This randomized clinical trial assesses if epigallocatechin-3-gallate solution reduces the incidence of radiation-induced dermatitis in patients undergoing radiotherapy after breast cancer surgery.
Key Points
Question
Does epigallocatechin-3-gallate (EGCG) reduce the occurrence of radiation-induced dermatitis (RID) for patients with breast cancer receiving adjuvant radiotherapy?
Findings
In this phase 2 randomized clinical trial of 180 patients, grade 2 or worse RID occurred in 50.5% of participants treated with EGCG solution and 72.2% with placebo, a statistically significant difference. Furthermore, symptom indexes were also significantly lower in patients receiving EGCG with a safety profile.
Meaning
The safety profile and prophylactic effect of topical EGCG solution may offer a convenient, well-tolerated, and valid option for patients with breast cancer who are at risk for RID.
Abstract
Importance
Safe and effective prophylactic therapies for radiation-induced dermatitis (RID) remain an unmet need.
Objective
To determine if epigallocatechin-3-gallate (EGCG) solution reduces the incidence of RID in patients undergoing radiotherapy after breast cancer surgery.
Design, Setting, and Participants
This phase 2 double-blind, placebo-controlled randomized clinical trial enrolled 180 patients with breast cancer receiving postoperative radiotherapy at Shandong Cancer Hospital and Institute in Shandong, China, between November 2014 and June 2019. Data analysis was performed from September 2019 to January 2020.
Interventions
Participants were randomly assigned (2:1) to receive either EGCG solution (660 μmol/L) or placebo (0.9% NaCl saline) sprayed to the whole radiation field from day 1 of the radiation until 2 weeks after radiation completion.
Main Outcomes and Measures
The primary end point was incidence of grade 2 or worse RID, defined by the Radiation Therapy Oncology Group scale. The secondary end points included RID index (RIDI), symptom index, changes in the skin temperature measured by infrared thermal images, and safety.
Results
A total of 180 eligible patients were enrolled, of whom 165 (EGCG, n = 111; placebo, n = 54) were evaluable for efficacy (median [range] age, 46 [26-67] years). The occurrence of grade 2 or worse RID was significantly lower (50.5%; 95% CI, 41.2%-59.8%) in the EGCG group than in the placebo group (72.2%; 95% CI, 60.3%-84.1%) (P = .008). The mean RIDI in the EGCG group was significantly lower than that in the placebo group. Furthermore, symptom indexes were significantly lower in patients receiving EGCG. Four patients (3.6%) had adverse events related to the EGCG treatment, including grade 1 pricking skin sensation (3 [2.7%]) and pruritus (1 [0.9%]).
Conclusions and Relevance
In this randomized clinical trial, prophylactic use of EGCG solution significantly reduced the incidence and severity of RID in patients receiving adjuvant radiotherapy for breast cancer. It has the potential to become a new choice of skin care for patients receiving radiotherapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02580279
Introduction
Breast cancer is the leading malignant neoplasm in women worldwide.1 Radiotherapy is essential for patients with breast cancer who received mastectomy or breast-conserving surgery, to prolong local control time and overall survival.2,3,4 The most common adverse event associated with breast cancer radiotherapy is radiation-induced dermatitis (RID). Even though RID is very rarely life-threatening, its bothersome symptoms may cause treatment interruptions and even earlier treatment terminations without the delivery of the prescribed total radiation dose, which might compromise the outcome. There is no recognized standard approach to prevent RID.5,6,7,8 As stated in the guidelines of the Multinational Association of Supportive Care in Cancer Skin Toxicity Study Group,9 the strongest evidence was from the Miller trial,5 in which prophylactic mometasone showed only significant reduction in discomfort or burning and itching, not RID grade. More recently, the guidelines of the Oncology Nursing Society recommended the use of topical steroids to minimize RID, but strength of recommendation is conditional, and certainty of evidence is low.10 An American Society for Radiation Oncology editorial also stated that the guideline authors found many flaws in the studies performed that limited the strength of the recommendations.11 Therefore, more clinical strategies need to be established to prevent RID.
Epigallocatechin-3-gallate (EGCG), the major and most highly bioactive constituent in green tea, is responsible for its biochemical and pharmacological effects.12,13,14,15 A retrospective study suggested that green tea extracts helped to restore the skin integrity in patients with grades 2 or higher RID receiving head and neck radiotherapy.16 We performed a phase 1 dose-escalating trial for treating RID using EGCG in breast cancer radiotherapy, which demonstrated that the topical administration of EGCG solution is well tolerated, and the maximum tolerated dose was not found.17 Subsequently, we conducted a single-arm phase 2 trial and demonstrated that topical EGCG reduced 71.4% to 89.8% of RID symptoms during adjuvant radiotherapy after modified radical mastectomy.18 There was no study to investigate whether topical EGCG could prevent RID. Based on these encouraging results, we performed the current trial. The purpose of this placebo-controlled phase 2 randomized clinical trial was to investigate the prevention effects of EGCG solution vs placebo in patients with breast cancer who received postmastectomy adjuvant radiotherapy.
