Abstract
This study aimed to evaluate the effects of cutting height, heterofermentative microbial inoculants, and storage length on the fermentation profile and nutrient composition of whole-plant corn silage. The experiment was a completely randomized design with a 2 (cutting height) × 3 (microbial inoculation) × 5 (storage length) factorial arrangement of treatments. Corn forage was harvested at two cutting heights: either 25 cm (REG) or 65 cm (HI). Then, forage was inoculated with one of three microbial inoculants: (1) 300,000 CFU/g of fresh forage of Pediococcus acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074 (LBLD; Bonsilage Speed inoculant, Provita Supplements Inc., Mendota Heights, MN), (2) 500,000 CFU/g of fresh forage of Lactiplantibacillus plantarum DSM 12837 and L. buchneri DSM 16774 (LPLB; Bonsilage Corn + WS inoculant, Provita Supplements Inc., Mendota Heights, MN), or (3) distilled water (CON). Last, forage was randomly assigned to ferment for 5, 7, 14, 28, or 56 d of storage in vacuum-sealed bags. Silage pH was affected by a three-way interaction (P = 0.01), where CON treatments decreased continually over time while LPLB and LBLD began to increase at later storage lengths. Acetic acid concentration was greater (P = 0.001) in LPLB and LBLD than CON silage after 56 d of storage. Silage treated with LBLD did not have detectable levels of propionic acid (P > 0.05), although 1-propanol concentration was greater (P = 0.001) in LBLD treatments after 56 d of storage. The concentrations of total acids and acetic acid were greater (P = 0.01 and P = 0.001, respectively) for REG silage compared to HI. Additionally, HI silage had greater (P = 0.001 and P = 0.001, respectively) concentrations of dry matter (DM) and starch, while neutral detergent fiber (aNDF) and lignin concentrations were lower (P = 0.001 and P = 0.001, respectively) in HI silage compared to REG silage. Last, HI silage had a greater (P = 0.001) NDF digestibility than REG silage. The results of this study demonstrate that increasing cutting height can improve nutrient composition of whole-plant corn silage. Additionally, results demonstrate that heterofermentative microbial inoculants can be used to shift silage fermentation to the production of lactic and acetic acids.
Keywords: 1-propanol, heterofermentative, Lentilactobacillus buchneri, Lentilactobacillus diolivorans, neutral detergent fiber digestibility, propionic acid
INTRODUCTION
Whole-plant corn silage (WPCS) is one of the most prevalent forage sources for dairy producers worldwide because it contains the energy and physically effective fiber required to support lactation. Because of its importance, producers constantly seek practices to improve the quality and nutritive value of WPCS by increasing digestibility, altering nutrient composition, and manipulating fermentation profile.
There are many management practices that can increase digestibility and improve nutrient composition of WPCS (Ferraretto et al., 2018; Adesogan et al., 2019). For example, producers can increase cutting height to improve silage nutritive value through increased starch concentrations and NDF digestibility (NDFD), increasing milk production in dairy cows (Ferraretto et al., 2018). Moreover, despite potential decreases in dry matter (DM) yield since more stover is left in the field, the greater predicted milk yield from high cut WPCS has the potential to offset the lower yields (Ferraretto et al., 2018). Increasing cutting height may also impact fermentation profile. Although increasing cutting height did not change the concentration of organic acids, increased ethanol concentration and improved aerobic stability were reported by Aoki et al. (2013). Additionally, Mendonça et al. (2020) observed lower yeast counts and acetic acid concentration in high cut WPCS. It is possible the use of different microbial inoculants may improve fermentation, by either decreasing pH or producing antifungal acids that improve aerobic stability (Muck et al., 2018) when cutting silage at greater heights, but investigations into the interaction between these factors are scarce in the literature.
Heterofermentative microbial inoculants are well-known to shift fermentation toward antifungal acids thereby improving aerobic stability (Arriola et al., 2021). The most common heterofermentative bacterium is Lentilactobacillus buchneri which has consistently improved aerobic stability in a variety of silage types (Arriola et al., 2021) by converting a portion of lactic acid into acetic acid and 1,2-propanediol (Oude Elferink et al., 2001). Recently, the use of L. diolivorans has been revisited (Diepersloot et al., 2021) as it can convert 1,2-propanediol into propionic acid or 1-propanol (Krooneman et al., 2002). It is possible that these two bacteria could complement each other and modulate silage fermentation to a greater extent than individually, but to the best our knowledge, this information is unavailable in the literature.
Therefore, the objective of this experiment was to evaluate the effects of cutting height, microbial inoculation, and storage length on the fermentation profile and nutrient composition of WPCS. We hypothesized that microbial inoculation would increase the production of acetic and propionic acid, especially when inoculating with the combination containing Lentilactobacillus diolivorans and that these effects would increase with storage length. Additionally, we hypothesized that increasing cutting height would improve nutrient composition and NDFD but would reduce the extent of fermentation.
MATERIALS AND METHODS
Silage Preparation and Treatments
Whole-plant corn forage (DS-4816AMXT; Dairyland Seed, Kewaskum, WI) was obtained from the University of Wisconsin-Madison Arlington Agricultural Research Station (Arlington, WI) on September 8, 2020 at approximately 33% DM. Forage was harvested with a self-propelled forage harvester (Claas of America, Omaha, NE) set with a theoretical length of cut of 26 mm, a kernel processor with a roll gap setting of 2 mm, and no inoculant applied at the harvester. Corn silage was cut at a height of either 25 or 65 cm (REG and HI, respectively) from four random locations within the same field (each location was considered a replication). To prevent contamination of forage from inoculant residue of previous harvests, approximately 3 m of forage from eight rows were harvested and discarded prior to harvesting experimental samples. After harvest, forage from each location (replicate) and cutting height was homogenized, and subsamples were collected (n = 8) for nutrient characterization. Subsamples of approximately 700 g each from each location and cutting height were randomly assigned to 1 of 15 treatments which were a combination of three microbial inoculant treatments and five storage lengths. Microbial inoculant treatments were applied by hand and consisted of: (1) 300,000 CFU/g of fresh forage of Pediococcus acidilactici DSM 16243, L. buchneri DSM 12856, and L. diolivorans DSM 32074 (LBLD; Bonsilage Speed, Provita Supplements Inc., Mendota Heights, MN), (2) 500,000 CFU/g of fresh forage of Lactiplantibacillus plantarum DSM 12837 and L. buchneri DSM 16774 (LPLB; Bonsilage Corn + WS inoculant, Provita Supplements Inc., Mendota Heights, MN), or 15 mL distilled water (CON). Inoculation rates were based on counts determined by pour plating on Man, Rogosa, and Sharpe agar (Oxoid, Basingstoke, United Kingdom) by incubating under anaerobic conditions at 32°C for 48 h and followed manufacturer recommendations of application rate. After microbial inoculation, whole-plant corn forage was packed into nylon-polyethylene standard barrier vacuum pouches (0.09 mm thick, 25.4 × 35.6 cm; Doug Care Equipment Inc., Springerville, CA) and sealed using an external clamp vacuum machine (Bestvac; distributed by Doug Care Equipment) before being randomly assigned to storage lengths of 5, 7, 14, 28, or 56 d. Mini-silos were stored in the dark at room temperature (approximately 22°C), until the appropriate storage length was achieved. Thus, this experiment consisted of 30 treatments (2 cutting heights × 3 microbial inoculants × 5 storage lengths) in quadruplicate (total of 120 mini-silos).
