Abstract
Despite the fact that carbamates and organophosphates cause acute poisoning via different mechanisms and require disparate management, they are indistinguishable by current clinical assays. Herein, direct immersion solid-phase microextraction (DI-SPME) plus thermal desorption-electrospray ionization tandem mass spectrometry (TD-ESI/MS/MS) was developed to discern them. Both pesticides spiked in human serum were extracted by SPME and analyzed by TD-ESI/MS/MS. This is a promising emergency care platform as rapid analyses could be done in tiny sample volumes with satisfactory recovery (89.46%–116.32%), precision (covariance <20%), sensitivity (LOD <0.1 μg/mL), turnaround time (<5 minutes), and linearity (R2 = 0.9827–0.9992) within 0.1–100 μg/mL.
Keywords: Carbamate, Emergency care, Organophosphate, Pesticides, Solid-phase microextraction, Thermal desorption-electrospray ionization tandem mass spectrometry
1. Introduction
Carbamate and organophosphate insecticides are commonly used in the agricultural sector for pest control. Due to their easy accessibility (especially in Asia), both insecticides have been consumed in self-poisoning attempts by suicidal patients. Since both pesticides can drastically reduce free cholinesterase in the bloodstream, clinical manifestations of organophosphate and carbamate poisoning are virtually indistinguishable [1]. While carbamate binding to cholinesterase is reversible, organophosphate binding to cholinesterase is irreversible since the organophosphate-cholinesterase bond is much stabler than the carbamate-cholinesterase bond. Binding of a carbamate molecule with cholinesterase eventually leads to slow hydrolysis via spontaneous decarbamylation, resulting in enzyme reactivation. In addition, the aging process does not occur as readily in the carbamate-cholinesterase complex than in the organophosphate-cholinesterase complex [2]. Furthermore, carbamates generally do not cross the blood-brain barrier as easily as organophosphates, so that brain damage usually occurs less frequently and severely in carbamate-poisoned patients than in organophosphate-poisoned patients [3,4]. Unlike symptoms of carbamate poisoning, symptoms of organophosphate poisoning do not resolve within 24 hours [5]. Consequently, the corresponding clinical treatments for intoxication by either carbamate and organophosphate insecticides can differ significantly in terms of antidote or supportive modality selection. For instance, the use of oximes to manage carbamate insecticide poisoning remains a controversial safety issue, particularly in adequately atropinized patients [6]; the harmful effects of oximes used to treat carbaryl poisoning have been reported in animal studies, where clinical practice recommends against the use of oximes to manage carbamate insecticide poisoning [7–11]. Therefore, when carbamates are the intoxicating agent, the administration of oximes is contraindicated in guidelines for the clinical management of poisoning by cholinesterase inhibitors, except during poisoning by both organophosphates and carbamates [12].
Since the clinical manifestations of organophosphate intoxication are similar to those of carbamate intoxication, emergency physicians are confronted with the challenging choice of correct clinical management. To facilitate that choice, an analytical approach is needed that can rapidly and precisely differentiate between carbamates and organophosphates and expedite the prognoses of poisoned patients (which is usually more severe in cases of organophosphate poisoning). Therefore, early and rapid distinction between different pesticide types is crucial for informing the appropriate clinical management. The objective of this study is to develop an efficient analytical platform for distinguishing carbamates and organophosphates in human serum, solid-phase microextraction (SPME) was integrated with thermal desorption-electrospray ionization tandem mass spectrometry (TD-ESI/MS/MS) for rapid analysis and quantification of trace insecticides found in human serum.
2. Materials and methods
2.1. Chemicals and materials
Standards for the carbamate insecticides methomyl, carbaryl, and methiocarb, and the organophosphate insecticides fenthion, chlorpyrifos, and ethion were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). HPLC-grade methanol and acetone were purchased from Merck (Darmstadt, Germany), while hexane and ethyl acetate (EA) were purchased from J.T. Baker (Phillipsburg, NJ, U.S.A.). Acetic acid was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Distilled deionized water (purified with a PURE-LAB Classic UV from ELGA, Marlow, U.K.) was used to prepare the electrospray solution. All chemical reagents and solvents were used without additional purification. Human serum was purchased from the Innovative Research Incorporation (Oakland, CA, U.S.A.) and diluted 1000 times in methanol for analysis.
