Abstract
Interest in understanding the role of biocrusts as ecosystem engineers in drylands has substantially increased during the past two decades. Mosses are a major component of biocrusts and dominate their late successional stages. In general, their impacts on most ecosystem functions are greater than those of early-stage biocrust constituents. However, it is common to find contradictory results regarding how moss interactions with different biotic and abiotic factors affect ecosystem processes. This review aims to (i) describe the adaptations and environmental constraints of biocrust-forming mosses in drylands, (ii) identify their primary ecological roles in these ecosystems, and (iii) synthesize their responses to climate change. We emphasize the importance of interactions between specific functional traits of mosses (e.g. height, radiation reflectance, morphology, and shoot densities) and both the environment (e.g. climate, topography, and soil properties) and other organisms to understand their ecological roles and responses to climate change. We also highlight key areas that should be researched in the future to fill essential gaps in our understanding of the ecology and the responses to ongoing climate change of biocrust-forming mosses. These include a better understanding of intra- and interspecific interactions and mechanisms driving mosses’ carbon balance during desiccation–rehydration cycles.
Keywords: Abiotic interactions, biological soil crusts, biotic interactions, bryophytes, global change, hydrology, microbial community, nutrient cycles, plant interactions, soil properties
This review synthesizes our understanding of key topics related to the ecology of biocrust-forming mosses in drylands and the plausible effects of climate change on them.
Introduction
Drylands represent the largest terrestrial biome, occupying ~ 41% of the global land area (Cherlet et al., 2018). A heterogeneous cover composed of patches of vascular plants surrounded by rocks and bare or biocrust-dominated soils characterizes these water-limited landscapes (Viles, 2008). Biocrusts, diverse communities of organisms (heterotrophic and photoautotrophic bacteria, archaea, protists, algae, fungi, lichens, mosses, liverworts, nematodes, microarthropods) living within the first centimetres of the soil surface (Weber et al., 2016), constitute a significant feature of stressful environments such as drylands. These communities, whose global coverage is estimated at around 12% of Earth’s terrestrial surface (Rodriguez-Caballero et al., 2018), can dominate the plant interspaces in many drylands due to their specific adaptations to cope with high insolation, low rainfall, and drought. In these systems, the species richness of mosses is low compared with other wetter regions: only about 250 of the approximately 11 000 species of mosses known have been recorded as a biocrust component, and most of these species are from the families Pottiaceae, Grimmiaceae, and Bryaceae (Zhao et al., 2009; Geffert et al., 2013; Seppelt et al., 2016). However, biocrust-forming mosses are common in drylands worldwide (Bowker et al., 2016; Maestre et al., 2021), where they typically form part of late-successional biocrusts (Belnap and Eldridge, 2003, Deng et al., 2020).
During the past three decades, a relevant body of literature underpinning the importance of vascular plant diversity for ecosystem functions and services has emerged (e.g. Hector et al., 2010; Liang et al., 2016; Duffy et al., 2017). Mosses also provide critical ecosystem services, but their study has largely been ignored until recently (Cornelissen et al., 2007). In drylands, mosses can act as ecosystem engineers regulating soil properties, microbial communities, and key ecosystem processes such as infiltration, nutrient cycling, and carbon (C) sequestration (Bowker et al., 2011; Delgado-Baquerizo et al., 2016, 2018; Bao et al., 2019). In addition, they can also promote the establishment of vascular vegetation during ecosystem restoration (Havrilla et al., 2019, 2020; Chen et al., 2020). However, it is also possible to find contradictory information about their effects on ecosystem processes and interactions with other organisms. Several reviews and meta-analyses have attempted to clarify better the ecological roles of biocrusts. Recent reviews have focused on their influence on the hydrological (Belnap, 2006; Eldridge et al., 2020) and nutrient (Barger et al., 2016; Sancho et al., 2016) cycles, their roles as soil stabilizers (Belnap and Büdel, 2016), their interactions with vascular plants (Y. Zhang et al., 2016; Havrilla et al., 2019), or their physiological ecology (Coe et al., 2014). However, most of these reviews are not specifically focused on mosses, and their main goals are often to find general patterns without considering the importance of local environmental factors, linkages with other ecosystem processes, or species-specific traits. Besides, as in most organisms, an alteration of the current distribution of mosses is expected under future climate scenarios (Coe et al., 2014, Rodriguez-Caballero et al., 2018). To contribute to filling these gaps in the literature, here we review the adaptations of biocrust-forming mosses to drylands, their ecological roles in these ecosystems, and the potential impacts of climate change on these organisms and the ecosystem processes that rely on them.
Adaptations of biocrust-forming mosses to dryland environments and their main biotic and abiotic constraints
Moss species are typically linked to humid habitats (Geffert et al., 2013). However, a smaller group of species can thrive in harsh environments such as drylands. To do so, they have developed a unique variety of physiological and morphological strategies that allow them to survive in extreme habitats such as the Sahara, Mojave, or Atacama deserts (Ros et al., 1999; Stark and Whittemore, 2000; Warren-Rhodes et al., 2007). Water is the primary limiting factor for plant growth in drylands worldwide. Still, desiccation tolerance, i.e. the ability to dry to equilibrium with moderate to extremely dry air and to recover the normal metabolic functions after rehydration (Alpert, 2005), is relatively common in dryland mosses (Alpert and Oliver, 2002; Oliver et al., 2005; Wood, 2007; Zhao et al., 2015). Mosses have a maximal water content higher than other poikilohydric organisms such as lichens, which implies longer hydration periods after receiving water pulses (from rain, snow, dew, or fog) and the possibility of gaining more C through photosynthesis (Green et al., 2011). However, they also have higher respiration rates and a lower ability to fix C below their optimum water content (Green et al., 2011). These physiological traits can be a double-edged sword, and the result of having a positive C balance is highly determined by the frequency and magnitude of the water pulses that shape the desiccation–rehydration cycles (Coe et al., 2014). In addition, and unlike green algal lichens that can reach positive net photosynthesis when the relative humidity is near saturation, most mosses rely on the presence of liquid water for activating their photosynthetic machinery (Rundel and Lange, 1980; Green and Lange, 1995). To mitigate these handicaps, mosses have physiological and morphological traits to take advantage of non-rainfall water. For example, their exposure to high atmospheric relative humidity prior to inputs of liquid water has positive effects on the recovery of the photosynthetic apparatus (Slate et al., 2020a). Also, their leaves, which typically have recurved margins, papillose surfaces, and tips with excurrent hair-points (Longton, 1988), can act as condensation points of water vapour and divert the water toward the shoot apex or leaf itself, where it can finally provide metabolic activation and maintenance. This phenomenon has been recently described in the desert moss Syntrichia caninervis, which has a hierarchical water collection and storage system that comprises multiscale structures in the hairs for maximizing the exploitation of water inputs derived from dew, fog, and rainfall (Tao and Zhang, 2012; Pan et al., 2016). Morphological functional traits also operate at several scales (i.e. at the leaf, shoot, and clump level) in bryophytes worldwide (Stanton and Coe, 2021). For example, Moore et al. (2016) found that the higher robusticity of shoots and taller clumps in female Bryum argenteum lend them greater water-holding capacity than male clumps.
These physiological and morphological characteristics of biocrust-forming mosses largely determine their frequency and distribution patterns in drylands. In part due to their dependency on liquid water, their presence and abundance in hyperarid and arid habitats are lower than those of other biocrust constituents (Belnap et al., 2001; Maestre et al., 2021), and their clustered distribution increases proportionally with the aridity of the environment (Navas Romero et al., 2020). This distribution indicates the requirement of an environmental niche narrower than that of other biocrust members for success in drylands, which is generally determined by higher soil moisture and shade levels. For this reason, mosses are more frequent on the north slopes of arid and semiarid landscapes (Nash et al., 1977; Kidron, 2014a; Zhou et al., 2020), and their richness and dominance within biocrust communities can significantly increase with precipitation (Li et al., 2017). Also, when vascular plants colonize dryland areas, they usually create microhabitats more suitable for mosses than for lichens or cyanobacteria (Martínez et al., 2006; Hernandez and Knudsen, 2012; Li et al., 2017; Blanco-Sacristán et al., 2021). Nevertheless, the preference for these microhabitats can differ among moss species due to their different adaptations to light intensity (Fig. 1). Those species that can take advantage of variable light and brief sun flecks for photosynthesis can also increase their hydration time as the surface evaporation is lower under the canopy of vascular plants than in open sites. This prolongation of hydration time is especially true when the vascular plant that provides shade does not exploit the subsurface water soil content, avoiding in this way direct competition with mosses for this resource. An example of this is the association of the moss S. caninervis with the predominant shrubs of dryland areas of North America, and its competitive relationship for surface water and space with the annual invasive species Bromus rubens (Bowker et al., 2000; Stark et al., 2005). However, in Mediterranean drylands, mosses are quite common under the canopy of the perennial grass Macrochloa tenacissima, a species with a shallow root system (Martínez-Sánchez et al., 1994; Maestre et al., 2001). Some shrub species could also negatively interact with bryophytes due to their high litterfall rates (Thompson et al., 2005), although this effect is still unclear as another study found developed moss-dominated biocrusts in habitats with high litter coverage (Briggs and Morgan, 2008). Thus, the relations between vascular plants and biocrust-forming mosses driven by litter could be complex because litter cover may affect several microenvironmental variables such as light intensity, temperature, moisture, and soil nutrient status (Y. Zhang et al., 2016). In other biocrust constituents, such as cyanobacteria, green algae, and lichens, the relationship is negative due to a burial effect (Szyja et al., 2019). However, some species of mosses could have particular adaptations to cope with this adverse effect (e.g. high shade tolerance or greater capacity to grow through the litter layer) and to take advantage of the positive effects provided by litter (e.g. greater concentration of nutrients and lower soil water evaporation).
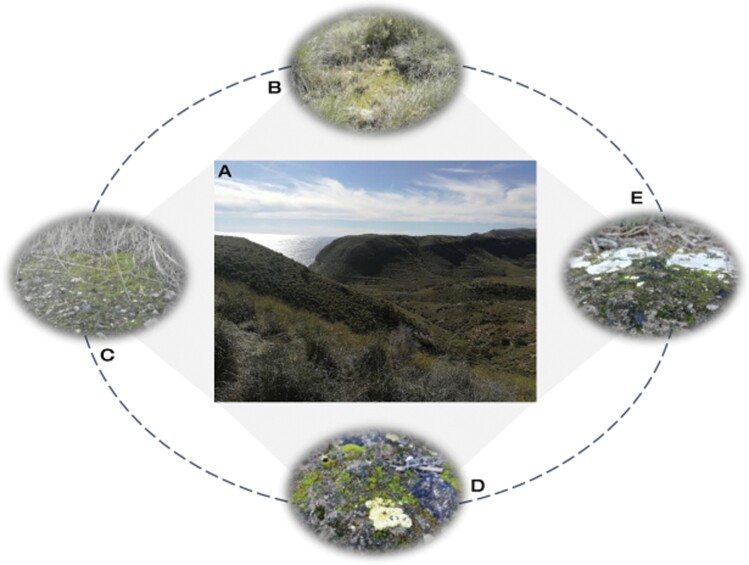
Fig. 1.
