Abstract
This study aimed to evaluate the possible protective effects of quercetin, a natural flavonoid, against nephrotoxicity induced by Di (2-ethylhexyl) phthalate (DEHP) in kidney tissue of rats and human embryonic kidney (HEK) 293 cell line. The HEK-293 cells were treated with different concentrations of quercetin 24 h before treatment with monoethylhexyl phthalate (MEHP). Male rats were treated with 200-mg/kg DEHP, 200-mg/kg DEHP plus quercetin (50 and 100 mg/kg), and 200-mg/kg DEHP plus vitamin E (20 mg/kg) for 45 days by gavage. Quercetin treatment reduced cytotoxicity and oxidative damage inducing by MEHP in HEK-293 cells. The in vivo findings showed that 100-mg/kg quercetin significantly suppressed DEHP-induced kidney damage. For exploring the involved mechanisms, the expressions of nuclear factor E2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), nuclear factor kappa B (NFκB), and tumor necrosis factor alpha (TNFα) genes were determined via real-time Polymerase chain reaction (PCR) assay. High dose of quercetin significantly decreased the gene expressions of NF-κB and TNFα, whereas the alternations of Nrf2 and HO-1 gene expressions were not significant in quercetin groups in compared with DEHP group. These findings suggested that the suppression of DEHP-induced nephrotoxicity via quercetin is correlated, at least in part, with its potential to regulate NF-κB signaling pathway.
Keywords: Di (2-ethylhexyl) phthalate, quercetin, mitochondria, HEK293 cell line, nephrotoxicity, nuclear factor kappa B
Graphical Abstract
Graphical Abstract.
Introduction
Di-(2-ethylhexyl) phthalate (DEHP) is a commonly used plasticizer in plastic manufacturing industry, especially in the manufacture of medical devices and food packaging components. DEHP bound to the matrix of plastics weakly, and due to such connecting, it can easily separate from plastics and contaminate the milieu.1 The most exposure route of DEHP is oral route in both human and rodents.2 Monoethylhexyl phthalate (MEHP) is one of the main metabolites of DEHP produced by intestinal lipases and esterase.3 The daily exposure of DEHP for the general population is between 5 and 100 μg/kg and this amount is higher, more than 4 mg/kg, in hemodialysis patients who are in exposure with some certain medical devices.4
In recent years, the potential of DEHP to change renal function has been reported. Glomerular 5 and tubular damage,6 progression of renal cancer,7 and disruption of kidney development8 were observed following exposure to DEHP/MEHP. Oxidative stress and inflammation are among the main involved mechanisms in DEHP-induced nephrotoxicity that regulate by nuclear factor E2-related factor 2 (Nrf2)9 and Nuclear factor kappa B (NFκB)7 transcription factors, respectively.
Furthermore, in our previous study, mitochondrial dysfunction was observed following exposure to DEHP in kidney of rats,10 which shown the importance of the mentioned organelle in nephrotoxic effects induced by phthalates. Mitochondria are the both of source and target of free radicals in cells because of its specific structural and functional features.11,12 The free radicals can attack to the thiols of mitochondrial permeability transition pore (MPTP), which lead to MPTP opening and subsequent the entrance of water and ions to the cells which result in mitochondrial swelling and mitochondrial membrane potential (MMP) collapse.12 In this condition, the apoptosis cell death occurs by releasing the proapoptotic factors such as cytochrome c from mitochondria.13
Nrf2 is a main transcription factor stimulating in response to oxidative stress conditions to reduce oxidative damage by expression of antioxidant genes such as NAD(P)H quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and glutathione (GSH).14 The expression of Nrf2 and its downregulate genes have been decreased following exposure to nephrotoxic agents.15,16 The inhibition of the mentioned signaling was also observed in phthalates-induced toxicity.17–20
NF-κB is regarded as a vital transcription factor due to its role in regulation of genes involving in proliferation, survival, inflammation, and apoptosis.21 NF-κB, as an important transcription factor, is made of five various proteins such as p50/p105 (NF-κB1), p52/p100 (NF-κB2), p65 (RelA), RelB, and c-Rel.22 Under basal conditions, the proteins of NF-κB are kept in the cytosol by the inhibitor κB family (IκBα, IκBβ, and IκBε) proteins.23 IκB phosphorylation is vital for NF-κB activation, which is carried out by IkB kinase (IKK-α, IKK-β, and IKK-ϓ).24 The activation of NF-κB signaling pathway has been indicated in toxicities inducing by phthalates.25–27
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a flavonoid agent distributing in numerous foods such as apples, tomatoes, grapes, onions, berries, nuts, and tea.28 The pharmacological applications of quercetin including anticarcinogen, antioxidant, and anti-inflammation have been indicated in various studies.29,30 Furthermore, the beneficial effects of quercetin have been determined on mitochondrial toxicity induced by toxic agents.31,32 One of the main mechanisms by which quercetin inhibits inflammation is its potency to suppress NF-κB activity and its downstream genes, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8.28,33,34 In addition, quercetin has been shown its antioxidant activity by influencing on Nrf2 signaling pathway.35,36
The kidney is one of the main targets for quercetin accumulation in the body.37 Quercetin has shown nephroprotective effects by reduction of renal injury biomarkers, histopathologic changes, inhibition of oxidative stress, and inflammation.38–41 However, the possible protective effect of quercetin against DEHP/MEHP-induced nephrotoxicity is not determined so far. Therefore, this study was designed to evaluate the protective effects of quercetin against DEHP-induced nephrotoxicity in both in vitro and in vivo models with trying to reveal its underlying mechanisms.
Materials and methods
Chemicals
Embryonic human kidney cells (HEK293 cell line) were obtained from the Institut Pasteur (Iran, Tehran). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), penicillin, and streptomycin were purchased from Gibco company. The other used chemical components were purchased from Sigma-Aldrich company.
In vitro
Cell culture
The HEK-293 cell line was cultured in DMEM supplemented with 10% FBS and 100-IU/ml penicillin–streptomycin in a 37 °C incubator (5% CO2). The HEK293 cells were treated with the 256.6-μM MEHP as IC50 concentration (dissolved in %1 DMSO), and different concentrations of quercetin (25, 50, 100, and 200 μM) 2 h before MEHP treatment. The used concentrations of quercetin were selected based on previous studies.42,43
MTT assay
The MTT assay was used to evaluate the viability of cells.44,45 The 3-(4, 5-dimethylthiazol-2-yl)-2, the 5-diphenyltetrazolium bromide (MTT) solution (0.5 mg/mL) was added to the cells treated with MEHP alone and MEHP plus quercetin. The cells were maintained for 4 h in a 37 °C incubator. Supernatant was removed and 200-μL DMSO was added to the cells. An ELISA reader (Tecan, Rainbow Thermo, Austria) was used to measure the absorbance of samples.
Oxidative stress parameters assay
In the current study, ROS, Malondialdehyde (MDA), and GSH levels were measured in HEK-293 cells by the methods described previously46–48 via using 20,70-Dichlorofluorescein diacetate (DCFHDA), thiobarbituric acid (TBA), and 50,50-dithio-bis (2-nitrobenzoic acid) (DTNB), respectively.
