Abstract
Background:
To support innovative trial designs in a regulatory setting for pediatric-onset multiple sclerosis (MS), the study aimed to perform a systematic literature review and meta-analysis of relapse rates with interferon β (IFN β), fingolimod, and natalizumab and thereby demonstrate potential benefits of Bayesian and non-inferiority designs in this population.
Methods:
We conducted a literature search in MEDLINE and EMBASE from inception until 17 June 2020 of all studies reporting annualized relapse rates (ARR) in IFN β-, fingolimod-, or natalizumab-treated patients with pediatric-onset relapsing–remitting MS. These interventions were chosen because the literature was mainly available for these treatments, and they are currently used for the treatment of pediatric MS. Two researchers independently extracted data and assessed study quality using the Cochrane Effective Practice and Organization of Care – Quality Assessment Tool. The meta-analysis estimates were obtained by Bayesian random effects model. Data were summarized as ARR point estimates and 95% credible intervals.
Results:
We found 19 articles, including 2 randomized controlled trials. The baseline ARR reported was between 1.4 and 3.7. The meta-analysis-based ARR was significantly higher in IFN β-treated patients (0.69, 95% credible interval: 0.51–0.91) versus fingolimod (0.11, 0.04–0.27) and natalizumab (0.17, 0.09–0.31). Based on the meta-analysis results, an appropriate non-inferiority margin versus fingolimod could be in the range of 2.29–2.67 and for natalizumab 1.72–2.29 on the ARR ratio scale. A Bayesian design, which uses historical information for a fingolimod or natalizumab control arm, could reduce the sample size of a new trial by 18 or 14 patients, respectively.
Conclusion:
This meta-analysis provides evidence that relapse rates are considerably higher with IFNs versus fingolimod or natalizumab. The results support the use of innovative Bayesian or non-inferiority designs to avoid exposing patients to less effective comparators in trials and bringing new medications to patients more efficiently.
Keywords: annualized relapse rate, clinical trial design, fingolimod, interferon, natalizumab, pediatric-onset multiple sclerosis, systematic review
Introduction
Multiple sclerosis (MS), an autoimmune disorder of the central nervous system, is characterized by inflammation and neurodegeneration, leading to disability accumulation. 1 The onset of disease is generally observed in adulthood (age 20–40 years); however, the global incidence (0.05–2.85 per 100,000 children) and prevalence (0.69–26.92 per 100,000 children) rates in pediatric-onset MS have been on the rise. 2
Although the underlying pathology is similar to adult-onset MS, pediatric-onset MS is associated with higher relapse rates and radiological activity. 3 Compared with adult-onset patients, children with pediatric-onset MS reach the secondary progressive disease stage and manifest prominent levels of cognitive impairment at younger age, leading to longer time lived with disability. 4 Furthermore, the symptoms of depression and fatigue impact the daily functioning and psychosocial well-being of children at school. 5 These symptoms may be associated with poor academic performance, social interactions, behavioral aspects, and quality of life of the child during the key formative years of development, impacting the attainment of educational and career milestones. 6
Historically, most pediatric MS patients have been treated with first-line injectable therapies – interferon (IFN) β and glatiramer acetate – as evidenced by the data available from retrospective and observational studies. 7 The efficacy of these treatments has never been demonstrated in a controlled clinical trial in pediatric patients, and up to 30% of the treated patients experience breakthrough disease activity and require disease-modifying therapies (DMTs) that have demonstrated higher efficacy in adult patients. 8 The available literature for DMTs in pediatric MS is mainly for IFN β, fingolimod, natalizumab, or teriflunomide, which are largely based on the observational or retrospective studies except the phase III randomized, double-blind clinical trials – PARADIGMS in fingolimod 9 and TERIKIDS in teriflunomide. 10 Fingolimod is a sphingosine-1-phosphate receptor modulator and demonstrated superior reduction in annualized relapse rate (ARR) and magnetic resonance imaging activity versus IFN β in patients with MS, including pediatric-onset MS.4,9,11 Another treatment, natalizumab, is an anti-very late antigen-4, humanized monoclonal antibody which has been shown to be effective in adult MS, including pediatric-onset MS patients with highly active MS based on observational/retrospective studies;12–16 however, there are no pediatric data available from phase III randomized controlled trials. Recently, TERIKIDS study in 166 relapsing MS patients (aged 10–17 years) investigating efficacy of safety of teriflunomide, a dihydroorotate dehydrogenase inhibitor, 17 did not meet its primary endpoint (reduction in relapse rate: 34% vs placebo, i.e. rate ratio of 0.66 [95% confidence interval from 0.39 to 1.11], p = 0.29; NCT02201108). 10 In addition to the above studies, several clinical trials in pediatric MS are ongoing to determine the effect of DMTs in pediatric-onset MS, including LemKids (NCT03368664) for alemtuzumab, CONNECT (NCT02283853) for dimethyl fumarate, and an open-label phase II study (NCT04075266) for ocrelizumab. Overall, there remains a strong medical need for phase III trials for new treatment options in pediatric MS with proven efficacy based on controlled clinical trials.
Current regulations by the US Food and Drug Administration (FDA) and European Union require that any new product or drug developed for the treatment of MS in the adult population must also be investigated for efficacy and safety in the pediatric patient population. 18 Several planned or ongoing trials in pediatric MS patients compete for a small pool of available patients, which causes feasibility constraints. 19 Moreover, the testing of new drugs in classical randomized controlled clinical trials poses ethical and feasibility challenges and calls for more innovative trial designs. 20 At the start of a new trial in pediatric participants, data from adult patients are often available. As biology of the disease is similar between adult- and pediatric-onset MS, a new trial in pediatric patients could make use of the data from adult patients, which is often available, in a Bayesian framework to reduce sample size requirements and gain efficiency for a pediatric trial. In addition, it would be desirable to minimize the use of placebo and low-efficacy treatment control groups to reduce the risk of relapse. One way to achieve this is to demonstrate that the relapse rates are equally low on treatment with a new drug as on a concurrent efficacious control and distinctly lower than those historically reported in untreated or IFN β-treated patients.