Example of distribution and biotic interactions of moss-dominated biocrusts in drylands. Cabo de Gata-Níjar Natural Park (A) is a Mediterranean coastal steppe ecosystem in southeast Spain and one of the driest sites in Europe. The long-term average rainfall is around 200 mm, but an important source of water for vegetation comes from fog and dew. This allows a well-developed grassland vegetation dominated by sparse tussocks of Macrochloa tenacissima with open spaces where mosses are abundant (B). A preferential microhabitat for biocrust-forming mosses is located under the canopy of these tussocks (C). In Mediterranean drylands is very common to find biocrusts where mosses coexist with lichens such as Fulgensia spp. (D) or Squamarina lentigera (E).
In drylands with cold winters, snow can also cover moss crusts for prolonged times. However, snow is a critical source of water for biocrust-forming mosses during the melting period in spring and forms a layer protecting from subfreezing temperatures in winter. For example, in the Gurbantunggut Desert (China), several positive effects of an increase in snowfall have been reported: it can reduce the oxidative, temperature, and desiccation stresses during winter and spring (Zhang and Zhang, 2020), and the greater water availability it provides when it melts enables higher growth from spring to early autumn (Zhao et al., 2016). Hui et al. (2018) also observed a positive effect of a moderate increase in snow depth on the chlorophyll content and photochemical efficiency after an individual snow event.
It is also possible to find dryland mosses with preferences for open, sun-exposed spaces (Soliveres and Eldridge, 2020). They also possess some morphological and physiological strategies for confronting the challenge of receiving intense solar radiation, especially when they are dry and cannot dissipate energy through photosynthesis. For example, S. caninervis can rapidly adjust the leaf angles to minimize or maximize light interception depending on its hydration level by employing biophysical turgor-driven reversible changes led by strategically located leaf cells (Zheng et al., 2011; Wu et al., 2014). Also, the acclimation capacity of desert mosses to different degrees of UV radiation is remarkable. A major mechanism is the ability to adjust photoprotective compounds according to the risks of suffering light damage by using pigmentation plasticity (Ekwealor and Fisher, 2020). As UV stress increases, mosses can reduce the chlorophyll to total pigment content ratio and increase the levels of zeaxanthin (a potential antioxidant) and chlorophyll a:b and carotenoid:chlorophyll ratios (Hamerlynck et al., 2002; Ekwealor et al., 2021). In addition to their presence, the location of photoprotective compounds in the cells can be essential for their functionality. For example, Ceratodon purpureus, a cosmopolitan species that can persist and be dominant in arid ecosystems (Weber et al., 2018), has a lower total quantity of photoprotective compounds than Bryum pseudotriquetrum, another cosmopolitan species more abundant in wetter areas (Clarke and Robinson, 2008). However, Ceratodon has its ultraviolet screening compounds mainly located in the cell wall rather than inside the cells, which is why it has greater UV tolerance (Clarke and Robinson, 2008). Another central photoprotective mechanism in plants is the dissipation of excess energy as heat through a set of processes known as non-photochemical quenching. The relative importance of this mechanism in mosses is not evident. However, a recent study suggests that desert mosses can undergo a sustained form of non-photochemical quenching, and its relaxation after hydration is the main modulator of photosynthetic recovery, rather than the repair of damaged or inactivated photosynthetic systems (Ekwealor et al., 2021). Finally, the different photoprotective mechanisms found in bryophytes seem to be more effective as their ability to tolerate desiccation increases, with the result that some dryland mosses can withstand both stresses (Takács et al., 1999).
Along with climatic determinants such as aridity, edaphic factors also influence the distribution of biocrusts (Bowker et al., 2017). Climate and soil properties are closely linked in drylands; as an example, aridity is the main factor responsible for soil salinity in continental ecosystems (Mota et al., 2011). One type of saline soil typical of drylands is gypsum soil, which covers large arid and semiarid regions worldwide (Herrero, 2004). The high levels of calcium (Ca) in the form of gypsum are not a limiting factor for the distribution of mosses, and a high taxonomic and functional richness of mosses has been reported on gypsum soils in the USA, Europe, and Australia (Salmerón et al., 2011; Aleffi et al., 2014; Seppelt et al., 2016; Bowker et al., 2017). Some specialized gypsum species (e.g. S. caninervis var. gypsophila, Didymodon nevadensis, and Tortula revolvens), strongly calcicolous species (e.g. Aloina aloides, Crossidium crassinerve, and Weissia controversa), and outcrop colonizers that can also be terricolous and require greater moisture and shade (e.g. Gymnostomum calcareum, Eucladium verticillatum, and Pellia endiviifolia; Aleffi et al., 2014) appear to converge in gypsiferous areas. However, some species only reported in gypsum areas are at isolated sites and far from each other, meaning that gypseous substratum alone might not determine their colonization of new areas, but a set of microenvironmental conditions present in restricted sites within gypsum ecosystems (Guerra et al., 1995; Aleffi et al., 2014).
The fact that some of these species are also found on soils enriched with Ca carbonate (CaCO3) suggests that they require or tolerate high Ca levels. For example, the abundance of mosses has been related to high soil pH, electrical conductivity, and Ca levels in Australia (Downing and Selkirk, 1993). A certain degree of soil stability is also necessary for mosses, and soils with a finer texture, which provide an inherent stability and increase water retention, can favour their growth (Downing and Selkirk, 1993; Bowker et al., 2006). In conclusion, the richness and diversity of biocrust-forming mosses in drylands are determined by a convergence of large- and local-scale environmental variables. Mosses are moisture-limited at a large scale (Concostrina-Zubiri et al., 2014b), but the positive effect on water availability of biotic and abiotic variables at the microhabitat scale (e.g. soil texture and radiation interception by vascular plants and topography) and the physiological and morphological adaptations explained in this section, allow them to penetrate the more arid regions of the world.
Biocrust-forming mosses as ecosystem engineers in drylands
Building the foundations: effects of mosses on soil properties and their conservation
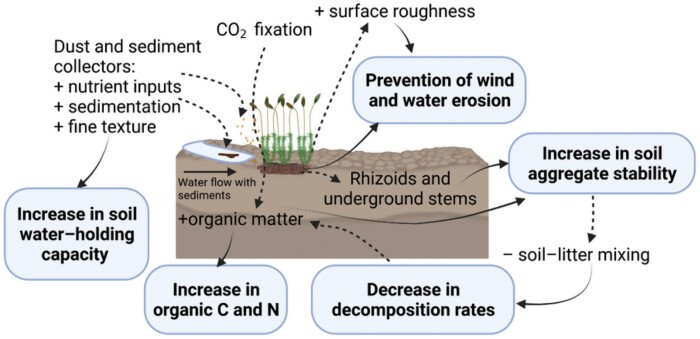
Soil erosion is a primary determinant of land degradation and desertification in drylands (Kidane et al., 2019; Chen et al., 2021). Sediment capture by biocrusts is a key functional trait of these communities that directly influences their capacity to aggregate soil particles and thus control erosion (Mallen-Cooper and Eldridge, 2016). There is a strong consensus that biocrust-forming mosses prevent both wind and water erosion (Yang et al., 2014; Bu et al., 2015) and are more effective than other biocrust constituents in doing so (Mallen-Cooper and Eldridge, 2016; Gao et al., 2020a, b). Their use to prevent erosion has even provided better results than those of vascular plants in some cases (Zhao and Xu, 2013; Wang et al., 2021). Biocrusts can act against erosion through several mechanisms. The most evident is the physical barrier, but other physical properties, such as their effects on soil surface roughness, can also play a primary role in energy dispersion processes (Eldridge and Rosentreter, 1999; Wang et al., 2017). However, the effectiveness of this property is intrinsically linked to water content. Many mosses shrivel, fold, or curl during dry periods, losing a relevant volume, but upon rehydration, they can rapidly recover their volume and increase soil roughness (Danin and Ganor, 1991; Warren, 2001). This wetting-induced roughness is much greater in mosses than in other biocrust constituents (Wang et al., 2017) and can explain the high capacity of mosses to absorb raindrop and runoff kinetic energy, and thus to reduce erosion associated with rainfall splash and overland water flow (Kidron et al., 2003; Y. Zhao et al., 2014). Besides, the increase in surface roughness provided by mosses reduces wind speed and allows the capture of dust and nutrients (Danin and Ganor, 1991; Williams et al., 2012, 2013). In addition to physical mechanisms, changes in soil properties induced by mosses, such as increases in soil organic matter, cohesion, and fine soil texture, also protect the soil against erosive forces (Gao et al., 2020b; Fig. 2).
Fig. 2.
Effects of mosses on soil properties. Soil variables and processes increased and decreased by the presence of mosses are indicated by + and – signs, respectively. Created with BioRender.com.
Biocrust-forming mosses as modulators of biogeochemical cycles
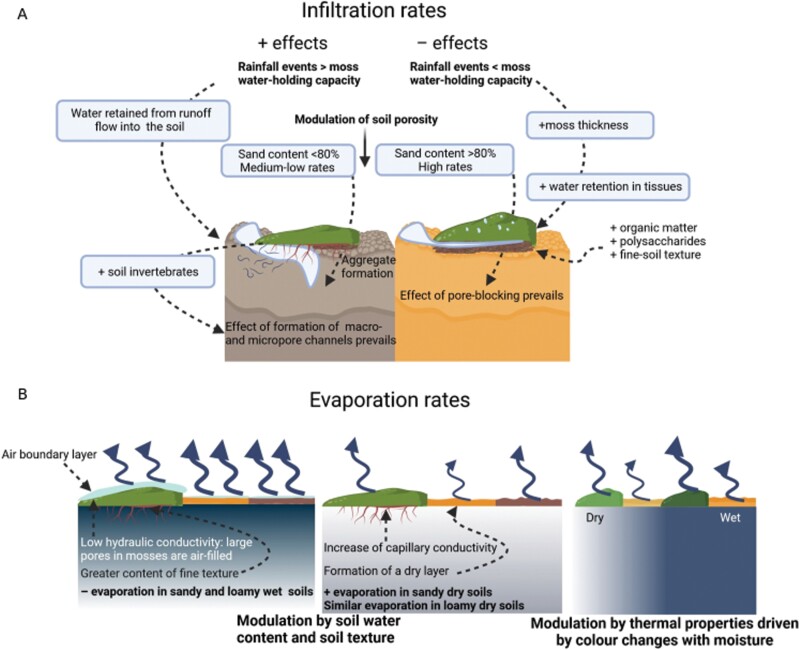
In general, biocrusts are crucial to soil functioning in drylands as they influence the concentration of elements essential for the metabolism of soil organisms and vascular plants (Moreno-Jiménez et al., 2020). It is well known that plant growth and ecological processes in drylands are strongly constrained by water availability, and biocrusts can significantly influence the distribution and preservation of water throughout the soil profile (Eldridge et al., 2020). Biocrust-forming mosses can modulate both horizontal (runoff) and vertical (infiltration and evaporation) fluxes of water as well as soil moisture and water holding capacity (Eldridge et al., 2020). However, there are inconsistent results regarding the effects of biocrust-forming mosses on hydrological processes due to the interactions of species-specific traits with site-specific characteristics and legacies, such as soil texture, climate, or previous disturbances. Factors such as surface roughness (Fig. 3) enhance surface soil vapour sorption and deposition of non-rainfall water (Tao and Zhang, 2012; Pan et al., 2016; S. Li et al., 2021a, b). Hence, biocrust-forming mosses can increase soil moisture in the first centimetres but decrease it at deeper horizons in sandy soils (Yang et al., 2014; Xiao et al., 2016). These organisms can also prevent water losses through evaporation after rainfall events and, therefore, increase soil moisture during this time (Y.-F. Zhang et al., 2016). However, hydric conditions can modulate this response since the capacity of mosses to mobilize water by capillarity has a negative relationship with their moisture level (Voortman et al., 2014). Thus, the water that mosses retain by adsorption after a rain event can be easily lost 3 or 4 d after that, especially if the mosses are dark green coloured (Xiao et al., 2010; Y.-F. Zhang et al., 2016). Altogether, the final balance of moss effects on soil moisture depends on local features (e.g. characteristics of rainfall events and soil properties) and the traits of the moss species. This could explain results in which moss cover either decreased the soil moisture in the first layers of the soil (e.g. Bu et al., 2015; Xiao and Hu, 2017) or enhanced it (e.g. Xiao et al., 2015, 2016; F. Sun et al., 2021a).
