Abstract
Topiramate-induced acute angle closure (TiAAC) is a potentially vision-threatening side effect of topiramate (TPM) use. The purpose of this article is to review demographic characteristics, clinical features, and management options of TiAAC. A systematic literature search of all reported cases and case series of TiAAC was conducted in the following search engines: PubMed, Web of Science, Google Scholar, Elsevier, and EBSCO. Seventy-three publications describing 77 cases were included. 58 (75.3%) patients were female, and the mean age was 34.88 ± 11.21 years (range, 7–57). The most commonly reported indication of TPM use was migraine headache (59.7%), and the mean duration from starting treatment until the onset of angle closure was 14.1 ± 31.5 days. All cases were managed by immediate cessation of TPM and topical therapy. In addition, systemic medications (carbonic anhydrase inhibitors, hyperosmotic agents, and steroids) were used in 51 patients (66.2%). A laser and/or surgical intervention was performed in 10 patients (13%). After commencement of treatment, the mean duration until the resolution of TiAAC was 3.9 ± 3.6 days (range, 1–18). The findings of our study present a summary of the current body of evidence provided by case reports and case series on TiAAC. In conclusion, the onset of angle closure following TPM use peaks at 2 weeks after initiating treatment, and in most cases, successful management can be achieved by discontinuing TPM and initiating appropriate medical therapy.
Keywords: Angle-closure, glaucoma, topiramate
Topiramate (TPM) is a sulfamate-substituted monosaccharide and an antiepileptic drug that was approved by the United States Food and Drug Administration for its efficacy in treating epilepsy and preventing migraine headache. The drug exerts its effect through several mechanisms of action, including sodium and L-type calcium channels blockage, enhancement of gamma-aminobutyric acid (GABA) receptors, a-amino-3-hydroxy-5-methylisoxazole- 4-propionic acid (AMPA) and kainite current suppression, and carbonic anhydrase inhibition.[1] Following its widespread use, several systemic adverse effects were documented following treatment with TPM, such as weight loss,[2] cognitive dysfunction,[3] and kidney stones.[4] In addition, TPM can also lead to a wide range of ophthalmologic side effects such as acute angle closure, acute onset myopia, uveitis, scleritis, visual field defects, suprachoroidal effusions, oculogyric crisis, and retinal hemorrhage.[5]
TPM-induced acute angle closure (TiAAC) is a potentially vision-threatening side effect of TPM use. It occurs secondary to a drug-induced ciliochoroidal effusion that is associated with a forward movement of the lens-iris diaphragm which subsequently leads to acute angle closure.[6] The exact mechanism underlying ciliary body effusion is not clearly understood; however, it is thought to be related to pharmacological stimulation of prostaglandins release leading to vasodilation and increased permeability in the ciliary body.[7] The literature describing TiAAC primarily consists of a large number of case reports, making it difficult to review such reports and draw clinically relevant conclusions. In this article, we conducted a systematic review of these case reports in an attempt to summarize the current body of evidence on clinical features, management options, and prognosis of TiAAC.
Methods
Protocol
This review was written per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[8]
Eligibility criteria
Inclusion criteria comprised of published case reports and case series reporting original data of confirmed acute angle closure secondary to the use of TPM in either adult or pediatric patients. Exclusion criteria were as follows: articles written in a language other than English, research papers on non-human subjects, studies other than case reports and case series, and reports of other TPM-related side effects.
Information sources
A literature search of the following search engines was conducted: PubMed, Web of Science, Google Scholar, Elsevier, and EBSCO was performed on January 2021.
Search strategy
The search was conducted using the term “topiramate” combined with either “glaucoma” OR “acute” OR “angle closure”. Search filters were used to narrow the results down to meet the inclusion criteria. The same strategy was used to search all the aforementioned online databases.
Study selection
Two investigators independently screened the search results for papers that meet the required inclusion criteria in two steps: First, the screening was focused on the titles and abstracts of the seemingly relevant articles; then, the full-text articles of the initially selected papers were retrieved, independently reviewed, and ultimately decided for compatibility according to the inclusion criteria. Disagreements were discussed and resolved between the two investigators.
Data collection process
The extracted data included patient demographics (age and gender), the indication of TPM use, dosage, duration of usage until the onset of clinical manifestations, presenting symptoms, ophthalmic examination findings, treatment modalities, and the period until the intraocular pressure (IOP) was controlled. An electronic form was used to facilitate data collection from the selected case reports and series.
Summary measures
The extracted data was revised, coded, and entered into IBM SPSS, Version 22 (SPSS, Inc. Chicago, IL) for analysis. Descriptive analysis was performed for all variables in which categorical data were presented as frequencies and percentages and continuous variables were presented as mean ± SD.
Results
Study selection
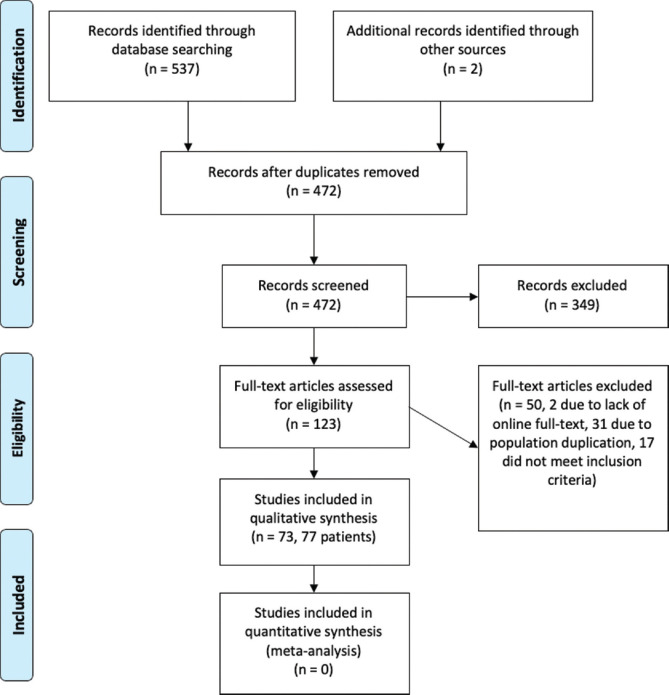
Our search through online databases yielded 537 citations. Furthermore, two additional citations were added through manual search. After removing duplicate records, 472 citations remained; 349 of these were excluded after the initial screening of titles and abstracts. After that, the full text of 123 citations was screened for inclusion criteria. Of these, 50 studies were excluded due to lack of full text (n = 2), duplicate population (n = 31), and failure to meet inclusion criteria (n = 17). Finally, a total of 73 articles (77 patients) were included in the review [Fig. 1].[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]
Figure 1.
PRISMA Flow Diagram of the systematic review
Study characteristics
The years of publication ranged from 2001 to 2020. Tables 1 and 2 present a summary of the baseline and clinical characteristics of the 77 patients included in this review.
Table 1.
