestly after rmTBI, there were no significant differences between groups (Supplementary Fig. 1). These results indicate the presence of ketotic state in mice fed with KD.
rmTBI Caused Neurobehavioral Dysfunction, Which Was Ameliorated by KD
The neurobehavioral assessments were performed on 7th day post-injury. The balance and motor coordination of mice were assessed using the beam walk test. Average crossing time and hind-leg foot slips significantly increased in TCG mice, as compared with SCG mice. Moreover, KD decreased average crossing time and hind-leg foot slips in TKG mice compared with TCG mice (Fig. 3A, B). Moreover, the memory dysfunction as assessed by the Y-maze test indicated that rmTBI caused significant impairment of spatial working memory in TCG mice, compared with that in SCG mice; TKG mice showed significantly improved memory performance in the Y maze test (Fig. 3C). These observations indicate that KD treatment could ameliorate neurobehavioral dysfunction caused due to rmTBI.
Fig. 3.
rmTBI caused functional impairments, which were ameliorated by KD. A Average crossing time; B Number of foot slips in the beam walk task. Both SCG and TKG mice showed marked improvement compared with the TCG mice. Data in the bar graphs represent mean ± SD. Average crossing time: n = 10; one-way ANOVA; **p = 0.0012 SCG vs TCG, p = 0.5878 SCG vs TKG, *p = 0.0143 TCG vs TKG; F2, 27 = 0.2959, p = 0.7462; hind-leg foot slips: n = 10; one-way ANOVA; ***p = 0.0005 SCG vs TCG, p = 0.3471 SCG vs TKG, **p = 0.0060 TCG vs TKG; F2, 27 = 1.518, p = 0.2372. C Memory function assessment with the Y-maze test. A significant increase of wrong alteration was seen in TCG mice compared to those in SCG mice, and this was markedly reduced in TKG mice. Data in the bar graphs represent mean ± SD. N = 10; one-way ANOVA; *p = 0.0106 SCG vs TCG, p = 0.7856 SCG vs TKG, *p = 0.0489 TCG vs TKG; F2, 27 = 2.626, p = 0.0908
rmTBI Caused Axonal Injury, Which Was Alleviated by KD
Silver staining revealed abnormalities of white matter in the brains of TCG and TKG mice. The density of silver staining in the corpus callosum of TCG mice on 7th day post-injury showed significant axonal damage compared with that in SCG mice. However, there was a significant reduction of corpus callosum silver staining density in TKG mice (Fig. 4A, C).
Fig. 4.

rmTBI caused axonal injury, which was alleviated by KD. A Representative silver staining of the corpus callosum in different groups. Placement of the regions of interest in the corpus callosum (white box). Red scale bar = 50 μm; black scale bar = 20 μm. B Representative T2WI and DTI images of the corpus callosum in different groups. Placement of the regions of interest in the corpus callosum (yellow area). C Analysis of intensity of silver staining in the corpus callosum from SCG, TCG, TKG mice. Data in the bar graphs represent mean ± SD. N = 6; one-way ANOVA; ****p < 0.0001 SCG vs TCG, *p = 0.0230 SCG vs TKG, **p = 0.0066 TCG vs TKG; F2, 15 = 0.4987, p = 0.6170. D Evaluation of FA in the corpus callosum from SCG, TCG, TKG mice. Data in the bar graphs represent mean ± SD. N = 6; one-way ANOVA; *p = 0.0112 SCG vs TCG, p = 0.9643 SCG vs TKG, *p = 0.0186 TCG vs TKG; F2, 15 = 0.6393, p = 0.5415. E Evaluation of MD in the corpus callosum from SCG, TCG, TKG mice. Data in the bar graphs represent mean ± SD. N = 6; one-way ANOVA; **p = 0.0011 SCG vs TCG, p = 0.3988 SCG vs TKG, *p = 0.0153 TCG vs TKG; F2, 15 = 0.4786, p = 0.6288
FA and MD are the most commonly used DTI-derived metrics, which are believed to reflect overall white matter health. On 7th day after rmTBI, TCG mice showed increased FA and MD values in the corpus callosum compared with those in SCG. Meanwhile, TKG mice showed decreased FA and MD values, as compared with TCG mice (Fig. 4B, D and E). Thus, axonal damage on 7th day after rmTBI, was significantly alleviated by KD.