Methods
Study Design and Participants
This trial was conducted at Shandong Cancer Hospital and Institute in Shandong, China. Eligibility criteria included women 18 years or older who had histologically confirmed breast cancer; with 2 to 3 weeks after the completion of adjuvant chemotherapy; Eastern Cooperative Oncology Group performance status of 0 to 1; adequate hematologic, hepatic, and kidney function profile; and no prior radiotherapy to the thorax. Exclusion criteria included patients with unhealed wounds in the radiation area; receiving other anticancer therapies except concurrent endocrine therapy or anti-ERBB2 (formerly HER2) therapy; with severe or uncontrolled medical conditions, pregnancy, or lactation; and a known allergy or hypersensitivity to EGCG (trial protocol in Supplement 1). There was no restriction regarding neoadjuvant or adjuvant chemotherapy regimens. The study protocol and informed consent were reviewed and approved by local institutional review and ethical committees. Written informed consent was provided by each participant. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Masking
Considering the good effect of EGCG in previous studies, a 2:1 randomization (EGCG:placebo) was conducted based on patient benefit and adherence.18,19 The allocation was performed by telephone with a computer-generated list, using a randomly permuted block design. Participants, investigators, and research staff assessing the participants were blinded to the treatment allocation. Just before the intervention, the investigator contacted the data center by telephone to receive the allocation group.
Radiotherapy
The simulation was performed by a big-bore spiral computed tomography (Philips Medical Systems) on BreastBoards (CIVCO Radiotherapy). Eclipse treatment planning system (Eclipse 8.6, Varian Medical Systems) was used for radiotherapy planning. The plan was designed using 6-MV beams to the chest/breast, supraclavicular area, and the internal mammary nodes according to N stage. Additional boluses were used in light of chest-wall thickness variation of all patients receiving postmastectomy radiotherapy. Patients with modified radical mastectomy received irradiation at a dose of 50 Gy in 25 fractions. Patients receiving breast-conserving surgery underwent simultaneous integrated boost (50-Gy whole-breast irradiation and 57.5-Gy tumor bed boost), sequential boost (50 Gy plus 10 Gy boost), or only 50 Gy whole-breast irradiation, at the physician’s discretion. The protocol allowed for dose variations between 95% and 105% of the reference point on the central axis.20,21
Procedures
EGCG or Placebo Administration
The EGCG (95% purity by high-performance liquid chromatography) was purchased from HEP Biotech Co, Ltd, and its solution was freshly prepared (660 μmol/L). The application of EGCG solution or placebo (0.9% saline solution) was initiated from day 1 of radiotherapy until 2 weeks after radiotherapy completion. The EGCG or placebo solutions were uniformly sprayed on the whole radiation field using a sterilized medical sprayer, 3 times a day at 0.05 mL/cm2. The skin folds, such as the armpits, required a full stretch and exposure before spraying. The patients followed general good skin-care practices at the start of radiotherapy. No deodorant, lotion, cream, perfume, or any topical agent was allowed during radiotherapy.
RID Evaluation
Evaluation of RID was performed weekly by 2 investigators who were unaware of the patient’s clinical history and treatment allocation according to the Radiation Therapy Oncology Group (RTOG) scale (0 = no change; 4 = the worst impairment; eTable 1 in Supplement 2).6 The grade is closely related to the degree of impairment. Higher grade indicates more severe impairment. Grade 2 or higher RID at any assessment time point during EGCG or placebo application is considered as having grade 2 or worse RID.
The RID grades for each time point also were plotted on a graph against time. The area under the curve, as RID index (RIDI), was calculated for each patient’s graph using the trapezoidal method. Therefore, RIDI are quantitative data, with higher score indicating longer duration of high-grade skin RID.
RID-Related Symptom Evaluation
Patients reported RID-related symptoms (including pain, burning feeling, itching, pulling, and tenderness) using the Skin Toxicity Assessment Tool (0 = no symptom; 5 = the worst symptoms) once a week.17,18,22 The highest score at each assessment time point was considered as having personal highest symptom score. A symptom score 2 or greater at any assessment time point was regarded as the symptom score of the individual patient greater than or equal to grade 2.
The same RIDI calculation method was used for the 5 RID-related symptom index.19 The higher the index, the longer the severe symptoms persisted.
Skin Temperature Measurement
Skin temperature was measured weekly from March 2019 with the approval of the ethics committee owing to its ability to provide relatively objective measurement for skin injury. Difference in skin temperature (DST) was defined as the value of the irradiated breast or the chest wall minus that of the contralateral region. High-grade RID was associated with an additional increase in DST, which is probably associated with the intense inflammatory reaction.23,24 The maximum increase in DST was determined by subtracting the baseline value from the maximum value during the entire observation period. A frontal thermal image of the torso from the neck to upper abdomen was obtained 2 hours before routine radiotherapy using a digital infrared thermal imager (FLIR E5 Serial No. 63985976).