A summary of baseline characteristics
| SN | Reference | Age | Sex | Indication | Drug Dose mg/day) | Duration of Use Prior to Onset |
|---|---|---|---|---|---|---|
| 1 | Agarwal.[10] | 25 | F | weight loss | 23 | 11 days |
| 2 | Aminlari et al.[21] | 48 | F | pain control | - | 2 weeks |
| 3 | Aminlari et al.[21] | 53 | M | cluster headache | - | 6 weeks |
| 4 | Arun et al.[32] | 25 | F | migraine headache | 50 | 1 week |
| 5 | Baloch et al.[43] | 20 | F | migraine headache | - | - |
| 6 | Banta et al.[54] | 51 | M | bipolar affective disorder | 150 | 2 weeks |
| 7 | Behl et al.[65] | 20 | F | migraine headache | 50 | 8 days |
| 8 | Bhattacharyya et al.[76] | 40 | F | migraine headache | 25 | 4 days |
| 9 | BMR DEO et al.[16] | 29 | F | weight loss | 100 | - |
| 10 | Boentert et al.[80] | 23 | F | epilepsy | 50 | 6 days |
| 11 | Boonyaleephan.[81] | 23 | F | migraine headache | 25 | 1 week |
| 12 | Braganza et al.[9] | 19 | M | migraine headache | 50 | 2 weeks |
| 13 | Caglar et al.[11] | 36 | F | migraine headache | 25 | 1 day |
| 14 | Chalam et al.[12] | 34 | F | migraine headache | 100 | 1 week |
| 15 | Cole et al.[13] | 56 | F | migraine headache | 50 | - |
| 16 | Craig et al.[14] | 25 | F | epilepsy and depression | 100 | 1 week |
| 17 | Craig et al.[14] | 45 | F | epilepsy | 50 | 10 days |
| 18 | Czyz et al.[15] | 40 | F | migraine headache | 100 | 262 days |
| 19 | Desai et al.[17] | 36 | F | migraine headache | 25 | 10 days |
| 20 | Diaz-Cespedes et al.[18] | 45 | M | headache | 50 | 2 weeks |
| 21 | Giuliari et al.[19] | 13 | F | migraine headache | - | 1 week |
| 22 | Grewal et al.[20] | 39 | F | weight loss | 23 | 1 week |
| 23 | Guier.[22] | 27 | F | migraine headache | 50 | 2 weeks |
| 24 | Joshi et al.[24] | 47 | M | migraine headache | 25 | 10 days |
| 25 | Kamal et al.[25] | 32 | F | migraine headache | 50 | - |
| 26 | Katsimpris et al.[26] | 36 | F | migraine headache | 100 | 2 weeks |
| 27 | Kulkarni et al.[27] | 25 | F | migraine headache | 25 | 3 days |
| 28 | Kumar et al.[28] | 25 | F | headache and insomnia | 100 | 2 weeks |
| 29 | Kumar et al.[28] | 18 | F | headache | 100 | - |
| 30 | Lan et al.[29] | 43 | F | weight loss | 50 | 1 month |
| 31 | Lan et al.[29] | 34 | F | weight loss | 25 | 3 weeks |
| 32 | Latini et al.[30] | 13 | F | headache | 25 | 1 week |
| 33 | Levy et al.[31] | 35 | F | migraine headache | 100 | 1 week |
| 34 | Lin et al.[33] | 41 | F | migraine headache | 50 | 1 week |
| 35 | Mahendradas et al.[34] | 36 | F | migraine headache | 100 | 5 days |
| 36 | Mansoor et al.[35] | 51 | F | migraine headache | 25 | 1 week |
| 37 | Mazumdar et al.[36] | 38 | M | migraine headache | 25 | 1 week |
| 38 | Medeiros et al.[37] | 44 | M | bipolar affective disorders | - | 3 days |
| 39 | Mitra et al.[38] | 31 | F | migraine headache | 25 | 1 week |
| 40 | Morales-Leon et al.[58] | 25 | F | lack of satiety (side effect of olanzapine) | 25 | 10 days |
| 41 | Natesh et al.[39] | 23 | M | migraine headache | 25 | 5 days |
| 42 | Nizamani et al.[40] | 24 | F | migraine headache | 25 | A few hours |
| 43 | Osaba et al.[41] | 45 | F | migraine headache | - | 1 week |
| 44 | Paciuc-Beja et al.[42] | 39 | F | migraine headache | 50 | 1 week |
| 45 | Pai et al.[44] | 40 | M | alcohol use disorders | 100 | 1 week |
| 46 | Palomares et al.[45] | 29 | F | migraine headache | - | 1 week |
| 47 | Parikh et al.[46] | 51 | M | epilepsy | 50 | 15 days |
| 48 | Pikkel.[47] | 45 | M | weight loss | 50 | 1 week |
| 49 | Prakash et al.[48] | 34 | M | alcohol use disorders | 50 | 1 week |
| 50 | Quagliato et al.[49] | 55 | F | migraine headache | 25 | 1 week |
| 51 | Raj et al.[50] | 37 | F | weight loss | 25 | 2 weeks |
| 52 | Rajjoub et al.[51] | 36 | F | migraine headache | - | - |
| 53 | Rapoport et al.[52] | 7 | F | epilepsy and headache | 25 | 2 weeks |
| 54 | Reis et al.[53] | 40 | F | alcohol use disorders | 50 | 10 days |
| 55 | Rewri et al.[55] | 43 | F | migraine headache | 50 | 9 days |
| 56 | Rhee et al.[57] | 35 | F | migraine headache | 50 | 2 months |
| 57 | Rhee et al.[56] | 43 | F | migraine headache | - | 1 day |
| 58 | Rosenberg et al.[59] | 29 | F | depression, anxiety, obesity, and migraine headache | - | 1 week |
| 59 | Sachi et al.[60] | 33 | F | migraine headache | 25 | 3 weeks |
| 60 | Saffra et al.[61] | 36 | F | migraine headache | 25 | 1 week |
| 61 | Salim[62] | 14 | M | migraine headache | 12.5 | 1 week |
| 62 | Santos-Nevarez et al.[63] | 34 | M | substance abuse-associated anxiety | 100 | 10 days |
| 63 | Sbeity et al.[64] | 59 | F | migraine headache | 100 | 11 days |
| 64 | Senthil et al.[66] | 28 | F | migraine headache | 50 | 4 days |
| 65 | Senthilkumar et al.[67] | 42 | F | vascular headache | 50 | 1 week |
| 66 | Sierra-Rodríguez et al.[68] | 29 | F | epilepsy | 50 | 9 days |
| 67 | Singh et al.[69] | 33 | F | migraine headache | 50 | - |
| 68 | Sorkhabi et al.[70] | 35 | F | epilepsy | 200 | 2 weeks |
| 69 | Spaccapelo et al.[71] | 34 | M | migraine headache | 100 | 1 week |
| 70 | Stangler et al.[72] | 40 | F | migraine headache | - | 10 days |
| 71 | Tambe et al.[73] | 54 | F | pain control | - | 2 weeks |
| 72 | Vahdani et al.[74] | 26 | F | migraine headache | 25 | 2 weeks |
| 73 | van Issum et al.[23] | 34 | M | epilepsy | - | 2 weeks |
| 74 | Verma et al.[75] | 17 | M | migraine headache | 25 | 10 days |
| 75 | Viet Tran et al.[77] | 57 | M | bipolar affective disorders | 50 | 1 week |
| 76 | Willett et al.[78] | 39 | M | migraine headache | 50 | 1 week |
| 77 | Ybarra et al.[79] | 47 | F | migraine headache | 100 | - |
SN, serial number; M, male; F, female
Table 2.
