Abstract
Epilepsy is a neurological disorder characterized by a hyperexcitable state in neurons from different brain regions. Much is unknown about epilepsy and seizures development, depicting a growing field of research. Animal models have provided important clues about the underlying mechanisms of seizure-generating neuronal circuits. Mammalian complexity still makes it difficult to define some principles of nervous system function, and non-mammalian models have played pivotal roles depending on the research question at hand. Mollusks and the Helix land snail have been used to study epileptic-like behavior in neurons. Neurons from these organisms confer advantages as single-cell identification, isolation, and culture, either as single cells or as physiological relevant monosynaptic or polysynaptic circuits, together with amenability to different protocols and treatments. This review’s purpose consists in presenting relevant papers in order to gain a better understanding of Helix neurons, their characteristics, uses, and capabilities for studying the fundamental mechanisms of epileptic disorders and their treatment, to facilitate their more expansive use in epilepsy research.
Keywords: Epilepsy, Animal models, Helix pomatia, Helix aspersa, Drug screening, Ion channels, Synaptic vesicles
Introduction
Epilepsy and neuron behavior
Epilepsy is one of the oldest recognized neurological disorders, first described by Hippocrates in the fifth century BC (Pitkänen and Lukasiuk 2011). The International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) defined it as a disease characterized by an abnormally increased predisposition to generate epileptic seizures, which are signs and/or symptoms derived from abnormal excessive or synchronous neuronal activity in the brain (Fisher et al. 2005, 2014).
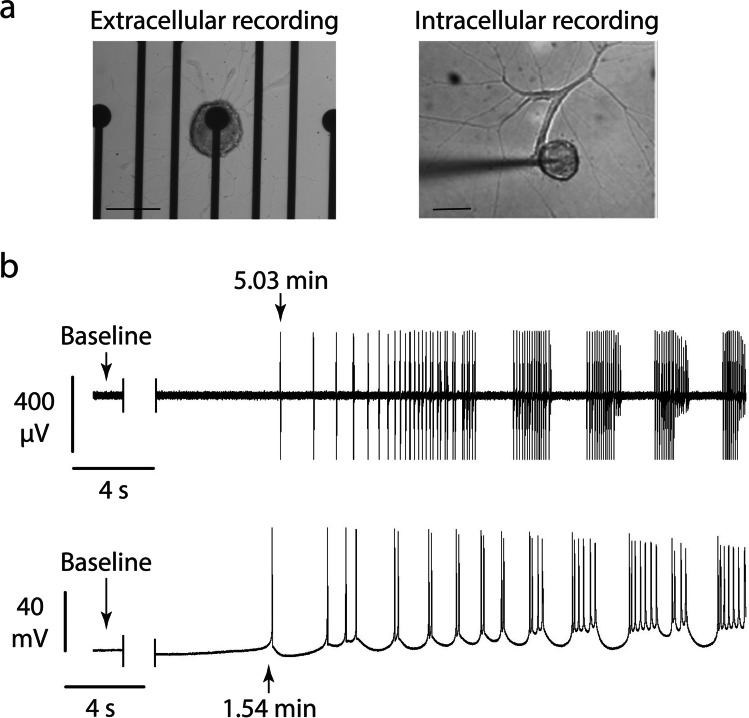
At the cellular level, epileptic behavior displays as different electric manifestations, such as (1) instability of resting membrane potential and oscillations, (2) high-frequency spike discharges including doublets of action potentials with short interspike intervals, and (3) paroxysmal depolarization shifts (PDS), consisting of steep depolarizations of the membrane voltage, followed by a plateau potential with superimposed APs that terminate with steep repolarizations (Fig. 1) (Altrup 2004).
Fig. 1.
a Drug-induced epileptic-like activity in Helix neurons can be recorded through extracellular microelectrodes arrays culturing one single cell in each electrode, and through intracellular borosilicate fine electrodes. b C1 neurons of Helix do not present intrinsic pacemaker potentials (flat baseline signal, in the absence of stimuli, is shown), the epileptic-like activity was induced with 40 mM PTZ. The activity recorded with extracellular electrodes presented a latency of ~5 min, and the activity recorded with high-resistance intracellular electrodes started ~1.5 min after PTZ bath application, mean latency is about 4.4 ± 1.4 min (mean ± sem). In both recordings, it is possible to observe single spikes, action potential doublets, and paroxysmal depolarization shifts. Scale bar, 100 μm
These events are termed altogether as cellular epileptic-like activity. And, when events such as PDSs are synchronized within many neurons in the brain, seizures will be apparent in an electroencephalogram or behavioral manifestations will occur (Altrup 2004).
In 2010, ILAE classified epilepsies from an origin point of view (etiology) into three broad categories: genetic, structural/metabolic, and unknown (Berg et al. 2010). The congenital factor associated with genetic epilepsies comprises mutations in proteins involved in circuitry (inducing abnormal neural development and synaptogenesis), synaptic transmission (un-balancing circuits toward excitatory synapses), and membrane excitability (increasing cell excitability affecting ion channels and their modulators) (Noebels 2003; Noebels et al. 2010; Pitkänen and Lukasiuk 2011; Steinlein 2004). Some acquired factors associated with structural/metabolic epilepsies are neuronal damage from trauma, stroke, and infections (Berg et al. 2010).
Animal models have provided some clues to the contributing mechanisms of seizure-generating neuronal circuit formation, for example, epileptogenesis cannot be induced in humans due to ethical reasons, as a consequence have been studied in animals as rodents through technics such as repeated electrical stimulation in limbic structures or neonatal hypoxia/ischemia; genetically tractable epileptic seizures have been studied in animals like mice, flies, and other animals where genomic modification can be performed, ictogenesis and seizures characteristics can also be electrically or chemically induced for studying seizure susceptibility or for drug screening assays in several in vitro and in vivo animal models (Campos et al. 2018; Johan Arief et al. 2018; Löscher 2016; Nirwan et al. 2018; Pitkänen and Lukasiuk 2011). Depending on the research question, the epilepsy model organisms can span from complex species like baboons (Papio hamadryas), dogs (Canis familiaris), and mice (Mus musculus) to simple models like zebrafish (Dario rerio), fruit fly (Drosophila melanogaster), roundworms (Caenorhabditis elegans), and flatworms (planaria) (reviewed in Grone and Baraban 2015; Johan Arief et al. 2018).
In addition to these models, mollusks have been used extensively to study neuron physiology and circuitry. For example, the Loligo squid was used to describe the ionic basis of action potentials (Hodgkin and Huxley 1952), the Aplysia sea slug and the Lymnaea pond snail have been used to study neurotransmitter release and synaptic plasticity (Dyakonova et al. 2019; Ghirardi et al. 1995; Klein and Kandel 1978; Pinsker et al. 1970), and cultured neurons from the land snails Helix aspersa and Helix pomatia have been used to study neurite outgrowth, synaptogenesis (Ghirardi et al. 1996), neurotransmitter release (Fiumara et al. 2001; Ghirardi et al. 2001; Giachello et al. 2010), and the formation of neuronal circuits (Massobrio et al. 2013). Moreover, Helix neurons have been used as a simple model system for studying the mechanisms underlying epileptic activity (Altrup 2004; Brenes et al. 2016; Giachello et al. 2013).
Helix neurons as a model system
The Helix land snail can be found in America, Europe, and part of Russia, mostly due to human intervention (Barrientos 2010; Ierusalimsky et al. 1994). This land mollusk provides several advantages over mammalian models and marine mollusks, being easy to maintain in a restricted space like net-covered pens, and with simple housing conditions including a controlled environment with temperatures of 20–32 °C, light:dark cycles of 12:12 h, and feeding on green leaves, fruits, and tubers (i.e., lettuce, cucumber, and carrot), making their care a cheap and low labor practice (Casadio et al. 2004; Joshi and Pandey 2019; Sallam and El-Wakeil 2012).
One specific advantage of studying neuronal behavior in mollusks like Helix is the presence of specific, identifiable, and individual neurons that can be isolated from their synaptic inputs. In this way, single cells or physiological relevant synaptic connections can be reliably reproduced in vitro, recapitulating in vivo features. In vitro, these neurons facilitate the study of their intrinsic properties, while avoiding potential non-specific effects resulting from surrounding tissue or random connections (Cibelli et al. 1996; Fiumara et al. 2001, 2005).
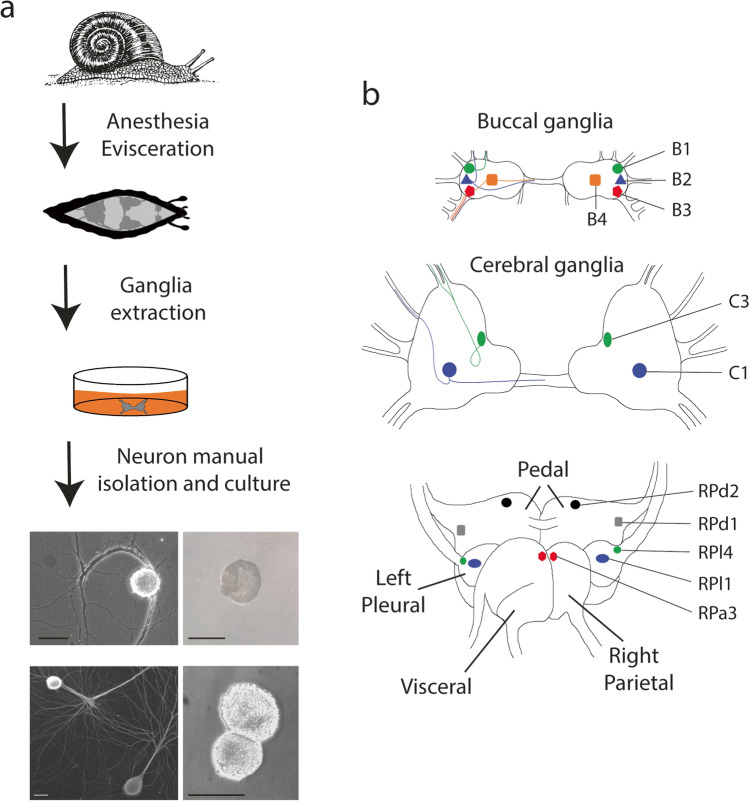
The isolation of these neurons begins by anesthetizing the snail and relaxing their muscles through isotonic MgCl2 injection in the foot, followed by shell remotion (Ghirardi et al. 1996). Helix nervous system is composed of the buccal, cerebral, and subesophageal ring ganglia, which can be easily dissected under a stereomicroscope. Ganglia can be used for direct recordings in superficial neurons, recognized by size and location after removing the soft outer connective tissue (Altrup 2004), or each neuron can be isolated one by one if the connective tissue and the cell-cell attachments are softened by proteolytic enzyme digestion (Fig. 2a) (Ghirardi et al. 1996).