Sample Preparation and Analysis
Approximately 50 g of fresh samples were dried in duplicate at 60°C for 48 h in a forced air oven to determine DM concentration, and 250 g was frozen for later analysis. Frozen fresh samples were submitted to a commercial laboratory (Rock River Laboratory Inc.; Watertown, WI) for analysis of crude protein (CP), neutral detergent fiber using α-amylase and sodium sulfite (aNDF), water-soluble carbohydrates (WSC), starch, 30 h in vitro ruminal NDF digestibility (NDFD), 240 h in vitro ruminal undigested NDF (uNDF), and 7 h in vitro ruminal starch disappearance (StarchD) using near-infrared reflectance spectroscopy (NIRS). Samples were dried as described before and ground to 1-mm (Cyclone sample mill; UDY Corporation, Fort Collins, CO) before being analyzed by NIRS using a using a Foss 5000 (Foss North America Inc., Eden Prairie, MN). Calibration equations for NIRS analysis of CP, aNDF, WSC, and starch were based on procedures described in AOAC method 990.03 (AOAC International, 2012), AOAC method 2002.04 (AOAC International, 2012), Dubois et al. (1956), AOAC method 920.39 (AOAC International, 2012), and Hall (2015), respectively. Calibration equations for NIRS analysis of starch disappearance were based on ruminal in situ incubation procedures described by Vanzant et al. (1998). Calibration equations for NIRS analysis of NDFD and uNDF were based on in vitro incubation procedures described by Goeser et al. (2009). Samples were also analyzed for concentration of borate-phosphate-soluble crude protein (SCP) by wet chemistry according to the method by Krishnamoorthy et al. (1982).
When mini-silos reached their assigned storage lengths, vacuum pouches were opened and duplicate 50 g samples were dried to determine DM concentration as described before. In addition, approximately 250 g samples were frozen and later sent to a commercial laboratory (Rock River Laboratory Inc.; Watertown, WI) for determination of fermentation profile (organic acids, alcohols, ammonia-N, and pH) by wet chemistry and nutrient composition via NIRS, as described previously. Fermented samples were analyzed for pH, ammonia-N, organic acids (lactic acid, acetic acid, butyric acid, and propionic acid), and alcohols (ethanol, 1,2-propanediol, 1-propanol, 2,3-butanediol, and 2-butanol). Twenty grams of undried, unground sample was diluted 10-fold (mass basis) in double-distilled water, blended for 30 s in a high-speed blender, and filtered through a filter funnel with a screen (2-mm pore size). The extract was collected and analyzed for pH using a pH meter (Thermo-Orion Dual Star; Thermo Fisher Scientific Inc., Waltham, MA) fitted with a glass pH electrode (Thermo-Orion 9172BNWP; Thermo Fisher Scientific Inc., Waltham, MA). After pH was measured, the extract was centrifuged (750 × g) for 20 min at 25°C, and the supernatant was combined with 1.0 mL of calcium hydroxide solution and 0.5 mL of copper sulfate solution and re-centrifuged as described previously. The supernatant was analyzed for organic acids and alcohols using high-performance liquid chromatography with an isocratic pump, auto sampler, column heater, and refractive index detector (1515, 2707, heater, and 2414, respectively; Waters Corporation, Milford, MA) and a reverse-phase ion exclusion column (Bio-Rad Aminex HPX-876H; Bio-Rad Laboratories, Hercules, CA). Flow rate and temperature were set at 0.6 mL/min and 35°C, respectively. The mobile phase used was 0.015 N H2SO4/0.25 mM EDTA. For ammonia-N analysis, fresh sample (5 g) was diluted in 100 mL of distilled water and mixed for 30 min using a magnetic stir plate. Measurements were performed using a pH/ion selective electrode meter (Thermo-Orion 9172BNWP; Thermo Fisher Scientific Inc.) fitted with an ammonia-specific electrode (Orion High-Performance Ammonia Electrode; Thermo Fisher Scientific Inc.) equipped with a hydrophobic gas permeable membrane. The membrane allows dissolved ammonia to diffuse across, separating ammonia-N from the rest of the solution. The probe was submerged into the solution, 1 mL of 10 N NaOH was added, and ammonia-N was recorded. Total acid production was calculated as the sum of all organic acids measured in this experiment.
Statistical Analysis
Data were analyzed as a completely randomized design with a 2 (cutting height) × 3 (microbial inoculant) × 5 (storage length) factorial arrangement of treatments using PROC GLIMMIX of SAS (version 9.4; SAS Institute Inc., Cary, NC). Fixed effects included cutting height, microbial inoculation, storage length, and their two- and three-way interactions. Means were determined using the LSMEANS statement and were compared using the sequentially rejective Bonferroni t-test adjustment after an overall significant F-test. Interaction effects were partitioned using the SLICE option to examine the effects of cutting height and microbial inoculation within each storage length. Significance was declared when P ≤ 0.05.
RESULTS
Nutrient composition of fresh, uninoculated whole-plant corn forage for both HI and REG are in Table 1. Briefly, REG forage had lower DM and starch concentrations, NDFD, and predicted milk yield, but greater aNDF concentration.
Table 1.
The nutrient composition of regular cut and high cut fresh, uninoculated whole-plant corn forage.
| Item1 | Regular cut2 | High cut3 | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| DM, % as fed | 33.5 | 1.10 | 35.8 | 2.24 |
| CP, % DM | 6.3 | 0.41 | 6.2 | 0.41 |
| aNDF, % DM | 38.9 | 3.07 | 34.6 | 2.16 |
| Lignin, % DM | 3.3 | 0.38 | 2.8 | 0.20 |
| Starch, % DM | 39.0 | 3.43 | 44.1 | 2.57 |
| WSC, % DM | 8.9 | 0.69 | 8.1 | 0.39 |
| NDFD 30 h, % aNDF | 65.2 | 2.94 | 69.0 | 1.76 |
| uNDF 240 h,% DM | 7.8 | 1.66 | 6.1 | 0.81 |
| StarchD 7 h,% Starch | 76.5 | 1.78 | 74.4 | 0.69 |
| Milk yield4, kg/Mg | 1236 | 59 | 1306 | 43 |
DM: dry matter; CP: crude protein; ADF: acid detergent fiber; aNDF: neutral detergent fiber; WSC: water soluble carbohydrates; EE: ether extract; NDFD: neutral detergent fiber digestibility after 30 h; uNDF: undigested neutral detergent fiber after 240 h; StarchD: starch digestibility after 7 h.
25 cm cutting height.
65 cm cutting height.
Predicted milk yield (per unit forage) according to the Milk 2006 spreadsheet.
Probability significance values (P values) for cutting height, microbial inoculation, storage length, and their two- and three-way interactions are in Table 2. Two-way interactions are only presented and discussed if a three-way interaction was not significant (P > 0.05), whereas main effects are presented and discussed if no interaction effects were detected (P > 0.05). Even though measurements of propionic and butyric acids, 2,3-butanediol, and 2-butanol were performed, these fermentation end-products were not detected among any treatment combinations. Therefore, these variables will not be presented or discussed.
Table 2.