Safety Assessments
Safety assessments were performed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Adverse events were monitored using laboratory tests, such as complete blood cell count, chemistry profile, and liver function tests. Chest computed tomography images were acquired at baseline and 1 month after the end of radiotherapy to evaluate lung toxicity.
Rescue Treatment
Radiotherapy was interrupted in patients who developed grade 3 skin reactions characterized by confluent moist desquamation. Administration of EGCG or placebo was discontinued at the physician’s discretion. The wound dressing with normal saline was performed daily under sterile conditions, and antibiotics were given when necessary.
Outcomes
The primary end point was incidence of grade 2 or worse RID. The secondary end points included RIDI, the RID-related symptoms, the maximum increase in DST, and safety.
Statistical Analysis
Based on documentations during RID prevention,5,8,25,26,27,28 the incidence of grade 2 or higher RID observed during breast irradiation was about 75% in historical studies and 53% in topical steroids trials. According to the previous research experience of EGCG,17,18 we hypothesized that its preventive effect should be no worse than that of topical steroids at least. To detect this difference with a power of 0.80 using a 2-sided test at significance level of .05, it was necessary to recruit 162 patients. Assuming a 10% attrition rate, a total of 180 patients would be required.
The RTOG scores, symptom scores, and safety assessments were recorded weekly from the start of radiotherapy to 2 weeks after its completion. The efficacy analysis set included patients in group and subgroup who completed the composite assessment by the investigators.
Statistical analyses were performed using SPSS Statistics for Windows, version 17.0 (SPSS Inc). Measurement data of the different groups are expressed as mean and SD and analyzed by t test. The qualitative measures were compared by the χ2 test or Fisher exact test, as appropriate. Scores of 2 or more RID symptoms and their hazard ratios were calculated and compared with Mantel-Haenszel analysis. All the P values are 2-sided. The statistical analysis plan is detailed in Supplement 3.
Results
Between November 2014 and June 2019, 191 patients were screened, and 180 eligible patients were enrolled. Among them, 165 (EGCG, n = 111; placebo, n = 54) were evaluable for efficacy (Figure 1). The characteristics of the fully eligible patients were similar between the 2 groups (Table 1). The median (range) age of the 165 evaluable patients was 46 (26-67) years; 2 (1.2%) and 114 (69.1%) patients had smoking history and Eastern Cooperative Oncology Group performance status of 1, respectively.
Figure 1. Enrollment and Randomization of the Study Patients.
EGCG indicates epigallocatechin-3-gallate.
Table 1. Demographic and Clinical Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| EGCG (n = 111) | Placebo (n = 54) | |
| Age, mean (SD), y | 46.5 (8.1) | 48.4 (9.5) |
| BMI, mean (SD) | 25.8 (3.5) | 25.6 (2.8) |
| Breast sizea | ||
| Small | 22 (19.8) | 6 (11.1) |
| Medium | 66 (59.5) | 39 (72.2) |
| Large | 23 (20.7) | 9 (16.7) |
| Tumor location | ||
| Left | 63 (56.8) | 25 (46.3) |
| Right | 48 (43.3) | 29 (53.7) |
| Type of surgery | ||
| Breast-conserving | 19 (17.1) | 9 (16.7) |
| Mastectomy | 92 (82.9) | 45 (83.3) |
| Menopause | ||
| No | 82 (73.9) | 32 (59.3) |
| Yes | 29 (26.1) | 22 (40.7) |
| ECOG performance status | ||
| 0 | 33 (29.7) | 18 (33.3) |
| 1 | 78 (70.3) | 36 (66.7) |
| Smoking history | ||
| No | 110 (99.1) | 53 (98.1) |
| Yes | 1 (0.9) | 1 (1.9) |
| pT stage | ||
| T1 | 47 (42.3) | 16 (29.6) |
| T2 | 53 (47.7) | 28 (51.9) |
| T3 | 7 (6.3) | 5 (9.3) |
| T4 | 4 (3.6) | 5 (9.3) |
| pN stage | ||
| N0 | 14 (12.6) | 8 (14.8) |
| N1 | 46 (41.4) | 17 (31.5) |
| N2 | 28 (25.2) | 12 (22.2) |
| N3 | 23 (20.7) | 17 (31.5) |
| Pathological type | ||
| Ductal | 98 (88.3) | 46 (85.2) |
| Lobular | 12 (10.8) | 6 (11.1) |
| Other | 1 (0.9) | 2 (3.7) |
| Estrogen receptor status | ||
| Negative | 29 (26.1) | 16 (29.6) |
| Positive | 82 (73.9) | 38 (70.4) |
| Progesterone receptor status | ||
| Negative | 45 (40.5) | 20 (37.0) |
| Positive | 66 (59.5) | 34 (63.0) |
| ERBB2 status | ||
| Negative | 77 (69.4) | 37 (68.5) |
| Positive | 32 (28.8) | 17 (31.5) |
| Unknown | 2 (1.8) | 0 |
| Ki-67 value, mean (SD) | 33.5 (24.0) | 35.9 (24.0) |
| Adjuvant systemic therapy during study | ||
| None | 37 (33.3) | 23 (42.6) |
| Tamoxifen | 52 (46.8) | 25 (46.3) |
| Trastuzumab | 22 (19.8) | 6 (11.1) |
| Chemotherapy | 0 | 0 |
| Radiotherapy field | ||
| Chest + supraclavicular area | 42 (37.8) | 17 (31.5) |
| Chest + supraclavicular + IM | 50 (45.0) | 28 (51.9) |
| Whole breast + tumor bed | 18 (16.2) | 7 (13.0) |
| Whole breast + tumor bed + IM | 0 | 1 (1.9) |
| Whole breast | 1 (1.9) | 1 (1.9) |
| Radiotherapy dose | ||
| 50 Gy in 25 fractions | 92 (82.9) | 45 (83.3) |
| 57.5 Gy in 25 fractions | 5 (4.5) | 3 (5.6) |
| 60 Gy in 30 fractions | 14 (12.6) | 6 (11.1) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; EGCG, epigallocatechin-3-gallate; ECOG, Eastern Cooperative Oncology Group; IM, internal mammary lymph nodes.
