Abstract
Autoimmune disease of the head and neck (H&N) could be primary or secondary to systemic diseases, medications, or malignancies. Immune-mediated diseases of the H&N are not common in daily practice of radiologists; the diagnosis is frequently delayed because of the non-specific initial presentation and lack of familiarity with some of the specific imaging and clinical features. In this review, we aim to provide a practical diagnostic approach based on the specific radiological findings for each disease. We hope that our review will help radiologists expand their understanding of the spectrum of the discussed disease entities, help them narrow the differential diagnosis, and avoid unnecessary tissue biopsy when appropriate based on the specific clinical scenarios.
Keywords: Head and neck, Imaging, Autoimmune disease, Head and neck autoimmune disease
Introduction
Biomarkers in medicine may be defined as quantifiable parameters which assist with diagnosing disease, guiding therapy, and predicting prognosis and outcome. In this age of modern “personalized” medicine, most biomarkers are with the realm of biological science, that is, DNA, microRNA, proteins, cell receptors, and cytokines. Similarly with imaging, biomarkers may be defined and considered to help reach a diagnosis, evaluate the effects of treatment, and provide some idea of the long-term evolution of disease in any particular patient. There is a gap in radiology literature on the topic of comprehensive reviews of autoimmune diseases of the head and neck. In this review of autoimmune conditions which affect the orbit and head and neck, we discuss the status of neuroimaging along with commonly recognized clinical and biological parameters and MRI features of the disease processes that could help suggest a specific diagnosis (Table 1: Categorization of the H&N Autoimmune Diseases).
Table 1.
Categorization of the H&N autoimmune diseases.
| I. Orbit | I–A: Optic neuritis in NMO-spectrum disorders
and MOG- associated disease I–B: Orbital involvement in multiple sclerosis I–C: Tolosa-Hunt syndrome I–D: Orbital inflammatory pseudotumor I–E: Uveitis I–F: Optic perineuritis I–G: Lacrimal gland inflammation I–H: Nasolacrimal duct |
| II: Hypophysis, Cavernous Sinus, and Sella | II–A: Autoimmune hypophysitis II–B: IgG-4-related hypophysitis II–C: Immune check point inhibitors hypophysitis II–D: Secondary cause of hypophysitis II–D1: Sarcoidosis II–D2: Granulomatosis with polyangiitis II–D3: Tolosa-Hunt |
| III: Audiovestibular | III–A: Cogan syndrome III–B: Autoimmune inner ear disease III–C: Susac syndrome III-D: Vogt-Koyanagi-Harada III–E: Meniere’s disease |
| IV: Lymph nodes and salivary glands | IV–A: Castleman disease IV–B: Sjögren syndrome IV–C: Chronic sclerosing adenitis (Kuttner’s tumor) IV–D: Mikulicz |
| V: Thyroid | V–A: Graves’ disease V–B: Hashimoto thyroiditis V–C: Riedel thyroiditis V–D: Postpartum thyroiditis |
| VI: Large vessel vasculitis | VI–A: Takayasu arteritis VI–B: Giant cell arteritis VI–C: Paraneoplastic arteritis |
| VII: Miscellaneous | VII–A: Granulomatosis with polyangiitis
(Wegner’s Granulomatosis) VII–B: Sarcoidosis VII–C: Relapsing polychondritis VII–D: Eosinophilic granulomatous with polyangiitis |
ORBIT
Optic neuritis in Neuromyelitis Optica Spectrum Disorders (NMOSD) and Myelin Oligodendrocyte Glycoprotein Antibody Associated Disease (MOGAD)
NMOSD and MOGAD are autoimmune inflammatory demyelinating disorders of the brain and spinal cord. They dominantly involve the optic nerve (optic neuritis, bilateral or rapidly sequential) and spinal cord (myelitis) with less extensive involvement of the brain compared to multiple sclerosis (MS), though cerebral involvement is seen in all of these conditions. 1 Pathogenic anti-aquaporin-4 antibodies are found in the majority of NMOSD patients and MOGAD is associated with anti-myelin oligodendrocyte glycoprotein antibody. Despite common clinical features, differentiation from each other and from MS is crucial for clinical management. 2 NMOSD patients have more severe disease with multiple relapses. Transverse myelitis and optic neuritis are the most common neurological involvement in both NMOSD and MOGAD. Both are slightly more frequent in females. However, MOGAD has a better long-term prognosis 2 (Table 2: ON in NMSOD vs MOGAD).
Table 2.
ON in NMSOD versus MOGAD.
| NMOSD | MOGAD |
|---|---|
| • Bilateral or rapidly sequential | • More commonly bilateral |
| • Long segment, less swelling | • Short or long segment, enlarged edematous optic nerves |
| • Posterior segment (intracranial optic nerve, optic chiasm [OC], and retrochiasmatic optic pathway [RCOP]) | • Anterior segment (spares OC and RCOP) |
| • Other: *More involvement of area postrema and medulla oblongata | • Perineural sheath and retro-orbital fat enhancement more than NMOSD |
| • LETM—commonly involves cervical and thoracic spinal cord | • Other: *Lumbosacral myelitis, conus medullaris involvement, and meningeal enhancement |
NMOSD
Magnetic Resonance Imaging (MRI) features of optic neuritis (ON) in NMOSD are long segment involvement of optic nerve with milder swelling compared to MOGAD. It is severe, most commonly bilateral with more involvement of posterior pathway including the intracranial portion of optic nerve, optic chiasm, and retro chiasmatic optic pathway.3,4 There is also more involvement of area postrema and medulla oblongata compared to MS. 3 The acute phase of ON presents with swelling, increased T2 and FLAIR signal, and associated abnormal enhancement of the optic nerves on MRI (Figure 1A and B). In the chronic phase of ON, there is long segment atrophy with abnormal increased signal of optic nerves on T2-weighted images. In NMOSD, the spinal cord involvement is classically longitudinally extensive transverse myelitis (LETM) which presents with ≥3 vertebral segments involvement on imaging. It could be located centrally or peripherally with more than 50% of the cord involved.5,6 It commonly involves cervical and thoracic spinal cord and shows more enhancement and edema compared to MOGAD.3,4,7
Figure 1.
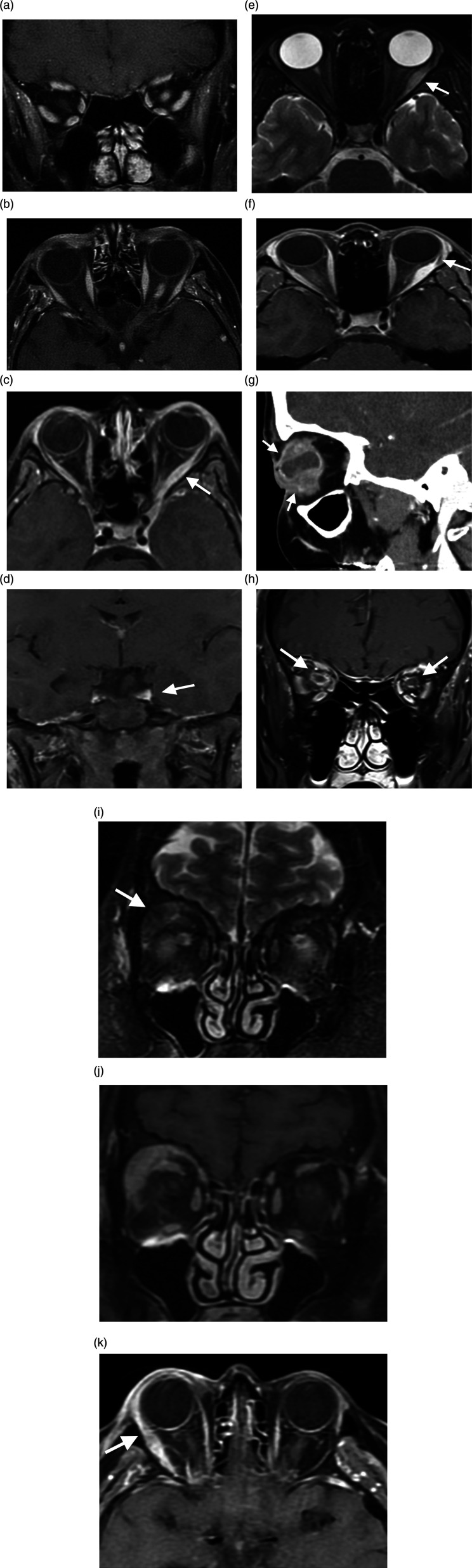
The Orbit. (A, B). Neuromyelitis optica. Mild enhancement and edema in the left retrobulbar optic nerve on coronal and axial contrast-enhanced T1WI (A, B). The Orbit. (C, D) Tolosa-Hunt Syndrome. Axial (C) and coronal (D) contrast-enhanced T1WI demonstrates left lateral rectus muscle enlargement and enhancement (arrow, C) which extends to the orbital apex and cavernous sinus (arrow, D). The Orbit. (E, F) Orbital inflammatory pseudotumor. Enlarged and edematous left lateral rectus muscle on axial T2WI (arrow, E) demonstrates homogenous enhancement which spares the insertion on axial contrast-enhanced T1WI (arrow, F). The Orbit. (G) Uveitis. Sagittal contrast-enhanced CT soft tissue window image of the face demonstrates globe irregularity, marked irregular thickening/enhancement of the uvea and sclera (arrows), inhomogeneous vitreous density, and globe rupture with a small air focus (not shown). The Orbit. (H) Optic perineuritis. Coronal contrast-enhanced T1WI shows right greater than left retro-orbital optic nerve sheath enhancement (arrows). The Orbit. (I–K) Lacrimal gland inflammation. Coronal T2WI demonstrates right lacrimal gland enlargement (arrow, I), which enhances on coronal (J) and axial (K) contrast-enhanced fat sat T1WI; adjacent periorbital stranding and enhancement of the right lateral rectus muscle (arrow, K) related to local inflammation.