The statistical analysis (P-values) of the effect of cutting height (CH), microbial inoculation (MI), storage length (SL), and their interactions on the fermentation profile and nutrient composition of whole-plant corn silage1
| Item2 | CH | MI | SL | CH × MI | CH × SL | MI × SL | CH × MI × SL |
|---|---|---|---|---|---|---|---|
| pH | 0.82 | 0.001 | 0.001 | 0.02 | 0.001 | 0.001 | 0.01 |
| Total acids, % DM | 0.001 | 0.01 | 0.001 | 0.51 | 0.10 | 0.01 | 0.20 |
| Lactic acid, % DM | 0.01 | 0.01 | 0.001 | 0.63 | 0.01 | 0.001 | 0.22 |
| Acetic acid, % DM | 0.001 | 0.001 | 0.001 | 0.91 | 0.07 | 0.001 | 0.15 |
| 1,2-Propanediol, % DM | 0.09 | 0.001 | 0.001 | 0.01 | 0.11 | 0.001 | 0.001 |
| 1-Propanol, % DM | 0.18 | 0.01 | 0.001 | 0.001 | 0.01 | 0.001 | 0.02 |
| Ethanol, % DM | 0.47 | 0.001 | 0.001 | 0.02 | 0.25 | 0.001 | 0.01 |
| DM, % as fed | 0.001 | 0.81 | 0.10 | 0.74 | 0.15 | 0.85 | 0.97 |
| WSC, % DM | 0.21 | 0.15 | 0.001 | 0.001 | 0.62 | 0.001 | 0.17 |
| CP, % DM | 0.001 | 0.85 | 0.74 | 0.50 | 0.05 | 0.41 | 0.70 |
| SCP, % CP | 0.001 | 0.001 | 0.001 | 0.001 | 0.02 | 0.02 | 0.07 |
| Ammonia-N, % CP | 0.37 | 0.01 | 0.001 | 0.16 | 0.75 | 0.87 | 0.35 |
| aNDF, % DM | 0.001 | 0.99 | 0.06 | 0.20 | 0.62 | 0.43 | 0.53 |
| Lignin, % DM | 0.001 | 0.65 | 0.07 | 0.06 | 0.15 | 0.08 | 0.63 |
| NDFD 30 h, % aNDF | 0.001 | 0.34 | 0.59 | 0.10 | 0.06 | 0.12 | 0.25 |
| uNDF 240 h, % DM | 0.001 | 0.84 | 0.20 | 0.02 | 0.15 | 0.08 | 0.58 |
| Starch, % DM | 0.001 | 0.15 | 0.05 | 0.75 | 0.68 | 0.13 | 0.52 |
| StarchD 7 h, % Starch | 0.21 | 0.001 | 0.001 | 0.001 | 0.34 | 0.001 | 0.04 |
CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/ g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage.
DM: dry matter; CP: crude protein; SCP: soluble crude protein; aNDF: neutral detergent fiber; WSC: water soluble carbohydrates; NDFD: neutral detergent fiber digestibility after 30 h; uNDF: undigested neutral detergent fiber after 240 h; StarchD: starch digestibility after.
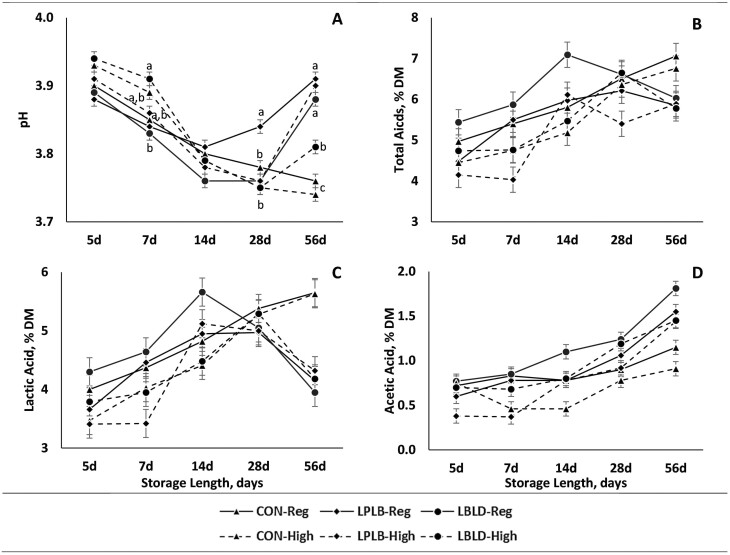
A three-way interaction was observed for pH (Figure 1A; P = 0.01). At 7 d of storage, HI-LBLD was greater than REG-LBLD, while no other treatment combinations differed. At 28 d of storage, pH was greater for REG-LPLB than all other treatment combinations. At 56 d of storage REG-LPLB, REG-LBLD, and HI-LPLB had the greatest pH values, while HI-LBLD was intermediate, and REG and HI treatments in CON silage were the lowest. The concentration of total acids was affected by the interaction of microbial inoculation × storage length and by the main effect of cutting height (Figure 1B; P = 0.01 and P = 0.001, respectively). Even though an interaction of microbial inoculation × storage length was detected, no differences among microbial inoculants were observed at any storage length after the Bonferroni correction. Total acid concentration was 0.5 percentage-units lower for HI silage compared to REG silage. Lactic acid concentration was affected by interactions of microbial inoculation × storage length and cutting height × storage length (Figure 1C; P = 0.001 and P = 0.01, respectively). For the interaction of microbial inoculation × storage length, the concentration of lactic acid was 1.5 percentage-units greater for CON than LPLB and LBLD at 56 d of storage. However, no other differences among treatments were observed for any other storage lengths. For the interaction of cutting height × storage length, REG silage had a 0.7 percentage-units greater concentration of lactic acid than HI cut silage at 7 d of storage. However, no differences in cutting heights were observed at any other storage length. Acetic acid was affected by the interaction of microbial inoculation × storage length and the main effect of cutting height (Figure 1D; P = 0.001 and P = 0.001, respectively). For the interaction of microbial inoculation × storage length, at 14 and 28 d of storage LBLD had a 0.4 percentage-unit greater concentration of acetic acid than CON, while LPLB did not differ from either. At 56 d of storage, CON was 0.6 percentage-units lower than LPLB and LBLD silage, on average. Additionally, HI silage had 0.2 percentage-units lower acetic acid concentration than REG silage.
Figure 1.
The effect of cutting height (CH), microbial inoculant (MI), and storage length (SL) on pH, total acids, lactic acid, and acetic acid. a,b,c Different superscripts denote differences in sliced effects within day, if present. Treatments consist of: CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage. (A) pH; P = 0.01; SEM = 0.01. (B) Total acids, % DM (Dry Matter); P = 0.20; SEM = 0.31. (C) Lactic acid, % DM; P = 0.22; SEM = 0.24. (D) Acetic acid, % DM; P = 0.15; SEM = 0.08.