Breast size was grouped according to the Radiation Therapy Oncology Group 97-13 study.6
The incidence of grade 2 or worse RID was significantly lower in the EGCG group at 50.5% (95% CI, 41.2%-59.8%) than that in the placebo group (72.2%; 95% CI, 60.3%-84.1%; P = .008). There was a tendency that grade 3 or worse RID in the EGCG group was lower (4 of 111 [3.6%] vs 5 of 54 [9.3%]), but it did not reach statistical significance (P = .16). The mean (SD) RIDI of patients in the EGCG group was also significantly lower (5.22 [1.60]) than that in the placebo group (5.22 [1.60] vs 6.21 [1.56]; t = −3.79; P < .001). The highest scores of RID-related symptoms were also significantly lower after EGCG treatment compared with those of the placebo group at the end of the study, except with the pulling score (Table 2). The incidence of grade 2 or worse burning feeling, itching, pain, and tenderness scores were all significantly lower in the EGCG group than in the placebo group (Figure 2A). Differences were statistically significant in all symptom indexes (Figure 3).
Table 2. The Highest Score of RID and Symptoma.
| Score | No. (%) | P value | |
|---|---|---|---|
| EGCG (n = 111) | Placebo (n = 54) | ||
| RID | |||
| 1 | 55 (49.5) | 15 (27.8) | .01 |
| 2 | 52 (46.8) | 34 (63.0) | |
| 3 | 4 (3.6) | 5 (9.3) | |
| Burning feeling | |||
| 0 | 24 (21.6) | 3 (5.6) | .001 |
| 1 | 65 (58.6) | 27 (50.0) | |
| 2 | 22 (19.8) | 24 (44.4) | |
| Itching | |||
| 0 | 4 (3.6) | 0 | <.001 |
| 1 | 59 (53.2) | 13 (24.1) | |
| 2 | 46 (41.4) | 39 (72.2) | |
| 3 | 2 (1.8) | 2 (3.7) | |
| Pulling | |||
| 0 | 5 (4.5) | 1 (1.9) | .27 |
| 1 | 95 (85.6) | 43 (79.6) | |
| 2 | 11 (9.9) | 10 (18.5) | |
| Pain | |||
| 0 | 34 (30.6) | 9 (16.7) | .03 |
| 1 | 62 (55.9) | 30 (55.6) | |
| 2 | 15 (13.5) | 13 (24.1) | |
| 3 | 0 | 2 (3.7) | |
| Tenderness | |||
| 0 | 58 (52.3) | 15 (27.8) | .002 |
| 1 | 29 (26.1) | 14 (25.9) | |
| 2 | 24 (21.6) | 25 (46.3) | |
Abbreviations: EGCG, epigallocatechin-3-gallate; RID, radiation-induced dermatitis.
The highest score of RID (or RID-related symptom) at each assessment time point is considered as having personal highest RID (symptom) score.
Figure 2. Forest Plots of Grade 2 or Greater Radiation-Induced Symptoms.
