Abstract
Background
Early identification of patients at risk of psoriatic arthritis (PsA) is essential to facilitate early diagnosis and improve clinical outcomes. Severe cutaneous psoriasis has been proposed to be associated with PsA, but a recent assessment of the evidence is lacking. Therefore, in this systematic review, we address the association of psoriasis skin severity with the presence and development of PsA.
Summary
We included articles from a review published in 2014 and supplemented these with recent literature by performing an additional systematic search to identify studies published between 1 January 2013 and 11 February 2021. A meta-analysis was performed when sufficient comparable evidence was available. Of 2,000 screened articles, we included 29 in the analysis, of which 16 were identified by our updated search. Nineteen studies reported psoriasis severity as psoriasis area and severity index (PASI), ten studies as body surface area (BSA), and two studies as “number of affected sites.” Most studies show that more extensive skin disease is associated with the presence of PsA. The quantitative pooled analyses demonstrate higher PASI (mean difference [Δ] 1.59; 95% confidence interval [CI] 0.29–2.89) and higher BSA (Δ 5.31; 95% CI 1.78–8.83) in patients with PsA as compared to psoriasis patients without PsA. Results from prospective studies − that assess the risk of future development of PsA in psoriasis patients − were inconclusive.
Key Messages
In patients with psoriasis, more severe skin involvement is associated with the presence of PsA, underpinning the importance of optimal dermatology-rheumatology collaboration in clinical care. There are insufficient data to support the use of psoriasis skin severity to predict the future development of PsA in psoriasis patients.
Keywords: Psoriatic arthritis, Psoriasis, Skin manifestations, Review
Introduction
Psoriatic arthritis (PsA) is a musculoskeletal disorder characterized by inflammation of the skin, nail deformities, arthritis, axial spondyloarthritis, enthesitis, and dactylitis [1]. PsA develops in 6–41% of psoriasis patients, but it is unknown why only a subset of patients transits to PsA [1, 2, 3]. Psoriatic skin disease precedes PsA in 85% of the cases (on average, 10 years), which opens a window of opportunity for early recognition, treatment initiation, and possibly delaying or even prevention of the onset of PsA [1, 4]. Early diagnosis and treatment of PsA are essential because irreversible joint damage can develop within 6 months and delayed diagnosis is associated with long-term adverse outcomes [5, 6, 7, 8]. Therefore, defining patients at risk of PsA transition has been a topic of interest [9, 10].
Multiple clinical predictors for PsA in psoriasis patients have been suggested, including obesity, trauma, nail dystrophy, and psoriasis localization [9, 10]. Moreover, a meta-analysis published in 2014 reported a trend for an association between the extent of psoriasis and the presence of PsA [9]. The extent of cutaneous disease − commonly expressed as psoriasis area and severity index (PASI; range 0–72) or body surface area (BSA; range 0–100) − is a relatively quick and noninvasive clinical outcome and could therefore function as a useful predictor for transition to PsA in psoriasis patients that can readily be applied in clinical practice [11]. However, a meta-analysis investigating this potential predictor for PsA development is lacking [10]. We aimed to update and complement the prior meta-analysis by Rouzaud et al. [9] with current knowledge of the association of psoriatic skin disease severity with PsA by assessing not only the association of psoriasis severity with the presence of PsA but also the association with later development of PsA. Furthermore, we postulate that defining the association between the skin disease severity and the development of PsA may support our understanding of shared pathogenic features within the psoriatic spectrum of disease [12].
Methods
Search
We conducted a systematic literature search in PubMed and Embase on 11 February 2021 (PICO question: “Is psoriasis skin severity predictive of transition to PsA in psoriasis patients”?). We used a combination of synonym terms in the title/abstract and MesH/Emtree terms for “psoriasis,” “psoriatic arthritis,” “severity,” “PASI,” and “BSA” (online suppl. Table S1; for all online suppl. material, see). We screened studies using predefined eligibility criteria in line with Rouzaud et al. [9] (online suppl. Table S2). We included original studies published after 1 January 2013, that studied human subjects aged >18 years old and compared psoriasis severity between psoriasis patients without PsA (Pso-PsA), patients with PsA (PsA), and/or psoriasis patients that developed PsA. We focused on publications after 2012 to supplement the comprehensive meta-analysis by Rouzaud et al. [9] (search period 1980 to January 2013).
Data Extraction
Eligibility of selected studies for qualitative and quantitative analysis was discussed by two authors (M.E.J. and J.N.P.) and a quality assessment was reported. After study selection, we identified estimators for the association of PsA and psoriasis severity (PASI, BSA, affected sites): mean and standard deviation (±) in (sub)groups, mean difference between groups (Δ), median and interquartile range in (sub)groups, odds ratio (OR), risk ratio, and hazard ratio (HR) for the association of psoriasis severity and PsA (development) with confidence intervals (95% CI). We calculated missing OR and CI and requested the corresponding authors to provide additional data if information to perform quantitative analyses was lacking.
Differentiation by Research Question
We differentiated between studies that report the association of cutaneous psoriasis severity with the presence of PsA and studies that report the association with later development of PsA in patients with psoriasis because these studies answer different clinical questions. Articles that report the extent of skin disease at a certain baseline and subsequently study conversion to PsA (prospective design) are important to support the potential use of psoriasis severity as a biomarker to identify psoriasis patients at risk for PsA transition. On the other hand, studies that compare skin disease severity between Pso-PsA and PsA (cross-sectional design) enable us to study the association of psoriasis severity and the present risk of PsA. Although these studies do not address our PICO, we reckon that they do answer a clinically relevant question and therefore we included them in our analyses.
Meta-Analysis
We performed quantitative meta-analyses if ≥3 studies used a homogenous study design, reported similar psoriasis severity measures, and used the same association measures. For quantitative analyses, we used random effects models and evaluated heterogeneity with the I2 statistic. Meta-analyses were performed using review manager (Version 5.4) and meta-regression with comprehensive meta-analysis (Version 3). We considered a p value <0.05 to be statistically significant.