Fig. 3.
The effects of mosses on the soil water balance are determined by water infiltration into the soil (A) and evaporative processes (B). Positive signs on biotic and abiotic variables indicate their positive correlations with the presence of biocrust-forming mosses in (A). The increase of the variables located in the soil profile on the left positively affects the infiltration rate, whereas those located on the right have a negative effect on this rate. The representation of mosses in (B) varies in colour to indicate the effect of radiation reflectance on evaporation. Created with BioRender.com.
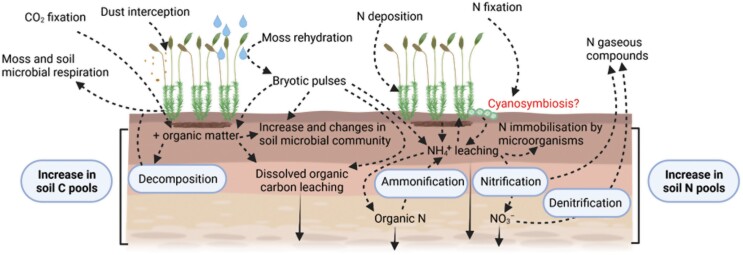
Just like vascular plants, mosses need nitrogen (N) for their growth. Uptake from wet or dry deposition has traditionally been considered their primary means of N acquisition (Brown and Bates, 1990). However, and despite a lack of developed roots and a vascular system, mosses can uptake N from soil and transport it to their shoots (Ayres et al., 2006), and establish symbiotic associations with cyanobacteria, a group of N-fixer organisms, to obtain this macronutrient (Belnap, 2001). Cyanobacteria in soil crusts, as free-living organisms or as symbionts in lichens and mosses, significantly impact the N cycle as their N-fixing activity ranges from 40% to 85% of the total fixed biologically in terrestrial ecosystems at a global scale (Rodriguez-Caballero et al., 2018). Unfortunately, there is still little known about the role of cyanosymbiosis in the nutrient status of moss-dominated biocrusts and how it interacts with soil N availability (Coe et al., 2014). A recent study suggests that all these ways of acquiring N allow mosses to have enough N reserves even to transfer some of them to vascular plants through fungal loops without compromising their survival (Carvajal Janke and Coe, 2021). Mosses can also enrich the soil through direct N leakage during the cyanobacterial N fixation, the decomposition of moss tissues (Evans and Lange, 2001), or the phenomenon of ‘bryotic pulses’ (Slate et al., 2019; Fig. 4). These pulses occur when mosses are rehydrated after a rainfall event and the cells damaged during the dehydration–rehydration process lose their intracellular content (carbohydrates, inorganic N, amino acids, and ionic compounds), which can ultimately be leached to the soil. Despite this, the effects of biocrust-forming mosses on the N cycle are poorly studied, particularly when compared with other biocrust constituents that are N-fixers (e.g. cyanobacteria or cyanolichens). The direction of N mobilization between the moss–soil interface is unclear and could depend on several biotic and abiotic factors. The population dynamics of the moss community could be one of them. In incipient communities, where mosses are colonizing new places and need to grow faster than their competitors for space, their N demand could be much higher than in stable communities. Thus, there is a mobilization of available N—ammonium (NH4+) or nitrate (NO3−)—from soil to mosses in these situations. This factor in the direction of N mobilization agrees with results from Slate et al. (2020b), who found that the establishment of a moss-dominated biocrust for 3 years after its inoculation caused a decrease of NH4+ concentration in the soil beneath. However, the NO3− concentration in the soil was not affected by moss cover, also supporting the idea that NH4+ instead of NO3− is energetically much more efficient for generating new moss biomass (Ruan and Giordano, 2017). An alternative explanation of the decrease of ammonium caused by mosses is that the ‘bryotic pulses’ enhance the microbiome responsible for N immobilization (Slate et al., 2020b). However, more research is needed to elucidate the main mechanism of ammonium decrease in soils colonized by mosses. In mature communities, N can be transferred from mosses to the soil, and abiotic factors such as rainfall inputs drive the magnitude of this transfer (Slate et al., 2021). Events causing high mortality of mosses can also significantly alter N pools in soils (Reed et al., 2012). After these events, Reed et al. (2012) observed a switch from NH4+ to NO3− dominance in a dryland ecosystem in Utah. This shift in the soil N pools has important implications for ecosystem functioning. On the one hand, although NO3− is energetically less effective in plant nutrition, its greater mobility in most soils and lower use by microorganisms could increase its availability for plants (Austin et al., 2006). But on the other hand, the easier loss of this N component through gaseous emissions (McCalley and Sparks, 2009; Weber et al., 2015) could reduce its content in drylands.
Fig. 4.
Effects of mosses on nutrient cycles. An interaction worth investigating in moss-dominated biocrusts is highlighted in red. Created with BioRender.com.
Since N is a primary limiting element for C acquisition by plants, mosses can act as modulators of the C cycle through their impacts, as explained, on the different forms of N and their links with other organisms. Nevertheless, mosses also have a direct role by C fixation through photosynthesis. The functional traits that govern the C balance in biocrust-forming mosses are still not well understood. Different moss species have a broad range of responses to environmental conditions, especially to water input patterns (Coe et al., 2019). Among the different biocrust constituents, mosses usually have the highest photosynthetic efficiency in optimal moisture conditions (Lan et al., 2017). Therefore, they have a higher potential to sequester C (Yang et al., 2019; Xu et al., 2022). However, they also have greater physiological limitations than lichens as aridity increases. For example, mosses have lower net photosynthetic rates when annual precipitation is below 200 mm (Raggio et al., 2018).
Recent studies have highlighted the relevant role of the microbial communities within or below biocrusts as modulators of nutrient cycling (Liu et al., 2017; Concostrina-Zubiri et al., 2021; Qi et al., 2021). Specifically, biocrust-forming mosses can increase the abundance and diversity of bacteria and fungi beneath them (Liu et al., 2018; Maier et al., 2018). The fungi:bacteria ratio and the functional genes involved in C and N cycles also increase under moss-dominated biocrusts compared with other less-developed biocrust types (Liu et al., 2018). These genes were linked to C degradation and N denitrification, causing, for example, an increase in respiration and nitrous oxide (N2O) emissions in moss-crusted soils (Maier et al., 2018; Slate et al., 2019). However, the nitric oxide (NO) and nitrous acid (HONO) effluxes in biocrust-forming mosses are lower than in cyanobacteria-dominated biocrusts (Weber et al., 2015; Maier et al., 2018). These different efflux dynamics reflect the necessity of studying multiple N compounds to unravel the role of mosses in N cycling. In conclusion, the net effect on soil nutrient content will be modulated by abiotic factors that drive processes such as leaching and microbial activity.
Environmental gradients modulate biotic interactions involving mosses
Biocrust-forming mosses have a large capacity to alter the soil’s physical and chemical properties, and by doing so, mosses can affect the performance of vascular plants. However, the impacts of mosses on vascular plants are not clear-cut and seem to be strongly modulated by environmental gradients (Doxford et al., 2013). A recent meta-analysis (Havrilla et al., 2019) found that, in general, the positive effects of mosses on the performance of vascular plants (i.e. germination, survival, growth, cover, nutrient uptake, phenology, reproduction, and diversity) prevails over the negative ones. This result contrasted with the effects of cyanobacteria and lichens on vascular plants, as they came out as negative (Havrilla et al., 2019). Biocrust-forming mosses can promote vascular plant growth by forming soil fertility islands (Ferrenberg et al., 2018). As commented above, these nutrients can be mobilized from open spaces to vascular plants through fungal loops. However, it is also possible to find examples where mosses undermine vascular plant growth due to their competition for water and their capacity to reduce water infiltration through the soil profile (Guan and Liu, 2019; X. Li, Yu et al., 2021).
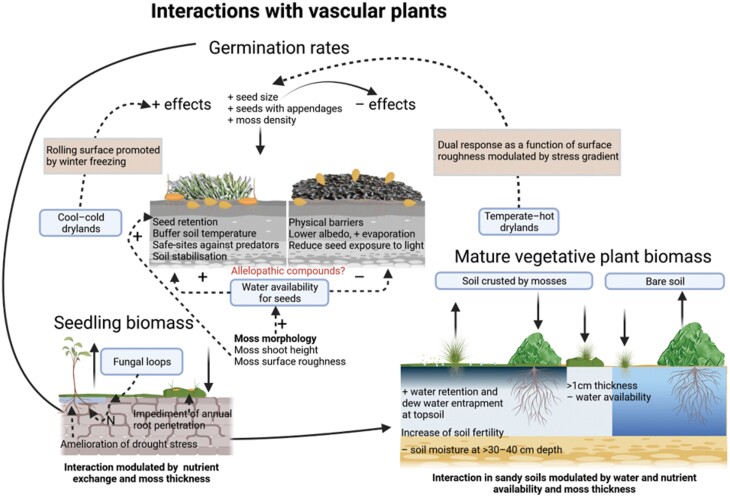
This competition can be highly modulated by environmental conditions, especially precipitation. For example, in the Negev desert, the highest densities of vascular plants under near-average precipitation conditions can be found at the base of dunes. In these sites, moss-dominated biocrusts are dominant, and the water supply is higher than in the dunes themselves (Tielbörger, 1997). However, in years of extreme drought, water availability is higher in non-crusted mobile dunes, so the bloom of annual species takes place in these areas (Kidron, 2014b). The effect of mosses on the germination of vascular plants can range from positive to negative (e.g. Y. Zhang et al., 2016; Ferrenberg et al., 2018; Havrilla et al., 2019; X. Li, Yu et al., 2021), a range of responses likely driven by the species-specific requirements for seed germination. Mosses have a high capacity to modulate the soil microenvironment beneath them through their morphology and light reflectance. Consequently, the degree to which the optimum environment for seed germination meets the temperature and moisture ranges influenced by moss traits will determine the result of this moss–plant interaction (Fig. 5). For example, Schlatterer and Tisdale (1969) found a possible inhibitory effect of moss litter on seed germination in only one of three grass species tested. In some cases of very well-developed crusts, germination can be prevented because seeds do not reach the soil surface (McIlvanie, 1942). Thus, the morphology and dispersal mechanisms of seeds could also be crucial to ensure their germination within moss-dominated biocrusts.