A summary of clinical characteristics
| SN | BCVA | IOP (mmHg) | SE (D) | ACD (mm) | AL (mm) | Associated Findings | Systemic Medications | Interventions | Time Until IOP Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||
| OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | |||||
| 1 | 20/125 | 20/200 | 42 | 44 | - | - | - | - | - | - | ciliochoroidal effusion | - | - | 2 weeks |
| 2 | 20/50 | 20/50 | 67 | 78 | - | - | - | - | - | - | - | Hyperosmotic agent | - | 1 week |
| 3 | 20/400 | 20/400 | 72 | 68 | - | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI | LPI OU | - |
| 4 | 20/800 | 20/400 | 56 | 38 | - | - | - | - | - | - | - | Hyperosmotic agent, CAI | - | 3 days |
| 5 | 20/30 | 20/30 | 14 | 14 | -6.5 | -6.5 | - | - | - | - | - | - | - | 4 days |
| 6 | 20/200 | 20/200 | 32 | 38 | - | - | 0.9 | 0.9 | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI | LPI OD | - |
| 7 | LP | LP | 64 | 48 | - | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, steroids | - | 1 day |
| 8 | - | - | 22 | 20 | -6 | -5.5 | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent | - | 5 days |
| 9 | 20/50 | 20/50 | 32 | 32 | -7 | -7 | 2.64 | 2.55 | - | - | - | - | - | 2 days |
| 10 | 20/50 | 20/25 | 50 | 50 | -6 | -4.5 | - | - | - | - | - | CAI | - | 18 days |
| 11 | CF | CF | 33 | 32 | -7.5 | -7.5 | - | - | - | - | - | Hyperosmotic agent, CAI | - | 5 days |
| 12 | 20/400 | 20/400 | 40 | 42 | - | - | - | - | - | - | ciliochoroidal effusion | CAI, steroids | - | 3 days |
| 13 | 20/400 | 20/400 | 68 | 70 | -6 | -6 | 1.75 | 1.72 | 22.21 | 22.31 | ciliochoroidal effusion | Hyperosmotic agent, CAI | - | 3 days |
| 14 | CF | CF | 49 | 51 | - | - | - | - | - | - | ciliochoroidal effusion | - | LPI OU | 5 days |
| 15 | 20/50 | 20/70 | 70 | 70 | - | - | - | - | - | - | - | Hyperosmotic agent, CAI, steroids | - | - |
| 16 | 20/40 | 20/40 | 40 | 39 | -5.75 | -5.25 | 2 | 1.8 | - | - | ciliochoroidal effusion | - | - | 1 week |
| 17 | 20/20 | 20/20 | 14 | 14 | -2.75 | -2 | 2.1 | 2 | - | - | ciliochoroidal effusion | - | - | - |
| 18 | CF | CF | 38 | 37 | -6.5 | -7.5 | - | - | - | - | - | - | - | - |
| 19 | 20/20 | 20/20 | 19 | 20 | -4.5 | -5 | - | - | - | - | ciliochoroidal effusion | - | - | - |
| 20 | HM | 20/160 | 36 | 32 | - | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent | - | 8 hours |
| 21 | 20/20 | 20/20 | 45 | 45 | - | - | - | - | - | - | papilledema | CAI | - | 1 day |
| 22 | 20/25 | 20/25 | 50 | 52 | -3.5 | -3.5 | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, steroids | - | 1 day |
| 23 | 20/30 | 20/25 | 33 | 26 | -5.5 | -4.5 | - | - | - | - | ciliochoroidal effusion | - | - | 1 day |
| 24 | 20/100 | 20/100 | 50 | 50 | -5 | -5 | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, steroids | - | 3 days |
| 25 | 20/400 | 20/800 | 37 | 43 | - | - | 1.22 | 1.16 | - | - | ciliochoroidal effusion, uveitis | Hyperosmotic agent, CAI | - | 1 day |
| 26 | 20/400 | 20/400 | 60 | 60 | - | - | - | - | - | - | ciliochoroidal effusion | - | - | 5 days |
| 27 | 20/400 | 20/400 | 34 | 32 | - | - | - | - | - | - | - | CAI | - | 1 day |
| 28 | 20/20 | 20/20 | 10 | 6 | -5 | -5 | - | - | - | - | ciliochoroidal effusion | CAI | - | - |
| 29 | 20/20 | 20/20 | 25 | 25 | -4.5 | -4.5 | - | - | - | - | ciliochoroidal effusion | - | - | - |
| 30 | CF | CF | 56 | 60 | - | - | 2.02 | 1.94 | 23.13 | 23.12 | ciliochoroidal effusion | Hyperosmotic agent, CAI | - | <1 day |
| 31 | 20/20 | 20/20 | 26 | 23 | - | - | 2.33 | 2.3 | 22.86 | 22.76 | - | CAI | - | <1 day |
| 32 | 20/20 | 20/20 | 24 | 26 | -8.5 | -7.5 | - | - | - | - | - | - | - | 2 days |
| 33 | 20/200 | 20/200 | 57 | 56 | - | - | 2.2 | 2.2 | 23.8 | 23.78 | ciliochoroidal effusion | Hyperosmotic agent, CAI | - | 5 days |
| 34 | 20/20 | 20/20 | 44 | 49 | -5.25 | -4.75 | 0.8 | 0.8 | - | - | ciliochoroidal effusion | Hyperosmotic agent | - | 2 days |
| 35 | 20/30 | 20/30 | 28 | 30 | -5 | -4.75 | - | - | - | - | uveitis | steroids | - | 1 week |
| 36 | CF | CF | 38 | 44 | - | - | - | - | - | - | uveitis | CAI | - | 1 day |
| 37 | 20/20 | 20/20 | 38 | 40 | -5 | -5 | - | - | - | - | ciliochoroidal effusion, exudative RD | - | LPI OU | 4 days |
| 38 | 20/100 | 20/80 | 60 | 60 | - | - | - | - | - | - | ciliochoroidal effusion | CAI | - | 4 days |
| 39 | 20/20 | 20/20 | 28 | 32 | -3.75 | -3.25 | - | - | - | - | - | CAI | - | 1 day |
| 40 | 20/400 | 20/400 | 32 | 33 | -9.15 | -7.75 | - | - | - | - | - | - | - | 30 min |
| 41 | 20/20 | 20/20 | 24 | 24 | -6 | -6 | - | - | - | - | ciliochoroidal effusion | - | - | 1 day |
| 42 | CF | CF | 32 | 32 | - | - | - | - | - | - | - | Hyperosmotic agent, CAI | LPI OU | 4 days |
| 43 | 20/50 | 20/50 | 40 | 45 | - | - | - | - | - | - | uveitis | CAI | - | - |
| 44 | 20/70 | 20/70 | 36 | 40 | -1.87 | -2 | 0.9 | 1.1 | 21.8 | 21.8 | ciliochoroidal effusion | CAI | - | 1 day |
| 45 | 20/80 | 20/60 | 48 | 46 | - | - | - | - | - | - | - | CAI | - | 1 day |
| 46 | 20/200 | 20/200 | 32 | 30 | -7 | -7 | - | - | - | - | - | CAI | - | 1 day |
| 47 | HM | HM | 57 | 57 | - | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI, steroids | AC paracentesis OD, choroidal drainage OD | 2 weeks |
| 48 | 20/100 | 20/100 | 70 | 64 | - | - | - | - | - | - | ciliochoroidal effusion, uveitis | Hyperosmotic agent, CAI, steroids | - | 1 week |