MOGAD
MRI of ON in MOGAD illustrates enlarged, edematous tortuous optic nerve with short or long segment signal abnormality on T2-weighted images. It is more commonly bilateral with less clinical relapse and better prognosis. Optic nerve swelling and retrobulbar involvement are more common in this entity. Findings are more predominant in the anterior segment of optic nerve and usually spare the optic chiasm and retro chiasmatic pathway. Enhancement of perioptic nerve sheath and surrounding orbital fat is present in more than half of patients with MOGAD and less likely in NMOSD. Lumbosacral myelitis, conus medullaris involvement, and meningeal enhancement are also more common in MOGAD.1,5,7–9
NMOSD have greater reduction in apparent diffusion coefficient (ADC) values and more reduced diffusion than MS. 9
Orbital involvement in Multiple sclerosis (MS)
MS is an inflammatory demyelinating disease of the central nervous system and spinal cord with a typical onset in the third or fourth decade of life and a strong female predominance (>2:1).10–12 ON is one of the most common first presentations of MS. 13 Typical optic neuritis is defined as idiopathic demyelinating disease related to MS, while atypical optic neuritis is due to other inflammatory, infective, or other autoimmune disease. In MS, optic neuritis is typically unilateral, painful, and more common in women with average age of 32 years at presentation. It shows anterior optic nerve enhancement with normal optic disc and no enhancement in the optic nerve sheet. 14 Features of atypical ON in MS are bilateral, painless, and extensive ON with optic nerve sheath enhancement in a male patient. Presence of chiasm involvement and diffusion restriction is more in favor of atypical ON. Atypical ON is also seen in NMOSD, MOGAD, sarcoidosis, Sjogren syndrome, granulomatosis with polyangiitis, systemic lupus erythematous (SLE), antiphospholipid antibody syndrome, and primary CNS vasculitis.1,14
On MRI, the ON is seen as swollen and high signal of optic nerve on T2-weighted sequences. This is more apparent in orbital segment of nerve and enhances with contrast in more than 90% of cases, which persists for 3 weeks11,13 Orbital MRI should be acquired with high special resolution and rapid sequences due to small caliber optic nerve and ocular motion. Routine orbit MRI protocol includes T1, T2 fat sat and T1 fat sat after Gadolinium (Gd) administration. Axial images best demonstrate orbital apex and optic canal while coronal images best depict optic nerve, optic chiasm, and optic tract. In patients with dental hardware, fat suppressed images show more artifact, and short tau inversion recovery (STIR) images could be helpful but with lower speed and more motion artifact. 11 There are also promising MRI sequences for the future use. For example: fat-suppressed T1-weighted 3D radial gradient-recalled echo-volumetric interpolated breath hold examination (Radial VIBE) or Half-Fourier acquisition single-shot turbo spin echo (HASTE). 3 Tesla MRI scanner theoretically increases the signal to noise ratio (SNR), but the indication is not always as simple as it seems and has some limitations. 11
Tolosa-Hunt Syndrome
Tolosa-Hunt Syndrome is a rare autoimmune disease which presents with nonspecific chronic inflammation of wall and septa of the cavernous sinus, superior orbital fissure, and orbit. 15 Typically, clinical signs and symptoms involve unilateral orbital pain, ipsilateral cranial nerve palsies (III, IV, VI), and granulomatous inflammation of cavernous sinus. Pain often subsides within 72 hours after treatment of glucocorticoids,16,17 the mainstay of treatment for decades, whereas the resolution of cranial nerve paresis is more variable.15,18,19 Granulomatosis is shown on MRI or biopsy. 18 It is typically considered a benign condition, but it may involve the optic nerve and cause blindness. 18 Some authors consider pseudotumor of orbit and Tolosa Hunt Syndrome identical entities but in different anatomic locations. 16 Tolosa-Hunt Syndrome has more retro-orbital location and orbital inflammatory pseudotumor is more intra-orbital location.16,20 Differential diagnoses of painful ophthalmoplegia are inflammatory conditions (Tolosa-Hunt Syndrome, orbital pseudotumor, and sarcoidosis), tumors, vascular, infections, diabetes, migraine, or giant cell arteritis. 16
On MRI, Tolosa-Hunt Syndrome shows abnormal unilateral enlargement of cavernous sinus with associated abnormal enhancement, extends via the superior orbital fissure to the orbital apex (Figure 1C and D). There is a wide range of signal abnormalities reported in Tolosa-Hunt syndrome (T1 Iso-hyper-signal, T2 hypo- iso- or hypersignal).17,21 There are reports of reversible vascular abnormalities of internal carotid artery on CT or MR angiography (focal narrowing of intra-cavernous segment) related to Tolosa-Hunt syndrome after treatment with steroids. 22 High resolution CT (HRCT) may also show soft tissue changes but with low sensitivity.17,23 The lesion will be disappeared or diminished in 1 week to 1 year on follow-up MRI. 21
Orbital Inflammatory Pseudotumor
Orbital inflammatory pseudotumor is a benign, space occupying inflammatory lesion of orbit which may extend to the periorbital area. The mean age of orbital inflammatory pseudotumor is from the third to sixth decade and has a female predominance. 24 Orbital inflammatory pseudotumor is categorized into subgroups depending on the site of involvement: lacrimal or dacryoadenitis (most common), anterior (in the fat immediately behind the globe), posterior (in the fat at orbital apex), diffuse, myositis, and optic perineuritis.24,25 The most common muscle involved is the medial rectus followed by superior, lateral, and inferior recti muscles. Response to corticosteroid is typically within 24–48 hours. 24 However, lymphoma may also respond well to steroids and may have similar appearance on MRI, albeit, with less acute course in older patients.24,25 Other differential diagnoses are orbital cellulitis, sarcoidosis, granulomatosis with polyangiitis, thyroid eye disease (thyroid ophthalmopathy), and Tolosa-Hunt syndrome. 26
Orbital ultrasound, CT scan, and MRI are helpful for diagnosis of orbital inflammatory pseudotumor when combined with clinical data and response to treatment. On MRI, orbital inflammatory pseudotumor is hypointense in T1 and T2 sequences with marked enhancement and no bony destruction. CT scan shows muscle enlargement of extraocular muscles including tendons (Figure 1E and F). There are also inflammatory changes throughout involved areas. Involvement of tendinous insertion and irregular contour of muscle help to differentiate it from thyroid ophthalmopathy.24,26 Pseudotumor may present as an enhancing mass in dacryoadenitis, diffuse ill-defined enhancing retrobulbar mass involving fascial planes, or thickening of muscle and tendons with enhancement. On the contrary, Tolosa-Hunt syndrome mainly involves the orbital apex with extension to cavernous sinus.25,26 There is some literature that shows promise in differentiating lymphoma from inflammatory conditions. Intravoxel incoherent motion diffusion-weighted MRI (IVIM-DWI) in comparison with DWI alone is a more valuable method to differentiate orbital inflammatory tumor from orbital lymphoma. IVIM-DWI parameters such as true diffusion coefficient (D) and perfusion fraction (f) as well as mean ADC values are significantly lower in lymphoma.27,28
Uveitis
Uveitis could be idiopathic or due to systemic inflammatory diseases such as ankylosing spondylitis, sarcoidosis, Behcet’s disease, ulcerative colitis, relapsing polychondritis, RA, Vogt-Koyanagi-Harada disease, and MS.29–31 The diagnosis of uveitis is clinical with the help of non-radiologic imaging. However, MRI is useful for evaluating and detecting pathologies of uveal tract. The uvea is a highly vascularized coating tissue which enhances normally and cannot be differentiated from the retina unless it has been detached. 31
On MRI, the normal uveal tract is difficult to separate from bright signal of the vitreous on T2 and it is demonstrated as a very thin, slightly hyperintense layer on T1. The sclera is composed of fibrous tissue and hypointense in both T1 and T2. 32 Due to normal enhancement of the uvea, comparison with a normal contralateral eye is helpful. MRI findings include enhancement and thickening of the involved uvea. In panuveitis, MRI shows thickening and enhancement of the uvea and marked hyperintensity of vitreous (Figure 1G). Vogt-Koyanagi-Harada disease KH demonstrates the same changes on MRI. 31 There is also detached retina on T2 sequences with diffusion restriction within the uvea. In anterior uveitis, enhancement and thickening of iris ciliary body with local enhanced vitreous humor are noted. 31 In Relapsing Polychondritis, enhancement of uveoscleral coating is seen with calcification of posterior pole. 31
Optic perineuritis
Optic perineuritis is a form of idiopathic orbital inflammatory disease. It can be idiopathic (primary) or part of a systemic inflammatory disease (secondary). It affects the unilateral optic nerve sheath rather than optic nerve and is more common in middle-aged women. 33
On MRI, Optic perineuritis reveals a typical appearance of circumferential optic nerve sheath enhancement on coronal fat-saturated T1-weighted images (Figure 1H). On axial images, this enhancement appears as “a tram track sign” which can be also seen in other etiologies of perineural involvement such as meningioma, leukemia, orbital pseudotumor, metastases, and even perioptic hemorrhage. 33 Optic nerve sheath enhancement is also seen in MS, but it is always accompanied by optic nerve enhancement. 33 It is important to recognize normal enhancement of the dural layer on MRI from abnormal enhancement. Secondary Optic perineuritis has been reported in sarcoidosis, Behcet’s, Granulomatosis with polyangiitis, and Crohn’s disease. 33
Lacrimal gland inflammation
Inflammation of the lacrimal gland is called dacryoadenitis. 34 Specific cause includes Sjogren syndrome, sarcoidosis, IgG4-related disease, granulomatosis with polyangiitis (GPA), Churg-Strauss syndrome, and other systemic autoimmune inflammatory conditions. Clinical and radiologic signs are non-specific and there can be considerable overlap of symptoms with neoplastic disease such as lymphoma. 35