Fig. 5.
Different moss–vascular plant interactions. In the germination rates section, biotic factors with positive (+) signs and an arrow pointing to the soil profile on the left indicate positive correlations with germination rate, whereas those pointing to the right indicate negative correlations with this rate. An interaction worth investigating in moss-dominated biocrusts is highlighted in red. Created with BioRender.com.
Competition for space can act as a powerful structuring force of biocrust communities in drylands (Maestre et al., 2008; Bowker et al., 2010). Dominant moss species in these communities are often large-sized species with a high growth rate, as has been detected for the species Pleurochaete squarrosa across an environmental gradient in Spain (Bowker and Maestre, 2012). Those moss species are also more effective in the retention of water and the uptake of nutrients such as N and phosphorus (P) (Li et al., 2019). One of their competitors for space, lichens, can produce around 800 different secondary compounds (Asplund and Wardle, 2017). Thus, it is not unreasonable to think that some of them can be used as chemical weapons against bryophytes. For example, Gardner and Mueller (1981) examined in the lab the toxicity potential of several lichen acids on the germination and sporeling development of Funaria hygrometrica. Some of them had negative effects on these parameters, although their relative toxicity was highly dependent on their concentration and the pH of the growing medium. Thus, it is still difficult to interpret the potential of the results in the real world. Also, a non-hierarchical competition (also called intransitive competition), where there are no clear dominant or winner species, can exist within the members of biocrust communities (Bowker and Maestre, 2012). Soliveres and Eldridge (2020) found a microenvironment modulation of intransitive competition between moss and lichen species since this mechanism of community assembly has a higher role under shrub canopies than in open areas. These situations allow greater richness within the biocrust community as no one member is displaced and each can coexist with others.
Several studies also provide evidence of positive interactions between mosses and other biocrust constituents. Using a culturing approach, Bowker and Antoninka (2016) found a higher cover increase in a combination of lichens and mosses than in their respective monocultures. Colesie et al. (2012) found that moss-associated thalli of Peltigera rufescens had a higher net photosynthetic rate, thallus thickness and growth rate than those growing in isolation, providing a clear example of facilitation between mosses and lichens. Facilitative interactions in dryland biocrust communities seem to be less relevant as aridity increases (Bowker et al., 2010). This fact is likely explained by the effects of mosses on water availability. In environments with very few rainfall events that allow poikilohydric organisms to reach their water holding capacity, mosses usually compete strongly for water. But under wetter conditions, mosses promote the infiltration and retention of water that can be used more gradually through capillarity by their neighbours (Eldridge et al., 2010). However, the switch between competitive and facilitative interactions is not linear throughout stress gradients, but rather follows a U-shaped curve, with the maximum competition levels at the extremes (J. Sun et al., 2021b). This curvilinear relationship can be observed when the analysis of the interactions includes all the main biocrust constituents and a wide stress gradient. In the wettest situations, cyanobacteria and algae are strongly displaced by lichens and mosses, although the specific mechanisms that provide these competitive advantages are still unclear. Mosses also interact with other members of the microbial communities in soils. The positive effects of mosses on soil stability and organic matter promote favourable microhabitats for microbial communities (Bao et al., 2019) and, therefore, boost the diversity of bacteria and fungi. This biodiversity is higher when compared with bare and cyanobacterial crusted soils (Maier et al., 2018; Tian et al., 2021). However, these studies did not find significant differences when they compared the moss microbial communities with those located beneath lichens.
The fate of biocrust-forming mosses in a changing world
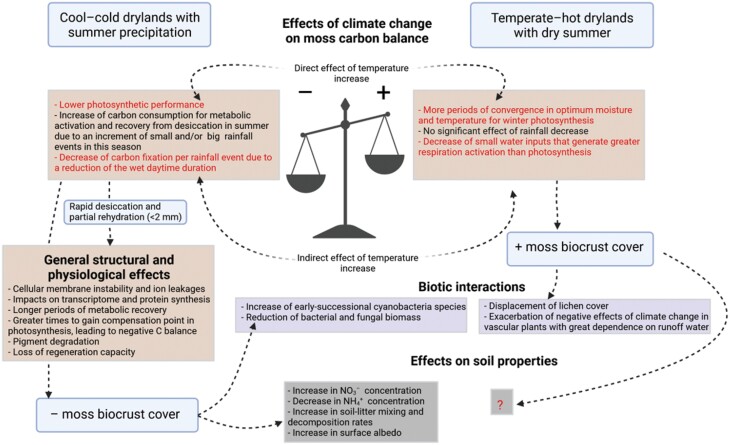
The effects of global warming and altered rainfall regimes on biocrust-forming mosses are closely linked to their impacts on the water balance and desiccation–rehydration cycles of mosses. In those areas where large water inputs are dominant (versus non-rainfall water inputs such as fog and dew), a negative effect of elevated temperature through accelerated drying rates is expected in biocrusts dominated by mosses and lichens (Tucker et al., 2019). However, a recent study using a long-term (53 years) record of biocrust surveys has found negative impacts of warming (~0.27 °C per decade) only in lichens, mosses being more sensitive to changes in precipitation (Finger-Higgens et al., 2022). Mosses can fix more C than other biocrust types after large rainfall events. Still, they perform worst, and even have a negative C balance due to respiration, when subjected to small rainfall events (Zhang et al., 2018) and/or prolonged desiccation periods (Coe et al., 2012). If the pattern of small rainfall pulses during summer is prolonged over time, as several models forecast for some dryland regions (Miller et al., 2021), this can lead to moss C starvation and a significant loss of their cover (Reed et al., 2012; Ferrenberg et al., 2015). The break of dormancy during summer, even with large rainfall events, can negatively impact on moss biomass (Stark et al., 2011). However, warming can also reduce the frequency of very small events of water condensation and fasten the soil surface desiccation. These effects on water availability could also decrease the frequency of metabolic activations of mosses with a final negative C balance and shorten their respiration periods after photosynthetically unproductive small rainfalls (Fig. 6). In this case, an increase in temperatures can promote the development of moss-dominated biocrusts (Ladrón de Guevara et al., 2018). Also, if the maximum photosynthetic period is determined by winter and spring rainfalls, an increase in temperature could favour a lengthening of the optimal temperature for photosynthesis in mosses, which ranges from 10 °C to 20 °C (Coe et al., 2014). In fact, a favourable effect of spring precipitation on mosses was recently observed in a cool desert (Finger-Higgens et al., 2022).
Fig. 6.
Loss of moss-dominated biocrusts driven by negative carbon balances in two climate change field experiments (Reed et al., 2012; X. Li, Hui et al., 2021) and in a mesocosm experiment (Tucker et al., 2019) in cool drylands; and increasing (but not significant) trends of moss cover in two field experiments in temperate–hot drylands (Escolar et al., 2012; Maestre et al., 2015; Ladrón de Guevara et al., 2018). Effects related to temperature are highlighted in red. Biotic and abiotic effects found or suggested in these experiments are shown. The effects of mosses on soil properties are still inconclusive in temperate–hot drylands as lichens are the dominant biocrust component of these studies. In addition, several physiological effects derived from the desiccation–rehydration cycles caused by the treatments are presented as complementary mechanisms that could explain the observed changes in moss cover. Created with BioRender.com.
Bryophytes have evolved to use water when this resource is available aboveground (Proctor, 1982). It is thus reasonable to think that the desiccation–rehydration cycles that determine the C economy of mosses are more influenced by the frequency of water inputs than by their magnitude when the rainfall events surpass the water holding capacity of the upper soil layer. However, large rainfall events that cause water infiltration into deeper soil layers could positively affect the moss C balance as they could increase the period of optimum water content for photosynthesis through capillary processes (Ladrón de Guevara et al., 2014). Although mosses in drylands are considered resistant to drought, multiyear drought events can overcome their resistance threshold and cause a population decline (Belnap et al., 2006). Even for a single drought event, its duration can influence the speed of photosynthetic reactivation. This recovery speed could be key in determining the outcome of biotic interactions within the moss communities in regions such as the Mediterranean basin, as most climatic models converge in forecasting longer drought events in this area. For example, Munzi et al. (2019) found a slower photosynthetic reactivation in the dominant Mediterranean moss P. squarrosa after a 2-month drought than after a 2-week drought period. However, this effect was not observed in the alien moss Campilopus introflexus. Thus, climate change could also promote changes in the composition of moss communities in this area.
As mentioned above, seasonal snow can play a fundamental role in the physiological performance and biomass production of mosses in cold drylands. There is a consensus that the snowpack started to decline at the end of the last century along with an earlier spring melting in the northern hemisphere (Bormann et al., 2018; Zhang and Ma, 2018). However, there is also a high regional variability, and in places where mosses are dominant, such as in the Gurbantunggut Desert, these trends are inverted (Tan et al., 2019). In a scenario of a snow increase to twice that of the current regime, the physiological characteristics of mosses would allow them to increase their growth (Zhao et al., 2016, 2018). Despite this, there are uncertainties about the impacts of snow changes due to its interaction with the expected increase in temperatures. For example, in the past decades, the trends of the snow/precipitation ratio modulated by temperature changes did not have the same direction across the Gurbantunggut Desert (Li et al., 2018), and the forecasted trend in snow cover depth is a general decrease in almost all the area (Shi et al., 2011). In this scenario, organisms with lower hydric requirements, such as cyanobacteria and algae, will likely displace mosses.
Mosses as a tool for creating more resistant and resilient dryland ecosystems
Interest in using biocrusts for restoring ecosystem functions in drylands after relevant disturbance events has increased exponentially during the past decade. Biocrust-forming mosses have received particular attention because they usually have a greater impact on the ecological processes described above than the other biocrust members (Xiao et al., 2019) and their ex situ culture for generating enough biomass for restoration work is feasible (e.g. Xu et al., 2008; Y.-M. Zhao et al., 2014; Antoninka et al., 2016; Grover et al., 2020). However, as this review has elucidated, mosses do not have a single effect on several ecosystem functions and it is important to consider site idiosyncrasy to predict restoration trajectories better. The level of degradation is an essential factor to consider when restoration goals are defined. When native populations persist in their natural locations, it is feasible to implement a passive recovery of moss populations in a time frame of 20 years, especially in grasslands and grassy woodlands with shaded areas where mosses have competitive advantages over other organisms (Read et al., 2011; Concostrina-Zubiri et al., 2014a). However, active restoration is required if the site has suffered an intense disturbance and there are not enough sources of propagules (Condon et al., 2020). A first step for overcoming propagule limitations is the development of ex situ cultivation. For example, mosses grow well in trays with an organic substrate under greenhouse conditions (Grover et al., 2020). Then, the translocation of the moss biomass obtained to the sites targeted for restoration is necessary and, nowadays, several techniques show promise for improving moss survival under field conditions in drylands (e.g. Blankenship et al., 2020; Doherty et al., 2020).
