Abstract
OBJECTIVE
To describe antibiotic susceptibilities for Staphylococcus aureus and Pseudomonas aeruginosa among pediatric institutions in 2018. To assess correlations between antibiotic utilization and susceptibilities.
METHODS
Institutional antibiograms from 2018 were compiled among 13 institutions via a survey. Resistant pathogens and antibiotic days of therapy/1000 patient days (PD) were collected from 6 institutions over 5 years. Correlations were assessed as pooled data among all institutions and relative changes within individual institutions.
RESULTS
All 8552 S aureus isolates in 2018 were vancomycin susceptible and 40.1% were methicillin resistant (MRSA). Among MRSA, 96.3% and 78.8% were susceptible to trimethoprim/sulfamethoxazole and clindamycin, respectively. Pooled yearly MRSA/1000 PD decreased from 2014–2018 and correlated with pooled yearly decreases in vancomycin utilization (R = 0.983, p = 0.003). Institutional relative decreases in vancomycin utilization from 2014–2018 did not correlate with institutional relative decreases in MRSA susceptibility (R = −0.659, p = 0.16). Susceptibility to meropenem was 90.9% among 2315 P aeruginosa isolates in 2018. Antipseudomonal beta-lactam susceptibility ranged from 89.4% to 92.3%. Pooled yearly meropenem-resistant P aeruginosa/1000 PD and meropenem utilization did not significantly decrease over time or correlate (both p > 0.6). Institutional relative change in meropenem utilization from 2013–2017 correlated with the institutional relative change in P aeruginosa susceptibility to meropenem from 2014–2018 (Rs = −0.89, p = 0.019).
CONCLUSIONS
Among included institutions, the burden of MRSA decreased over time. Institutional MRSA prevalence did not consistently correlate with institutional vancomycin utilization. Institutional changes in meropenem utilization correlated with P aeruginosa susceptibility the following year. Pooled analyses did not illustrate this correlation, likely owing to variability in utilization between institutions.
Keywords: antibiotic resistance, antimicrobial stewardship, meropenem, pediatrics, Pseudomonas aeruginosa, Staphylococcus aureus, vancomycin
Introduction
There is significant variation in antimicrobial utilization among pediatric institutions in the United States.1,2 It is unclear if the variation is due to differences in geographic susceptibility patterns or practice differences. It is also unclear if variation in antimicrobial utilization results in different susceptibility patterns between pediatric institutions.
Staphylococcus aureus is among the most common infectious pathogens found in pediatric institutions and clinics,3,4 with methicillin- (or oxacillin-)resistant S aureus (MRSA) having worse outcomes than methicillin-susceptible S aureus.5,6 Treatment of severe infections caused by MRSA typically requires the use of vancomycin or other intravenous agents with MRSA activity.7 Changes in S aureus susceptibility could impact institutional utilization of agents with MRSA activity, although data are limited.8
Another resistant organism, Pseudomonas aeruginosa, is a common health care–associated pathogen.4 Treatment of P aeruginosa usually requires the use of broad-spectrum intravenous therapy, and multidrug resistance can occur.9 P aeruginosa is considered a serious antibiotic resistance threat to public health by the Centers for Disease Control and Prevention.10 Single center studies have illustrated a correlation between meropenem utilization in pediatric institutions and institutional meropenem susceptibility, possibly with greater correlation between utilization and susceptibilities the following year.11,12 The correlation of meropenem utilization with susceptibilities among multiple pediatric institutions is not well studied.
The first aim of this analysis was to describe the susceptibilities of S aureus and P aeruginosa to common antibiotics among a cohort of institutions caring for pediatric patients in 2018. We also aimed to determine if S aureus and P aeruginosa susceptibilities changed over a 5-year period and if these changes correlated with utilization of anti-MRSA antibiotics and meropenem.
Methods
Data Sources. This was a multicenter retrospective cohort analysis with methods approved by the University of Tennessee Health Science Center Institutional Review Board. Participant institutions were identified by using a survey that was sent to the Pediatric Pharmacy Association (PPA) membership to collect institutional antibiogram data. The project was approved by the PPA Practice Based Research Network (PBRN), and the survey was developed by the authors and reviewed for validation by multiple members of the PPA PBRN not directly involved in the project before release of the survey.13 The survey was created and administered using the REDCap data collection tool.14 Participation was voluntary and participants did not receive any form of incentive for completing the survey. Survey respondents who finished the survey answered at least 22 questions, but the number of questions answered varied based on answers provided from the respondent and the ability to upload a version of the antibiogram (i.e., many questions included skip logic). Questions in the survey ascertained institutional characteristics including number of pediatric beds, susceptibility breakpoints utilized, and antimicrobial stewardship program (ASP) activities. Antibiograms were submitted via the survey software for available years, and survey questions also determined if the antibiogram including cystic fibrosis isolates and if it included inpatient and outpatient isolates.