In sarcoidosis, the most involved orbital tissue is the lacrimal gland, and 50% of orbital sarcoidosis present with dacryoadenitis without previous sign of sarcoidosis. 35 It presents most commonly with isolated painless enlargement of lacrimal gland. Involvement of optic nerve, ocular fat, and extraocular muscle may be seen.35–38
IgG4-related disease is a generalized fibroinflammatory disease. It has been characterized by IgG4-positive plasma cell infiltration. Female-to-male ratio is variably reported, and the mean age of involvement is 50 years. 39 The lacrimal gland is the most common type of ocular involvement. 40 On imaging, bilateral enlargement of the lacrimal glands is seen. Other findings are enlargement of extra ocular muscles, especially lateral rectus, 41 infiltration of orbital fat, enlargement of infraorbital nerve, 41 and less commonly, involvement of cavernous sinus and Meckel cave.35,42 The main differential diagnoses are Sjögren syndrome, sarcoidosis, lymphoma, orbital inflammatory pseudotumor, and thyroid-related orbitopathy. 35
Granulomatosis with polyangiitis is an autoimmune small vessel vasculitis. Ocular involvement is seen in 50% of cases. 43 Commonly, there is diffuse involvement of orbit with extension to the lacrimal gland. 43 The most common ophthalmic manifestations are orbital masses, episcleritis, scleritis, and conjunctivitis. 43 It is usually bilateral and involves a wide range of ages from 12 to 80 years. 35 Sinus and upper respiratory tract involvement is widespread. Isolated lacrimal involvement is rare. The affected lacrimal gland typically presents with acute signs of dacryoadenitis. CT may demonstrate a diffuse inflammatory mass molded to orbit, proptosis, and involvement of paranasal sinus. On MRI, granulomas have hypointense on T2-weighted images.24,35,43,44
On MRI, a normal lacrimal gland has intermediate signal intensity and on CT scan it is isodense with muscle. It also enhances symmetrically. 34 MRI is the investigation of choice for dacryoadenitis and demonstrates enlargement of the gland with moderate isointense enhancement, with or without lateral rectus enhancement. Inflammatory changes of adjacent fat may be seen (Figure 1I, 1J, 1K). There is no bony change on CT except for chronic sclerosing variety.34,35
Nasolacrimal duct disease
Acquired nasolacrimal duct disease obstruction can be primary or secondary. Acute or chronic dacryocystitis is seen following the obstruction. Primary obstruction seems to be associated with infections. 45 Causes of secondary Nasolacrimal duct disease obstruction include inflammatory disease such as sarcoidosis, granulomatosis with polyangiitis, Sjögren disease, orbital inflammatory pseudotumor, and IgG4-related disease.46–48 Most nasolacrimal lesions present with high T2 signal intensity, so low T2 signal with bone erosion suggests granulomatosis with polyangiitis. 49
Imaging of the nasolacrimal drainage system includes MRI, CT, ultrasound, and nuclear scintigraphy. CT is the initial imaging of choice and shows enlargement of the lacrimal sac with pressure effect on orbital structure. 50 MRI has the advantage of showing the lesion extension to the adjacent structures such as the orbital fat and differentiation of cystic and solid components of nasolacrimal drainage system lesions. Contrast is used for better evaluation. 50
Hypophysis, cavernous sinus, and Sella
Autoimmune Hypophysitis
Inflammatory lesions of the pituitary (hypophysitis) are divided into primary and secondary groups. Primary hypophysitis refers to inflammation confined to hypophysis. Primary hypophysitis is differentiated into lymphocytic types (88%) and granulomatous types. The most common causes of the secondary groups are Rathke’s cleft cysts (48%) followed by tumors, adenomas, infections, previous operations, and systemic disorders such as sarcoidosis and IgG4-related disease. 51 Differential diagnosis should include pituitary adenoma in adults and Langerhans cell histiocytosis and germinoma in adolescents.49–51 Lymphocytic types are more frequent in women and often associate with late pregnancy and postpartum hypophysitis. 53
Hypophysitis may present with anterior or posterior hypophysis symptoms. Diagnosis of hypophysitis is based on clinical data, radiology, and circulating antibodies. MRI with contrast is the investigation of choice. Lymphocytic type should be differentiated from non-functioning adenoma. Subacute lymphocytic type is more common in pregnancy or postpartum. In this situation, it presents with signs and symptoms of mass which resolve gradually. MRI demonstrates enlargement of the pituitary gland with uniform enhancement.49,51 Chronic cases may present with pituitary gland atrophy and empty Sella making diagnosis difficult.49,51
On MRI, Lymphocytic type is seen as a T1 isointense mass in shape of dumbbell or triangle. It has often suprasellar extension. 53 In posterior lobe hypophysitis, MRI features are loss of normal posterior hypophyseal high signal in precontrast images with alteration of enhancement pattern due to vascular alterations and pituitary stalk thickening. 53 Features leading to diagnosis of lymphocytic hypophysitis versus adenoma include no mass effect, rapid enhancement, symmetric enlargement, intact seller floor, and rare cystic change as well as dural enhancement. The diagnosis is supported with absence of bright spots of neurohypophysis on T1 in those with DI.50–52 In an adenoma, there is cystic change, contralateral deviation of stalk, and presence of bright spot of neurohypophysis. 51
IgG4-related hypophysitis
This is a rare disease due to a lymphoplasmacytic IgG4 positive infiltration of the pituitary gland. It is more common in Japan and Korea and has male predominance at the 7th decade of life.50,53
On MRI, there is T2 heterogenous hyperintense signal of hypophysis and sometimes hyperintense signal of the chiasm due to edema. 54 Post-contrast enhancement is inconsistent depending on dose and timing of contrast injection. 54 The thickening and enhancement of the perisellar dura is almost a constant feature. 54 Some specific signs are also described in the literature, for instance: pachymeningitis and thickening of pachymeninges (T2 hypo signal intensity) or elephant face appearance on post-contrast coronal MRI images (enlarged pituitary gland and cavernous sinuses with stenosis of internal carotid arteries. 53
Immune checkpoint inhibitor hypophysitis
Immune checkpoint inhibitors are types of monoclonal antibodies for malignancy treatment. Male-to-female ratio of patients using ICIs is 4:1, the and mean age of onset is 60 years.52,54 Two classes of ICIs currently FDA approved are anti-CTLA4 and anti-PD1/PDL1. Hypophysitis can be diagnosed with low levels of TSH, ACTH, FSH/LH, and hypothalamic hormone and supportive evidence on MRI. 55 MRI shows hypophysitis in 77% of patients. In 50%, MRI abnormalities may precede the clinical symptoms. 52 18F-FDG PET for staging of underlying malignancy may show the intense radiotracer uptake. Contrast enhanced MRI is needed in order to exclude metastasis in these patients.52,54,55
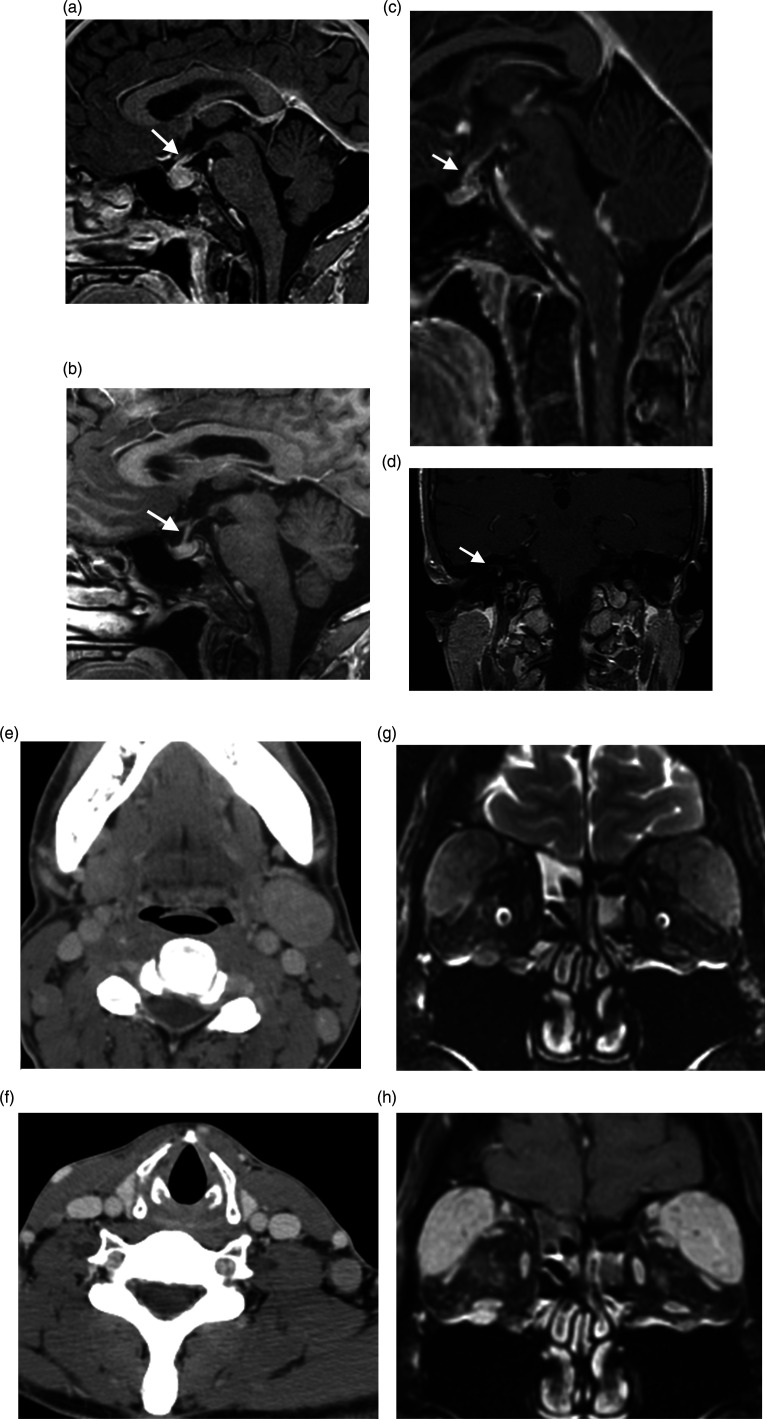
MRI findings include diffuse, mild-to-moderate enlargement of the pituitary gland, (up to 60–100%), homogenous or heterogenous mild-to-moderate enhancement, thick pituitary stalk without deviation (Figure 2A and B), and enhanced dural tail.52,55 Mass effect is not common. 52 The posterior pituitary is preserved. MRI shows decreasing gland size with subsequent atrophy after 4–12 weeks following withdrawal and treatment.52,54,55
Figure 2.
Miscellaneous Head and Neck Diseases. (A, B) Immune checkpoint inhibitor hypophysitis. Sagittal contrast enhanced T1WI demonstrates pituitary gland enlargement and infundibular thickening (arrow, A) in a patient on immunologic therapy for melanoma (ipilimumab and nivolumab), which resolved following medication cessation (arrow, B). Miscellaneous Head and Neck Diseases. (C) Neurosarcoidosis. Sagittal contrast-enhanced fat sat T1WI shows nodular thickening and enhancement of the brainstem leptomeninges and infundibulum (arrow). Miscellaneous Head and Neck Diseases. (D) Cogan Syndrome. Right inner ear saccule enhancement on coronal contrast enhanced T1WI (arrow). Miscellaneous Head and Neck Diseases. (E–H) Castleman Disease. Axial contrast-enhanced neck CT shows cervical lymphadenopathy at the level II (E) and V (F) stations. In a separate patient, lacrimal gland enlargement and enhancement on coronal T2WI (G) and coronal contrast-enhanced T1WI (H), causing exophthalmos. Also note infraorbital nerve enhancement.
Some secondary causes of hypophysitis
Sarcoidosis
The central nervous system involvement is about 5–15% in sarcoidosis, and it affects the infundibular stalk and the hypothalamus in 0.5%. 52 Male preponderance is reported with a mean age of 30–40 years.52,56,57 MRI findings are plaque-like or nodular thickening in the infundibular stalk (Figure 2C) and optic chiasm. 58 Lesions may be T1 iso- and T2 hypo-signal with enhancement on post-contrast images. MRI findings can disappear or improve, but endocrine abnormalities are permanent. 59
Granulomatosis with polyangiitis (GPA, Old name Wegner Granulomatosis)
Granulomatosis with polyangiitis is a small size vessel vasculitis characterized by necrotizing noncaseating granulomatous formation in upper and lower respiratory tract and involvement of kidneys. 60 73–99% of granulomatosis with polyangiitis cases present with head and neck symptoms 61 which may be misdiagnosed with infective diseases and allergies. 60 More than 90% of patients are white. 60 It is more common in the 40–50-years-old people. Male-to-female ratio is 1:1 in the United States.58–60
Pituitary involvement is a late manifestation of granulomatosis with polyangiitis. 52 MRI findings are a thick stalk, diffuse enlargement of the pituitary gland, and normal to mildly enlarged sella with enhancement of optic chiasma. 62 There is also involvement of the paranasal sinuses. The differential diagnosis on MRI includes an aggressive neoplasm and infection.52,63
Tolosa-Hunt syndrome
Tolosa-Hunt syndrome is described in greater detail in section 1C of this paper. However, pituitary involvement has symptoms of hypopituitarism.