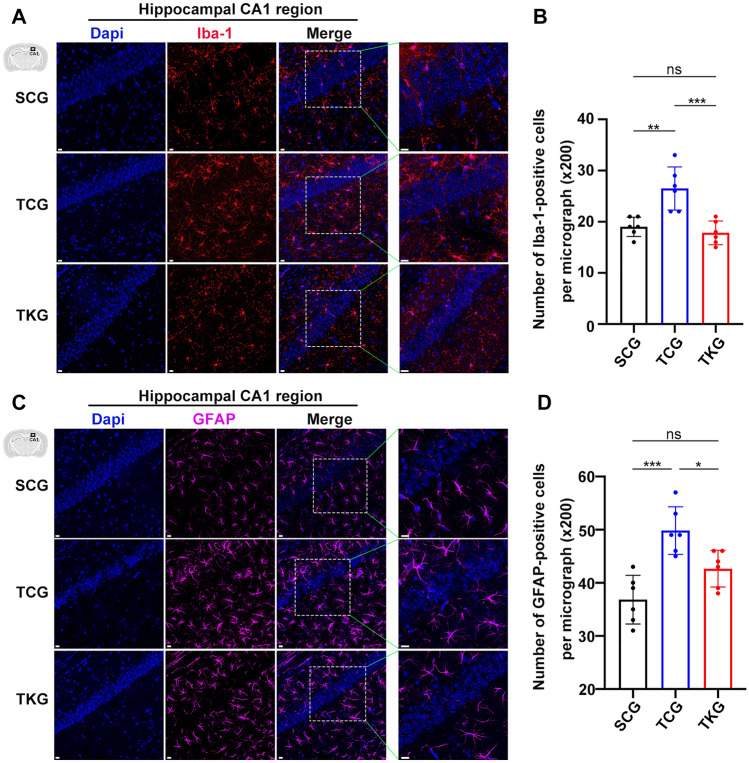
rmTBI Led to Microglial Activation and Astrogliosis, Which Were Inhibited by KD
rmTBI is followed by microglial activation and other neuroinflammatory responses. Change in microglial activation was evaluated by the number of Iba-1 positive cells in hippocampus by immunohistochemical staining. We found that the number of Iba-1 positive cells was dramatically increased in TCG mice, as compared with SCG mice. Meanwhile, the number of Iba-1 positive cells were decreased in TKG mice, as compared with TCG mice (Fig. 5A, B).
Fig. 5.
rmTBI led to microglial activation and astrogliosis, which were inhibited by KD. A and C Representative immunohistochemical staining of Iba-1 or GFAP from the hippocampal CA1 region. Scale bar = 20 μm. B and D Quantification of Iba-1 or GFAP positive cells in hippocampus. Data in the bar graphs represent mean ± SD. Iba-1: n = 6; one-way ANOVA; **p = 0.0016 SCG vs TCG, p = 0.7811 SCG vs TKG, ***p = 0.0004 TCG vs TKG; F2, 15 = 1.837, p = 0.1934; GFAP: n = 6; one-way ANOVA; ***p = 0.0002 SCG vs TCG, p = 0.0717 SCG vs TKG, *p = 0.0252 TCG vs TKG; F2, 15 = 0.3737, p = 0.6944
Immunohistochemical staining of astrocyte marker-glial fibrillary acidic protein (GFAP) was used to evaluate astrogliosis in hippocampus. Mice in TCG had significantly increased number of GFAP-positive cells, as compared with SCG mice, and mice in TKG had fewer GFAP-positive cells, as compared with TCG mice (Fig. 5C, D).
The morphological analyses of hippocampal microglia and astrocytes showed a shortening of microglia processes, an elongating of astrocytes processes, and no change of cell body size after rmTBI. Meanwhile, we also found considerably morphological changes of hippocampal microglia and astrocytes in KD-fed mice (Supplementary Fig. 2). These observations indicate that rmTBI caused microglial activation and astrogliosis, which were suppressed by KD.
rmTBI Activated L. reuteri Mediated Indole/AHR Signaling Pathway, Which Was Inhibited by KD
Standard curve obtained by plotting the average Ct values against the estimated log10 CFU for L. reuteri is shown in Fig. 6A. The amounts of L. reuteri (Log10 CFU/gram of feces) were determined in mice feces with qRT-PCR. The results showed that the amount of L. reuteri (Log10 CFU/gram of feces) in TCG mice was markedly increased compared with that in SCG mice. KD reduced the amount of L. reuteri in TKG mice, compared with that in TCG mice (Fig. 6B).
Fig. 6.
KD downregulated microbial tryptophan metabolism by GM after rmTBI. A Representative standard curve obtained by plotting the average Ct values against the estimated log10 CFU/PCR for L. reuteri. B Quantity of L. reuteri in the feces of different groups. Data in the bar graphs represent mean ± SD. N = 6; one-way ANOVA; ****p < 0.0001 SCG vs TCG, *p = 0.0149 SCG vs TKG, **p = 0.0027 TCG vs TKG; F2, 15 = 0.03846, p = 0.9962. C, D and E Concentration of 3-IAA in the large intestines, sera and brains, as determined by ELISA. Data in the bar graphs represent mean ± SD. 3-IAA in large intestines: n = 6; one-way ANOVA; ***p = 0.0002 SCG vs TCG, p = 0.9938 SCG vs TKG, ***p = 0.0002 TCG vs TKG; F2, 15 = 1.094, p = 0.3601; 3-IAA in serum: n = 6; one-way ANOVA; ***p = 0.0008 SCG vs TCG, p = 0.9812 SCG vs TKG, **p = 0.0011 TCG vs TKG; F2, 15 = 0.1263, p = 0.8823; 3-IAA in brains: n = 6; one-way ANOVA; *p = 0.0139 SCG vs TCG, p = 0.2686 SCG vs TKG, ***p = 0.0006 TCG vs TKG; F2, 15 = 3.351, p = 0.0626
To determine the role of indole/AHR pathway after rmTBI, we measured the levels of 3-IAA with ELISA, a potential endogenous AHR ligands in serum, large intestines, and brains in mice. The results showed that the levels of 3-IAA levels in TCG mice were markedly increased compared with those in SCG mice. Moreover, KD decreased the levels of 3-IAA in TKG mice (Fig. 6C-E). There were no significant differences in the levels of 3-IAA between SCG and TKG mice.