2.2. Direct immersion-solid phase microextraction (DI-SPME)
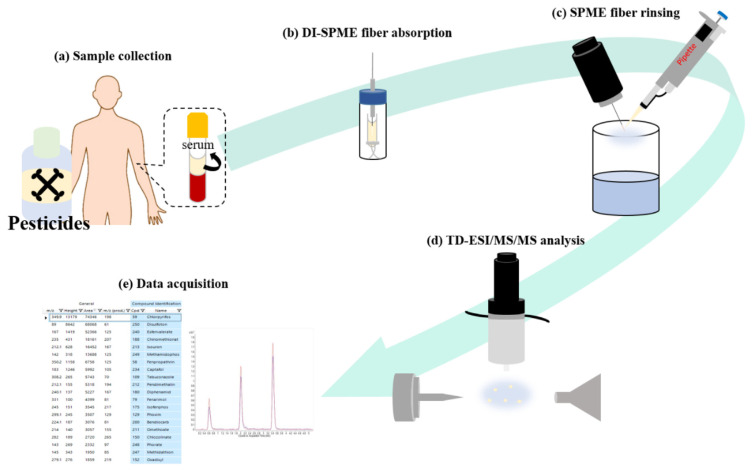
The SPME fiber coating comprised of polydimethylsiloxane/ divinylbenzene (PDMS/DVB, Bellefonte, PA, U.S.A.), which required fiber pretreatment such as conditioning prior to its combination with the TD-ESI system. The total ion chromatogram indicates a strong increase in ion signals (Fig. S1a), where the extracted ion chromatogram indicates strong ion signals of m/z 371 + 74n detected when a new SPME fiber was inserted into the TD-ESI/MS system (Fig. S1c). The difference of m/z 74 [(CH3)2SiO] between detected ions indicates the presence of a series of polysiloxanes — one of the major components of the SPME fiber coating. Within 2 min, each ion signal in the polysiloxane ion series decreased dramatically (Fig. S1d) to background levels before any SPME fibers were inserted in the TD-ESI/MS system (Fig. S1b). Therefore, to remove residual polysiloxanes, each SPME fiber was inserted into the TD-ESI source for 2 min prior to sampling (and the subsequent extraction and characterization of carbamate and organophosphate pesticides in diluted human serum using DI-SPME and TD-ESI/ MS/MS). To simulate acute poisoning in real patients, ethion and carbaryl were individually spiked in diluted human serum, which were extracted via DI-SPME prior to TD-ESI/MS/MS analysis as shown in the schematic diagram (Fig. 1).
Fig. 1.
Schematic diagram of the analytical processes in this study: (a) pesticides were spiked in human serum, (b) an SPME fiber was used to extract pesticides from diluted human serum, (c) the SPME fiber was attached to the sampling probe and rinsed with pure water, (d) TD-ESI/MS/MS analysis was performed, (e) acquired MS data were compared with data in pesticide databases to identify analytes.
2.3. Thermal desorption-electrospray ionization tandem mass spectrometry (TD-ESI/MS/MS)
A TD-ESI source was constructed to be compatible with a triple quadrupole mass analyzer (Ultivo, Agilent, Santa Clara, CA, U.S.A.); details of the source have been described in previous publication [13]. To conduct TD-ESI/MS/MS, a conditioned SPME fiber was immersed in samples for 1 min to concentrate analytes on the fiber. The SPME fiber was then rinsed with pure water and attached to the sample holder. The raw data acquired from DI-SPME-TD-ESI/MS/MS were further processed using the MassHunter qualitative analysis software, which includes mass spectral data for the pesticides analyzed in this study. To reduce the possibility of false-positive or false-negative results during MRM analysis, pesticide matching and identification required the detection of two major product ions of each protonated pesticide ion, where signal durations and intensity ratios must also match those from MS/MS spectra of individual pesticide standards.
2.4. Gas chromatography tandem mass spectrometry (GC/MS/MS) and liquid chromatography tandem mass spectrometry (LC/ MS/MS) analyses
The results obtained by DI-SPME-TD-ESI/MS/ MS were validated using GC/MS/MS and LC/MS/ MS. The pesticides were extracted from spiked human serum using equal extraction volumes of EA. The EA-extracted solution was placed in a heated sample concentrator and dried with a gentle nitrogen stream to evaporate the EA. The dried residue was reconstituted in 100 μL MeOH and divided into two aliquots for subsequent GC/MS/MS and LC/ MS/MS analyses. Residual pesticides in the extract were separated and detected using a GC/MS/MS (GCMS-TQ8040, Shimadzu, Japan) and LC/MS/MS (LCMS-8045, Shimadzu, Japan) system; details of the separation parameters have been described in previous publication [14].
2.5. Mass spectral database matching
Once the pesticide-spiked human serum samples were analyzed using SPME-TD-ESI/MS/MS, the mass spectrometric data were uploaded to a free online mass spectral database such as the Agilent Ultivo tMRM database libraries and an analytical protocol of the Taiwan Food and Drug Administration (TFDA) to match and identify analyte peaks for the spiked pesticides.
