Abstract
Purpose:
Cereblon (CRBN), a substrate receptor of the E3 ubiquitin ligase complex CRL4CRBN, is the target of the small molecules lenalidomide and avadomide. Upon binding of the drugs, Aiolos and Ikaros are recruited to the E3 ligase, ubiquitylated, and subsequently degraded. In diffuse large B-cell lymphoma (DLBCL) cells, Aiolos and Ikaros are direct transcriptional repressors of interferon-stimulated genes (ISG) and degradation of these substrates results in increased ISG protein levels resulting in decreased proliferation and apoptosis. Herein, we aimed to uncover the mechanism(s) Aiolos and Ikaros use to repress ISG transcription and provide a mechanistic rationale for a combination strategy to enhance cell autonomous activities of CRBN modulators (CELMoD).
Experimental Design:
We conducted paired RNA sequencing with histone modification and Aiolos/Ikaros chromatin immunoprecipitation sequencing to identify genes regulated by these transcription factors and to elucidate correlations to drug sensitivity. We confirmed Aiolos/Ikaros mediated transcriptional complex formation in DLBCL patient samples including those treated with avadomide.
Results:
In DLBCL, the repression of ISG transcription is accomplished in part through recruitment of large transcriptional complexes such as the nucleosome remodeling and deacetylase, which modify the chromatin landscape of these promoters. A rational combination approach of avadomide with a specific histone deacetylase inhibitor leads to a significant increase in ISG transcription compared with either single agent, and synergistic antiproliferative activity in DLBCL cell lines.
Conclusions:
Our results provide a novel role for lineage factors Aiolos and Ikaros in DLBCL as well as further insight into the mechanism(s) of Aiolos and Ikaros–mediated transcriptional repression and unique therapeutic combination strategies.
Translational Relevance.
Single-agent therapies rarely exhibit broad clinical responses in patients with diffuse large B-cell lymphoma (DLBCL) and are frequently combined with other targeted agents. Through an integrated approach of chromatin immunoprecipitation sequencing, transcriptomic, and proteomic analyses, we identified Aiolos and Ikaros mediate transcriptional repression of interferon-stimulated genes (ISG) by recruiting the nucleosome remodeling and deacetylase complex to their transcriptional start sites. A rational combination of an Aiolos/Ikaros degrading cereblon modulator (CELMoD) with a selective histone deacetylase inhibitor leads to greater ISG production and synergistic inhibition of DLBCL proliferation. These data support a strategy for identifying epigenetic agents to combine with Aiolos/Ikaros degradation to further enhance cell autonomous activity of CELMoDs in patients with DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents approximately 40% of all non-Hodgkin lymphoma and presents with an aggressive clinical course with few therapeutic options. DLBCL arises from genetic abnormalities and epigenetic reprogramming that occur in the germinal center (GC) reaction, which is normally a finely tuned program involving lineage factors such as BCL6, PAX5, and EZH2. These programs regulate cycling, proliferation, and expansion of centroblasts and centroctyes within the dark and light zones of the GC to allow somatic hypermutation, interaction with T follicular helper cells and follicular dendritic cells, and finally differentiation of naïve B cells to high affinity memory and plasma cells. DLBCL is a genetically heterogeneous disease with over 400 described recurrent somatic mutations thought to drive the disease (1, 2). Some of the most frequent mutations and structural variants involve chromatin modifiers such as CREBBP, KMT2D, EZH2, and transcription factors including BCL6. Although targeting epigenetic modifiers in DLBCL has been limited, some therapeutics have demonstrated clinical activity including inhibitors of EZH2 and histone deacetylase (HDAC; ref. 3, 4). These data provide a proof of concept that agents with the ability to target epigenetic and transcriptional dysregulation are viable therapeutic strategies. Aside from somatically mutated genes, there are lineage factors that are important in DLBCL disease biology which are not recurrently mutated but are integral for maintenance of the disease.
Normal lymphocyte development and differentiation occurs through a series of tightly regulated events governed by a number of lineage transcription factors. Two such factors are Aiolos and Ikaros, both with transcriptional repressor and activator activities, which orchestrates a transcriptional reprogramming, potentiating lymphocyte differentiation from hematopoietic stem cells in mice (5). The preponderance of evidence for the activity of this family of transcription factors in lymphocyte development has been generated through the use of genetically engineered mouse models by inserting mutations in or deletions of specific zinc finger domains leading to dysfunctional dominant negative isoforms leading to a lack of B- and T-cell differentiated cells from lymphoid progenitors (6, 7). While Aiolos and Ikaros are important lymphoid lineage factors, they are not frequently mutated in DLBCL and therefore are not considered drivers of the disease and consequently have been understudied in lymphoma. We have previously demonstrated that degradation of Aiolos and Ikaros by lenalidomide and avadomide results in upregulation of interferon-stimulated genes (ISG) such as IRF7, IFIT3, and DDX58 leading to apoptosis of DLBCL cell lines in an interferon secretion independent manner (8). Preclinical studies have demonstrated that the antiproliferative activity of lenalidomide is preferentially observed in ABC-DLBCL cells while avadomide is active in both ABC- and GCB-DLBCL cell lines. The molecular basis for this differential activity is unknown and has only been correlated to a deeper and more rapid kinetics of the degradation of Aiolos and Ikaros from avadomide compared with lenalidomide. Multiple clinical trials have demonstrated that lenalidomide and avadomide both as monotherapy and in combination with chemoimmunotherapy has demonstrated significant clinical benefit in subsets of patients with DLBCL (9–12).