Mechanistically, AHR can modulate neuroinflammation via competitive binding with RelB. Therefore, we further investigated the expression and nuclear localization of AHR and RelB in microglia and astrocytes on 7th day after rmTBI, using immunofluorescent staining (Vogel and Matsumura 2009; Chen et al. 2019). Immunofluorescence analysis revealed that TCG mice showed a marked increase in the expression of AHR/RelB and co-localization in the nucleus of Iba-1 positive microglia and GFAP positive astrocytes, as compared with SCG mice. In TKG mice, compared with TCG mice, the AHR/RelB co-localization was more pronounced in the cytosol than in the nucleus (Fig. 7A). The negative control images have been added to supplementary materials (Supplementary Fig. 3).
Fig. 7.
rmTBI activated the L. reuteri mediated indole/AHR signaling pathway, which was inhibited by KD. A Representative confocal image of AHR/RelB colocalization in microglia and astrocytes from the hippocampal CA1 region. Solid arrows indicate co-localization of AHR and RelB in the nucleus. Hollow arrows indicate co-localization of AHR and RelB in the cytosol. Scale bar = 20 μm. B and C ELISA of CCL1 and IRF3 from hippocampus after rmTBI. Data in the bar graphs represent mean ± SD. CCL1: n = 6; one-way ANOVA; *p = 0.0122 SCG vs TCG, p = 0.2096 SCG vs TKG, ***p = 0.0004 TCG vs TKG; F2, 15 = 0.2224, p = 0.8032; IRF3: n = 6; one-way ANOVA; *p = 0.0163 SCG vs TCG, p = 0.9715 SCG vs TKG, *p = 0.0103 TCG vs TKG; F2, 15 = 0.09401, p = 0.9108
CCL1 and IRF3 are involved in the neuroinflammation after rmTBI. Thus, the levels of CCL1 and IRF3 in hippocampus were determined with ELISA. The results showed that the levels of CCL1 and IRF3 in TCG mice were markedly increased compared with those in SCG mice. KD attenuated the expressions of CCL1 and IRF3 in TKG mice (Fig. 7B, C). Collectively, these results suggest that L. reuteri mediated indole/AHR signaling pathway might participate in the pathological development of rmTBI by modulating inflammatory activation and KD played an important role in suppressing L. reuteri mediated indole/AHR signaling pathway.
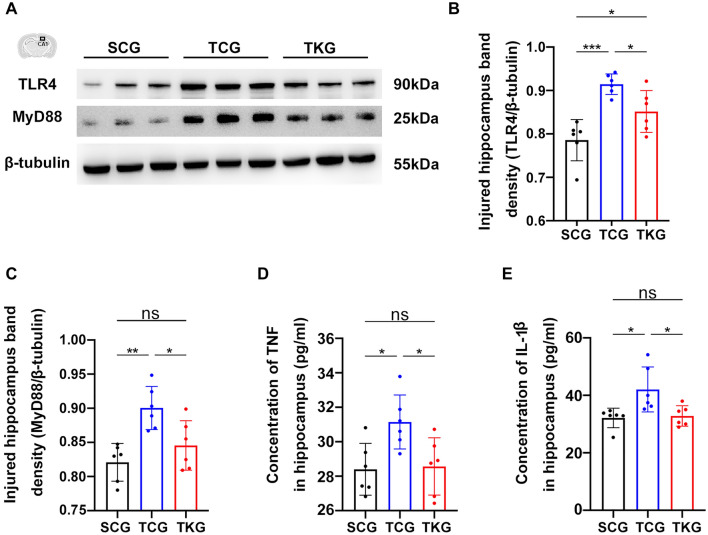
rmTBI Activated the TLR4/MyD88 Mediated NF-κB Pathway, Which Was Inhibited by KD
Expression levels of TLR4 and MyD88 in hippocampus were measured with Western blotting. Increased levels of TLR4 and MyD88 were observed in the hippocampus of mice in TCG on 7th day post-rmTBI, compared with those in SCG mice. Moreover, KD decreased levels of TLR4 and MyD88 in TKG mice (Fig. 8A-C and Supplementary materials).