Results
Search Results
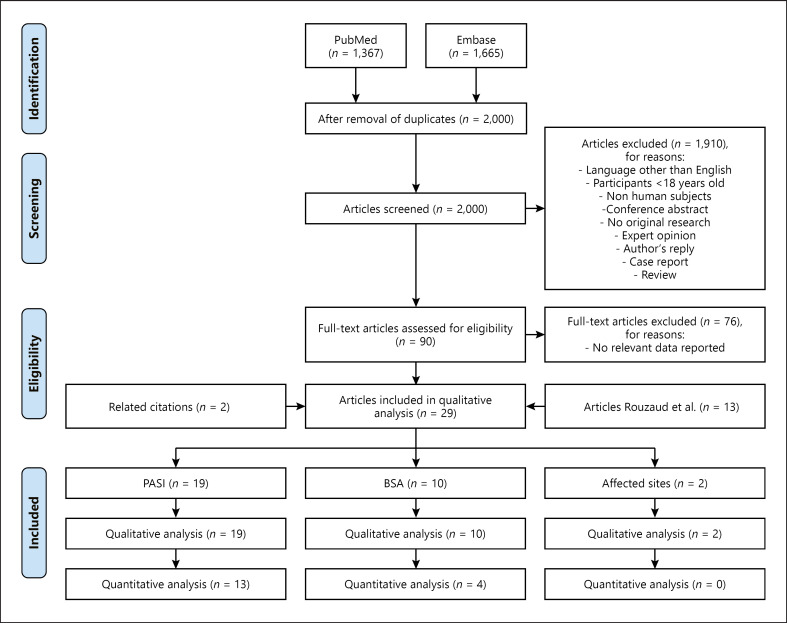
The search yielded 2,000 unique studies. One author performed title/abstract screening and thereafter screening of 90 studies in full-text (M.E.J.). Selection of 14 studies was discussed by three authors (M.E.J., J.N.P., and E.F.A.L.) (Fig. 1). Two articles were retrieved via reference and related citations in PubMed and supplemented with 13 studies selected by Rouzaud et al. [9]. Of the 29 articles included in our final analysis, three studies assessed the extent of skin disease in Pso-PsA patients and the later development of PsA (PASI n = 2, affected sites n = 1). The other 26 studies reported psoriasis severity and the presence of PsA in Pso-PsA and PsA patients: either the highest value from repeated measures over a period of time (PASI n = 1, BSA n = 2) or a single measurement (PASI n = 14, BSA n = 6, PASI and BSA n = 2, affected sites n = 1) (Table 1).
Fig. 1.
Flowchart. A literature search was conducted to identify original articles that reported psoriasis severity in patients with psoriasis and PsA. PubMed and Embase were searched on 11 February 2021. A combination of synonym terms in title/abstract and MesH/Emtree terms for “psoriasis,” “psoriatic arthritis,” “severity,” “PASI,” and “BSA” was used (online suppl. Table S1). In total, 3,032 articles were identified. Duplicates were removed and 2,000 articles were screened on title and abstract based on predefined eligibility criteria. Consequently, 90 selected articles were screened full-text for relevancy to be included in the analysis. The search was supplemented with 13 articles by Rouzaud et al. [9] and 2 articles via related citations in PubMed and reference citations of the identified articles in the initial search. In total, 29 studies were included in the qualitative analyses. These studies reported the following outcome measures for skin disease severity: PASI (n = 17), PASI and BSA (n = 2), BSA (n = 8) and number of affected sites (n = 2). We included 13, 4, and 0 of these studies in the quantitative analyses, respectively.
Table 1.
Studies that report the association of cutaneous psoriasis severity and PsA
| Psoriasis measure | Studiesa | Severity assessmentb | Patients Pso-PsA/PsA | Severity stratification | Results (Pso-PsA vs. PsA unless otherwise specified) |
|---|---|---|---|---|---|
| PASI | Present PsA | ||||
|
|
|||||
| Choi et al. 2017 [13] | Cross | 173/27 | <10: mild 10–20: moderate >20: severe | (I) Mean 6.8±4.3 versus 9.5±6.3; p = 0.014* (II) Stratified Mild: 78.5% versus 61.9%; p NR Moderate: 21.5% versus 33.3%; p NR Severe: 0.0% versus 4.8%; p NR (III) ORc PASI > 10: 2.24 (CI 0.86–5.86); p = 0.099 |
|
|
|
|||||
| Cinar et al. 2015 [14] | Cross | 94/32 | <3: mild 3–15: moderate >15: severe |
(I) Median 2.8 (0.3–30.0) versus 3.6 (0.8–37.7); p = 0.032* (II) Stratified Mild: 59.6% versus 40.6%; NS Moderate: 33.0% versus 53.1%; NS Severe: 7.4% versus 6.3%; NS (III) ORc PASI > 15: 0.83 (95% CI 0.16–4.21); p = 0.821 |
|
|
|
|||||
| Dağdelen et al. 2020 [33] | Cross | 80/40 | na | Mean 10.2±9.9 versus 4.9±4.8; p NR | |
|
|
|||||
| Eder et al. 2011 [30] | Cross | 159/159 | Highest during first 3 yr FU <10: non-severe ≥10: severe |
(I) Mean 7.1±7.2 versus 7.3±9.6 (II) Multivariable logistic regression Severe: OR 0.89 (95% CI 0.49–1.61); NS |
|
|
|
|||||
| El Miedany et al. 2015 [43] | Cross | 112/126 | na | Mean 11.7±11.8 versus 12.4±10.4; NS |
|
|
|
|||||
| Gladman and Chandran 2011 [44] |
Cross | 438/1,066 | na | Mean 5.7±5.8 versus 7.0±8.9; p NR |
|
|
|
|||||
| Haroon et al. 2013 [15] | Cross | 71/29 | na | (I) Mean 1.89±1.14 versus 2.40±1.13; p = 0.04* (II) Multivariable logistic regression PASI: OR 1.61 (95% CI 1.06–2.44); p = 0.02* |
|
|
|
|||||
| Henes et al. 2013 [16] | Cross | 48/50 | 0–1: not active 2–10: mild 11–15: moderate >15: severe |
(I) Median 1 (0–3) versus 1 (0–3); NS (II) Stratified Not active: 26.2% versus 23.9%; NS Mild: 42.9% versus 45.7%; NS Moderate: 21.4% versus 28.3%; NS Severe: 20.8% versus 10.0%; p NR (III) ORc PASI > 10: 0.86 (95% CI 0.38–1.94); p = 0.715 PASI > 15: 0.42 (95% CI 0.13–1.34); p = 0.144 |