In vivo
Animals
Male Wistar rats (250–300 g) were obtained from the Animal Laboratory of Mazandaran University of Medical Sciences (Sari, Iran). The rats were maintained under standard conditions at a temperature of 23 ± 2 °C and humidity 55 ± 5% with access to water and food. The examinations of study were performed based on the approved instructions by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences (Sari, Iran) that was in accordance with the National Institutes of Health guide for the care and use of laboratory animals.
Experimental design
The rats were divided into 4 groups with 6 animals in each as follows: control group (corn oil); DEHP group (200 mg/kg); DEHP (200 mg/kg) plus quercetin (50 and 100 mg/kg); and DEHP (200 mg/kg) plus vitamin E (20 mg/kg) as a positive control group. The used doses were selected based on previous studies.10,49,50 The treatment processes were carried out by gavage for 45 days; 24 h after last treatment, the rats were sacrificed by the chloroform and the blood gathering was performed for biochemical analyzes, and the tissue of the kidneys were removed for further assessments.
Biochemical assay
The blood serum was gathered by centrifuging at 3,000 rpm for 15 min. The renal injury biomarkers, such as blood urea nitrogen (BUN), and creatinine were determined in serum through using a commercial kit (Parsazmoon, Iran).
Isolation of kidney mitochondria
The isolation of kidney mitochondria was performed by a method described previously.10 In brief, the kidney was washed with a cold solution known as mannitol buffer containing 0.225-M D-mannitol, 75-mM sucrose, and 0.2-mM EDTA. The homogenizing of kidney tissue was done by a homogenizer device. The homogenized tissue was centrifuged at 1,000 × g for 10 min to remove nuclei and cell remains. The supernatant was centrifuged at 15,000 × g for 10 min to participate the mitochondria. The mannitol buffer was added to the mitochondria and was centrifuged at 10,000 × g for 10 min (repeat for twice). By considering the kind of test, the mitochondria were suspended in the each specific buffer, including Tris buffer contained 0.05-M Tris–HCl, 0.25-M sucrose, 20-mM KCl, 2.0-mM MgCl2, and 1.0-mM Na2HPO4, pH = 7.4, respiration buffer contained 125-mM sucrose, 65-mM KCl, 10-mM HEPES, 20-mM Ca2+, 5-mM sodium succinate, swelling buffer contained 125-mM sucrose, 65-mM KCl, 10-mM Hepes-KOH, 20-mM Ca2+, and MMP buffer contained 68-mM Mannitol, 220-mM Sucrose, 10-mM KCl, 5-mM KH2PO4, 50-μM EGTA, 2-mM MgCl2, and 10-mM HEPES. The mitochondria isolation was carried out by maintaining the cold chain in all processes.
Protein concentration assay
The protein concentration of samples was measured by the Bradford method expressed previously.51 Briefly, the samples of mitochondria and bovine serum albumin were incubated with coomassie for 10 min in darkness. After that, the samples absorbance was measured via spectrophotometry (UV-1601 PC, Shimadzu, Japan).
Mitochondrial function assay
The succinate dehydrogenase activity was measured as mitochondrial function index by using the (MTT) method.10 The mitochondrial fractions were incubated with 0.4% of MTT at 37 °C for 30 min. The supernatant was removed and 1-mL DMSO was added to the cells. The purple formazan was produced in the presence of succinate dehydrogenase enzyme, which could measure by ELISA reader at 570 nm (Tecan, Rainbow Thermo, Austria).
MMP assay
The MMP was measured by using Rhodamine 123.52 In brief, the suspended mitochondrial samples in MMP buffer (pH 7.2) were incubated with 10 μM of Rhodamine 123 at 37 °C for 15 min. The samples were centrifuged at 16,000 × g for 5 min in 4 °C. After that, the supernatant fluorescence was determined at 490 and 535 nm for excitation and emission wavelengths, respectively, by using a spectrofluorometer (Jasco, FP6200, Japan).
Mitochondrial swelling assay
The MPTP opening was determined by mitochondrial swelling evaluation. The mitochondria fractions were suspended in swelling buffer (pH 7.2) and the absorbance of samples was determined during 60 min via an ELISA reader at 540 nm (Tecan, Rainbow Thermo, Austria).10
Mitochondrial oxidative stress assay
The 20,70-dichlorofluorescein diacetate (DCFHDA) was used to evaluate the ROS levels.53 The MDA levels were measured as a lipid peroxidation (LPO) index by using TBA described previously.51 The GSH contents were measured by using DTNB.54
Real-time Polymerase chain reaction
Total RNA from the kidney tissue was extracted using total RNA extraction kit (Yekta tajhiz company, Iran). cDNA was synthesized from total RNA by a commercial kit provided by yekta tajhiz company (Iran). After that, real-time quantitative PCR was carried out to determine the mRNA expressions of Nrf2, HO-1, p65 NFκB, and TNFα via using a StepOne Plus real-time PCR system (Applied Biosystems, USA) with RealQ Plus 2× Master Mix Green (Ampliqon, Denmark). All amplifications were done in duplicate. β-actin was applied to normalize. The primers sequences synthesized by metabion (Germany) were as follows:
Nrf2: Forward: 5´-CACCCACATTCCCAAACAAGATG-3´.
Reverse: 5´-TATCCAGGGCAAGCGACTCA-3´.
HO-1: Forward: 5´-GCACAGGGTGACAGAAGAGG-3´.
Reverse: 5´-AGGTAGTATCTTGAACCAGGCT-3´.
TNFα: Forward: 5´-ATGGGCTCCCTCTCATCAGT-3´.
Reverse: 5´-GCTTGGTGGTTTGCTACGAC-3′.
p65 NFκB: Forward: 5´-CCTCATCTTTCCCTCAGAGCC-3´.
Reverse: 5´-TGCTTCTCTCCCCAGGAATAC-3′.
β-actin: 5´-ACGGTCAGGTCATCACTATCG-3´.
Reverse: 5´-CCACAGGATTCCATACCCAG-3´.
Step One Software version 2.3 was used to analyze data and fold change was achieved by using 2−ΔΔCt formula.55,56
Histopathological assay
To evaluate kidney damage, samples were fixed in 10% formalin for 24 h. Then, tissues were cut with a standard method. Kidney sections with 4-μm thick were stained with hematoxylin and eosin stain (H&E) for kidney damage evaluation.57 The slides were evaluated with a light microscope (Olympus, Japan) by an unaware pathologist to the treatment groups.
Statistical analysis
The data were analyzed through one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. The prism (version 8.2) statistical software was used to analyze the all data. The results are indicated as mean ± SEM. P < 0.05 was considered as the statistical significance level.