3. Results and discussion
Fig. 2a shows the DI-SPME-TD-ESI mass spectrum of diluted human serum without spiked pesticides (after the SPME fiber was conditioned). Ion signals from several unknown biological compounds in human serum were detected at an adsorption time of 1 min (i.e. the duration of SPME fiber immersion in diluted human serum). Fig. 2b shows the experimental results for analysis of diluted human serum spiked with six pesticides (5 μg/mL each). The DI-SPME-TD-ESI mass spectrum clearly shows the ion peaks of the protonated pesticides (MH+, M: analyte) for methomyl (m/z 163), carbaryl (m/z 202), methiocarb (m/z 226), fethion (m/z 279), chlorpyrifos (m/z 350), and ethion (m/z 385). The time required to complete a DI-SPME-TD-ESI/MS/MS analysis was less than 5 min (including sample concentration and characterization), where results indicate that at a sufficiently high concentration of pesticides in serum (such as 5 μg/mL), carbamates and organophosphates can be rapidly distinguished using DI-SPME-TD-ESI/MS. Fig. 2c shows the results from analysis of diluted human serum spiked with different pesticides (0.5 μg/mL each). No protonated pesticide ions (MH+) were detected using SPME-TD-ESI/MS, indicating that more sensitive analytical approaches are necessary to detect trace pesticides in human serum.
Fig. 2.
DI-SPME-TD-ESI/MS mass spectra of (a) diluted human serum, (b) diluted human serum from (a) spiked with six carbamate (■) and organophosphate (●) insecticides (5 μg/mL each), and (c) diluted human serum spiked with six insecticides (0.5 μg/mL each).
Since the detection sensitivity of MS/MS analysis (MRM mode) is much higher than that of MS analysis (full scan mode), the use of DI-SPME-TD-ESI/MS/MS may be a viable analytical strategy for detecting trace pesticides in diluted serum like that analyzed in Fig. 2c. Herein, each protonated pesticide ion and its two main product ions were used to match and identify pesticides, where the m/z ratios for these analyte ions were obtained via DI-SPME-TD-ESI/MS/MS analysis on diluted human serum spiked with six pesticides. The two major product ions for each pesticide are as follows: m/z 163 (methomyl) → m/z 88 and 106, m/z 202 (carbaryl) → m/z 127 and 145, m/z 226 (methiocarb) → m/z 121 and 169, m/z 279 (fethion) → m/z 105 and 169, m/z 350 (chlorpyrifos) → m/z 198 and 97, and m/z 385 (ethion) → m/z 199 and 171. The protonated pesticides and their product ions were further confirmed by comparing their DI-SPME-TD-ESI/MS and DI-SPME-TD-ESI/MS/MS spectra with those in the TFDA analytical protocol. Table 1 summarizes the precursor and two major product ions of the pesticides that were detected using TD-ESI/MS/MS, where the product ion mass spectra of the pesticide standards are shown in Fig. S2.
Table 1.
Results of MS and MS/MS analyses of six pesticides used in this study.
| Pesticide type | Analyte | Precursor ion (m/z) | Product ion (m/z) | LOD/LOQ (μg/mL) |
|---|---|---|---|---|
| Carbamate | Methomyl | 163 (MH+) | 88a, 106 | 1/5 |
| Carbaryl | 202 (MH+) | 127a, 145 | 0.5/1 | |
| Methiocarb | 226 (MH+) | 121a, 169 | 0.1/0.5 | |
| Organophosphate | Fenthion | 279 (MH+) | 105a, 169 | 0.05/0.1 |
| Chlorpyrifos | 350 (MH+) | 198a, 97 | 0.1/0.5 | |
| Ethion | 385 (MH+) | 199a, 171 | 0.1/0.5 |
Product ion for quantitative analysis.