|
|
|
|||||
| Jamshidi et al. 2008 [17] | Cross | 291/29 | na | Mean 10.70±8.44 versus 24.33±10.36; p = <0.05* |
|
|
|
|||||
| PASI | Leijten et al. 2017 [18] | Cross | 68/18 | na | Mean 5.69±4.84 versus 4.65±5.75; p = 0.478 |
|
|
|||||
| Maejima et al. 2010 [45] | Cross | 23/23 | na | Mean 9.5±9.4 versus 9.5±13.3; NS |
|
|
|
|||||
| Pietrzak et al. 2019 [34] | Cross | 62/31 | na | Mean 26.00±6.54 versus 28.07±5.87; p = <0.05* |
|
|
|
|||||
| Reich 2009 [46] | Cross | 1,055/312 | na | Mean 11.5 versus 14.3; p = <0.0001* |
|
|
|
|||||
| Salvarini et al. 1995 [20] | Cross | 130/75 | na | Mean 4.7±3.5 versus 5.4±5.1; NS |
|
|
|
|||||
| Schons et al. 2015 [47] | Cross | 49/16 | na | Mean 8.3±7.44 versus 11.3±9.6; NS |
|
|
|
|||||
| Soy et al. 2008 [48] | Cross | 40/49 | na | Mean 6.2±8.2 versus 8.6±12.2; NS |
|
|
|
|||||
| Yang et al. 2011 [49] | Cross | 1,816/112 | na | Mean 6.0±5.6 versus 9.7±10.4; p = <0.001* |
|
|
|
|||||
| Later development of PsA | |||||
|
|
|||||
| Eder et al. 2016 [31] | Pro | Baseline: 464/0 After 8 yr FU: 404/60 |
At baseline <10: mild 10–20: moderate >20: severe |
(I) Stratified Mild: 88.1% versus 78.3%; p NR Moderate: 10.1% versus 15.0%; p NR Severe: 1.7% versus 6.7%; p NR (II) Cox regression Moderate versus mild: RR 1.16 (95% CI 0.50–2.64); NS Severe versus mild: RR 5.39 (95% CI 1.64–17.7); p = 0.006* |
|
|
|
|||||
| Zenke et al. 2017 [21] | Pro | 974/118 | At first, visit dermatology clinic <10: non-severe ≥10: severe | (I) Mean 4.5±7.5 versus 9.3±10.2; p = <0.01* (II) Multivariable logistic regression Severe: OR 1.55 (95% CI 0.89–2.71); NS |
|
|
| |||||
| BSA | Present PsA | ||||
|
|
|||||
| Choi et al. 2017 [13] | Cross | 173/27 | <3: mild 3–10: moderate >10: severe | (I) Mean 6.7±6.6 versus 11.0±16.4; p = 0.029* (II) Stratified Mild: 27.8% versus 14.3%; p NR Moderate: 55.7% versus 66.7%; p NR Severe: 16.5% versus 19.0%; p NR (III) ORc BSA > 10: 1.19 (95% CI 0.37–3.84); p = 0.765 |
|
|
|
|||||
| Christophers et al. 2010 [23] | Cross | 1,434/126 | na | (I) Mean 17.2±16.9 versus 26.6±19.9; p = <0.0005* (II) Multivariable logistic regression BSA: OR 1.020 (95% CI 1.012–1.029); p = <0.0005* |
|
|
|
|||||
| BSA | Gelfand et al. 2005 [24] | Cross | 530/71 | <1: no or little 1–2: mild 3–10: moderate >10: severe | (I) Stratified No or little: 75.7% versus 30.8%; p NR Mild: 14.5% versus 30.8%; p NR Moderate: 8.0% versus 21.5%; p NR Severe: 1.8% versus 16.9%; p NR (II) ORc BSA > 10: 11.06 (95% CI 4.11–29.75); p < 0.001* |
|
|
|||||
| Ogdie et al. 2013 [25] | Cross | 3,699/365 | ≤2: mild 3–10: moderate >10: severe |
Multivariable logistic regression Moderate versus mild: OR 1.49 (95% CI 1.1–1.99); p < 0.001* Severe versus mild: OR 3.34 (95% CI 2.40–4.65); p < 0.001* |
|
|
|
|||||
| Pietrzak et al. 2019 [34] | Cross | 62/31 | na | Mean 35.83±15.59 versus 38.64±13.95; p = 0.2438 |
|
|
|
|||||
| Soltani-Arabshahi et al. 2010 [26] | Cross | 693/250 | Highest ever <5: mild 5–10: moderate >10: severe |
Cox regression Worst BSA ever: OR 1.01 (95% CI 1.00–1.01); p < 0.05 |
|
|
|
|||||
| Stern 1985 [27] | Cross | 1,019/266 | na | Mean 31% versus 37%; p = <0.01* |
|
|
|
|||||
| Tey et al. 2010 [22] | Cross | 266/134 | Max. in 1 yr FU 0–25%: I 26–50%: II 51–75%: III 76–100%: IV |
Multivariable logistic regression II versus I: OR 1.53 (95% CI 0.86–2.71); NS III versus I: OR 1.64 (95% CI 0.85–3.19); NS IV versus I: OR 2.52 (95% CI 1.33–4.75); p = 0.004* |
|
|
|
|||||
| Truong et al. 2015 [19] | Cross | 399/169 | na | Mean 13.4±17.4 versus 16.7±21.3; p = 0.05 |
|
|
|
|||||
| Yan et al. 2018 [28] | Cross | 497/175 | Mild Mild to moderate Moderate to severe Severec |
(I) Stratified Mild: 6.0% versus 3.6%; NS Mild to moderate: 21.5% versus 13.1%; p = 0.021 * Moderate to severe: 37.2% versus 30.9%; NS Severe: 32.0% versus 50.3%; p = 2.39−5* (II) ORc BSA “severe”: OR 2.15 (95% CI 1.51–3.06); p < 0.001 * (III) Univariate logistic regression Severe: OR 2.15 (95% CI 1.51–3.05); p NR (IV) Multivariable logistic regression Severe: OR 1.92 (95% CI 0.88–4.21); NS |