| 49 | 20/400 | 20/400 | 47 | 43 | -8 | -9 | - | - | - | - | - | CAI, steroids | - | 3 days |
| 50 | 20/25 | 20/40 | 48 | 48 | -2 | -2 | - | - | - | - | - | Hyperosmotic agent, CAI | LPI OU | 2 hours |
| 51 | 20/50 | 20/50 | 50 | 56 | -4.37 | -4.5 | 1.9 | 1.9 | - | - | - | Hyperosmotic agent | - | 1 week |
| 52 | 20/30 | CF | 31 | 53 | - | - | - | - | - | - | uveitis | - | LPI OU, AC paracentesis OS, trabeculectomy OS | - |
| 53 | 20/20 | 20/20 | 40 | 41 | - | - | - | - | - | - | - | CAI | - | 8 days |
| 54 | CF | 20/400 | 30 | 30 | - | - | - | - | - | - | ciliochoroidal effusion | CAI | - | 2 days |
| 55 | CF | CF | 28 | 18 | - | - | 0.8 | 0.9 | 20.2 | 20.7 | ciliochoroidal effusion | - | - | - |
| 56 | 20/100 | 20/100 | 88 | 82 | -3.5 | -3.5 | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI, steroids | - | 1 day |
| 57 | 20/20 | 20/20 | 29 | 30 | -5 | -5 | - | - | - | - | - | - | - | 5 days |
| 58 | HM | HM | 40 | 40 | - | - | - | - | - | - | exudative RD | Hyperosmotic agent, CAI | LPI OU | 1 day |
| 59 | 20/200 | 20/200 | 55 | 34 | -2.75 | -3.5 | 2.14 | 2.28 | 22.21 | 22.31 | ciliochoroidal effusion | CAI | - | 3 days |
| 60 | 20/30 | 20/40 | 24 | 25 | -6.25 | -5.5 | - | - | - | - | ciliochoroidal effusion | steroids | - | 1 day |
| 61 | 20/20 | 20/20 | 29 | 29 | -9 | -9 | - | - | - | - | ciliochoroidal effusion | - | - | - |
| 62 | 20/20 | 20/20 | 56 | 38 | -4.5 | -4.5 | - | - | - | - | - | CAI | 1 week | |
| 63 | 20/30 | 20/100 | 45 | 43 | - | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI | iridoplasty | 1 week |
| 64 | 20/30 | 20/30 | 34 | 34 | -5 | -5 | - | - | - | - | - | - | - | 3 days |
| 65 | 20/80 | 20/100 | 60 | 64 | - | - | - | - | - | - | ciliochoroidal effusion, uveitis | - | - | 2 weeks |
| 66 | CF | CF | 38 | 38 | -13 | -13 | - | - | - | - | - | Hyperosmotic agent | - | a few hours |
| 67 | LP | LP | 48 | 48 | - | - | - | - | - | - | - | Hyperosmotic agent, CAI | - | 3 days |
| 68 | 20/30 | 20/30 | 29 | 31 | -0.75 | -0.75 | 1.1 | 1 | - | - | ciliochoroidal effusion | CAI | - | 1 week |
| 69 | - | - | 40 | 40 | -5.5 | -5 | - | - | - | - | - | CAI | - | 2 days |
| 70 | CF | 20/400 | 40 | 38 | -4.5 | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI | - | 3 days |
| 71 | - | - | 55 | 53 | - | - | - | - | - | - | - | - | - | 1 day |
| 72 | CF | CF | 30 | 30 | -5 | -4 | - | - | - | - | ciliochoroidal effusion | CAI | - | 5 days |
| 73 | 20/20 | 20/20 | 34 | 40 | -6 | -6 | - | - | - | - | ciliochoroidal effusion | - | - | 4 days |
| 74 | - | - | 36 | 28 | -3.5 | -4 | - | - | - | - | ciliochoroidal effusion | - | - | 4 days |
| 75 | 20/140 | 20/80 | 28 | 28 | -3.5 | -2.75 | - | - | - | - | ciliochoroidal effusion, uveitis | - | - | 1 week |
| 76 | 20/100 | 20/100 | 70 | 70 | - | - | - | - | - | - | ciliochoroidal effusion | Hyperosmotic agent, CAI, steroids | - | 1 week |
| 77 | 20/200 | 20/100 | 36 | 38 | - | - | - | - | - | - | - | - | - | 2 hours |
SN, serial number; BCVA, best corrected visual acuity; IOP, intraocular pressure; SE, spherical equivalent; ACD, anterior chamber depth; AL, axial length; OD, right eye; OS, left eye; OU, both eyes; CF, counting fingers; HM, hand movement; LP, light perception; RD, retinal detachment; CAI, carbonic anhydrase inhibitor; LPI, laser peripheral iridotomy; AC, anterior chamber
Demographics
Out of the 77 patients, 58 (75.3%) were female. The mean age of patients was 34.88 ± 11.21 years (range, 7–57).
TPM usage
The most common indication for TPM usage was migraine headache (59.7%) followed by epilepsy (10.4%) and weight loss (9.1%). Other less common indications were alcohol use disorder, bipolar affective disorder, cluster headache, anxiety, and pain control. In the cases that reported TPM dosage, the daily dose ranged from 12.5 to 200 mg/day. The mean time that elapsed from the initiation of TPM therapy until the onset of TiAAC was 14.1 ± 31.5 days (range, 0–262).
Clinical presentation
The visual acuity at presentation widely ranged from light perception to 20/20. The mean IOP at presentation was 41.04 ± 15.76 mmHg (range, 8–88) in the right eye and 41 ± 15.3 mmHg (range, 6–82) in the left eye. The amount of refractive error at presentation was reported in 42 cases (54.5%), and the mean spherical equivalent in these patients was -5.37 ± 2.2 diopters (range, (-13)–(-0.75)) in the right eye and -5.31 ± 2.18 diopters (range, (-13)–(-0.75)) in the left eye. Only a few cases had further biometric measurements (anterior chamber depth (ACD) reported in 15 cases (19.5%), and axial length (AL) reported in 7 cases (9.1%)). The mean ACD was 1.65 ± 0.63 mm (range, 0.8–2.64) in the right eye and 1.64 ± 0.6 mm (range, 0.8–2.55) in the left eye, whereas the mean AL was 22.32 ± 1.15 mm (range, 20.2–23.8) in the right eye and 22.31 ± 0.98 mm (range, 20.7–23.78) in the left eye. In addition to acute angle closure, other ophthalmologic features reported include ciliochoroidal effusion in 43 cases (55.8%) [Figs. 2 and 3], uveitis in 8 cases (10.4%), exudative retinal detachment in 2 cases (2.6%), and papilledema in 1 case (1.3%).
Figure 2.
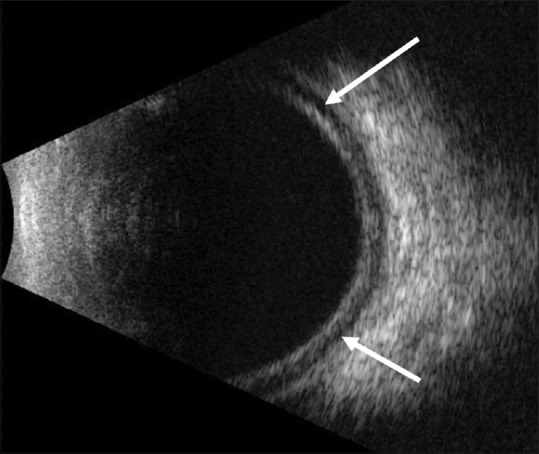
B-scan ultrasonography of a patient with topiramate-induced acute angle closure showing choroidal effusion (arrows)
Figure 3.