To support innovative trial designs in a regulatory setting for patients with pediatric-onset MS, this study aimed to (1) perform a systematic literature review and meta-analysis of studies investigating relapse rates in pediatric MS patients treated with IFN β, fingolimod, or natalizumab and (2) demonstrate potential benefits of Bayesian and non-inferiority designs in this population using the meta-analysis results.
Methods
Study population and intervention selection
For a study to be considered for inclusion, the study population and outcomes had to be typical of pediatric-onset MS or at least informative for a new regulatory study in pediatric MS. For instance, studies conducted in special populations, such as patients with highly active MS or children below the age of 10 years, or with unusual endpoint definitions were excluded because such studies would not be informative in the context of a new regulatory trial. Interventions considered in the literature search were in line with the regulatory context and included (1) fingolimod because of its proven efficacy and safety in a randomized controlled phase III study; (2) IFN β because it is the de facto standard of care among platform therapies and has been used as a comparator to demonstrate efficacy against new treatment as per regulatory guidelines (intramuscular IFN β-1a was tested in a limited number of pediatric patients 21 ); and (3) natalizumab because of its high efficacy in adult MS patients and its de facto use in pediatric patients. Although natalizumab is not approved yet in pediatric patients, it was considered informative to summarize relapse rates as a potential alternative control treatment in a non-inferiority design.
Data sources and literature search strategy
A systematic review and a meta-analysis of studies were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 22 We conducted a literature search of all studies in MEDLINE and EMBASE through the Ovid platform from inception to 17 June 2020, reporting relapse rates in patients with pediatric-onset relapsing–remitting MS. Search terms included MS AND (adolescents OR children OR juvenile OR pediatric OR early onset) AND (interferon OR betaseron OR betaferon OR Avonex OR Rebif OR fingolimod OR Gilenya OR natalizumab OR Tysabri OR placebo OR untreated OR no treatment).
Selection criteria
Eligible records were screened first by title and abstract, and those that did not meet the search criteria, such as case reports, conference material, editorials/letters, short surveys, or review papers, were excluded. The remaining publications were excluded if they did not report any information about relapses (e.g. ARR, total number of relapses, or patient-level information about relapses). An overview of the inclusion criteria for the systematic search is summarized in Table S1.
Data extraction
The literature search was conducted by a researcher (A.S.) based on the described search methodology. All studies that were retrieved from the databases were evaluated for study design, patient population, intervention, and outcomes. Titles and abstracts at the first stage and full articles at the second stage were evaluated by two researchers (M.T. and A.S.) independently to determine their potential relevance. The included studies were validated and clinically confirmed by another researcher (J.L.). Any disagreements or discrepancies were resolved through joint article review and discussion.
Quality assessment
The level of evidence and quality assessment were evaluated using the Cochrane Effective Practice and Organization of Care – Quality Assessment Tool for the included studies. 23 In this analysis, we assigned total scores of ⩽ 4, 5–7, and 8–10 for low, moderate, and high quality of studies, respectively.
Outcomes
The ARR, defined as the mean number of confirmed relapses per patient per year, that is, adjusted for the individual follow-up time, was assessed in this study. ‘Relapse’ was commonly defined as ‘new or worsening symptoms that last 24 h, occurring in the absence of fever or infection’. 24 Definitions of ‘relapse’ varied across pediatric studies that used the Poser criteria, 25 modified McDonald criteria,26–28 IPMSSG criteria,29,30 and/or Lublin criteria 31 and are summarized in Table S2. Uncertainty in ARR estimates was captured based on confidence interval (CI) or standard error (SE) if these were available. However, not all studies reported measures of uncertainty (in the form of either CI or SE) for the ARRs.
Data analyses
Meta-analysis
Meta-analysis estimates were obtained using a Bayesian random effects model fitted to the log ARRs and SEs from individual studies, under the assumption of equal between-trial variability for all treatments. If the SE (or CI) was not available from the individual study, the values were calculated based on the reported relapse rates and follow-up times, with assumption of similar over-dispersion of relapses as in the PARADIGMS study. 9 For a study reporting zero relapses, 32 a non-zero ARR estimate derived from a Bayesian negative binomial model 33 was used in the meta-analysis.
For the meta-analysis, weakly informative normal (0, 5) priors were used for the population means for each treatment. For the between-trial standard deviation, a half-normal prior with a scale parameter of 0.5 was used. 34 Results were summarized in forest plots showing ARR point estimates and 95% credible intervals for individual studies and meta-analysis estimates. Heterogeneity across the studies was assessed by the estimated between-trial variability parameter in the model.
Sensitivity analyses were performed to assess the robustness of the meta-analysis results to the model assumptions and priors. These analyses included fitting the model, assuming different between-trial variability for IFN β versus natalizumab and fingolimod, and a less informative prior scale for the between-trial standard deviation. In addition, the meta-analysis was repeated without some outlier studies that reported ARRs > 1 for IFN β.
To supplement the data from the literature review, individual patient data from adult fingolimod phase III studies (24-month FTY720 Research Evaluating Effects of Daily Oral therapy in MS (FREEDOMS; NCT00289978), 35 FREEDOMS II (NCT00355134), 36 and 12-month Trial Assessing Injectable Interferon versus FTY720 Oral in Relapsing–Remitting MS (TRANSFORMS; NCT00340834) 37 ) were used in a supplementary analysis to obtain extrapolated relapse rate estimates in children on fingolimod and IFNs using negative binomial models adjusting for age and baseline number of relapses on individual data. All analyses were performed using R version 3.6.1 (R Core Team, Vienna, Austria). 38
Informing future innovative trial designs
Based on historical data from meta-analysis results, features of innovative designs for future pediatric MS studies were explored. These included non-inferiority trials versus highly effective treatments (which would show superiority over placebo or low-efficacy treatments indirectly based on historical control data) and Bayesian designs using the historical information (e.g. based on completed trials for the same medication) to reduce the number of patients required in the new study. 39
To explore the possibility of the use of completed adults study data to inform the newly planned pediatric trial, a supplementary meta-analysis, including adult studies, was performed. Non-inferiority margins for a non-inferiority study of a new test drug versus fingolimod or natalizumab were derived using the inverse of the upper bound of the credible intervals for the ARR ratios of fingolimod/natalizumab versus IFN β. Showing non-inferiority of a new test drug versus a concurrent fingolimod or natalizumab control using these margins would guarantee superior efficacy over IFN β (and placebo). In addition, the meta-analytic predictive (MAP) approach 40 was used to obtain prior distributions for the ARR in a new pediatric study with a Bayesian design. Effective sample size (ESS) can be used to quantify the information included in the priors and represents the number of patients by which the sample size for a trial can be reduced, when using MAP-priors for the control arm. The ESS for MAP priors was calculated using the expected local information ratio approach proposed by Neuenschwander et al. 41
Data availability statement
Anonymized data that support the findings of this study will be made available to qualified external researchers, with requests reviewed and approved by an independent review panel on the basis of scientific merit.