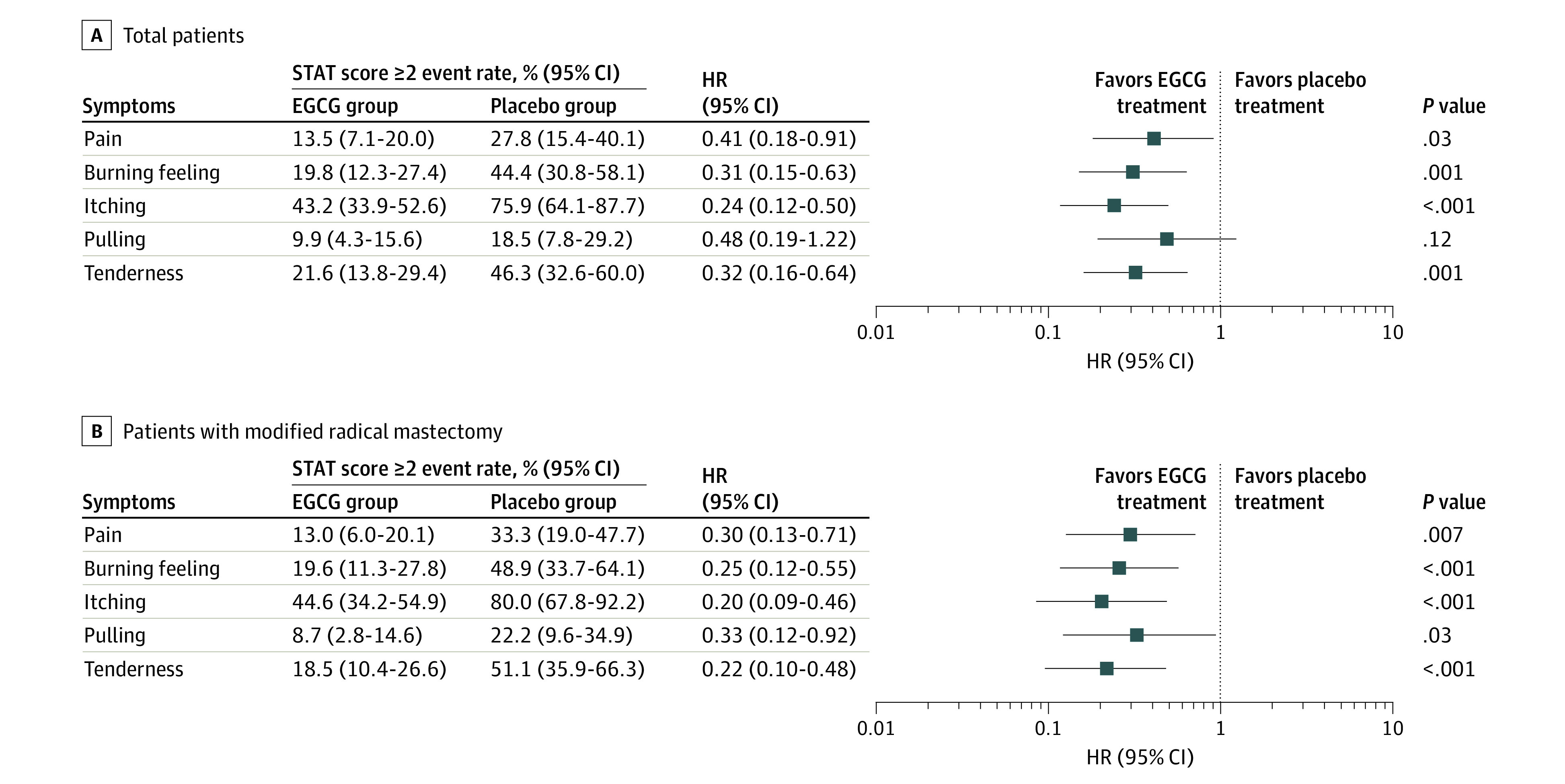
Positions of the squares in the forest plot show the estimate of the hazard ratio (HR) describing the relative effect of epigallocatechin-3-gallate (EGCG) compared with the placebo, with the 95% CI represented by the horizontal lines. Squares to the left of the vertical line indicate when the adverse effects rates were lower in the EGCG group compared with the placebo. A, In patients who were enrolled and eligible; B, in patients who had modified radical mastectomy only. STAT indicates Skin Toxicity Assessment Tool.
Figure 3. Comparison of Radiation-Induced Dermatitis Index (RIDI) and Symptom Indexes Between the Epigallocatechin-3-Gallate (EGCG) and Placebo Groups.
Error bars indicate SDs.
The distributions of the maximum RID and symptom score for the 2 groups are detailed in eFigure 1 in Supplement 2. As radiation dose increased, the average RID-related scores of patients in the EGCG group gradually reduced compared with those in the placebo group, and the difference reached the peak in the sixth week. For most patients, RID began to occur 2 to 3 weeks after the beginning of radiotherapy, but the mean (SD) appearance time of RID in the EGCG group was delayed (3.27 [0.86] weeks) compared with that in the placebo group (2.89 [0.60] weeks) (P = .001).
We conducted a subgroup analysis of patients with modified radical mastectomy (n = 137; eTable 2 in Supplement 2). The incidence of grade 2 or worse RID, burning feeling, itching, pulling, pain, and tenderness scores were all significantly lower in the EGCG group than in the placebo group (Figure 2B). The maximum scores of RID and radiation-induced symptoms were also significantly lower after EGCG treatment compared with the placebo (eTable 3 in Supplement 2). Differences were also statistically significant in RIDI and all symptom indexes (eFigure 2 in Supplement 2).