A three-way interaction was observed for 1,2-propanediol (Figure 2A; P = 0.001). At 14 d of storage, REG-LBLD was greater than all other treatment combinations, except for HI-LBLD which did not differ from any treatment combinations. At 28 d of storage, REG-LPLB had the greatest concentration of 1,2-propanediol, although HI-LBLD did not differ. Additionally, the concentration of 1,2-propnediol was intermediate for REG-LBLD, while REG-CON and HI-CON were lowest. Last, the concentration of 1-2-propanediol of HI-LPLB did not differ from REG-LBLD or either CON treatment. At 56 d of storage, REG-LPLB and HI-LPLB had the greatest concentration of 1,2-propanediol, followed by REG-LBLD and HI-LBLD, while REG-CON and HI-CON had the lowest concentrations. Similarly, a three-way interaction was observed for 1-propanol (Figure 2B; P = 0.01). At 56 d of storage, the concentration of 1-propanol in REG-LBLD was greatest, while HI-LBLD was intermediate, and HI-LPLB and both CON treatments were lowest. However, REG-LPLB did not differ from HI-LBLD, HI-LPLB or either CON treatment. There was a three-way interaction for ethanol concentration (Figure 2C; P = 0.01). At 5 d of storage, HI-LBLD was greater than HI-LPLB, while there were no differences among other treatment combinations. At 14 d of storage, HI-LPLB had greater concentration of ethanol than HI-CON, while there were no differences among other treatment combinations. At 28 d of storage, ethanol concentrations in HI-LPLB and HI-LBLD were greater than HI-CON, while there were no differences among other treatment combinations.
Figure 2.
The effect of cutting height (CH), microbial inoculant (MI), and storage length (SL) on 1,2-propanediol, 1-propanol, and ethanol. a,b,c Different superscripts denote differences in sliced effects within day, if present. Treatments consist of: CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage. (A) 1,2-Propanediol, % DM (dry matter); P = 0.001; SEM = 0.02. (B) 1-Propanol, % DM; P = 0.02; SEM = 0.02. (C) Ethanol, % DM; P = 0.01; SEM = 0.13.
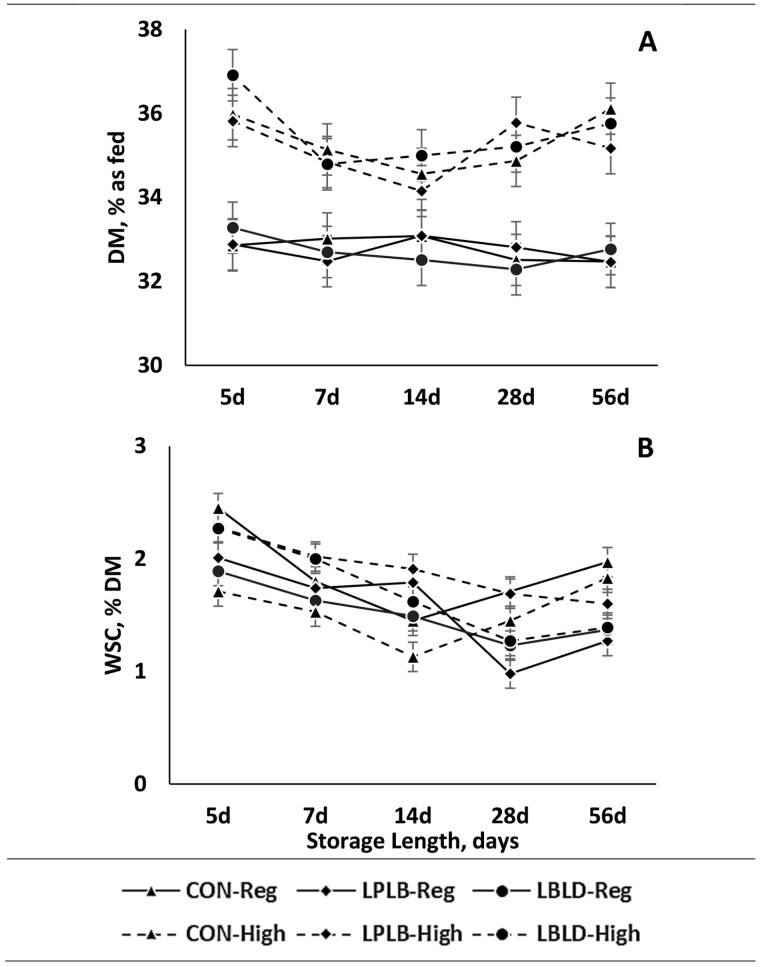
The concentration of DM was affected by cutting height (Figure 3A; P = 0.001). The HI silage had 2.5 percentage-units greater DM than REG silage. Interactions between cutting height × microbial inoculation and microbial inoculation × storage length were observed for WSC (Figure 3B; P = 0.001 and P = 0.001, respectively). For the interaction of cutting height × microbial inoculation, REG-CON and HI-LPLB were 0.4 percentage-units greater than other treatments, although HI-LBLD did not differ from REG-CON or HI-LPLB. Additionally, REG-LPLB, REG-LBLD and HI-CON had a 0.3 percentage-units lower concentration of WSC. For the interaction of microbial inoculation × storage length, at 14 d of storage LPLB had a 0.6 percentage-units greater concentration than CON while LBLD did not differ from either. Additionally, at 56 d of storage, CON had a 0.4 percentage-units greater WSC concentration than LBLD, while LPLB did not differ from either.
Figure 3.
The effect of cutting height (CH), microbial inoculant (MI), and storage length (SL) on DM (dry matter) and WSC (water-soluble carbohydrates). a,b,c Different superscripts denote differences in sliced effects within day, if present. Treatments consist of: CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage. (A) DM, % as fed; P = 0.97; SEM = 0.61. (B) WSC, % DM; P = 0.17; SEM = 0.13.