|
|
|
|||||
| Affected sites | Present PsA | ||||
|
|
|||||
|
|
Thumboo et al. 2002 [29] | Cross | 120/60 | ≤2: limited | (I) Generalized |
|
|
|||||
| >2: generalized | 38.3% versus 41.7%; p NR | ||||
|
|
|||||
| (II) Univariate logistic regression | |||||
| Generalized: OR 1.18 (95% CI 0.59–2.34); NS | |||||
| Later development of PsA | |||||
| Wilson et al. 2009 [32] | Pro | Baseline: 1,633/0 End of 20.936 person years FU: 1,593/57 | At baseline Unknown 1 site 2 sites ≥3 | (I) Cox regression univariate 2 versus 1 sites: HR 0.77 (95% CI 0.37–1.64); p NR ≥3 versus 1 sites: HR 2.24 (95% CI 1.23–4.08); p NR* (II) Cox regression multivariate NR (NS) | |
BSA, body surface area (1% is equivalent to the size of the palm of the patient's hand); CI, confidence interval; FU, follow-up; na, not applicable; NR, not reported; NS, not significant (p value not reported); OR, odds ratio; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; Pso-PsA, psoriasis without psoriatic arthritis; SD, standard deviation; RR, risk ratio.
Significant (p value <0.05).
Differentiation between studies that report the association of cutaneous psoriasis severity with the presence of PsA and studies that report the association with future development of PsA.
Assessment of psoriasis severity in a either a cross sectional (Cross) or prospective (Pro) design.
ORs calculated as follows: (a × d)/(b × d), with the standard error (SE) of the log OR being SE[ln(OR)] = √[(1/a] + [1/b] + [1/c] + [1/d]), and 95% CI = exp{ln(OR) − 1.95 ×SE[ln(OR)]} to exp{ln(OR) + 1.95 × SE[ln(OR)]}.
Study Quality
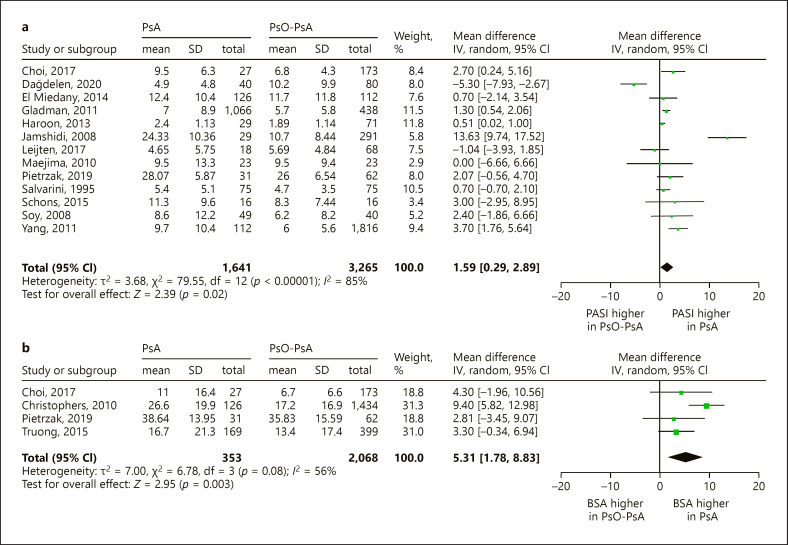
Concerning studies that investigated psoriasis severity and the presence of PsA, the overall quality was low. In seven studies, selection bias could have been introduced by patient selection (Choi et al. [13]; Cinar et al. [14]; Haroon et al. [15]; Henes et al. [16]; Jamshidi et al. [17]; Leijten et al. [18]; Truong et al. [19]), as they assessed previously undiagnosed PsA in cohorts of psoriasis patients (online suppl. Table S3: detailed study characteristics). The majority of studies recruited patients at dermatology departments of hospitals or dedicated dermatology clinics (15 studies). Five studies were performed in combined dermatology/rheumatology clinics, two in rheumatology departments, and of the remaining studies, the setting was unknown or of another category. Concerning the classification of PsA, one third of the studies applied validated criteria (CASPAR, Moll & Wright, ESSG), while 11 studies used either a clinical or self-reported diagnosis (Salvarani et al. [20]; Zenke et al. [21]; Tey et al. [22]; Christophers et al. [23]; Gelfand et al. [24]; Ogdie et al. [25]; Soltani-Arabshahi et al. [26]; Stern [27]; Truong et al. [19]; Yan et al. [28]; Thumboo et al. [29]). Whether psoriasis severity was determined by an experienced dermatologist was not described in more than half of the studies. All studies assessed psoriasis severity at a single time point, except for three studies that measured repeatedly over a period of time and reported the highest value during follow-up (Eder et al. [30], Soltani-Arabshahi et al. [26]; Tey et al. [22]). Most studies reported psoriasis duration. As expected, because psoriasis precedes PsA in most cases, psoriasis duration was longer in PsA patients compared to Pso patients (range 0.2–9.5 years) [1, 4]. Details of therapies were not well described in most studies and varied greatly between studies. With regards to confounding, only two studies (Haroon et al. [15] and Eder et al. [31]) corrected for the use of (topical or systemic) psoriasis therapy. After selection based on our criteria of homogeneity, we included 15 studies in two meta-analyses to compare ΔPASI (n = 13) and ΔBSA (n = 4) between Pso-PsA and PsA patients (Fig. 2).