For assessment of 2018 susceptibilities, all responses were reviewed and responses were excluded if they were duplicates from the same institution or did not contain antibiogram data from 2018 for S. aureus or P. aeruginosa. Only pediatric specific data was used when available if the institution also cared for adult patients. Pooled MRSA susceptibility data included vancomycin, clindamycin, trimethoprim/sulfamethoxazole, and tetracyclines. Antibiotic susceptibilities pooled for P. aeruginosa included ceftazidime, cefepime, meropenem, piperacillin/tazobactam, and ciprofloxacin. Aminoglycoside susceptibilities were not included due to possible inferiority seen in the adult population compared to beta-lactams as monotherapy for P. aeruginosa.15,16
To assess correlations with antibiotic utilization and susceptibilities, additional responses were excluded if they lacked antibiogram data from 2014 to 2018 and if it was not possible to obtain antibiotic utilization data from that institution. Relevant institutional antibiotic utilization was obtained from institutions that participate in the claims-based Pediatric Health Information System (PHIS) Database. The PHIS hospitals are 51 of the largest and most advanced children's hospitals in America, and constitute the most demanding standards of pediatric service in America. For each identified institution, anti-MRSA agent DOT (i.e., vancomycin, daptomycin, ceftaroline, linezolid, tedizolid) was obtained from 2014–2018 and meropenem days of therapy (DOT) was obtained from 2013–2018. Institutional patient days (PD) were also collected for each included year and institution from PHIS.
Data Reporting. Susceptibilities were pooled amongst the total number of isolates from all institutions. If only percentages were available, the number of resistant isolates were calculated using percentages and total isolates provided in the antibiogram and rounded when applicable. Inpatient and outpatient isolates were combined if institutions provided both separately. The number of resistant isolates (either MRSA or meropenem resistant P. aeruginosa) were normalized to 1000 patient days (PD) to estimate the rate or burden of MRSA and meropenem resistant P. aeruginosa, as recommended for epidemiologic analysis of resistance.17 Yearly antibiotic utilization data was pooled for all institutions and calculated as days of therapy (DOT) per 1000 PD, which is the recommended measurement for antimicrobial utilization.18,19 The relative percentage change from baseline ([reference point-baseline]/baseline*100) to the end of the study period for each institution was calculated for antibiotic utilization and percent susceptibility. For MRSA, the relative percentage change calculation was based on the years 2014 and 2018 for both utilization and percent susceptibility. The relative percentage change calculation was based on the years 2013 and 2017 for meropenem utilization and the years 2014 and 2018 for percent meropenem susceptible P. aeruginosa. This timeframe was selected based on prior reports suggesting resistance changes based on meropenem utilization are delayed approximately 1 year.11,12
Statistical analysis. Percentages were used for descriptive statistics for all included institutions and survey respondents. Ranges were provided to describe variability present in the data. The chi-squared test was used to assess proportional changes in pooled susceptibility between years. Pearson's or Spearman's rho correlations were used (based on data normality) to assess for yearly trends, burden of resistance trends, and correlations between antibiotic utilization and changes in susceptibilities. Pooled yearly MRSA burden (MRSA isolates/1000 PD) was correlated over time. All correlations between MRSA occurrence and anti-MRSA agent utilization were done with utilization data from the same year. Pooled yearly meropenem DOT/1000 PD was assessed for correlations over time. All correlations between P. aeruginosa susceptibilities and meropenem utilization and were done with utilization data from the year prior. Statistical analyses were performed using IBM SPSS Statistics version 27 (IBM Corp, 2018, Armonk, NY).
Results
There were 26 responses from 23 institutions, of which 13 unique institutions submitted antibiogram data for 2018 with S aureus susceptibilities and 12, with P aeruginosa susceptibilities. Of the submitted 2018 institutional antibiograms, 12 contained solely pediatric isolates, 11 removed P aeruginosa isolates from cystic fibrosis (CF) patients, and 7 removed S aureus isolates from CF patients. Additional characteristics of the institutions are presented in Table 1. Importantly, there was a mix of freestanding children's institutions and academic medical centers, and most were large institutions with >200 pediatric-specific beds (>150 general ward beds and >50 intensive care unit beds). The institutions were found in the Northeast, South, and Midwest regions of the United States.