In order to evaluate the parameters and analytical capabilities from TD-ESI/MS/MS. The recovery, repeatability, limit of detection (LOD), and limit of quantification (LOQ) were determined in this study. The averaged recoveries of carbamates (1 μg/mL each) were around 89.46%–116.32%, while organophosphates (1 μg/mL each) were ranged from 92.11% to 104.82%, respectively (data not shown). The value was calculated by comparing the acquired average peak area from the spiked diluted human serum extracted by DI-SPME and pure standard solutions in TD-ESI/MS/MS analysis. The repeatability of DI-SPME-TD-ESI/MS/MS was examined by analyzing ten replicates of diluted human serum spiked with both ethion and carbaryl (5 μg/mL each); Fig. S3 shows that the coefficients of variation of ethion and carbaryl ions were 18.74% and 18.4%, respectively. The detection limit and quantification limit of DI-SPME-TD-ESI/MS/MS for the six carbamate and organophosphate pesticides were observed through serial dilutions of diluted human serum spiked with pesticide standards. The limit of detection (LOD) for carbamates and organophosphates was estimated at 0.1 μg/mL and 0.05 μg/mL, respectively. The LODs of these compounds should be sufficiently low to be applicable for analyzing real blood samples from emergency room patients that usually contain much higher pesticide concentrations. In addition, the limit of quantification (LOQ) for carbamates and organophosphates was determined on 0.5 μg/mL and 0.1 μg/mL, respectively (Table 1). Triplicate measurements of the pesticides at concentrations between 0.1 and 100 μg/mL were performed with DI-SPME-TD-ESI/MS/MS. The matrix-matched calibration curves for the six pesticides show high linearity (R2 = 0.9827–0.9992) for concentrations ranging between 0.1 and 100 μg/mL (Fig. S4).
The adsorption time is a major factor in the sensitivity of an SPME-based approach. Herein, diluted human serum spiked with 5 μg/mL of carbaryl was used to optimize the SPME extraction time before TD-ESI/MS/MS analysis. Fig. 3 shows the results of carbaryl ion signals detected from diluted human serum using DI-SPME-TD-ESI/MS/ MS at different adsorption times (0.25, 0.5, 1, 2, 5, and 10 min), indicating that extraction efficiency — and therefore signal intensity — increased with adsorption time. The minimum adsorption time required to obtain a sufficient analyte signal intensity (S/N > 10) was 1 min, which was the adsorption time used throughout this study.
Fig. 3.
Carbaryl ion intensity vs. SPME adsorption time for 5 μg/mL of carbaryl spiked in diluted human serum. Six replicates were performed for each adsorption duration.
The applicability of DI-SPME-TD-ESI/MS/MS for screening multiple pesticides was studied by analyzing diluted human serum with or without spiking carbaryl, methiocarb, methomyl, chlorpyrifos, ethion and fenthion (5 μg/mL each). Herein, 558 ion pairs for 279 pesticides were monitored within each MRM scan, where the presence of each pesticide in each sample was confirmed by similar signal durations and intensity ratios for two major product ions for each protonated pesticide ion. For instance, the presence and duration of two major MRM transitions for the protonated carbaryl ion (m/z 202 → m/z 127, in red; and m/z 202 → m/z 145, in blue) shown in Fig. 4a was well-matched (1.2–1.5 min), indicating that both transitions m/z 202 → m/z 127 and m/z 202 → m/z 145 resulted from fragmentation of the protonated carbaryl ion. Similar results were obtained from MS/MS analysis of other pesticide standards (Fig. 4b–f). In comparison, Fig. 4g–l shows the MRM results of six randomly selected pesticides — acephate, carbofuran, isoprocarb, methidatihion, terbufos, and thiobencarb — which were not present in the sample solution; no protonated ion signals for these random pesticides were detected in diluted human serum samples from DI-SPME-TD-ESI/MS/MS analysis.
Fig. 4.
Triplicate DI-SPME-TD-ESI/MS/MS analyses of diluted human serum spiked with a mixture of 6 pesticide standards (0.5 μg/mL each), where two ion pairs were monitored for each pesticide in MRM mode (a–f). Pesticide ion signals detected in the sample: a) carbaryl, b) methiocarb, c) methomyl, d) chlorpyrifos, e) ethion, and f) fenthion. (g–l) Six randomly selected pesticide ion signals which were absent in the sample: g) acephate, h) carbofuran, i) isoprocarb, j) methidatihion, k) terbufos, and l) thiobencarb.
Solvent-based extraction and DI-SPME-TD-ESI/ MS/MS analysis of samples followed by GC/MS/ MS and LC/MS/MS validation can be used to quantify pesticides as needed. To compare the detection capabilities of these different analytical approaches for spiked pesticides in diluted human serum, samples spiked with six pesticide standards (5 μg/mL each) were extracted in equal volumes of EA as those of sample volumes followed by GC/MS/ MS or LC/MS/MS analyses. Table 2 shows the results of six spiked pesticides (three carbamates and three organophosphates) detected using GC/MS/ MS (Fig. S5) and LC/MS/MS analyses (Fig. S6).
Table 2.