Results
Identified studies
A total of 1751 studies were identified during the initial database search (Figure 1). After removing the duplicates (n = 264) and screening based on titles and abstracts, full texts of 82 studies were considered. Of these, 19 studies met the inclusion criteria as defined in Table S1. The average study duration follow-up ranged from 0.72 to 6.7 years. Among these studies, 2 were randomized controlled clinical trials in patients with pediatric MS, while 17 were observational studies. An overview of 19 studies summarizing the study design, patient population, and treatment interventions is presented in Table S2.
Figure 1.
PRISMA diagram for study selection.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Patient disposition and baseline characteristics
The key demographic and baseline characteristics of patients from the 19 studies that met the selection criteria are presented in Table 1. The mean age of the patients ranged from 11.0 to 16.7 years across studies. Approximately 44%–85% of patients were female. The mean disease duration ranged from 0.4 to 5.1 years. The ARR reported at baseline before observation was between 1.4 and 3.7 when available. In most studies (11/19), patients were treatment-naïve at study entry. In one study, the presence or absence of prior treatment was not reported, and in the remaining seven studies, 37%–100% of patients had received prior MS treatment.
Table 1.
Patient demographics and baseline characteristics of trial populations.
| Study author (Study acronym) |
Treatment (Sample size, N) |
Mean age ± SD (and/or range) (years) | Female (n, %) | Mean ± SD disease duration (and/or range) (years) | Mean ARR ± SD (and/or range) | Prior treatment (n, %) |
|---|---|---|---|---|---|---|
| Randomized controlled clinical trials | ||||||
| Chitnis et al.
9
(PARADIGMS) |
Fingolimod (107)IFN β (107) | 15.3 ± 1.8 | 134 (62) | 2.1 ± 1.9 (0.2–10.9) | 1.2 ± 1.4 (0–4.5) | 79 (37) |
| Pakdaman et al. 42 | IFN β (8)No treatment (8) | 12.9 | 10 (63) | 1.72 | 1.42 | 0 (0) |
| Non-randomized controlled clinical trials | ||||||
| Margoni et al. 32 | Natalizumab (20) | 14.2 ± 2.5 | 13 (65) | 0.5 ± 0.33 | 2.1 ± 0.3 | 20 (100) |
| Huppke et al. 43 | IFN β (249) | 13.7 ± 2.7 | N/R | 1 | N/R | 0 (0) |
| GA (51) | ||||||
| Fragomeni et al. 44 | IFN β (45) | 15.0 (4.5–17.9) | N/R | N/R | N/R | 0 (0) |
| GA (15) | ||||||
| Ben Achour et al. 45 | IFN β (17) | 11 (3–17) | 13 (76) | N/R | 2.0 (0.25–4) | 0 (0) |
| Gärtner et al.
46
(BETAPAEDIC) |
IFN β (65) | 14.2 ± 1.3 | 50 (77) | 0.4 ± 1.4 (0–9.2) | 2.2 ± 1.4 | 0 (0) |
| Alroughani et al. 12 | Natalizumab (32) | 15.7 ± 1.9 (8–17) | 23 (72) | 5.1 ± 3.1 (1–11) | 1.66 ± 0.5 | 21 (66) |
| Fragoso et al. 47 | Fingolimod (17) | 16.1 (14–17) | 10 (59) | N/R | 2.8 (0–8) | 13 (76) |
| Ghezzi et al. 15 | Natalizumab (101) | 14.7 ± 2.4 | 69 (68) | 2.1 ± 1.94 | 2.3 ± 1.3 | 66 (65) |
| Arnal-Garcia et al. 13 | Natalizumab (9) | 15.3 (9.8–17.7) | 4 (44) | 4.0 (2–7) | 3.0 (1–8) | 8 (89) |
| Kornek et al. 14 | Natalizumab (20) | 16.7 ± 1.1 | 16 (80) | 1.5 ± 0.33 | 3.7 | 19 (95) |
| Tenembaum et al. 48 | IFN β (307) | 14.0 ± 3.0 | 190 (62) | 0.8 | 1.79 | 0 (0) |
| Basiri et al. 49 | IFN β (13) | 14.7 ± 1.9 | 11 (85) | N/R | N/R | N/R |
| Ghezzi et al. 50 | IFN β (Avonex®) (77) | 11.4 ± 3.1 | 47 (61) | 1.94 ± 1.33 | 2.5 ± 1.9 | 0 (0) |
| Ghezzi et al. 50 | IFN β (Rebif®/Betaferon®) (39) | 12.6 ± 2.6 | 25 (64) | 1.6 ± 1.43 | 3.2 ± 2.5 | 0 (0) |
| Tenembaum et al. 51 | IFN β (19) | 15.9 (11.3–17.9) | N/R | 3.4 (0.3–13.9) | 1.8 (1–3) | 0 (0) |
| Pohl et al. 52 | IFN β (51) | 14.6 (8.1–17.9) | 36 (71) | 2.0 (0.1–6.7) | 1.9 (0.4–7.6) | 0 (0) |
| Waubant et al. 53 | IFN β (9) | 12.7 (8–15) | 7 (78) | N/R | 1.4 | 0 (0) |
ARR, annualized relapse rate; GA, glatiramer acetate; IFN, interferon; N/R, not reported; SD, standard deviation.