Fig. 8.
rmTBI activated the TLR4/MyD88 mediated NF-κB pathway, which was inhibited by KD. A Representative western blotting of TLR4 and MyD88 from hippocampus. B and C Analysis the expression of TLR4 and MyD88 from hippocampus, with β-tubulin as the loading control. TLR4: n = 6; one-way ANOVA; ***p = 0.0002 SCG vs TCG, *p = 0.0363 SCG vs TKG, *p = 0.0462 TCG vs TKG; F2, 15 = 0.9181, p = 0.4206; MyD88: n = 6; one-way ANOVA; **p = 0.0017 SCG vs TCG, p = 0.3947 SCG vs TKG, *p = 0.0244 TCG vs TKG; F2, 15 = 0.5038, p = 0.6141. D and E Concentrations of TNF and IL-1β in hippocampus after rmTBI, as determined by ELISA. Data in the bar graphs represent mean ± SD. TNF: n = 6; one-way ANOVA; *p = 0.0131 SCG vs TCG, p = 0.9113 SCG vs TKG, *p = 0.0294 TCG vs TKG; F2, 15 = 0.01170, p = 0.9884; IL-1β: n = 6; one-way ANOVA; *p = 0.0162 SCG vs TCG, p = 0.9141 SCG vs TKG, *p = 0.0115 TCG vs TKG; F2, 15 = 1.858, p = 0.1901
TNF and IL-1β are involved in neuroinflammatory responses after rmTBI. Hence, the levels of TNF and IL-1β in hippocampus were determined with ELISA. Our results revealed that the expression levels of TNF and IL-1β in TCG mice were markedly increased compared with those in SCG mice. KD attenuated the expressions of TNF and IL-1β in TKG mice, compared with those in TCG mice (Fig. 8D, E). Taken together, these findings suggested that rmTBI activated the TLR4/MyD88 mediated NF-κB pathway, which was inhibited by KD.
Discussion
It was found that rmTBI induced by CCI caused axonal damage, microglial activation and astrogliosis, and impaired neurological functions. KD given immediately post rmTBI in adolescent mice significantly increased the blood β-HB levels and led to functional neurological benefits. KD, compared with control diet, inhibited the microgliosis, astrogliosis, and pro-inflammatory activation after rmTBI. Furthermore, rmTBI also activated TLR4/MyD88 mediated NF-κB pathway and 3-IAA mediated AHR pathway, which were attenuated by KD.
Spatial reference and working memory are mainly hippocampus-based (Garrett et al. 2020). CA1 subcircuit-dependent spatial working memory and reference memory are commonly impaired in brain-damaged patients (Ameen-Ali et al. 2015). Earlier studies indicated that selective removal of parvalbumin interneurons from the CA1 region of the hippocampus induced selective alterations in spatial working memory (Murray et al. 2011). Consequently, the hippocampal CA1 region was selected for further investigation in our study. Moreover, the Y-maze task is particularly used to evaluate the attention and spatial working memory of mice because of their natural ability to find an escape through small holes (Walrave et al. 2016). Here we show that experimental rmTBI causes behavioral abnormalities, including motor coordination and spatial memory impairment. Meanwhile, KD ameliorated rmTBI-induced neurobehavioral dysfunction.
rmTBI triggers axonal injury, which is associated with post-traumatic neurobehavioral impairment. Silver staining and DTI are two reliable approaches for the estimation of axonal damage after rmTBI. Several studies have confirmed that acute axonal injury in various white matter regions in rmTBI is indicated by argyrophilic structures and significant silver uptake compared to sham (Winston et al. 2016). Of all regions, the corpus callosum may have the most prominent and persistent course of silver staining abnormalities at 7 days (Hylin et al. 2013). FA and MD are the most commonly used DTI-derived metrics, which are believed to reflect overall white matter health, maturation, and organization (Hofstetter and Assaf 2017). In this study, results of silver staining and DTI showed significant axonal injury 7 days post-rmTBI. The increased intensity of silver staining in the injured mice correlated with their impaired motor coordination, as assessed by the beam walking test. We found that KD could ameliorate axonal damage and improve motor coordination in mice with rmTBI.
KD protects myelinated axons by increasing myelination that could improve axonal energy support (Stumpf et al. 2019). TBI-induced changes in cerebral glucose metabolism occur as a series of neurochemical events (Prins and Matsumoto 2016). When cerebral metabolism of glucose is compromised following brain injury, ketones can fulfill the energy requirements of the brain cells. Classical KD, with high-fat and low-carbohydrate content, can increase ketone levels and act as alternative substrates for all cell types. These KB stimulate mitochondrial metabolism and increase its metabolic efficiency, reduce the production of reactive oxygen species, and supply up to 70% of the energy required for brain function (Thau-Zuchman et al. 2021). Therefore, KD may be beneficial for the treatment for axonal damage following rmTBI.