Results from DI-SPME-TD-ESI/MS/MS, GC/MS/MS, and LC/ MS/MS analyses of diluted human serum spiked with six pesticide standards (5 μg/mL each).
| Pesticide type | Analyte | DI-SPME-TD-ESI/MS/MS | GC/MS/MS | LC/MS/MS |
|---|---|---|---|---|
| Carbamate | Carbaryl | ✓ | -a | ✓ |
| Methiocarb | ✓ | -a | ✓ | |
| Methomyl | ✓ | -a | ✓ | |
| Organophosphate | Chlorpyrifos | ✓ | ✓ | -b |
| Ethion | ✓ | ✓ | -b | |
| Fenthion | ✓ | -a | ✓ | |
| 6/6 (100%) | 2/6(33.3%) | 4/6(66.7%) |
Analyte not in the TFDA GC/MS/MS pesticide database.
Analyte not in the TFDA LC/MS/MS pesticide database.
In emergency settings, it is difficult to find out rapidly whether carbamates, or organophosphates, or both insecticides have be consumed by the patients by using conventional serum assays that determine free cholinesterase levels in blood. For instance, Rotenberg et al. proposed a novel and simple assay for the real-time differentiation between carbamate and organophosphate inhibition of cholinesterase, based on the observed kinetic behavior of the inhibited enzymes over time [15]. Carbamylated cholinesterase activity was found to follow a non-linear kinetic pattern compared to a linear kinetic pattern of phosphorylated enzyme activity; this key distinction enabled differentiation between cholinesterase inhibition by carbamate or organophosphate pesticides. However, this time-consuming method (2.5 hrs) is merely suitable for confirming patient exposure to cholinesterase inhibitors but is unable to inform timely emergency care. In this study, we have successfully developed an analytical technique to 1) rapidly characterize pesticides in human serum using ambient mass spectrometry and 2) efficiently distinguish between carbamate and organophosphate pesticides through precise matching with a pesticide database.
Moreover, traditional chromatographic mass spectrometric methods such as GC/MS/MS or LC/MS/MS can be time- and effort-intensive. Alternatively, ambient ionization mass spectrometry enables rapid analyte characterization, where more than 40 different ambient mass spectrometric techniques have been reported [16]. Ambient mass spectrometric techniques usually possess sampling, desorption, and ionization capabilities, making sample preparation unnecessary and facilitating high-throughput analysis.
Solid-phase microextraction, a sample pretreatment method, has been successfully applied in bioanalytical and clinical analyses of complex matrices to facilitate the analysis of a large number of samples [17–19]. Compared to traditional chromatographic approaches, SPME considerably simplifies and expedites the extraction and concentration processes, while requiring much less solvent and sample amounts. Naturally, SPME has been combined with different ambient mass spectrometric techniques for rapid sample analysis [20], where reproducible and sensitive results have been obtained for phthalates, prescription drugs, and illegal substances [21–29]. In these studies, trace chemical compounds adsorbed on SPME fibers were rapidly characterized using direct electrospray probe (DEP) [21], direct analysis in real time (DART) [22,23], desorption electrospray ionization (DESI) [24], direct ionization [25], dielectric barrier discharge ionization (DBDI) [26], wooden-tip electrospray ionization (WT-ESI) [27,28], and coated blade spray-mass spectrometry [29].
As demonstrated in this study, thermal desorption-electrospray ionization/mass spectrometry is an ambient mass spectrometric technique that could potentially bypass the analytical limitations of conventional GC/MS/MS and LC/MS/MS techniques to rapidly characterize trace volatile and semi-volatile chemical compounds without sample pretreatment or chromatographic separation. TD-ESI/MS consists of a direct sampling probe, thermal desorption unit, electrospray ionization device, and mass analyzer [13]. Sampling with a direct metallic probe is demonstrably a rapid and efficient approach for analyzing liquid or solid samples. Analytes collected on a sampling probe are rapidly desorbed after it is loaded into a heated thermal desorption unit. The desorbed analytes are subsequently carried by heated nitrogen gas into an ESI plume where they are ionized through ion-molecule reactions (IMRs) with charged solvent species. TD-ESI/MS/MS combined with probe sampling has been developed to detect trace compounds like residual pesticides on fruits and vegetables, preservatives in cosmetics, phthalates on various object surfaces, and illicit drugs in adulterated samples. Given the easy sampling, efficient thermal desorption and ionization, and rapid detection features of TD-ESI/MS/MS, this technique can also be applied to rapidly characterize hazardous substances in biofluids [30].
As shown in our study, a useful bioanalytical method like TD-ESI/MS/MS can cover a dynamic quantitative range with a lower limit of quantification (LLOQ) below analyte concentrations. However, TD-ESI/MS analysis biofluids with highly complex compositions is complicated by serious matrix interferences, requiring a sample extraction and concentration method like SPME. Since the objective of this study is to develop an efficient analytical platform for distinguishing carbamates and organophosphates in human serum, SPME was thus integrated with TD-ESI/MS/MS for rapid analysis and quantification of trace insecticides spiked in human serum. Since sampling and desorption and ionization are independent events of TD-ESI/MS analysis, SPME was easily integrated with TD-ESI/MS and TD-ESI/MS/MS by simply using an SPME fiber to collect samples that was then inserted into the source. To demonstrate the applicability of this technique, results obtained from SPME-TD-ESI/MS/MS analyses were validated by traditional GC/MS/MS and LC/MS/MS.