Of the 165 patients, 80 (48.5%; EGCG, n = 54; placebo, n = 26) underwent skin temperature measurements at baseline until 2 weeks after radiotherapy completion (eTable 4 in Supplement 2). The mean (SD) maximum increase in DST of 1.18 (0.73) (EGCG group) and 1.51 (0.99) (placebo group) showed no significant difference (t = −1.68; P = .10). A representative patient per group, with thermography and photography weekly, is shown in eFigure 3 in Supplement 2. The DST increased significantly as the radiotherapy progressed, as the thermography showed, and RID was also generally aggravated, as shown in the photograph. The RID score was the highest 1 week after the end of radiotherapy, and DST also reached the peak.
No severe adverse events were judged to be related to EGCG or placebo application (eTable 5 in Supplement 2). For the local skin of drug application, a total of 6 (3.6%) patients expressed discomfort within 10 minutes after drug application, which was considered to be related to radiotherapy and local drug application. Among them, 2 (3.7%) patients in the control group had grade 1 and grade 2 pricking skin sensation, respectively, and 3 (2.7%) patients in the EGCG group had grade 1 pricking skin sensation. One (0.9%) patient with EGCG application had grade 1 pruritus. The 2 symptoms were temporary and self-remitting and were not treated with other drugs.
Discussion
Based on phases 1 to 2 clinical studies,17,18 we conducted this phase 2 randomized clinical trial, which demonstrated that prophylactic use of EGCG solution significantly reduced the incidence and severity of RID in patients with breast cancer receiving adjuvant radiotherapy.17,18 These findings may establish EGCG as an inexpensive and broadly available preventive agent for skin toxicity caused by ionizing radiation.
An increasing amount of clinical evidence provides guidance for RID prophylactic strategies.5,6,7 The phase 3 RTOG 97-13 trial revealed that an emollient cream, Biafine, did not reduce the skin toxicity or improve the quality of life compared with best supportive care during adjuvant radiotherapy for breast cancer.6 There is no evidence to support the prophylactic application of either the sucralfate or the aqueous cream tested for the prevention of radiation skin reactions.7 Local steroids have some positive outcomes for the prevention of acute RID in patients with breast cancer treated with adjuvant radiotherapy.5,29 However, some recent clinical trials from India and Japan had contradictory results in patients with head and neck cancer receiving radiation. These studies suggest that the effect of topical steroids is strongly advantageous for symptom management for RID, rather than prophylactic intervention.30,31 Therefore, many experts state that there is currently no high-level evidence-based standard for RID.32 Recently, some bioactive components extracted from natural plants have gradually stood out among many potential new radiation protective agents.
Green tea extract and its principal active ingredient, EGCG, are gaining attention with increased usage for radiation-induced damage, owing to their healthful properties. However, the molecular mechanisms underlying the beneficial effects of EGCG are complex. Treatment of human skin with EGCG inhibits UV radiation–induced oxidative stress.13 Topical EGCG can protect human skin against UV-B–induced reactive oxygen species–associated inflammatory dermatoses, photoaging, and photocarcinogenesis.14 Zhu et al15 found that pretreatment with EGCG significantly enhanced the viability of the human skin cells that were irradiated with x-rays, with decreased apoptosis induced by x-ray irradiation. Use of EGCG induced the expression of the cytoprotective molecule heme oxygenase-1 in a dose-dependent manner via transcriptional activation.
Many sophisticated techniques are applied to breast cancer adjuvant radiotherapy.33 In the past decade, different hypofractionated radiotherapy schemes were introduced, and patients receiving hypofractionated radiotherapy showed less skin toxicity compared with those receiving conventional fractionated radiotherapy.34 To standardize the patient characteristics, the present study only enrolled patients receiving conventional fractional radiotherapy. Because hypofractionated radiotherapy is more widely used in whole-breast radiotherapy after breast-conserving surgery, fewer patients were enrolled in this study who received conventional fractionated radiotherapy; hence, further subgroup analysis was not performed. We plan to conduct further prospective studies in patients with breast cancer receiving hypofractionated radiotherapy to determine the effectiveness of topical EGCG.
In the process of developing the clinical preparations for the external use of EGCG, there are several important problems to be solved. First, its safety must be verified. A previous study showed that topical EGCG preparations caused minor dermal irritation in rats and guinea pigs, but not in rabbits, and was a moderate dermal sensitizing agent in the guinea pig maximization test.35 We conducted a dose-escalating study for topical EGCG (140 μmol/L to 660 μmol/L) in breast cancer radiotherapy, which demonstrated that the topical administration of EGCG was well tolerated.17 Because the maximum tolerated dose was not known, the highest dose in the phase 1 trial (660 μmol/L) was defined as the recommended dose in the phase 2 trial18 and in the current study. In addition, it is necessary to evaluate the human skin penetration of EGCG in certain formulations. A previous study showed that EGCG can reach all the skin layers when the green tea extract was vehiculated in a cosmetic formulation.36 Topical application of EGCG in hydrophilic ointment USP to human or mouse skin resulted in substantial intradermal uptake of up to 1% to 20% of the applied dose.37 Freshly prepared EGCG saline solution was used in the present study, which might limit its actions. Ointment formulation is being developed to improve the skin penetration and intradermal uptake and facilitate further multicenter studies.