Astrocytes are crucial for brain metabolism and are involved in many processes such as regulating glucose metabolism, participating in fatty oxidation (Gzielo et al. 2019). Under KD conditions, astrocytes mitochondria may spare glucose utilization, do not increase their insulin sensitivity, and provide trophic support to neurons (Koppel et al. 2021). Meanwhile, astrocytes are highly sensitive to a wide range of injuries. Astrogliosis is also a lingering impact seen in mice following rmTBI. Axonal damage and the related release of inflammatory cytokines by activated microglia can induce astrogliosis (Davenport et al. 2016). Our investigation showed that rmTBI caused reactive astrogliosis, and KD could attenuate astrocytes reactivity. We hypothesize these outcomes may be primarily related to KD-induced metabolic changes. Thus, modulation of metabolism in astrocytes might be a potential therapeutic target for rmTBI.
Studies on KD have shown that a fat-rich and carbohydrate-poor diet can inhibit microglial activation after moderate-severe TBI (Thau-Zuchman et al. 2021). Studies examining the mechanisms of therapeutic effects of KD on rmTBI are lacking. In this study, we have demonstrated that rmTBI caused neuroinflammation, which was ameliorated by KD. In addition to reducing the number of activated microglia and astrocytes, KD may also directly affect the pro-inflammatory function of inflammatory cells (Guan et al. 2020). This negative regulatory effect of KD may play an important role in reducing neuroinflammation mediated by inflammatory cells after rmTBI. Therefore, further explorations were conducted to elucidate underlying mechanisms of KD-regulated neuroinflammatory activation.
Since inflammatory damage is the key pathophysiological feature of rmTBI, we chose microglial activation and astrogliosis as the focus in our research to study the mechanisms underlying the beneficial effects of KD on rmTBI. Multiple molecular pathways, such as STAT and nuclear factor-κB (NF-κB) are involved in the regulation of neuroinflammation (Qin et al. 2012; Kobayashi et al. 2013; Tanaka et al. 2015). Previous studies have reported that moderate-severe TBI activated the TLR4/MyD88 mediated NF-κB pathway in microglial cells (Zhang et al. 2018). Our results show that KD could suppress the expression of TLR4 and MyD88 and negatively modulate the level of the inflammatory cytokines TNF and IL-1β 7 days after rmTBI. However, the mechanism by which KD regulates the TLR4/MyD88 mediated NF-κB pathway after rmTBI is not clear.
AHR could be activated by numerous exogenous compounds. Indole and its derivates such as 3-IAA and indole-3-propionic acid (3-IPA) have been identified as high-affinity endogenous ligands to the AHR (Gao et al. 2020). GM can impact levels of various neuroactive molecules in the peripheral nervous system and the brain (Vuong et al. 2017). Tryptophan, an essential amino acid in the diet, can be converted to 3-IAA by some bacteria of genus Lactobacillus and Bifidobacterium through aromatic amino acid aminotransferase and indole lactic acid dehydrogenase–dependent pathways (Cervantes-Barragan et al. 2017; Gao et al. 2020). Moreover, it has been reported that KD altered the GM and significantly decreased L. reuteri in mice fed with KD (Olson et al. 2018). However, whether L. reuteri mediated indole/AHR signaling pathway is involved in the regulation of KD on inflammatory activation after rmTBI was unclear.
Mechanistically, activated AHR translocates into the nucleus, interacts with RelB, and occupies RelB/AHR-response elements (RelB/AHRE) of promoters, resulting in activation of downstream target genes, like CCL1, IRF3 (Vogel and Matsumura 2009). It has been reported that AHR signaling potentially mediates post-stroke gliosis and ischemic brain injuries (Chen et al. 2019). In this study, we have shown that L. reuteri-produced tryptophan metabolite, 3-IAA, may be associated with neuroinflammation. Our study found that there were reduced co-localization of AHR/RelB in the nucleus of inflammatory cells and lower level of CCL1 and IRF3 in mice fed with KD, post-rmTBI. Thus, L. reuteri may participate in the modulation of KD on inflammatory activation after rmTBI through indole/AHR signaling.
Conclusion
Axonal damage, microglial activation and astrogliosis induced by rmTBI could be ameliorated in adolescent mice by giving KD immediately after rmTBI. KD significantly increased blood β-HB levels and provided functional neurological benefits. L. reuteri mediated indole/AHR and TLR4/MyD88 mediated NF-κB signaling pathways in the hippocampus were inhibited after KD intervention, which indicated a suppressed neuroinflammation related to microglial activation and astrogliosis. Our study provides insights into the potential mechanisms of beneficial effects of KD in rmTBI in adolescent mice.