4. Conclusion
In this study, DI-SPME combined with TD-ESI/MS/ MS and precise database matching was used to rapidly characterize and distinguish between trace carbamate and organophosphate pesticides in diluted human serum for emergency toxicology applications. The analytical time required to complete an analysis is less than 5 min, which includes the durations of analyte preconcentration via DI-SPME and analyte characterization via triplicate analysis using TD-ESI/MS/MS. The application of DI-SPME-AIMS herein heralds a breakthrough in toxicant identification and marks a new era in emergency diagnostics. While this study demonstrates the detection and distinction of six pesticides spiked in diluted human serum, the analytical platform developed herein demonstrates potential for rapidly analyzing pesticides in biofluids like saliva, urine, and whole blood for other clinical applications. Furthermore, big data acquisition by AIMS followed by subsequent analysis by machine-learning algorithms would eventually bring about highly efficient toxicological diagnosis in the field of emergency medicine.
Acknowledgements
This work was partially supported by the NSYSUKMU Joint Research Project (NSYSUKMU 109-I009), the Ministry of Science and Technology of Taiwan (108-2320-B-110-002-) and the Research Center for Environmental Medicine (Kaohsiung Medical University, Kaohsiung, Taiwan) from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by the Kaohsiung Medical University Research Center Grant (KMU-TC109A01).
Appendix
(a) Total DI-SPME-TD-ESI/MS ion current during SPME fiber conditioning. (b) TD-ESI mass spectrum of background signals before inserting the SPME fiber in the TD-ESI source, and TD-ESI mass spectra recorded immediately (c) after inserting a new SPME fiber, and (d) after the SPME fiber had remained in the TD source for 2 min.
MS/MS spectra of six pesticide standards each showing the precursor ion (●) and two major product ions (○): (a) fenthion, (b) chlorpyrifos, (c) ethion, (d) methomyl, (e) carbaryl, and (f) methiocarb.
Repeatability tests for ten consecutive DI-SPME-TD-ESI/MS/MS analyses of (a) ethion and (b) carbaryl spiked in diluted human serum (5 μg/mL each). The coefficients of variation of ethion and carbaryl ions were 18.74% and 18.4%, respectively.
The linear responses of the detection of six pesticides by SPME-TD-ESI/MS/MS: (a) fenthion, (b) chlorpyrifos, (c) ethion, (d) methomyl, (e) carbaryl, and (f) methiocarb with concentrations between 0.1 μg/mL and 100 μg/mL and R2 between 0.9827 and 0.9992.
TIC for (a) chlorpyrifos and (b) ethion standards spiked in diluted human serum that was solvent-extracted and concentrated prior to GC/MS/ MS analysis.
TIC for (a) carbaryl, (b) methiocarb, (c) fenthion, and (d) methomyl standards spiked in diluted human serum that was solvent-extracted and concentrated prior to LC/MS/MS analysis.
Funding Statement
This work was partially supported by the NSYSUKMU Joint Research Project (NSYSUKMU 109-I009), the Ministry of Science and Technology of Taiwan (108-2320-B-110-002-) and the Research Center for Environmental Medicine (Kaohsiung Medical University, Kaohsiung, Taiwan) from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by the Kaohsiung Medical University Research Center Grant (KMU-TC109A01).