Results
Quercetin improved cytotoxicity induced by MEHP
The MTT assay was used to evaluate cytotoxic and cytoprotective behavior of MEHP and quercetin, respectively, in HEK-293 cells. The cells were treated with different concentrations (50, 100, 200, and 400 μM) of MEHP for 24 h and IC50 was determined. Then, IC50 concentration (256.6 μM) was used for other assessments in this study. To determine the cytoprotective potential of quercetin, various concentrations of it were added to the cells MEHP-treated cells. As shown in Fig. 1, the significant (P < 0.01) decrease was observed in MEHP group compared with the control group, which confirmed the cytotoxic effect of MEHP in HEK-293 cells. In contrast, quercetin concentrations of 100 and 200 μM significantly (P < 0.05, P < 0.01, respectively) decreased cytotoxicity in comparison with the MEHP alone.
Fig. 1.

The cytoprotective effect of quercetin against MEHP-induced cytotoxicity in HEK-293 cells. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. ##P < 0.01 versus control group. *P < 0.05, **P < 0.01 versus MEHP group.
Quercetin decreased MEHP-oxidative damage in HEK293 cells
ROS, MDA, and GSH contents as oxidative stress parameters were measured in HEK-293 cells. As shown in Fig. 2a and b, the ROS and MDA levels were significantly (P < 0.01) increased in MEHP group compared with the control group. Pretreatment with quercetin significantly decreased ROS and MDA levels in high concentrations of quercetin compared with the MEHP group. In addition, GSH content was significantly (P < 0.01) decreased in MEHP group compared with the control group and was significantly increased in high concentrations of quercetin compared with the MEHP group (Fig. 2c).
Fig. 2.
The effect of quercetin pretreatment on ROS a), MDA b), and GSH c) levels in HEK293 cells exposed MEHP. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. ##P < 0.01 versus control group. *P < 0.05, **P < 0.01 versus MEHP group.
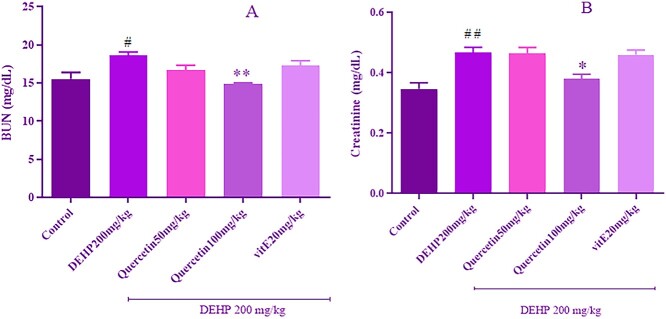
Quercetin improved DEHP-induced renal dysfunction
BUN and creatinine levels as renal function biomarkers were shown in Fig. 3a and b. The levels of BUN and creatinine significantly (P < 0.05, P < 0.01, respectively) increased in the DEHP treatment group compared with the control group. Quercetin at a dose of 100 mg/kg significantly decreased BUN and creatinine levels compared with the DEHP group. However, the mentioned biomarkers had no remarkable change in the vitamin E treatment group compared with DEHP group.
Fig. 3.
The effect of quercetin treatment on level of serum BUN, and creatinine in rats received DEHP. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. #P < 0.05, ##P < 0.01 versus control group. *P < 0.05, **P < 0.01 versus DEHP group.
Quercetin improved DEHP-induced mitochondrial dysfunction
The succinate dehydrogenase activity is evaluated as mitochondrial function index. As shown in Fig. 4, DEHP treatment significantly (P < 0.05) decreased succinate dehydrogenase activity in isolated kidney mitochondria compared with the control group. However, 100-mg/kg quercetin significantly (P < 0.05) increased the activity of succinate dehydrogenase compared with the DEHP group. Also, administration of vitamin E significantly (P < 0.05) increased the activity of the mentioned enzyme compared with the DEHP alone.
Fig. 4.
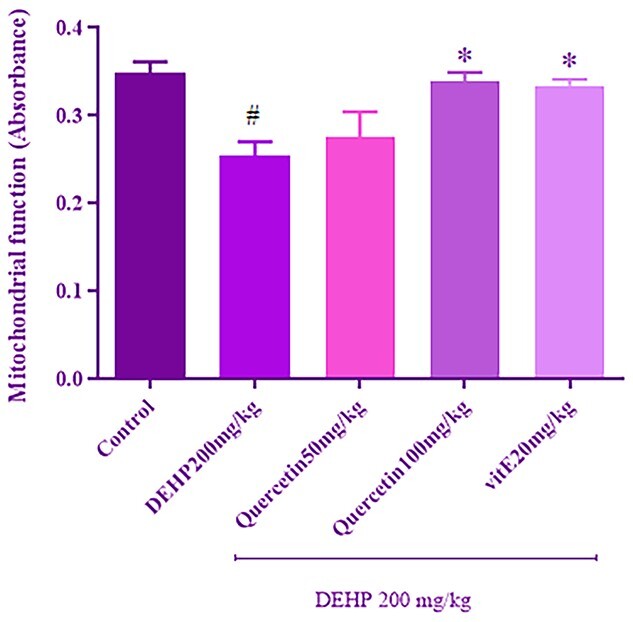
The effect of quercetin treatment on kidney mitochondrial function (by MTT assay) in rats received DEHP. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. #P < 0.05 versus control group. *P < 0.05 versus DEHP group.
Quercetin improved DEHP-induced MMP collapse
As shown in Fig. 5, the fluorescence intensity was significantly (P < 0.05) decreased in the DEHP treatment group compared with the control group. Pretreatment with 100-mg/kg quercetin significantly (P < 0.05) increased fluorescence intensity compared with DEHP alone. There was not the remarkable change in the fluorescence intensity between vitamin E group and DEHP group.
Fig. 5.

The effect of quercetin treatment on kidney mitochondria membrane potential (MMP) in rats received DEHP. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. #P < 0.05 versus control group. *P < 0.05 versus DEHP group.
Quercetin improved DEHP-induced mitochondrial swelling
DEHP significantly (P < 0.05) increased the mitochondrial swelling compared with the control group. Quercetin at a dose of 100 mg/kg significantly (P < 0.05) decreased mitochondrial swelling compared with DEHP group. Also, mitochondrial swelling was significantly (P < 0.05) decreased following treatment with vitamin E compared with the DEHP treatment alone (Fig. 6).
Fig. 6.

The effect of quercetin treatment on kidney mitochondria swelling in rats received DEHP. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. #P < 0.05 versus control group. *P < 0.05 versus DEHP group.
Quercetin improved DEHP-induced mitochondrial oxidative damage
As indicated in Fig. 7a, the levels of ROS were significantly (P < 0.01) enhanced after treatment with DEHP alone compared with the control group. Quercetin at a dose of 100 mg/kg and vitamin E significantly (P < 0.05, P < 0.01, respectively) decreased ROS levels compared with DEHP group.
Fig. 7.
The effect of quercetin treatment on kidney mitochondria levels of ROS a), MDA b), and GSH c) in rats received DEHP. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. ##P < 0.01 versus control group. *P < 0.05, **P < 0.01 versus DEHP group.
The MDA levels were measured as LPO index and the results are shown in Fig. 7b. The significant (P < 0.01) enhance was observed in the levels of MDA following exposure to DEHP compared with the control group. Pretreatment with 100-mg/kg quercetin significantly (P < 0.05) decreased MDA levels compared with the DEHP treatment group. The same result was shown in the vitamin E group.