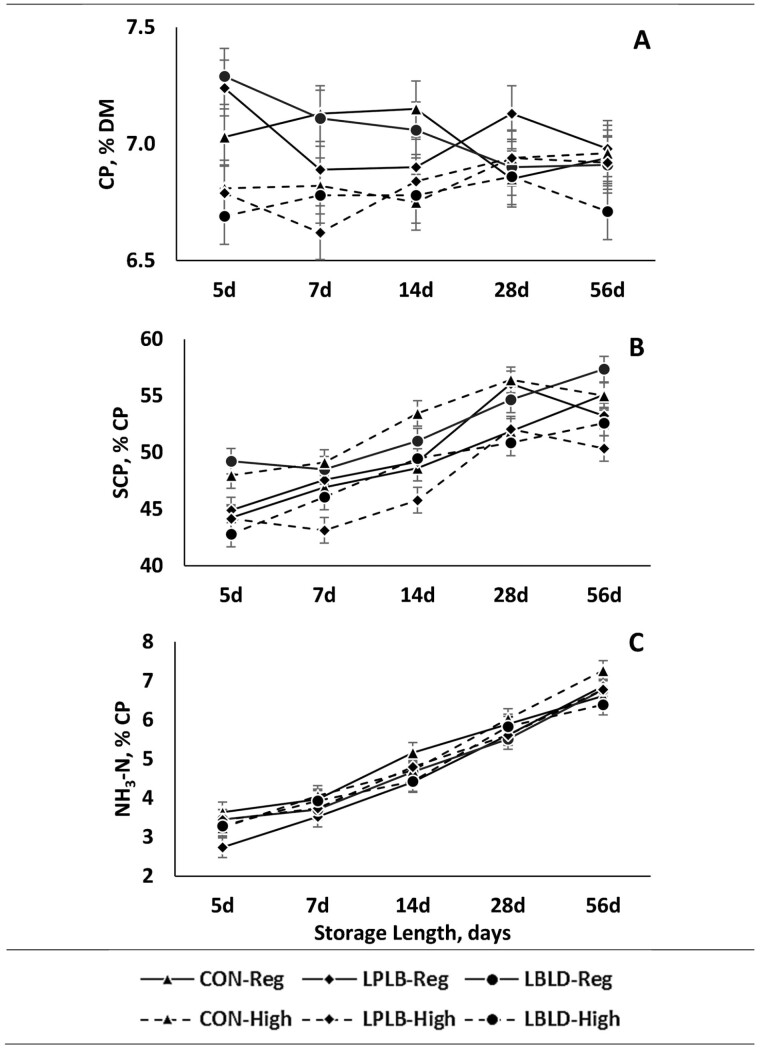
Crude protein concentration was affected by the interaction of cutting height × storage length (Figure 4A; P = 0.01). At 5 d of storage, REG silage was 0.3 percentage-units greater than HI silage. However, there were no differences between cutting heights for other storage lengths. The concentration of SCP was affected by the interactions of cutting height × microbial inoculation, cutting height × storage length, and microbial inoculation × storage length (Figure 4B; P = 0.001, P = 0.02, and P = 0.02, respectively). For the interaction of cutting height × microbial inoculation, HI-CON and REG-LBLD were on average 3.4 percentage-units greater than other treatments, although REG-LPLB did not differ from HI-CON or REG-LBLD. Additionally, HI-LPLB was on average 3.0 percentage-units lower than other treatments, although REG-CON and HI-LBLD did not differ from HI-LPLB. Last, there were no differences among treatments REG-LPLB, REG-CON, and HI-LBLD. Although interactions of cutting height × storage length and microbial inoculation × storage length were detected for SCP, there were no differences among cutting heights or microbial inoculants for any storage lengths after Bonferroni corrections. Ammonia was affected by both microbial inoculation and storage length (Figure 4C; P = 0.01 and P = 0.001, respectively). The concentration of ammonia was 0.3 percentage-units greater in CON silage than LPLB or LBLD. Additionally, the concentration of ammonia increased 3.5 percentage-units over time, from 5 d to 56 d of storage.
Figure 4.
The effect of cutting height (CH), microbial inoculant (MI), and storage length (SL) on CP (crude protein), SCP (soluble crude protein), and NH3-N. a,b,c Different superscripts denote differences in sliced effects within day, if present. Treatments consist of: CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage. (A) CP, % DM (dry matter); P = 0.70; SEM = 0.12. (B) SCP, % CP; P = 0.07; SEM = 1.13. (C) NH3-N, % CP; P = 0.35; SEM = 0.26.
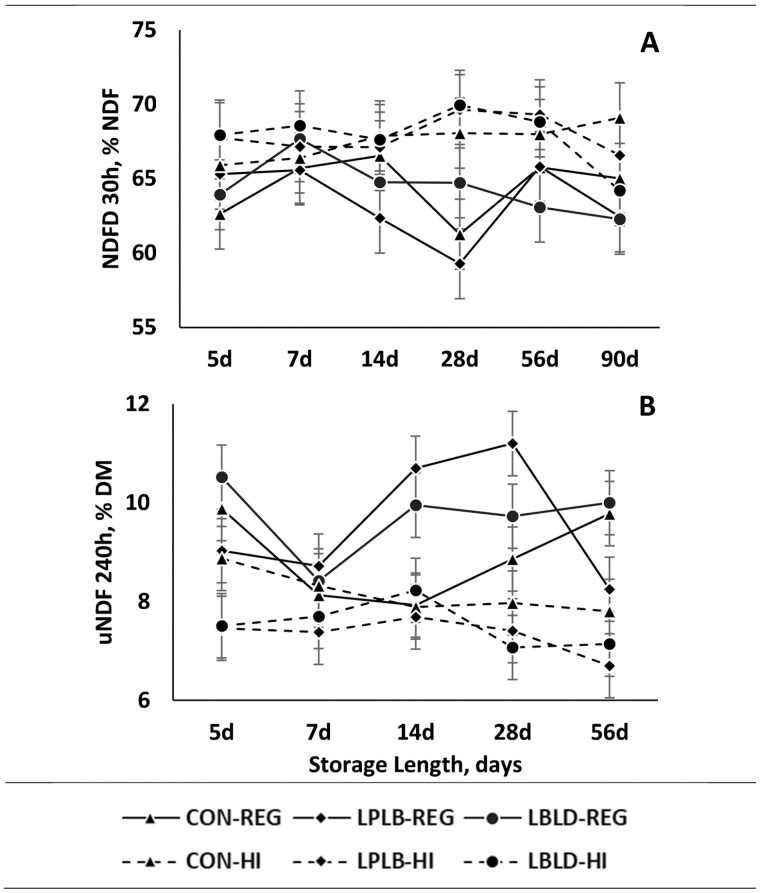
The concentrations of aNDF and lignin were affected by cutting height (data not presented in figures; P = 0.001, and P = 0.001, respectively). The concentrations of aNDF and lignin were 3.0 and 0.5 percentage-units greater, respectively, in REG silage compared to HI silage. Conversely, NDFD was 3.6 percentage-units greater (Figure 5A; P = 0.001) in HI silage compared to REG silage. The concentration of uNDF was affected by the interaction of cutting height × microbial inoculation (Figure 5B; P = 0.02). The concentration of uNDF was 1.7 percentage-units greater in REG-LBLD and REG-LPLB than other treatments, although REG-CON did not differ from REG-LBLD or REG-LPLB. Additionally, HI-LBLD and HI-LPLB had a 1.6 percentage-units lower uNDF concentration than other treatments, although HI-CON did not differ HI-LBLD or HI-LPLB. Lastly, HI-CON and REG-CON did not differ from each other.
Figure 5.
The effect of cutting height (CH), microbial inoculant (MI), and storage length (SL) on 30 h in vitro NDF digestibility (NDFD) and 240 h in vitro undigested NDF (uNDF). a,b,c Different superscripts denote differences in sliced effects within day, if present. Treatments consist of: CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage. (A) NDFD 30 h, % NDF (neutral detergent fiber); P = 0.25; SEM = 0.24. (B) uNDF 240 h, % DM (dry matter); P = 0.04; SEM = 0.58.
Starch concentration was affected by cutting height and storage length (Figure 6A; P = 0.001 and P = 0.05, respectively). Starch concentration was 4.1 percentage-units greater in HI cut silage compared to REG cut silage. However, despite the observed effect of storage length on starch concentration, there were no differences after Bonferroni corrections were applied. For starchD, a three-way interaction was observed (Figure 6B; P = 0.04). At 14 d of storage, starchD in HI-LBLD was greater than CON-REG, while no other treatments differed. At 28 d of storage, REG-LPLB had the greatest starchD, although REG-LBLD, HI-LPLB, and HI-LBLD did not differ. Additionally, REG-CON had the lowest starchD, although no HI silage treatments differed. Lastly, REG-LPLB did not differ from any HI silage treatments.
Figure 6.