Limitations
The exact mechanism by which KD affects the synthesis of indole compounds in the GM remains unclear. Inhibition of the indole/AHR pathway in inflammatory cells is the next logical step for confirmation of the role of GM produced indole metabolites in mediating the inhibitory effect of KD on neuroinflammatory responses. Several studies have reported age-related differences in the neuroprotection provided by KD and MCT1 expression after TBI (Appelberg et al. 2009). We have chosen adolescent mice for this study, further studies are required to verify the beneficial effect of KD on adult mice with rmTBI.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1 Serum levels of total bilirubin, biliverdin and hemin in mice of different groups. Bilirubin: n = 6; one-way ANOVA; *p = 0.0211 SCG vs TCG, *p = 0.0489 SCG vs TKG, p = 0.9017 TCG vs TKG; F2, 15 = 0.2121, p = 0.8113; biliverdin: n = 6; one-way ANOVA; p = 0.2009 SCG vs TCG, p = 0.0710 SCG vs TKG, p = 0.8210 TCG vs TKG; F2, 15 = 0.08621, p = 0.9179; hemin: n = 6; one-way ANOVA; p = 0.3920 SCG vs TCG, p = 0.5662 SCG vs TKG, p = 0.9483 TCG vs TKG; F2, 15 = 0.01908, p = 0.9811. Supplementary file3 (TIF 4196 KB)
Supplementary Figure 2 The morphological changes of microglia and astrocytes. A, C Fluorescence images showing microglia and astrocytes in the hippocampal CA1 region. Scale bar = 10 μm.B Graphs showing the morphological analyses in Iba-1+ microglia. Soma area: n = 6; one-way ANOVA; p = 0.2099 SCG vs TCG, p = 0.9224 SCG vs TKG, p = 0.1106 TCG vs TKG; F2, 15 = 1.281, p = 0.3065; process length: n = 6; one-way ANOVA; *p = 0.0102 SCG vs TCG, p = 0.7394 SCG vs TKG, *p = 0.0444 TCG vs TKG; F2, 15 = 1.452, p = 0.2653.D Graphs showing the morphological analyses in GFAP+ astrocytes. Soma area: n = 6; one-way ANOVA; p = 0.3629 SCG vs TCG, p = 0.2174 SCG vs TKG, *p = 0.0167 TCG vs TKG; F2, 15 = 0.09059, p = 0.9139; process length: n = 6; one-way ANOVA; *p = 0.0152 SCG vs TCG, p = 0.9847 SCG vs TKG, *p = 0.0212 TCG vs TKG; F2, 15 = 0.2645, p = 0.7711. Supplementary file4 (TIF 6632 KB)
Supplementary Figure 3 Negative control immunofluorescence images of AHR/RelB colocalization in microglia from the hippocampal CA1 region. Scale bar = 20 μm. Supplementary file5 (TIF 13068 KB)
Acknowledgements
We thank the entire the laboratory of Neurosurgery Department for supporting experimental space and technical services.
Abbreviations
- 3-IAA
Indole-3-acetic-acid
- AHR
Aryl hydrocarbon receptor
- β-HB
Beta-hydroxybutyrate
- CXCL1
Chemokine (C-X-C motif) ligand 1
- CCI
Controlled cortical impact
- DTI
Diffusion tensor imaging
- ELISA
Enzyme-linked immunosorbent assay
- FA
Fractional anisotropy
- GFAP
Glial fibrillary acidic protein
- HE
Hematoxylin and eosin
- IL-1β
Interleukin β1
- IRF-3
Interferon regulatory Factor 3
- Iba-1
Ionized calcium-binding adapter molecule 1
- KD
Ketogenic diet
- MyD88
Myeloid differentiation primary response 88
- MCT1
Monocarboxylate transporter 1
- MD
Mean diffusivity
- NF-κB
Nuclear factor kappa light chain enhancer of activated B cells
- OCT
Optimal cutting temperature compound
- PBS
Phosphate-buffered saline
- rmTBI
Repetitive mild traumatic brain injury
- TLR4
Toll-like receptor 4
- TNF
Tumor necrosis factor
Author Contributions
DD, FZ and YJ designed the experiments. DD, FZ and SS performed and analyzed most experiments with the help of TL, MC, QL. DD and FZ were major contributors in writing this manuscript. FJ and XZ participated in the discussions and revised the manuscript. YJ supervised the entire project and was responsible for finalizing and submitting the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82071359), the Shanghai Rising-Star Program (21QA1405600), the Natural Science Foundation of Shanghai (21ZR1439000) and Ren ji New Star Program (F. Jia).
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Animal protocols were approved by the Animal Care and Experimental Committee of the School of Medicine of Shanghai Jiao Tong University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dilirebati Dilimulati, Fengchen Zhang, and Shuai Shao have contributed equally to this work.
Contributor Information
Yichao Jin, Email: honam612@163.com, Email: alexmason@sjtu.edu.cn.
Feng Jia, Email: projiafeng@163.com.
Xiaohua Zhang, Email: zxh1969@aliyun.com.