Fig. 2.
a Helix anesthetized animals are sacrificed by removing the shell that contains most of the visceral organs; ganglia and cells are surgically isolated. Neurons can be cultured with (upper left panel) or without (upper right panel) axons. In addition, cells can be cultured with their physiological partner in the presence of axons and neurites (bottom left panel) or through direct contact of axon-reabsorbed somata (bottom right panel). b Scheme of Helix central nervous system, showing the position of some relevant neurons. Each neuron has a contralateral counterpart. In the buccal and cerebral ganglia, left neurons are shown with their axon’s trajectories
The buccal ganglion of Helix contains about 100 neurons. Four giant neurons in each hemi-ganglion can be easily identified at the surface, named B1 to B4, and are related to control of esophagus circular muscles (B1), control of salivary epithelium (B2), kidney modulation (B3), and control of muscles from pharynx and radula (B4) (Fig. 2b) (Altrup 2004; Altrup and Speckmann 1994). The cerebral ganglion is divided into the procerebrum, which contains more than 20,000 neurons, most of them with sensory functions; the mesocerebrum with about 140 neurons, most of them with a role in the control of mating behavior; and the metacerebrum, which contains two giant neurons: the dorsal C3 neuron, a motoneuron related with tentacle withdrawal, and the ventral C1 neuron, also called metacerebral giant cells (MGC), which is the alimentary command neuron of the salivary reflex (Chase 2000; Cottrell and Macon 1974). The subesophageal ganglion ring is composed of one visceral, two pedal, two pleural, and two parietal ganglia, which have different functions, and their contained cells are named as right and left pedal cells (RPd and LPd), right and left pleural cells (RPl and LPl), right and left parietal cells (RPa, LPa or F neurons), and among others (Fig. 2b) (Azanza et al. 2007, 2008; Ierusalimsky et al. 1994).
After isolation by sharp borosilicate microelectrodes, the neurons can be plated with the initial segment of their axons still present, in plates pre-treated to promote cell attachment and growth. They can also be cultured in suspension for 24 h, allowing for the retraction of their processes and yielding a spherical cell called “soma configuration” (Fig. 2a). These two arrangements can be used for different purposes since, for example, soma configuration minimizes capacitance and allows a better space clamp for whole-cell patch-clamp recordings (Brenes et al. 2015a, b), soma-to-soma paired cells ensure cell contact even in absence of neurite growth-promoting factors (Fiumara et al. 2005), and in this configuration, presynaptic neurites project onto postsynaptic neurons maximizing cellular contact and allowing the development of presynaptic structures thereby the study of distribution patterns of molecules under different conditions through the presynaptic compartments (Giachello et al. 2010).
Helix neurons also hold advantages for studying cells and synapses in terms of dimensions, synaptic specificity, and amenability to a variety of experimental manipulations that are not feasible with mammalian neurons (Fiumara et al. 2001). For example, intranuclear DNA, cytoplasmic RNA, and protein microinjection procedures can be performed in single components of neuronal circuits (for example, injecting presynaptic cells and leaving postsynaptic cells unaltered). This is an experimental design that is difficult to achieve through classical cell culture transfection (Brenes et al. 2015a, b; Fiumara et al. 2001, 2004, 2007; Giachello et al. 2010). In addition to intracellular recording and patch-clamp, Helix neurons have been used to study circuitry through microelectrode arrays (MEA) recordings. Since single identified cells can be isolated, each cell can be plated in specific microelectrodes within MEA chips and cell-by-cell network reconstruction is feasible. This 1:1 coupling between cells and microelectrodes is not easy to perform with mammalian neurons and facilitates activity interpretation in neuronal networks (Massobrio et al. 2009, 2013). Moreover, Helix neurons start to create synaptic connections a few hours after plating (6 to 24 h) and complex circuits are useful within 48 to 72 h (Massobrio et al. 2013), much faster than cortical and hippocampal mammalian neurons, where cultures are maintained at least 10 days and up to 41 days in culture before recordings (Chiappalone et al. 2008; Kapkaeva et al. 2017). One final advantage of Helix neurons is that they are isolated from a cold blood animal and their normal physiological activity occurs at temperatures close to room temperature, which allows for reliable and stable recordings at room temperature for long periods (Altrup 2004; Brenes et al. 2016).
On the other hand, the principal disadvantage of invertebrate models is their phylogenetic distance to the human nervous system; however, they can be used to uncover fundamental biological principles and key mechanisms of neuronal functions conserved in evolution. Vertebrates and invertebrates keep important similarities at the cellular and subcellular levels, and many parallels exist, particularly at the level of excitable membrane component and its biophysical properties; at the level of synapse formation and functioning; and in basic network modulation (reviewed in Clarac and Pearlstein 2007; Humeau et al. 2011; Parker et al. 2011; Schmold and Syed 2012).
Epileptic-like activity in Helix neurons
Helix neurons have been used as a model of chemical-induced epileptiform activity, where the abnormal epileptic discharges are usually induced through local extracellular application of epileptogenic drugs. Pentylenetetrazol (PTZ) is a central nervous system stimulant with anxiogenic properties at low concentrations (Rodin and Calhoun 1970) and convulsant properties (Squires et al. 1984). Because of this, PTZ has been widely employed in both vertebrates and invertebrates for the induction of epileptic activity (Campos et al. 2018). In cultured Helix neurons, the epileptiform activity has been evoked by PTZ application over 30 mM, using whole ganglia, isolated monosynaptic circuits, and single neurons. For example, bath application of PTZ over complete buccal ganglia evokes the development of PDSs in B1, B2, and B3 neurons, but not in B4 neurons (Altrup et al. 2006; Altrup and Wiemann 2003; Walden et al. 1988), and PTZ applied over cerebral ganglia induces PDSs in C1 neurons (Fehér et al. 1988).
It is known that PTZ is a gamma-aminobutyric acid receptors GABAA antagonist (Meilleur et al. 2003), therefore PTZ may induce epileptic-like activity in whole ganglia Helix preparations through GABAA blockage, unbalancing activity to overexcitation. In agreement with this, it has been observed that PTZ induces suppression of inhibitory postsynaptic potentials (IPSPs) in buccal B1 neurons (Altrup et al. 2006). The presence of a GABAA receptor-like structure has been shown in Helix cells through the application of GABA and through the differential effects of the application of GABAA agonist muscimol and GABAB agonist baclofen. However, GABA application had no effects in PTZ-sensitive B1 neurons and, on the other hand, had inhibitory effects in the PTZ-insensitive B4 neurons (Haarmeier et al. 1994), suggesting additional mechanisms.
PTZ also induced epileptiform activities on isolated neurons, such as unidentified neurons from Helix parietal and visceral ganglia (Speckmann and Caspers 1973), B2-B2 isolated monosynaptic connections (Giachello et al. 2013), C1 isolated neurons, and C1-B2 isolated monosynaptic connections (Brenes et al. 2016). In these conditions, lacking inhibitory and excitatory afferent connections, or any source of GABA neurotransmitter, the PTZ mechanism is possibly related to membrane currents modifications.
In invertebrate neurons (from Helix, Euhadra, and Aplysia) it has been suggested that PTZ inhibits K+ currents, by a mechanism related to Ca2+/calmodulin and PKA phosphorylation. According to this, PTZ reduces the voltage-dependent and Ca2+-dependent K+ currents (KV and BK, respectively), slowing the action potential repolarization and reverting the after-hyperpolarization to an after-depolarization. In addition, PTZ evokes a non-specific inward current (containing Na+ and Ca2+), which is responsible for maintaining sustained depolarizations (Fehér et al. 1988; Klee et al. 1973; Onozuka et al. 1983, 1991; Onozuka and Tsujitani 1991; Walden et al. 1988). Similar PTZ effects on intrinsic membrane properties could be present in some mammalian neuron types as a secondary mechanism, for example, cerebellar Purkinje cells have been reported to develop PTZ-induced PDSs through effects on synaptic transmission, however also developed spontaneous spikes in presence of a broad-spectrum excitatory amino acid receptor antagonists, suggesting possible effects in membrane properties (Haghdoost-Yazdi et al. 2011). In addition, K+ currents through rat neuronal KV1.1 can be depressed by PTZ application (Madeja et al. 1996), Na+/K+ pump directly interacts and is inhibited by PTZ (Dubberke et al. 1998), and mice lacking Ca2+ channels (as CaV2.3) are less susceptible to PTZ-induced seizure activity (Weiergräber et al. 2006), results that could associate K+ and Ca2+ currents to PTZ effects in mammalian cells. Similarly, less KV and BK channel activation, together with an inward current carried by Ca2+ was associated with a slower return to baseline during action potentials and bursting firing in mammalian chromaffin cells (Vandael et al. 2015), with a burst waveform similar to the PDSs induced by PTZ.
The dose-dependent convulsivant effect of PTZ was evaluated in C1 single neurons, which are silent in the absence of electrical or chemical stimulation. In this cell, the extracellular application of PTZ at 10 mM concentrations results in long latency (more than 10 min) and short duration (less than 10% of the recording period) spiking in around 30% of the treated cells. A total of 20 mM concentrations induced activity in around 50% of treated neurons, also with long latencies of even 8 min. The absence of PDSs allowed for the classification of these dosages (10–20 mM) as sub-convulsivant. Whereas, at higher concentrations (30–50 mM), the response pattern changes rapidly from a pre-convulsive state, with an acceleration of the spontaneous spiking activity, to PDSs in the convulsive phase, with almost 80% of the cells showing this activity, with latencies of about 1 min and abnormal activity for around 70% of the total recording (Brenes et al. 2016; Speckmann and Caspers 1973). The PDSs developed by invertebrate cells resemble the PDSs evoked by PTZ administration in mammals (Haghdoost-Yazdi et al. 2011; Speckmann and Caspers 1973).