Fig. 2.
Meta-analysis: psoriasis severity in PsO-PsA and PsA patients Forrest plots of studies that measure psoriasis severity as PASI (a) or BSA (b) and compare mean values between PsO-PsA and PsA patients. BSA, body surface area; CI, confidence interval; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; Pso-PsA, psoriasis without psoriatic arthritis; SD, standard deviation.
With regards to the three studies that reported psoriasis severity and later development of PsA, the overall risk of bias was low. However, heterogeneity with regards to the reported psoriasis severity measures and estimators impeded pooling of results in quantitative analyses (Eder et al. [31]; Zenke et al. [21]; Wilson et al. [32]).
Psoriasis Severity and Presence of PsA
Sixteen cross-sectional studies reported PASI from one single measurement, of which 12 studies observed a higher mean or median PASI in PsA compared to Pso-PsA. We included 13 studies in our meta-analysis, that showed a significantly higher PASI in PsA (Δ 1.59 [95% CI 0.29–2.89]) with a high level of heterogeneity (I2 85%) (Fig. 2a). Given the high heterogeneity and possible publication bias (online suppl. Fig. S1), we performed a sensitivity analysis by removing the studies by Dağdelen et al. [33] and Jamshidi et al. [17]. The result of the adjusted meta-analysis showed a smaller but still significant difference (Δ 1.25 [95% CI 0.55–1.95]) and with acceptable heterogeneity (I2 42%) (online suppl. Fig. S2). Further, three studies (Choi et al. [13]; Cinar et al. [14]; Henes et al. [16]) compared PASI between Pso-PsA and PsA by stratification into mild, moderate, or severe psoriasis. Although two studies found that moderate-severe psoriasis was more prevalent amongst PsA patients, these results were not statistically significant. One study assessed psoriasis severity repeatedly over time and compared the highest PASI (dichotomized <10 vs. ≥10) during 3 years of follow-up (Eder et al. [30]), but these results too were not significantly different.
Eight cross-sectional studies reported BSA, of which five studies reported mean or median BSA. All studies (Choi et al. [13]; Christophers et al. [23]; Pietrzak et al. [34]; Stern [27]; Truong et al. [19]) showed that PsA patients have higher BSA compared to psoriasis patients. Our meta-analysis confirms that BSA is significantly higher in PsA patients (Δ 5.31 [95% CI 1.78–8.83]) with an intermediate level of heterogeneity (I2 56%) (Fig. 2b). Furthermore, three studies that stratified patients into mild, moderate, and severe psoriasis showed that patients with severe skin disease (BSA > 10) were more likely to have PsA than those with non-severe psoriasis. This association was significant in two studies (Gelfand et al. [24] OR 11.06 p < 0.001; Yan et al. [28] OR 2.15 p < 0.001). Moreover, severe psoriasis was a predictor of present PsA in two studies that performed multivariable regression analysis (Ogdie et al. [25] OR 3.34 p < 0.001; Yan et al. [28] OR 1.92 p = NS). Furthermore, two studies measured BSA severity repeatedly over time and compared the highest value during follow-up between PsA and psoriasis patients. “Highest BSA ever” (OR 1.01 [95% CI 1.00–1.01]; Soltani-Arabshahi et al. [26]) and “very severe skin disease” (as defined by BSA ≥76%) (OR 2.25 [95% CI 1.33–4.75]; Tey et al. [22]), were significantly associated with PsA diagnosis.
Only one cross-sectional study compared the number of affected psoriasis sites between psoriasis and PsA patients (Table 1) [29]. The number of patients with generalized psoriasis (>2 affected sites) was higher in PsA (41.4% vs. 38.3%; OR 1.18), but these results were not significant.
Psoriasis Severity and Future Development of PsA
We identified three prospective studies that reported psoriasis severity in Pso-PsA patients and assessed later development of PsA (Eder et al. [31]; Zenke et al. [21]; Wilson et al. [32]). One study showed with multivariable logistic regression that severe psoriasis (PASI ≥ 10) at psoriasis onset is not a statistically significant predictor for PsA transition (OR 1.55, p value not reported), after correction for young age, sex, scalp psoriasis, and nail dystrophy [21]. The second study reported that severe psoriasis (PASI ≥ 20) is significantly associated with PsA transition within 8 years (risk ratio 5.39; p = 0.006) [31]. Finally, one study indicated using univariate Cox regression that patients with ≥3 affected sites were significantly more at risk to develop PsA (HR 2.24 [95% CI 1.23–4.08]), but this effect was not sustained in multivariate analysis after correction for age, sex, calendar year, scalp psoriasis, intergluteal psoriasis, and nail dystrophy (HR not reported) [32].
Discussion
To our knowledge, this is the first systematic review and meta-analysis in 8 years to provide both qualitative and quantitative answers as to whether psoriasis severity is associated with the presence and development of PsA. This is a clinically relevant question because skin severity measurement could aid in identifying those psoriasis patients at risk for PsA transition and thus serve as an easily implementable clinical measurement to facilitate early PsA diagnosis and improve clinical outcomes. Our results confirm that in patients with psoriasis, the presence of slightly more extensive skin disease, as measured by higher PASI and BSA, is associated with concurrent PsA. We were unable to draw a definite conclusion about the association of psoriasis severity with later development of PsA.