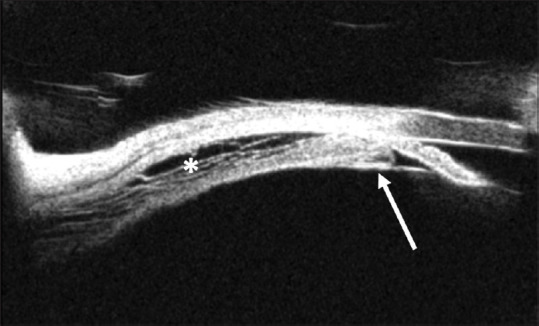
Ultrasound biomicroscopy of a patient with topiramate-induced acute angle closure showing an edematous, anteriorly displaced ciliary process (arrow) and uveal effusion (asterisk)
Medical management
All cases were managed initially by immediately discontinuing TPM. In addition, topical IOP-lowering medications, cycloplegics, and steroids were used. Some patients were also given systemic medications such as oral carbonic anhydrase inhibitors (CAIs) in 40 cases (51.9%), hyperosmotic agents in 28 cases (36.4%), and steroids in 12 cases (15.6%).
Interventions
The majority of cases were managed medically without the need for laser and/or surgical intervention. Of the few cases that underwent an intervention, laser peripheral iridotomy (LPI) was performed in 8 cases (10.4%), anterior chamber paracentesis in 2 cases (2.6%), laser iridoplasty in 1 case (1.3%), choroidal drainage in 1 case (1.3%), and trabeculectomy in 1 case (1.3%).
Resolution of TiAAC
Following initiation of treatment, control of IOP associated with the resolution of TiAAC occurred in half of the cases (50.6%) during the first three days. The mean duration from presentation until the resolution of TiAAC was 3.9 ± 3.6 days (range, 1–18). Our analysis showed that the use of an intravenous hyperosmotic agent was significantly associated with a shorter period of recovery (4.4 ± 3.9 days vs 2.7 ± 2.1 days, P = 0.0261).
Discussion
Summary of evidence
The current review describes the demographic and clinical findings of the 77 TiAAC case reports described in scientific literature during the searched period. For the vast majority of cases, the condition was successfully managed by prompt identification of the underlying cause, discontinuing TPM, and initiating appropriate medical therapy. Further surgical intervention was only required in two refractory cases that could not be managed medically.[51,56] Below we present a brief discussion of salient findings in our review that are important to physicians involved in the prescription of TPM or the management of TiAAC.
A great proportion of reported patients with TiAAC were females (75.3%); however, this does not necessarily imply that females are at a higher risk of TiAAC. A possible explanation of the higher incidence among females is that migraine headaches, the most common indication of TPM usage in our review, predominantly affects women.[82] The majority of cases (93.5%) were adults above the age of 18 years, and only a small proportion of cases were children, with the youngest patient in our review being a 7-year-old girl who was prescribed TPM for seizures and headache.[57] Although TiAAC usually occurs during the first two weeks after initiating TPM therapy, the onset of angle closure may occur after that time frame. In our review, Czyz CN, et al.[23] reported a case of delayed-onset TiAAC 262 days after starting TPM. The authors speculate that the advancement of a subclinical angle closure could be the causal mechanism behind this delayed presentation.
Visual acuity at presentation was widely variable ranging from cases that had a vision as poor as light perception to patients with 20/20 vision. In patients with poor vision at presentation, it was mainly attributed to severe corneal edema induced by extremely high IOP. Most reported cases had high IOP at presentation as a result of the induced angle closure; however, five cases had normal IOP that was explained by the usage of IOP-lowering medications before presentation,[35,59] concomitant use of furosemide which is known to lower the IOP,[22] and early presentation prior to an impending attack of angle closure.[13,25] New-onset myopia was another clinical feature reported in association with TiAAC. It is thought to be a result of the forward movement of the lens-iris diaphragm. Another postulated mechanism is a disturbance in the osmotic state of the lens leading to swelling and subsequently a change in the refractive lens power.[83] Future studies including specific measurements of the lens thickness during and after the attack would be useful to support this mechanism. Uveitis, exudative retinal detachment, and papilledema were three other clinical signs reported in a few patients. The mechanism underlying uveitis is presumed to be an idiosyncratic reaction to TPM in which drug metabolites bind with intraocular proteins leading to a complex that is recognized as a foreign body stimulating an immune reaction,[69] whereas the occurrence of retinal detachment is thought to be related to a TPM-induced effusion of fluids into the subretinal space.[63] Finally, in the case that had TiAAC associated with papilledema, the patient was later diagnosed with pseudotumor cerebri to which the papilledema was attributed.[27]
Management of TiAAC is primarily medical consisting of IOP-lowering medications, cycloplegia, and steroids. The use of CAIs was controversial between studies. Around half of cases (51.9%) were treated with a systemic CAI to provide a further reduction in IOP, whereas CAIs were avoided in the remaining cases to prevent the occurrence of an idiosyncratic reaction that may lead to progression of the ciliochoroidal effusion and subsequently a paradoxically increased IOP.[84] Although systemic hyperosmotic therapy was only used in slightly above one-third of cases (36.4%), our analysis showed a faster resolution of TiAAC with the use of hyperosmotic treatment (P = 0.0261). Therefore, we recommend the use of a systemic hyperosmotic agent in TiAAC especially in refractory cases with high IOP and impending flat anterior chamber.
Given that the mechanism of TiAAC does not involve pupillary block, there is no theoretical justification for performing a LPI. Despite that, eight cases (10.4%) in our review underwent LPI during the course of treatment for various reasons. Chalam K. V., et al.[20] Aminlari A., et al.[11] and Banta J. T., et al.[14] performed LPI because they presumed that a relative pupillary block mechanism was present. Secondly, Rosenberg K., et al.[63] proceeded with LPI in their patient since visual acuity did not improve despite IOP control; however, as it eventually turned out, the patient had concomitant macular neurosensory retinal detachment that explained the visual decline. Thirdly, a plateau iris configuration was noted in the case reported by Rajjoub L. Z., et al.[56] for which LPI was performed. Fourthly, the choice of LPI in the cases presented by Nizamani N. B., et al.[46] and Quagliato L. B., et al.[54] was justified by attempting to provide further IOP lowering and prevent synechiae formation, consecutively. Finally, the case reported by Mazumdar S., et al.[42] underwent LPI before establishing a diagnosis of TiAAC as the patient was initially thought to have acute angle closure. In our opinion, LPI does not provide any benefit in cases with an established diagnosis of TiAAC and treatment should be targeted toward managing the underlying ciliary effusion. In the presence of an untreated effusion, an LPI would neither be expected to provide further IOP lowering nor will it prevent synechiae formation.