Audiovestibular
Cogan syndrome
Cogan syndrome is a rare autoimmune vasculitis and more common in young adults. 64 It is characterized by inflammatory ocular manifestations and audiovestibular dysfunction. Audiovestibular symptoms present similarly to Meniere’s disease. 64 The immunologic basis of disease is due to presence of autoantibodies against inner ear, corneal, and endothelial antigens.64–66
MRI and CT show narrowing or obliteration of the vestibular labyrinth in T2-weighted images. Contrast-enhanced T1-weighted imagining shows enhancement of membranous labyrinth (Figure 2D). 66 Infarction and ischemic changes, cranial neuropathy, cerebral venous sinus thrombosis, and meningoencephalitis are other findings on imaging. 66
Autoimmune inner ear disease
Autoimmune inner ear disease is a bilateral sensorineural hearing loss (SNHL) caused by an uncontrolled response of the immune system. Autoimmune inner ear disease is a progressive bilateral asymmetric SNHL disease. It is more common in women. 67 There is no standard diagnostic test. Clinical history, antibodies, or activated T-cells against inner ear antigen and favorable response to immunosuppression are helpful for diagnosis. 68 Three-dimensional MRI with intratympanic contrast injection demonstrates endolymphatic hydrops in primary autoimmune inner ear disease. 67
Susac syndrome
Susac syndrome is a rare autoimmune-mediated disease. It usually affects young or middle-aged women with the clinical triad of acute or subacute encephalopathy, bilateral sensorineural hearing impairment, and branch retinal artery occlusions.69,70 The definite diagnosis is dependent on fulfilling all three criteria. Although it mimics MS as a differential diagnosis, demyelination is not characteristic of Susac syndrome.69,70
On MRI, Leptomeningeal enhancement, and lesions in basal ganglia, frequently occurs and can be a clue to diagnosis. 71 Fluorescein angiography is used for diagnosis of retinal artery branch occlusion. 72 The main differential diagnoses are demyelinating conditions (MS, ADEM, and MOGAD), infarction (vasculitis and thromboembolic disease), and infection. Round T2 lesions, central location, and leptomeningeal enhancement help for differentiation from MS and ADEM. 73 Brian parenchymal findings of Susac have been described elsewhere—Neuroradiol J.2021 Sep 7; 19,714,009,211,042,879. Doi: 10.1177/19714009211042879. Online ahead of print. Autoimmune disease of the brain, imaging and clinical review.
Vogt-Koyanagi-Harada disease
Vogt-Koyanagi-Harada disease is a rare multisystemic autoimmune disease which affects melanin-containing organs and tissue like the inner ear, eye, meninges, and skin. It is more common in people with darker skin. Asians, Hispanics, and native Americans are affected more frequently. This disease is more common in women aged 20–50. 74 Clinically it is characterized by bilateral panuveitis, hypoacusis, and meningitis and skin involvement.74–76
On MRI, a few cases reports have shown abnormal enhancement of cochlea and vestibule with the same pattern as in acute otitis media. Gd-enhanced 3D FLAIR shows the changes better than Gd-enhanced 3D T1 images. 76
Meniere’s disease
Meniere’s disease is a chronic condition with recurrent attacks of vertigo, tinnitus, and sensorineural hearing loss. Its cause is not clear, but there is excessive fluid in the endolymphatic spaces of the inner ear. This is more common at the age of 30–70. 77 Proteomic analyses in blood have recently shown increased expression of some protein biomarkers such as complement factors H and B, and reduction of the plasma beta-2-glycoprotein and vitamin D binding protein. 78 There is no definitive test for diagnosis. The endolymphatic hydrops is the structural abnormality of Meniere’s disease, which is distention of endolymphatic compartment. 79
MRI imaging after 4 hours of IV contrast injection with a 3 Tesla scanner is helpful. Previous technique, intratympanic contrast injection, is invasive. MRI demonstrates endolymphatic hydrops. This is characterized by distention of cochlear and vestibular endolymph areas (increase >33–50% of vestibular space) and diminishing peri-lymphatic areas. However, these current imaging techniques are insufficient to diagnose Meniere’s disease.79,80
Lymph node and Salivary glands
Castleman disease
Castleman disease is an uncommon benign lymphoproliferative disease and presents with enlarged lymph nodes. There are two types of Castleman disease histologically, hyalin vascular variant and plasma cell variant. It is also divided into unicentric and multicentric types. 81 The range of patient age in unicentric Castleman disease is 10–56 years. Unicentric is more frequent and is mostly hyalin vascular variant type. 82 It is most commonly present in thoracic areas followed by the abdomen and neck. Multicentric Castleman disease is more difficult to be differentiated from lymphoma; avid contrast enhancement helps in differentiation. 83 Multicentric disease is associated with plasma variant histologic type, human herpes virus 8 infection, HIV, and other autoimmune diseases.81–83
On CT scan, solitary or multiple homogenous mild hypodense lymph nodes are seen. Lymph nodes are well defined and lobulated showing moderate to avid enhancement with contrast (Figure 2E and F). On MRI, there is mild hypo to slightly hyper T1 signal intensity as well as rapid, avid enhancement (Figure 2G and H). T2-weighted images show iso to hypersignal intensity relative to skeletal muscle. DWI shows hypersignal intensity.79,81 On double phase CT scan, mild nonhomogeneous enhancement in arterial phase and gradual uniform enhancement in arteriovenous phase could be a clue for differentiation from other diseases.81,84 In FDG-PET, avid uptake is noted which also helps to differentiate it from the goiter continuous with thyroid tissue or ectopic goiter which rarely have avid enhancement. Lymphoma, thyroid lesions, and metastasis are other differential diagnoses.81,84
Sjögren syndrome
Sjögren syndrome is an autoimmune disease of exocrine glands. Clinical findings include dry eyes and mouth, with painful enlarged parotid glands. It is the second most common autoimmune disorder after rheumatoid arthritis. Peak incidence is at the age of 50. Female-to-male ratio is 9:1. 85 Sjögren syndrome is classified to primary and secondary types. Secondary Sjögren syndrome is associated with another systemic disease such as SLE, RA, and dermatomyositis.85–87 Risk of lymphoma in primary Sjögren syndrome is about 5–10%. 88
Ultrasound may show multiple hypoechoic or anechoic areas in the parotid and submandibular glands, sometimes with hypervascularity. 86 On MRI, there is enlargement of the parotid gland with cystic change on T2. 89 On T1, there are characteristic findings in the parotid gland including high signal intensity of fat tissue, which can be confirmed by fat suppression techniques (STIR, fat suppression). Fat deposition correlates well with the impaired salivary flow in Sjögren syndrome patients. 90 On MRI of lacrimal gland, ADCs are lower than normal which suggests measuring ADCs as a tool to assess the lacrimal gland in Sjögren syndrome. 91
Chronic sclerosing sialadenitis (Küttner tumor)
Chronic sclerosing sialadenitis is a fibroinflammatory disease of mainly the submandibular gland. In some studies, it is considered part of IgG4-related disease. It produces a hard swelling of submandibular gland and may mimic neoplasia. 92
MRI shows intermediate T1 signal intensity with homogenous enhancement and low-to-intermediate signal intensity on T2. On CT, it is homogenously attenuated. On unilateral lesions malignancy is in the differential diagnosis and in bilateral involvement lymphoma and Sjogren are differentials. 92
Mikulicz syndrome
This disease is characterized by painless idiopathic swelling of salivary glands. It was previously considered part of Sjögren disease; however, it is now classified under IgG4 disease. 39
CT shows homogenous attenuation and enhancement. On MRI, it is homogenous, low-to-intermediate T2 signal intensity, and low signal on T1 sequence with homogeneous enhancement. Viral infections such as mump, Sjögren syndrome, sarcoidosis, and lymphoma are differential diagnosis. 39
Thyroid
Graves’ disease
Graves’ disease is an autoimmune disorder of thyroid due to autoantibodies causing activation of thyroid stimulating hormone receptor. Incidence of graves’ ophthalmopathy is more common in women. Graves’ disease presents with hyperthyroidism as well as ophthalmopathy, acropachy, and localized dermopathy. The thyroid is moderately enlarged diffusely. Ophthalmic signs are proptosis, lid retraction, double vision, Chemosis, and periorbital edema. Most common dermopathy is peritibial dermopathy. Acropachy occurs in form of finger or toe clubbing.93,94
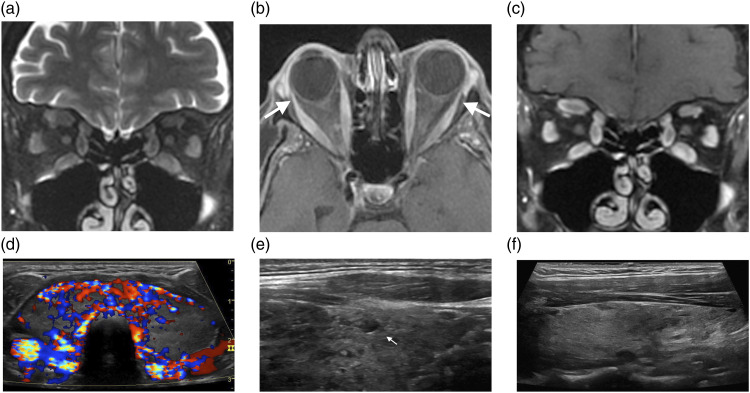
MRI demonstrates enlargement of extraocular muscles belly with tendon insertion sparing. Classically the inferior rectus is the first involved muscle, followed by medical, superior, and lateral rectus muscles (Figure 3A–3C). Involvement is bilateral in 90% of cases. Other orbital manifestations are orbital fat expansion, engorgement of superior ophthalmic vein, and displacement of lacrimal gland. 95 Ultrasound findings in diffuse thyroid disease are characterized by a diffuse enlarged thyroid or a small thyroid in later stages of disease. There is increased or decreased parenchymal echogenicity and coarse echotexture. Thyroid inferno is defined as increased color flow in active Graves’ disease due to shunting and increased vascularity of thyroid (Figure 3D). No definite clinical indication is available for elastography in diffuse thyroid disease.95,96
Figure 3.
The Thyroid. (A–D) Graves ophthalmopathy. Coronal T2WI (A) shows diffuse enlargement and edema of the extraocular muscles, which enhance on axial (B) and coronal (C) contrast-enhanced T1WI; mild proptosis and relative sparing of the orbital muscular insertions (arrows, B), consistent with Graves' ophthalmopathy. On transverse color Doppler sonography (D), the gland is diffusely enlarged and hyperemic. The Thyroid. (E) Hashimoto’s thyroiditis. Longitudinal grayscale sonographic image shows glandular enlargement and heterogenous echogenicity; note a small hypoechoic nodule surrounded by echogenic fibrous septa (arrow, E). The Thyroid. (F) Postpartum thyroiditis. Longitudinal grayscale sonographic image shows diffuse hypoechoic glandular parenchyma.