References
- Agus A et al (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23(6):716–724. 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Ameen-Ali K et al (2015) Moving beyond standard procedures to assess spontaneous recognition memory. Neurosci Biobehav Rev 53:37–51. 10.1016/j.neubiorev.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Appelberg KS et al (2009) The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma 26(4):497–506. 10.1089/neu.2008.0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MMB et al (2016) Cerebral metabolism and the role of glucose control in acute traumatic brain injury. Neurosurg Clin 27(4):453–463. 10.1016/j.nec.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L et al (2017) Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science 357(6353):806–810. 10.1126/science.aah5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-C et al (2019) Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflammation 16(1):1–13. 10.1186/s12974-019-1572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constante M et al (2021) Saccharomyces boulardii CNCM I-745 modulates the microbiota–gut–brain axis in a humanized mouse model of Irritable Bowel Syndrome. Neurogastroenterol Motil 33(3):e13985. 10.1111/nmo.13985 [DOI] [PubMed] [Google Scholar]
- Davenport EM et al (2016) Abnormalities in diffusional kurtosis metrics related to head impact exposure in a season of high school varsity football. J Neurotrauma 33(23):2133–2146. 10.1089/neu.2015.4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M et al (2021) Microbiota–gut–brain axis and epilepsy: a review on mechanisms and potential therapeutics. Front Immunol 4131. 10.3389/fimmu.2021.742449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling C, Allen NJ (2018) Mice lacking glypican 4 display juvenile hyperactivity and adult social interaction deficits. Brain Plasticity 4(2):197–209. 10.3233/BPL-180079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K et al (2020) Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr 11(3):709–723. 10.1093/advances/nmz127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett L et al (2020) A truncating Aspm allele leads to a complex cognitive phenotype and region-specific reductions in parvalbuminergic neurons. Transl Psychiatry 10(1):1–14. 10.1038/s41398-020-0686-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y-F et al (2020) Anti-depression effects of ketogenic diet are mediated via the restoration of microglial activation and neuronal excitability in the lateral habenula. Brain Behav Immun 88:748–762. 10.1016/j.bbi.2020.05.032 [DOI] [PubMed] [Google Scholar]
- Gzielo K et al (2019) The impact of the ketogenic diet on glial cells morphology. A quantitative morphological analysis. Neuroscience 413:239–251. 10.1016/j.neuroscience.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Hofstetter S, Assaf Y (2017) The rapid development of structural plasticity through short water maze training: A DTI study. Neuroimage 155:202–208. 10.1016/j.neuroimage.2017.04.056 [DOI] [PubMed] [Google Scholar]
- Hylin MJ et al (2013) Behavioral and histopathological alterations resulting from mild fluid percussion injury. J Neurotrauma 30(9):702–715. 10.1089/neu.2012.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomehzadeh N et al (2020) Quantification of intestinal lactobacillus species in children with functional constipation by quantitative real-time PCR. Clin Exp Gastroenterol 13:141. 10.2147/CEG.S250755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MS et al (2016) Metabolomic modeling to monitor host responsiveness to gut microbiota manipulation in the BTBRT+ tf/j mouse. J Proteome Res 15(4):1143–1150. 10.1021/acs.jproteome.5b01025 [DOI] [PubMed] [Google Scholar]
- Kobayashi K et al (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4(3):e525–e525. 10.1038/cddis.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel SJ et al (2021) A ketogenic diet differentially affects neuron and astrocyte transcription. J Neurochem 157(6):1930–1945. 10.1111/jnc.15313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel SJ, Swerdlow RH (2018) Neuroketotherapeutics: a modern review of a century-old therapy. Neurochem Int 117:114–125. 10.1016/j.neuint.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A-K et al (2019) The Y-maze for assessment of spatial working and reference memory in mice. Pre-Clinical Models. 10.1007/978-1-4939-8994-2_10 [DOI] [PubMed] [Google Scholar]
- Lee YH et al (2015) Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia 63(7):1138–1154. 10.1002/glia.22805 [DOI] [PubMed] [Google Scholar]
- Maas AI et al (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16(12):987–1048. 10.1016/S1474-4422(17)30371-X [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Cervenka MC (2018) The expanding role of ketogenic diets in adult neurological disorders. Brain Sci 8(8):148. 10.3390/brainsci8080148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A (2005) Cerebral ketone body metabolism. J Inherit Metab Dis 28(2):109–121. 10.1007/s10545-005-5518-0 [DOI] [PubMed] [Google Scholar]
- Murray AJ et al (2011) Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci 14(3):297–299. 10.1038/nn.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Rho JM (2017) Genetic modifications associated with ketogenic diet treatment in the BTBRT+ Tf/J mouse model of autism spectrum disorder. Autism Res 10(3):456–471. 10.1002/aur.1682 [DOI] [PubMed] [Google Scholar]