The study of intrinsic cellular excitability in Helix neurons
As was pointed out before, perhaps the most useful characteristic of invertebrate models, such as Helix, over mammalian systems is the capacity of single-cell identification, isolation, and culture, allowing for the study of intrinsic neuronal excitability and its modulation in the same cell and in absence of synaptic inputs, in such a way that the changes due to modification in the biophysical properties of membrane components, like ion channels, can be studied straightly. On this matter, Helix neuron subtypes express different populations of ionic channels that resemble the channels expressed by mammalian neurons (Table 1) and can be selected according to the experimental question to be addressed. As far as we know, sequence similarities between Helix and mammalian channels have not been studied; however, some comparison can be found in the literature. For example, the Aplysia NaV α subunit had 45–50% of amino acid identity when compared with vertebrate (rat and eel) channels and the presence of alternatively spliced channels was detected by reverse transcription (Dyer et al. 1997; Johnston et al. 1996). Lymnaea present Ca2+ channels that cluster with vertebrate CaV1, CaV2, and CaV3 channel, called LCaV1 to 3, all with a high degree of similarity (62.2%, 62.0%, and 55.3% with human sequence). The sequence conservation was especially high in the transmembrane domains and the regions associated with interactions with β subunits, calcium and calmodulin, and others (Huang et al. 2010; Senatore and Spafford 2010; Spafford et al. 2003; Spafford et al. 2006). The differential recognition by antibodies developed for mammalian channels and the similarities reported confer construct validity and allow invertebrate cell selection dependent on the experimental question.
Table 1.
Ionic channels reported on Helix neurons
| Channel+ | Evidence* | Cells | References |
|---|---|---|---|
| Na transient | Ephys | RPa3, LPa3 | (Kiss 2003) |
| Na persistent | Ephys | RPa3, LPa3 | (Kiss 2003) |
| NaV1.2 | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| NaV1.7 | Ab/Ephys | C1, C3, B1, B2, B4, Pd2 | (Kiss et al. 2012) |
| NaV1.8 | Ab/Ephys | Buccal and cerebral neurons, B3, Pd1, PC | Kiss et al. 2012, 2014; Pirger et al. 2014) |
| NaV1.9 | Ab/Ephys | PC, RPa2, RPa3, LPa2, LPa3, other. | (Kiss et al. 2012, 2014; Pirger et al. 2014) |
| NaV | Ephys | A cell | (Standen 1975) |
| NaV and CaV | Ephys | Subesophageal ganglia | (Lee et al. 1978) |
| NaV and CaV | Ephys | B1, B2, B3 | (Walden et al. 1984) |
| CaV1 (L) | Ab/Ephys | F1, F2, F34, F53, F54, F26, F1 | (Azanza et al. 2008; Ghasemi et al. 2011) |
| CaV2.1 (P/Q) | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| CaV2.2 (N) | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| CaV3 | Ephys | Unidentified, F1 | (Ghasemi et al. 2011; Kits and Mansvelder 1996) |
| HVA CaV | Ephys | C1, pedal ganglia nuerons | (Brenes et al. 2015b; Lukyanetz et al. 2002) |
| CaV | Ephys | Unidentified, Visceral 2 and 4, circun-esophagic neurons, C1, parietal ganglia | (Akaike et al. 1978; Brown et al. 1978, 1981, 1983; Lesser et al. 1997; Lux and Brown 1984; Marom and Dagan 1987) |
| KV1.5 | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| KV2.1 | Ab y Ephys | PC | (Battonyai et al. 2014; Pirger et al. 2014) |
| KV3.4 | Ab y Ephys | B2 | (Battonyai et al. 2014) |
| KV4.3 | Ab y Ephys | Unidentified, PC | (Battonyai et al. 2014; Pirger et al. 2014) |
| KV | Ephys | C1 | (Brenes et al. 2015b) |
| K A-type | Ephys/Ab | B2, D1, F1, F2, F34, F53, F54, F26, F76, F77 | (Azanza et al. 2008; Bal et al. 2001; Battonyai et al. 2014; Denton et al. 2007; Walden et al. 1984) |
| SK2 y SK3 | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| SK | Ephys | Unidentified | (Sotkis et al. 1998) |
| BK | Ab/Ephys | B3, C1, E1, E2, E5, F1, F2, F34, F53, F54, F26, F76, F77 | (Akaike et al. 1983; Azanza et al. 2008; Brenes et al. 2015b; Denton et al. 2007; Ghasemi et al. 2011; Sotkis et al. 1998; Walden et al. 1984) |
| Khy: hyperpolarization-activated K channels | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| GIRK2 (G protein activated) | Ab | F1, F2, F34, F53, F54, F26 | (Azanza et al. 2008) |
| IKDR: K delayed rectifier | Ephys | F76, F77 | (Denton et al. 2007) |
| ClC | Ephys | C1 | (Reece et al. 2008) |
| Cx26 | Ab | F77, F53, F2, F54 | (Azanza et al. 2007) |
| GABAA receptor | Ephys | B4 | (Haarmeier et al. 1994) |
| 5-HT receptor | Ephys | E5, E6 | (Green et al. 1996; Massobrio et al. 2013) |
| Dopamine receptor | Ephys | C2 | (Green and Cottrell 1997) |
| AMPA/kainate | Ephys | C1, F30 | (Piggott et al. 1975; Radovic et al. 1998) |
| Cl− and K+ GluR | Ephys | C1, E4, F1 | (Piggott et al. 1975; Radovic et al. 1998) |
+Channels are presented as reported by the authors, if possible specific isoforms are presented. *Ephys electrophysiological recordings, Ab antibody staining
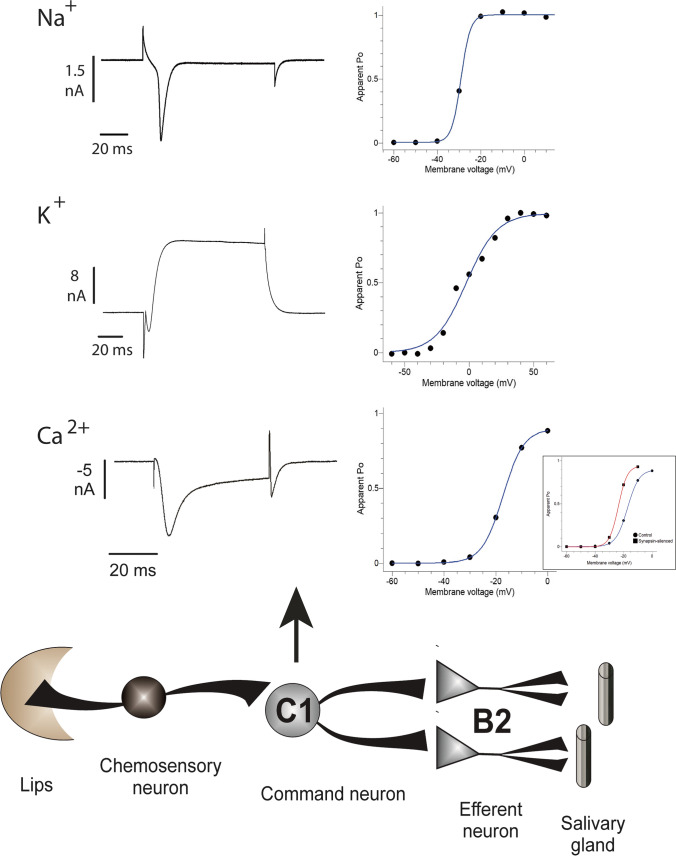
C1 neuron from the ventral side of the cerebral ganglia and the B2 from the dorsal side of the buccal ganglia are two of the cells used to study epileptic behavior and modulation of cellular excitability. These neurons form an in vivo physiologically relevant synapse, related to the salivary reflex of the snail, where C1 is the alimentary command neuron, and B2 is the efferent secretory neuron that contacts the salivary glands (Fiumara et al. 2005) (Fig. 3). In vivo, each symmetrically placed C1 neuron is monosynaptically coupled to both B2 neurons and the B2 neurons from contralateral ganglia are coupled to each other (Altrup and Speckmann 1994; Cottrell and Macon 1974). Functional and anatomical studies using electrophysiology and antibodies described the presence of channels similar to the voltage-gated Na+ channel 1.7 (NaV1.7) on the C1 and B2 neurons (Kiss et al. 2012), channels similar to KV3.4 and A-type on B2 neuron (Battonyai et al. 2014; Walden et al. 1984), BK channels on C1 neurons (Brenes et al. 2015b), one chloride current on C1 (Reece et al. 2008), and several calcium currents are currently under study on C1. Examples of some of the C1 currents and their voltage dependency are shown in Fig. 3.
Fig. 3.
Schematic representation of Helix salivary reflex circuit (lower panel) made up of chemosensory neurons that innervate snail lips and C1 command neuron, which in turn innervates bilaterally the efferent B2 neurons with outputs to salivary glands. Sample currents (upper panels) elicited by Na+, K+, and Ca2+ channels (at −20 mV, 30 mV, and −10 mV, respectively) in C1 neuron are shown. The upper right panels show the apparent open probability (Po) of depicted ion channels. Insert in Ca2+ apparent Po allow comparing the control (black circle and blue line) and synapsin-silenced (black squares and red line) voltage dependency of Ca2+ currents
The knowledge of the population of channels expressed in specific neurons allows the development of studies regarding the origin of some epileptic phenotypes. For example, the relationship between synapsin mutations and epilepsy was traditionally associated with abnormal circuitry during maturation and disruption of synaptic vesicle pools. In particular, the literature focuses on the rate of neurotransmitter release, which is differentially affected in excitatory and inhibitory synapses in synapsin-deficient organisms, and thus causes an excitation/inhibition imbalance in network excitability (Baldelli et al. 2007; Chiappalone et al. 2009; Farisello et al. 2013; Gitler et al. 2004a, b; Ketzef et al. 2011; Lignani et al. 2013; Medrihan et al. 2013; Noebels et al. 2010; Pitkänen and Lukasiuk 2011; Terada et al. 1999). However, when the electrical characteristics of hippocampal CA1 pyramidal neurons obtained from synapsin knock-out mice were analyzed, a more depolarized resting membrane potential, a smaller rheobase, and faster action potential frequencies were reported, regarding control aged-matched neurons (Farisello et al. 2013). The use of Helix C1 neurons cultured in the absence of excitatory or inhibitory inputs (Fig. 2) allowed the study of synapsin silencing influence on intrinsic cell excitability, and similarly, these cells showed a slightly depolarized resting membrane potential, more negative threshold for action potential firing and higher firing rates with different stimulation intensities. This increased intrinsic excitability was related to changes in Ca2+ and K+ currents (Brenes et al. 2015b).