Two patients (2.6%) in our review required a surgical intervention to control their condition. The first patient[56] required trabeculectomy with mitomycin in the left eye to achieve adequate IOP control. Surgical intervention was chosen because the patient had uncontrolled IOP despite maximum medical therapy associated with clinically evident glaucomatous disc damage. Furthermore, the patient had reported a history of intermittent headache prior to starting TPM and upon examination, a plateau iris configuration was noted. These findings suggest that this patient might have had pre-existing angle closure before developing TiAAC. In the second patient, choroidal drainage was performed in the right eye because the patient progressed to a flat anterior chamber and corneal edema that necessitated urgent surgical intervention to drain the effusion and reform the anterior chamber.[51]
Limitations
Our review has some limitations. First, given that case reports only capture short periods of follow-up, our review lacks long-term clinical findings that may occur after an attack of TiAAC, such as persistent corneal edema and cataract. Second, the quality of reporting certain biometric measurements in the cited articles was low. Only a few papers presented data on ACD and AL measurements. Furthermore, the refractive error at presentation was only reported in 54.5% of cases. The presence of such information would have provided more insight into the mechanisms underlying TiAAC. Finally, given the anecdotal nature of case reports, our systematic review could not provide data on the frequency of angle closure among patients treated with TPM.
Conclusion
In conclusion, our systematic review provides an updated summary on the reported cases of TiAAC. Practitioners involved in prescribing TPM (e.g., neurologists, psychiatrists) should be alert to this possible adverse effect, especially during the first two weeks of therapy. From an ophthalmologic perspective, TiAAC should be ruled out in any patient presenting with acute angle closure by inquiring about their drug history. If diagnosed, TiAAC can be successfully managed by stopping TPM and initiating appropriate medical treatment. Surgical intervention is rarely needed as the majority of cases can be managed medically and resolved within the first few days.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge Dr. Ohoud Owaidhah for providing the clinical images used in the manuscript.
References
- 1.Minton GC, Miller AD, Bookstaver PB, Love BL. Topiramate: Safety and efficacy of its use in the prevention and treatment of migraine. J Cent Nerv Syst Dis. 2011;3:155–68. doi: 10.4137/JCNSD.S4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: A review. Epilepsy Res. 2011;95:189–99. doi: 10.1016/j.eplepsyres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005;6:373–81. doi: 10.1016/j.yebeh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoud AAH, Rizk T, El-Bakri NK, Riaz M, Dannawi S, Al Tannir M. Incidence of kidney stones with topiramate treatment in pediatric patients. Epilepsia. 2011;52:1890–3. doi: 10.1111/j.1528-1167.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 5.Abtahi MA, Abtahi SH, Fazel F, Roomizadeh P, Etemadifar M, Jenab K, et al. Topiramate and the vision: A systematic review. Clin Ophthalmol. 2012;6:117–31. doi: 10.2147/OPTH.S27695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovino JA, Marcus DF. The mechanism of transient myopia induced by sulfonamide therapy. Am J Ophthalmol. 1982;94:99–102. doi: 10.1016/0002-9394(82)90199-4. [DOI] [PubMed] [Google Scholar]
- 7.Krieg PH, Schipper I. Drug-induced ciliary body oedema: A new theory. Eye. 1996;10:121–6. doi: 10.1038/eye.1996.21. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adrian B, Heda A, Kharbanda V, Shetty R. Topiramate induced bilateral simultaneous angle closure glaucoma in a steroid responder. Clin Ophthalmol Res. 2015;1:00–3. [Google Scholar]
- 10.Agarwal A. Ciliochoroidal effusion in topiramate-induced bilateral acute angle closure glaucoma. Indian J Ophthalmol. 2019;67:1466–7. doi: 10.4103/ijo.IJO_245_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aminlari A, East M, Wei W, Quillen D. Topiramate induced acute angle closure glaucoma. Open Ophthalmol J. 2008;2:46–7. doi: 10.2174/1874364100802010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arun V, Sirohi Y, Dixit Y. Topiramate-induced angle closure glaucoma in a case of chronic migraine. Med J Dr DY Patil Univ. 2017;10:482–4. [Google Scholar]
- 13.Baloch M, Siddiqui MAR. Topiramate induced sudden loss of vision. J Pak Med Assoc. 2012;62:1092–3. [PubMed] [Google Scholar]
- 14.Banta JT, Hoffman K, Budenz DL, Ceballos E, Greenfield DS. Presumed topiramate-induced bilateral acute angle-closure glaucoma. Am J Ophthalmol. 2001;132:112–4. doi: 10.1016/s0002-9394(01)01013-3. [DOI] [PubMed] [Google Scholar]
- 15.Behl S, Fasahtay A. Topiramate-induced bilateral angle closure glaucoma and myopic shift. Neurol India. 2016;64:1040–2. doi: 10.4103/0028-3886.190232. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya KB, Basu S. Acute myopia induced by topiramate: Report of a case and review of the literature. Neurol India. 2005;53:108–9. doi: 10.4103/0028-3886.15074. [DOI] [PubMed] [Google Scholar]
- 17.Boentert M, Aretz H, Ludemann P. Acute myopia and angle-closure glaucoma induced by topiramate. Neurology. 2003;61:1306. doi: 10.1212/01.wnl.0000091425.43083.c1. [DOI] [PubMed] [Google Scholar]
- 18.Boonyaleephan S. Bilateral acute onset myopia and angle closure glaucoma after oral topiramate: A case report. J Med Assoc Thail. 2008;91:1904–8. [PubMed] [Google Scholar]
- 19.Caglar C, Yasar T, Ceyhan D. Topiramate induced bilateral angle-closure glaucoma: Low dosage in a short time. J Ocul Pharmacol Ther. 2012;28:205–7. doi: 10.1089/jop.2011.0161. [DOI] [PubMed] [Google Scholar]
- 20.Chalam KV, Tillis T, Syed F, Agarwal S, Brar VS. Acute bilateral simultaneous angle closure glaucoma after topiramate administration: A case report. J Med Case Rep. 2008;2:1. doi: 10.1186/1752-1947-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole KL, Wang EE, Aronwald RM. Bilateral acute angle-closure glaucoma in a migraine patient receiving topiramate: A case report. J Emerg Med. 2012;43:e89–91. doi: 10.1016/j.jemermed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Craig JE, Ong TJ, Louis DL, Wells JM. Mechanism of topiramate-induced acute-onset myopia and angle closure glaucoma. Am J Ophthalmol. 2004;137:193–5. doi: 10.1016/s0002-9394(03)00774-8. [DOI] [PubMed] [Google Scholar]
- 23.Czyz CN, Clark CM, Justice JD, Pokabla MJ, Weber PA. Delayed topiramate-induced bilateral angle-closure glaucoma. J Glaucoma. 2014;23:577–8. doi: 10.1097/IJG.0b013e3182948491. [DOI] [PubMed] [Google Scholar]