ARR on different DMTs
The ARR reported in the individual publications for 19 studies are summarized in Table 2.
Table 2.
ARR in pediatric patients with MS.
| Study | Treatment group (N) |
Comparator group (N) |
ARR (treatment group) (95% CI) |
ARR (comparator group) (95% CI) |
|---|---|---|---|---|
| I. Randomized controlled clinical trials | ||||
| Chitnis et al.
9
(PARADIGMS) |
Fingolimod (107) | IFNs (107) | 0.12 (0.08–0.19) | 0.67 (0.52–0.89) |
| Pakdaman et al. 42 | IFN β (8) | No treatment (8) |
0.59 (0.38–0.93) a | 1.09 (N/R) a |
| II. Non-randomized controlled clinical trials | ||||
| Margoni et al. 32 | Natalizumab (20) | – | 0.0 (N/R) | – |
| Huppke et al. 43 | IFN β (249) | GA (51) |
0.79 (0.70–0.90) | 0.89 (0.7–1.1) |
| Fragomeni et al. 44 | IFN β (45; 32 on high dose, 13 on low dose) | GA (15) |
1.38 (N/R) on high dose 1.24 (N/R) on low dose |
0.53 (N/R) |
| Alroughani et al. 12 | Natalizumab (32) | – | 0.06 (0.01–0.25) b | – |
| Ben Achour et al. 45 | IFN β (17) | – | 0.42 (N/R) | – |
| Gärtner et al.
46
(BETAPAEDIC) |
IFN β (65) | – | 0.70 (0.51–0.96) b | – |
| Fragoso et al. 47 | Fingolimod (17) | – | 0.08 (N/R) | – |
| Ghezzi et al. 15 | Natalizumab (101) | – | 0.10 (0.06–0.18) b | |
| Arnal-Garcia et al. 13 | Natalizumab (9) | – | 0.38 (N/R) | – |
| Kornek et al. 14 | Natalizumab (20) | – | 0.40 (N/R) | – |
| Tenembaum et al. 48 | IFN β (307) | – | 0.47 (N/R) | – |
| Basiri et al. 49 | IFN β (13) | – | 0.41 (0.15–1.09) a | – |
| Ghezzi et al. 50 | IFN β (Avonex) (77) |
– | 0.40 (0.32–0.49) b | – |
| Ghezzi et al. 50 | IFN β (Rebif/Betaferon) (39) |
– | 0.90 (0.63–1.3) b | |
| Tenembaum et al. 51 | IFN β (19) | – | 0.21 (N/R) | – |
| Pohl et al. 52 | IFN β (51) | – | 0.80 (N/R) | – |
| Waubant et al. 53 | IFN β (9) | – | 1.60 (N/R) a | – |
ARR, annualized relapse rate; CI, confidence interval; GA, glatiramer acetate; IFN, interferon; MS, multiple sclerosis; N/R, not reported.
Avonex® (intramuscular IFN β-1a (Biogen Netherlands BV, Badhoevedorp, Netherlands)). Betaferon® (IFN β-1b (Bayer AG, Leverkusen, Germany)). Rebif® (subcutaneous IFN β-1a (Merck Europe BV, Amsterdam, Netherlands)). Betaseron® (IFN β-1b (Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA)).
ARR calculated from reported individual patient data.
CI was derived from reported standard deviation/standard error.
In the randomized controlled trials (n = 2), ARR with IFN β was 0.679 9 and 0.5942 42 (Table 2). In the remaining trials of varying study designs (n = 11), ARR ranged from 0.21 to 1.6 in the IFN β group. The ARR with fingolimod was 0.12 in the randomized trial of PARADIGMS9 and 0.08 in the non-randomized trial based on a Brazilian database. 44 In non-randomized studies (n = 5), ARR with natalizumab treatment ranged from 0.00 to 0.4.
Meta-analysis
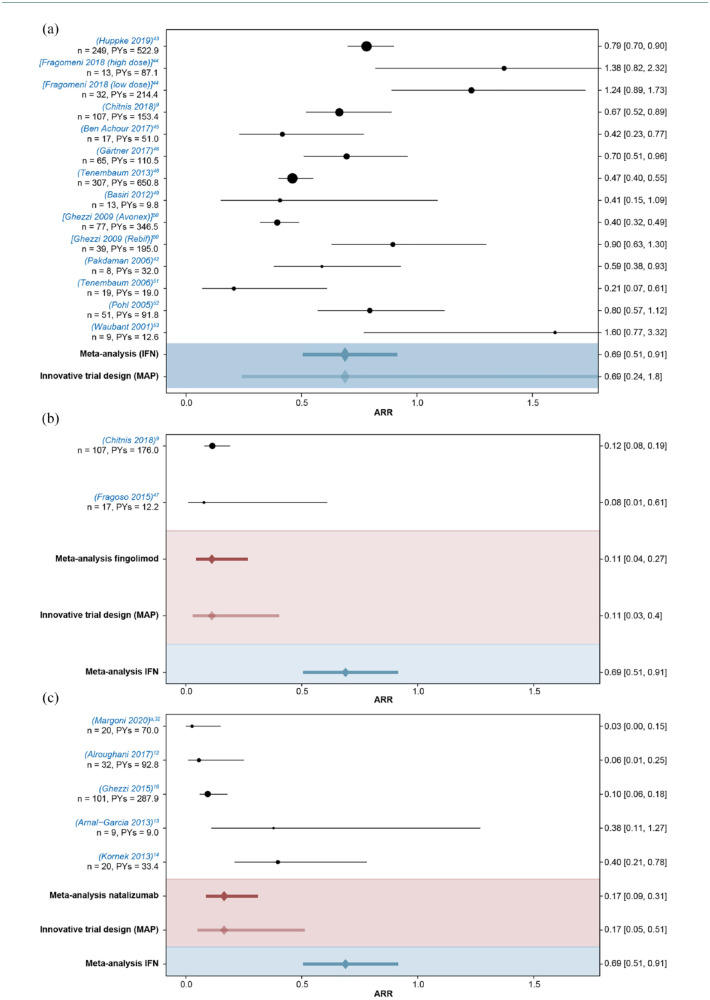
The results of the meta-analysis based on the literature review of 19 studies (IFN β, 12; fingolimod, 2; and natalizumab, 5) are depicted in Figure 2. These studies were considered informative for a new trial in pediatric MS. The combined estimate for the ARR (95% credible interval) in IFN β-treated patients was 0.69 (0.51–0.91) across all studies versus 0.11 (0.04–0.27) with fingolimod and 0.17 (0.09–0.31) with natalizumab. ARRs reported in the fingolimod and natalizumab studies were lower than all ARRs reported in the IFN β studies, with the exception of one IFN β study. 51 The between-trial standard deviation on the log-ARR scale was estimated to be 0.45 (0.27–0.72), indicating considerable between-trial heterogeneity.
