Abstract
Background
Epidermal growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs) are recommended as first‐line treatment in non‐small cell lung cancer (NSCLC) patients with sensitizing EGFR mutations. The sequential use of different EGFR‐TKIs has been reported to demonstrate improvement in overall survival of NSCLC patients with EGFR mutations. There are limited reports on comparisons between regimens with first‐line use of afatinib, gefitinib or erlotinib, followed by osimertinib upon disease progression with acquired T790M mutation.
Methods
A retrospective cohort study of Chinese patients with metastatic NSCLC harboring EGFR mutations who received first‐line gefitinib, erlotinib or afatinib treatment, followed by osimertinib upon disease progression with acquired T790M mutation, was conducted. The differences in overall survival (OS) and progression‐free survival (PFS) with first‐line EGFR‐TKI (PFS1) and time to second objective disease progression (PFS2) were compared among patients on different first‐line EGFR‐TKIs.
Results
Among 155 patients, 101 (65.2%), 38 (24.5%) and 16 (10.3%) patients were on first‐line gefitinib, erlotinib or afatinib, respectively. Patients treated with afatinib in the first‐line setting had significantly longer OS compared with those on gefitinib or erlotinib, while the PFS1 and PFS2 were longer for patients on afatinib but did not reach statistical significance.
Conclusions
First‐line afatinib, followed by osimertinib upon disease progression with T790M mutation, demonstrated significantly longer OS compared to that using other EGFR‐TKI in the first‐line setting.
Keywords: afatinib, erlotinib, gefitinib, osimertinib, T790M
First‐line afatinib, followed by osimertinib upon disease progression with T790M mutation, demonstrated significantly longer OS compared to that using other EGFR‐TKI in the first‐line setting.

INTRODUCTION
The management of locally advanced and metastatic non‐small cell lung cancer (NSCLC) relies on the presence of actionable driver mutations. Epidermal growth factor receptor (EGFR) mutations account for 55.4% of actionable driver mutations in NSCLC. 1 EGFR‐tyrosine kinase inhibitors (EGFR‐TKIs) are recommended as upfront treatment for patients with EGFR mutated NSCLC. 2 Afatinib, gefitinib, erlotinib, dacomitinib and osimertinib are available options of EGFR‐TKIs for NSCLC patients with EGFR mutations. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Afatinib, gefitinib and erlotinib have demonstrated a longer progression‐free survival (PFS) than platinum‐based chemotherapy in patients with sensitizing EGFR mutations. 10 , 11 , 12 , 13 Afatinib, dacomitinib and osimertinib were shown to give rise to longer PFS than gefitinib in clinical studies. 14 , 15 , 16 Survival benefits appeared to be even more prominent in patients and EGFR exon 19 deletion treated with afatinib, when compared with other EGFR‐TKIs. 17
The sequential use of different EGFR‐TKIs has been shown to improve the overall survival (OS) of NSCLC patients with EGFR mutations, as demonstrated in the pooled analysis of LUX‐Lung 3, 6 and 7 trials. 18 Patients who had previous treatment with afatinib followed by acquisition of EGFR T790M mutation had a median PFS with further osimertinib treatment for 20.2 months. This pooled analysis also showed that the median OS was not reached after a median follow‐up of 4.7 years. The evidence for sequential use of afatinib and osimertinib was further supported by a real‐world study, 19 with a median OS of 41.3 months. Patients with EGFR exon 19 deletion had a median OS of 45.7 months. From a real‐world study in South Korea, sequential afatinib and osimertinib treatment resulted in better survival rates than treatment with afatinib followed by other chemotherapies. 3 This study only, however, compared those with T790M mutation with those without T790M mutation or unknown resistant mechanisms upon progression with afatinib. Another real‐world study conducted in Taiwan suggested that afatinib offered longer OS than gefitinib or erlotinib. 20 However, patients without EGFR mutation were also included in that study. The study also did not mention the resistance mechanism to first‐line EGFR‐TKI and the subsequent treatment upon progression. A recently published global noninterventional study (UpSwinG) suggested that sequential afatinib and osimertinib demonstrated encouraging activity in patients with EGFR mutation‐positive NSCLC and acquired T790M. 21 However, there is lack of comparator arm in this study. As such, comparing the response to treatment among the three most commonly used EGFR‐TKIs, namely gefitinib, erlotinib or afatinib, in a cohort with lesser bias in clinical characteristics, resistance mechanism and treatment upon disease progression, will inform the treatment efficacy of these EGFR‐TKIs, especially on the OS in the real‐world setting
METHODS
Study design
This was a retrospective cohort study to compare the survival between patients with advanced NSCLC who had primary treatment with gefitinib, erlotinib or afatinib, and upon disease progression with acquisition of EGFR T790M mutations received second‐line osimertinib.