Limitations
There are some limitations to the present study. First, the study was a single-center, phase 2 trial. The results need to be validated in multicenter phase 3 national or international trials. Second, not all the patients received infrared skin temperature measurement; therefore, the reduction in DST did not reach statistical significance. Thermography was reported in 2018 to be used for the evaluation of acute skin toxicity due to breast radiotherapy.23 Our previous study also confirmed that RID was associated with thermographic response.24 This approach needs to be further optimized to be more suitable as a clinical application.
Conclusions
In this randomized clinical trial, prophylactic use of EGCG solution significantly reduced the incidence and severity of RID in patients with breast cancer receiving adjuvant radiotherapy. Use of EGCG has the potential to become a new standard of skin care for patients receiving radiotherapy.
Trial Protocol
eFigure 1. Change in RID and symptom scores between epigallocatechin-3-gallate and placebo groups
eFigure 2. The comparison of different RID-related indexes of patients with modified radical mastectomy between two groups
eFigure 3. The representative cases in the epigallocatechin-3-gallate (panel A) and placebo groups (panel B)
eTable 1. Radiation Therapy Oncology Group score
eTable 2. The characteristics of patients who received modified radical mastectomy
eTable 3. The highest RID-related scores of patients who received modified radical mastectomy
eTable 4. The characteristics of patients who underwent skin temperature measurements
eTable 5. Adverse events
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Oluwasanu M, Olopade OI. Global disparities in breast cancer outcomes: new perspectives, widening inequities, unanswered questions. Lancet Glob Health. 2020;8(8):e978-e979. doi: 10.1016/S2214-109X(20)30307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartelink H, Maingon P, Poortmans P, et al. ; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups . Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47-56. doi: 10.1016/S1470-2045(14)71156-8 [DOI] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-2106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-1241. doi: 10.1056/NEJMoa022152 [DOI] [PubMed] [Google Scholar]
- 5.Miller RC, Schwartz DJ, Sloan JA, et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys. 2011;79(5):1460-1466. doi: 10.1016/j.ijrobp.2010.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48(5):1307-1310. doi: 10.1016/S0360-3016(00)00782-3 [DOI] [PubMed] [Google Scholar]
- 7.Wells M, Macmillan M, Raab G, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? a randomised controlled trial. Radiother Oncol. 2004;73(2):153-162. doi: 10.1016/j.radonc.2004.07.032 [DOI] [PubMed] [Google Scholar]
- 8.Hindley A, Zain Z, Wood L, et al. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2014;90(4):748-755. doi: 10.1016/j.ijrobp.2014.06.033 [DOI] [PubMed] [Google Scholar]
- 9.Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933-2948. doi: 10.1007/s00520-013-1896-2 [DOI] [PubMed] [Google Scholar]
- 10.Gosselin T, Ginex PK, Backler C, et al. ONS guidelines for cancer treatment-related radiodermatitis. Oncol Nurs Forum. 2020;47(6):654-670. doi: 10.1188/20.ONF.654-670 [DOI] [PubMed] [Google Scholar]
- 11.Marquez CM, Wong W. ASTRO editorial: ONS guidelines for cancer treatment-related radiodermatitis. Pract Radiat Oncol. 2021;11(5):352-353. doi: 10.1016/j.prro.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Kada T, Kaneko K, Matsuzaki S, Matsuzaki T, Hara Y. Detection and chemical identification of natural bio-antimutagens. a case of the green tea factor. Mutat Res. 1985;150(1-2):127-132. doi: 10.1016/0027-5107(85)90109-5 [DOI] [PubMed] [Google Scholar]
- 13.Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22(2):287-294. doi: 10.1093/carcin/22.2.287 [DOI] [PubMed] [Google Scholar]
- 14.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69(2):148-153. doi: [DOI] [PubMed] [Google Scholar]
- 15.Zhu W, Xu J, Ge Y, et al. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J Radiat Res. 2014;55(6):1056-1065. doi: 10.1093/jrr/rru047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajonk F, Riedisser A, Henke M, McBride WH, Fiebich B. The effects of tea extracts on proinflammatory signaling. BMC Med. 2006;4:28. doi: 10.1186/1741-7015-4-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Zhu W, Jia L, et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br J Radiol. 2016;89(1058):20150665. doi: 10.1259/bjr.20150665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W, Jia L, Chen G, et al. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget. 2016;7(30):48607-48613. doi: 10.18632/oncotarget.9495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Jia L, Chen G, et al. A prospective, three-arm, randomized trial of EGCG for preventing radiation-induced esophagitis in lung cancer patients receiving radiotherapy. Radiother Oncol. 2019;137:186-191. doi: 10.1016/j.radonc.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 20.De Rose F, Fogliata A, Franceschini D, et al. Hypofractionation with simultaneous boost in breast cancer patients receiving adjuvant chemotherapy: a prospective evaluation of a case series and review of the literature. Breast. 2018;42:31-37. doi: 10.1016/j.breast.2018.08.098 [DOI] [PubMed] [Google Scholar]