Figure 2.
Meta-analysis of ARRs (95% CI) reported in pediatric patients with MS. (a) IFN β studies, (b) fingolimod studies, and (c) natalizumab studies. Point sizes of individual studies are proportional to the sample size. Meta-analysis is obtained using Bayesian random effects model for the log ARRs.
ARR, annualized relapse rate; CI, confidence interval; IFN, interferon; MAP, meta-analytic predictive; Meta, meta-analysis; MS, multiple sclerosis; PYs, patient-years.
aReported ARR for this study was 0. ARR estimate and interval given here are based on a Bayesian negative binomial model to allow inclusion into the meta-analysis.
For all sensitivity analyses conducted, results were consistent with the main analysis and showed no overlap in the estimated ARR credible intervals between IFNs and fingolimod/natalizumab (Table S3).
Innovative trial designs
The results of the meta-analysis, in particular the high relapse rates with IFNs compared with the higher efficacy therapies, justify the use of innovative trial designs to avoid unnecessary risk of relapse during the trial. Possible innovative design options we would like to highlight here include non-inferiority designs versus an effective treatment (e.g. fingolimod), where the objective of the trial is to indirectly demonstrate superiority to IFN β by showing that a new treatment is non-inferior (i.e. not worse in terms of relapse rate) to an established effective treatment within a prespecified margin. This margin can be chosen based on historical data so that superiority over IFN β is guaranteed when the objective of non-inferiority is reached. A second option is the use of a Bayesian trial design, where historical data on the control treatment can be directly incorporated in the form of priors. This would then allow for comparison against an IFN β to show superiority and for reduction in the number of patients required for a new study (as some information is already included in the priors).
When using historical data to inform non-inferiority margins for a new trial or for a Bayesian prior, all relevant data should be considered. In the pediatric setting, this includes data from adult patients, if accurate extrapolation of data from adults to children is possible. 54 In MS, it is possible to extrapolate adult data to children. 55 Therefore, in the setting of pediatric MS relevant, data from adult studies should be included when informing non-inferiority margins or forming Bayesian priors for a new trial.
Based on the estimated ARRs from the supplementary analysis, including the adult studies, approximately five times lower relapse rates were observed with fingolimod treatment compared with IFN β in children (Figure S1). The ARR ratio (95% credible interval) of fingolimod versus IFN β in children was estimated to be 0.21 (0.12–0.37). Using the upper bound of the 95% interval as a conservative estimate of the treatment effect and taking the inverse of this, a non-inferiority margin 2.67 versus fingolimod could ensure indirectly superiority over IFN β. Thus, if the ARR ratio of the investigational treatment versus fingolimod in a new trial would be smaller than 2.67, the treatment could be considered non-inferior to fingolimod and superior to interferons. Taking into account that the results are mostly based on observational data and the large between-trial variability, a smaller non-inferiority margin using the upper bound of the 99% interval could be recommended, which is approximately 2.29. Therefore, based on the historical data used for the meta-analysis, margins in the range from 2.29 to 2.67 on the ARR ratio scale could be considered to ensure indirectly superiority over IFNs. Similarly, a range of possible non-inferiority margins for natalizumab would be 1.72–2.29 on the ARR ratio scale.
To directly use the historical information on ARRs from a meta-analysis in a new study, MAP39,40 priors can be used to represent the range of values in the ARR that could be expected on the control treatment in the new study. A Bayesian design for a new study incorporates the historical information through the MAP priors and reduces the number of patients required in the control arm or even allows comparison only against historical data. Based on our meta-analysis, including adult studies, MAP prior estimates (95% credible interval; Table S3) would be 0.68 (0.27–1.66) for IFN β, 0.15 (0.06–0.38) for fingolimod, and 0.17 (0.06–0.47) for natalizumab. For a new 2-year study with these priors, the ESS would be 7 for IFN β, 18 for fingolimod, and 14 for natalizumab.
Discussion
This meta-analysis summarizes our knowledge of relapse rates in pediatric patients treated with IFNs, fingolimod, and natalizumab using currently available information and can inform new trials in pediatric MS. Pediatric patients treated with first-line IFN β had an ARR of 0.69 (95% CI: 0.51–0.91), which is an average of more than one relapse every other year for patients under treatment. The relapse rates were approximately fivefold higher than those observed in pediatric patients treated with either fingolimod or natalizumab, which raises concerns on the use of IFN β as a comparator in pediatric participants in future trials.
In this meta-analysis, fingolimod reduced the ARR by 83% (95% credible interval 55%–94%) and natalizumab by 76% (95% CI: 54–89) versus IFN β in pediatric-onset MS. The ARR reduction reported in this meta-analysis for fingolimod versus IFN β-1a (83%) is in line with that of the pediatric PARADIGMS study, which showed a reduction of 82% (95% CI: 0.11–0.30) in the relapse rate. 9 Similarly, results of natalizumab were in line with those of previous observational studies.12–16
Regulatory phase III studies in pediatric MS are typically conducted in patients aged between 10 and 18 years; for patients younger than 10 years, a waiver is usually granted based on the rarity of the disease at that age. In the targeted age group, the biology of pediatric MS is similar to that of adult MS patients, except for a higher level of inflammatory disease activity in the pediatric-onset patients. It is therefore possible to use an age-dependent extrapolation of relapse rates from adult patients to pediatric patients. Likewise, previously, data of adult-onset MS patients from the TRANSFORMS trial were extrapolated to pediatric patients in PARADIGMS by modeling relapse rates as a function of the patient’s age; this provided highly accurate predictions of the actual PARADIGMS results. 55 Similarly, comparing between the main analysis and the sensitivity analysis in this meta-analysis, extrapolated results from adults36,37 were in line with those obtained from pediatric patients. This is relevant for the planning of new trials in pediatric MS, as it demonstrates that extrapolation of data from adult MS trials (which are typically available at the time of the launch of a pediatric study) can in principle be used to inform a new study and help to reduce the overall sample size or the size of the control arm.