Patients
Between July 1, 2014 and December 31, 2020, all patients with metastatic NSCLC harboring common EGFR mutations (exon 19 deletion or exon 21 L858R substitution) fulfilling the above criteria in Queen Mary Hospital in Hong Kong were included. Patients with background brain metastasis were excluded as these three EGFR‐TKIs (gefitinib, erlotinib and afatinib), have different efficacy in patients with central nervous system involvement, and patients with brain metastasis were preferably treated with osimertinib in the first‐line setting.
Eligible patients were identified using the Clinical Data Analysis and Report System (CDARS) under the Hong Kong Hospital Authority. Demographic data (age, gender, smoking status), clinical data/investigations (driver mutation status, metastatic sites, and hepatitis B status), prescription details of EGFR‐TKI and the associated adverse effects were collected. The primary outcome of interest was OS. The secondary endpoint included PFS while on primary treatment with EGFR‐TKI (PFS1) and time to second objective disease progression (PFS2), which was the time from using primary EGFR‐TKIs to objective tumor progression on next‐line treatment or death from any cause. The response was graded according to the Response Evaluation Criteria In Solid Tumors (RECIST 1.1). Severity of adverse effects was graded according to the CTCAE V5.0 published by the National Cancer Institute (NCI) of the National Institutes of Health (NIH). 22
Statistical analysis
The demographic and clinical data are described in actual frequency or mean ± SD. Baseline demographic and clinical data were compared by independent t‐tests. Cox regression analysis was used to assess survival outcomes. The Kaplan–Meier method was used to estimate the cumulative progression and death rates; and the stratified log‐rank statistics to assess the differences between the groups with respect to the composite primary endpoint. The statistical significance was determined at the level of p = 0.05. All statistical analyses were done using the 26th version of SPSS statistical package. The study was approved by the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW) IRB (UW18‐555).
RESULTS
Baseline characteristics
A total of 155 patients who received primary treatment with gefitinib, erlotinib or afatinib for metastatic EGFR‐mutated NSCLC were included. The mean age of these patients was 68.4 ± 11.6 (range 41–94) years, with 110 (71.0%) females and 134 (86.5%) nonsmokers. In total, 84 (54.2%) of the patients had exon 19 deletion and 71 (45.8%) had exon 21 L858R. A total of 101 (65.2%), 38 (24.5%) and 16 (10.3%) patients had primary treatment with gefitinib, erlotinib or afatinib, respectively. The baseline demographics and clinical features are summarized in Table 1.
TABLE 1.
Baseline demographics and clinical characteristics of 155 NSCLC patients
| First‐line EGFR‐TKIs | p‐values | |||
|---|---|---|---|---|
| Gefitinib (n = 101) | Erlotinib (n = 38) | Afatinib (n = 16) | ||
| Gender, female | 80 (79.2%) | 22 (57.9%) | 8 (50.0%) | 0.007* |
| Age (years), mean ± SD | 70.5 ± 11.5 | 64.8 ± 12.1 | 63.7 ± 7.8 | 0.008* |
| Nonsmoker | 86 (85.1%) | 34 (89.5%) | 14 (87.5%) | 0.795 |
| EGFR mutations | 0.009* | |||
| Exon 19 deletion | 57 (56.4%) | 14 (36.8%) | 13 (81.2%) | |
| L858R | 44 (43.6%) | 24 (63.2%) | 3 (18.8%) | |
| Liver metastasis | 17 (16.8%) | 4 (10.5%) | 1 (6.3%) | 0.401 |
| Bone metastasis | 49 (48.5%) | 25 (65.8%) | 6 (37.5%) | 0.247 |
| Pleural effusion | 37 (36.6%) | 16 (42.1%) | 6 (37.5%) | 0.663 |
| Hepatitis B carrier | 9 (9.0%) | 5 (13.2%) | 2 (12.5%) | 0.901 |
| Performance status by ECOG at the start of first‐line EGFR‐TKI | 0.004* | |||
| 0 | 12 (11.9%) | 5 (13.5%) | 8 (50.0%) | |
| 1 | 83 (82.2%) | 30 (81.1%) | 8 (50.0%) | |
| 2 | 6 (5.9%) | 2 (5.4%) | 0 (0%) | |
| 3/4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Performance status by ECOG upon starting osimertinib | 0.129 | |||
| 0 | 11 (10.9%) | 8 (21.1%) | 3 (18.8%) | |
| 1 | 72 (71.3%) | 29 (76.3%) | 13 (81.2%) | |
| 2 | 9 (9.9%) | 1 (2.6%) | 0 (0%) | |
| 3 | 17 (16.8%) | 0 (0%) | 0 (0%) | |
Patients on afatinib as primary EGFR‐TKI were more likely to achieve partial response than patients on primary gefitinib or erlotinib. The best response of the included patients while on primary EGFR‐TKI and osimertinib are summarized in Table 2.