The content of GSH is shown in Fig. 7c; the remarkable (P < 0.01) decrease was indicated in GSH content in DEHP group compared with the control group. Also, the remarkable (P < 0.05) enhance was shown in GSH content after pretreatment with 100-mg/kg quercetin compared with DEHP alone. No remarkable effect on the GSH content was observed in the vitamin E group compared with DEHP group.
Taken together, quercetin at high dose could reduce mitochondrial oxidative damage induced by DEHP.
Quercetin improved nephrotoxic effects of DEHP through inhibition of NFκB signaling pathway
By considering that quercetin at a dose of 100 mg/kg was effective against DEHP-induced nephrotoxicity, therefore, the changes of Nrf2, HO-1, p65 NFκB, and TNFα gene expressions were evaluated only in 100-mg/kg quercetin group. The findings are shown in Fig. 8. DEHP significantly (P < 0.05) decreased Nrf2 and HO-1 gene expressions compared with the control group. Administration of quercetin increased gene expressions of Nrf2 and HO-1 compared with DEHP group; however, the changes were not statistically significant. Furthermore, the significant (P < 0.05) increase was observed in p65 NFκB and TNFα gene expressions in DEHP group compared with the control group. Quercetin could significantly (P < 0.05) decrease p65 NFκB and TNFα gene expressions in comparison with DEHP group. These findings indicated that quercetin probably induced protective effects against DEHP-induced nephrotoxicity partly by inhibiting of p65 NFκB signaling pathway.
Fig. 8.
The effects of DEHP and 100 mg/kg of quercetin on mRNA expressions of Nrf2 a), HO-1 b), NFκB c), and TNFα d) in kidney tissue of rats. Values are expressed as mean ± SEM for at least three experiments. Data were analyzed by one-way ANOVA multiple comparison test followed by a Tukey’s multiple comparisons test. #P < 0.05 versus control group. *P < 0.05 versus DEHP group.
Quercetin improved DEHP-renal histopathology changes
Alternations of kidney tissue following exposure to DEHP and 100-mg/kg quercetin are shown in Fig. 9. DEHP led to inflammation and congestion in the glomerulus and blood vessels of kidney tissue in comparison with the control group, which indicated almost normal structure. Furthermore, the appearance of kidney tissue in quercetin a dose of 100 mg/kg was approximately near the normal.
Fig. 9.

The appearance of kidney was normal in control group a). The severe inflammation (red arrow) and moderate conjunction (blue arrow) were observed in DEHP treatment group b). The kidney tissue in DEHP plus quercetin at dose of 100 mg/kg was near the normal appearance c).
Discussion
The various evidences indicate the potential of DEHP and its metabolite to induce or promote the renal injury through different mechanisms.5,7,58,59 The mitochondria as an important cellular organelle are one of the main targets for xenobiotics to induce deleterious impacts on cell.60 The literature reports that phthalates can induce toxic effects by influencing mitochondrial functions mostly through ROS formation.61,62 In our recent study, mitochondrial dysfunction and oxidative damage were observed in kidney tissue of rats and HEK-293 cells after exposure to DEHP and MEHP, respectively.10 Therefore, the use of agents with antioxidant features can be useful against nephrotoxic effects induced by DEHP/MEHP. Quercetin, as the potent antioxidant component, is not only effective to reduce oxidative stress but also potent to ameliorate inflammation and apoptosis.63,64 In the current study, the efficacy of quercetin against DEHP/MEHP-induced nephrotoxicity has been determined. Also, the main molecular mechanisms by which quercetin can decrease these effects have been evaluated.
The levels of BUN and creatinine were increased following subchronic exposure to DEHP in serum of rat; furthermore, some histopathological changes, including inflammation and congestion, were observed in rat’s glomerulus and blood vessels. However, treatment with quercetin remarkably protected rat against DEHP-induced kidney injury via decreasing serum BUN and creatinine levels and ameliorating kidney histological changes. These findings are in accordance with others showing the potent effect of quercetin in reducing kidney injury biomarkers and histopathological damage after exposure to nephrotoxic agents.39,65–67 Furthermore, the cytoprotective effect of quercetin has been shown in different cells68–70 that in agreement with others, in this study, quercetin attenuated the MEHP-induced cytotoxicity in HEK-293 cells.
Oxidative stress is an imbalance between production of free radicals and their neutralization though antioxidants,71 which is expressed as an involved mechanism in adverse effects inducing by DEHP and its metabolite in organism cells.72–74 In this study, the ROS levels were increased in MEHP treated cells, which can induce damage macromolecules of cells, such as lipids, proteins, and DNA.75 The level of MDA, which is the LPO index in cells, was increased, and GSH content as the main nonenzymatic antioxidant was decreased after treatment with MEHP in HEK-293 cells. These results are in accordance with the previous researches.73,76–78 The use of quercetin led to reduction of ROS and MDA levels and enhancement of GSH content in MEHP treated cells, which were in agreement with findings of other studies.79–81
It is reported that ROS can disrupt the hemostasis of mitochondria, which are the main regulating organelles for respiration, redox status, and apoptosis.82 The mitochondrial respiration chain consists of five complexes which are the main source of mitochondrial ROS. The complex II activity is dependent on succinate dehydrogenase enzyme activity which can be considered as mitochondrial function index measuring by MTT assay. 83 In the present study, the mitochondrial function was decreased in isolated kidney mitochondria treating with DEHP. This result was reversed by using quercetin, which was in agreement with previous findings.84,85 ROS can attack to lipids of mitochondrial membrane leading to mitochondrial membrane disruption and mitochondrial swelling which are involved in proapoptotic factors release such as cytochrome c.86,87 Fewer than 20% of GSH is present in the mitochondria that inhibit the oxidation of thiols of mitochondrial membrane proteins and suppress MPTP opening, which plays an important role in the apoptotic cell death initiation.88,89 In the present study, the mitochondrial MDA and ROS levels were increased and the mitochondrial GSH content was decreased in DEHP-treated group, which confirmed the mitochondrial oxidative damage in kidney of rat. Furthermore, the collapse of MMP and mitochondrial swelling were observed in isolated kidney mitochondria following treatment with DEHP. The same findings were observed in our and others previous studies following exposure to phthalates.10,76,90 These results were reversed through pretreatment with 100-mg/kg quercetin. Quercetin reduced nephrotoxicity in a dose of 100 mg/kg. The lack of quercetin ability to inhibit nephrotoxicity in less than this amount may be related to its low bioavailability. It is reported that quercetin bioavailability is 16% in rats.91