Despite these therapeutic benefits, scant data have been reported on the function of Aiolos and Ikaros in complex with transcriptional complexes and how they contribute to survival in the malignant clone. Herein, using DLBCL cell lines and primary DLBCL tissue, we demonstrate that Aiolos and Ikaros are members of the transcriptional repressor nucleosome remodeling and deacetylase (NuRD) complex and through their DNA binding capabilities recruits this complex to modify the chromatin landscape of promoters including ISGs. Aiolos and Ikaros function in concert with the NuRD complex to repress transcription of ISGs leading to their function in tumor cell survival and this repressive activity is greater in the GCB subtype than ABC contributing to the differential sensitivity of ABC-DLBCL cells to lenalidomide. In addition, we demonstrate that a rational combination of degrading Aiolos and Ikaros paired with targeting the NuRD complex via inhibition of histone deacetylase 1/2 (HDAC1/2), results in a significant increase in ISG transcription and a synergistic antiproliferative activity in a panel of DLBCL cells. Importantly, this combination was able to demonstrate synergistic activity in cell lines which are intrinsically resistant to degradation of Aiolos and Ikaros and provides an opportunity to overcome various degrees of epigenetic mediated silencing contributing to tumorigenesis. These studies provide mechanistic rational to investigate Aiolos and Ikaros degraders in combination therapies with epigenetic modifiers in the clinic.
Materials and Methods
Cell Culture
DLBCL (TMD8, U2932, WSU-DLCL2, SUDHL-4, OCI-LY18, RL, WILL1, Pfeiffer, SUDHL-8, Riva) were obtained from ATCC and DSMZ, authenticated through use of AmpFLSTR (ThermoFisher) and cultured in RPMI1640 containing 10% FBS, 1% Penicillin/Streptomycin, and 1 mmol/L sodium pyruvate.
Immunoblotting
Cells were lysed in NT2 buffer containing 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L MgCl2, 0.05% Nonidet P-40, 1 mmol/L sodium orthovanadate, 0.5 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 2 μg/mL pepstatin. Proteins from cell lysates were separated by 10% SDS-PAGE gel electrophoresis (Bio-Rad), and transferred to nitrocellulose membranes (Invitrogen). Immunoblots were probed with antibodies recognizing: Aiolos (Cell Signaling Technology), Ikaros (Millipore), IRF7 (Cell Signaling Technology), DDX58 (Thermo Scientific), IFIT3 (Novus Biologicals), c-myc (Abcam), IRF4 (Santa Cruz), p65 (Cell Signaling Technology), p-p65 Ser236 (Cell Signaling Technology), p105/p50 (Cell Signaling Technology), p100/p52 (Cell Signaling Technology), Tubulin (Cell Signaling Technology), TBP (Cell Signaling Technology), and β-actin (Li-Cor). Signals were detected with a Li-Cor Odyssey imager or WES (Protein Simple).
Flow cytometric analysis for apoptosis
U2932, TMD8, WSU-DLCL2, and SUDHL-4 (1×105 cells/mL) were treated with either DMSO or various concentrations of lenalidomide or avadomide for 7 days. On day 7, cells were collected, and apoptosis was analyzed through flow cytometric analysis of Annexin V and To-Pro 3 staining according to the manufacturer's protocol (Life Technologies). The gating strategy used for determining the quadrants in the flow cytometry data segregates viable cells in the DMSO control within each cell line as Annexin V negative/ToPro-3 negative. All drug treatments are measured relative to the gating employed in the DMSO control.
Immunoprecipitation
For immunoprecipitation of Aiolos and Ikaros protein:protein complexes, SUDHL-4, TMD8, and WSU-DLCL2 cells were collected, and NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) were used to extract nuclear lysates following the manufacturer's instructions. Co-immunprecipitation reactions were carried out using Dynabeads Co-Immunoprecipitation Kit (Thermo Scientific) following the manufacturer's protocol.
RNA preparation and qPCR
As previously described “Total RNA was harvested and reverse transcribed by using TaqMan Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) and the resulting cDNA was amplified by qPCR analysis using gene-specific primer pairs for IRF7, IFIT3, DDX58, and β-actin. An Applied Biosystems Viia 7 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA) and TaqMan fast Advanced Master Mix (Applied Biosystems, Carlsbad, CA) were used to carry out the qPCR analysis” (8).
Proximity ligation assay
DLBCL cell lines treated with DMSO or avadomide were cytospun onto L-polylysine coated glass slides (Thermo Fisher). Cells were then fixed with 4% formaldehyde (Thermo Fisher) and permeabilized with 0.2% triton X or ice-cold methanol for 30 minutes. Cells were then blocked in proximity ligation assay (PLA) blocking reagent for 30 minutes, followed by incubation with primary antibodies or corresponding isotype controls diluted in Duolink Antibody Diluent (Sigma) overnight at 4°C. Additional steps for detection and imaging were performed according to the manufacturer's procedures from Duolink In Situ Detection Reagents (Sigma).