TABLE 2.
Best response of the 155 NSCLC patients while on first‐line EGFR‐TKIs
| First‐line EGFR‐TKI | p‐values | |||
|---|---|---|---|---|
| Gefitinib (n = 101) | Erlotinib (n = 38) | Afatinib (n = 16) | ||
| Best response to first‐line EGFR‐TKI | 0.688 | |||
| Progressive disease | 0 (0%) | 0 (0%) | 0 (0%) | |
| Stable disease | 53 (52.5%) | 21 (56.8%) | 6 (37.5%) | |
| Partial response | 47 (46.5%) | 16 (43.2%) | 10 (62.5%) | |
| Complete remission | 1 (1.0%) | 0 (0%) | 0 (0%) | |
| Best response to osimertinib | 0.079 | |||
| Progressive disease | 10 (9.9%) | 10 (26.3%) | 1 (6.3%) | |
| Stable disease | 63 (62.4%) | 20 (52.6%) | 7 (43.8%) | |
| Partial response | 27 (26.7%) | 8 (21.1%) | 8 (50.0%) | |
| Complete remission | 1 (1.0%) | 0 (0%) | 0 (0%) | |
Overall survival (OS)
Patients treated with afatinib in the first‐line setting had significantly longer OS compared with those on gefitinib or erlotinib with median OS of being 44.6 months for patients on gefitinib and 48.6 months for patients on erlotinib, and 59.2 months for patients on afatinib. The hazard ratios (HR) were 0.407 (95% CI: 0.176–0.943, p = 0.036) when afatinib was compared with gefitinib and 0.739 (95% CI: 0.176–1.251, p = 0.261) when afatinib was compared with erlotinib. The results are in favor of afatinib (Figure 1). With multivariate analysis adjusted for age, gender, smoking status and the initial EGFR mutation, the result was statistically significant with HR of 0.373 (95% CI: 0.152–0.911, p = 0.031) for afatinib over gefitinib.
FIGURE 1.
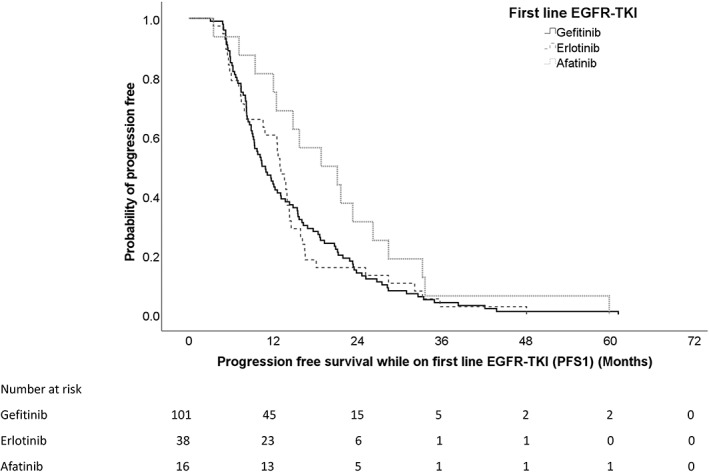
Overall survival for patients on different first‐line EGFR‐TKI.
Progression‐free survival while on first‐line EGFR‐TKI (PFS1)
Median PFS1 was longer for patients on afatinib than those on gefitinib or erlotinib, being 18.8 months (CI = 8.2–29.4), 10.4 months (CI = 8.4–12.4) and 13.0 months (CI = 11.5–14.5) for afatinib, gefitinib and erlotinib, respectively. However, the result was borderline statistical insignificant by Cox regression when comparing the PFS1 for patients on afatinib with gefitinib or erlotinib. The HR of 0.601 (95% CI: 0.353–1.023, p = 0.061) and 0.995 (95% CI: 0.684–1.450, p = 0.981) when afatinib or erlotinib was compared with gefitinib, respectively (Figure 2).