Table 1.
Institution Characteristics Obtained From Survey Responses (n = 13)
| Description | Value, n (%) |
|---|---|
| Institution category | |
| Freestanding children’s academic medical center* and associated clinics | 4 (30.8) |
| Academic medical center* and associated clinics (not a freestanding children’s hospital) | 6 (46.2) |
| Non-academic freestanding children’s medical center and associated clinics | 2 (15.4) |
| Non-academic medical center with pediatric services and associated clinics | 1 (7.7) |
| >150 total beds and >50 intensive care unit beds† | 10 (76.9) |
| Used updated CLSI meropenem and Pseudomonas aeruginosa breakpoints‡ | 7 (78) |
| Cystic fibrosis P aeruginosa isolates separated | 10 (76.9) |
| Inpatient and outpatient antibiogram | 7 (53.9) |
| Pediatric-specific antibiogram | 12 (92.3) |
| Antimicrobial stewardship | |
| Pediatric specific prior to 2017 | 8 (61.5) |
| Pediatric focused started after 2017, before 2019 | 4 (30.8) |
| Antimicrobial stewardship–dedicated pediatric pharmacists | |
| None | 3 (.1) |
| Partial FTE | 5 (38.5) |
| ≥1 FTE | 4 (30.8) |
| Unknown | 1 (7.7) |
| Antimicrobial stewardship activities | |
| Prospective audit with feedback | 10 (76.9) |
| Guideline development | 11 (84.6) |
| De-escalation | 13 (100) |
| Dose optimization | 9 (69.2) |
| Intravenous to oral conversions | 8 (61.5) |
| Restrictions | 7 (53.9) |
CLSI, Clinical Laboratory Standards Institute; FTE, full-time equivalent
* Academic medical centers were defined in the survey as “a teaching institution that uses medical residents in the majority of their service lines and is generally affiliated with a School of Medicine and/or School of Pharmacy.”
† Combination of all possible intensive care unit beds within an institution.
‡ Updated breakpoints were described in the survey as the 2012 breakpoint changes for P aeruginosa including a MIC of ≤2 mg/L being susceptible for meropenem and ≤16/4 mg/L being susceptible for piperacillin-tazobactam.
Table 2 describes the 2018 resistance patterns for S aureus and P aeruginosa. There were no vancomycin-intermediate or vancomycin-resistant S aureus isolates. Trimethoprim/sulfamethoxazole had the highest percent susceptibility among oral agents included with anti-MRSA activity. Susceptibilities for anti-MRSA antibiotics aside from those in Table 2 were not included owing to inconsistent reporting among institutions. For P aeruginosa, pooled susceptibilities among the cohort were between 89.4% and 92.4% for commonly used beta-lactams. Eleven institutions had at least 1 of the included beta-lactams with over 90% susceptibility. All institutions had all reported (some reported only 3 of the included antibiotics, others all 4) beta-lactams with 85% or higher susceptibility and 5 institutions had ceftazidime, cefepime, meropenem, and piperacillintazobactam all reported and having >90% susceptibility. Other antipseudomonal antibiotics and levofloxacin were not included owing to inconsistent reporting among institutions.
Table 2.
Pooled 2018 Susceptibilities for Staphylococcus aureus and Pseudomonas aeruginosa Isolates Among Institutions (n = 13) *
| Drug (Number of Isolates Tested) | % Susceptible (Range Among Institutions) |
|---|---|
| Staphylococcus aureus | |
| Methicillin or oxacillin (8552) | 59.9 (52.5–73) |
|
| |
| Methicillin-resistant S aureus | |
| Vancomycin (3428) | 100 |
| Trimethoprim-sulfamethoxazole (3156)† | 96.3 (87–100) |
| Tetracycline (2422)† | 95.7 (85–100) |
| Clindamycin (3156)† | 78.8 (49–87) |
|
| |
| Pseudomonas aeruginosa | |
| Ceftazidime (1617)‡ | 91.1 (86–95) |
| Cefepime (2220)‡ | 89.4 (85–96) |
| Meropenem (2315) | 90.9 (85–100) |
| Piperacillin-tazobactam (2163)‡ | 92.4 (87–99) |
| Ciprofloxacin (2172)‡ | 87.5 (82–97) |
* All data obtained from antibiograms collected via survey, with 13 institutions having S aureus data and 12 containing P aeruginosa data.