When currents’ voltage dependency was analyzed, it was evident that synapsin silencing correlates with a left shift to more negative resting potential in the open probability of Cav currents (insert in Fig. 3.) and no changes in KV currents (Brenes et al. 2015b). The calcium current amplitude increased, and the voltage-dependence was more stepped, changing from −4.06 in control neurons to −3.13 in synapsin-silenced neurons. The increased Ca2+ permeability at more negative potentials could be related to the slight depolarization in the resting membrane potential (Condliffe et al. 2010), since the resting membrane potential is dependent on the ions permeant in the membrane. In addition, since action potential depolarization in C1 Helix neuron depends on Na+ and Ca2+ currents (unpublished results), the increased Ca2+ currents may be associated with the faster depolarization observed in the pre-trigger phase of action potentials in synapsin-silenced neurons. And finally, the increased Ca2+ could be related to the consequent increase in BK currents in these cells (Brenes et al. 2015b).
Changes in voltage-dependent Ca2+ channels have been linked with epilepsy also in humans and mouse models, for example, reduced currents in CaV2.3 and increased currents in CaV3.2 were associated with epilepsy, and cerebellar ataxia, among other cognitive impairments (Damaj et al. 2015; Imbrici et al. 2004; Khosravani et al. 2005; Souza et al. 2019; Wang et al. 2015). Also, several studies in humans have shown increased BK currents in epilepsy and paroxysmal movement disorders that correlate with faster cellular spiking behavior (Diez-Sampedro 2006; Du et al. 2005; Lee and Cui 2009; Lorenz et al. 2007; Shruti et al. 2009; Wang et al. 2009; Yang et al. 2010). Consequently, even if part of the depolarization observed in hippocampal neurons from synapsin knock-out mice was due to a decreased GABA tonic current (Farisello et al. 2013), the possibility of changes in membrane properties as a secondary mechanism could be addressed in the future.
Single synapsin knock-down Helix cells also showed increased susceptibility to PTZ-induced epileptic-like activity. In this regard, synapsin-silenced Helix neurons developed drug-induced discharges with 80% shorter latencies and 2.6 longer activity duration when treated with 40 mM PTZ. Moreover, 10 mM PTZ induced paroxysmal activity in only 1 over 12 control neurons and induced this activity in 5 over 10 synapsin-silenced neurons, with increased duration of the paroxysmal activity to more than 20% of the total recording period. In addition, under recurrent drug-induced epileptic-like activity, Helix neurons developed spontaneous action potential firing in the absence of PTZ, an activity lacking in normal conditions, and this effect was more prominent in synapsin-silenced neurons. Ca2+ currents could also be related to this increased PTZ susceptibility since, as was pointed out before, a depolarizing Ca2+ current could drive action potential and PDSs firing during PTZ application, and synapsin-silenced Helix neurons, in which CaV currents are increased and negatively shifted, it is possible that the marked blocking of KV and BK channels by PTZ application could lead to an increased susceptibility to developing epileptic-like behavior, regarding neurons expressing synapsin (Brenes et al. 2016).
The specific relationship between Ca2+, BK currents, and the hyperexcitable phenotype developed when synapsin was silenced is a complex system, since many other currents can be affected, therefore this relationship should be studied in deeper detail in future research, for example through mathematical modeling, since this approach has been useful to study electrophysiological behavior in vertebrate (De Schutter and Bower 1994) and invertebrate neurons from animals as Aplysia (Canavier et al. 1991) and Hirudo (Meiser et al. 2019).
Studying neurotransmitter release in Helix neurons
Circuit hyperexcitability can be associated with increased neurotransmitter release, and as mentioned before, this can be tested in reconstructed Helix monosynaptic or polysynaptic circuits. In vivo and in vitro, C1 and B2 develop a unidirectional serotoninergic excitatory synapse, and presynaptic stimulation causes an individual excitatory postsynaptic potential (EPSP) in the B2 neuron with a duration of about 160 ms and an average amplitude of 0.5 to 6 mV (Brenes et al. 2015a; Fiumara et al. 2005). In addition, C1-B2 synapses show multiple forms of short-term plasticity that depend on presynaptic activity. For example, although the low-frequency presynaptic stimulation (0.01 to 0.2 Hz) does not cause significant changes in the amplitude of the EPSP, stimulation at frequencies of 2–3 Hz causes facilitation at the synapse. Short periods of high-frequency stimulation (10 Hz for 500 ms) lead to augmentation for 10–15 s, with a time constant (τ) of 5.52 s. Additionally, a longer period in the train of action potentials (10 Hz for 2 s) yields a strengthening of synapses that can last for more than 3 min, which indicates the generation of post-tetanic potentiation (PTP) (Fiumara et al. 2005).
Also, B2 neurons, when coupled together, develop a bidirectional synapse with EPSPs amplitudes of 3 to 12 mV. The bidirectionality of this synapse confers an additional advantage in this model since specific treatments can be applied to both cells simultaneously or to one of them by intracellular microinjection. These synapses also showed the capability to display distinct forms of activity-dependent synaptic plasticity, such as a particularly robust PTP with a τ of about 1 min when presynaptic cells were stimulated at 10 Hz for 10 s (Giachello et al. 2010).
In these synapses (C1-B2 and B2-B2), it is possible to estimate the size of the pool of vesicles with a higher probability of being released (RRP, for readily releasable pool) and the size of the pool with less probability (RP, for resting pool), using long high-frequency stimulations and cumulative amplitude analysis (Schneggenburger et al. 1999), therefore vesicle pool changes generated for a drug-induced activity or under different experimental conditions can be studied.
For example, neurotransmitter release was analyzed under PTZ-induced activity using B2-B2 synapses. In this monosynaptic model, the prolonged effect of the epileptiform activity over the synaptic strength was evidenced, even after PTZ washout. Much as 15 and 30 min after epileptic-like activity, the EPSP amplitude was increased in these synapses, suggesting a use-dependent potentiation in basal conditions. This was associated first with an increased probability of release (Pr) and subsequently with increased RRP at expenses of the RP, probably related to synapsin phosphorylation status, remaining high even 30 min after the end of the drug-induced epileptic-like activity (Giachello et al. 2013). Using B3 neurons still attached to the ganglia, PTZ application induced smaller EPSPs and etomidate induces similar amplitude, but slower EPSPs, possibly reflecting synaptic fatigue (Altrup et al. 2006), similar to the smaller RRP measured immediately post PTZ treatment in the B2-B2 synapse (Giachello et al. 2013).
The C1-B2 monosynaptic connection was used to study neurotransmitter release when synapsin was downregulated in the presynaptic compartment. In this case, similar EPSPs, but increased neurotransmitter release under high-frequency stimulation (10 Hz), were reported. These apparently discordant data were explained by the increased size of the RRP at the expense of the RP and increased Ca2+ currents (Brenes et al. 2015a, b). It is known that the amplitude of the EPSPs is determined by variables such as the number of releasing sites (N) and the probability of vesicle release (Pr) (Schneggenburger et al. 2002). And, the Pr is dependent on a balance between two processes, the calcium currents, and the size of the RRP (Saviane et al. 2002). The interplay between these processes at the moment of arrival of each action potential would determine the EPSP amplitude and the presence of facilitation or depression in the synaptic transmission (Saviane et al. 2002). Synapsin-silenced Helix neurons showed fewer releasing sites (varicosities), but increased Ca2+ currents and RRP, reflecting increased vesicle availability and release and allowing normal EPSPs at the beginning with an increased amplitude if the stimulus was prolonged in time until the depletion of the neurotransmitter (Brenes et al. 2015a).
Also, synapsin triple knock-out mice models described in the literature showed similar excitatory postsynaptic currents amplitude (Gitler et al. 2004a), increased RRP (Farisello et al. 2013; Kile et al. 2010), decreased RP (Gitler et al. 2004a; Orenbuch et al. 2012), and increased synaptic vesicle mobility (Orenbuch et al. 2012) were observed regarding wild-type mice.
Studying neuronal outgrowth and synaptogenesis
The correct outgrowth and formation of synapses are necessary for the function of brain circuits and aberrant patterns of brain development are a frequent substrate for inherited seizure syndromes (Noebels 2003). Helix neurons have been used to study neurites outgrowth in normal conditions, with different growth factors (Ghirardi et al. 1996), through modulation of biochemical pathways as MAPK/Erk cascade (Giachello et al. 2010), during the silencing of synapsin (Brenes et al. 2015a), overexpressing non-phosphorilatable synapsin mutants or mimicking its constitutive phosphorylation (Giachello et al. 2010) and after acute or recurrent PTZ-induced epileptic-like activity (Altrup and Speckmann 1988; Brenes et al. 2016). Highlighting its capacity to study processes that induce not just functional changes but also structural abnormalities.
For example, some synapsin deficient rodent neurons show delayed growth and decreased neurite branching (Chin et al. 1995), and long-term inhibition of Helix synapsin synthesis correlated with neurite outgrowth suppression at the level of neurite elongation and branching, the presynaptic varicosities also decreased, and the number of functional connections developed between pairs of C1-B2 neurons was impaired in a 50%, showing the decreased circuitry capacity of cells were synapsin was silenced (Brenes et al. 2015a). In addition, when normal neurons were exposed repeatedly to PTZ, the neurite density was unchanged, but connectivity was impaired, decreasing the number of functional connections to values similar to the connection developed by synapsin-silenced neurons (Brenes et al. 2016).
Drug screening in Helix neurons
As pointed before, invertebrate neurons are susceptible to epileptogenic drugs, such as PTZ, that has been effectively tested in B1, B2, B3, and C1 neurons from Helix, but also in R-2 neurosecretory cell of Aplysia (Klee et al. 1973), and in subesophageal neurons RC-2 and RC-3 of land snail Euhadra (Onozuka et al. 1983; Onozuka and Tsujitani 1991), all at concentrations from 10 to 70 mM. In addition, also etomidate showed its reactivity in B1, B2, and B3 neurons at concentrations above 200 and up to 500 μM (Altrup et al. 1991, 2006), and strychnine was tested in R-2, at a concentration range of 0.01 to 1.0 mM, where blocked all excitatory and inhibitory postsynaptic potentials (Klee et al. 1973). Ni2+ also have been used to induce epileptic-like behavior in Leech neurons (Pathak et al. 2010). These reports highlighted invertebrates’ value as models to study epileptic behavior and these drug-induced epileptic behavior models may be useful for the initial screening of antiepileptic drugs (AED) that act as anti-convulsants, even if they are not predictive of clinical response (Johan Arief et al. 2018; Kandratavicius et al. 2014).