Paraffin-embedded tissue blocks from patient tumor samples were sectioned at 5-μm thickness and floated in a 40°C water bath containing distilled water, prior to transferring onto glass slides. After dewaxing with xylene/ethanol titrations, slides were transferred to pre-warmed high pH target retrieval solution (Invitrogen eBioscience IHC Antigen Retrieval Solution) in a glass container in the water bath set at 95°C for 30 minutes. Background fluorescence was minimized by soaking slides in 0.2% Sodium Borohydride (Sigma) for 10 minutes. Permeabilization was carried out by soaking slides in 0.2% triton X for 5 minutes. PLA in situ Fluorescent detection was carried out following the Duolink PLA Fluorescent Detection instructions (Sigma) with the following additions: after the amplification process, slides were stained with 1:50 Alexa Fluo 488 conjugated anti-CD20 antibodies (clone L26, eBioscience). Finally, nuclei were stained with DAPI Solution (1 ug/mL, Invitrogen Thermo Fisher Scientific) prior to mounting slides with VECTASHIELD Antifade Mounting Medium(Fisher Scientific). Imaging analysis was carried out using NIS Elements on a Nikon Confocal Microscope A1R. Number of PLA-positive spots were quantified in each image using Fiji ImageJ software.
When assessing complex via flow cytometry, the manufacturer's protocol for Duolink PLA Flow Cytometry (Sigma) was followed with minor modification. Specifically, following the final wash step, cells were resuspended in stain buffer (BD) and Hoechst 33342 Ready Flow Reagent (Invitrogen) was added at 1:30 (vol/vol). Acquisition of at least 10,000 events per sample analysis was carried out using BDFortessa flow cytometer. Data were analyzed using FlowJo (v10).
See Supplementary Data file for additional details.
Data Availability
RNA sequencing (RNA-seq) data have been deposited in the Gene Expression Omnibus under accession number GSE201324.
Results
Induction of ISGs is correlated with antiproliferative activity of Aiolos and Ikaros degradation by lenalidomide and avadomide
DLBCL cells treated with CRBN-modulating compounds such as lenalidomide or avadomide, results in CRL4CRBN-mediated degradation of Aiolos and Ikaros and subsequent proliferative arrest by lenalidomide exerting cell autonomous activity in primarily ABC-DLBCL cells whereas avadomide demonstrates antiproliferative activity in both ABC- and GCB-DLBCL cell line models (8). To define a panel of cell lines with differential sensitivities to lenalidomide and avadomide, we measured the proportion of DLBCL cells entering apoptosis as measured by Annexin V/ToPro-3 following 7 days of treatment with lenalidomide and avadomide. The data are consistent with a previous report that ABC-DLBCL cells such as TMD8 and U2932 are sensitive to both lenalidomide and avadomide. Avadomide extends its cell autonomous effects to GCB-DLBCL cells such as WSU-DLCL2, in which lenalidomide does not demonstrate activity, and there exists a subset of GCB-DLBCL as demonstrated by SUDHL-4 which is intrinsically resistant to both molecules (Fig. 1A and B).
Figure 1.
Degradation of Aiolos and Ikaros and induction of ISGs is correlated with induction of apoptosis. A and B, DLBCL cell lines were treated with DMSO, lenalidomide, and avadomide (0.1–10 μmol/L) for 7 days, after which apoptosis was measured by Annexin V and To-Pro 3 flow cytometric analysis. Graphical representation of two independent experiments (mean ± SEM). C, DLBCL cells were treated with DMSO, lenalidomide (1–10 μmol/L), or avadomide (0.1–10 μmol/L) for 24 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, IRF7, IRF4, c-myc, and β-actin were assessed by immunoblot analysis.
To correlate these findings to downstream molecular mechanisms of the two drugs, we treated the same panel of sensitive and resistant DLBCL cell lines with DMSO, lenalidomide, and avadomide for 24 hours and measured degradation of substrates Aiolos and Ikaros, as well as modulation of ISGs such as IRF7. Interestingly, we observed that substrate degradation occurs independent of sensitivity to either lenalidomide or avadomide. Interestingly, IRF7 protein expression increases upon lenalidomide treatment only in TMD8 and U2932 sensitive cells but not in WSU-DLCL2 and SUDHL-4 resistant cell lines. Likewise, IRF7 is increased by avadomide in WSU-DLCL2, which is insensitive to lenalidomide but upon treatment with avadomide will exhibit an increased apoptotic fraction (Fig. 1C). In addition, the magnitude of IRF7 induction in ABC-DLBCL cell lines is greater with avadomide compared with lenalidomide in agreement with the difference in potency of Aiolos/Ikaros degradation between the two molecules. These data suggest that Aiolos/Ikaros degradation is necessary but not always sufficient for ISG upregulation and subsequent apoptosis in DLBCL.
Intrinsically resistant cell lines to lenalidomide and avadomide exhibit repressive chromatin modifications at the promoters of ISGs.
To define the mechanism underlying resistance to lenalidomide and avadomide as well as the necessity for faster kinetics and deeper degradation of Aiolos and Ikaros, we performed concomitant RNA-seq and chromatin immunoprecipitation sequencing (ChIP-seq) for multiple poised and repressive histone marks in the same panel of four DLBCL cell lines. In these paired experiments, DLBCL cells were treated with DMSO, lenalidomide, and avadomide for 18 hours and mRNA were extracted and subjected to RNA-seq. Differential gene expression pathways analysis revealed that the top functional gene categories included ISGs (HALLMARK Interferon alpha response genes). Similar to the protein data shown in Fig. 1C, transcription of ISGs is correlated with sensitivity of DLBCL cells to lenalidomide and avadomide, with SUDHL-4 demonstrating only a marginal increase in ISG transcription in response to either drug treatment (Fig. 2A; Supplementary Table S1).
Figure 2.