The majority of the cross-sectional studies found a positive association between severe psoriasis and the presence of PsA. Moreover, our meta-analyses revealed a statistically significant mean difference of both PASI and BSA between PsO-PsA and PsA patients, although the differences were relatively small. We speculate these results may be an underestimation because most studies included psoriasis patients that were treated in a hospital and patients with only mild psoriasis are typically less prone to visit a dermatologist. Unfortunately, we were unable to accurately assess the association of psoriasis severity with transition to PsA, as prospective studies were limited and heterogeneous. Although all point estimates were in the direction of a higher risk of developing PsA, the results were not always significant. Therefore, there is currently insufficient evidence to recommend dermatologists using psoriasis severity as a reliable biomarker for PsA development.
In the past, specific psoriasis localizations have been suggested to associate with PsA, including scalp and intergluteal psoriasis [9]. PASI and BSA capture all anatomically affected sites of psoriasis and therefore may not be the most suitable outcome measures to assess risk for PsA transition. Moreover, a PASI score of severe scalp psoriasis can be numerically comparable with that of only moderate psoriasis on the knees. Therefore, we recommend future studies to include an in-depth topographic assessment of psoriasis localization and report individual PASI components.
The difference in psoriasis severity between PsA and psoriasis patients could improve our understanding of the pathogenic link between skin and joint disease. From a pathophysiologic perspective, the association between severe psoriasis and PsA may be explained by the important role of the interleukin (IL)-23, IL-17, and tumor necrosis factor alpha (TNF) pathways in inflammation of both the skin and musculoskeletal apparatus [1]. Overlapping cytokines − including IL-17, IL-22, IL-23, and tumor necrosis factor alpha − play a role in immune-mediated inflammation of skin and synovium that involves infiltration of pathogenic CD8+ T cells, macrophages, dendritic cells, monocytes, and B cells [35]. It is hypothesized that local proinflammatory cytokine production and activated immune cells in psoriatic skin create a self-perpetuating inflammatory response that results in systemic inflammation and PsA [35]. However, this does not explain why in 15% of the patients, arthritis precedes skin lesions [1]. Moreover, cutaneous psoriasis severity has shown only a modest correlation with joint disease [36]. Thus, the exact relation between inflammation of the skin, joints, and other domains remains incompletely understood [35].
This review has several limitations. First, we have not repeated the systematic search performed by Rouzaud et al. [9], but as they employed validated methodology and even broader search methods, we assume to have included all relevant publications. Second, our meta-analyses were limited by heterogeneity and a relatively small number of included studies. Third, most studies were conducted in dermatology clinics, which may have resulted in an overestimation of psoriasis severity in PsA, since patients with “PsA sine psoriasis” and limited psoriasis − typically seen by rheumatologists − could have been missed. Fourth, it needs to be taken into account that the meta-analysis did not include high-quality studies. Most importantly, the use of therapies could have confounded the results. However, these studies do represent daily clinical practice, as psoriatic patients are frequently treated with topical and/or systemic treatment. Furthermore, we examined the effects of two potential confounders that are associated with PsA in psoriasis patients, i.e., the presence of nail psoriasis and psoriasis disease duration. Meta-regression analysis suggested that our results were not explained by confounding by nail psoriasis or psoriasis duration, although we could only analyze the effects in six and eight studies, respectively (online suppl. Table S4). Additional subgroup analyses to investigate potential confounders − including psoriasis localization, family history of PsA, obesity, history of trauma of fracture, and smoking status − could unfortunately not be performed in consequence of limited reporting of data [10, 37]. Overall, we deem that these results are the currently best available answer to a clinically relevant question.
Concluding Remarks
Our results demonstrate that psoriasis severity is associated with increased likelihood of concurrent PsA. The high extent of psoriasis skin activity in PsA patients reinforces the necessity of multidisciplinary collaboration between rheumatologists and dermatologists in PsA care.
Defining psoriasis patients at risk for PsA transition remains an important topic to facilitate early recognition and prevent irreversible joint damage. Long lasting follow-up studies are necessary to study predictors for the development of PsA in psoriasis patients. Given the complexity of PsA pathogenesis, we deem that prediction models that combine genotypic and phenotypic predictors are the most promising to identify psoriasis patients at risk for PsA transition [38, 39, 40, 41, 42].
Key Message
In patients with psoriasis, more severe skin involvement is associated with the presence of psoriatic arthritis.
Statement of Ethics
The paper is exempt from ethical committee approval because data were collected from published trials in which informed consent had been obtained by the trial investigators.
Conflict of Interest Statement
J.M.L. has received honoraria from Abbvie, Arxx Tx, Boerhinger Ingelheim, Galapagos, Gesyntha, Leadiant, Magenta, Roche, and Sanofi-Genzyme and research grants from Astra Zeneca, Boehringer Ingelheim, MSD, Pfizer, and Roche. All other authors have no conflicts of interest to declare.
Funding Sources
This work was not funded.