Through the years, several studies showed that drugs acting as anti-epileptic in mammals had inhibitory activity also in invertebrate neurons, both during intrinsic pacemaker potentials and during drug-induced epileptic-like activity. For example, in Aplysia, 0.05 mM diphenylhydantoin (DHP) or phenytoin (equivalent to 13.6 μg/ml, part of the therapeutic serum range in humans) blocks the normal bursting activity of pacemaker cells as L6. In addition, PTZ-induced bursting in normally silent R2 Aplysia neuron was reverted adding 0.1 mM DHP. And these actions were associated with a decrease in sodium inward currents, without effects on the resting membrane potentials, by the AED (Ayala and Johnston 1977; Johnston and Ayala 1975). Similar blockage of sodium channels was found in bullfrog nerve preparation and mammals (Courtney and Etter 1983; Macdonald and Kelly 1995).
Like DHP, lamotrigine (LTG, 80 μM) also showed the capacity to decrease the spike frequency of the intrinsic activity of B3 Helix neuron, and when added in the presence of 40 mM PTZ, the activity frequency and PDS generation decreased (Altrup 2004). LTG now has been used in primary generalized epilepsies and is generally accepted that block sodium channels (Aneja and Sharma 2013; Macdonald and Kelly 1995; Mccabe 2000). Similar inhibition of PTZ-induced activity was observed using carbamazepine (50 mg/l), levetiracetam (500 mg/l), and verapamil (50 μM) (Altrup 2004). In the case of phenobarbital, it was tested in Aplysia pacemaker neurons L2-6 and R15, and it also suppresses the bursting pacemaker potential, at concentrations as 50 mM (Johnston 1978). This AED is capable to block sodium channels (Courtney and Etter 1983) and it increases GABA receptor chloride currents (Twyman et al. 1988).
The drugs with possible synaptic activity can be tested using monosynaptic connections or whole ganglia preparations. In the case of previously mentioned R2 neurons, they receive cholinergic inhibitory inputs from L10 interneurons and the application of DHP during L10 stimulation facilitated the IPSPs. Similar results were also observed with DHP in GABAergic transmission over the slow adapting stretch receptor neuron of crayfish (Ayala et al. 1977). In the case of valproate (VPA), it was tested in the buccal ganglia of Helix during PTZ- and etomidate-induced paroxysmal activity. VPA applied extracellularly at concentrations of 0.5–10 mM had an early effect (within minutes) consisting of hyperpolarization accompanied by a decrease in PDS frequency, and a late effect (1 h later, at the highest concentration) consisting of a decrease in steepness, amplitude, and duration of each PDS and a marked desynchronization in the physiological circuit between left and right B3 neurons, an effect even more pronounced several hours after VPA washout. The late effect was likely related to the slow internalization of VPA, and this was tested injecting VPA intracellularly and the late effect was evident with a delay, dependent on the injection rate (Altrup et al. 1992). VPA effect was associated with increased transient potassium currents and BK currents (Walden et al. 1993). A similar increase in repolarizing potassium currents has been suggested in rats, together with depressed Ca2+ currents (Franceschetti et al. 1986; Kelly et al. 1990), and with the potentiation of the inhibitory responses to GABA (Kerwin et al. 1980).
The potential screening of modified AED was employed to study if the membrane permeability and antiepileptic activity of VPA could be improved. In this matter, applying the VPA metabolite trans-2-en-VAP with increased membrane permeability decreased the late effect of VPA, decreasing its antiepileptic potential (Altrup et al. 1992). In a bigger study, twelve VPA derivates were tested in parallel in Helix neurons and rat cortex. Seven increased duration and/or repetition rate of PDS in Helix and let stable or increase field potentials in rats. Nevertheless, five derivates showed some degree of antiepileptic activity, decreasing PDS duration and amplitude in Helix neurons. From them, two mannitol esters derivates were classified as new AED and one of them was selected and tested in rat cerebral cortex in vivo, depressing epileptiform activity when applied via intraperitoneal injection (Redecker et al. 2000a). Lately, one selected molecule was structurally modified, and twenty-eight compounds were tested. Twenty-two modifications had similar effects, four had less effect, and two improved (Redecker et al. 2000b).
A particularly useful conformation of Helix neurons is the culture of soma configuration in multielectrode arrays. Neurons can be isolated and cultured cell-by-cell in single MEA electrodes allowing single-cell recordings from all the electrodes during one single drug application. This conformation can be useful for drug screenings since each molecule can be tested in tens of cells of the same kind, selected according to their membrane characteristics, and during periods that can be extended several days. In addition, the same physiological monosynaptic connection can be reproduced several times in different pairs of electrodes and tested in parallel. Finally, a key advantage of these monosynaptic connections is that the presynaptic and postsynaptic compartments can be selectively targeted by cytosol and nuclear injections of DNA, RNA, or proteins before cell culture, increasing the mechanisms that can be tested during drug perfusion in the MEA.
Conclusions
In conclusion, invertebrate models and Helix neurons, as other invertebrate neurons, have probed their capacities as a simple and useful model to study different phenomena related to epileptic behavior. Despite their phylogenetic distance to the human nervous system, this model has proven several similarities with mammalian models and has been successfully used in studies related to neurites outgrowth and branching, varicosities formation, development of functional synapses, assembly and maintenance of synaptic vesicle pools, modulatory mechanisms of single action potential firing and high-frequency spiking, and modulatory mechanisms of single-cell excitable states, among other possibilities that can be reliable and reproducibly studied in these neurons.
Acknowledgements
The author thanks María Laura Ríos, Silvia Calvo, Galit Akerman, and Delicia Ríos for their work and support in the laboratory during experiments, data analysis, literature search, and valuable discussion and Catherine Ellis Wegley for language correction.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akaike N, Lee KS, Brown AM. The Calcium Current of Helix Neuron. J Gen Physiol. 1978;71(1975):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N, Brown A, Dahl G, Higashi H, Isenberg G, Tsuda Y, Yatani A. Voltage-dependent activation f potassium currents in Helix neurones by endogenous celluar calcium. J Physiol. 1983;334:309–324. doi: 10.1113/jphysiol.1983.sp014496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altrup U. Epileptogenicity and epileptic activity: mechanisms in an invertebrate model nervous system. Curr Drug Targets. 2004;5(5):473–484. doi: 10.2174/1389450043345344. [DOI] [PubMed] [Google Scholar]
- Altrup U, Speckmann EJ. Epileptic discharges induced by pentylenetetrazol: changes of shape of dendrites. Brain Res. 1988;456:401–405. doi: 10.1016/0006-8993(88)90248-X. [DOI] [PubMed] [Google Scholar]
- Altrup U, Speckmann EJ. Identified neuronal individuals in the buccal ganglia of Helix pomatia. Neurosci Behav Physiol. 1994;24(1):23–32. doi: 10.1007/BF02355649. [DOI] [PubMed] [Google Scholar]
- Altrup U, Wiemann M. Paroxysmal depolarization shifts (PDS) induce non-synaptic responses in neighboured neurons (buccal ganglia, Helix pomatia) Brain Res. 2003;972(1–2):186–196. doi: 10.1016/S0006-8993(03)02532-0. [DOI] [PubMed] [Google Scholar]
- Altrup U, Lehmenkuhler A, E, S. J. Effects of the hypnotic drug etomidate in a model nervous system (buccal ganglia, Helix pomatia) Comp Biochem Physiol Part - C Toxicol Pharmacol. 1991;99(3):579–587. doi: 10.1016/0742-8413(91)90290-a. [DOI] [PubMed] [Google Scholar]
- Altrup U, Reith H, Speckmann E-J. Effects of valproate in a model nervous system (Buccal Ganglia of Helix Pomatia): II. Epileptogenic Actions. Epilepsia. 1992;33(4):753–759. doi: 10.1111/j.1528-1157.1992.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Altrup U, Häder M, Cáceres JL, Malcharek S, Meyer M, Galla HJ. Epileptogenic drugs in a model nervous system: electrophysiological effects and incorporation into a phospholipid layer. Brain Res. 2006;1122(1):65–77. doi: 10.1016/j.brainres.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Aneja S, Sharma S. Newer anti-epileptic drugs. Indian Pediatr. 2013;50:1033–1040. doi: 10.1007/s13312-013-0284-9. [DOI] [PubMed] [Google Scholar]
- Ayala GF, Johnston D. The mechanisms of action of diphenylhydantoin on invertebrate neurons. I. Effects on basic membrane properties. Brain Res. 1977;121:245–258. doi: 10.1016/0006-8993(77)90150-0. [DOI] [PubMed] [Google Scholar]
- Ayala GF, Johnston D, Dichter HN. The mechanisms of action of diphenylhydantoin on invertebrate neurons. II. Effects on synaptic mechanisms. Brain Res. 1977;121:259–270. doi: 10.1016/0006-8993(77)90151-2. [DOI] [PubMed] [Google Scholar]
- Azanza MJ, Pes N, Pérez-Bruzón RN, Aisa J, Raso M, Junquera C, Lahoz JM, Maestú C, Martínez-Ciriano C, Pérez-Castejón C, Vera-Gil A, Del Moral A. Localization of connexins in neurons and glia cells of the Helix aspersa suboesophageal brain ganglia by immunocytochemistry. Histol Histopathol. 2007;22:497–504. doi: 10.14670/HH-22.497. [DOI] [PubMed] [Google Scholar]
- Azanza MJ, Pérez-Castejón C, Pes N, Pérez-Bruzón RN, Aisa J, Junquera C, Maestú C, Lahoz M, Martínez-Ciriano C, Vera-Gil A, Del Moral A. Characterization by immunocytochemistry of ionic channels in Helix aspersa suboesophageal brain ganglia neurons. Histol Histopathol. 2008;23(4):397–406. doi: 10.14670/HH-23.397. [DOI] [PubMed] [Google Scholar]