Sensitivity to lenalidomide and avadomide is associated with baseline chromatin state at Aiolos and Ikaros–regulated genes. A, mRNA from indicated DLBCL cells were purified following treatment with DMSO, lenalidomide, or avadomide (1 μmol/L) for 18 hours and gene expression via RNA-seq was performed. Gene set variation analysis (GSVA) Hallmark Interferon-α response genes enrichment plot is shown for a category identified as positively enriched with treatment. Values are scaled as Z-scores. Negative and positive values do not have a specific meaning, but they are rather part of the same continuous scale. B, Comparison of chromatin states (poised: H3K27ac, H3K4me3; repressive: H3K27me3, H3K9me3) for promoters of Interferon-α response genes in indicated DLBCL cells. C, Scatterplot demonstrating correlation between average log2 fold change in gene expression and log2 fold change in H3K27ac, H3K4me3 abundance at promoters of Interferon-α response genes after 18 hours treatment with avadomide in indicated DLBCL cells. D, ChIP-seq peaks demonstrating direct binding of Aiolos and Ikaros at representative promoters of Interferon-α response genes in TMD8 DLBCL cells.
To understand this differential gene expression, we performed ChIP-seq of poised (H3K27ac and H3K4me3) and repressive (H3K27me3 and H3K9me3) histone modifications. In a focused analysis of the transcription start site (TSS) of genes within the HALLMARK Interferon alpha response gene signature, we identified that in cells sensitive to avadomide (TMD8, U2932, and WSU-DLCL2) there is an enrichment for poised/active histone marks compared with intrinsically resistant SUDHL-4. And conversely, SUDHL-4 exhibits the highest enrichment of repressive histone modifications at the TSS and least transcriptionally responsive to Aiolos/Ikaros degradation compared with sensitive cells lines (Fig. 2B). Furthermore, we observed a positive correlation between changes in gene expression and changes in H3K27ac/H3K4me3 at the TSS of ISGs (HALLMARK Interferon alpha response gene) post-administration of avadomide in the sensitive TMD8, U2932, and WSU-DLCL2 but not the insensitive SUDHL-4 (Fig. 2C; Supplementary Fig. S1A). We next examined ChIP-seq performed using Aiolos and Ikaros and were able to confirm that both substrates bind to the TSS of ISGs such as IRF9, IRF7, and ISG20 (Fig. 2D). Interestingly, the abundance of Aiolos and Ikaros at ISG promoters was not found to be significantly different across cells lines that are sensitive or resistant to degradation of Aiolos or Ikaros (P > 0.05). In addition, there was a significant overlap of Aiolos and Ikaros bound promoters suggesting redundancy of regulatory effects with these two transcription factors (Supplementary Fig. S1B). These data further support the hypothesis that the basal chromatin landscape as defined by histone modifications at the TSS of Aiolos and Ikaros–regulated ISGs is a major determinant of sensitivity to lenalidomide and avadomide.
Aiolos and Ikaros interact with the NuRD transcriptional repressor complex in DLBCL
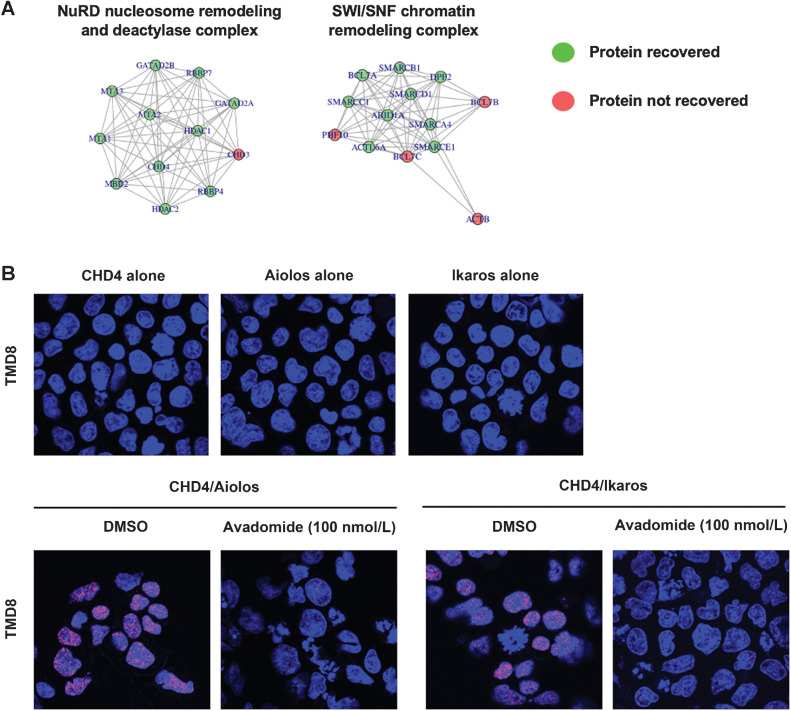
To gain insight into the transcriptional complexes with which Aiolos and Ikaros are interacting and contributing to this poised or repressive chromatin state at the TSS of ISGs, TMT-based proteomics of immunoprecipitate reactions for Aiolos and Ikaros was applied in TMD8, WSU-DLCL2, and SUDHL-4 DLBCL cells. Comparison of Aiolos and Ikaros with isotype control resulted in 125 proteins being significantly associated with the two transcription factors (PAdj < 0.05; Table S2). Through this, we were able to identify transcriptional complexes associated with Aiolos and Ikaros such as the NuRD complex and the SWI/SNF ATP-dependent chromatin remodeling complex (Fig. 3A; Supplementary Table S3).