To determine the involved molecular mechanisms of quercetin in reduction of nephrotoxic effects of DEHP, the authors evaluated Nrf2 and NF-κB signaling pathways. Nrf2 transcription factor plays an important role against oxidative damage by stimulating expression of phase II detoxification enzymes genes, including superoxide dismutase, catalase, GSH, NQO1, and HO-1.92 HO-1 enzyme converts heme into biliverdin, carbon monoxide, and iron ions which have a key role against oxidative stress, inflammation, and apoptosis.93,94 HO-1 suppressed inflammatory mediators by suppression of NF-κB.95,96 The nephroprotective effects of HO-1 were shown previously.94 The present study showed that DEHP significantly decreased the expression of Nrf2 and HO-1 in the rat kidneys. Nrf2 and HO-1 expressions were reduced in testis of DEHP treated rats.17 DEHP induced testicular damage via Nrf2 pathway inhibition in rat demonstrating by reduction of Nrf2 protein and its downstream antioxidant enzymes.18 In this study, quercetin at dose of 100 mg/kg increased the expressions of Nrf2 and HO-1 genes in compared with DEHP; however, the changes were not significant. Different studies had reported quercetin potential to the activation of Nrf2 and the induction of HO-1 expression.97,98
The NF-κB pathway is a main cellular signaling in response to harmful component such as free radicals, cytokines, bacterial toxin, and viruses.99 NF-κB regulates the expression of numerous genes contributing in inflammation, apoptosis, and cancer, such as TNFα, iNOS, COX-2, and interleukins.21 DEHP induced apoptosis in hepatocyte cells by influencing on NF-κB pathway.61 NF-κB expression and its transcriptional activity were upregulated by MEHP; actually, p65 NF-κB pathway was responsible for MEHP-induced migration and invasion.7 MEHP increased RelB/p52 migration into the nucleus in cytotrophoblast cultured from term human placenta.25 p65 NF-κB were enhanced in endometrial stromal cells following DEHP treatment.100 DEHP significantly enhanced p65 NF-κB migration into the nucleus and enhanced cytokines IL-8, IL-1β, IL-6, and TNFα in human macrophage such as THP-1 cells.26 The findings of this study in agreement with previous studies indicated that DEHP significantly increased the expression of p65 NF-κB and TNFα in kidney of rat. Quercetin significantly reduced the expressions of p65 NF-κB and TNFα genes in the rat kidney exposed to DEHP. The suppression effect of quercetin on NF-κB signaling pathway is well shown previously.101,102 Quercetin inhibited TNF-α caused apoptosis and inflammation in part via suppressing NF-κB signaling pathway.103 Quercetin acts as an inflammatory component in liver by inhibiting NF-κB signaling pathway.104
The findings of this study suggested that quercetin improved mitochondrial dysfunction, mitochondrial oxidative damage, and overall kidney injury by influencing on NF-κB signaling pathway. There are some evidences that activated NF-κB can translocate to the mitochondria and influence on mitochondrial function by affecting on mtDNA105; however, such effects were not determined in the present study. Future studies are needed to evaluate whether mitochondria are affected directly by NF-κB or not.
Conclusion
In conclusion, the present study indicates for the first time that quercetin has nephroprotective effects against DEHP/MEHP-induced kidney damage revealing by improvement of mitochondrial function, and oxidative damage through its potential to inhibit NF-κB pathway. Therefore, quercetin can be considered as a potent protective agent against nephrotoxicity induced by DEHP and its metabolite.
Funding
The present study was supported by a PhD student grant from the research council of Mazandaran University of Medical Sciences, Sari, Iran [Reference number: IR.MAZUMS.REC.1397.3411].
Conflicts of interest statement. There are no conflicts of interest to declare.
Contributor Information
Sorour Ashari, Student Research Committee, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran; Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
Mohammad Karami, Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran; Pharmaceutical Science Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
Mohammad Shokrzadeh, Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran; Pharmaceutical Science Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
Abouzar Bagheri, Department of Clinical Biochemistry and Medical Genetics, Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Morteza Ghandadi, Pharmaceutical Science Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
Mohammad Ranaee, Clinical Research Development Center, Rouhani Hospital, Babol University of Medical Sciences, Babol, Iran; Department of Pathology, Rouhani Hospital, Babol University of Medical Sciences, Babol, Iran.
Ayat Dashti, Student Research Committee, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran; Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
Hamidreza Mohammadi, Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran; Pharmaceutical Science Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
References
- 1. Wang W, Craig Z, Basavarajappa M, Gupta R, and Flaws J. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol. 2012:258(2):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aydemir D, Karabulut G, Şimşek G, Gok M, Barlas N, and Ulusu N. Impact of the Di (2-ethylhexyl) phthalate administration on trace element and mineral levels in relation of kidney and liver damage in rats. Biol Trace Elem Res. 2018:186(2):474–488. [DOI] [PubMed] [Google Scholar]
- 3. Dong J, Ma Y, Leng K, Wei L, Wang Y, Su C, Liu M, Chen J. Associations of urinary di-(2-ethylhexyl) phthalate metabolites with the residential characteristics of pregnant women. Sci Total Environ. 2020:707(10):135671. [DOI] [PubMed] [Google Scholar]
- 4. Chen H, Zhang W, Rui B, Yang S, Xu W, Wei W. Di (2-ethylhexyl) phthalate exacerbates non-alcoholic fatty liver in rats and its potential mechanisms. Environ Toxicol Pharmacol. 2016:42(2):38–44. [DOI] [PubMed] [Google Scholar]
- 5. Kamijo Y, Hora K, Nakajima T, Kono K, Takahashi K, Ito Y, Higuchi M, Kiyosawa K, Shigematsu H, Gonzalez F, Aoyama T. Peroxisome proliferator–activated receptor α protects against glomerulonephritis induced by long-term exposure to the plasticizer Di-(2-Ethylhexyl) phthalate. J Am Soc Nephrol. 2007:18(1):176–188. [DOI] [PubMed] [Google Scholar]