- Bal R, Janahmadi M, Green GGR, Sanders DJ. Two kinds of transient outward currents, IA and IAdepol, in F76 and D1 soma membranes of the subesophageal ganglia of Helix aspersa. J Membr Biol. 2001;179(1):71–78. doi: 10.1007/s002320010038. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Fassio A, Valtorta F, Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci. 2007;27(49):13520–13531. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos Z. Los moluscos terrestres (Mollusca: Gastropoda) de Costa Rica: Clasificación, distribución y conservación. Rev Biol Trop. 2010;58(4):1165–1175. doi: 10.15517/rbt.v58i4.5402. [DOI] [PubMed] [Google Scholar]
- Battonyai I, Krajcs N, Serfőző Z, Kiss T, Elekes K. Potassium channels in the central nervous system of the snail, Helix pomatia: localization and functional characterization. Neuroscience. 2014;268:87–101. doi: 10.1016/j.neuroscience.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Brenes O, Giachello CNG, Corradi A, Ghirardi M, Montarolo PG. Synapsin knockdown is associated with decreased neurite outgrowth, functional synaptogenesis impairment, and fast high-frequency neurotransmitter release. J Neurosci Res. 2015;93(10):1492–1506. doi: 10.1002/jnr.23624. [DOI] [PubMed] [Google Scholar]
- Brenes O, Vandael DHF, Carbone E, Montarolo PG, Ghirardi M. Knock-down of synapsin alters cell excitability and action potential waveform by potentiating BK and voltage-gated Ca2+ currents in Helix serotonergic neurons. Neuroscience. 2015;311:430–443. doi: 10.1016/j.neuroscience.2015.10.046. [DOI] [PubMed] [Google Scholar]
- Brenes O, Carabelli V, Gosso S, Romero A, Carbone E, Montarolo PG, Ghirardi M. Subconvulsant doses of pentylenetetrazol uncover the epileptic phenotype of cultured synapsin-deficient Helix serotonergic neurons in the absence of excitatory and inhibitory inputs. Epilepsy Res. 2016;127:241–251. doi: 10.1016/j.eplepsyres.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Brown AM, Akaike N, Lee KS. The Calcium Conductance of Neurons. Ann N Y Acad Sci. 1978;307(1):330–344. doi: 10.1111/j.1749-6632.1978.tb41960.x. [DOI] [PubMed] [Google Scholar]
- Brown BYAM, Morimoto K, Tsuda Y, Wilson DL. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BYAM, Tsuda Y, Wilson DL. A description of inactivation and conduction in calcium channels based on tail and turn-on current measurements in the snail. J Physiol. 1983;344(1983):549–583. doi: 10.1113/jphysiol.1983.sp014956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos G, Fortuna A, Falcão A, Alves G. In vitro and in vivo experimental models employed in the discovery and development of antiepileptic drugs for pharmacoresistant epilepsy. Epilepsy Res. 2018;146(July):63–86. doi: 10.1016/j.eplepsyres.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Canavier CC, Clark JW, Byrne JH. Simulation of the bursting activity of neuron R15 in Aplysia: Role of ionic currents, calcium balance, and modulatory transmitters. J Neurophysiol. 1991;66(6):2107–2124. doi: 10.1152/jn.1991.66.6.2107. [DOI] [PubMed] [Google Scholar]
- Casadio A, Fiumara F, Sonetti D, Montarolo PG, Ghirardi M. Distribution of sensorin immunoreactivity in the central nervous system of Helix pomatia: functional aspects. J.Neurosci.Res. 2004;75(1):32–43. doi: 10.1002/jnr.10841. [DOI] [PubMed] [Google Scholar]
- Chase R. Structure and function in the cerebral ganglion. Microsc Res Tech. 2000;49:511–520. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- Chiappalone M, Massobrio P, Martinoia S. Network plasticity in cortical assemblies. Eur J Neurosci. 2008;28(1):221–237. doi: 10.1111/j.1460-9568.2008.06259.x. [DOI] [PubMed] [Google Scholar]
- Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, Martinoia S, Benfenati F. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin i-deficient cortical networks. Cereb Cortex. 2009;19(6):1422–1439. doi: 10.1093/cercor/bhn182. [DOI] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli G, Ghirardi M, Onofri F, Casadio A, Benfenati F, Montarolo PG, Vitiello F. Synapsin-like molecules in Aplysia punctata and Helix pomatia: identification and distribution in the nervous system and during the formation of synaptic contacts in vitro. Eur J Neurosci. 1996;8(12):2530–2543. doi: 10.1111/j.1460-9568.1996.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Clarac F, Pearlstein E. Invertebrate preparations and their contribution to neurobiology in the second half of the 20th century. Brain Res Rev. 2007;54(1):113–161. doi: 10.1016/j.brainresrev.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Condliffe SB, Corradini I, Pozzi D, Verderio C, Matteoli M. Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J Biol Chem. 2010;285(32):24968–24976. doi: 10.1074/jbc.M110.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell BYGA, Macon JB. Synaptic connexions of two symmetrically placed giant serotonin-containing neurones. J Physiol. 1974;236:435–464. doi: 10.1113/jphysiol.1974.sp010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K, Etter E. Modulated anticonvulsant block sodium channels in nerve and muscle. Eur J Pharmacol. 1983;88:1–9. doi: 10.1016/0014-2999(83)90386-2. [DOI] [PubMed] [Google Scholar]
- Damaj L, Lupien-meilleur A, Lortie A, Riou É, Ospina LH, Gagnon L, Vanasse C, Rossignol E. CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur J Hum Genet. 2015;23:1505–1512. doi: 10.1038/ejhg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter E, Bower JM. An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses. J Neurophysiol. 1994;71(1):401–419. doi: 10.1152/jn.1994.71.1.401. [DOI] [PubMed] [Google Scholar]
- Denton JS, McCann FV, Leiter JC. CO2 chemosensitivity in Helix aspersa: Three potassium currents mediate pH-sensitive neuronal spike timing. Am J Physiol Cell Physiol. 2007;292(1):292–305. doi: 10.1152/ajpcell.00172.2006. [DOI] [PubMed] [Google Scholar]
- Diez-Sampedro A. Mechanism of increased open probability by a mutation of the BK channel. J Neurophysiol. 2006;96(3):1507–1516. doi: 10.1152/jn.00461.2006. [DOI] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You S-A, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37(7):733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Dubberke R, Vasilets LA, Schwarz W. Inhibition of the Na+,K+ pump by the epileptogenic pentylenetetrazole. Arch Eur J Physiol. 1998;437(1):79–85. doi: 10.1007/s004240050750. [DOI] [PubMed] [Google Scholar]
- Dyakonova TL, Sultanakhmetov GS, Mezheritskiy MI, Sakharov DA, Dyakonova VE. Storage and erasure of behavioural experiences at the single neuron level. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-51331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer J, Johnston W, Castellucci VF, Dunn RJ. Cloning and tissue distribution of the Aplysia Na+ channel a-subunit cDNA. DNA Cell Biol. 1997;16(3):347–356. doi: 10.1089/dna.1997.16.347. [DOI] [PubMed] [Google Scholar]
- Farisello P, Boido D, Nieus T, Medrihan L, Cesca F, Valtorta F, Baldelli P, Benfenati F. Synaptic and extrasynaptic origin of the excitation/inhibition imbalance in the hippocampus of synapsin I/II/III knockout mice. Cerebral Cortex (New York, NY: 1991) 2013;23(3):581–593. doi: 10.1093/cercor/bhs041. [DOI] [PubMed] [Google Scholar]
- Fehér O, Erdélyi L, Papp A. The effect of pentylenetetrazol on the metacerebral neuron of Helix pomatia. Gen Physiol Biophys. 1988;7:505–516. [PubMed] [Google Scholar]
- Fisher RS, Van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshé SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Onofri F, Benfenati F, Montarolo PG, Ghirardi M. Intracellular injection of synapsin I induces neurotransmitter release in C1 neurons of Helix pomatia contacting a wrong target. Neuroscience. 2001;104(1):271–280. doi: 10.1016/s0306-4522(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Giovedì S, Menegon A, Milanese C, Merlo D, Montarolo PG, Valtorta F, Benfenati F, Ghirardi M. Phosphorylation by cAMP-dependent protein kinase is essential for synapsin-induced enhancement of neurotransmitter release in invertebrate neurons. J Cell Sci. 2004;117(Pt 21):5145–5154. doi: 10.1242/jcs.01388. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Leitinger G, Milanese C, Montarolo PG, Ghirardi M. In vitro formation and activity-dependent plasticity of synapses between Helix neurons involved in the neural control of feeding and withdrawal behaviors. Neuroscience. 2005;134(4):1133–1151. doi: 10.1016/j.neuroscience.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Milanese C, Corradi A, Giovedì S, Leitinger G, Menegon A, Montarolo PG, Benfenati F, Ghirardi M. Phosphorylation of synapsin domain A is required for post-tetanic potentiation. J Cell Sci. 2007;120(Pt 18):3228–3237. doi: 10.1242/jcs.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S, Hamon B, Heinemann U. The action of valproate on spontaneous epileptiform activity in the absence of synapsin transmission and or evoked changes in [Ca2+]o and [K+]o in the hippocampal slice. Brain Res. 1986;386:1–11. doi: 10.1016/0006-8993(86)90135-6. [DOI] [PubMed] [Google Scholar]
- Ghasemi Z, Hassanpour-Ezatti M, Kamalinejad M, Janahmadi M. Functional involvement of Ca 2+ and Ca 2+-activated K + channels in anethol-induced changes in Ca 2+ dependent excitability of F1 neurons in Helix aspersa. Fitoterapia. 2011;82(5):750–756. doi: 10.1016/j.fitote.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of aplysia. Neuron. 1995;14(2):413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Casadio A, Santarelli L, Montarolo PG. Aplysia hemolymph promotes neurite outgrowth and synaptogenesis of identified Helix neurons in cell culture. Invertebr Neurosci: IN. 1996;2(1):41–49. doi: 10.1007/BF02336659. [DOI] [PubMed] [Google Scholar]
- Ghirardi M, Naretto G, Fiumara F, Vitiello F, Montarolo PG. Target-dependent modulation of neurotransmitter release in cultured Helix neurons involves adhesion molecules. J Neurosc Res. 2001;65(2):111–120. doi: 10.1002/jnr.1134. [DOI] [PubMed] [Google Scholar]
- Giachello CNG, Fiumara F, Giacomini C, Corradi A, Milanese C, Ghirardi M, Benfenati F, Montarolo PG. MAPK/Erk-dependent phosphorylation of synapsin mediates formation of functional synapses and short-term homosynaptic plasticity. J Cell Sci. 2010;123:881–893. doi: 10.1242/jcs.056846. [DOI] [PubMed] [Google Scholar]