† Three institutions did not report tetracycline susceptibilities, 1 institution only had methicillin-resistant S aureus susceptibilities for vancomycin.
‡ Eleven, 10, 11, and 10 institutions reported ceftazidime, cefepime, piperacillin-tazobactam, and ciprofloxacin susceptibilities, respectively. All 12 institutions had meropenem susceptibilities for P aeruginosa.
Six institutions had anti-MRSA antibiotic utilization data and S aureus susceptibilities available from 2014–2018 in these specific institutions. All of these institutions were freestanding children's hospitals, had over 200 inpatient beds, submitted pediatric-specific antibiograms, and had a pediatric-specific antimicrobial stewardship program (ASP) started in 2016 or earlier. Three institutions had CF MRSA isolates included in the antibiogram. Figure 1a contains pooled MRSA prevalence and vancomycin utilization data over time for the included institutions. Generally, pooled proportional MRSA and pooled vancomycin utilization significantly decreased from 2014 to 2018 (Figure 1a). There were minimal changes in utilization of other anti-MRSA agents during the study period, with pooled utilization of 7.5 days of therapy (DOT)/1000 patient days (PD) in 2014 and 7.3 DOT/1000 PD in 2018 (R = −0.408, p = 0.496). All institutions had a reduction in MRSA burden from 2014 to 2018 (Figure 1b). The reductions in pooled vancomycin therapy significantly correlated with reductions in pooled MRSA burden (Figure 2a). However, the institutional relative reduction in MRSA from 2014 to 2018 did not correlate with relative institutional reductions in vancomycin utilization within the same years (Figure 2b).
Figure 1.
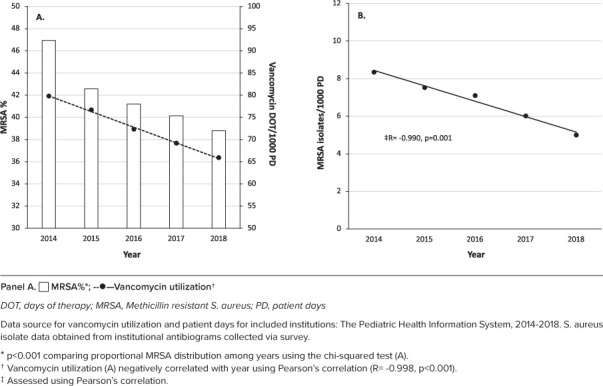
Pooled MRSA percentage and vancomycin utilization over time (A) and pooled MRSA burden versus time (B) among 6 pediatric institutions.
Figure 2.

Pooled yearly MRSA burden versus vancomycin utilization (A) and institutional relative changes from 2014 to 2018 in MRSA proportion versus vancomycin utilization (B) among 6 pediatric institutions.
The same 6 institutions had antibiotic utilization data from 2013–2017 for meropenem and P aeruginosa susceptibilities available for 2014–2018. All 6 institutions excluded P aeruginosa isolates from CF patients. There was a significant increase in pooled distribution among the included years in P aeruginosa susceptibility to meropenem (Figure 3a), but this difference was largely due to the year 2014 (p = 0.3 when comparing 2015–2018 only). Pooled meropenem utilization did not significantly decrease over time (Figure 3a), and the pooled meropenem-resistant P aeruginosa/1000 PD did not significantly decrease over time (Figure 3b). There was not a significant correlation found between pooled preceding-year utilization and resistance burden (Figure 4a). Institutional relative change in meropenem utilization from 2013 to 2017 correlated with the relative change in institutional P aeruginosa susceptibility to meropenem from 2014 to 2018 (Figure 4b).
Figure 3.

Pooled PSA susceptibility and meropenem utilization over time (A*,†) and pooled meropenem resistant PSA burden versus time (B) among 6 pediatric institutions.
Figure 4.

Pooled meropenem resistant PSA burden versus prior year meropenem utilization (A) and institutional relative changes from 2014 to 2018 in meropenem susceptibility versus meropenem utilization from 2013–2017 (B) among 6 pediatric institutions.