The effect of cutting height (CH), microbial inoculant (MI), and storage length (SL) on starch and starchD (starch digestibility after 7 h). a,b,c Different superscripts denote differences in sliced effects within day, if present. Treatments consist of: CH: Regular (25 cm) or High (65 cm); MI: LBLD (P. acidilactici DSM 16243, Lentilactobacillus buchneri DSM 12856, and L. diolivorans DSM 32074; 300,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), LPLB (L. plantarum DSM 12837 and Lentilactobacillus buchneri DSM 16774; 500,000 CFU/g forage; Provita Supplements Inc., Mendota Heights, MN), or CON (distilled water); SL: 5, 7, 14, 28, 56, or 90 d of storage. (A) Starch, % DM (dry matter); P = 0.52; SEM = 1.25. (B) StarchD 7h, % Starch; P = 0.04; SEM = 0.98.
DISCUSSION
The pH of silage inoculated with LPLB and LBLD decreased up until 14 or 28 d of storage before beginning to increase at either 28 or 56 d of storage, depending on the treatment combination. However, the pH of CON silage remained low up to 56 d of storage, regardless of cutting height. The pH of REG-LPLB began to increase more than other treatments starting at 28 d, while HI-LPLB, HI-LBLD and REG-LBLD began increasing at 56 d, although HI-LBLD increased less than others. This is likely due to the decrease in lactic acid concentration as it was degraded into acetic acid, as lactic acid is the strongest acid found in the silo and heavily influences pH (Kung et al., 2018). Additionally, at 7 d of fermentation the pH of HI-LBLD was greater than REG-LBLD, suggesting there may have been a delay in acid production for the HI compared to REG-LBLD treated silage. Total acid concentration was also greater for REG cut silage than HI cut silage. This may be due to the more rapid or thorough proliferation of epiphytic or inoculant bacteria in REG silage with greater WSC, as greater substrate encourages microbial growth (Pahlow et al., 2003). Lactic acid concentration was also affected by cutting height through an interaction with storage length. However, REG cut silage had greater lactic acid than HI only at 7 d of storage. Possibly, beginning after 5 d of storage there was a greater proliferation of LAB in REG silage due to greater WSC. High cut silage also had lower concentration of acetic acid than REG silage, but the small difference is unlikely to be biologically meaningful.
The production of 1,2-propanediol is an important marker of L. buchneri activity because it is produced in equal proportions to acetic acid (Oude Elferink et al., 2001). However, Schein et al. (2018) suggests L. diolivorans may also be capable of producing 1,2-propanediol. At 14 d of storage, REG-LBLD had a greater concentration of 1,2-propanediol than other treatments, although HI-LBLD did not differ. This, combined with the numerically greater acetic acid concentration in LBLD at 28 d of storage, suggests there may be a more rapid onset of heterofermentative activity in LBLD than LPLB (Oude Elferink et al., 2001; Schein et al., 2018). The cause of the potentially more rapid onset of heterofermentative activity in LBLD cannot be determined based on these results, however, as there could be a stimulation of L. buchneri DSM 12856 activity or production of acetic acid and 1,2-propanediol by L. diolivorans DSM 32074. The stimulus of L. buchneri DSM 12856 could be caused by LBLD depleting WSC or increasing competition among bacteria for WSC which could initiate heterofermentative activity sooner. However, at later storage lengths, there was greater 1,2-propanediol concentrations for LPLB treatments, which imply LBLD treatments had a lower concentration of 1,2-propanediol because of degradation by L. diolivorans DSM 32074, as supported by greater concentrations of 1-propanol (Zielińska et al., 2017).
For ethanol concentration, there were no differences among cutting height and microbial inoculant combinations for later storage lengths, while several differences were detected in early and intermediate storage lengths. Ethanol is produced by heterofermentative bacteria during the degradation of lactic acid into acetic acid and therefore can serve as a marker of L. buchneri activity, or other bacteria utilizing the same fermentation pathway, such as L. diolivorans (Oude Elferink et al., 2001; Schein et al., 2018). In addition to heterofermentative bacteria, undesirable microorganisms, such as enterobacteria species, yeasts, and Clostridia species, can also produce ethanol (Rooke and Hatfield, 2003). While normal levels of ethanol can range from 0.5 to 1.5% DM in corn silages, very high levels of ethanol may be indicative of high numbers of yeasts, which are undesirable because they promote greater DM losses and more rapid aerobic deterioration (Kung et al., 2018). At 5 d of storage HI-LBLD was greater than HI-LPLB. Similarly, at 14 d of storage HI-LPLB was greater than HI-CON. Last, at 28 d of storage both HI-LPLB and HI-LBLD were greater than HI-CON. Because LPLB and LBLD both contained L. buchneri, they were expected to produce more ethanol than CON treatments since epiphytic organisms capable of producing ethanol are expected to be similar among treatments. Unexpectedly, only HI treatments differed from each other, while REG treatments did not differ from any combinations. The greater ethanol concentration may be related to lower production of acids (decreasing dilution effects) in HI silage or may be explained by undesirable microorganisms contributing to ethanol production in HI silage as described before due to less competition from lactic acid bacteria. Despite the greater concentration of ethanol for HI-LBLD than HI-LPLB at 5 d, by 7 d of storage there was no difference among LPLB and LBLD so the results for ethanol from 5 d of storage are likely mitigated as fermentation length progresses.
Heterofermentative inoculants are used in silage to increase the production of antifungal acids, such as acetic and propionic acid, that can inhibit yeast and mold growth after exposure to oxygen, improving aerobic stability (Kung et al., 2018). Lentilactobacillus buchneri has effectively increased acetic acid production and aerobic stability under a variety of conditions and in a variety of crops (Kleinschmidt and Kung, 2006; Muck et al., 2018), including those in this study for LPLB and LBLD silages. However, further research is warranted to evaluate if these effects are sufficient to improve aerobic stability in corn silage under the conditions of the present study. Conversely, petri dish studies show low potential for propionic acid production by L. diolivorans in the presence of WSC (Zhang et al., 2010; Zielińska et al., 2017; Schein et al., 2018). Similarly, Diepersloot et al. (2021) did not observe greater propionic acid concentration after inoculation of sorghum silage with L. diolivorans up to 56 d of storage. In agreement with these results, there were no detectable concentrations of propionic acid in any samples from the current study, suggesting the heterofermentative pathway of L. diolivorans DSM 32074 that produces propionic acid may not be utilized before 56 d of storage, or may be strain dependent. However, an increase in 1-propanol concentration, another by-product of L. diolivorans heterofermentative activity, was observed in sorghum silage inoculated with a microbial inoculant containing L. diolivorans (Diepersloot et al., 2021). Additionally, Schein et al. (2018) observed acetic acid, 1,2-propanediol, and ethanol production after petri dishes containing lactic acid were inoculated with L. diolivorans. Therefore, other heterofermentative pathways of L. diolivorans should be considered when evaluating heterofermentative activity of this bacteria. There was a three-way interaction observed for 1-propanol concentration in the current experiment. At 56 d of storage, 1-propanol concentration in REG-LBLD was greatest, although HI-LBLD did not differ, demonstrating L. diolivorans DSM 32074 was involved in heterofermentative activity (Krooneman et al., 2002). Diepersloot et al. (2021) also reported greater 1-propanol concentration in inoculated sorghum silage, in agreement with current results. Additionally, the elevated 1,2-propanediol concentrations observed at early storage lengths in the current study suggests there could potentially be production by L. diolivorans DSM 32074. This reinforces the premise proposed by Diepersloot et al. (2021), that some strains of L. diolivorans may have pathway preferences that favor the production of 1-propanol, and possibly the pathway producing acetic acid and 1,2-propanediol, over the pathway that yields propionic acid.