Hashimoto thyroiditis
Hashimoto thyroiditis is the most common autoimmune disease of the thyroid and the most common cause of hypothyroidism. Primary Hashimoto thyroiditis is the most common type and comprises some subgroups like IgG4-related variants. 97 Women are at least 8 times more affected than men. It is also more common in whites and Asians than in African American. Secondary type is due to an etiologic cause. For example, following the treatment of hepatitis C with interferon alpha. Differential diagnosis is thyroid lymphoma and subacute granulomatous thyroiditis. 97
On ultrasound, the thyroid shows marked decrease of thyroid echogenicity and in IgG4-related Hashimoto thyroiditis this finding is more prominent (Figure 3E). Some authors suggest shear wave elastography to estimate the degrees of fibrosis. 98
Riedel thyroiditis
Riedel thyroiditis is a rare inflammatory disease of the thyroid with resultant fibrosis of the thyroid gland and hypothyroidism. The gland is hard and nontender. The etiology of Riedel thyroiditis is not clear. Possible theories include autoimmune disease, an IgG4-related disease, or part of a systemic fibrosing disorder. 99 It is more common in adult females between 30 and 50 years of age. Diagnosis is made by clinical evaluation and biopsy. In Riedel thyroiditis, fibrosis extends to adjacent soft tissue and cause obstructive symptoms in adjacent organs. Dyspnea, stridor (involvement of the recurrent laryngeal nerve), dysphagia, and venous thrombosis are seen due to extension of fibrosis. Differential diagnoses are anaplastic thyroid carcinoma, thyroid lymphoma, thyroid sarcoma, and fibrosing types of Hashimoto thyroiditis. 99
On ultrasound, it is a hypoechoic vascular mass invading and fibrosing adjacent structures and may even occasionally encase carotid arteries. On CT, it is a hypo to iso dense heterogenous mass with no enhancement in post-contrast images. PET shows intense uptake and can identify remote areas of inflammation and fibrosis.99,100 On MRI, it is hypointense on T1 and T2 images with minimal enhancement in contrast study.99–101
Postpartum Thyroiditis
Postpartum thyroiditis is an autoimmune thyroid disorder. Postpartum thyroiditis is defined as development of hypo or hyperthyroidism in the first year after pregnancy in a pregnant woman with no clinical history of previous thyroid disorder. It presents with hyperthyroidism followed by hypothyroidism. The condition is usually transient. Its differential diagnosis is Grave’s disease. Ultrasound demonstrates hypoechogenic thyroid (Figure 3F).102,103
Large vessel vasculitis
Takayasu arteritis
Takayasu arteritis is a chronic inflammatory granulomatous vasculitis involving large size arteries. The main involved arteries are the aorta and its main branches. Pulmonary artery may also be affected. It is more common in young women aged 20–40. Takayasu arteritis is also more common in Asian countries. 104 The thickening of arterial walls is the most characteristic early sign of the disease. Vessel inflammation leads to wall thickening and stenosis which lead to ischemia and thrombotic events. Diagnosis is based on imaging.104,105 Clinical manifestations are divided into the early phase, vascular phase, and the late phase. At the early phase, there are generalized symptoms like fever, fatigue, and weight loss. In the late phase, vascular symptoms dominate. These include claudication, angina, syncope, and visual symptoms.106,107
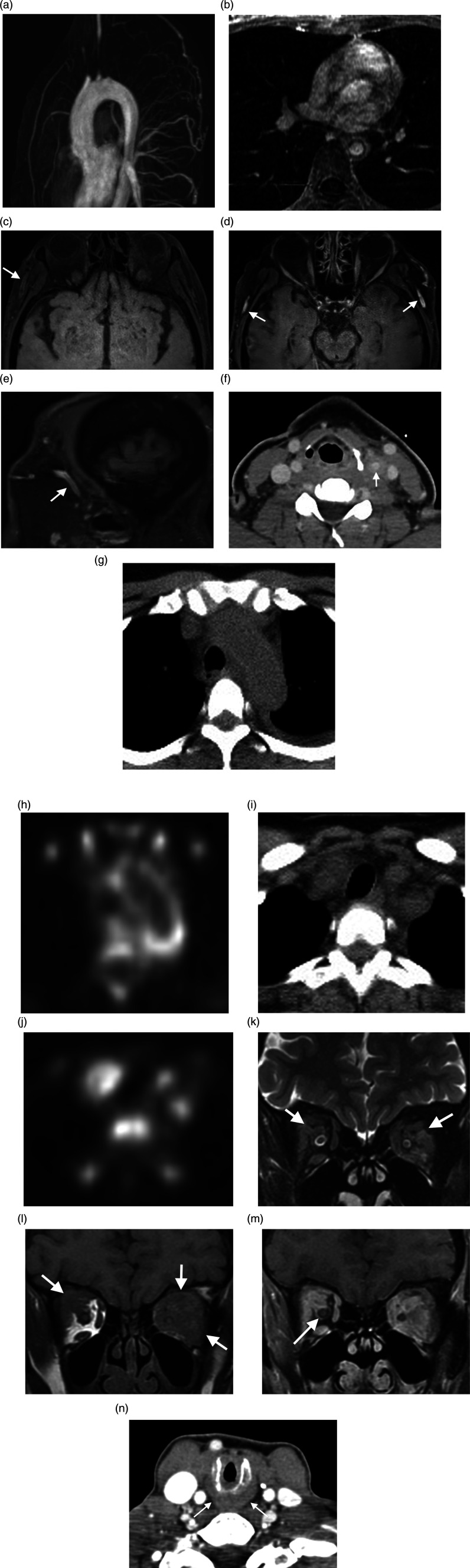
Imaging includes color Doppler ultrasound, CT, and MRI (including angiography). FDG-PET is a sensitive and effective method for detecting disease activity. Angiography is used for angioplasty and stent placement. It usually shows mild to severe long, smooth tapered stenosis. On ultrasound, circumferential homogenous thickening is seen indistinguishable from atherosclerosis. On CT, in the later stage of Takayasu arteritis, there is concentric narrowing with extensive vascular calcification.106,107 MRI in acute phase shows thickening of the vessel wall in the aorta or pulmonary artery which enhances with contrast on T1 images. Obstruction of aortic branches or pulmonary artery can be seen in the acute phase. In the later stage, the most common finding is stenosis which is typically seen in proximal segments of aortic branches (Figure 4A and B). Stenosis is most common in the common carotid and subclavian arteries. The second most common sign of late Takayasu arteritis is occlusion in which collateral vessels are seen. Renal artery occlusion is the most common occluded artery, causing hypertension.106,107 Aneurysms also happen in the late stage of disease. Aneurysm can be saccular or fusiform. 108 On MRI, brain atrophy and hypersignal intensity of white matter are also seen due to stenosis of aortic branches and small infarction, respectively. 106
Figure 4.
Vascular and Inflammatory Diseases. (A, B) Takayasu’s arteritis. MR angiography (A) and axial contrast-enhanced T1WI (B) show descending thoracic aorta circumferential wall thickening and enhancement, consistent with active inflammation. Severe narrowing with smooth tapering in the aortic arch branch vessel ostia. Vascular and Inflammatory Diseases. (C–E) Giant cell arteritis. Vessel wall axial pre-contrast fat sat T1WI demonstrates superficial temporal artery long segment symmetric mural thickening (arrow, C), with vessel wall enhancement on axial and sagittal contrast-enhanced sequences (arrow, D, E). Vascular and Inflammatory Diseases. (F–J) Paraneoplastic carotid arteritis. Axial contrast-enhanced neck CT shows left common carotid artery circumferential wall thickening and perivascular fat stranding (arrow, F). Axial PET/CT shows abnormal FDG avidity in the aortic arch (G, H) and branch vessels (I, J) related to paraneoplastic arteritis in the setting of myelodysplastic syndrome / myeloproliferative disorder. Vascular and Inflammatory Diseases. (K–N) Granulomatosis with polyangiitis (Wegener’s). Coronal T2WI (K) and coronal T1WI (L) show intra-orbital hypointense infiltrating mass replacing the intraconal fat (arrows), which avidly enhances on coronal contrast-enhanced fat sat T1WI (M). While inflammation is contiguous with the extraocular fat and extraocular muscles, the optic nerve sheath complex is preserved (arrow). Axial contrast-enhanced neck CT shows subglottic mucosal edema and wall thickening (arrow, N).
Giant cell arteritis (GCA)
GCA is a vasculitis of large and medium vessels. GCA predominately affects white populations, though it can be seen in all ethnic groups. 107 It occurs exclusively in people over 50. There is a predilection for women. GCA involves extracranial branches of the carotid arteries, specifically the temporal artery and other arteries such as aorta, axillary, femoral, and iliac arteries. Inflammation of arterial walls is the cause of vessel occlusion and symptoms of the patient.109,110 Jaw claudication is present due to involvement of the maxillary artery. The ophthalmic artery is the most critical vessel involved, which can cause temporary (30–50%) or permanent visual loss (14–20%). Non-ophthalmic manifestations are also present such as neuropathy, large vessels disease (decrease or absent pulse), vestibular dysfunction, and polymyalgia rheumatica.109–111 Complications are aortic aneurysm and dissection. 112 Differential diagnoses of GCA are Takayasu arteritis, arteriosclerosis, and thoracic outlet syndrome. 111 ESR and CRP are usually increased, but low values do not exclude the diagnosis. Temporal artery biopsy is the gold standard method of diagnosis. 113 Prompt diagnosis and treatment are essential. Imaging modalities are not usually performed for diagnosis, but they are helpful for diagnosis when required. 114
Color Doppler ultrasound shows characteristic halo sign (superficial temporal artery diffuse hypoechoic wall thickening) with high specificity especially if it is bilateral (100% specificity). Superficial temporal artery and its frontal and parietal branches should be evaluated bilaterally. Thickness persists even after compression (positive compression sign). 112 CT angiographies demonstrate attenuated superficial temporal artery. On MRI, using contrast enhanced T1 fat saturated images, there are wall thickening and mural enhancement in the superficial temporal and extracranial arteries (Figure 4C–E). Arterial stenosis is also demonstrated. FDG-PET is a sensitive tool for large vessel vasculitis.109,115
Paraneoplastic vasculitis
Paraneoplastic syndromes are secondary to malignant disease. One type of paraneoplastic syndrome is vasculitis. There is a strong association between hematologic malignancies and vasculitis. Vasculitis as a paraneoplastic syndrome mostly involves small or medium size vessels. Involvement of large vessel is rare.116–118 Among a few reported cases of large vasculitis, one is described in the literature as related to chronic myelomonocytic leukemia. It shows involvement of the aorta and its major branches (Figure 4F–4J) and responds well to corticosteroid with follow-up imaging showing complete resolution of large vessel wall thickening.116,118,119
Miscellaneous
Granulomatosis with polyangiitis (GPA)
On imaging, features of sinonasal GPA are bony erosion and destruction, mucosal thickening, and neo-osteogenesis. About 85% of patients show nonspecific mucosal thickening in nasal cavity or paranasal sinus. Mucosal thickening is most common in the maxillary sinus, and nodularity of mucosa may suggest GPA rather than chronic sinusitis. 75% of GPA cases have bone erosion. Erosion mostly occurs in the anterior ethmoid sinuses, but other sinuses are also involved. CT demonstrates erosion by punctate osseous destruction. It starts in the midline from septum and turbinate and symmetrically spreads, eventually making a large single sinus cavity. The differential diagnosis for sinonasal GPA is sarcoidosis, lymphoma, and cocaine use.58–60 Sparing of the hard palate helps to differentiate it from lymphoma and cocaine use. Although erosion is seen in other diseases, involvement of septum and turbinate with mass formation are more frequently seen in GPA. Soft tissue masses in GPA are typically nasal granulomas which enhance after contrast administration.58–60 Neo osteogenesis occurs in 50% of cases. It makes a distinctive sclerotic edge of well corticated bone which is parallel to the sinus wall over a less dense bone. In chronic cases, paranasal sinus volumes decrease due to marked residual wall thickening mimicking ground glass appearance on CT scan (Figure 4N).