Conclusions
Biocrust-forming mosses have relevant roles within and beyond biocrusts. Their distributions along dryland habitats are more constrained by environmental factors than those of early-stage biocrust constituents. However, they can displace other biocrust members when they successfully occupy spaces in their optimal environmental ranges. The distribution of mosses is more linked to vascular plants than that of lichens or cyanobacteria, especially when aridity increases. We have highlighted the critical role of biocrust-forming mosses in the hydrological cycle in drylands and in preventing soil loss, improving soil structure, and enhancing nutrient status in these areas. The interactions of biocrust-forming mosses with vascular plants are complex and, in most cases, species- and site-specific. The influence of mosses on the soil water content throughout the soil profile, and their radiation reflectance, morphology, and degree of development, can determine their effects on vascular plants. It is also difficult to forecast a general response of biocrust-forming mosses to climate change since the few existing field studies show divergent effects depending on local climatic characteristics. Some areas that require further research include a better understanding of the impacts of climate change on moss populations at the global scale, their biotic interactions within and outside biocrust communities, their effects on microbial communities and nutrient cycles, and the impacts of desiccation–rehydration cycles on their C economy. Identifying the best species to be used in restoration work and the best way of growing them are also key topics for future research.
Acknowledgements
We thank Roberto Lázaro for his valuable comments on the manuscript.
Contributor Information
Mónica Ladrón de Guevara, Departamento de Desertificación y Geoecología. Estación Experimental de Zonas Áridas (CSIC), Carretera de Sacramento, s/n, 04120 La Cañada de San Urbano-Almería, Spain.
Fernando T Maestre, Instituto Multidisciplinar para el Estudio del Medio ‘Ramón Margalef’, Universidad de Alicante, Carretera de San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Spain; Departamento de Ecología, Universidad de Alicante, Carretera de San Vicente del Raspeig s/n, 03690 San Vicente del Raspeig, Spain.
Alessandro Petraglia, University of Parma, Italy.
Author contributions
MLG conceived the original review focus and wrote the manuscript with critical edits, inputs and discussion from FTM. All authors read and approved the final content of the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
MLG was supported by the Andalusian Research, Development and Innovation Plan (PAIDI 2020, DOC_01041). FTM was supported by the European Research Council (ERC Grant agreement 647038 [BIODESERT]) and Generalitat Valenciana (CIDEGENT/2018/041).
Data availability
No new data have been analyzed or created in this review.
References
- Aleffi M, Pellis G, Puglisi M.. 2014. The bryophyte flora of six gypsum outcrops in the northern Apennines (Nature 2000 Network, Emilia Romagna region, Italy). Plant Biosystems 148, 825–836. [Google Scholar]
- Alpert P. 2005. The limits and frontiers of desiccation-tolerant life. Integrative and Comparative Biology 45, 685–695. [DOI] [PubMed] [Google Scholar]
- Alpert P, Oliver MJ.. 2002. Drying without dying. In: Black M, Pritchard HW, eds. Desiccation and survival in plants: drying without dying. Wallingford: CAB International, 3–43. [Google Scholar]
- Antoninka A, Bowker MA, Reed SC, Doherty K.. 2016. Production of greenhouse-grown biocrust mosses and associated cyanobacteria to rehabilitate dryland soil function. Restoration Ecology 24, 324–335. [Google Scholar]
- Asplund J, Wardle DA.. 2017. How lichens impact on terrestrial community and ecosystem properties. Biological Reviews 92, 1720–1738. [DOI] [PubMed] [Google Scholar]
- Austin AT, Sala OE, Jackson RB.. 2006. Inhibition of nitrification alters carbon turnover in the Patagonian steppe. Ecosystems 9, 1257–1265. [Google Scholar]
- Ayres E, Van der Wal R, Sommerkorn M, Bardgett RD.. 2006. Direct uptake of soil nitrogen by mosses. Biology Letters 2, 286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao T, Zhao Y, Gao L, Yang Q, Yang K.. 2019. Moss-dominated biocrusts improve the structural diversity of underlying soil microbial communities by increasing soil stability and fertility in the Loess Plateau region of China. European Journal of Soil Biology 95, 103120. [Google Scholar]
- Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J.. 2016. Patterns and controls on nitrogen cycling of biological soil crusts. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226. Cham: Springer International Publishing, 257–285. [Google Scholar]
- Belnap J. 2001. Factors influencing nitrogen fixation and nitrogen release in biological soil crusts. In: Belnap J, Lange OL, eds. Biological soil crusts: structure, function, and management. Ecological Studies Series 150. Berlin, Heidelberg: Springer-Verlag, 241–261. [Google Scholar]
- Belnap J. 2006. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrological Processes: An International Journal 20, 3159–3178. [Google Scholar]
- Belnap J, Büdel B.. 2016. Biological soil crusts as soil stabilizers. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226. Cham: Springer International Publishing, 305–320 [Google Scholar]
- Belnap J, Büdel B, Lange OL.. 2001. Biological soil crusts: characteristics and distribution. In: Belnap J, Lange OL, eds. Biological soil crusts: structure, function, and management. Ecological Studies Series 150. Berlin, Heidelberg: Springer-Verlag, 3–30. [Google Scholar]
- Belnap J, Eldridge DJ.. 2003. Disturbance and recovery of biological soil crusts. In: Belnap J, Lange OL, eds. Biological soil crusts: structure, function, and management. Ecological Studies Series 150. Berlin, Heidelberg: Springer-Verlag, 364–383. [Google Scholar]
- Belnap J, Phillips SL, Troxler T.. 2006. Soil lichen and moss cover and species richness can be highly dynamic: the effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Applied Soil Ecology 32, 63–76. [Google Scholar]
- Blanco-Sacristán J, Panigada C, Gentili R, Tagliabue G, Garzonio R, Martín MP, Ladrón de Guevara M, Colombo R, Dowling TPF, Rossini M.. 2021. UAV RGB, thermal infrared and multispectral imagery used to investigate the control of terrain on the spatial distribution of dryland biocrust. Earth Surface Processes and Landforms 46, 2466–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship WD, Condon LA, Pyke DA.. 2020. Hydroseeding tackifiers and dryland moss restoration potential. Restoration Ecology 28, S127–S138. [Google Scholar]
- Bormann KJ, Brown RD, Derksen C, Painter TH.. 2018. Estimating snow-cover trends from space. Nature Climate Change 8, 924–928. [Google Scholar]
- Bowker MA, Antoninka AJ.. 2016. Rapid ex situ culture of N-fixing soil lichens and biocrusts is enhanced by complementarity. Plant and Soil 408, 415–428. [Google Scholar]
- Bowker MA, Belnap J, Büdel B, Sannier C, Pietrasiak N, Eldridge DJ, Rivera-Aguilar V.. 2016. Controls on distribution patterns of biological soil crusts at micro-to global scales. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226. Cham: Springer International Publishing, 173–197. [Google Scholar]
- Bowker MA, Belnap J, Miller ME.. 2006. Spatial modeling of biological soil crusts to support rangeland assessment and monitoring. Rangeland Ecology & Management 59, 519–529. [Google Scholar]
- Bowker MA, Büdel B, Maestre FT, Antoninka A, Eldridge DJ.. 2017. Bryophyte and lichen diversity on arid soils: determinants and consequences. In: Steven B, ed. The biology of arid soils. Berlin: De Gruyter, 73–96. [Google Scholar]
- Bowker MA, Maestre FT.. 2012. Inferring local competition intensity from patch size distributions: a test using biological soil crusts. Oikos 121, 1914–1922. [Google Scholar]
- Bowker MA, Mau RL, Maestre FT, Escolar C, Castillo-Monroy AP.. 2011. Functional profiles reveal unique ecological roles of various biological soil crust organisms. Functional Ecology 25, 787–795. [Google Scholar]
- Bowker MA, Soliveres S, Maestre FT.. 2010. Competition increases with abiotic stress and regulates the diversity of biological soil crusts. Journal of Ecology 98, 551–560. [Google Scholar]
- Bowker MA, Stark LR, McLetchie DN, Mishler BD.. 2000. Sex expression, skewed sex ratios, and microhabitat distribution in the dioecious desert moss Syntrichia caninervis (Pottiaceae). American Journal of Botany 87, 517–526. [PubMed] [Google Scholar]
- Briggs A, Morgan JW.. 2008. Morphological diversity and abundance of biological soil crusts differ in relation to landscape setting and vegetation type. Australian Journal of Botany 56, 246–253. [Google Scholar]
- Brown DH, Bates JW.. 1990. Bryophytes and nutrient cycling. Botanical Journal of the Linnean Society 104, 129–147. [Google Scholar]
- Bu C, Wu S, Han F, Yang Y, Meng J.. 2015. The combined effects of moss-dominated biocrusts and vegetation on erosion and soil moisture and implications for disturbance on the Loess Plateau, China. PLoS One 10, e0127394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal Janke N, Coe KK.. 2021. Evidence for a fungal loop in shrublands. Journal of Ecology 109, 1842–1857. [Google Scholar]
- Chen J, Li Z, Xiao H, Ning K, Tang C.. 2021. Effects of land use and land cover on soil erosion control in southern China: Implications from a systematic quantitative review. Journal of Environmental Management 282, 111924. [DOI] [PubMed] [Google Scholar]
- Chen N, Yu K, Jia R, Teng J, Zhao C.. 2020. Biocrust as one of multiple stable states in global drylands. Science Advances 6, eaay3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherlet M, Hutchinson C, Reynolds J, Hill J, Sommer S, von Maltitz G.. 2018. World Atlas of desertification: rethinking land degradation and sustainable land management. Luxembourg: Publication Office of the European Union. [Google Scholar]
- Clarke LJ, Robinson SA.. 2008. Cell wall-bound ultraviolet-screening compounds explain the high ultraviolet tolerance of the Antarctic moss, Ceratodon purpureus. New Phytologist 179, 776–783. [DOI] [PubMed] [Google Scholar]
- Coe KK, Belnap J, Sparks JP.. 2012. Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology 93, 1626–1636. [DOI] [PubMed] [Google Scholar]
- Coe KK, Howard NB, Slate ML, Bowker MA, Mishler BD, Butler R, Greenwood J, Stark LR.. 2019. Morphological and physiological traits in relation to carbon balance in a diverse clade of dryland mosses. Plant, Cell and Environment 42, 3140–3151. [DOI] [PubMed] [Google Scholar]