Discussion
This multicenter study, implemented by the PPA PBRN, described pooled antibiotic susceptibilities in 2018 among the pediatric population and analyzed pooled and institutional correlations with antibiotic susceptibilities and antibiotic utilization over time. Importantly, MRSA incidence decreased over time. Pooled decreases in vancomycin utilization correlated with pooled MRSA decreases, although institutional relative reductions in MRSA prevalence did not correlate with relative vancomycin utilization reduction. Pooled P aeruginosa susceptibilities changed during the study period and compared with prior publications. Pooled changes in meropenem utilization did not correlate with pooled susceptibilities. This lack of correlation is likely due to differences in institutional use as evidenced by the correlation between relative changes in institutional P aeruginosa susceptibility and relative institutional changes in prior year meropenem utilization. These data can help guide empiric treatment decisions and illustrates the possible impact of and areas for improvement in pediatric ASP.
Previous studies have illustrated decreased MRSA proportional occurrence over the past decade with peak MRSA occurrence between 2005 and 2008 and decreases since.8,20,21 A study using the Pediatric Health Information System (PHIS) database, which is based on International Classification of Diseases coding, illustrated a decrease in MRSA infections from 2009 to 2016.8 The present study verifies this finding via a different methodology, pooling of antibiograms among institutions. The present study also included 2 additional years to illustrate the trend has continued. Based on our study design, a causal relationship could not be determined. There is some evidence that decolonization efforts and hygiene interventions could prevent hospital and community-acquired infections.22–24 Increased implementation of these measures could result in lower MRSA occurrence over time, but cannot be proven by the current study. Prior antibiotic exposure has been associated with MRSA isolation, and as such changes in antibiotic use could impact MRSA occurrence.25,26 The reason behind the epidemiologic changes in MRSA occurrence requires further investigation.
The present study also found that changes in vancomycin utilization correlated with changes in MRSA occurrence, and there was no increase in other anti-MRSA agents used primarily for severe MRSA infections. We did not assess utilization of other possible oral MRSA agents (e.g., clindamycin, trimethoprim/sulfamethoxazole) owing to the possibility of use for numerous other indications at an institutional level. The differences described appear to correlate specifically with MRSA prevalence, but it is possible vancomycin use for treatment of coagulase-negative Staphylococcus or Enterococcus species impacted our findings. Decreased pooled vancomycin utilization is an important finding regardless of the cause. Therapeutic drug monitoring to optimize dosing and minimize nephrotoxicity is often necessary in the pediatric population and there is a concern for emergence of vancomycin-resistant Enterococcus species with vancomycin overutilization.27
Changes in anti-MRSA therapy have been previously described, however with a focus on patients with use for S aureus versus whole institution utilization.8 The present study illustrated that the MRSA changes are impacting the pooled utilization of vancomycin among multiple institutions. It is unclear if the changes in MRSA prevalence from antibiograms are impacting empiric or definitive therapy for MRSA. Owing to an over 20% prevalence of MRSA among all institutions included in our study, it is less likely these changes are due to empiric therapy changes, because MRSA occurrence of >10% to 20% suggests empiric coverage for MRSA is usually indicated for severe diseases such as osteomyeltis.28 An adult Canadian study has illustrated MRSA proportions as low as 16%, which would certainly impact the need for empiric MRSA coverage.29 The relative institutional change analysis during the study period illustrated that while the changes in MRSA occurred in all institutions, resulting changes in vancomycin utilization among institutions varied. Variability in antibiotic utilization between pediatric institutions is a well-known issue and combined with the current study illustrates the continual need for ASP efforts targeting vancomycin use.1,2
The present study did not find any MRSA resistant to vancomycin. The Clinical Laboratory Standards Institute (CLSI) breakpoint for vancomycin MRSA susceptibility is ≤2 mcg/mL, but some studies and guidelines have questioned clinical utility of vancomycin with minimum inhibitory concentration (MIC) of 2 mg/L owing to concerns for requiring larger dosing to achieve efficacy and resultant increased risk of nephrotoxicity.6,27 We were unable to obtain actual MIC data to determine the occurrence of MRSA with a vancomycin MIC of 2 mg/L. The need for additional agents aside from vancomycin may still exist for certain patients with higher MIC values, toxicity, or highly virulent S aureus pathogens.