As expected, the DM of HI silage was greater than REG silage, which has also been observed by other authors (Neylon and Kung, 2003). This was expected as manipulating cutting height changes the portion of plants harvested, affecting DM concentration. The WSC concentration was affected by the interaction of microbial inoculation × cutting height, where REG-CON and HI-LPLB had the greatest concentrations, although they were not different from HI-LBLD. The concentration of WSC is an important factor in silage because the simple sugars that compose the WSC fraction are the main fermentable substrates of lactic acid bacteria (Pahlow et al., 2003). Therefore, greater WSC concentration may promote a more substantial fermentation and greater residual concentrations of WSC imply a lower extent of fermentation. Although this suggests that REG-CON and HI-LPLB were not fermented as extensively as other treatments, the difference among treatments were minor (0.3 percentage-units). The concentration of WSC was also affected by an interaction between microbial inoculation × storage length, but only differed at 14 and 56 d of storage, the small difference (<0.6 percentage-units) at two storage lengths is not a good indicator of the extent of fermentation among inoculants.
Although CP concentration was affected by the interaction of cutting height × storage length, differences among cutting heights were only detected at 5 d of storage. Additionally, there was a small difference between treatments (0.4 percentage-units), which is unlikely to have biological significance in dairy cows or affect ration formulation. The concentration of SCP was affected by the interactions of cutting height × microbial inoculation, where HI-CON and REG-LBLD were lower than REG-CON and HI-LBLD. The concentration of SCP is expected to increase over time, or with more extensive fermentation, because it is produced during proteolysis in silage, including the degradation of the protein matrix that surrounds starch granules and inhibits microbial fermentation of starch in the rumen (Hoffman et al., 2011). This would suggest that there were higher levels of proteolysis in HI-CON and REG-LBLD, although those treatments did not have a greater ammonia-N or starchD, both of which would also increase with breakdown of the protein matrix surrounding starch granules. The concentration of ammonia increased with storage length, demonstrating that proteolysis occurred in the silo, and ultimately increasing starchD over time. Ammonia was also affected by microbial inoculation, where CON was greater than LPLB or LBLD; however, the difference among treatments was small (0.3 percentage-units). Therefore, there was a little difference in levels of proteolysis among microbial inoculants, which is unlikely to be biologically significant.
As expected, the concentration of aNDF was greater in REG silage than HI silage. This is because increasing cutting heights leaves more of the fibrous stalk (Tolera and Sundstøl, 1999) in the field, which increases the leaf to stem ratio and grain to stem ratio (Neylon and Kung, 2003). Additionally, since the stalk contains greater proportion of lignin than other components, such as the leaves and grain (Tolera and Sundstøl, 1999), lignin concentration was lower for HI silage. Lignin concentration is known to negatively affect NDFD (Adesogan et al., 2019), and the lower lignin concentration in HI silage increased NDFD as expected. Unexpectedly, uNDF concentration was affected by the interaction of cutting height × microbial inoculation. The concentration of uNDF was expected to be lower in HI cut silage due to the lower lignin concentration, which improves digestibility (Adesogan et al., 2019). However, while HI and REG silage differed for LPLB and LBLD, the uNDF of CON silages did not differ. The reason for the lack of differences among CON treatments is not clear, as NDF concentration and digestibility are not usually affected by fermentation (Kung et al., 2018).
Starch concentration was also affected by cutting height as expected, where starch was greater in HI silage due to the greater proportion of grain in silage with greater cutting height (Ferraretto et al., 2018). StarchD was affected by a three-way interaction, but treatment differences occurred only after 14 and 28 d of storage. At each of these storage lengths, REG-CON had the lowest starchD, although not all treatments differed from it. It has been suggested that heterofermentative inoculants may encourage the growth of proteolytic bacteria (Junges et al., 2017; Saylor et al., 2020), possibly explaining the lower starchD of REG-CON. However, this does not explain why HI-CON was also not lower than other inoculant and cutting height combinations. Possibly silo conditions, or greater extent of fermentation, favored proteolytic bacteria in some silos over others, and further research examining these potential mechanisms are warranted. As expected, starchD increased over time confirming proteolysis occurred throughout the storage lengths tested.
CONCLUSION
In the current study, corn silage with greater cutting height had greater starch concentration and NDFD combined with a lower NDF and lignin concentration, improving nutrient composition. However, these benefits are at the expense of reduced yield. Additionally, silage fermentation was manipulated through inoculation with heterofermentative microbial inoculants in this study, as demonstrated by the shift from lactic to acetic acid production in LPLB and LBLD inoculant combinations. Conversely, increasing cutting height had little effect on fermentation profile. However, the results from this experiment demonstrate further research is necessary to better understand the actions of L. diolivorans DSM 32074 in silage, and potential preferences for different pathways of heterofermentative activity, especially when combined with other LAB. Additional research should also be conducted to evaluate the potential for delayed production of propionic acid by L. diolivorans DSM 32074. However, regardless of L. diolivorans DSM 32074 heterofermentative activity, the results of this study suggest that inoculating silage with heterofermentative inoculant combinations after increasing cutting height promotes a fermentation that could improve aerobic stability and possibly create conditions that favor proteolysis, also increasing starch digestibility. Further research is warranted to evaluate the effect of these treatments on aerobic stability, as well as yeast and mold counts.
Acknowledgments
We would like to acknowledge Mike Bertram and his team at UW-Madison Arlington Agricultural Research Station (Arlington, WI) for their assistance and harvesting whole-plant corn forage for this experiment. This work was supported by the USDA National Institute of Food and Agriculture, Hatch project 1024417. Partial funding for this study was also provided by Provita Supplements Inc. (Mendota Heights, MN). Sponsors had no role in the collection, analyses, and interpretation of data. Provita Supplements Inc. representatives reviewed the original draft of this manuscript.
Conflict of Interest Statement
None declared.