Author Contributions
M.E.J., J.N.P., and E.F.A.L. were responsible for conceptualization. J.N.P. and M.E.J. extracted the data and performed the analyses. All the authors contributed substantially to reviewing and editing the manuscript before submission.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its online supplemental information files.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Funding Statement
This work was not funded.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017 Mar;376((10)):957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 2.Edson-Heredia E, Zhu B, Guo J, Maeda-Chubachi T, Lebwohl M. Disease burden and quality of life in psoriasis patients with and without comorbid psoriatic arthritis: results from National Psoriasis Foundation panel surveys. Cutis. 2015 Mar;95((3)):173–178. [PubMed] [Google Scholar]
- 3.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015 Nov 1;41((4)):545–568. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21–37. doi: 10.1016/j.jaut.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015 Jun 1;74((6)):1045–1050. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]
- 6.Gladman DD, Thavaneswaran A, Chandran V, Cook RJ. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis. 2011 Dec 1;70((12)):2152–2154. doi: 10.1136/ard.2011.150938. [DOI] [PubMed] [Google Scholar]
- 7.Tillett W, Jadon D, Shaddick G, Cavill C, Korendowych E, de Vries CS, et al. Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann Rheum Dis. 2013 Aug;72((8)):1358–1361. doi: 10.1136/annrheumdis-2012-202608. [DOI] [PubMed] [Google Scholar]
- 8.Theander E, Husmark T, Alenius G-M, Larsson PT, Teleman A, Geijer M, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA) Ann Rheum Dis. 2014 Feb;73((2)):407–413. doi: 10.1136/annrheumdis-2012-201972. [DOI] [PubMed] [Google Scholar]
- 9.Rouzaud M, Sevrain M, Villani AP, Barnetche T, Paul C, Richard MA, et al. Is there a psoriasis skin phenotype associated with psoriatic arthritis? Systematic literature review. J Eur Acad Dermatology Venereol. 2014;28 Suppl 5:17–26. doi: 10.1111/jdv.12562. [DOI] [PubMed] [Google Scholar]
- 10.Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019 Mar;15((3)):153–166. doi: 10.1038/s41584-019-0175-0. [DOI] [PubMed] [Google Scholar]
- 11.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005 Mar;64((Suppl 2)):ii65–73. doi: 10.1136/ard.2004.031237. discussion ii69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehncke W. Psoriasis and psoriatic arthritis: flip sides of the coin? Acta Derm Venereol. 2016 May;96((4)):436–441. doi: 10.2340/00015555-2385. [DOI] [PubMed] [Google Scholar]
- 13.Choi JW, Kim BR, Seo E, Youn SW. Could psoriatic arthritis be easily diagnosed from current suspicious physical findings in the dermatology clinic? Ann Dermatol. 2017 Feb;29((1)):48–54. doi: 10.5021/ad.2017.29.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çinar N, Bodur H, Eser F, Gül Ü, Gönül M, Oğuz ID. The prevalence and characteristics of psoriatic arthritis in patients with psoriasis in a tertiary hospital. Arch Rheumatol. 2015;30((1)):23–27. [Google Scholar]
- 15.Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis. 2013 May;72((5)):736–740. doi: 10.1136/annrheumdis-2012-201706. [DOI] [PubMed] [Google Scholar]
- 16.Henes JC, Ziupa E, Eisfelder M, Adamczyk A, Knaudt B, Jacobs F, et al. High prevalence of psoriatic arthritis in dermatological patients with psoriasis: a cross-sectional study. Rheumatol Int. 2014 Feb;34((2)):227–234. doi: 10.1007/s00296-013-2876-z. [DOI] [PubMed] [Google Scholar]
- 17.Jamshidi F, Bouzari N, Seirafi H, Farnaghi F, Firooz A. The prevalence of psoriatic arthritis in psoriatic patients in Tehran, Iran. Arch Iran Med. 2008;11((2)):162–165. [PubMed] [Google Scholar]
- 18.Leijten EFA, Sigurdsson V, Wenink MH, Radstake TRDJ. Screening for psoriatic arthritis using the psoriasis epidemiology screening tool questionnaire: examining the optimal cut-off. Br J Dermatol. 2017;176((5)):1357–1359. doi: 10.1111/bjd.14953. [DOI] [PubMed] [Google Scholar]
- 19.Truong B, Rich-Garg N, Ehst BD, Deodhar AA, Ku JH, Vakil-Gilani K, et al. Demographics, clinical disease characteristics, and quality of life in a large cohort of psoriasis patients with and without psoriatic arthritis. Clin Cosmet Investig Dermatol. 2015;8:563–569. doi: 10.2147/CCID.S90270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvarani C, Lo Scocco G, Macchioni P, Cremonesi T, Rossi F, Mantovani W, et al. Prevalence of psoriatic arthritis in Italian psoriatic patients. J Rheumatol. 1995 Aug;22((8)):1499–1503. [PubMed] [Google Scholar]
- 21.Zenke Y, Ohara Y, Kobayashi D, Arai S, Kishimoto M, Okada M, et al. Nail findings in patients with psoriatic arthritis: a cross-sectional study with special reference to transverse grooves. J Am Acad Dermatol. 2017 Nov;77((5)):863–867. doi: 10.1016/j.jaad.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Tey HL, Ee HL, Tan AS, Theng TS, Wong SN, Khoo SW. Risk factors associated with having psoriatic arthritis in patients with cutaneous psoriasis. J Dermatol. 2010;37((5)):426–430. doi: 10.1111/j.1346-8138.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 23.Christophers E, Barker J, Griffiths C, Daudén E, Milligan G, Molta C, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J Eur Acad Dermatology Venereol. 2010;24((5)):548–554. doi: 10.1111/j.1468-3083.2009.03463.x. [DOI] [PubMed] [Google Scholar]
- 24.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53((4)):573.e1–3. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Ogdie A, Langan SS, Love T, Haynes K, Shin D, Seminara N, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatol. 2013 Mar;52((3)):568–575. doi: 10.1093/rheumatology/kes324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soltani-Arabshahi R, Wong B, Feng BJ, Goldgar DE, Callis Duffin K, Krueger GG. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol. 2010;146((7)):721–726. doi: 10.1001/archdermatol.2010.141. [DOI] [PubMed] [Google Scholar]