- 6. Ward J, Diwan B, Ohshima M, HU H, Schuller H, Rice J. Tumor-initiating and promoting activities of di (2-ethylhexyl) phthalate in vivo and in vitro. Environ Health Perspect. 1986:65(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Z, Shao M, Liu Y. Promotion of Wilms' tumor cells migration and invasion by mono-2-ethyhexyl phthalate (MEHP) via activation of NF-κB signals. Chem Biol Interact. 2017:270(10):1–8. [DOI] [PubMed] [Google Scholar]
- 8. Wei Z, Wei Z, Song L, Wei J, Chen T, Chen J, Lin Y, Xia W, Xu B, Li X, Chen X, Li Y, Xu S. Maternal exposure to di-(2-ethylhexyl) phthalate alters kidney development through the renin–angiotensin system in offspring. Toxicol Lett. 2012:212(2):212–221. [DOI] [PubMed] [Google Scholar]
- 9. Amara I, Amara I, Timoumi R, Graiet I, Ben Salem I, Adelou K, Abid-Essefi S. Di (2-ethylhexyl) phthalate induces cytotoxicity in HEK-293 cell line, implication of the Nrf-2/HO-1 antioxidant pathway. Environ Toxicol. 2019:34(9):1034–1042. [DOI] [PubMed] [Google Scholar]
- 10. Ashari S, Karami M, Shokrzadeh M, Ghandadi M, Ghassemi-Barghi N, Dashti A, Ranaee M, Mohammadi H. The implication of mitochondrial dysfunction and mitochondrial oxidative damage in di (2-ethylhexyl) phthalate induced nephrotoxicity in both in vivo and in vitro models. Toxicol Mech Methods. 2020:30(6):427–437. [DOI] [PubMed] [Google Scholar]
- 11. Dong P, Li J, Xu S, Xu X, Wu X, Xiang X, Yang Q, Jin J, Liu Y, Jiang F. Mitochondrial dysfunction induced by ultra-small silver nanoclusters with a distinct toxic mechanism. J Hazard Mater. 2016:308(8):139–148. [DOI] [PubMed] [Google Scholar]
- 12. Marchi S, Giorgi C, Suski J, Agnoletto Ch, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, Rimessi A, Duszynski J. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012:2012(2):329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016:1863(12):2977–2992. [DOI] [PubMed] [Google Scholar]
- 14. Oh CJ, Kim J, Choi Y, Kim H, Jeong J, Bae K, Park K, Lee I. Dimethylfumarate attenuates renal fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. PLoS One. 2012:7(10):e45870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, Kucuk O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010:87(7–8):240–245. [DOI] [PubMed] [Google Scholar]
- 16. Elsherbiny NM, El-Sherbiny M. Thymoquinone attenuates doxorubicin-induced nephrotoxicity in rats: role of Nrf2 and NOX4. Chem Biol Interact. 2014:223(17):102–108. [DOI] [PubMed] [Google Scholar]
- 17. Zhang L, Li H, Gao M, Zhang T, Wu Z, Wang Z, Chong T. Genistein attenuates di-(2-ethylhexyl) phthalate-induced testicular injuries via activation of Nrf2/HO-1 following prepubertal exposure. Int J Mol Med. 2018:41(3):1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao T-X, et al. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ Pollut. 2020:259(4):113911. [DOI] [PubMed] [Google Scholar]
- 19. Sun X, Huang Q, Shi J, Qiu L, Kang M, Chen Y, Fang C, Ye T, Dong S. Di (2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015:19(3):581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohammadi H, Ashari SJES, Research P. Mechanistic insight into toxicity of phthalates, the involved receptors, and the role of Nrf2, NF-κB, and PI3K/AKT signaling pathways. Environ Sci Pollut Res Int 2021:28(27):35488–35527. [DOI] [PubMed] [Google Scholar]
- 21. Gupta SC, Sundaram C, Reuter S, Aggarwal B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2010:1799(10–12):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siomek A. NF-κB signaling pathway and free radical impact. Acta Biochim Pol. 2012:59(3):323–31. [PubMed] [Google Scholar]
- 23. Bhatt D, Ghosh S. Regulation of the NF-κB-mediated transcription of inflammatory genes. Front Immunol. 2014:25(5):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008:132(3):344–362. [DOI] [PubMed] [Google Scholar]
- 25. Wang XK, Agarwal M, Parobchak N, Rosen A, Vetrano A, Srinivasan A, Wang B, Rosen T. Mono-(2-ethylhexyl) phthalate promotes pro-labor gene expression in the human placenta. PLoS One. 2016:11(1):e0147013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishioka J, Iwahara C, Kawasaki M, Yoshizaki F, Nakayama H, Takamori K, Ogawa H, Iwabuchi K. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflamm Res. 2012:61(1):69–78. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, et al. Up regulation of IL-6 is involved in di (2-ethylhexyl) phthalate (DEHP) induced migration and invasion of non small cell lung cancer (NSCLC) cells. Biomed Pharmacother. 2017:89(5):1037–1044. [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Yao J, Han C, Yang J, Chaudhry M, Wang S, Liu H, Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016:8(3):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lesjak M, Beara I, Simin N, Pintać D, Majkić T, Bekvalac K, Orčić D, Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods. 2018:40(1):68–75. [Google Scholar]
- 30. Ezzati M, Yousefi B, Velaei K, Safa A. A review on anti-cancer properties of quercetin in breast cancer. Life Sci. 2020:248(9):117463. [DOI] [PubMed] [Google Scholar]
- 31. Shirani M, Alizadeh S, Mahdavinia M, Dehghani M. The ameliorative effect of quercetin on bisphenol A-induced toxicity in mitochondria isolated from rats. Environ Sci Pollut Res. 2019:26(8):7688–7696. [DOI] [PubMed] [Google Scholar]
- 32. Waseem M, Tabassum H, Bhardwaj M, Parvez S. Ameliorative efficacy of quercetin against cisplatin-induced mitochondrial dysfunction: study on isolated rat liver mitochondria. Mol Med Rep. 2017:16(3):2939–2945. [DOI] [PubMed] [Google Scholar]
- 33. Vicentini FT, He T, Shao Y, Fonseca M, Verri Jr W, Fisher G, Xu Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J Dermatol Sci. 2011:61(3):162–168. [DOI] [PubMed] [Google Scholar]
- 34. Min Y-D, Choi C, Bark H, Son H, Park H, Lee S, Park J-w, Park E-K, Shin H, Kim S. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007:56(5):210–215. [DOI] [PubMed] [Google Scholar]
- 35. Carrasco-Pozo C, Castillo R, Beltran R, Miranda A, Fuentes J, Gotteland M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: role of NF-κB and Nrf2. J Nutr Biochem. 2016:27(1):289–298. [DOI] [PubMed] [Google Scholar]
- 36. Jin Y, Huang Z, Li L, Yang Y, Wang C, Wang Zh, Ji L. Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol Sin. 2019:40(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batiha GE-S, Beshbishy A, Ikram M, Mulla Z, Abd El Hack A, Taha A, Algammal A, Elewa Y. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. 2020:9(3):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan RZ, Wang C, Deng C, Zhong X, Yan Y, Lou Y, Lan H, He T,Wang L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother Res. 2019:34(1):139–152. [DOI] [PubMed] [Google Scholar]
- 39. Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009:32(1):61–67. [DOI] [PubMed] [Google Scholar]