LITERATURE CITED
- Adesogan, A. T., Arriola K. G., Jiang Y., Oyebade A., Paula E. M., Pech-Cervantes A. A., Romero J. J., Ferraretto L. F., and Vyas D.. . 2019. Symposium review: Technologies for improving fiber utilization. J. Dairy Sci. 102:5726–5755. doi: 10.3168/jds.2018-15334. [DOI] [PubMed] [Google Scholar]
- Arriola, K. G., Vyas D., Kim D., Agarussi M. C. N., Silva V. P., Flores M., Jiang Y., Yanlin X., Pech-Cervantes A. A., Ferraretto L. F., . et al. 2021. Effect of Lactobacillus hilgardii, Lactobacillus buchneri, or their combination on the fermentation and nutritive value of sorghum silage and corn silage. J. Dairy Sci. 104:9664–9675. doi: 10.3168/jds.2020-19512. [DOI] [PubMed] [Google Scholar]
- AOAC International. 2012. Official methods of analysis, 19 ed. Arlington, VA: AOAC International. [Google Scholar]
- Aoki, Y., Oshita T., Namekawa H., Nemoto E., and Aoki M. . . 2013. Effect of cutting height on the chemical composition, nutritional value and yield, fermentative quality and aerobic stability of corn silage and relationship with plant maturity at harvest. Grassland Sci 59:211–220. doi: 10.1111/grs.12033. [DOI] [Google Scholar]
- Diepersloot, E. C., Pupo M. R., Ghizzi L. G., Gusmão J. O., C.Heinzen, Jr, McCary C. L., Wallau M. O., and Ferraretto L. F.. . 2021. Effects of microbial inoculation and storage length on fermentation profile and nutrient composition of whole-plant sorghum silage of different varieties. Front. Microbiol. 12:31–45. doi: 10.3389/fmicb.2021.660567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois, M., Gilles K. A., Hamilton J. K., Rebers P. A., and Smith F.. . 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ferraretto, L. F., Shaver R. D., and Luck B. D.. . 2018. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 101:3937–3951. doi: 10.3168/jds.2017-13728. [DOI] [PubMed] [Google Scholar]
- Goeser, J. P., Hoffman P. C., and Combs D. K.. . 2009. Modification of a rumen fluid priming technique for measuring in vitro neutral detergent fiber digestibility. J. Dairy Sci. 92:3842–3848. doi: 10.3168/jds.2008-1745. [DOI] [PubMed] [Google Scholar]
- Hall, M. B. 2015. Determination of dietary starch in animal feeds and pet food by an enzymatic-colorimetric method: collaborative study. J. AOAC Int. 98:397–409. doi: 10.5740/jaoacint.15-012. [DOI] [PubMed] [Google Scholar]
- Hoffman, P. C., Esser N. M., Shaver R. D., Coblentz W. K., Scott M. P., Bodnar A. L., Schmidt R. J., and Charley R. C.. . 2011. Influence of ensiling time and inoculation on alteration of the starch-protein matrix in high-moisture corn. J. Dairy Sci. 94:2465–2474. doi: 10.3168/jds.2010-3562. [DOI] [PubMed] [Google Scholar]
- Junges, D., Morais G., Spoto M. H. F., Santos P. S., Adesogan A. T., Nussio L. G., and Daniel J. L. P.. . 2017. Short communication: influence of various proteolytic sources during fermentation of reconstituted corn grain silages. J. Dairy Sci. 100:9048–9051. doi: 10.3168/jds.2017-12943. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt, D. H., and Kung L. Jr. 2006. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J. Dairy Sci. 89:4005–4013. doi: 10.3168/jds.S0022-0302(06)72444-4. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy, U., Muscato T. V., Sniffen C. J., and Van Soest P. J.. . 1982. Nitrogen fractions in selected feedstuffs. J. Dairy Sci. 65:217–225. doi: 10.3168/jds.S0022-0302(82)82180-2. [DOI] [Google Scholar]
- Krooneman, J., Faber F., Alderkamp A. C., Oude Elferink S. J. H. W., Driehuis F., Cleenwerck I., Swings J., Gottschal J. C., and Vancanneyt M.. . 2002. Lactobacillus diolivorans sp. nov., a 1,2-propanediol-degrading bacterium isolated from aerobically stable maize silage. Int. J. Syst. Evol. Microbiol. 52:639–646. doi: 10.1099/00207713-52-2-639. [DOI] [PubMed] [Google Scholar]
- Kung, L.Jr., Shaver R. D., Grant R. J., and Schmidt R. J.. . 2018. Silage review: interpretation of chemical, microbial, and organoleptic components of silage. J. Dairy Sci. 101:4020–4033. doi: 10.3168/jds.2017-13909. [DOI] [PubMed] [Google Scholar]
- Mendonça de R. de C. A., Cardoso M. V. S. B., Pantoja S. O. S., de Souza M. S., Domingues F. N., Faturi C., da Silva T. C., and do Rêgo A. C.. 2020. Effects of cutting height and bacterial inoculant on corn silage aerobic stability and nutrient digestibility by sheep. R. Bras. Zootec. 49. doi: 10.37496/rbz4920190231. [DOI] [Google Scholar]
- Muck, R. E., Nadeau E. M. G., McAllister T. A., Contreras-Govea F. E., Santos M. C., and L.Kung, Jr. 2018. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 101:3980–4000. doi: 10.3168/jds.2017-13839. [DOI] [PubMed] [Google Scholar]
- Neylon, J. M., and L.Kung, Jr. 2003. Effects of cutting height and maturity on the nutritive value of corn silage for lactating cows. J. Dairy Sci. 86:2163–2169. doi: 10.3168/jds.S0022-0302(03)73806-5. [DOI] [PubMed] [Google Scholar]
- Oude Elferink S. J. H. W., Krooneman J., Gottschal J. C., Spoelstra S. F., Faber F. and Driehuis F.. . 2001. Anaerobic conversion of lactic acid to acetic acid and 1,2-propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 67:125–132. doi: 10.1128/AEM.67.1.125-132.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlow, G., Muck R. E., Driehuis F., Oude Elferink S. J. W. H., and Spoelstra S. F.. . 2003. Microbiology of ensiling. In: Buxton D. R., Muck R. E., and Harrison J. H., editors, Silage science and technology, doi: 10.2134/agronmonogr42.c2. [DOI] [Google Scholar]
- Rooke, J. A. and Hatfield R. D.. . 2003. Biochemistry of ensiling. In: Buxton D. R., Muck R. E., and Harrison J. H., editors, Silage science and technology, doi: 10.2134/agronmonogr42.c3. [DOI] [Google Scholar]
- Saylor, B. A., Casale F., Sultana H., and Ferraretto L. F.. . 2020. Effect of microbial inoculation and particle size on fermentation profile, aerobic stability, and ruminal in situ starch degradation of high-moisture corn ensiled for a short period. J. Dairy Sci. 103:379–395. doi: 10.3168/jds.2019-16831. [DOI] [PubMed] [Google Scholar]
- Schein, H., Hirz M., Buchebner M., and Kramer W.. . 2018. The use of Lactobacillus diolivorans as silage inoculant. In: Kuoppala, K., M. Rinne, and A. Vannhatalo, editors. XVIII International Silage Conference Proceedings. (Abstr.), p. 166–167. [Google Scholar]
- Tolera, A., and Sundstøl F.. . 1999. Morphological fractions of maize stover harvested at different stages of grain maturity and nutritive value of different fractions of the stover. Anim. Feed Sci. Tech. 81:1–16. doi: 10.1016/S0377-8401(99)00072-3. [DOI] [Google Scholar]
- Vanzant, E. S., Cochran R. C., and Titgemeyer E. C.. . 1998. Standardization of in situ techniques for ruminant feedstuff evaluation. J. Anim. Sci. 76:2717–2729. doi: 10.2527/1998.76102717x. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Brandt M. J., Schwab C., and Gänzle M. G.. . 2010. Propionic acid production by cofermentation of Lactobacillus buchneri and Lactobacillus diolivorans in sourdough. Food Microbiol. 27:390–395. doi: 10.1016/j.fm.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Zielińska, K., Fabiszewska A., Świątek M., and Szymanowska-Powałowska D.. . 2017. Evaluation of the ability to metabolize 1,2-propanediol by heterofermentative bacteria of the genus Lactobacillus. Electron. J. Biotechnol. 26:60–83. doi: 10.1016/j.ejbt.2017.01.002. [DOI] [Google Scholar]