- 27.Stern RS. The epidemiology of joint complaints in patients with psoriasis. J Rheumatol. 1985 Apr;12((2)):315–320. [PubMed] [Google Scholar]
- 28.Yan D, Ahn R, Leslie S, Liao W. Clinical and genetic risk factors associated with psoriatic arthritis among patients with psoriasis. Dermatol Ther. 2018;8((4)):593–604. doi: 10.1007/s13555-018-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thumboo J, Uramoto K, Shbeeb MI, O'Fallon WM, Crowson CS, Gibson LE, et al. Risk factors for the development of psoriatic arthritis: a population based nested case control study. J Rheumatol. 2002;29((4)):757–762. [PubMed] [Google Scholar]
- 30.Eder L, Law T, Chandran V, Shanmugarajah S, Shen H, Rosen CF, et al. Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis Care Res. 2011;63((8)):1091–1097. doi: 10.1002/acr.20496. [DOI] [PubMed] [Google Scholar]
- 31.Eder L, Haddad A, Rosen CF, Lee KA, Chandran V, Cook R, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68((4)):915–923. doi: 10.1002/art.39494. [DOI] [PubMed] [Google Scholar]
- 32.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61((2)):233–239. doi: 10.1002/art.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dağdelen D, Karadag AS, Kasapoğlu E, Wang JV, Erman H. Correlation of metabolic syndrome with serum omentin-1 and visfatin levels and disease severity in psoriasis and psoriatic arthritis. Dermatol Ther. 2020;33((6)):e14378. doi: 10.1111/dth.14378. [DOI] [PubMed] [Google Scholar]
- 34.Pietrzak A, Chabros P, Grywalska E, Kicinski P, Pietrzak-Franciszkiewicz K, Krasowska D, et al. Serum lipid metabolism in psoriasis and psoriatic arthritis: an update. Arch Med Sci. 2019 Mar;15((2)):369–375. doi: 10.5114/aoms.2018.74021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391((10136)):2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 36.Mease PJ, Etzel CJ, Huster WJ, Muram TM, Armstrong AW, Lisse JR, et al. Understanding the association between skin involvement and joint activity in patients with psoriatic arthritis: experience from the Corrona Registry. RMD Open. 2019;5((1)):e000867. doi: 10.1136/rmdopen-2018-000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabotti A, De Lucia O, Sakellariou G, Batticciotto A, Cincinelli G, Giovannini I, et al. Predictors, risk factors, and incidence rates of psoriatic arthritis development in psoriasis patients: a systematic literature review and meta-analysis. Rheumatol Ther. 2021;8((4)):1519–1534. doi: 10.1007/s40744-021-00378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Muijen ME, van Hal TW, Groenewoud HMM, van den Reek JMPA, de Jong EMGJ. The skin may clear but the arthritis won't disappear: focusing on concomitant and new-onset psoriatic arthritis in a daily practice cohort of psoriasis patients on biologic therapy. Psoriasis. 2020 Oct;10:29–37. doi: 10.2147/PTT.S270619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winchester R, FitzGerald O. The many faces of psoriatic arthritis: their genetic determinism. Rheumatology. 2020 Mar;59((Suppl 1)):i4–9. doi: 10.1093/rheumatology/kez325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winchester R, FitzGerald O. MHC class I associations beyond HLA-B27: the peptide binding hypothesis of psoriatic arthritis and its implications for disease pathogenesis. Curr Opin Rheumatol. 2020 Jul;32((4)):330–336. doi: 10.1097/BOR.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 41.Hile G, Kahlenberg JM, Gudjonsson JE. Recent genetic advances in innate immunity of psoriatic arthritis. Clin Immunol. 2020;214:108405. doi: 10.1016/j.clim.2020.108405. [DOI] [PubMed] [Google Scholar]
- 42.Patrick MT, Stuart PE, Raja K, Gudjonsson JE, Tejasvi T, Yang J, et al. Genetic signature to provide robust risk assessment of psoriatic arthritis development in psoriasis patients. Nat Commun. 2018 Oct 9;9((1)):4178. doi: 10.1038/s41467-018-06672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Miedany Y, El Gaafary M, Youssef S, Ahmed I, Nasr A. Tailored approach to early psoriatic arthritis patients: clinical and ultrasonographic predictors for structural joint damage. Clin Rheumatol. 2015;34((2)):307–313. doi: 10.1007/s10067-014-2630-2. [DOI] [PubMed] [Google Scholar]
- 44.Gladman DD, Chandran V. Observational cohort studies: lessons learnt from the University of Toronto Psoriatic Arthritis Program. Rheumatology (Oxford) 2011;50((1)):25–31. doi: 10.1093/rheumatology/keq262. [DOI] [PubMed] [Google Scholar]
- 45.Maejima H, Taniguchi T, Watarai A, Katsuoka K. Evaluation of nail disease in psoriatic arthritis by using a modified nail psoriasis severity score index. Int J Dermatol. 2010;49((8)):901–906. doi: 10.1111/j.1365-4632.2009.04452.x. [DOI] [PubMed] [Google Scholar]
- 46.Reich K, Krüger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. 2009;160((5)):1040–1047. doi: 10.1111/j.1365-2133.2008.09023.x. [DOI] [PubMed] [Google Scholar]
- 47.Schons KRR, Beber AAC, Beck MO, Monticielo OA. Nail involvement in adult patients with plaque-type psoriasis: prevalence and clinical features. An Bras Dermatol. 2015;90((3)):314–319. doi: 10.1590/abd1806-4841.20153736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soy M, Karaca N, Umit EU, Bes C, Piskin S. Joint and nail involvement in Turkish patients with psoriatic arthritis. Rheumatol Int. 2008;29((2)):223–225. doi: 10.1007/s00296-008-0686-5. [DOI] [PubMed] [Google Scholar]
- 49.Yang Q, Qu L, Tian H, Hu Y, Peng J, Yu X, et al. Prevalence and characteristics of psoriatic arthritis in Chinese patients with psoriasis. J Eur Acad Dermatol Venereol. 2011;25((12)):1409–1414. doi: 10.1111/j.1468-3083.2011.03985.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its online supplemental information files.