- Giachello CNG, Premoselli F, Montarolo PG, Ghirardi M. Pentylenetetrazol-induced epileptiform activity affects basal synaptic transmission and short-term plasticity in monosynaptic connections. PLoS One. 2013;8(2):17. doi: 10.1371/journal.pone.0056968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24(50):11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Xu Y, Kao H, Lin D, Lim S, Feng J, Greengard P, Augustine GJ. Molecular determinants of synapsin targeting to presynaptic terminals. J Neurosci. 2004;24(14):3711–3720. doi: 10.1523/JNEUROSCI.5225-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Cottrell GA. Modulation of ligand-gated dopamine channels in Helix neurones. Pflugers Arch. 1997;434(3):313–322. doi: 10.1007/s004240050402. [DOI] [PubMed] [Google Scholar]
- Green KA, Lambert JJ, Cottrell GA. Ligand-gated ion channels opened by 5-HT in molluscan neurones. Br J Pharmacol. 1996;119(3):602–608. doi: 10.1111/j.1476-5381.1996.tb15715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone BP, Baraban SC. Animal models in epilepsy research: legacies and new directions. Nat Neurosci. 2015;18(3):339–343. doi: 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- Haarmeier T, Altrup U, Speckmann EJ. Attenuation of a voltage-dependent sodium current by GABA (identified neurons, buccal ganglia, Helix pomatia. Brain Res. 1994;663(1):131–139. doi: 10.1016/0006-8993(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Haghdoost-Yazdi H, Rajaei F, Janahmadi M. Cerebellar Purkinje cells fire paroxysmal depolarization shift (PDS)-like events in response to epileptogenic drugs. Neurol Res. 2011;33(1):50–55. doi: 10.1179/016164110X12816242542454. [DOI] [PubMed] [Google Scholar]
- Hodgkin A, Huxley A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Senatore A, Dawson TF, Quan Q, Spafford JD. G-proteins modulate invertebrate synaptic calcium channel ( LCav2 ) differently from the classical voltage-dependent regulation of mammalian Cav2.1 and Cav2.2 channels. J Exp Biol. 2010;213:2094–2103. doi: 10.1242/jeb.042242. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Candiani S, Ghirardi M, Poulain B, Montarolo P. Functional roles of synapsin: lessons from invertebrates. Semin Cell Dev Biol. 2011;22(4):425–433. doi: 10.1016/j.semcdb.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Ierusalimsky VN, Zakharov IS, Palikhova TA, Balaban PM. Nervous system and neural maps in gastropod Helix lucorum L. Neurosci Behav Physiol. 1994;24(1):13–22. doi: 10.1007/BF02355648. [DOI] [PubMed] [Google Scholar]
- Imbrici P, Jaffe SL, Eunson LH, Davies NP, Herd C, Robertson R, Kullmann DM, Hanna MG. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–2692. doi: 10.1093/brain/awh301. [DOI] [PubMed] [Google Scholar]
- Johan Arief MF, Choo BKM, Yap JL, Kumari Y, Shaikh MF (2018) A systematic review on non-mammalian models in epilepsy research. Front Pharmacol 9(June). 10.3389/fphar.2018.00655 [DOI] [PMC free article] [PubMed]
- Johnston D. Phenobarbital: concentration-dependent biphasic effects on Aplysia burst-firing neurons. Neurosci Lett. 1978;10:175–180. doi: 10.1016/0304-3940(78)90031-9. [DOI] [PubMed] [Google Scholar]
- Johnston D, Ayala GF. Diphenylhydantoin: action of a common anticonvulsant on bursting pacemaker cells in Aplysia. Science. 1975;189(4207):1009–1011. doi: 10.1126/science.1220006. [DOI] [PubMed] [Google Scholar]
- Johnston WL, Dyer JR, Castelluci V, Dunn R. Clustered Na+ channels in Aplysia axons. J Neurosci. 1996;76(5):1730–1739. doi: 10.1523/JNEUROSCI.16-05-01730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N, Pandey S. Meat demand-snailed it: a comprehensive review on snail rearing, to meet the meat demand in future India. J Entomol Zool Stud. 2019;7(6):396–400. [Google Scholar]
- Kandratavicius L, Alves Balista P, Lopes-Aguiar C, Ruggiero RN, Umeoka EH, Garcia-Cairasco N, Soares Bueno-Junior L, Leite JP. Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat. 2014;10:1693–1705. doi: 10.2147/NDT.S50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapkaeva MR, Popova OV, Kondratenko RV, Rogozin PD, Genrikhs EE, Stelmashook EV, Skrebitsky VG, Khaspekov LG, Isaev NK. Effects of copper on viability and functional properties of hippocampal neurons in vitro. Exp Toxicol Pathol. 2017;69(5):259–264. doi: 10.1016/j.etp.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Kelly K, Gross R, Macdonald R. Valproic acid selectively reduces the low-threshold ( T ) calcium current in rat nodose neurons. Neurosci Lett. 1990;116:233–238. doi: 10.1016/0304-3940(90)90416-7. [DOI] [PubMed] [Google Scholar]
- Kerwin RW, Olpe H, Schmutz M. The effect of sodium-n-dipropyl acetate on y-amino-butyric acid-dependent inhibition in the rat cortex and substantia nigra in relation to its anticonvulsant activity. Br J Pharmacol. 1980;71:545–551. doi: 10.1111/j.1476-5381.1980.tb10971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketzef M, Kahn J, Weissberg I, Becker AJ, Friedman A, Gitler D. Compensatory network alterations upon onset of epilepsy in synapsin triple knock-out mice. Neuroscience. 2011;189:108–122. doi: 10.1016/j.neuroscience.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Bladen C, Parker D, Snutch T, McRoy J, Zamponi G. Effects of Ca v 3 . 2 channel mutations linked to idiopathic generalized epilepsy. Ann Neurol. 2005;57:745–749. doi: 10.1002/ana.20458. [DOI] [PubMed] [Google Scholar]
- Kile B, Guillot T, Venton B, Wetsel W, Augustine GJ, Wightman R. Synapsins differentially control dopamine and serotonin release. J Neurosci. 2010;30(29):9762–9770. doi: 10.1016/j.drugalcdep.2008.02.002.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T. Evidence for a persistent Na-conductance in identified command neurones of the snail, Helix pomatia. Brain Res. 2003;989(1):16–25. doi: 10.1016/S0006-8993(03)03316-X. [DOI] [PubMed] [Google Scholar]
- Kiss T, László Z, Pirger Z. Cellular localization and kinetic properties of Na V1.9-, Na V1.8-, and Na V1.7-like channel subtypes in Helix pomatia. Neuroscience. 2012;203:78–90. doi: 10.1016/j.neuroscience.2011.11.045. [DOI] [PubMed] [Google Scholar]
- Kiss T, Battonyai I, Pirger Z. Down regulation of sodium channels in the central nervous system of hibernating snails. Physiol Behav. 2014;131:93–98. doi: 10.1016/j.physbeh.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Kits KS, Mansvelder HD. Voltage gated calcium channels in molluscs: Classification, Ca2+ dependent inactivation, modulation and functional roles. Invertebr Neurosci. 1996;2(1):9–34. doi: 10.1007/BF02336657. [DOI] [PubMed] [Google Scholar]
- Klee MR, Faber DS, Heiss W. Strychnine- and pentylenetetrazol-induced changesnof excitability in Aplysia Neurons. Science. 1973;179:1133–1136. doi: 10.1126/science.179.4078.1133. [DOI] [PubMed] [Google Scholar]
- Klein M, Kandel ER. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee US, Cui J. {beta} subunit-specific modulations of BK channel function by a mutation associated with epilepsy and dyskinesia. J Physiol. 2009;587(Pt 7):1481–1498. doi: 10.1113/jphysiol.2009.169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Akaike N, Brown AM. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser W, Falconer SWP, Cottrell GA. Actions of FMRFamide-related peptides on the gCa2+ of the C1 neuron in Helix aspersa. Peptides. 1997;18(6):909–911. doi: 10.1016/S0196-9781(97)00010-7. [DOI] [PubMed] [Google Scholar]
- Lignani G, Raimondi A, Ferrea E, Rocchi A, Paonessa F, Cesca F, Orlando M, Tkatch T, Valtorta F, Cossette P, Baldelli P, Benfenati F. Epileptogenic Q555X SYN1 mutant triggers imbalances in release dynamics and short-term plasticity. Hum Mol Genet. 2013;22(11):2186–2199. doi: 10.1093/hmg/ddt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S, Heils A, Kasper JM, Sander T. Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. Am J Med Genet B Neuropsychiatr Genet. 2007;144(December 2005):10–13. doi: 10.1002/ajmg.b.30369. [DOI] [PubMed] [Google Scholar]
- Löscher W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–184. doi: 10.1016/j.eplepsyres.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Lukyanetz EA, Sotkis AV, Kostyuk PG. Mechanisms of up-regulation of single calcium channels by serotonin in Helix pomatia neurons. Biochem Biophys Res Commun. 2002;293(1):132–138. doi: 10.1016/S0006-291X(02)00195-X. [DOI] [PubMed] [Google Scholar]
- Lux HD, Brown AM. Patch and whole cell calcium currents recorded simultaneously in snail neurons. J Gen Physiol. 1984;83(5):727–750. doi: 10.1085/jgp.83.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36:S2–S12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- Madeja M, Mußhoff U, Lorra C, Pongs O, Speckmann E-J. Mechanism of action of the epileptogenic drug pentylenetetrazol on a cloned neuronal potassium channel. Brain Res. 1996;722(1–2):59–70. doi: 10.1016/0006-8993(96)00181-3. [DOI] [PubMed] [Google Scholar]
- Marom S, Dagan D. Calcium current in growth balls from islated Helix aspersa neuronal growth cones. Arch Eur J Physiol. 1987;409(6):578–581. doi: 10.1007/BF00584656. [DOI] [PubMed] [Google Scholar]
- Massobrio P, Tedesco M, Giachello C, Ghirardi M, Fiumara F, Martinoia S. Helix neuronal ensembles with controlled cell type composition and placement develop functional polysynaptic circuits on micro-electrode arrays. Neurosci Lett. 2009;467(2):121–126. doi: 10.1016/j.neulet.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Massobrio P, Giachello CN, Ghirardi M, Martinoia S. Selective modulation of chemical and electrical synapses of Helix neuronal networks during in vitro development. BMC Neurosci. 2013;14(1):22. doi: 10.1186/1471-2202-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe PH. New anti-epileptic drugs for the 21st century. Expert Opin Pharmacother. 2000;1(4):633–674. doi: 10.1517/14656566.1.4.633. [DOI] [PubMed] [Google Scholar]
- Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F. Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun. 2013;4:1512. doi: 10.1038/ncomms2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilleur S, Aznavour N, Descarries L, Carmant L, Mamer OA, Psarropoulou C. Pentylenetetrazol-induced seizures in immature rats provoke long-term changes in adult hippocampal cholinergic excitability. Epilepsia. 2003;44(4):507–517. doi: 10.1046/j.1528-1157.2003.44402.x. [DOI] [PubMed] [Google Scholar]