Furthermore, the findings from this systematic review suggest that it is possible to design new, feasible studies in pediatric MS that use efficacious active control treatment. This corroborates with recommendations from the International Pediatric Multiple Sclerosis Study Group that suggest ensuring high-quality evidence-based treatment for children and adolescents with MS. 20
The meta-analysis provides insight on how non-inferiority margins or Bayesian priors could be used to inform designs for future pediatric MS studies based on the historical data and adult phase III studies. A recent example of a study design in pediatric MS making use of the innovative elements proposed here is the phase III NEOS study comparing ofatumumab and siponimod with fingolimod (NCT04926818). NEOS uses innovative design elements by incorporating historical data to define a non-inferiority margin versus fingolimod and also robustly borrows information from historical studies in adults and children for the analysis using MAP priors, avoiding the use of placebo or IFN comparator and optimizing sample size. The innovative design elements for this study have been discussed and agreed with the FDA and the European Committee for Medicinal Products for Human Use (CHMP), and the study started recruitment of patients in 2021.
Limitations: Most studies in pediatric-onset MS are observational. The literature review and the subsequent meta-analysis revealed high between-study heterogeneity in the ARRs reported across the published studies. Although meta-regression using baseline variables (age, ARR, disease duration, and percentage of treatment-naïve patients) on the available information reveals no large variability, there could be variability resulting from (partly unknown) differences in study design, patient population, treatments, and ‘relapse’ definition.
Our analysis also calculated non-inferiority margins and MAP priors for natalizumab, even though natalizumab is not an approved treatment in pediatric MS. The demonstration of non-inferiority to natalizumab could theoretically be considered an adequate proof of efficacy of a new therapy; however, the acceptability and practicability of such an approach would need to be evaluated with regulatory agencies and ethical bodies before initiating such a study.
Our literature review and meta-analyses focus on efficacy with ARR as the typical primary endpoint in MS and do not consider safety endpoints. While safety is naturally also of concern in pediatric trials, due to the limited sample size, it is generally possible to only detect strong imbalances in common adverse events, irrespective of the design of the trial. It would not be feasible to design a pediatric MS trial to detect possibly rare safety signals. Data from larger adult trials, which will typically be available at the time of pediatric development, and real-world data collected after approval can be used to assess safety topics on a more granular level.
Conclusion
Our meta-analysis suggests that relapse rates are approximately fivefold higher with IFNs than with fingolimod or natalizumab. Thus, innovative trials will improve the feasibility of recruitment and avoid the use of low-efficacy treatments as comparators in pediatric studies. Based on our results, novel trial design in pediatric patients can reduce the number of required patients using Bayesian designs or employing non-inferiority designs, which will help to establish superiority by indirect comparison to historical IFN β data.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211070449 for Improving pediatric multiple sclerosis interventional phase III study design: a meta-analysis by Jennifer S. Graves, Marius Thomas, Jun Li, Anuja R. Shah, Alexandra Goodyear, Markus R. Lange, Heinz Schmidli, Dieter A. Häring, Tim Friede and Jutta Gärtner in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864211070449 for Improving pediatric multiple sclerosis interventional phase III study design: a meta-analysis by Jennifer S. Graves, Marius Thomas, Jun Li, Anuja R. Shah, Alexandra Goodyear, Markus R. Lange, Heinz Schmidli, Dieter A. Häring, Tim Friede and Jutta Gärtner in Therapeutic Advances in Neurological Disorders
Acknowledgments
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, have critically reviewed the article for intellectual content, and have given final approval for the version to be published. All authors are responsible for intellectual content and data accuracy. The authors thank Gillipsie Minhas (Novartis Healthcare Ltd, Hyderabad, India) for providing technical assistance for submission of the article, under the direction of the authors. The article development and submission were supported by Novartis.
Footnotes
Author contributions: J.S.G. contributed to the study concept, design, data acquisition, and outline review and critical revision of the article; M.T. contributed to the study concept, design, execution, data acquisition, analysis and interpretation, outline review and critical revision of the article, and statistical analysis; J.L. contributed to the study concept, design, execution, interpretation of results, and outline review and critical revision of the article; A.S. contributed to the systematic literature search, analysis and interpretation, and article development, including revising and finalizing the draft for submission; A.G. contributed to the study concept, design, execution, data acquisition, interpretation of results, and outline review and critical revision of the article; M.R.L. contributed to the analysis and interpretation and the critical revision of the article and statistical analysis; H.S. contributed to the analysis and interpretation, critical revision of the article, and statistical analysis; D.A.H. contributed to the study concept, design, execution, analysis and interpretation, outline review and the critical revision of the article, and statistical analysis; T.F. contributed to the analysis and interpretation, outline review and the critical revision of the article, and statistical analysis; J.G. contributed to the study concept, design, data acquisition, and outline review and critical revision of the article. All authors approved the final article as submitted and agree to be accountable for all aspects of the work.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.S.G. over the past year received grant/contract research support from the National Multiple Sclerosis Society, Biogen, and Octave Biosciences; she serves on a steering committee for a trial supported by Novartis; she has received honoraria for a non-promotional, educational activity for Sanofi-Genzyme; she has received speaker fees from Alexion and Bristol Myers Squibb (BMS) and served on an advisory board for Genentech. M.T. and J.L. are the employees of Novartis Pharma AG. A.S. is an employee of Novartis Healthcare Pvt. Ltd. A.G. was an employee of Novartis at the time of article development. M.R.L., H.S., and D.A.H. are the employees of Novartis Pharma AG. T.F. reports personnel fees for consultancies (including data monitoring committees and steering committees) from Bayer, Biosense Webster, Boehringer Ingelheim, Coherex Medical, CSL Behring, Daiichi Sankyo, Fresenius Kabi, Galapagos, Janssen, LivaNova, Novartis, Penumbra, Roche, and Vifor. J.G. reports consultant fees for research, lectures, and advisory boards from Bayer, Biogen, Novartis, Sanofi, and Teva; she also received financial support for a research project from Novartis.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study sponsor (Novartis Pharma AG, Basel, Switzerland) participated in the design and conduct of the study, data collection, data management, data analysis, and data interpretation; in the preparation, review, and approval of the article and writing of the report; and in the decision to submit the paper for publication. All authors had full access to all study data and had final responsibility for the decision to submit for publication. The article processing charges for this publication were funded by Novartis Pharma AG, Basel, Switzerland.