- 40. Yuksel Y, Yuksel R, Yagmurca M. Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum Exp Toxicol. 2017:36(1):51–61. [DOI] [PubMed] [Google Scholar]
- 41. Yang H, Song H, Liang Y, Li R. Quercetin treatment improves renal function and protects the kidney in a rat model of adenine-induced chronic kidney disease. Med Sci Monit. 2018:24(1):4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee Y-K, Park S, Kim Y-A, Lee W, Park O. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009:41(3):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duo J, Ying G-G, Wang G-W, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep. 2012:5(6):1453–1456. [DOI] [PubMed] [Google Scholar]
- 44. Hassani M, Ghassemi-Barghi N, Modanloo M, Mohammadpour A. Cytotoxic effects of duloxetine on MKN45 and NIH3T3 cell lines and genotoxic effects on human peripheral blood lymphocytes. Arq Gastroenterol. 2019:56(4):372–376. [DOI] [PubMed] [Google Scholar]
- 45. Shokrzadeh M, Mohammadpour A, Modanloo M, Hassani M, Ghassemi-Barghi N, Niroomand P. Cytotoxic effects of aripiprazole on MKN45 and NIH3T3 cell lines and genotoxic effects on human peripheral blood lymphocytes. Arq Gastroenterol. 2019:56(2):155–159. [DOI] [PubMed] [Google Scholar]
- 46. Shokrzadeh M, Ghassemi-Barghi N. Antioxidant and Genoprotective effects of Amifostine against irinotecan toxicity in human hepatoma cells. Int J Cancer Res Ther. 2018:3(1):1–5. [Google Scholar]
- 47. Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol In Vitro. 2009:23(5):808–815. [DOI] [PubMed] [Google Scholar]
- 48. Cheng B, Yang X, An L, Gao B, Liu X. Arsenic trioxide-induced apoptosis of Hep-2 cell line through modulating intracellular glutathione (GSH) level. Auris Nasus Larynx. 2010:37(1):89–94. [DOI] [PubMed] [Google Scholar]
- 49. Gelen V, Şengül E, Gedikli S, Atila G, Uslu H, Makav M. The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac J Trop Biomed. 2017:7(7):647–653. [Google Scholar]
- 50. Şengül E, Gelen V, Gedikli S, Özkanlar S, Gür C, Çelebi F, Çınara A. The protective effect of quercetin on cyclophosphamide-induced lung toxicity in rats. Biomed Pharmacother. 2017:92(8):303–307. [DOI] [PubMed] [Google Scholar]
- 51. Shaki F, Ashari S, Ahangar N. Melatonin can attenuate ciprofloxacin induced nephrotoxicity: involvement of nitric oxide and TNF-α. Biomed Pharmacother. 2016:84(8):1172–1178. [DOI] [PubMed] [Google Scholar]
- 52. Ahmad A, Kumari P, Ahmad M. Apigenin attenuates edifenphos-induced toxicity by modulating ROS-mediated oxidative stress, mitochondrial dysfunction and caspase signal pathway in rat liver and kidney. Pestic Biochem Physiol. 2019:159(7):163–172. [DOI] [PubMed] [Google Scholar]
- 53. Shokrzadeh M, Etebari M, Ghassemi-Barghi N. An engineered non-erythropoietic erythropoietin-derived peptide, ARA290, attenuates doxorubicin induced genotoxicity and oxidative stress. Toxicol In Vitro. 2020:66(5):104864. [DOI] [PubMed] [Google Scholar]
- 54. Khan H, Khan M-F, Jan S-U, Ullah N. Effect of aluminium metal on glutathione (GSH) level in plasma and cytosolic fraction of human blood. Pak J Pharm Sci. 2011:24(1):13–18. [PubMed] [Google Scholar]
- 55. Sreedharan SP, Kumar A, Giridhar PJB. Primer design and amplification efficiencies are crucial for reliability of quantitative PCR studies of caffeine biosynthetic N-methyltransferases in coffee. 3 Biotech. 2018:8(11):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shokrzadeh M, Mohammadpour A, Ghassemi-Barghi N, Hoseini V, Abediankenari S, Saleh Tabari Y. Metallothionein-2A (RS1610216&RS28366003) gene polymorphisms and the risk of stomach adenocarcinoma. Arq Gastroenterol. 2019:56(4):367–371. [DOI] [PubMed] [Google Scholar]
- 57. Mohammadian M, Mianabadi M, Zargari M, Karimpour A, Khalafi M, Talebpour Amiri F. Effects of olive oil supplementation on sodium arsenate-induced hepatotoxicity in mice. Int J Prev Med. 2018::6(9):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Di Lorenzo M, Forte M, Valiante S, Laforgia V, De Falco M. Interference of dibutylphthalate on human prostate cell viability. Ecotoxicol Environ Saf. 2018:147(1):565–573. [DOI] [PubMed] [Google Scholar]
- 59. Zhao Y, Du Z-H, Talukder M, Lin J, Li X-N, Zhang C, Li J-L. Crosstalk between unfolded protein response and Nrf2-mediated antioxidant defense in Di-(2-ethylhexyl) phthalate-induced renal injury in quail (Coturnix japonica). Environ Pollut. 2018:242(11):1871–1879. [DOI] [PubMed] [Google Scholar]
- 60. Meyer JN, Leung M-C K, Rooney J-P, Sendoel A, Hengartner M-O, Kisby G-E, Bess A-S. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013:134(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ghosh J, Das J, Manna P, Sil P-C. Hepatotoxicity of di-(2-ethylhexyl) phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF-κB pathway activation. Free Radic Biol Med. 2010:49(11):1779–1791. [DOI] [PubMed] [Google Scholar]
- 62. Zhang G, Yang W, Jiang F, Zou P, Zeng Y, Ling X, Zhou Z, Cao J, Ao L. PERK regulates Nrf2/ARE antioxidant pathway against dibutyl phthalate-induced mitochondrial damage and apoptosis dependent of reactive oxygen species in mouse spermatocyte-derived cells. Toxicol Lett. 2019:308(9):24–33. [DOI] [PubMed] [Google Scholar]
- 63. Roslan J, Giribabu N, Karim K, Salleh N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharmacother. 2017:86(2):570–582. [DOI] [PubMed] [Google Scholar]
- 64. Lu X-L, Zhao C-H, Yao X-L, Zhang H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed Pharmacother. 2017:85(1):658–671. [DOI] [PubMed] [Google Scholar]
- 65. Aldemir M, Okulu E, Kösemehmetoğlu K, Ener K, Topal F, Evirgen O, Gürleyik E, Avcı A. Evaluation of the protective effect of quercetin against cisplatin-induced renal and testis tissue damage and sperm parameters in rats. Andrologia. 2014:46(10):1089–1097. [DOI] [PubMed] [Google Scholar]
- 66. Sánchez-González PD, López-Hernández F-J, Dueñas M, Prieto M, Sánchez-López E, Thomale J, Ruiz-Ortega M, López-Novoa J-M, Morales A-I. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem Toxicol. 2017:107(9):226–236. [DOI] [PubMed] [Google Scholar]
- 67. Gomes IB, Porto M-L, Santos M-C L F S, Campagnaro B-P, Pereira T-M C, Meyrelles S-S, Vasquez E-C. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014:13(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bao D, Wang J, Pang X, Liu H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules. 2017:22(7):1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu X, Li N, Wang Y, Ding L, Chen H, Yu Y, Shi X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol Rep. 2017:37(1):209–218. [DOI] [PubMed] [Google Scholar]
- 70. Nishimura K, Matsumoto R, Yonezawa Y, Nakagawa H. Effect of quercetin on cell protection via erythropoietin and cell injury of HepG2 cells. Arch Biochem Biophys. 2017:636(22):11–16. [DOI] [PubMed] [Google Scholar]