P aeruginosa often requires treatment with broad-spectrum agents. A prior study also based on pooled antibiogram data from 2005–2011 in the Unites States described higher levels of P aeruginosa resistance in the pediatric population among common beta-lactams, with no commonly used beta-lactams having pooled susceptibilities over 90% susceptibility.30 A multicenter surveillance study31 reported bloodstream pathogen susceptibilities from 1997 to 2016 and found that multidrug-resistant P aeruginosa rates were highest in 2005–2008 in North America and have slightly decreased in 2013–2016, with beta-lactam susceptibilities ranging from 79.5% to 84.7%. This prior study did not delineate specific data for pediatric isolates. The present study provides data suggesting susceptibilities may be higher than previously reported in the pediatric population, because all but 1 beta-lactam included had over 90% pooled susceptibility.30 These susceptibility changes may have occurred around or before 2015, as 2014 was the only year with pooled meropenem susceptibility less than 90% in our study. These differences compared with previous studies could also be due to a difference in the number of included institutions or could be due to the high presence of ASP among the included institutions. The susceptibilities described in the present study could impact empiric use of antipseudomonal beta-lactams and the possible need for dual Gram-negative empiric coverage. Over 80% to 90% coverage for P aeruginosa may be used to determine empiric therapy choices for resistant Gram-negative organisms and the possible need for dual Gram-negative coverage.32,33 It is important to note that despite the positive findings of this current study, there are likely still some institutions or specific patients with risk factors for beta-lactam Gram-negative resistance, which could warrant broadened empiric Gram-negative coverage.
Meropenem utilization has been shown to correlate with P aeruginosa susceptibility in prior single institution studies, likely more related to prior year utilization.11,12 The present study did not find a significant correlation in the pooled analysis. Upon individual institutional analysis, a significant correlation was found between relative changes in meropenem utilization from 2013 to 2017 and relative change in susceptibilities from 2014 to 2018. This finding validates that utilization changes the prior year could impact institutional resistance the following year both positively and negatively (e.g., increased utilization decreased susceptibilities). This also suggests that differences in patient populations between institutions and different baseline resistance and utilization within the included institutions impacted the correlations for the pooled analysis. While pooled meropenem utilization did not significantly decrease over the study period, there is a possibility that greater changes in institutional utilization occurred prior to 2014, as many of the included institutions had active ASP that started before 2014 and previous studies have shown changes in utilization within 1 to 2 years of ASP initiation.11,12,34 The findings of this study are supportive of ASP efforts, but the impact of meropenem utilization changes due to ASP and resultant P aeruginosa resistance among multiple pediatric institutions requires further investigation.
The present study had various limitations. All of the institutions included in this study had at least some amount of pediatric ASP involvement. Many of the institutions implemented an ASP before the study period began, thus preventing a direct association with ASP involvement. But the changes in utilization and susceptibilities described could be due to ASP practices at these institutions. While a large number of isolates were included, the number of included institutions was still relatively small and may not reflect all geographic patterns of antimicrobial resistance. The small number of included institutions also leads to the possibility of the associations or lack of associations due to larger changes in a few of the institutions. Thus, these findings may not be applicable to all pediatric institutions. Institutional antibiotic utilization and antibiograms were included in this study. While this does assess the burden of resistance in an institution, there could be alternative factors impacting the correlation of utilization and pathogen susceptibility. We also relied on institutional antibiogram data that combined inpatient and outpatient isolates, because not all institutions stratified the antibiograms between inpatient and outpatient isolates. Inpatient-acquired isolates may not have the same patterns or resistance described and could impact susceptibilities reported. Some institutions included CF isolates in the S aureus antibiograms, which could impact our S aureus findings. This would not likely impact our P aeruginosa analyses because only 1 institution included P aeruginosa isolates from CF patients, and that institution was not included in the correlation analyses. These findings would not apply to the CF population. Finally, PHIS database is claims based, thus data coding irregularities are possible. The database is also primarily inpatient focused. However, the PHIS database has been used previously in antimicrobial utilization studies.1,2
Conclusion
This multicenter study described changes in MRSA and P aeruginosa susceptibilities from 2014–2018. Among all the included institutions, the burden of MRSA in the pediatric population has decreased over time. These susceptibility changes likely impacted pooled vancomycin utilization. However, the institutional level analysis suggests this was not the case in all institutions and illustrates that vancomycin use is still an important area for improvements in ASP practices. P aeruginosa susceptibilities were higher than previously reported among commonly used beta-lactams. This may suggest ASP efforts are having an impact and changes seen may impact empiric therapy decisions at some institutions. Changes in institutional meropenem utilization can impact P aeruginosa susceptibility the following year. These data highlight the importance and potential impact of ASP efforts focused on broad-spectrum Gram-negative agents like carbapenems.