References
- 1.Ellenhorn MJ, Barceloux DG. Diagnosis and treatment of human poisoning. Amsterdam: Elsevier/ North Holland; 1988. Medical toxicology; pp. 1077–8. [Google Scholar]
- 2.Rumack BH, Spoerke DG, editors. POISINDEX(R) information system. 1986. Consensus: pesticide specialty board. [Google Scholar]
- 3.Aquilonius SM, Eckernas SA, Hartvig P, Hultman J, Lindstrom B, Osterman PO. A pharmacokinetic study of neostigmine in man using gas chromatography-mass spectroscopy. Eur J Clin Pharmacoi. 1979;15:367–71. doi: 10.1007/BF00558442. [DOI] [PubMed] [Google Scholar]
- 4.Unni LK, Hannant ME, Bccker RE, Giacobini E. Determination of physostigmine in plasma and cerebrospinal fluid by liquid chromatography with electrochemical detection. Clin Chem. 1989;35:292–5. [PubMed] [Google Scholar]
- 5.Ellin RJ, Zvirblis P, Wilson MR. Method for isolation and determination of pyridostigmine and metabolites in urine and blood. J Chromatogr. 1982;22g:235–44. doi: 10.1016/s0378-4347(00)80436-3. [DOI] [PubMed] [Google Scholar]
- 6.Chan K, Williams NE, Baty JD, Calvey TN. A quantitative gas-liquid chromatographic method for the determination of neostigmine and pyridostigmine in human plasma. J Chromatogr. 1976;120:349–58. doi: 10.1016/s0021-9673(76)80012-x. [DOI] [PubMed] [Google Scholar]
- 7.Natoff IL, Reiff B. Effect of oximes on the toxicity of anti-choline esterase carbamates. Toxicol Appl Pharmacol. 1973;25:569–75. doi: 10.1016/0041-008x(73)90026-4. [DOI] [PubMed] [Google Scholar]
- 8.Sterri SH, Rognerud B, Fiskum SE, Lyngaas S. Effect oftoxogonin and P2S on the toxicity of carbamates and organophosphorous compounds. Acta Pharmacol Toxicol. 1979;45:9–15. doi: 10.1111/j.1600-0773.1979.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 9.Farago A. Suicidal fatal sevin poisoning. Arch Toxicol. 1969;24:309–15. [PubMed] [Google Scholar]
- 10.Dawson RM. Rate constants of carbamylation and decarbamylation of acetylcholinesterase for physostigmine and carbaryl in the presence of an oxime. Neurochem lnt. 1994;24:173–82. doi: 10.1016/0197-0186(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 11.Lieske CN, Clark JH, Maxwell DM, Zoeffel LD, Sultan WE. Studies on the amplification of carbaryl toxicity by various oximes. Toxicol Lett. 1992;62:127–37. doi: 10.1016/0378-4274(92)90016-d. [DOI] [PubMed] [Google Scholar]
- 12.Rosman Y, Makarovsky I, Bentur Y, Shrot S, Dushnistky T, Krivoy A. Carbamate poisoning: treatment recommendations in the setting of a mass casualties event. Am J Emerg Med. 2009;27(9):1117–24. doi: 10.1016/j.ajem.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Huang MZ, Zhou CC, Liu DL, Jhang SS, Cheng SC, Shiea J. Rapid characterization of chemical compounds in liquid and solid states using thermal desorption electrospray ionization mass spectrometry. Anal Chem. 2013;85:8956–63. doi: 10.1021/ac401364k. [DOI] [PubMed] [Google Scholar]
- 14.Jeng JY, Jiang ZH, Cho YT, Su H, Lee CW, Shiea J. Obtaining molecular imagings of pesticide residues on strawberry surfaces with probe sampling followed by ambient ionization mass spectrometric analysis. J Mass Spectrom. 2021;56(4):e4644. doi: 10.1002/jms.4644. [DOI] [PubMed] [Google Scholar]
- 15.Rotenberg M, Shefi M, Dany S, Dore I, Tirosh M, Almog S. Differentiation between organophosphate and carbamate poisoning. Clin Chim Acta. 1995;234:11–21. doi: 10.1016/0009-8981(94)05969-y. [DOI] [PubMed] [Google Scholar]
- 16.Huang MZ, Yuan CH, Cheng SC, Cho YT, Shiea J. Ambient ionization mass spectrometry. Annu Rev Anal Chem. 2010;3:43–65. doi: 10.1146/annurev.anchem.111808.073702. [DOI] [PubMed] [Google Scholar]
- 17.Souza-Silva ÉA, Reyes-Garcés N, Gómez-Ríos GA, Boyac E, Bojko B, Pawliszyn J. A critical review of the state of the art of solid-phase microextraction of complex matrices III. Bioanalytical and clinical applications. Trends Anal Chem. 2015;71:249–64. [Google Scholar]
- 18.Wang CH, Su H, Chou JH, Huang MZ, Lin HJ, Shiea J. Solid phase microextraction combined with thermal-desorption electrospray ionization mass spectrometry for high-throughput pharmacokinetics assays. Anal Chim Acta. 2018;1021:60–8. doi: 10.1016/j.aca.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Wang CH, Su H, Chou JH, Lin JY, Huang MZ, Lee CW, et al. Multiple solid phase microextraction combined with ambient mass spectrometry for rapid and sensitive detection of trace chemical compounds in aqueous solution. Anal Chim Acta. 2020;1107:101–6. doi: 10.1016/j.aca.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Fang L, Deng J, Yang Y, Wang X, Chen B, Liu H, et al. Coupling solid-phase microextraction with ambient mass spectrometry: strategies and applications. Trends Anal Chem. 2016;85:61–72. [Google Scholar]