Figure 3.
Aiolos and Ikaros interact with NuRD repressive transcriptional complex. A, Visual representation of networks of protein interactions with Aiolos and Ikaros revealed through immunoprecipitation of Aiolos and Ikaros followed by TMT-based proteomics. B, Confocal images of PLA reaction between Aiolos or Ikaros and CHD4, core component of NuRD complex, in TMD8 cells treated with either DMSO or avadomide (100 nmol/L) for 3 days. PLA signal (red), DAPI (blue).
To confirm these findings, we employed a PLA which produces an excitation signal when two proteins are in close proximity and therefore contained within the same complex. As shown in Fig. 3B, in DLBCL cell lines, Aiolos and Ikaros are in complex with CHD4 which is a core nonredundant member of the NuRD complex. This interaction of Aiolos and Ikaros with the NuRD complex was demonstrated to be specific as the single antibodies of Aiolos, Ikaros, or CHD4 alone do not produce a signal and the treatment of TMD8 and SUDHL-4 cells with avadomide greatly diminishes the signal due to degradation of Aiolos and Ikaros protein (Fig. 3B; Supplementary Fig. S2). Given previous reports demonstrating that treatment of DLBCL cell lines with an Aiolos/Ikaros degrading molecule results in cell-cycle arrest in a G1 state (12), we examined these complex formations in each phase of the cell cycle by using the PLA assay in a flow cytometry format. The specificity of the interactions was confirmed through comparison to rabbit and mouse isotype antibodies, as well as to a nonspecific protein UBC9. In this way, we can observe that Aiolos and Ikaros form a complex with each other in all phases of the cell cycle (Supplementary Fig. S3). We next examined the interaction of Aiolos and Ikaros with HDAC1/HDAC2, the two deacetylase enzymes responsible for NuRD chromosomal remodeling activity. As shown in Supplementary Fig. S4, the interaction between Aiolos and Ikaros with the NuRD complex occurs in all stages of the cell cycle. While the reported effects of ISG induction involve a G1 arrest of the target cell, we observe induction of IRF7 in all phases of the cell cycle (Supplementary Fig. S4C). It remains to be determined if this is merely a pharmacodynamic effect of the drug mechanism or if ISGs exert their activity in other parts of the cell cycle not yet discovered.
We subsequently investigated if this complex could be detected in primary DLBCL tissue. Representative images shown in Fig. 4A demonstrate that there is a robust association of Ikaros with CHD4 in the nucleus of CD20+ DLBCL cells within a primary tumor. To further these observations, we probed a tissue microarray (TMA) with 111 DLBCL tumors for the association of Aiolos and Ikaros with HDAC1 and HDAC2. Through quantification of these foci, we establish that Aiolos and Ikaros each are in complex with HDAC1 and HDAC2 with a slight trend towards greater interaction between Aiolos and HDAC1/2 compared with Ikaros (Fig. 4B; Supplementary Fig. S5A). We also characterized the TMA for cell of origin using the Han's staining method and found there to be no difference in complex formation between GCB (n = 32) and non-GCB (n = 79) for Aiolos/HDAC1 (P = 0.908), Ikaros/HDAC1 (P = 0.184), Aiolos/HDAC2 (P = 0.482), and Ikaros/HDAC2 (P = 0.771). Finally, we were able to confirm that the interaction between Aiolos/Ikaros and HDAC1/2 is disrupted in patients with relapsed and/or refractory (R/R) DLBCL that were administered single-agent avadomide treatment (NCT01421524). In the representative images obtained from paired tumor biopsies collected during the screening period prior to the first dose of avadomide and an on-treatment biopsy collected at day 10 in the first cycle of avadomide treatment from the same patient, there is a demonstrable decrease in complex formation between Aiolos/Ikaros and HDAC1/2 in the on-treatment biopsy compared with the screening biopsy (Fig. 4C), confirming our in vitro observations in the clinic.
Figure 4.
Confirmation of the interaction of Aiolos and Ikaros with NuRD complex proteins in primary DLBCL tumor biopsies. A, Representative fields of FFPE tumor samples from patients with DLBCL were immunohistochemically stained with Dapi (blue), CD20 (green), and Ikaros, Isotype and CHD4. Positive interactions are shown via PLA (red foci). B, Graphical representation of interactions formed as measured by PLA foci for Aiolos/Isotype, Aiolos/HDAC1, Ikaros/HDAC1, Aiolos/HDAC2, and Ikaros/HDAC2 in DLBCL tumors (n = 111). C, Representative fields of FFPE samples from a patient with relapsed/refractory DLBCL administered with 3 mg avadomide on an intermittent schedule. Screening and on-treatment (cycle 1 day 10) biopsies were obtained. Tissues were then subjected to FFPE PLA immunofluorescence for Aiolos/Ikaros in complex with HDAC1/2. Dapi (blue), CD20 (green), positive interactions are shown via PLA (red foci).
Combination of avadomide with an HDAC inhibitor results in synergistic antiproliferative activity.