MRI demonstrates high (matching fat) T1 signal intensity in areas of new bone formation. Post-contrast images show increased enhancement of lining of affected nose and sinus cavities. In 30% of cases, invasion of the orbit and orbital mass is present. Imaging especially helps in the diagnosis of localized form of GPA when the result of biopsy is not conclusive. In a patient with no history of operation, destruction, and new bone formation on CT with mentioned fat signal of sclerotic sinus wall is almost diagnostic of GPA.120–122 Ophthalmic manifestation of GPA is common including conjunctivitis, episcleritis, scleritis, uveitis, nasolacrimal obstruction, and direct orbital involvement or extension from paranasal sinuses and nasopharynx.121,122 On CT, involvement of sinuses with mass in the orbit, infiltration of fat planes, and osseous erosion are often seen. On MRI at the beginning, mucosal changes cannot be differentiated from other mucosal inflammation, but gradually signal intensity changes to low on T1 and particularly T2-weighted sequences (Figure 4K–M). Management of orbital involvement is challenging and need surgical decompression in case of optic nerve compromise due to mass effect. Some differential diagnoses are infectious disease, neoplasm, thyroid orbitopathy, sarcoidosis, and idiopathic sclerosing orbital inflammation.123,124
Sarcoidosis
Sarcoidosis can involve all parts of head and neck. Diagnosis usually requires pathologic and radiologic evidence. Nasal and sinus disease is considered uncommon manifestation of sarcoidosis. Most common nasal symptom is nasal obstruction and rhinitis. The most common presentation of salivary gland involvement is painless enlargement. Diabetes mellitus and hyperprolactinemia are the most common signs of pituitary involvement.55,123 The most common ocular involvement is anterior uveitis. Lacrimal gland involvement causes abnormal enlarged gland and enhancement on CT and MRI. Involvement of extraocular muscles and orbital fat is uncommon but results in abnormal enhancement and enlargement of muscles and tendons and infiltrative heterogenous mass in retro orbital fat.124,125 Optic nerve involvement may cause nonspecific findings such as high signal intensity on T2-weighted images of optic nerve and nodular enhancement and thickening on T1-weighted images. Differential diagnosis includes optic neuritis, lymphoma, and carcinomatosis.
Parotid gland involvement causes enlargement and enhancement of gland as well as increased signal intensity on T2 images. This may lead to facial nerve palsy. Differentials are lymphoma and Sjögren syndrome.46,88 Thyroid involvement causes nonspecific enlargement and nodularity. Differentials are multinodular goiter and thyroid cancers.125,126
Involvement of vessel of head and neck such as carotid is characterized by soft tissue thickening and enhancement around the vessel due to vasculitis. Nasal involvement appears similar to other idiopathic granulomatous diseases such as GPA and Churg-Strauss disease. Lymph node involvement in neck is mostly in anterior triangle with mobile nontender discrete lymph nodes.57,126,127
Relapsing polychondritis
Relapsing polychondritis is a rare autoimmune disease that presents with recurrent inflammation and destruction of the cartilage in many different regions. Commonly affected areas are tracheobronchial and laryngeal tree (subglottic stenosis), auricular pinna (prominent ear), nasal cartilages (saddle nose), eyes (episcleritis), and peripheral joints. No sex predilection is seen. Relapsing polychondritis is more common at the age of 40–50 years.128–130
On CT, the findings are increased attenuation and thickening of the anterior and lateral wall of the large airways with sparing of posterior wall, large airway stenosis, subglottic stenosis, tracheomalacia, and dense tracheal cartilage calcification. PET-CT is useful for targeted biopsy. External auditory canal calcification should suggest the diagnosis of relapsing polychondritis. There is thickening and train track appearance due to cricoid calcification. 128 MRI demonstrates hyperintensity in fluid sensitive sequences and enhancement after gadolinium administration in perichondrium and chondroephiphysis which especially help for differentiation from joint disease in children. In relapsing polychondritis, there is no joint effusion or abnormal bone marrow signal.128,131–133
Eosinophilic granulomatosis with polyangiitis
Eosinophilic granulomatosis with polyangiitis is a rare small-medium size vessel vasculitis. It is characterized by asthma, rhinosinusitis, nasal polyps, peripheral eosinophilia, and at last the systemic vasculitis phase. Most granulomatosis disease of sinonasal cavities are granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis.131–133
On imaging, polyps are more common in Eosinophilic granulomatosis with polyangiitis, whereas nasal septal preformation is more common in granulomatosis with polyangiitis. High attenuated polypoid shape mucosal thickening is seen on CT which is suggestive of condensed, inspissated mucus. Mastoid and middle ear opacification is seen in some patients on MRI. A wide range of ophthalmic manifestations are also reported.121,134,135
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Alireza Paydar https://orcid.org/0000-0001-5795-3345
Zachary B Jenner https://orcid.org/0000-0001-7488-0512
Tyrell J Simkins https://orcid.org/0000-0002-6063-174X
Yu-Ming Chang https://orcid.org/0000-0003-2431-1164
References
- 1.Akaishi T, Nakashima I, Sato DK, et al. Neuromyelitis Optica Spectrum Disorders. Neuroimaging Clin North America. 2017;27(2):251–265. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1052514916301344 [DOI] [PubMed] [Google Scholar]
- 2.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol 2021; 20(9): 762–772. [DOI] [PubMed] [Google Scholar]
- 3.Weinshenker BG, Wingerchuk DM. Neuromyelitis Spectrum Disorders. Mayo Clinic Proc 2017; 92(4): 663–679. [DOI] [PubMed] [Google Scholar]
- 4.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler J 2016; 22(4): 470–482. [DOI] [PubMed] [Google Scholar]
- 5.Dutra BG, da Rocha AJ, Nunes RH, et al. Neuromyelitis optica spectrum disorders: Spectrum of MR imaging findings and their differential diagnosis. Radiographics 2018; 38(1). [DOI] [PubMed] [Google Scholar]
- 6.Pekcevik Y, Mitchell CH, Mealy MA, et al. Differentiating neuromyelitis optica from other causes of longitudinally extensive transverse myelitis on spinal magnetic resonance imaging. Mult Scler J 2016; 3: 22–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akaishi T, Sato DK, Nakashima I, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: A comparative study. J Neurol Neurosurg Psychiat 2016; 87: 446–448. [DOI] [PubMed] [Google Scholar]
- 8.Biotti D, Bonneville F, Tournaire E, et al. Optic neuritis in patients with anti-MOG antibodies spectrum disorder: MRI and clinical features from a large multicentric cohort in France. J Neurol 2017; 264: 2173–2175. [DOI] [PubMed] [Google Scholar]
- 9.Wan H, He H, Zhang F, et al. Diffusion-weighted imaging helps differentiate multiple sclerosis and neuromyelitis optica-related acute optic neuritis. J Magn Reson imag 2017; 45(6): 1780–1785. [DOI] [PubMed] [Google Scholar]
- 10.Amato MP, Derfuss T, Hemmer B, et al. Environmental modifiable risk factors for multiple sclerosis: Report from the 2016 ECTRIMS focused workshop. Mult Scler J 2018; 24(5): 590–603. [DOI] [PubMed] [Google Scholar]
- 11.Hoch MJ, Bruno MT, Shepherd TM. Advanced MRI of the Optic Nerve, Vol 37, Journal of Neuro-Ophthalmology. Lippincott Williams and Wilkins, 2017, pp. 187–196. [DOI] [PubMed] [Google Scholar]
- 12.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States. Neurology 2019; 92(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel A, McClelland C, Lee MS. Critical review: Typical and atypical optic neuritis. Surv Ophthalmol 2019; 64(6): 770–779. [DOI] [PubMed] [Google Scholar]
- 14.Clark D, Kebede W, Eggenberger E. Optic Neuritis. Neurol Clin 2010; 28(3): 573–580. [DOI] [PubMed] [Google Scholar]
- 15.Mantia L la, Curone M, Rapoport AM, et al. Tolosa-Hunt Syndrome: critical literature review based on IHS 2004 criteria. [DOI] [PubMed]
- 16.Gladstone JP, Dodick DW. Painful Ophthalmoplegia: Overview with a Focus on Tolosa-Hunt Syndrome, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Vallejo SS. . MRI findings in Tolosa-Hunt syndrome (THS) Images in…. BMJ Case Rep [Internet]. Available from:, http://casereports.bmj.com/ (2014).
- 18.Yuliati A, Rajamani K. Images in Clinical Neurology Tolosa-Hunt Syndrome. The Neurohospitalist 2018; 8(2): 104–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang W, Liu R, et al. Factors that influence Tolosa–Hunt syndrome and the short-term response to steroid pulse treatment. J Neurol Sci 2014; 341(1–2): 13–16. [DOI] [PubMed] [Google Scholar]
- 20.Wasmeier C, Pfadenhauer K, Rösler A. Original communication. J Neurol 2002; 249: 1237–1241. [DOI] [PubMed] [Google Scholar]
- 21.Jain R, Sawhney S, Koul RL, et al. Tolosa-Hunt syndrome: MRI appearances. [DOI] [PubMed]
- 22.Ravindran K, Schmalz P, Torun N, et al. Angiographic Findings in the Tolosa-Hunt Syndrome and Resolution after Corticosteroid Treatment. Neuro-ophthalmology (Aeolus Press 2018; 42(3): 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosa A, Filho G, Faccenda PG, et al. Os autores declaram não haver conflito de interesses Tolosa-Hunt Syndrome. Rev Bras Oftalmol 2018; 77(5): 289–291. [Google Scholar]
- 24.Ronquillo Y, Patel BC. Nonspecific Orbital Inflammation. 2021.
- 25.Neems L, Echalier EL, Subramanian PS. Orbital Tumors and Inflammatory Disorders: Diagnosis and Management. Int Ophthalmol Clin 2018; 58(2): 181–195. [DOI] [PubMed] [Google Scholar]
- 26.Mysore N, Gonçalves FG, Chankowsky J, et al. Adult Orbital Masses: A Pictorial Review. Can Assoc Radiologists J 2012; 63(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Wang S, Li Z, et al. Improving diagnostic performance of differentiating ocular adnexal lymphoma and idiopathic orbital inflammation using intravoxel incoherent motion diffusion-weighted MRI. Eur Journal Radiology 2020; 130: 109191. [DOI] [PubMed] [Google Scholar]
- 28.Sun B, Song L, Wang X, et al. Lymphoma and inflammation in the orbit: Diagnostic performance with diffusion-weighted imaging and dynamic contrast-enhanced MRI. J Magnetic Resonance Imaging : JMRI 2017; 45(5): 1438–1445. [DOI] [PubMed] [Google Scholar]
- 29.Abraham A, Nicholson L, Dick A, et al. Intermediate uveitis associated with MS. Neurol - Neuroimmunology Neuroinflammation 2021; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Commodaro AG, Bueno V, Belfort R, et al. Autoimmune uveitis: The associated proinflammatory molecules and the search for immunoregulation. Autoimmun Rev 2011; 10(4): 205–209. [DOI] [PubMed] [Google Scholar]
- 31.Li CQ, Cho AA, Edward NJ, et al. Magnetic resonance imaging of uveitis. [DOI] [PubMed]
- 32.Radhakrishnan R, Cornelius R, Cunnane MB, et al. MR imaging findings of endophthalmitis. Available from, www.abmedica.it [DOI] [PMC free article] [PubMed]
- 33.Hickman SJ. Optic Perineuritis. [DOI] [PubMed]
- 34.Gao Y, Moonis G, Cunnane ME, et al. Lacrimal Gland Masses. Am J Roentgenology 2013; 201(3): W371–W381. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Selva D. Non-infectious Dacryoadenitis. Surv Ophthalmol 2021. [DOI] [PubMed] [Google Scholar]
- 36.Rabinowitz MP, Halfpenny CP, Bedrossian EH. Orbit The International Journal on Orbital Disorders, Oculoplastic and Lacrimal Surgery The Frequency of Granulomatous Lacrimal Gland Inflammation as a Cause of Lacrimal Gland Enlargement in Patients Without a Diagnosis of Systemic Sarcoidosis The Frequency of Granulomatous Lacrimal Gland Inflammation as a Cause of Lacrimal Gland Enlargement in Patients Without a Diagnosis of Systemic Sarcoidosis. Available from, https://www.tandfonline.com/action/journalInformation?journalCode=iorb20 (2013). [DOI] [PubMed]
- 37.Wu C, Zeng Y-P. Mikulicz Disease. Available from, https://jamanetwork.com/ (2020). [DOI] [PubMed]
- 38.Vahdani K, Rose GE. Sarcoid-like granulomatous orbitopathy—presentation, systemic involvement and clinical outcome. Eye 2021; 35: 470–476, Available from:, DOI: 10.1038/s41433-020-0874-4 10.1038/s41433-020-0874-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita A, Sakai O, Chapman MN, et al. IgG4-related Disease of the Head and Neck: CT and MR Imaging Manifestations. RadioGraphics 2012; 32(7): 1945–1958. [DOI] [PubMed] [Google Scholar]
- 40.Koizumi S, Kamisawa T, Kuruma S, et al. Inflammatory Disorders.