Acknowledgments
Some of the data in this manuscript were presented as a research platform and poster presentation at the 29th Annual Pediatric Pharmacy Association Annual Virtual Meeting on May 2020. The authors would like to acknowledge the following and others not named who responded to the survey and whose submitted survey data were used for this study: Joshua D. Courter, PharmD, Cincinnati Children's, Cincinnati, OH; Lark Dunton, RPh, Carilion Clinic, Roanoke Memorial Hospital, Roanoke, VA; Lisa Garavaglia PharmD, West Virginia University Medicine Children's, Morgantown, WV; Lizbeth Hansen, PharmD, University of Minnesota Masonic Children's Hospital, Minneapolis, MN; Christina Schwarz, PharmD, Driscoll Children's Hospital, Corpus Christi, TX; Erin Weslander, PharmD, University of Pittsburgh Medical Center, Children's Hospital of Pittsburgh, Pittsburgh, PA. The authors would also like to acknowledge Allison Chung, PharmD, Auburn University Harrison School of Pharmacy, Mobile, AL, and other not named members of the PPA PBRN who critically reviewed the study proposal, survey, and the draft manuscript.
ABBREVIATIONS
- ASP
antimicrobial stewardship program
- CF
cystic fibrosis
- CLSI
Clinical Laboratory Standards Institute
- DOT
days of therapy
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- PBRN
Practice Based Research Network
- PD
patient days
- PHIS
Pediatric Health Information System
- PPA
Pediatric Pharmacy Association
Footnotes
Disclosures. Three authors (JSS, EB, FB) are members of the PPA PBRN. Otherwise, the authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. JSS and KRL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. The study methods were approved by the University of Tennessee Health Science Center Institutional Review Board. Given the retrospective and de-identified nature of this study, institutional review board determined patient-related informed consent was not possible or required. Consent to use the submitted survey data for research purposes was obtained from each survey participant before starting the survey, and each participant agreed to obtain permission from their institution when starting the survey.
References
- 1.Griffith HG, Dantuluri K, Thurm C et al. Considerable variability in antibiotic use among US children's hospitals in 2017–2018. Infect Control Hosp Epidemiol . 2020;41(5):571–578. doi: 10.1017/ice.2019.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber JS, Newland JG, Coffin SE et al. Variability in antibiotic use at children's hospitals. Pediatrics . 2010;126(6):1067–1073. doi: 10.1542/peds.2010-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lake JG, Weiner LM, Milstone AM et al. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011–2014. Infect Control Hosp Epidemiol . 2018;39(1):1–11. doi: 10.1017/ice.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larru B, Gong W, Vendetti N et al. Bloodstream infections in hospitalized children: epidemiology and antimicrobial susceptibilities. Pediatr Infect Dis J . 2016;35(5):507–510. doi: 10.1097/INF.0000000000001057. [DOI] [PubMed] [Google Scholar]
- 5.Hamdy RF, Dona D, Jacobs MB, Gerber JS. Risk factors for complications in children with Staphylococcus aureus bacteremia. J Pediatr . 2019;208:214–220.e2. doi: 10.1016/j.jpeds.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Canty E, Carnahan B, Curley T et al. Reduced vancomycin susceptibility, MRSA and treatment failure in pediatric Staphylococcus aureus bloodstream infections. Pediatr Infect Dis J . 2021;40(5):429–433. doi: 10.1097/INF.0000000000002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis . 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 8.Spaulding AB, Thurm C, Courter JD et al. Epidemiology of Staphylococcus aureus infections in patients admitted to freestanding pediatric hospitals, 2009–2016. Infect Control Hosp Epidemiol . 2018;39(12):1487–1490. doi: 10.1017/ice.2018.259. [DOI] [PubMed] [Google Scholar]
- 9.Gerber JS, Hersh AL, Kronman MP et al. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns across hospitals. Infect Control Hosp Epidemiol . 2017;38(8):993–997. doi: 10.1017/ice.2017.94. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. Antibiotic resistance threats in the United States. Accessed February 16, 2021. www.cdc.gov/DrugResistance/Biggest-Threats.html. [Google Scholar]