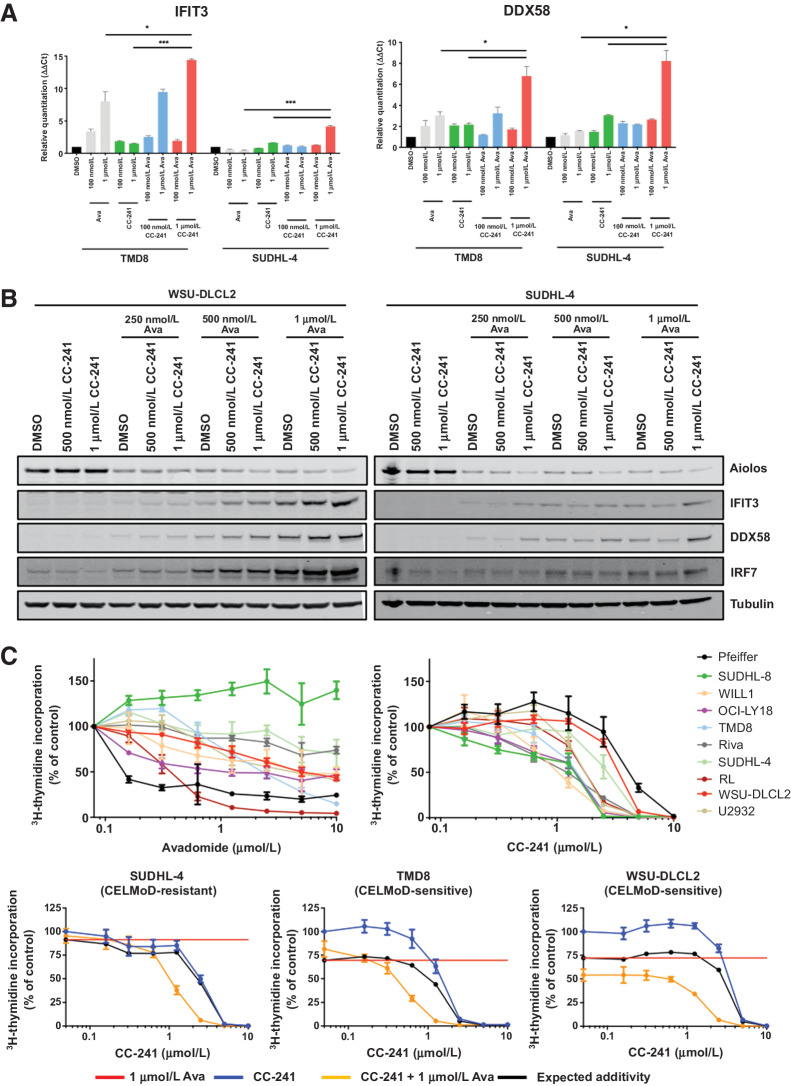
Given the interaction of Aiolos/Ikaros with the NuRD complex, we hypothesized that a combination treatment of avadomide with citarinostat (CC-241), a HDAC 1/2/3/6 inhibitor, would result in enhanced ISG expression and synergistic cell autonomous activity in DLBCL. To understand the effect of the combination on ISG transcription, we treated TMD8 (avadomide-sensitive) and SUDHL-4 (avadomide-resistant) with single-agent avadomide and CC-241 and the combination for 18 hours and measured mRNA expression of ISGs IFIT3, DDX58, and IRF7. In the avadomide-sensitive TMD8, we observed induction of IFIT3, DDX58, and IRF7 to 14, 6.8, and 4.4-fold higher than DMSO in the combination, while avadomide alone resulted in 8, 3, and 2.7-fold and CC-241 produced 1.7, 2.1, and 0.7-fold compared with DMSO (Fig. 5A; Supplementary Fig. S6A). Interestingly, in the avadomide-resistant SUDHL-4 cells, the combination treatment resulted in upregulation of ISGs. The induction of IFIT3, DDX58, and IRF7 for the avadomide/CC-241 combination was 4.2, 8.2, and 1.3-fold higher than DMSO, while avadomide alone resulted in 0.5, 1.5, and 1.0-fold and CC-241 produced 1.6, 3, and 0.4-fold compared with DMSO. Increased ISG production was also observed at the protein level in WSU-DLCL2 and SUDHL-4 cells treated with single agents and avadomide/CC-241 combination for 72 hours (Fig. 5B).
Figure 5.
Targeting NuRD complex through targeting Aiolos/Ikaros and HDAC1/2 induces synergistic antiproliferative effect in DLBCL cells. A, TMD8 and SUDHL-4 DLBCL cells were treated with DMSO, avadomide (0.1–1 μmol/L), CC-241 (0.1–1 μmol/L), or a combination of both for 18 hours and gene expression was assayed for IFIT3, DDX58, and β-actin by qPCR. B, WSU-DLCL2 and SUDHL-4 GCB-DLBCL cells treated with DMSO, avadomide (0.25–1 μmol/L), CC-241 (0.5–1 μmol/L), or a combination of both for 3 days. Cell lysates were separated by SDS-PAGE and levels of Aiolos, IFIT3, DDX58, IRF7, and Tubulin were assessed. C, Top, indicated DLBCL cell lines were treated with DMSO, avadomide, and CC-241 (0.01–10 μmol/L) for 5 days. Proliferation for all cell lines was determined using the (3)H-thymidine incorporation method. Results of three independent experiments are shown (mean ± SEM). Bottom, TMD8, WSU-DLCL2, and SUDHL-4 (left to right) were treated with DMSO, CC-241 (0.01–10,000 nmol/L), avadomide (1 μmol/L), or a combination of citarinostat (CC-241) with avadomide for 5 days. Proliferation for all cell lines was determined using the (3)H-thymidine incorporation method. Results of three independent experiments are shown (mean ± SEM).