- 24.DE Oliveira BMR, Ferrari PV, Herrerias BT, Hirai FE, Gracitelli CPB. The use of topiramate for weight loss causing acute glaucoma: A case report and literature review. Med Hypothesis Discov Innov Ophthalmol. 2019;8:116–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Desai C, Ramchandani S, Bhopale S, Ramchandani S. Acute myopia and angle closure caused by topiramate, a drug used for prophylaxis of migraine. Indian J Ophthalmol. 2006;54:195–7. doi: 10.4103/0301-4738.27072. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Cespedes RA, Toro-Giraldo D, Olate-Perez A, Hervas-Ontiveros A, Garcia-Delpech S, Udaondo-Mirete P. Contribution of the Visante®OCT and B-scan ultrasound in the diagnosis and follow up of a topiramate-induced bilateral ciliochoroidal effusion syndrome. Arch Soc Esp Oftalmol. 2019;94:391–5. doi: 10.1016/j.oftal.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Giuliari GP, Banda RM, Vann VR, Gonzalez VH, McMillin RB. Closed-angle glaucoma after topiramate therapy for migraine in a patient with undiagnosed pseudotumor cerebri. Can J Ophthalmol. 2008;43:371. doi: 10.3129/i08-052. [DOI] [PubMed] [Google Scholar]
- 28.Grewal DS, Goldstein DA, Khatana AK, Tanna AP. Bilateral angle closure following use of a weight loss combination agent containing topiramate. J Glaucoma. 2015;24:e132–6. doi: 10.1097/IJG.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 29.Guier CP. Elevated intraocular pressure and myopic shift linked to topiramate use. Optom Vis Sci. 2007;84:E1070–3. doi: 10.1097/OPX.0b013e31815b9e38. [DOI] [PubMed] [Google Scholar]
- 30.Issum C van, Mavrakanas N, Schutz JS, Shaarawy T. Topiramate-induced acute bilateral angle closure and myopia: Pathophysiology and treatment controversies. Eur J Ophthalmol. 2011;21:404–9. doi: 10.5301/EJO.2010.5979. [DOI] [PubMed] [Google Scholar]
- 31.Joshi AK, Pathak AH, Patwardhan SD, Kulkarni AN. A rare case of topiramate induced secondary acute angle closure glaucoma. J Clin Diagnostic Res. 2017;11 doi: 10.7860/JCDR/2017/28093.10052. ND01-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamal S, Yadava U, Kumar S, Goel R. Topiramate-induced angle-closure glaucoma: Cross-sensitivity with other sulphonamide derivatives causing anterior uveitis. Int Ophthalmol. 2014;34:345–9. doi: 10.1007/s10792-013-9793-8. [DOI] [PubMed] [Google Scholar]
- 33.Katsimpris JM, Katsimpris A, Theoulakis PE, Lepidas J, Petropoulos IK. Bilateral severe anterior uveitis and acute angle-closure glaucoma following topiramate use for migraine crisis. Klin Monbl Augenheilkd. 2014;231:439–41. doi: 10.1055/s-0034-1368282. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni C, Chaudhuri UR, Jagathesan A. Bilateral acute angle-closure glaucoma following treatment with topiramate for headache. Neurol Ther. 2013;2:57–62. doi: 10.1007/s40120-013-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar M, Kesarwani S, Rao A, Garnaik A. Macular folds: An unusual association in topiramate toxicity. Clin Exp Optom. 2012;95:449–52. doi: 10.1111/j.1444-0938.2012.00722.x. [DOI] [PubMed] [Google Scholar]
- 36.Lan YW, Hsieh JW. Bilateral acute angle closure glaucoma and myopic shift by topiramate-induced ciliochoroidal effusion: Case report and literature review. Int Ophthalmol. 2018;38:2639–48. doi: 10.1007/s10792-017-0740-y. [DOI] [PubMed] [Google Scholar]
- 37.Latini MF, Romano LM. Topiramate-induced acute myopia with MRI contrast enhancement. Acta Neurol Belg. 2012;112:81–4. doi: 10.1007/s13760-012-0022-4. [DOI] [PubMed] [Google Scholar]
- 38.Levy J, Yagev R, Petrova A, Lifshitz T. Topiramate-induced bilateral angle-closure glaucoma. Can J Ophthalmol. 2006;41:221–5. doi: 10.1139/I06-012. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Tseng PC, Chen CC, Woung LC, Liou SW. Topiramate-induced bilateral secondary angle closure and myopia shift. Taiwan J Ophthalmol. 2014;4:45–8. [Google Scholar]
- 40.Mahendradas P, Parab S, Sasikumar R, Kawali A, Shetty B. Topiramate-induced acute angle closure with severe panuveitis: A challenging case report. Indian J Ophthalmol. 2018;66:1342–4. doi: 10.4103/ijo.IJO_1192_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansoor Q, Jain S. Bilateral angle-closure glaucoma following oral topiramate therapy. Acta Ophthalmol Scand. 2005;83:627. doi: 10.1111/j.1600-0420.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 42.Mazumdar S, Tripathy K, Sarma B, Agarwal N. Acquired myopia followed by acquired hyperopia due to serous neurosensory retinal detachment following topiramate intake. Eur J Ophthalmol. 2019;29:21–4. doi: 10.1177/1120672118797286. [DOI] [PubMed] [Google Scholar]
- 43.Medeiros FA, Zhang XY, Bernd AS, Weinreb RN. Angle-closure glaucoma associated with ciliary body detachment in patients using topiramate. Arch Ophthalmol. 2003;121:282–5. doi: 10.1001/archopht.121.2.282. [DOI] [PubMed] [Google Scholar]
- 44.Mitra A, Ramakrishnan R, Kader MA. Anterior segment optical coherence tomography documentation of a case of topiramate induced acute angle closure. Indian J Ophthalmol. 2014;62:619–22. doi: 10.4103/0301-4738.129784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Natesh S, Rajashekhara S, Rao A, Shetty B. Topiramate-induced angle closure with acute myopia, macular straie. Oman J Ophthalmol. 2010;3:26. doi: 10.4103/0974-620X.60018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nizamani NB, Talpur KI. Idiosyncratic topiramate-induced high myopic shift with angle closure glaucoma. Pak J Ophthalmol. 2012;28:224–6. [Google Scholar]
- 47.Osaba M, Reviglio VE. Case report: The role of OCT in examination of a patient with topiramate-induced acute angle closure, acute myopia and macular striae. Oxf Med Case Reports 2018. 2018 doi: 10.1093/omcr/omy030. omy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paciuc-Beja M, Retchkiman-Bret M, Velasco-Barona CF, Galicia-Alfaro VH. Secondary bilateral angle closure glaucoma due to topiramate. Case Rep Ophthalmol Med. 2011;2011:1–3. doi: 10.1155/2011/594051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pai K, Rajashekaran P. Glaucoma: Adverse event on use of topiramate in alcohol de-addiction. Indian J Psychiatry. 2011;53:163–5. doi: 10.4103/0019-5545.82552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palomares P, Amselem L, Diaz-Llopis M. Optical coherence tomography for diagnosis and monitoring of angle-closure glaucoma induced by topiramate. Can J Ophthalmol. 2007;42:633–4. [PubMed] [Google Scholar]
- 51.Parikh R, Parikh S, Das S, Thomas R. Choroidal drainage in the management of acute angle closure after topiramate toxicity. J Glaucoma. 2007;16:691–3. doi: 10.1097/IJG.0b013e318098739b. [DOI] [PubMed] [Google Scholar]