- Coe KK, Sparks JP, Belnap J.. 2014. Physiological ecology of dryland biocrust mosses. In: Hanson DT, Rice SK, eds. Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration, vol. 37. Dordrecht: Springer Science+Business Media, 291–308. [Google Scholar]
- Colesie C, Scheu S, Green TA, Weber B, Wirth R, Büdel B.. 2012. The advantage of growing on moss: facilitative effects on photosynthetic performance and growth in the cyanobacterial lichen Peltigera rufescens. Oecologia 169, 599–607. [DOI] [PubMed] [Google Scholar]
- Concostrina-Zubiri L, Huber-Sannwald E, Martínez I, Flores JF, Reyes-Agüero JA, Escudero A, Belnap J.. 2014a. Biological soil crusts across disturbance–recovery scenarios: effect of grazing regime on community dynamics. Ecological Applications 24, 1863–1877. [DOI] [PubMed] [Google Scholar]
- Concostrina-Zubiri L, Martínez I, Rabasa SG, Escudero A.. 2014b. The influence of environmental factors on biological soil crust: from a community perspective to a species level approach. Journal of Vegetation Science 25, 503–513. [Google Scholar]
- Concostrina-Zubiri L, Valencia E, Ochoa V, Gozalo B, Mendoza BJ, Maestre FT.. 2021. Species-specific effects of biocrust-forming lichens on soil properties under simulated climate change are driven by functional traits. New Phytologist 230, 101–115. [DOI] [PubMed] [Google Scholar]
- Condon LA, Pietrasiak N, Rosentreter R, Pyke DA.. 2020. Passive restoration of vegetation and biological soil crusts following 80 years of exclusion from grazing across the Great Basin. Restoration Ecology 28, S75–S85. [Google Scholar]
- Cornelissen JH, Lang SI, Soudzilovskaia NA, During HJ.. 2007. Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Annals of Botany 99, 987–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danin A, Ganor E.. 1991. Trapping of airborne dust by mosses in the Negev Desert, Israel. Earth Surface Processes and Landforms 16, 153–162. [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Bowker MA, Jeffries TC, Singh BK.. 2018. Biocrust-forming mosses mitigate the impact of aridity on soil microbial communities in drylands: observational evidence from three continents. New Phytologist 220, 824–835. [DOI] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Bowker MA, Ochoa V, Gozalo B, Berdugo M, Val J, Singh BK.. 2016. Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytologist 209, 1540–1552. [DOI] [PubMed] [Google Scholar]
- Deng S, Zhang D, Wang G, Zhou X, Ye C, Fu T, Ke T, Zhang Y, Liu Y, Chen L.. 2020. Biological soil crust succession in deserts through a 59-year-long case study in China: how induced biological soil crust strategy accelerates desertification reversal from decades to years. Soil Biology and Biochemistry 141, 107665. [Google Scholar]
- Doherty KD, Grover HS, Bowker MA, Durham RA, Antoninka AJ, Ramsey PW.. 2020. Producing moss-colonized burlap fabric in a fog chamber for restoration of biocrust. Ecological Engineering 158, 106019. [Google Scholar]
- Downing AJ, Selkirk PM.. 1993. Bryophytes on the calcareous soils of Mungo National Park, an arid area of southern central Australia. The Great Basin Naturalist 53, 13–23. [Google Scholar]
- Doxford SW, Ooi MK, Freckleton RP.. 2013. Spatial and temporal variability in positive and negative plant–bryophyte interactions along a latitudinal gradient. Journal of Ecology 101, 465–474. [Google Scholar]
- Duffy JE, Godwin CM, Cardinale BJ.. 2017. Biodiversity effects in the wild are common and as strong as key drivers of productivity. Nature 549, 261–264. [DOI] [PubMed] [Google Scholar]
- Ekwealor JT, Clark TA, Dautermann O, Russell A, Ebrahimi S, Stark LR, Niyogi KK, Mishler BD.. 2021. Natural ultraviolet radiation exposure alters photosynthetic biology and improves recovery from desiccation in a desert moss. Journal of Experimental Botany 72, 4161–4179. [DOI] [PubMed] [Google Scholar]
- Ekwealor JTB, Fisher KM.. 2020. Life under quartz: hypolithic mosses in the Mojave desert. PLoS One 15, e0235928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge DJ, Bowker MA, Maestre FT, Alonso P, Mau RL, Papadopoulos J, Escudero A.. 2010. Interactive effects of three ecosystem engineers on infiltration in a semi-arid Mediterranean grassland. Ecosystems 13, 499–510. [Google Scholar]
- Eldridge DJ, Reed S, Travers SK, et al. 2020. The pervasive and multifaceted influence of biocrusts on water in the world’s drylands. Global Change Biology 26, 6003–6014. [DOI] [PubMed] [Google Scholar]
- Eldridge DJ, Rosentreter R.. 1999. Morphological groups: a framework for monitoring microphytic crusts in arid landscapes. Journal of Arid Environments 41, 11–25. [Google Scholar]
- Escolar C, Martínez I, Bowker MA, Maestre FT.. 2012. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: implications for ecosystem structure and functioning. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Lange OL.. 2001. Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Belnap J, Lange OL, eds. Biological soil crusts: structure, function, and management. Ecological Studies Series 150. Berlin, Heidelberg: Springer-Verlag, 263–279. [Google Scholar]
- Ferrenberg S, Faist AM, Howell A, Reed SC.. 2018. Biocrusts enhance soil fertility and Bromus tectorum growth, and interact with warming to influence germination. Plant and Soil 429, 77–90. [Google Scholar]
- Ferrenberg S, Reed SC, Belnap J.. 2015. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proceedings of the National Academy of Sciences, USA 112, 12116–12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger-Higgens R, Duniway MC, Fick S, Geiger EL, Hoover DL, Pfennigwerth AA, Van Scoyoc MW, Belnap J.. 2022. Decline in biological soil crust N-fixing lichens linked to increasing summertime temperatures. Proceedings of the National Academy of Sciences, USA 119, e2120975119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Bowker MA, Sun H, Zhao J, Zhao Y.. 2020a. Linkages between biocrust development and water erosion and implications for erosion model implementation. Geoderma 357, 113973. [Google Scholar]
- Gao L, Sun H, Xu M, Zhao Y.. 2020b. Biocrusts resist runoff erosion through direct physical protection and indirect modification of soil properties. Journal of Soils and Sediments 20, 133–142. [Google Scholar]
- Gardner CR, Mueller DM.. 1981. Factors affecting the toxicity of several lichen acids: effect of pH and lichen acid concentration. American Journal of Botany 68, 87–95. [Google Scholar]
- Geffert JL, Frahm JP, Barthlott W, Mutke J.. 2013. Global moss diversity: spatial and taxonomic patterns of species richness. Journal of Bryology 35, 1–11. [Google Scholar]
- Green TGA, Lange OL.. 1995. Photosynthesis in poikilohydric plants: a comparison of lichens and bryophytes. In: Schulze ED, Caldwell MM, eds. Ecophysiology of photosynthesis. Berlin, Heidelberg: Springer-Verlag, 319–341. [Google Scholar]
- Green TGA, Sancho LG, Pintado A.. 2011. Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In: Lüttge U, Beck E, Bartels D, eds. Plant desiccation tolerance. Berlin, Heidelberg: Springer-Verlag, 89–120. [Google Scholar]
- Grover HS, Bowker MA, Fulé PZ.. 2020. Improved, scalable techniques to cultivate fire mosses for rehabilitation. Restoration Ecology 28, S17–S24. [Google Scholar]
- Guan H, Liu X.. 2019. Does biocrust successional stage determine the degradation of vascular vegetation via alterations in its hydrological roles in semi-arid ecosystem? Ecohydrology 12, e2075. [Google Scholar]
- Guerra J, Ros RM, Cano MJ, Casares M.. 1995. Gypsiferous outcrops in SE Spain, refuges of rare, vulnerable and endangered bryophytes and lichens. Cryptogamie, Bryologie et Lichènologie 16, 125–135. [Google Scholar]
- Hamerlynck EP, Csintalan Z, Nagy Z, Tuba Z, Goodin D, Henebry GM.. 2002. Ecophysiological consequences of contrasting microenvironments on the desiccation tolerant moss Tortula ruralis. Oecologia 131, 498–505. [DOI] [PubMed] [Google Scholar]
- Havrilla C, Chaudhary V, Ferrenberg S, et al. 2019. Towards a predictive framework for biocrust mediation of plant performance: A meta-analysis. Journal of Ecology 107, 2789–2807. [Google Scholar]
- Havrilla C, Leslie AD, Di Biase JL, Barger NN.. 2020. Biocrusts are associated with increased plant biomass and nutrition at seedling stage independently of root-associated fungal colonization. Plant and Soil 446, 331–342. [Google Scholar]
- Hector A, Hautier Y, Saner P, et al. 2010. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Hernandez RR, Knudsen K.. 2012. Late-successional biological soil crusts in a biodiversity hotspot: an example of congruency in species richness. Biodiversity and Conservation 21, 1015–1031. [Google Scholar]
- Herrero J. 2004. Revisiting the definitions of gypsic and petrogypsic horizons in Soil Taxonomy and World Reference Base for Soil Resources. Geoderma 120, 1–5. [Google Scholar]
- Hui R, Zhao RM, Liu LC, Li YX, Yang HT, Wang YL, Xie M, Wang XQ.. 2018. Changes in winter snow depth affects photosynthesis and physiological characteristics of biological soil crusts in the Tengger Desert. Photosynthetica 56, 1304–1312. [Google Scholar]
- Kidane M, Bezie A, Kesete N, Tolessa T.. 2019. The impact of land use and land cover (LULC) dynamics on soil erosion and sediment yield in Ethiopia. Heliyon 5, e02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidron GJ. 2014a. Do mosses serve as sink for rain in the Negev Desert? A theoretical and experimental approach. Catena 121, 31–39. [Google Scholar]
- Kidron GJ. 2014b. The negative effect of biocrusts upon annual-plant growth on sand dunes during extreme droughts. Journal of Hydrology 508, 128–136. [Google Scholar]
- Kidron GJ, Yair A, Vonshak A, Abeliovich A.. 2003. Microbiotic crust control of runoff generation on sand dunes in the Negev Desert. Water Resources Research 39, doi: 10.1029/2002WR001561. [DOI] [Google Scholar]
- Ladrón de Guevara M, Gozalo B, Raggio J, Lafuente A, Prieto M, Maestre FT.. 2018. Warming reduces the cover, richness and evenness of lichen-dominated biocrusts but promotes moss growth: insights from an 8 yr experiment. New Phytologist 220, 811–823. [DOI] [PubMed] [Google Scholar]
- Ladrón de Guevara M, Lázaro R, Quero JL, Ochoa V, Gozalo B, Berdugo M, Uclés O, Escolar C, Maestre FT.. 2014. Simulated climate change reduced the capacity of lichen-dominated biocrusts to act as carbon sinks in two semi-arid Mediterranean ecosystems. Biodiversity and Conservation 237, 1787–1807. [Google Scholar]
- Lan S, Ouyang H, Wu L, Zhang D, Hu C.. 2017. Biological soil crust community types differ in photosynthetic pigment composition, fluorescence and carbon fixation in Shapotou region of China. Applied Soil Ecology 111, 9–16. [Google Scholar]