To understand if this increased ISG expression translated into enhanced phenotypic effects, we profiled 10 cell lines for their response to avadomide and CC-241 as single agents for 5 days in tritiated thymidine incorporation assays to measure proliferation. The response to avadomide is varied across the panel with antiproliferative activity in both ABC and GCB cell lines. CC-241 demonstrated an inhibition of cell proliferation in a dose dependent and cell of origin independent manner (1–4 μmol/L) in the panel of DLBCL cell lines (Fig. 5C, top). We next assessed the potential of the combination by adding either DMSO or a constant 1 μmol/L concentration of avadomide to a dose titration of CC-241. The combination resulted in a synergistic antiproliferative activity in 6 of the 10 cell lines including avadomide-resistant SUDHL-4 (Fig. 5C, bottom; Supplementary Fig. S6B). These data confirm that a combination strategy targeting the Aiolos/Ikaros/NuRD complex can boost ISG expression translating into a greater than expected cell autonomous effect.
Discussion
Recent genomic analyses of tumors from patients with DLBCL have highlighted that a number of the most prevalent genes with mutations or deletions control epigenetic and transcriptional programs such as EZH2, KMT2D, ARID1A, CREBBP, and EP300 (1, 13). In addition, approximately 20% of DLBCL tumors have translocations involving BCL6 which can result in upregulated protein expression and suspension of normal differentiation of GC B-cells into memory B-cells or plasma cells (14). The transcription factors, Aiolos and Ikaros, also participate in B-cell differentiation and development through promotion of IL7R, FLT3, and EBF expression, while repressing genes which could determine alternative cell fates (15).
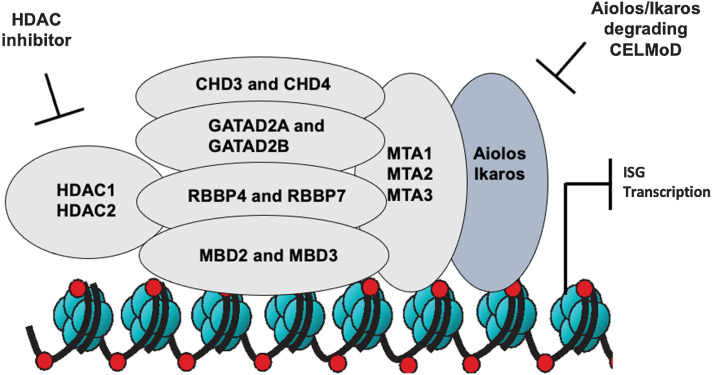
In this study, we describe a novel role for hematopoietic lineage factors Aiolos and Ikaros in DLBCL. While these two transcription factors are not generally identified as disease drivers, given they are rarely mutated or deleted in DLBCL, they do play an important role in maintaining DLBCL cell survival through recruitment of repressive chromatin remodeling complexes to the TSS of ISGs inhibiting their transcription. While we have previously reported that CRL4CRBN E3 ligase mediated ubiquitylation and subsequent proteasomal degradation of Aiolos and Ikaros was necessary for cell autonomous activity of Aiolos/Ikaros degrading cereblon modulators (CELMoD), this event was not always sufficient for cell death of all DLBCL cells especially GCB-DLBCL. Here, we demonstrate that increased kinetics and greater depth of degradation would result in a broader apoptotic induction across a DLBCL cell line panel, including activity in intrinsically resistant cells. Through a combination of ChIP-seq and proteomics technologies, we were able to confirm that the differential sensitivity was associated with increased abundance of repressive histone marks at the promoters of ISGs, at least in part through recruitment of the NuRD transcriptional complex and increased enzymatic activity of HDAC1 and HDAC2. Given the additional inhibition of HDAC3 and HDAC6 with CC-241, additional future work will be needed to understand the impact of these two enzymes on the cellular activity presented. To date, there has been scant evidence for the importance of the NuRD complex in DLBCL outside of the possible interaction and cooperation with BCL6 through binding of MTA3 (16, 17). Our data provide novel insight into the pro-survival activity of the NuRD complex in DLBCL and the importance of its interaction with Aiolos and Ikaros in governing this activity. Previous literature has reported that suppression of ISGs was through coordinated repression by either IRF4 or NFkB signaling (18, 19). In Supplementary Fig. S7A, we demonstrate in ABC-DLBCL TMD8 cells that neither lenalidomide nor avadomide treatment resulted in effects on NFkB signaling as measured by translocation of transcriptional subunits from the nucleus to the cytoplasm, while phorbol 12-myristate 13-acetate (PMA) induced p100/p52 cleavage and p65 relocalization to the nucleus. These data, in addition to upregulation of ISG in GCB-DLBCL cells, where IRF4 expression is not present lead us to model of Aiolos and Ikaros directly regulating expression of ISGs through recruitment of the NuRD complex to the promoter region of these genes and induction of a repressive chromatin environment (Fig. 6).
Figure 6.
Model of avadomide and HDAC1/2 inhibition of NuRD complex transcriptional repression to upregulate expression of ISGs. Multipronged approach to inhibition of the NuRD complex through CELMoD-mediated degradation of Aiolos and Ikaros in combination with HDAC1/2 inhibitor, resulting in increased ISG transcription and synergistic antiproliferative activity in DLBCL cells.