- Olson CA et al (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173(7):1728–1741. 10.1016/j.cell.2018.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J et al (2017) Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol Biol Cell 28(20):2623–2636. 10.1091/mbc.E17-06-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M et al (2005) Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res 82(3):413–420. 10.1002/jnr.20633 [DOI] [PubMed] [Google Scholar]
- Prins M et al (2004) Increased cerebral uptake and oxidation of exogenous βHB improves ATP following traumatic brain injury in adult rats. J Neurochem 90(3):666–672. 10.1111/j.1471-4159.2004.02542.x [DOI] [PubMed] [Google Scholar]
- Prins ML, Matsumoto J (2016) Metabolic response of pediatric traumatic brain injury. J Child Neurol 31(1):28–34. 10.1177/0883073814549244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H et al (2012) Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci USA 109(13):5004–5009. 10.1073/pnas.1117218109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Stafstrom CE (2012) The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 3:59. 10.3389/fphar.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22(6):586–597. 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salberg S et al (2019) The behavioural and pathophysiological effects of the ketogenic diet on mild traumatic brain injury in adolescent rats. Behav Brain Res 376:112225. 10.1016/j.bbr.2019.112225 [DOI] [PubMed] [Google Scholar]
- Shitaka Y et al (2011) Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 70(7):551–567. 10.1097/NEN.0b013e31821f891f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf SK et al (2019) Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus-Merzbacher disease. Acta Neuropathol 138(1):147–161. 10.1007/s00401-019-01985-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L et al (2012) Microglial cathepsin B contributes to the initiation of peripheral inflammation-induced chronic pain. J Neurosci 32(33):11330–11342. 10.1523/JNEUROSCI.0677-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T et al (2015) Interferon regulatory factor 7 participates in the M 1-like microglial polarization switch. Glia 63(4):595–610. 10.1002/glia.22770 [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O et al (2021) A new ketogenic formulation improves functional outcome and reduces tissue loss following traumatic brain injury in adult mice. Theranostics 11(1):346. 10.7150/thno.48995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboon LN et al (2021) The immune system’s role in the consequences of mild traumatic brain injury (concussion). Front Immunol 12:313. 10.3389/fimmu.2021.620698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Matsumura F (2009) A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-κB family. Biochem Pharmacol 77(4):734–745. 10.1016/j.bcp.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE et al (2017) The microbiome and host behavior. Annu Rev Neurosci 40:21–49. 10.1146/annurev-neuro-072116-031347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrave L et al (2016) Inhibition of connexin43 hemichannels impairs spatial short-term memory without affecting spatial working memory. Front Cell Neurosci 10:288. 10.3389/fncel.2016.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN et al (2016) Dendritic spine loss and chronic white matter inflammation in a mouse model of highly repetitive head trauma. Am J Pathol 186(3):552–567. 10.1016/j.ajpath.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J et al (2018) Impact of nutrition on inflammation, tauopathy, and behavioral outcomes from chronic traumatic encephalopathy. J Neuroinflammation 15(1):1–16. 10.1186/s12974-018-1312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T et al (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39(2):372–385. 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Zhang F et al (2018) Moderate hypothermia inhibits microglial activation after traumatic brain injury by modulating autophagy/apoptosis and the MyD88-dependent TLR4 signaling pathway. J Neuroinflamm 15(1):1–12. 10.1186/s12974-018-1315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Serum levels of total bilirubin, biliverdin and hemin in mice of different groups. Bilirubin: n = 6; one-way ANOVA; *p = 0.0211 SCG vs TCG, *p = 0.0489 SCG vs TKG, p = 0.9017 TCG vs TKG; F2, 15 = 0.2121, p = 0.8113; biliverdin: n = 6; one-way ANOVA; p = 0.2009 SCG vs TCG, p = 0.0710 SCG vs TKG, p = 0.8210 TCG vs TKG; F2, 15 = 0.08621, p = 0.9179; hemin: n = 6; one-way ANOVA; p = 0.3920 SCG vs TCG, p = 0.5662 SCG vs TKG, p = 0.9483 TCG vs TKG; F2, 15 = 0.01908, p = 0.9811. Supplementary file3 (TIF 4196 KB)
Supplementary Figure 2 The morphological changes of microglia and astrocytes. A, C Fluorescence images showing microglia and astrocytes in the hippocampal CA1 region. Scale bar = 10 μm.B Graphs showing the morphological analyses in Iba-1+ microglia. Soma area: n = 6; one-way ANOVA; p = 0.2099 SCG vs TCG, p = 0.9224 SCG vs TKG, p = 0.1106 TCG vs TKG; F2, 15 = 1.281, p = 0.3065; process length: n = 6; one-way ANOVA; *p = 0.0102 SCG vs TCG, p = 0.7394 SCG vs TKG, *p = 0.0444 TCG vs TKG; F2, 15 = 1.452, p = 0.2653.D Graphs showing the morphological analyses in GFAP+ astrocytes. Soma area: n = 6; one-way ANOVA; p = 0.3629 SCG vs TCG, p = 0.2174 SCG vs TKG, *p = 0.0167 TCG vs TKG; F2, 15 = 0.09059, p = 0.9139; process length: n = 6; one-way ANOVA; *p = 0.0152 SCG vs TCG, p = 0.9847 SCG vs TKG, *p = 0.0212 TCG vs TKG; F2, 15 = 0.2645, p = 0.7711. Supplementary file4 (TIF 6632 KB)
Supplementary Figure 3 Negative control immunofluorescence images of AHR/RelB colocalization in microglia from the hippocampal CA1 region. Scale bar = 20 μm. Supplementary file5 (TIF 13068 KB)
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.