Ethical statement: Our study did not require an ethical board approval because it is a systemic review and meta-analysis of studies identified through literature search. The authors acknowledge the patients, investigators, and staff at participating sites who participated in the trials that are included in the meta-analysis study.
ORCID iDs: Marius Thomas  https://orcid.org/0000-0003-2790-829X
https://orcid.org/0000-0003-2790-829X
Tim Friede  https://orcid.org/0000-0001-5347-7441
https://orcid.org/0000-0001-5347-7441
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jennifer S. Graves, Department of Neurosciences, University of California, San Diego, Box 0662 ACTRI, 9452 Medical Center Drive, Suite 4W-222, San Diego, CA 92037, USA.
Marius Thomas, Novartis Pharma AG, Basel, Switzerland.
Jun Li, Novartis Pharma AG, Basel, Switzerland.
Anuja R. Shah, Novartis Healthcare Pvt. Ltd., Hyderabad, India
Alexandra Goodyear, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA at the time of article development.
Markus R. Lange, Novartis Pharma AG, Basel, Switzerland
Heinz Schmidli, Novartis Pharma AG, Basel, Switzerland.
Dieter A. Häring, Novartis Pharma AG, Basel, Switzerland
Tim Friede, Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany.
Jutta Gärtner, Department of Pediatrics and Adolescent Medicine, German Center for Multiple Sclerosis in Childhood and Adolescence, University Medical Center Göttingen, Göttingen, Germany.
References
- 1. Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opin Neurol 2018; 31: 752–759. [DOI] [PubMed] [Google Scholar]
- 2. Jeong A, Oleske DM, Holman J. Epidemiology of pediatric-onset multiple sclerosis: a systematic review of the literature. J Child Neurol 2019; 34: 705–712. [DOI] [PubMed] [Google Scholar]
- 3. Fisher KS, Cuascut FX, Rivera VM, et al. Current advances in pediatric onset multiple sclerosis. Biomedicines 2020; 8: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macaron G, Feng J, Moodley M, et al. Newer treatment approaches in pediatric-onset multiple sclerosis. Curr Treat Options Neurol 2019; 21: 50. [DOI] [PubMed] [Google Scholar]
- 5. Storm Van’s Gravesande K, Blaschek A, Calabrese P, et al. Fatigue and depression predict health-related quality of life in patients with pediatric-onset multiple sclerosis. Mult Scler Relat Disord 2019; 36: 101368. [DOI] [PubMed] [Google Scholar]
- 6. McKay KA, Ernstsson O, Manouchehrinia A, et al. Determinants of quality of life in pediatric- and adult-onset multiple sclerosis. Neurology 2020; 94: e932–e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghezzi A, Amato MP, Makhani N, et al. Pediatric multiple sclerosis: conventional first-line treatment and general management. Neurology 2016; 87: S97–S102. [DOI] [PubMed] [Google Scholar]
- 8. Chitnis T, Ghezzi A, Bajer-Kornek B, et al. Pediatric multiple sclerosis: escalation and emerging treatments. Neurology 2016; 87: S103–S109. [DOI] [PubMed] [Google Scholar]
- 9. Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 2018; 379: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 10. Chitnis T, Banwell B, Kappos L, et al. Safety and efficacy of teriflunomide in paediatric multiple sclerosis (TERIKIDS): a multicentre, double-blind, phase 3, randomised, placebo-controlled trial. Lancet Neurol 2021; 12: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 11. Deiva K, Huppke P, Banwell B, et al. Consistent control of disease activity with fingolimod versus IFN beta-1a in paediatric-onset multiple sclerosis: further insights from PARADIGMS. J Neurol Neurosurg Psychiatry 2020; 91: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alroughani R, Ahmed SF, Behbehani R, et al. The use of natalizumab in pediatric patients with active relapsing multiple sclerosis: a prospective study. Pediatr Neurol 2017; 70: 56–60. [DOI] [PubMed] [Google Scholar]
- 13. Arnal-Garcia C, Garcia-Montero MR, Malaga I, et al. Natalizumab use in pediatric patients with relapsing-remitting multiple sclerosis. Eur J Paediatr Neurol 2013; 17: 50–54. [DOI] [PubMed] [Google Scholar]
- 14. Kornek B, Aboul-Enein F, Rostasy K, et al. Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol 2013; 70: 469–475. [DOI] [PubMed] [Google Scholar]
- 15. Ghezzi A, Pozzilli C, Grimaldi LM, et al. Natalizumab in pediatric multiple sclerosis: results of a cohort of 55 cases. Mult Scler 2013; 19: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 16. Ghezzi A, Moiola L, Pozzilli C, et al. Natalizumab in the pediatric MS population: results of the Italian registry. BMC Neurol 2015; 15: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 18. Rose K. The challenges of pediatric drug development. Curr Ther Res Clin Exp 2019; 90: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose K, Muller T. Children with multiple sclerosis should not become therapeutic hostages. Ther Adv Neurol Disord 2016; 9: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waubant E, Banwell B, Wassmer E, et al. Clinical trials of disease-modifying agents in pediatric MS: opportunities, challenges, and recommendations from the IPMSSG. Neurology 2019; 92: e2538–e2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. European Medicines Agency. Avonex interferon beta-1a, https://www.ema.europa.eu/en/medicines/human/EPAR/avonex (accessed 22 December 2020).