- Li Q, Yang T, Qi Z, Li L.. 2018. Spatiotemporal variation of snowfall to precipitation ratio and its implication on water resources by a regional climate model over Xinjiang, China. Water 10, 1463. [Google Scholar]
- Li S, Bowker MA, Xiao B.. 2021a. Biocrusts enhance non-rainfall water deposition and alter its distribution in dryland soils. Journal of Hydrology 595, 126050. [Google Scholar]
- Li S, Xiao B, Sun F, Kidron GJ.. 2021b. Moss-dominated biocrusts enhance water vapor sorption capacity of surface soil and increase non-rainfall water deposition in drylands. Geoderma 388, 114930. [Google Scholar]
- Li X, Hui R, Zhang P, Song N.. 2021. Divergent responses of moss-and lichen-dominated biocrusts to warming and increased drought in arid desert regions. Agricultural and Forest Meteorology 303, 108387. [Google Scholar]
- Li X, Yu MH, Ding GD, He Y, Liu W, Wang CY.. 2021. Soil biocrusts reduce seed germination and contribute to the decline in Artemisia ordosica Krasch. shrub populations in the Mu Us Sandy Land of North China. Global Ecology and Conservation 26, e01467. [Google Scholar]
- Li XR, Song G, Hui R, Wang ZR.. 2017. Precipitation and topsoil attributes determine the species diversity and distribution patterns of crustal communities in desert ecosystems. Plant and Soil 420, 163–175. [Google Scholar]
- Li YG, Zhou XB, Zhang YM.. 2019. Moss patch size and microhabitats influence stoichiometry of moss crusts in a temperate desert, Central Asia. Plant and Soil 443, 55–72. [Google Scholar]
- Liang J, Crowther TW, Picard N, et al. 2016. Positive biodiversity-productivity relationship predominant in global forests. Science 354, aaf8957. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao L, Wang Z, Liu L, Zhang P, Sun J, Wang B, Song G, Li X.. 2018. Changes in functional gene structure and metabolic potential of the microbial community in biological soil crusts along a revegetation chronosequence in the Tengger Desert. Soil Biology and Biochemistry 126, 40–48. [Google Scholar]
- Liu YR, Delgado-Baquerizo M, Trivedi P, He JZ, Wang JT, Singh BK.. 2017. Identity of biocrust species and microbial communities drive the response of soil multifunctionality to simulated global change. Soil Biology and Biochemistry 107, 208–217. [Google Scholar]
- Longton RE. 1988. Life-history strategies among bryophytes of arid regions. The Journal of the Hattori Botanical Laboratory 64, 15–28. [Google Scholar]
- Maestre FT, Bautista S, Cortina J, Bellot J.. 2001. Potential for using facilitation by grasses to establish shrubs on a semiarid degraded steppe. Ecological Applications 11, 1641–1655. [Google Scholar]
- Maestre FT, Benito BM, Berdugo M, et al. 2021. Biogeography of global drylands. New Phytologist 231, 540–558. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Escolar C, Bardgett RD, Dungait JA, Gozalo B, Ochoa V.. 2015. Warming reduces the cover and diversity of biocrust-forming mosses and lichens, and increases the physiological stress of soil microbial communities in a semi-arid Pinus halepensis plantation. Frontiers in Microbiology 6, 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Escolar C, Martínez I, Escudero A.. 2008. Are soil lichen communities structured by biotic interactions? A null model analysis. Journal of Vegetation Science 19, 261–266. [Google Scholar]
- Maier S, Tamm A, Wu D, Caesar J, Grube M, Weber B.. 2018. Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. The ISME Journal 12, 1032–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallen-Cooper M, Eldridge DJ.. 2016. Laboratory-based techniques for assessing the functional traits of biocrusts. Plant and Soil 406, 131–143. [Google Scholar]
- Martínez I, Escudero A, Maestre FT, de la Cruz A, Guerrero C, Rubio A.. 2006. Small-scale patterns of abundance of mosses and lichens forming biological soil crusts in two semi-arid gypsum environments. Australian Journal of Botany 54, 339–348. [Google Scholar]
- Martínez-Sánchez JJ, Casares-Porcel M, Guerra J, Gutiérrez-Carretero L, Ros RM, Hernández-Bastida J, Cano MJ.. 1994. A special habitat for bryophytes and lichens in the arid zones of Spain. Lindbergia 19, 116–121. [Google Scholar]
- McCalley CK, Sparks JP.. 2009. Abiotic gas formation drives nitrogen loss from a desert ecosystem. Science 326, 837–840. [DOI] [PubMed] [Google Scholar]
- McIlvanie SK. 1942. Grass seedling establishment, and productivity—overgrazed vs. protected range soils. Ecology 23, 228–231. [Google Scholar]
- Miller OL, Putman AL, Alder J, Miller M, Jones DK, Wise DR.. 2021. Changing climate drives future streamflow declines and challenges in meeting water demand across the southwestern United States. Journal of Hydrology X 11, 100074. [Google Scholar]
- Moore JD, Kollar LM, McLetchie DN.. 2016. Does selection for gamete dispersal and capture lead to a sex difference in clump water-holding capacity? American Journal of Botany 103, 1449–1457. [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez E, Ochoa-Hueso R, Plaza C, Aceña-Heras S, Flagmeier M, Elouali FZ, Ochoa V, Gozalo B, Maestre FT.. 2020. Biocrusts buffer against the accumulation of soil metallic nutrients induced by warming and rainfall reduction. Communications Biology 3, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota JF, Sánchez-Gómez P, Guirado Romero JS (eds).. 2011. Diversidad vegetal de las yeseras ibéricas. El reto de los archipiélagos edáficos para la biología de la conservación. Almería: ADIF-Mediterráneo Asesores Consultores. [Google Scholar]
- Munzi S, Varela Z, Paoli L.. 2019. Is the length of the drying period critical for photosynthesis reactivation in lichen and moss components of biological soil crusts? Journal of Arid Environments 166, 86–90. [Google Scholar]
- Nash TH III, White SL, Marsh JE. 1977. Lichen and moss distribution and biomass in hot desert ecosystems. Bryologist 80, 470–479. [Google Scholar]
- Navas Romero AL, Herrera Moratta MA, Martinez Carretero E, Rodriguez RA, Vento B.. 2020. Spatial distribution of biological soil crusts along an aridity gradient in the central-west of Argentina. Journal of Arid Environments 176, 104099. [Google Scholar]
- Oliver MJ, Velten J, Mishler BD.. 2005. Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integrative and Comparative Biology 45, 788–799. [DOI] [PubMed] [Google Scholar]
- Pan Z, Pitt WG, Zhang Y, Wu N, Tao Y, Truscott TT.. 2016. The upside-down water collection system of Syntrichia caninervis. Nature Plants 2, 16076. [DOI] [PubMed] [Google Scholar]
- Proctor MCF. 1982. Physiological ecology: water relations, light and temperature responses, carbon balance. In: Smith AJE, ed. Bryophyte ecology. Dordrecht: Springer, 333–381. [Google Scholar]
- Qi J, Liu Y, Wang Z, Zhao L, Zhang W, Wang Y, Li X.. 2021. Variations in microbial functional potential associated with phosphorus and sulfur cycling in biological soil crusts of different ages at the Tengger Desert, China. Applied Soil Ecology 165, 104022. [Google Scholar]
- Raggio J, Green TA, Pintado A, Sancho LG, Büdel B.. 2018. Environmental determinants of biocrust carbon fluxes across Europe: possibilities for a functional type approach. Plant and Soil 429, 147–157. [Google Scholar]
- Read CF, Duncan DH, Vesk PA, Elith J.. 2011. Surprisingly fast recovery of biological soil crusts following livestock removal in southern Australia. Journal of Vegetation Science 22, 905–916. [Google Scholar]
- Reed SC, Coe KK, Sparks JP, Housman DC, Zelikova TJ, Belnap J.. 2012. Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nature Climate Change 2, 752–755. [Google Scholar]
- Rodriguez-Caballero E, Belnap J, Budel B, Crutzen PJ, Andreae MO, Poschl U, Weber B.. 2018. Dryland photoautotrophic soil surface communities endangered by global change. Nature Geoscience 11, 185–189. [Google Scholar]
- Ros RM, Cano MJ, Guerra J.. 1999. Bryophyte checklist of northern Africa. Journal of Bryology 21, 207–244. [Google Scholar]
- Ruan Z, Giordano M.. 2017. The use of NH4+ rather than NO3− affects cell stoichiometry, C allocation, photosynthesis and growth in the cyanobacterium Synechococcus sp. UTEX LB 2380, only when energy is limiting. Plant, Cell and Environment 40, 227–236. [DOI] [PubMed] [Google Scholar]
- Rundel PW, Lange OL.. 1980. Water relations and photosynthetic response of a desert moss. Flora 169, 329–335. [Google Scholar]
- Salmerón E, Merlo ME, Mota JF.. 2011. Los briófitos de los afloramientos de yeso. Diversidad vegetal de las yeseras ibéricas. In: Mota JF, Sanchez P, Guirado Romero JS, eds. Diversidad vegetal de las yeseras ibéricas. El reto de los archipiélagos edáficos para la biología de la conservación. Almería: ADIF-Mediterráneo Asesores Consultores, 549–567. [Google Scholar]
- Sancho LG, Belnap J, Colesie C, Raggio J, Weber B.. 2016. Carbon budgets of biological soil crusts at micro-, meso-, and global scales. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226. Cham: Springer International Publishing, 287–304. [Google Scholar]
- Schlatterer EF, Tisdale EW.. 1969. Effects of litter of Artemisia, Chrysothamnus, and Tortula on germination and growth of three perennial grasses. Ecology 50, 869–873. [Google Scholar]
- Seppelt RD, Downing AJ, Deane-Coe KK, Zhang Y, Zhang J.. 2016. Bryophytes within biological soil crusts. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226. Cham: Springer International Publishing, 101–20. [Google Scholar]
- Shi Y, Gao X, Wu J, Giorgi F.. 2011. Changes in snow cover over China in the 21st century as simulated by a high resolution regional climate model. Environmental Research Letters 6, 045401. [Google Scholar]
- Slate ML, Brinda JC, Coe KK, Greenwood JL, Stark LR.. 2020a. Prehydration mitigates damage accrued from prolonged periods of desiccation in cultured shoot apices of Syntrichia ruralis. Journal of Bryology 43, 138–149. [Google Scholar]
- Slate ML, Durham RA, Pearson DE.. 2020b. Strategies for restoring the structure and function of lichen-moss biocrust communities. Restoration Ecology 28, S160–S167. [Google Scholar]
- Slate ML, Luce McLeod M, Callaway RM.. 2021. Positive interactions between an exotic invader and moss biocrusts vary across life-stage and correspond with the effect of water pulses on soil nitrogen. Functional Ecology 35, 2108–2118. [Google Scholar]
- Slate ML, Sullivan BW, Callaway RM.. 2019. Desiccation and rehydration of mosses greatly increases resource fluxes that alter soil carbon and nitrogen cycling. Journal of Ecology 107, 1767–1778. [Google Scholar]
- Soliveres S, Eldridge DJ.. 2020. Dual community assembly processes in dryland biocrust communities. Functional Ecology 34, 877–887. [Google Scholar]
- Stanton DE, Coe KK.. 2021. 500 million years of charted territory: functional ecological traits in bryophytes. Bryophyte Diversity and Evolution 43, 234–252. [Google Scholar]