- 41.Kim N, Yang HK, Kim JH, et al. IgG4-related ophthalmic disease involving extraocular muscles: case series. BMC Ophthalmol 2018; 18(1): 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goulam-Houssein S, Grenville JL, Mastrocostas K, et al. IgG4-related intracranial disease. Neuroradiology J 2019; 32(1): 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismailova DS, Abramova J v, Novikov PI, et al. Clinical features of different orbital manifestations of granulomatosis with polyangiitis. Available from:, DOI: 10.1007/s00417-018-4014-9 10.1007/s00417-018-4014-9 [DOI] [PubMed]
- 44.Provenzale JM, Mukherji S, Allen NB, et al. Orbital involvement by Wegener’s granulomatosis: imaging findings. Am J Roentgenology 1996; 166(4): 929–934. [DOI] [PubMed] [Google Scholar]
- 45.Avdagic E, Phelps PO. Nasolacrimal duct obstruction as an important cause of epiphora. Disease-a-Month 2020; 66(10): 101043. [DOI] [PubMed] [Google Scholar]
- 46.Naeser E, Friis P, Hansen IT, et al. External dacryocystorhinostomy in Wegener’s granulomatosis. Acta Ophthalmologica 2013; 91(8): 776–778. [DOI] [PubMed] [Google Scholar]
- 47.Nassif SJ, Ruiz D, Callahan A, et al. Nasolacrimal Duct Obstruction: An Unusual Presentation of Sarcoidosis. Available from:, https://us.sagepub.com/en-us/nam/open-access-at-sage [DOI] [PubMed]
- 48.Ishikawa S, Shoji T, Nishiyama Y, et al. A case with acquired lacrimal fistula due to Sjögren’s syndrome. Am J Ophthalmol Case Rep 2019: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alsoudi A, Copperman TS, Idowu OO, et al. Occult Nasolacrimal Duct Obstruction Secondary to IgG4-Related Ophthalmic Disease. Ophthalmic Plastic Reconstructive Surgery 2019; 35(3): e62–e64. [DOI] [PubMed] [Google Scholar]
- 50.Raslan OA, Ozturk A, Pham N, et al. A Comprehensive Review of Cross-Sectional Imaging of the Nasolacrimal Drainage Apparatus: What Radiologists Need to Know. Am J Roentgenology 2019; 213(6): 1331–1340. [DOI] [PubMed] [Google Scholar]
- 51.Warmbier J, Lüdecke DK, Flitsch J, et al. Typing of inflammatory lesions of the pituitary. Pituitary; 1: 3, DOI: 10.1007/s11102-021-01180-1 10.1007/s11102-021-01180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prete A, Salvatori R. Hypophysitis. 2000.
- 53.Falorni A, Minarelli V, Bartoloni E, et al. Diagnosis and classification of autoimmune hypophysitis. Autoimmun Rev 2014; 13(4–5): 412–416. [DOI] [PubMed] [Google Scholar]
- 54.Lojou M, Bonneville JF, Ebbo M, et al. IgG4 hypophysitis: Diagnosis and management. La Presse Médicale 2020; 49(1): 104016. [DOI] [PubMed] [Google Scholar]
- 55.Castillero F, Castillo-Fernández O, Fernández F, et al. Cancer immunotherapy-associated hypophysitis. Available from:, www.futuremedicine.com [DOI] [PubMed]
- 56.Sharma OP. Neurosarcoidosis: a personal perspective based on the study of 37 patients. Neurosarcoidosis Chest 1997; 112(1): 220–228. [DOI] [PubMed] [Google Scholar]
- 57.Badhey AK, Kadakia S, Carrau RL. et al. Sarcoidosis of the Head and Neck. [DOI] [PMC free article] [PubMed]
- 58.Midyett FA, Mukherji SK. Skull Base Imaging. Cham: Springer International Publishing, 2020, pp. 35–39. Neurohypophyseal Sarcoidosis. [Google Scholar]
- 59.Langrand C, Bihan H, Raverot G, et al. Hypothalamo-pituitary sarcoidosis: a multicenter study of 24 patients. Q J Med. 2012;105:981–995. Available from: https://academic.oup.com/qjmed/article/105/10/981/1527143 [DOI] [PubMed] [Google Scholar]
- 60.Carnevale C, Arancibia-Tagle D, Sarría-Echegaray P, et al. Head and Neck Manifestations of Granulomatosis with Polyangiitis: A Retrospective analysis of 19 Patients and Review of the Literature. Int Arch Otorhinolaryngol 2019; 23: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pakalniskis MG, Berg AD, Policeni BA, et al. The Many Faces of Granulomatosis With Polyangiitis: A Review of the Head and Neck Imaging Manifestations. Am J Roentgenology 2015; 205(6): W619–W629. [DOI] [PubMed] [Google Scholar]
- 62.Lloyd G, Lund VJ, Beale T, et al. Rhinologic changes in Wegener’s granulomatosis. The J Laryngol Otology 2002; 116(7): 565–569. [DOI] [PubMed] [Google Scholar]
- 63.Goyal M, Kucharczyk W, Keystone E. Granulomatous hypophysitis due to Wegener’s granulomatosis. AJNR Am Journal Neuroradiology 2000; 21(8): 1466–1469. [PMC free article] [PubMed] [Google Scholar]
- 64.Chung CH, Song MS, Cho HD, et al. A Case of Idiopathic Granulomatous Hypophysitis. Korean J Intern Med 2012; 27(3): 346, Available from:, http://kjim.org/journal/view.php?doi=10.3904/kjim.2012.27.3.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greco A, Gallo A, Fusconi M, et al. Cogan’s syndrome: an autoimmune inner ear disease. Autoimmun Reviews 2013; 12(3): 1033–1038. [DOI] [PubMed] [Google Scholar]
- 66.Kessel A, Vadasz Z, Toubi E. Cogan syndrome — Pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 2014; 13(4–5): 351–354. [DOI] [PubMed] [Google Scholar]
- 67.Abdel Razek AAK, Alvarez H, Bagg S, et al. Imaging Spectrum of CNS Vasculitis. RadioGraphics 2014; 34(4). [DOI] [PubMed] [Google Scholar]
- 68.Lobo D, Tuñón M, Villarreal I, et al. Intratympanic gadolinium magnetic resonance imaging supports the role of endolymphatic hydrops in the pathogenesis of immune-mediated inner-ear disease. J Laryngol Otol 2021; 132: 554–559. [DOI] [PubMed] [Google Scholar]
- 69.Goodall AF&, Siddiq MA. Current understanding of the pathogenesis of autoimmune inner ear disease: a review, 2015. [DOI] [PubMed] [Google Scholar]
- 70.Kleffner I, Dörr J, Ringelstein M, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiat 2016; 87: 1287–1295, Available from:, http://jnnp.bmj.com/ [DOI] [PubMed] [Google Scholar]
- 71.Papo T, Klein I, Sacré K, et al. Syndrome de Susac. La Revue de Médecine Interne 2012; 33(2): 94–98. [DOI] [PubMed] [Google Scholar]
- 72.Allmendinger AM, Spektor V, Destian S. CT and MR imaging of Susac syndrome in a young male presenting with acute disorientation. Clin imag 2010; 34(2): 138–142. [DOI] [PubMed] [Google Scholar]
- 73.Coulette S, Lecler A, Saragoussi E, et al. Diagnosis and Prediction of Relapses in Susac Syndrome: A New Use for MR Postcontrast FLAIR Leptomeningeal Enhancement. Am J Neuroradiology 2019; 40(7): 1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bitra RK, Eggenberger E. Review of Susac syndrome. Curr Opin Ophthalmol 2011; 22(6): 472–476. [DOI] [PubMed] [Google Scholar]
- 75.Greco A, Fusconi M, Gallo A, et al. Vogt-Koyanagi-Harada syndrome. Autoimmun Reviews 2013; 12(11): 1033–1038. [DOI] [PubMed] [Google Scholar]
- 76.O’Keefe GAD, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol 2017; 62(1). [DOI] [PubMed] [Google Scholar]
- 77.Hida K, Takano K, Yoshimitsu K, et al. Inner ear enhancement on gadolinium-enhanced 3D FLAIR images in a patient with Vogt-Koyanagi-Harada disease. BJR Case Rep 2017: 2, Available from:, DOI: 10.1259/bjrcr.20160090 10.1259/bjrcr.20160090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez-Escamez JA, Carey J, Chung W-H, et al. Diagnostic criteria for Menière’s disease. J Vestib Res 2015; 25: 1–7. [DOI] [PubMed] [Google Scholar]
- 79.Chiarella G, Saccomanno M, Scumaci D, et al. Proteomics in Mé niè re Disease. J Cell Physiol 2012; 227: 308–312. [DOI] [PubMed] [Google Scholar]
- 80.Connor SEJ, Pai I. Endolymphatic hydrops magnetic resonance imaging in Ménière’s disease. Clin Radiol 2021; 76(1): 76.e1–76.e19. [DOI] [PubMed] [Google Scholar]
- 81.Vassiliou A, Vlastarakos P v, Maragoudakis P, et al. Meniere’s disease: Still a mystery disease with difficult differential diagnosis. Ann Indian Acad Neurol 2011; 14(1): 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao S, Wan Y, Huang Z, et al. Imaging and clinical features of Castleman Disease. Available from, DOI: 10.1186/s40644-019-0238-0 10.1186/s40644-019-0238-0 [DOI] [PMC free article] [PubMed]
- 83.Madan R, Chen J-H, Trotman-Dickenson B, et al. The spectrum of Castleman’s disease: Mimics, radiologic pathologic correlation and role of imaging in patient management. Eur J Radiol 2012; 81(1): 123–131. [DOI] [PubMed] [Google Scholar]
- 84.Kligerman SJ, Auerbach A, Franks TJ, et al. Castleman Disease of the Thorax: Clinical, Radiologic, and Pathologic Correlation: From the Radiologic Pathology Archives. RadioGraphics 2016; 36(5): 1309–1332. [DOI] [PubMed] [Google Scholar]
- 85.Jiang XH, Song HM, Liu QY, et al. Castleman disease of the neck: CT and MR imaging findings. Eur J Radiol 2014; 83(11): 2041–2050. [DOI] [PubMed] [Google Scholar]
- 86.Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Available from: DOI: 10.1136/annrheumdis-2014-205375 10.1136/annrheumdis-2014-205375 [DOI] [PubMed]
- 87.Clinical Practice.