- Meiser S, Ashida G, Kretzberg J. Non-synaptic plasticity in leech touch cells. Front Physiol. 2019;10(November):1–14. doi: 10.3389/fphys.2019.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirwan N, Vyas P, Vohora D. Animal models of status epilepticus and temporal lobe epilepsy: a narrative review. Rev Neurosci. 2018;29(7):757–770. doi: 10.1515/revneuro-2017-0086. [DOI] [PubMed] [Google Scholar]
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26(1):599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Avoli M, Rogawski M, Olsen R, Delgado-Escueta AV. “Jasper’s basic mechanisms of the epilepsies” workshop. Epilepsia. 2010;51(SUPPL. 5):1–5. doi: 10.1111/j.1528-1167.2010.02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozuka M, Tsujitani M. Pentylenetetrazole suppresses the potassium current in Euhadra neurons which is coupled with Ca2+/calmodulin-dependent protein phosphorylation. Neurosci Res. 1991;11(2):146–153. doi: 10.1016/0168-0102(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Kishii K, Furuichi H, Sugaya E. Behavior of intracellular cyclic nucleotide and calcium in pentylenetetrazole-induced bursting activity in snail neurons. Brain Res. 1983;269(2):277–286. doi: 10.1016/0006-8993(83)90137-3. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Kubo K-Y, Ozono S. The molecular mechanism underlying pentylenetetrazole-induced bursting activity in Euhadra neurons: involvement of protein phosporilation. Comp Biochem Physiol Part - C Toxicol Pharmacol. 1991;100(3):423–432. doi: 10.1016/0742-8413(91)90019-P. [DOI] [PubMed] [Google Scholar]
- Orenbuch A, Shalev L, Marra V, Sinai I, Lavy Y, Kahn J, Burden JJ, Staras K, Gitler D. Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals. J Neurosci. 2012;32(12):3969–3980. doi: 10.1523/JNEUROSCI.5058-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Howlett IC, Rusan ZM, Tanouye MA. Seizure and epilepsy: studies of seizure disorders in drosophila. Int Rev Neurobiol. 2011;99:1–21. doi: 10.1016/B978-0-12-387003-2.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Lopicic S, Bratic-Stanojevic M, Pavlovic D, Andjus PR, Nedeljkov V. Modulation of nickel-induced bursting with 4-aminopyridine in leech Retzius nerve cells. Arch Biol Sci. 2010;62(4):1035–1045. doi: 10.2298/ABS1004035P. [DOI] [Google Scholar]
- Piggott SM, Kerkut GA, Walker RJ. Structure-activity studies on glutamate receptor sites of three identifiable neurones in the sub-oesophageal ganglia of Helix aspersa. Comp Biochem Physiol Part C Comp. 1975;51(1):91–100. doi: 10.1016/0306-4492(75)90044-1. [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167(3926):1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Pirger Z, Battonyai I, Krajcs N, Elekes K, Kiss T. Voltage-gated membrane currents in neurons involved in odor information processing in snail procerebrum. Brain Struct Funct. 2014;219(2):673–682. doi: 10.1007/s00429-013-0526-6. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10(2):173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- Radovic N, Gordana K, Nesic O. The presence of different glutamate receptors on the feeding modulatory interneuron. Logoslav Physiol Pharmacol Acta. 1998;34:221–230. [Google Scholar]
- Redecker C, Altrup U, Hoppe D, Düsing R, Speckmann EJ. Effects of valproate derivatives I. Antiepileptic efficacy of amides, structural analogs and esters. Neuropharmacology. 2000;39(2):254–266. doi: 10.1016/S0028-3908(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Redecker C, Altrup U, Hoppe D, Hense T, Kreier A, Rabe A, Düsing R, Speckmann EJ. Effects of valproate derivatives II. Antiepileptic efficacy in relation to chemical structures of valproate sugar esters. Neuropharmacology. 2000;39(2):267–281. doi: 10.1016/S0028-3908(99)00101-X. [DOI] [PubMed] [Google Scholar]
- Reece PJ, Dholakia K, Thomas RC, Cottrell GA. Green laser light (532 nm) activates a chloride current in the C1 neuron of Helix aspersa. Neurosci Lett. 2008;433(3):265–269. doi: 10.1016/j.neulet.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Rodin EA, Calhoun HD. Metrazol tolerance in a normal volunteer population. A ten year follow-up report. J Nerv Ment Dis. 1970;150(6):438–443. doi: 10.1097/00005053-197006000-00003. [DOI] [PubMed] [Google Scholar]
- Sallam A, El-Wakeil N. Biological and ecological studies on land snails and their control. In: Soloneski S, editor. Integrated Pest Management and Pest Control - Current and Future Tactics (Issue 1) InTech; 2012. [Google Scholar]
- Saviane C, Savtchenko LP, Raffaelli G, Voronin LL, Cherubini E. Frequency-dependent shift from paired-pulse facilitation to paired-pulse depression at unitary CA3-CA3 synapses in the rat hippocampus. J Physiol. 2002;544(Pt 2):469–476. doi: 10.1113/jphysiol.2002.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmold N, Syed NI. Molluscan neurons in culture: shedding light on synapse formation and plasticity. J Mol Histol. 2012;43(4):383–399. doi: 10.1007/s10735-012-9398-y. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23(2):399–409. doi: 10.1016/S0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25(4):206–212. doi: 10.1016/S0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Senatore A, Spafford JD. Transient and big are key features of an invertebrate T-type channel ( LCav3 ) from the central nervous system of Lymnaea stagnalis *. J Biol Chem. 2010;285(10):7447–7458. doi: 10.1074/jbc.M109.090753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruti S, Clem RL, Barth AL. NIH Public Access. Sci-New York. 2009;30(3):323–330. doi: 10.1016/j.nbd.2008.02.002.A. [DOI] [Google Scholar]
- Sotkis AV, Kostyuk PG, Lukyanetz EA. Diversity of single potassium channels in isolated snail neurons. NeuroReport. 1998;9(7):1413–1417. doi: 10.1097/00001756-199805110-00030. [DOI] [PubMed] [Google Scholar]
- Souza IA, Gandini MA, Zhang F, Mitchell WG, Matsumoto J, Lerner J, Pierson TM, Zamponi GW. Pathogenic Cav3 . 2 channel mutation in a child with primary generalized epilepsy. Mol Brain. 2019;12:5–10. doi: 10.1186/s13041-019-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spafford JD, Munno DW, Van Nierop P, Feng Z, Jarvis SE, Gallin WJ, Smit AB, Zamponi GW, Syed NI. Calcium channel structural determinants of synaptic transmission between identified invertebrate neurons *. J Biol Chem. 2003;278(6):4258–4267. doi: 10.1074/jbc.M211076200. [DOI] [PubMed] [Google Scholar]
- Spafford JD, Dunn T, Smit AB, Syed NI, Zamponi GW, David J, Dunn T, Smit AB, Syed NI, Zamponi GW. In vitro characterization of L-type calcium channels and their contribution to firing behavior in invertebrate respiratory neurons. J Neurophysiol. 2006;95:42–52. doi: 10.1152/jn.00658.2005. [DOI] [PubMed] [Google Scholar]
- Speckmann E-J, Caspers H. Paroxysmal depolarization and changes in action potentials induced by pentylenetetrazol in isolated neurons of Helix pomatia. Epilepsia. 1973;14(4):397–408. doi: 10.1111/j.1528-1157.1973.tb03979.x. [DOI] [PubMed] [Google Scholar]
- Squires RF, Saederup E, Crawley JN, Skolnick P, Paul SM. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984;35:1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Standen NB. Voltage-clmp studies of the calcium inward current in an identified snail neurone: comparison with the sodium inward current. J Physiol. 1975;249:253–268. doi: 10.1113/jphysiol.1975.sp011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5(5):400–408. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- Terada S, Tsujimoto T, Takei Y, Takahashi T, Hirokawa N. Impairment of inhibitory synaptic transmission in mice lacking synapsin I. J Cell Biol. 1999;145(5):1039–1048. doi: 10.1083/jcb.145.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of y-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1988;25(3):214–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Vandael DHF, Ottaviani MM, Legros C, Lefort C, Guérineau NC, Allio A, Carabelli V, Carbone E. Reduced availability of voltage-gated sodium channels by depolarization or blockade by tetrodotoxin boosts burst firing and catecholamine release in mouse chromaffin cells. J Physiol. 2015;593(4):905–927. doi: 10.1113/jphysiol.2014.283374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden J, Witte OW, Speckmann EJ, Elger CE. Reduction neurons of calcium currents in identified neurons of Helix pomatiia: intracellular injection of D890. Comp Biochem Physiol Part - C Toxicol Pharmacol. 1984;77(2):211–217. doi: 10.1016/0742-8413(84)90004-5. [DOI] [PubMed] [Google Scholar]
- Walden J, Speckmann EJ, Witte OW. Membrane currents induced by pentylenetetrazol in identified neurons of Helix pomatia. Brain Res. 1988;473(2):294–305. doi: 10.1016/0006-8993(88)90858-X. [DOI] [PubMed] [Google Scholar]
- Walden J, Altrup U, Reith H, Speckmann EJ. Effects of valproate on early and late potassium currents of single neurons. Eur Neuropsychopharmacol. 1993;3(2):137–141. doi: 10.1016/0924-977X(93)90265-N. [DOI] [PubMed] [Google Scholar]
- Wang B, Rothberg BS, Brenner R. Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J Gen Physiol. 2009;133(3):283–294. doi: 10.1085/jgp.200810141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Bochorishvili G, Chen Y, Salvati KA, Zhang P, Dubel SJ, Perez-reyes E, Snutch TP, Stornetta RL, Deisseroth K, Erisir A, Todorovic SM, Luo J, Kapur J, Beenhakker MP, Zhu JJ. CaV3.2 calcium channels control NMDA receptor-mediated transmission : a new mechanism for absence epilepsy. Genes Dev. 2015;29:1535–1551. doi: 10.1101/gad.260869.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiergräber M, Henry M, Krieger A, Kamp M, Radhakrishnan K, Hescheler J, Schneider T. Altered seizure susceptibility in mice lacking the Cav2.3 E-type Ca2+ channel. Epilepsia. 2006;47(5):839–850. doi: 10.1111/j.1528-1167.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Krishnamoorthy G, Saxena A, Zhang G, Shi J, Yang H, Delaloye K, Sept D, Cui J. An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing. Neuron. 2010;66(6):871–883. doi: 10.1016/j.neuron.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]