- 71. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018:26(13):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Y, Wang S, Zhao T, Yang L, Guo S, Shi Y, Zhang X, Zhou L, Ye L. Mono-2-ethylhexyl phthalate (MEHP) promoted lipid accumulation via JAK2/STAT5 and aggravated oxidative stress in BRL-3A cells. Ecotoxicol Environ Saf. 2019:184(18):109611. [DOI] [PubMed] [Google Scholar]
- 73. Park CG, Sung B, Ryu C-S, Kim Y-J. Mono-(2-ethylhexyl) phthalate induces oxidative stress and lipid accumulation in zebrafish liver cells. Comp Biochem Physiol C, Toxicol Pharmacol. 2020:230(4):108704. [DOI] [PubMed] [Google Scholar]
- 74. Wu M, Xu L, Teng C, Xiao X, Hu W, Chen J, Tu W. Involvement of oxidative stress in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis of mouse NE-4C neural stem cells. Neurotoxicology. 2019:70(1):41–47. [DOI] [PubMed] [Google Scholar]
- 75. Roy J, Galano J-M, Durand T, Guennec J-Y Le, Lee J C-Y. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017:31(9):3729–3745. [DOI] [PubMed] [Google Scholar]
- 76. Zhang Q, Zhao Y, Talukder M, Han Y, Zhang C, Li X-N, Li J-L. Di (2-ethylhexyl) phthalate induced hepatotoxicity in quail (Coturnix japonica) via modulating the mitochondrial unfolded protein response and NRF2 mediated antioxidant defense. Sci Total Environ. 2019:651(7):885–894. [DOI] [PubMed] [Google Scholar]
- 77. Ban J-B, Fan X-W, Huang Q, Li B-F, Chen C, Zhang H-C, Xu S-Q. Mono-(2-ethylhexyl) phthalate induces injury in human umbilical vein endothelial cells. PLoS One. 2014:9(5):e97607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen X, Wang J, Qin Q, Jiang Y, Yang G, Rao K, Wang Q, Xiong W, Yuan J. Mono-2-ethylhexyl phthalate induced loss of mitochondrial membrane potential and activation of Caspase3 in HepG2 cells. Environ Toxicol Pharmacol. 2012:33(3):421–430. [DOI] [PubMed] [Google Scholar]
- 79. Dong Y-S, Wang J-l, Feng D-Y, Qin H-Z, Wen H,Yin Z-M, Gao G-D, Li C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 2014:11(3):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ben Abdallah F, Zribi N, Ammar-Keskes L. Antioxidative potential of quercetin against hydrogen peroxide induced oxidative stress in spermatozoa in vitro. Andrologia. 2011:43(4):261–265. [DOI] [PubMed] [Google Scholar]
- 81. Kook D, Wolf A-H, Yu A-L, Neubauer A-S, Priglinger S-G, Kampik A, Welge-Lüssen U-C. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008:49(4):1712–1720. [DOI] [PubMed] [Google Scholar]
- 82. Singer M. Mitochondrial function. In: Hemodynamic monitoring. European Society of Intensive Care Medicine Springer; 2019. p. 97–106 [Google Scholar]
- 83. Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett. 2010:198(1):49–55. [DOI] [PubMed] [Google Scholar]
- 84. Dave A, Shukla F, Wala H, Pillai P. Mitochondrial electron transport chain complex dysfunction in MeCP2 knock-down astrocytes: protective effects of quercetin hydrate. J Mol Neurosci. 2019:67(1):16–27. [DOI] [PubMed] [Google Scholar]
- 85. Sandhir R, Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2013:1832(3):421–430. [DOI] [PubMed] [Google Scholar]
- 86. Anderson EJ, Katunga LA, Willis MS. Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin Exp Pharmacol Physiol. 2012:39(2):179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Al Maruf A, O'Brien P-J, Naserzadeh P, Fathian R, Salimi A, Pourahmad J. Methotrexate induced mitochondrial injury and cytochrome c release in rat liver hepatocytes. Drug Chem Toxicol. 2018:41(1):51–61. [DOI] [PubMed] [Google Scholar]
- 88. Zhang F, Xu Z, Gao J, Xu B, Deng Y. In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol. 2008:26(2):232–236. [DOI] [PubMed] [Google Scholar]
- 89. Feng Y, Wan M. Low intensity ultrasound induces apoptosis via MPT channel on mitochondrial membrane: target for regulating cancer therapy or not? In: AIP Conference Proceedings. China: AIP Publishing; 2017. [Google Scholar]
- 90. Zhou D, Wang H, Zhang J, Gao X, Zhao W, Zheng Y. Di-n-butyl phthalate (DBP) exposure induces oxidative damage in testes of adult rats. Syst Biol Reprod Med. 2010:56(6):413–419. [DOI] [PubMed] [Google Scholar]
- 91. Kaşıkcı MB, Bağdatlıoğlu N. Bioavailability of quercetin. Current research in nutrition and food science journal. In: Vol. 4(Special Issue Nutrition in Conference October 2016; 2016. p. 146–151. [Google Scholar]
- 92. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013:53(1):401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bolisetty S, Zarjou A, Agarwal A. Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis. 2017:69(4):531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Loboda A, Stachurska A, Podkalicka P, Sobczak M, Mucha O, Witalisz-Siepracka A, Jozkowicz A, Dulak J. Effect of heme oxygenase-1 on ochratoxin A-induced nephrotoxicity in mice. Int J Biochem Cell Biol. 2017:84(3):46–57. [DOI] [PubMed] [Google Scholar]
- 95. Park EJ, Kim Y-M, Park S-W, Kim H-J, Lee J-H, Lee D-U, Chang K-C. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem Toxicol. 2013:55(6):386–395. [DOI] [PubMed] [Google Scholar]
- 96. Ramyaa P, Padma VV. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochimica et Biophysica Acta (BBA)-General Subjects. 2014:1840(1):681–692. [DOI] [PubMed] [Google Scholar]
- 97. Kang C-H, Choi Y-H, Moon S-K, Kim W-J, Kim G-Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol. 2013:17(3):808–813. [DOI] [PubMed] [Google Scholar]
- 98. Panchal SK, Poudyal H, Brown L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J Nutr. 2012:142(6):1026–1032. [DOI] [PubMed] [Google Scholar]
- 99. Shih R-H, Wang C-Y, Yang C-M. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 2015:18(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cho YJ, Park SB, Han M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015:407(9):9–17. [DOI] [PubMed] [Google Scholar]
- 101. Chekalina N, Burmak Y, Petrov Y, Borisova Z, Manusha Y, Kazakov Y, Kaidashev I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018:70(5):593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Indra MR, Karyono S, Ratnawati R, Malik S-G. Quercetin suppresses inflammation by reducing ERK1/2 phosphorylation and NF kappa B activation in leptin-induced human umbilical vein endothelial cells (HUVECs). BMC Res Notes. 2013:6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen T, Zhang X, Zhu G, Liu H, Chen J, Wang Y, He X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine. 2020:99(38):e22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Granado-Serrano AB, Martín M-A, Bravo L, Goya L, Ramos S. Quercetin attenuates TNF-induced inflammation in hepatic cells by inhibiting the NF-κB pathway. Nutr Cancer. 2012:64(4):588–598. [DOI] [PubMed] [Google Scholar]
- 105. Albensi BC. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front Cell Dev Biol. 2019:7(7):154. [DOI] [PMC free article] [PubMed] [Google Scholar]