- 88.Pasoto SG, de Oliveira Martins A, Bonfa E. Sjögren’s syndrome and systemic lupus erythematosus: links and risks. 2019; Available from: DOI: 10.2147/OARRR.S167783 10.2147/OARRR.S167783 [DOI] [PMC free article] [PubMed]
- 89.Zintzaras E, Voulgarelis M, Moutsopoulos HM. . The Risk of Lymphoma Development in Autoimmune Diseases A Meta-analysis [Internet]. Available from:, https://jamanetwork.com/ [DOI] [PubMed]
- 90.Yousem DM, Kraut MA, Chalian AA. Major Salivary Gland Imaging. Radiology 2000; 216(1). [DOI] [PubMed] [Google Scholar]
- 91.Ren Y-D, Li X-R, Zhang J, et al. Original Article Conventional MRI techniques combined with MR sialography on T2-3D-DRIVE in Sjögren syndrome. Int J Clin Exp Med 2015; 8: 3974–3982, Available from, www.ijcem.com/ [PMC free article] [PubMed] [Google Scholar]
- 92.Kawai Y, Sumi Hideki Kitamori Yukinori Takagi Takashi Nakamura Kawai MY . . Diffusion-Weighted MR Microimaging of the Lacrimal Glands in Patients with Sjögren’s SyndromeAvailable from:, www.ajronline.org (2005. [DOI] [PubMed]
- 93.Geyer JT, Ferry JA, Harris NL, et al. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am Journal Surgical Pathology 2010; 34(2): 202–210, http://www.ncbi.nlm.nih.gov/pubmed/20061932 [DOI] [PubMed] [Google Scholar]
- 94.Li S, Yao J, Hao S, et al. Multifactor Analysis of Thyroid Stiffness in Graves Disease: A Preliminary Study. Am J Roentgenology 2019; 212(5): 1–8. [DOI] [PubMed] [Google Scholar]
- 95.Smith TJ, Hegedüs L. Graves’ Disease. New Engl J Med 2016; 16: 375. [DOI] [PubMed] [Google Scholar]
- 96.North VS, Freitag SK. A Review of Imaging Modalities in Thyroid-associated Orbitopathy. Int Ophthalmol Clin 2019; 59(4): 81–93. [DOI] [PubMed] [Google Scholar]
- 97.Dighe M, Barr R, Bojunga J, et al. Thyroid Ultrasound: State of the Art Part 1-Thyroid Ultrasound reporting and Diffuse Thyroid Diseases. Med Ultrason 2017; 19(1): 79–93. [DOI] [PubMed] [Google Scholar]
- 98.Caturegli P, de Remigis A, Rose NR. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun Rev 2014; 13(4–5): 391–397. [DOI] [PubMed] [Google Scholar]
- 99.Kara T, Ateş F, · Mehmet, et al. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto’s thyroiditis patients. J Ultrasound 2020; 23: 543–551, Available from:, DOI: 10.1007/s40477-020-00437-y 10.1007/s40477-020-00437-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gosi SKY, Nguyen M, Garla Vv. Riedel Thyroiditis, 2021. [PubMed] [Google Scholar]
- 101.Darouichi M, Constanthin PE. Riedel’s thyroiditis. Radiol Case Rep 2016; 11(3): 175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ozgen A, Cila A. Riedel’s thyroiditis in multifocal fibrosclerosis: CT and MR imaging findings. AJNR Am Journal Neuroradiology 2000; 21(2): 320–321. [PMC free article] [PubMed] [Google Scholar]
- 103.Stagnaro-Green A. Approach to the Patient with Postpartum Thyroiditis The Patient. J Clin Endocrinol Metab 2012; 97: 334–342, Available from:, https://academic.oup.com/jcem/article/97/2/334/2836278 [DOI] [PubMed] [Google Scholar]
- 104.Stagnaro-Green A. Postpartum thyroiditis. Best Pract Res Clin Endocrinol Metab 2004; 18(2). [DOI] [PubMed] [Google Scholar]
- 105.Quéméneur T, Hachulla É, Lambert M, et al. Maladie de Takayasu. La Presse Médicale 2006; 35(5): 847–856. [DOI] [PubMed] [Google Scholar]
- 106.Seyahi E. Takayasu arteritis: an update. Curr Opinion Rheumatology 2017; 29(1): 51–56. [DOI] [PubMed] [Google Scholar]
- 107.Sueyoshi E, Sakamoto I, Uetani M. MRI of Takayasu’s Arteritis: Typical Appearances and Complications. Am J Roentgenology 2006; 187(6): W569–W575. [DOI] [PubMed] [Google Scholar]
- 108.Gotway MB, Araoz PA, Macedo TA, et al. Imaging findings in Takayasu’s arteritis. AJR Am Journal Roentgenology 2005; 184(6): 1945–1950. [DOI] [PubMed] [Google Scholar]
- 109.Matsunaga N, Hayashi K, Sakamoto I, et al. Takayasu Arteritis: Protean Radio!ogic Manifestations and. [DOI] [PubMed]
- 110.Falardeau J. Giant Cell Arteritis. Neurol Clin 2010; 28(3): 581–591. [DOI] [PubMed] [Google Scholar]
- 111.Ciofalo A, Gulotta G, Iannella G, et al. Giant Cell Arteritis (GCA): Pathogenesis, Clinical Aspects and Treatment Approaches. Curr Rheumatology Reviews 2019; 15(4): 259–268. [DOI] [PubMed] [Google Scholar]
- 112.Stanson ’ AW, Klein RG, Hunder2 GG. Extracranial Angiographic Findings in Giant Cell (Temporal) Arteritis. Am J Roentgenol 1976; 127, Available from, www.ajronline.org [DOI] [PubMed] [Google Scholar]
- 113.Monti S, Floris A, Ponte C, et al. The use of ultrasound to assess giant cell arteritis: review of the current evidence and practical guide for the rheumatologist. Available from:, https://academic.oup.com/rheumatology/article/57/2/227/3772182 [DOI] [PMC free article] [PubMed]
- 114.Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5): 744–756. [DOI] [PubMed] [Google Scholar]
- 115.Waldman CW, Waldman SD, Waldman RA. Giant cell arteritis. The Med Clinics North America 2013; 97(2): 329–335. [DOI] [PubMed] [Google Scholar]
- 116.Bley TA, Wieben O, Uhl M, et al. High-Resolution MRI in Giant Cell Arteritis: Imaging of the Wall of the Superficial Temporal Artery. Am J Roentgenology 2005; 184(1): 283–287. [DOI] [PubMed] [Google Scholar]
- 117.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clinic Proceedings 2010; 85(9): 838–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kurzrock R, Cohen PR. Vasculitis and cancer. Clin Dermatology 1993; 11(1): 175–187. [DOI] [PubMed] [Google Scholar]
- 119.Sasinowska S, Traisak P, McCormack M, et al. A Rare Case of Paraneoplastic Aortitis Associated with Chronic Myelomonocytic Leukemia. Case Rep Hematol 2017; 2017: 3091973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mori M, Togami K, Fujita H, et al. Successful allogeneic bone marrow transplantation for chronic myelomonocytic leukemia complicated by refractory aortitis. Available from, www.nature.com/bmt (2010. [DOI] [PubMed]
- 121.Nakamaru Y, Takagi D, Suzuki M, et al. Otologic and Rhinologic Manifestations of Eosinophilic Granulomatosis with Polyangiitis. Audiol Neurotology 2016; 21(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 122.Nakamaru Y, Takagi D, Suzuki M, et al. E-Mail Otologic and Rhinologic Manifestations of Eosinophilic Granulomatosis with Polyangiitis. Audiol Neurotol 2016; 21: 45–53, Available from, www.karger.com/aud [DOI] [PubMed] [Google Scholar]
- 123.Muller K, Lin JH. Orbital Granulomatosis With Polyangiitis (Wegener Granulomatosis) Clinical and Pathologic Findings. [DOI] [PMC free article] [PubMed]
- 124.Pakrou N, Selva D, Leibovitch I. Wegener’s Granulomatosis: Ophthalmic Manifestations and Management. Semin Arthritis Rheum 2006; 35(5): 284–292. [DOI] [PubMed] [Google Scholar]
- 125.Cereceda-Monteoliva N, Rouhani MJ, et al. Maral |, Rouhani J, Maughan EF, Rotman | Anthony, Orban N, et al. Sarcoidosis of the ear, nose and throat: A review of the literature. Clin Otolaryngol 2021; 46: 935–940. [DOI] [PubMed] [Google Scholar]
- 126.Ganeshan D, Menias CO, Lubner MG, et al. Sarcoidosis from Head to Toe: What the Radiologist Needs to Know. RadioGraphics 2018; 38(4): 1180–1200. [DOI] [PubMed] [Google Scholar]
- 127.Koyama T, Ueda H, Togashi K, et al. Radiologic Manifestations of Sarcoidosis in Various Organs. RadioGraphics 2004; 24(1). [DOI] [PubMed] [Google Scholar]
- 128.Vitale A, Sota J, Rigante D, et al. Relapsing Polychondritis: an Update on Pathogenesis, Clinical Features, Diagnostic Tools, and Therapeutic Perspectives. [DOI] [PubMed]
- 129.Khalek AA, Razek A. Imaging of connective tissue diseases of the head and neck. [DOI] [PMC free article] [PubMed]
- 130.Furuya N, Oshitari T, Yotsukura J, et al. International Medical Case Reports Journal Dovepress Relapsing polychondritis with different types of ocular inflammations. Int Med Case Rep J 2015; 8: 193–197, www.dovepress.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung JH, Kanne JP, Gilman MD. CT of Diffuse Tracheal Diseases. Am J Roentgenology 2011; 196(3): W240–W246. [DOI] [PubMed] [Google Scholar]
- 132.Greco A, Rizzo MI, de Virgilio A, et al. Churg–Strauss syndrome. Autoimmun Rev 2015; 14(4): 341–348. [DOI] [PubMed] [Google Scholar]
- 133.Nwawka OK, Nadgir R, Fujita A, et al. Granulomatous Disease in the Head and Neck: Developing a Differential Diagnosis. RadioGraphics 2014; 34(5): 1240–1256. [DOI] [PubMed] [Google Scholar]
- 134.Manifestation of eosinophilic granulomatosis with polyangiitis in head and neck*. [DOI] [PubMed]
- 135.Akella SS, Schlachter DM, Black EH, et al. Ophthalmic Eosinophilic Granulomatosis With Polyangiitis (Churg–Strauss Syndrome): A Systematic Review of the Literature. Ophthalmic Plast Reconstr Surg 2019; 35(1): 7–16. [DOI] [PubMed] [Google Scholar]