- Stark LR, Brinda JC, McLetchie DN.. 2011. Effects of increased summer precipitation and N deposition on Mojave Desert populations of the biological crust moss Syntrichia caninervis. Journal of Arid Environments 75, 457–463. [Google Scholar]
- Stark LR, McLetchie DN, Mishler BD.. 2005. Sex expression, plant size, and spatial segregation of the sexes across a stress gradient in the desert moss Syntrichia caninervis. The Bryologist 108, 183–193. [Google Scholar]
- Stark LR, Whittemore AT.. 2000. Bryophytes from the northern Mojave Desert. The Southwestern Naturalist 45, 226–232. [Google Scholar]
- Sun F, Xiao B, Li S, Kidron GJ.. 2021. Towards moss biocrust effects on surface soil water holding capacity: Soil water retention curve analysis and modeling. Geoderma 399, 115120. [Google Scholar]
- Sun J, Li X, Jia R, Chen N, Zhang T.. 2021. Null-model analysis and changes in species interactions in biocrusts along a successional gradient in the Tengger Desert, northern China. Journal of Vegetation Science 32, e13037. [Google Scholar]
- Szyja M, Menezes AGDS, Oliveira FD, Leal I, Tabarelli M, Büdel B, Wirth R.. 2019. Neglected but potent dry forest players: Ecological role and ecosystem service provision of biological soil crusts in the human-modified Caatinga. Frontiers in Ecology and Evolution 17, 00482. [Google Scholar]
- Takács Z, Csintalan Z, Sass L, Laitat E, Vass I, Tuba Z.. 1999. UV-B tolerance of bryophyte species with different degrees of desiccation tolerance. Journal of Photochemistry and Photobiology B: Biology 48, 210–215. [Google Scholar]
- Tan X, Wu Z, Mu X, Gao P, Zhao G, Sun W, Gu C.. 2019. Spatiotemporal changes in snow cover over China during 1960–2013. Atmospheric Research 218, 183–194. [Google Scholar]
- Tao Y, Zhang YM.. 2012. Effects of leaf hair points of a desert moss on water retention and dew formation: implications for desiccation tolerance. Journal of Plant Research 125, 351–360. [DOI] [PubMed] [Google Scholar]
- Thompson DB, Walker LR, Landau FH, Stark LR.. 2005. The influence of elevation, shrub species, and biological soil crust on fertile islands in the Mojave Desert, USA. Journal of Arid Environments 61, 609–629. [Google Scholar]
- Tian C, Xi J, Ju M, et al. 2021. Biocrust microbiomes influence ecosystem structure and function in the Mu Us Sandland, northwest China. Ecological Informatics 66, 101441. [Google Scholar]
- Tielbörger K. 1997. The vegetation of linear desert dunes in the north-western Negev, Israel. Flora 192, 261–278. [Google Scholar]
- Tucker CL, Ferrenberg S, Reed SC.. 2019. Climatic sensitivity of dryland soil CO2 fluxes differs dramatically with biological soil crust successional state. Ecosystems 22, 15–32. [Google Scholar]
- Viles HA. 2008. Understanding dryland landscape dynamics: do biological crusts hold the key? Geography Compass 2, 899–919. [Google Scholar]
- Voortman BR, Bartholomeus RP, van Bodegom PM, Gooren H, van der Zee SE, Witte JPM.. 2014. Unsaturated hydraulic properties of xerophilous mosses: towards implementation of moss covered soils in hydrological models. Hydrological Processes 28, 6251–6264. [Google Scholar]
- Wang C, Hill RL, Bu C, Li B, Yuan F, Yang Y, Yuan S, Zhang Z, Cao Y, Zhang K.. 2021. Evaluation of wind erosion control practices at a photovoltaic power station within a sandy area of northwest, China. Land Degradation and Development 32, 1854–1872. [Google Scholar]
- Wang L, Zhang G, Zhu L, Wang H.. 2017. Biocrust wetting induced change in soil surface roughness as influenced by biocrust type, coverage and wetting patterns. Geoderma 306, 1–9. [Google Scholar]
- Warren SD. 2001. Synopsis: influence of biological soil crusts on arid land hydrology and soil stability. In: Belnap J, Lange OL, eds. Biological soil crusts: structure, function, and management. Ecological Studies Series 150. Berlin, Heidelberg: Springer-Verlag, 349–360. [Google Scholar]
- Warren-Rhodes K, Weinstein S, Piatek JL, et al. 2007. Robotic ecological mapping: Habitats and the search for life in the Atacama Desert. Journal of Geophysical Research, Biogeosciences 112, doi: 10.1029/2006JG000301. [DOI] [Google Scholar]
- Weber B, Büdel B, Belnap J (eds).. 2016. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226, Cham: Springer International Publishing. [Google Scholar]
- Weber B, Tamm A, Maier S, Rodríguez-Caballero E.. 2018. Biological soil crusts of the Succulent Karoo: a review. African Journal of Range and Forage Science 35, 335–350. [Google Scholar]
- Weber B, Wu D, Tamm A, et al. 2015. Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. Proceedings of the National Academy of Sciences, USA 112, 15384–15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Buck BJ, Beyene MA.. 2012. Biological soil crusts in the Mojave Desert, USA: micromorphology and pedogenesis. Soil Science Society of America Journal 76, 1685–1695. [Google Scholar]
- Williams AJ, Buck BJ, Soukup DA, Merkler DJ.. 2013. Geomorphic controls on biological soil crust distribution: a conceptual model from the Mojave Desert (USA). Geomorphology 195, 99–109. [Google Scholar]
- Wood AJ. 2007. The nature and distribution of vegetative desiccation-tolerance in hornworts, liverworts and mosses. The Bryologist 110, 163–177. [Google Scholar]
- Wu N, Zhang YM, Downing A, Aanderud ZT, Tao Y, Williams S.. 2014. Rapid adjustment of leaf angle explains how the desert moss, Syntrichia caninervis, copes with multiple resource limitations during rehydration. Functional Plant Biology 41, 168–177. [DOI] [PubMed] [Google Scholar]
- Xiao B, Hu K.. 2017. Moss-dominated biocrusts decrease soil moisture and result in the degradation of artificially planted shrubs under semiarid climate. Geoderma 291, 47–54. [Google Scholar]
- Xiao B, Hu K, Ren T, Li B.. 2016. Moss-dominated biological soil crusts significantly influence soil moisture and temperature regimes in semiarid ecosystems. Geoderma 263, 35–46. [Google Scholar]
- Xiao B, Hu K, Veste M, Kidron GJ.. 2019. Natural recovery rates of moss biocrusts after severe disturbance in a semiarid climate of the Chinese Loess Plateau. Geoderma 337, 402–412. [Google Scholar]
- Xiao B, Zhao Y, Wang Q, Li C.. 2015. Development of artificial moss-dominated biological soil crusts and their effects on runoff and soil water content in a semi-arid environment. Journal of Arid Environments 117, 75–83. [Google Scholar]
- Xiao B, Zhao YG, Shao MA.. 2010. Characteristics and numeric simulation of soil evaporation in biological soil crusts. Journal of Arid Environments 74, 121–130. [Google Scholar]
- Xu H, Zhang Y, Shao X, Liu N.. 2022. Soil nitrogen and climate drive the positive effect of biological soil crusts on soil organic carbon sequestration in drylands: A meta-analysis. Science of the Total Environment 803, 150030. [DOI] [PubMed] [Google Scholar]
- Xu S, Yin C, He M, Wang Y.. 2008. A technology for rapid reconstruction of moss-dominated soil crusts. Environmental Engineering Science 25, 1129–1138. [Google Scholar]
- Yang X, Xu M, Zhao Y, Gao L, Wang S.. 2019. Moss-dominated biological soil crusts improve stability of soil organic carbon on the Loess Plateau, China. Plant, Soil and Environment 65, 104–109. [Google Scholar]
- Yang Y, Bu C, Mu X, Shao H, Zhang K.. 2014. Interactive effects of moss-dominated crusts and Artemisia ordosica on wind erosion and soil moisture in Mu Us Sandland, China. The Scientific World Journal 2014, 649816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Niu D, Song M, Elser JJ, Okie JG, Fu H.. 2018. Effects of rainfall manipulations on carbon exchange of cyanobacteria and moss-dominated biological soil crusts. Soil Biology and Biochemistry 124, 24–31. [Google Scholar]
- Zhang J, Zhang Y.. 2020. Ecophysiological responses of the biocrust moss Syntrichia caninervis to experimental snow cover manipulations in a temperate desert of central Asia. Ecological Research 35, 198–207. [Google Scholar]
- Zhang Y, Aradottir AL, Serpe M, Boeken B.. 2016. Interactions of biological soil crusts with vascular plants. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological Studies Series 226. Cham: Springer International Publishing, 385–406. [Google Scholar]
- Zhang Y, Ma N.. 2018. Spatiotemporal variability of snow cover and snow water equivalent in the last three decades over Eurasia. Journal of Hydrology 559, 238–251. [Google Scholar]
- Zhang Y-F, Wang X-P, Pan Y-X, Hu R.. 2016. Comparison of diurnal dynamics in evaporation rate between bare soil and moss-crusted soil within a revegetated desert ecosystem of northwestern China. Journal of Earth System Science 125, 95–102. [Google Scholar]
- Zhao J, Zheng Y, Zhang B, Chen Y, Zhang Y.. 2009. Progress in the study of algae and mosses in biological soil crusts. Frontiers of Biology in China 4, 143–150. [Google Scholar]
- Zhao R, Hui R, Liu L, Xie M, An L.. 2018. Effects of snowfall depth on soil physical–chemical properties and soil microbial biomass in moss–dominated crusts in the Gurbantunggut Desert, Northern China. Catena 169, 175–182. [Google Scholar]
- Zhao R, Hui R, Wang Z, Liu L, Xie M, An L.. 2016. Winter snowfall can have a positive effect on photosynthetic carbon fixation and biomass accumulation of biological soil crusts from the Gurbantunggut Desert, China. Ecological Research 31, 251–262. [Google Scholar]
- Zhao X, Shi Y, Liu Y, Jia RL, Li XR.. 2015. Osmotic adjustment of soil biocrust mosses in response to desiccation stress. Pedosphere 25, 459–467. [Google Scholar]
- Zhao Y, Qin N, Weber B, Xu M.. 2014. Response of biological soil crusts to raindrop erosivity and underlying influences in the hilly Loess Plateau region, China. Biodiversity and Conservation 23, 1669–1686. [Google Scholar]
- Zhao Y, Xu M.. 2013. Runoff and soil loss from revegetated grasslands in the hilly Loess Plateau region, China: influence of biocrust patches and plant canopies. Journal of Hydrologic Engineering 18, 387–393. [Google Scholar]
- Zhao Y-M, Zhu Q, Li P, Zhao L, Wang L, Zheng X, Ma H.. 2014. Effects of artificially cultivated biological soil crusts on soil nutrients and biological activities in the Loess Plateau. Journal of Arid Land 6, 742–752. [Google Scholar]
- Zheng Y, Xu M, Zhao J, Zhang B, Bei S, Hao L.. 2011. Morphological adaptations to drought and reproductive strategy of the moss Syntrichia caninervis in the Gurbantunggut Desert, China. Arid Land Research and Management 25, 116–127. [Google Scholar]
- Zhou X, Ke T, Li S, Deng S, An X, Ma X, De Philippis R, Chen L.. 2020. Induced biological soil crusts and soil properties varied between slope aspect, slope gradient and plant canopy in the Hobq desert of China. Catena 190, 104559. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data have been analyzed or created in this review.