- 21.Kuo CP, Shiea J. Application of direct electrospray probe to analyze biological compounds and to couple to solid-phase microextraction to detect trace surfactants in aqueous solution. Anal Chem. 1999;71:4413–7. doi: 10.1021/ac990049r. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Wang H, Dong G, Musselman BD, Liu CC, Guo Y. Combination of solid-phase micro-extraction and direct analysis in real time-fourier transform ion cyclotron resonance mass spectrometry for sensitive and rapid analysis of 15 phthalate plasticizers in beverages. Chin J Chem. 2015;33:213–9. [Google Scholar]
- 23.Gómez-Ríos GA, Pawliszyn J. Solid phase microextraction (SPME)-transmission mode (TM) pushes down detection limits in direct analysis in real time (DART) Chem Commun. 2014;50:12937–40. doi: 10.1039/c4cc05301j. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy JH, Aurand C, Shirey R, Laughlin BC, Wiseman JM. Coupling desorption electrospray ionization with solid-phase microextraction for screening and quantitative analysis of drugs in urine. Anal Chem. 2010;82:7502–8. doi: 10.1021/ac101295g. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad S, Tucker M, Spooner N, Murnane D, Gerhard U. Direct ionization of solid-phase microextraction fibers for quantitative drug bioanalysis: from peripheral circulation to mass spectrometry detection. Anal Chem. 2015;87:754–9. doi: 10.1021/ac503706n. [DOI] [PubMed] [Google Scholar]
- 26.Mirabelli MF, Wolf JC, Zenobi R. Direct coupling of solid-phase microextraction with mass spectrometry: sub-pg/g sensitivity achieved using a dielectric barrier discharge ionization source. Anal Chem. 2016;88:7252–8. doi: 10.1021/acs.analchem.6b01507. [DOI] [PubMed] [Google Scholar]
- 27.Hu B, So PK, Chen H, Yao ZP. Electrospray ionization using wooden tips. Anal Chem. 2011;83:8201–7. doi: 10.1021/ac2017713. [DOI] [PubMed] [Google Scholar]
- 28.Deng J, Yang Y, Xu M, Wang X, Lin L, Yao ZP, et al. Surface-coated probe nanoelectrospray ionization mass spectrometry for analysis of target compounds in individual small organisms. Anal Chem. 2015;87:9923–30. doi: 10.1021/acs.analchem.5b03110. [DOI] [PubMed] [Google Scholar]
- 29.Tascon M, Gómez-Ríos GA, Reyes-Garcés N, Poole JJ, Boyaci E, Pawliszyn J. High-throughput screening and quantitation of target compounds in biofluids by coated blade spray-mass spectrometry. Anal Chem. 2017;89:8421–8. doi: 10.1021/acs.analchem.7b01877. [DOI] [PubMed] [Google Scholar]
- 30.Lee CW, Su H, Cai YD, Wu MT, Wu DC, Shiea J. Rapid identification of psychoactive drugs in drained gastric lavage fluid and whole blood specimens of drug overdose patients using ambient mass spectrometry. Mass Spectrom. 2017;6:S0056. doi: 10.5702/massspectrometry.S0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Total DI-SPME-TD-ESI/MS ion current during SPME fiber conditioning. (b) TD-ESI mass spectrum of background signals before inserting the SPME fiber in the TD-ESI source, and TD-ESI mass spectra recorded immediately (c) after inserting a new SPME fiber, and (d) after the SPME fiber had remained in the TD source for 2 min.
MS/MS spectra of six pesticide standards each showing the precursor ion (●) and two major product ions (○): (a) fenthion, (b) chlorpyrifos, (c) ethion, (d) methomyl, (e) carbaryl, and (f) methiocarb.
Repeatability tests for ten consecutive DI-SPME-TD-ESI/MS/MS analyses of (a) ethion and (b) carbaryl spiked in diluted human serum (5 μg/mL each). The coefficients of variation of ethion and carbaryl ions were 18.74% and 18.4%, respectively.
The linear responses of the detection of six pesticides by SPME-TD-ESI/MS/MS: (a) fenthion, (b) chlorpyrifos, (c) ethion, (d) methomyl, (e) carbaryl, and (f) methiocarb with concentrations between 0.1 μg/mL and 100 μg/mL and R2 between 0.9827 and 0.9992.
TIC for (a) chlorpyrifos and (b) ethion standards spiked in diluted human serum that was solvent-extracted and concentrated prior to GC/MS/ MS analysis.
TIC for (a) carbaryl, (b) methiocarb, (c) fenthion, and (d) methomyl standards spiked in diluted human serum that was solvent-extracted and concentrated prior to LC/MS/MS analysis.