Recently, clinical results from the single-agent avadomide in R/R DLBCL demonstrated that patients with DLBCL with a preexisting immune infiltrate of T cells and macrophages in the tumor microenvironment benefitted significantly more compared with patients with limited immune infiltrate and a tumor microenvironment primarily composed of the malignant DLBCL clone, 44% versus 19% overall response rate respectively (8). This clinical observation is in line with the dual mechanism of Aiolos/Ikaros degrading CELMoDs, resulting in both cell autonomous activity in the DLBCL cell and a pro-immunomodulatory activity. However, the observation of clinical benefit in the patients with limited preexisting immune infiltrate suggests that there is additional benefit to be gained in specifically targeting the malignant DLBCL clone. Our data indicate that additional clinical activity in R/R DLBCL may be harnessed through development of novel Aiolos/Ikaros degrading CELMoDs with even greater depth of degradation, as well as combination strategies with epigenetic agents such as HDAC inhibitors to further enhance production of ISGs.
What also seems apparent is that Aiolos and Ikaros are not traditional drivers of DLBCL lymphomagenesis which can be identified through mutations or variations in the copy numbers, but rather are important lineage factors that regulate differentiation of normal B cells that in the disease setting confer pro-survival signals in the malignant DLBCL clone. This indicates that therapeutic targets are not constrained only to those which are dysregulated through genetic means in the disease but may be lineage factors whose normal activity has been co-opted. Studies to further characterize the role of Aiolos and Ikaros in the GC of DLBCL and relationship to other driver genes will be critical to realize the full potential of Aiolos/Ikaros degrading CELMoDs.
Supplementary Material
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
P.R. Hagner reports other support from Bristol Myers Squibb during the conduct of the study, as well as other support from Bristol Myers Squibb outside the submitted work. H. Chiu reports other support from Bristol Myers Squibb during the conduct of the study, as well as other support from Bristol Myers Squibb outside the submitted work. M.O. Estevez reports other support from Bristol Myers Squibb during the conduct of the study, as well as other support from Bristol Myers Squibb outside the submitted work. M.F. Waldman reports other support from Bristol Myers Squibb during the conduct of the study, as well as other support from Bristol Myers Squibb outside the submitted work. R. Loos is an employee and equity holder of Bristol Myers Squibb. F. Towfic reports other support from Bristol Meyers Squibb during the conduct of the study. A.K. Gandhi reports other support from Bristol Myers Squibb outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
P.R. Hagner: Conceptualization, data curation, supervision, investigation, writing–original draft, project administration, writing–review and editing. H. Chiu: Data curation, formal analysis, investigation, methodology, writing–original draft. V.S. Chopra: Conceptualization, supervision, investigation, methodology, writing–original draft. M. Colombo: Data curation, software, formal analysis, writing–original draft. N. Patel: Formal analysis, investigation. M.O. Estevez: Data curation, software, supervision. M.F. Waldman: Investigation. R. Loos: Software, formal analysis, supervision. F. Towfic: Conceptualization, data curation, software, supervision, writing–original draft. A.K. Gandhi: Conceptualization, supervision, writing–original draft.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
References
- 1. Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell 2017;171:481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Italiano A, Soria J-C, Toulmonde M, Michot J-M, Lucchesi C, Varga A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumors: a first-in-human, open-label, phase I study. Lancet Oncol 2018;19:649–59. [DOI] [PubMed] [Google Scholar]
- 4. Assouline SE, Nielsen TH, Yu S, Alcaide M, Chong L, Macdonald D, et al. Phase II study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood 2016;128:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Jackson AF, Naito T, Dose M, Seavitt J, Liu F, et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat Immunol 2011;13:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J 1996;15:5358–69. [PMC free article] [PubMed] [Google Scholar]
- 7. Georgopoulos K, Bigby M, Wang J-H, Molnar A, Wu P, Winandy S, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell 1994;79:143–56. [DOI] [PubMed] [Google Scholar]
- 8. Hagner PR, Man H-W, Fontanillo C, Wang M, Couto S, Breider M, et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood 2015;126:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpio C, Bouabdallah R, Ysebaert L, Sancho J-M, Salles G, Cordoba R, et al. Avadomide monotherapy in relapsed/refractory DLBCL: safety, efficacy, and a predictive gene classifier. Blood 2020;135:996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nowakowski GS, Chiappella A, Gascoyne RD, Scott DW, Zhang Q, Jurckzac W, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol 2021;39:1317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nowakowski GS, Hong F, Scott DW, Macon WR, King RL, Habermann TM, et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US intergroup study ECOG-ACRIN E1412. J Clin Oncol 2021;39:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Czuczman MS, Trněný M, Davies A, Rule S, Linton KM, Wagner-Johnston N, et al. A phase II/III multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator's choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 2017;23:4127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 2018;378:1396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hatzi K, Melnick A. Breaking bad in the germinal center: how deregulation of BCL6 contributes to lymphomagenesis. Trends Mol Med 2014;20:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reynaud D, A Demarco I, L Reddy K, Schjerven H, Bertolino E, Chen Z, et al. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol 2008;9:927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 2003;113:207–19. [DOI] [PubMed] [Google Scholar]
- 17. Jaye D, Iqbal J, Fujita N, Geigerman C, Li S, Karanam S, et al. The BCL6-associated transcriptional co-repressor, MTA3, is selectively expressed by germinal center B cells and lymphomas of putative germinal center derivation. J Pathol 2007;213:106–15. [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Shaffer AL, Emre NCT, Ceribelli M, Zhang M, Wright G, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B-cell lymphoma. Cancer Cell 2012;21:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L-H, Kosek J, Wang M, Heise C, Schafer PH, Chopra R. Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 2013;160:487–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing (RNA-seq) data have been deposited in the Gene Expression Omnibus under accession number GSE201324.