- 11.Horikoshi Y, Suwa J, Higuchi H et al. Sustained pediatric antimicrobial stewardship program with consultation to infectious diseases reduced carbapenem resistance and infection-related mortality. Int J Infect Dis . 2017;64:69–73. doi: 10.1016/j.ijid.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Stultz JS, Arnold SR, Shelton CM et al. Antimicrobial stewardship impact on Pseudomonas aeruginosa susceptibility to meropenem at a tertiary pediatric institution. Am J Infect Control. 2019;47(12):1513–1515. doi: 10.1016/j.ajic.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Stultz JS, Knoderer CA, Manasco KB et al. Identification of factors associated with the desire to participate in a pediatric pharmacy practice-based research network. J Pediatr Pharmacol Ther . 2018;23(6):479–485. doi: 10.5863/1551-6776-23.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform . 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibovici L, Paul M, Poznanski O et al. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother . 1997;41(5):1127–1133. doi: 10.1128/aac.41.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phe K, Bowers DR, Babic JT, Tam VH. Outcomes of empiric aminoglycoside monotherapy for Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis . 2019;93(4):346–348. doi: 10.1016/j.diagmicrobio.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Schwaber MJ, De-Medina T, Carmeli Y. Epidemiological interpretation of antibiotic resistance studies—what are we missing? Nat Rev Microbiol . 2004;2(12):979–983. doi: 10.1038/nrmicro1047. [DOI] [PubMed] [Google Scholar]
- 18.Barlam TF, Cosgrove SE, Abbo LM et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis . 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber JS, Jackson MA, Tamma PD et al. Policy Statement: Antibiotic Stewardship in Pediatrics. J Pediatric Infect Dis Soc . 2021;10(5):641–649. doi: 10.1093/jpids/piab002. [DOI] [PubMed] [Google Scholar]
- 20.Sutter DE, Milburn E, Chukwuma U et al. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics . 2016;137(4):e20153099. doi: 10.1542/peds.2015-3099. [DOI] [PubMed] [Google Scholar]
- 21.Diekema DJ, Pfaller MA, Shortridge D et al. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY Antimicrobial Surveillance Program. Open Forum Infect Dis . 2019;6(suppl 1):S47–S53. doi: 10.1093/ofid/ofy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sroka S, Gastmeier P, Meyer E. Impact of alcohol hand-rub use on meticillin-resistant Staphylococcus aureus: an analysis of the literature. J Hosp Infect . 2010;74(3):204–211. doi: 10.1016/j.jhin.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Huang SS, Singh R, McKinnell JA et al. Decolonization to reduce postdischarge infection risk among MRSA carriers. N Engl J Med . 2019;380(7):638–650. doi: 10.1056/NEJMoa1716771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calfee DP, Salgado CD, Milstone AM et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol . 2014;35(7):772–796. doi: 10.1086/676534. [DOI] [PubMed] [Google Scholar]
- 25.Tacconelli E, De Angelis G, Cataldo MA et al. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation: a systematic review and meta-analysis. J Antimicrob Chemother . 2008;61(1):26–38. doi: 10.1093/jac/dkm416. [DOI] [PubMed] [Google Scholar]
- 26.Kanwar A, Cadnum JL, Jencson AL, Donskey CJ. Impact of antibiotic treatment on the burden of nasal Staphylococcus aureus among hospitalized patients. Antimicrob Agents Chemother . 2018;62(10):e00609–e00618. doi: 10.1128/AAC.00609-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rybak MJ, Le J, Lodise TP et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm . 2020;77(11):835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 28.Woods CR, Bradley JS, Chatterjee A et al. Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J Pediatric Infect Dis Soc . 2021;10(8):801–844. doi: 10.1093/jpids/piab027. [DOI] [PubMed] [Google Scholar]
- 29.Nichol KA, Adam HJ, Golding GR et al. Characterization of MRSA in Canada from 2007 to 2016. J Antimicrob Chemother . 2019;74(suppl 4):iv55–iv63. doi: 10.1093/jac/dkz288. [DOI] [PubMed] [Google Scholar]
- 30.Tamma PD, Robinson GL, Gerber JS et al. Pediatric antimicrobial susceptibility trends across the United States. Infect Control Hosp Epidemiol . 2013;34(12):1244–1251. doi: 10.1086/673974. [DOI] [PubMed] [Google Scholar]
- 31.Shortridge D, Gales AC, Streit JM et al. Geographic and temporal patterns of antimicrobial resistance in Pseudomonas aeruginosa over 20 years from the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect Dis . 2019;6(suppl 1):S63–S68. doi: 10.1093/ofid/ofy343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomkin JS, Mazuski JE, Bradley JS et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis . 2010;50(2):133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 33.Weiss SL, Peters MJ, Alhazzani W et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med . 2020;21(2):e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 34.Horikoshi Y, Higuchi H, Suwa J et al. Impact of computerized pre-authorization of broad spectrum antibiotics in Pseudomonas aeruginosa at a children's hospital in Japan. J Infect Chemother . 2016;22(8):532–535. doi: 10.1016/j.jiac.2016.05.001. [DOI] [PubMed] [Google Scholar]