- 21.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14(13):1269-1277. doi: 10.1016/S1470-2045(13)70497-2 [DOI] [PubMed] [Google Scholar]
- 22.Neben-Wittich MA, Atherton PJ, Schwartz DJ, et al. Comparison of provider-assessed and patient-reported outcome measures of acute skin toxicity during a phase III trial of mometasone cream versus placebo during breast radiotherapy: the North Central Cancer Treatment Group (N06C4). Int J Radiat Oncol Biol Phys. 2011;81(2):397-402. doi: 10.1016/j.ijrobp.2010.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maillot O, Leduc N, Atallah V, et al. Evaluation of acute skin toxicity of breast radiotherapy using thermography: results of a prospective single-centre trial. Cancer Radiother. 2018;22(3):205-210. doi: 10.1016/j.canrad.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Zhu W, Jia L, Chen G, et al. Relationships between the changes of skin temperature and radiation skin injury. Int J Hyperthermia. 2019;36(1):1160-1167. doi: 10.1080/02656736.2019.1685685 [DOI] [PubMed] [Google Scholar]
- 25.Freedman GM, Li T, Nicolaou N, Chen Y, Ma CC, Anderson PR. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys. 2009;74(3):689-694. doi: 10.1016/j.ijrobp.2008.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho AY, Olm-Shipman M, Zhang Z, et al. A randomized trial of mometasone furoate 0.1% to reduce high-grade acute radiation dermatitis in breast cancer patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2018;101(2):325-333. doi: 10.1016/j.ijrobp.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omidvari S, Saboori H, Mohammadianpanah M, et al. Topical betamethasone for prevention of radiation dermatitis. Indian J Dermatol Venereol Leprol. 2007;73(3):209. doi: 10.4103/0378-6323.32755 [DOI] [PubMed] [Google Scholar]
- 28.Shukla PN, Gairola M, Mohanti BK, Rath GK. Prophylactic beclomethasone spray to the skin during postoperative radiotherapy of carcinoma breast: a prospective randomized study. Indian J Cancer. 2006;43(4):180-184. doi: 10.4103/0019-509X.29424 [DOI] [PubMed] [Google Scholar]
- 29.Ulff E, Maroti M, Serup J, Nilsson M, Falkmer U. Prophylactic treatment with a potent corticosteroid cream ameliorates radiodermatitis, independent of radiation schedule: a randomized double blinded study. Radiother Oncol. 2017;122(1):50-53. doi: 10.1016/j.radonc.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 30.Menon A, Prem SS, Kumari R. Topical betamethasone valerate as a prophylactic agent to prevent acute radiation dermatitis in head and neck malignancies: a randomized, open-label, phase 3 trial. Int J Radiat Oncol Biol Phys. 2021;109(1):151-160. doi: 10.1016/j.ijrobp.2020.08.040 [DOI] [PubMed] [Google Scholar]
- 31.Yokota T, Zenda S, Ota I, et al. Phase 3 Randomized trial of topical steroid versus placebo for prevention of radiation dermatitis in patients with head and neck cancer receiving chemoradiation. Int J Radiat Oncol Biol Phys. 2021;111(3):794-803. doi: 10.1016/j.ijrobp.2021.05.133 [DOI] [PubMed] [Google Scholar]
- 32.Allali S, Kirova Y. Radiodermatitis and fibrosis in the context of breast radiation therapy: a critical review. Cancers (Basel). 2021;13(23):5928. doi: 10.3390/cancers13235928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coles CE, Griffin CL, Kirby AM, et al. ; IMPORT Trialists . Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390(10099):1048-1060. doi: 10.1016/S0140-6736(17)31145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagsi R, Griffith KA, Boike TP, et al. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015;1(7):918-930. doi: 10.1001/jamaoncol.2015.2590 [DOI] [PubMed] [Google Scholar]
- 35.Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44(5):636-650. doi: 10.1016/j.fct.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 36.dal Belo SE, Gaspar LR, Maia Campos PM, Marty JP. Skin penetration of epigallocatechin-3-gallate and quercetin from green tea and Ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol Physiol. 2009;22(6):299-304. doi: 10.1159/000241299 [DOI] [PubMed] [Google Scholar]
- 37.Dvorakova K, Dorr RT, Valcic S, Timmermann B, Alberts DS. Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother Pharmacol. 1999;43(4):331-335. doi: 10.1007/s002800050903 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Change in RID and symptom scores between epigallocatechin-3-gallate and placebo groups
eFigure 2. The comparison of different RID-related indexes of patients with modified radical mastectomy between two groups
eFigure 3. The representative cases in the epigallocatechin-3-gallate (panel A) and placebo groups (panel B)
eTable 1. Radiation Therapy Oncology Group score
eTable 2. The characteristics of patients who received modified radical mastectomy
eTable 3. The highest RID-related scores of patients who received modified radical mastectomy
eTable 4. The characteristics of patients who underwent skin temperature measurements
eTable 5. Adverse events
Statistical Analysis Plan
Data Sharing Statement