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pearson SD, Ollendorf DA, Chapman RH. New cost-effectiveness methods to determine value-based prices for potential cures: what are the options? Value Health 2019; 22: 656–660. [DOI] [PubMed] [Google Scholar]
- 24. Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci 1965; 122: 552–568. [DOI] [PubMed] [Google Scholar]
- 25. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983; 13: 227–231. [DOI] [PubMed] [Google Scholar]
- 26. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald criteria’. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 27. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 29. Krupp LB, Banwell B, Tenembaum S, et al. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007; 68: S7–S12. [DOI] [PubMed] [Google Scholar]
- 30. Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013; 19: 1261–1267. [DOI] [PubMed] [Google Scholar]
- 31. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 32. Margoni M, Rinaldi F, Riccardi A, et al. No evidence of disease activity including cognition (NEDA-3 plus) in naive pediatric multiple sclerosis patients treated with natalizumab. J Neurol 2020; 267: 100–105. [DOI] [PubMed] [Google Scholar]
- 33. Holzhauer B, Wang C, Schmidli H. Evidence synthesis from aggregate recurrent event data for clinical trial design and analysis. Stat Med 2018; 37: 867–882. [DOI] [PubMed] [Google Scholar]
- 34. Friede T, Rover C, Wandel S, et al. Meta-analysis of few small studies in orphan diseases. Res Synth Methods 2017; 8: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 36. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 37. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 38. R Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2019, https://www.R-project.org/ [Google Scholar]
- 39. Schmidli H, Gsteiger S, Roychoudhury S, et al. Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics 2014; 70: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 40. Neuenschwander B, Capkun-Niggli G, Branson M, et al. Summarizing historical information on controls in clinical trials. Clin Trials 2010; 7: 5–18. [DOI] [PubMed] [Google Scholar]
- 41. Neuenschwander B, Weber S, Schmidli H, et al. Predictively consistent prior effective sample sizes. Biometrics 2020; 76: 578–587. [DOI] [PubMed] [Google Scholar]
- 42. Pakdaman H, Fallah A, Sahraian MA, et al. Treatment of early onset multiple sclerosis with suboptimal dose of interferon beta-1a. Neuropediatrics 2006; 37: 257–260. [DOI] [PubMed] [Google Scholar]
- 43. Huppke B, Ellenberger D, Hummel H, et al. Association of obesity with multiple sclerosis risk and response to first-line disease modifying drugs in children. JAMA Neurol 2019; 76: 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fragomeni MO, Bichuetti DB, Oliveira EML. Pediatric-onset multiple sclerosis in Brazilian patients: clinical features, treatment response and comparison to pediatric neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2018; 25: 138–142. [DOI] [PubMed] [Google Scholar]
- 45. Ben Achour N, Rebai I, Raddadi S, et al. Pediatric multiple sclerosis in Tunisia: a retrospective study over 11 years. Biomed Res Int 2017; 2017: 4354826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gärtner J, Bruck W, Weddige A, et al. Interferon beta-1b in treatment-naive paediatric patients with relapsing-remitting multiple sclerosis: two-year results from the BETAPAEDIC study. Mult Scler J Exp Transl Clin 2017; 3: 2055217317747623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fragoso YD, Alves-Leon SV, Barreira AA, et al. Fingolimod prescribed for the treatment of multiple sclerosis in patients younger than age 18 years. Pediatr Neurol 2015; 53: 166–168. [DOI] [PubMed] [Google Scholar]
- 48. Tenembaum SN, Banwell B, Pohl D, et al. Subcutaneous interferon beta-1a in pediatric multiple sclerosis: a retrospective study. J Child Neurol 2013; 28: 849–856. [DOI] [PubMed] [Google Scholar]
- 49. Basiri K, Etemadifar M, Derakhshan F, et al. Interferon-beta in pediatric multiple sclerosis patients: safety in short-term prescription. Acta Med Iran 2012; 50: 97–100. [PubMed] [Google Scholar]
- 50. Ghezzi A, Amato MP, Annovazzi P, et al. Long-term results of immunomodulatory treatment in children and adolescents with multiple sclerosis: the Italian experience. Neurol Sci 2009; 30: 193–199. [DOI] [PubMed] [Google Scholar]
- 51. Tenembaum SN, Segura MJ. Interferon beta-1a treatment in childhood and juvenile-onset multiple sclerosis. Neurology 2006; 67: 511–513. [DOI] [PubMed] [Google Scholar]
- 52. Pohl D, Rostasy K, Gärtner J, et al. Treatment of early onset multiple sclerosis with subcutaneous interferon beta-1a. Neurology 2005; 64: 888–890. [DOI] [PubMed] [Google Scholar]
- 53. Waubant E, Hietpas J, Stewart T, et al. Interferon beta-1a in children with multiple sclerosis is well tolerated. Neuropediatrics 2001; 32: 211–213. [DOI] [PubMed] [Google Scholar]
- 54. Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics 2011; 128: e1242–e1249. [DOI] [PubMed] [Google Scholar]
- 55. Schmidli H, Häring DA, Thomas M, et al. Beyond randomized clinical trials: use of external controls. Clin Pharmacol Ther 2020; 107: 806–816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211070449 for Improving pediatric multiple sclerosis interventional phase III study design: a meta-analysis by Jennifer S. Graves, Marius Thomas, Jun Li, Anuja R. Shah, Alexandra Goodyear, Markus R. Lange, Heinz Schmidli, Dieter A. Häring, Tim Friede and Jutta Gärtner in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864211070449 for Improving pediatric multiple sclerosis interventional phase III study design: a meta-analysis by Jennifer S. Graves, Marius Thomas, Jun Li, Anuja R. Shah, Alexandra Goodyear, Markus R. Lange, Heinz Schmidli, Dieter A. Häring, Tim Friede and Jutta Gärtner in Therapeutic Advances in Neurological Disorders
Data Availability Statement
Anonymized data that support the findings of this study will be made available to qualified external researchers, with requests reviewed and approved by an independent review panel on the basis of scientific merit.