- 52.Pikkel YY. Acute bilateral glaucoma and panuveitis as a side effect of topiramate for weight loss treatment. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2014-203787. bcr2014203787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prakash J, Prabhu HRA, Srivastava K, Bhat PS, Kumar RS. Acute myopia and secondary angle closure glaucoma following topiramate medication. Delhi Psychiatry J. 2010;13:159–61. [Google Scholar]
- 54.Quagliato LB, Barella K, Neto JMA, Quagliato EMAB. Topiramate-associated acute, bilateral, angle-closure glaucoma: Case report. Arq Bras Oftalmol. 2013;76:48–9. doi: 10.1590/s0004-27492013000100014. [DOI] [PubMed] [Google Scholar]
- 55.Raj R, Raj A. Topiramate-induced bilateral acute angle closure glaucoma and myopic shift. Int J Basic Clin Pharmacol. 2014;3:562–5. [Google Scholar]
- 56.Rajjoub LZ, Chadha N, Belyea DA. Intermittent acute angle closure glaucoma and chronic angle closure following topiramate use with plateau iris configuration. Clin Ophthalmol. 2014;8:1351–4. doi: 10.2147/OPTH.S65748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapoport Y, Benegas N, Kuchtey RW, Joos KM. Acute myopia and angle closure glaucoma from topiramate in a seven-year-old: A case report and review of the literature. BMC Pediatr. 2014;14:96. doi: 10.1186/1471-2431-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reis GMSM, Lau OCF, Samarawickrama C, Heydon P, Goldberg I. Utility of ultrasound biomicroscopy in the diagnosis of topiramate-associated ciliochoroidal effusions causing bilateral acute angle closure. Clin Exp Ophthalmol. 2014;42:500–1. doi: 10.1111/ceo.12336. [DOI] [PubMed] [Google Scholar]
- 59.Rewri P, Rao N, Lingam V. Topiramate-induced secondary angle closure. J Heal Spec. 2014;2:26–7. [Google Scholar]
- 60.Rhee DJ, Goldberg MJ, Parrish RK. Bilateral angle-closure glaucoma and ciliary body swelling from topiramate. Arch Ophthalmol. 2001;119:1721–3. [PubMed] [Google Scholar]
- 61.Rhee DJ, Ramos-Esteban JC, Nipper KS. Rapid resolution of topiramate-induced angle-closure glaucoma with methylprednisolone and mannitol. Am J Ophthalmol. 2006;141:1133–4. doi: 10.1016/j.ajo.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez AG, Patiño LR, Enriquez ER, Morales-León J-E. Angle closure and myopic shift after topiramate used for appetite control. Pan-American J Ophthalmol. 2017;16:57–60. [Google Scholar]
- 63.Rosenberg K, Maguire J, Benevento J. Topiramate-induced macular neurosensory retinal detachment. Am J Ophthalmol Case Rep. 2017;7:31–7. doi: 10.1016/j.ajoc.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sachi D, Vijaya L. Topiramate induced secondary angle closure glaucoma. J Postgrad Med. 2006;52:72–3. [PubMed] [Google Scholar]
- 65.Saffra N, Smith SN, Seidman CJ. Topiramate-induced refractive change and angle closure glaucoma and its ultrasound bimicroscopy findings. BMJ Case Rep 2012. 2012 doi: 10.1136/bcr-2012-006509. bcr2012006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salim SF. A case of bilateral acute onset myopia and angle closure glaucoma following migraine therapy in a teenager. Univ J Surg Surg Spec. 2018. p. 4. Available from: http://ejournal-tnmgrmu.ac.in/index.php/surgery/article/view/7480 .
- 67.Santos-Nevarez V, Cantrell J, Gruosso P, Miller J, Culotta-Glynn T. Topiramate-induced acute bilateral angle closure glaucoma and transient myopia: A teaching case report. J Optom Educ. 2015;40:1–8. [Google Scholar]
- 68.Sbeity Z, Gvozdyuk N, Amde W, Liebmann JM, Ritch R. Argon laser peripheral iridoplasty for topiramate-induced bilateral acute angle closure. J Glaucoma. 2009;18:269–71. doi: 10.1097/IJG.0b013e31818159e9. [DOI] [PubMed] [Google Scholar]
- 69.Senthil S, Garudadri C, Rao H, Maheshwari R. Bilateral simultaneous acute angle closure caused by sulphonamide derivatives: A case series. Indian J Ophthalmol. 2010;58:248–52. doi: 10.4103/0301-4738.62657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senthilkumar V, Rajendrababu S. Aftermath of topiramate: An interesting case report and literature review. TNOA J Ophthalmic Sci Res. 2019;57:240–2. [Google Scholar]
- 71.Sierra-Rodríguez MA, Rodríguez-Vicente L, Chavarri-García JJ, del Río-Mayor JL. Acute narrow-angle glaucoma induced by topiramate with acute myopia and macular striae: A case report. Arch Soc Esp Oftalmol. 2019;94:130–3. doi: 10.1016/j.oftal.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Singh SK, Thapa SS, Badhu BP. Topiramate induced bilateral angle-closure glaucoma. Kathmandu Univ Med J. 2007;5:234–6. [PubMed] [Google Scholar]
- 73.Sorkhabi R, Taheri N. Topiramate induced bilateral angle-closure glaucoma. Iran J Ophthalmol. 2008;20:49–52. [Google Scholar]
- 74.Spaccapelo L, Leschiutta S, Aurea C, Ferrari A. Topiramate-associated acute glaucoma in a migraine patient receiving concomitant citalopram therapy: A case-report. Cases J. 2009;2:87. doi: 10.1186/1757-1626-2-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stangler F, Prietsch RF, Filho JBF. Bilateral acute angle closure glaucoma in a young patient receiving oral topiramate: Case report. Arq Bras Oftalmol. 2007;70:133–6. doi: 10.1590/s0004-27492007000100025. [DOI] [PubMed] [Google Scholar]
- 76.Tambe V, Goodman A, Tambe A, Hess M. Topiramate-associated acute angle closure glaucoma with myopic shift. Am J Ther. 2020;27:e537–8. doi: 10.1097/MJT.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 77.Vahdani K, Easto R, Shah A, Habib N. Topiramate-induced acute glaucoma. Pract Neurol. 2016;16:323–5. doi: 10.1136/practneurol-2015-001363. [DOI] [PubMed] [Google Scholar]
- 78.Verma N, Kumar A. Acute onset myopia and angle closure glaucoma after topiramate administration. Delhi J Ophthalmol. 2011;21:38–9. [Google Scholar]
- 79.Viet Tran H, Ravinet E, Schnyder C, Reichhart M, Guex-Crosier Y, Guex-Crosier Y. Blood-brain barrier disruption associated with topiramate-induced angle-closure glaucoma of acute onset. Klin Monbl Augenheilkd. 2006;223:425–7. doi: 10.1055/s-2006-926600. [DOI] [PubMed] [Google Scholar]
- 80.Willett MC, Edward DP. Refractory topiramate-induced angle-closure glaucoma in a man: A case report. J Med Case Rep. 2011;5:33. doi: 10.1186/1752-1947-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ybarra M, Rosenbaum T. Typical migraine or ophthalmologic emergency? J Emerg Med. 2012;30:831–e3-5. doi: 10.1016/j.ajem.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 82.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: Data from the American migraine study II. Headache. 2001;41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 83.Sen HA, O'Halloran HS, Lee WB. Case reports and small case series: Topiramate-induced acute myopia and retinal striae. Arch Ophthalmol. 2001;119:775–7. [PubMed] [Google Scholar]
- 84.Pathak-Ray V, Chandran P. Acetazolamide-associated idiosyncratic simultaneous bilateral angle closure and cross-sensitivity. Am J Ther. 2020;27:e680–2. doi: 10.1097/MJT.0000000000001045. [DOI] [PubMed] [Google Scholar]