FIGURE 2.
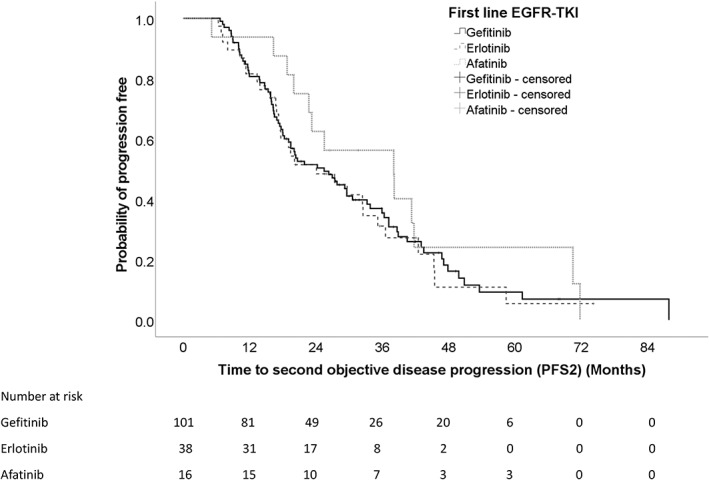
Progression‐free survival for patients on different first‐line EGFR‐TKI.
Time to second objective disease progression (PFS2)
Median PFS2 was longer for patients on afatinib than those on gefitinib and erlotinib, being 38.1 months (CI: 18.1–58.1), 25.5 months (CI:18.4–35.6) and 24.1 months (CI: 13.0–35.2) for afatinib, gefitinib and erlotinib, respectively. However, these results did not reach statistical significance, with HR of 0.34 (95% CI: 0.414–1.358, p = 0.341) and 1.052 (95% CI: 0.685–1.617, p = 0.817) when afatinib or erlotinib was compared with gefitinib, respectively (Figure 3).
FIGURE 3.
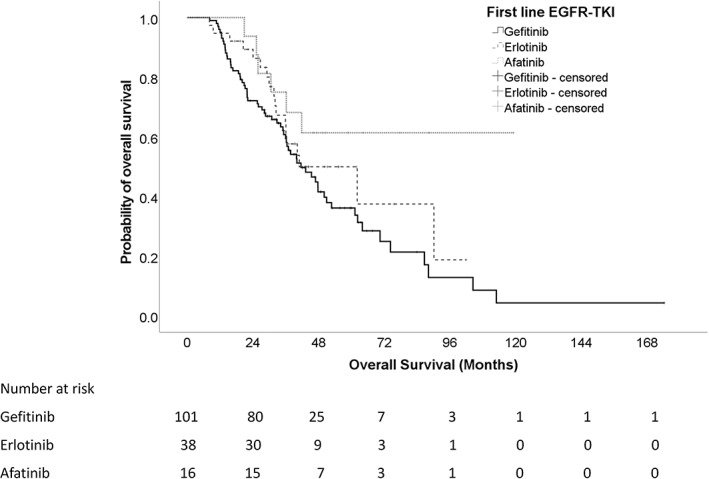
Time to second objective disease progression for patients on different first‐line EGFR‐TKI.
Adverse effects while on first‐line EGFR‐TKI
Patients on first‐line afatinib were more likely to develop grade 3 cutaneous adverse effects and grade 3 gastrointestinal adverse effects than those on first‐line gefitinib and erlotinib. The adverse effects of patients while on first‐line EGFR‐TKI and osimertinib are summarized in Tables 3 and 4.
TABLE 3.
Adverse effects in 155 NSCLC patients while on first‐line EGFR‐TKIs
| First‐line EGFR‐TKIs | ||||
|---|---|---|---|---|
| Gefitinib | Erlotinib | Afatinib | p‐values | |
| Cutaneous adverse effects | 0.001* | |||
| Nil | 18 (17.8%) | 3 (7.9%) | 1 (6.3%) | |
| Grade 1 | 68 (67.3%) | 17 (44.7%) | 9 (56.3%) | |
| Grade 2 | 13 (12.9%) | 17 (44.7%) | 4 (25.0%) | |
| Grade 3 | 2 (2.0%) | 1 (2.6%) | 2 (12.5%) | |
| Gastrointestinal adverse effects | 0.001* | |||
| Nil | 58 (57.4%) | 17 (44.7%) | 6 (37.5%) | |
| Grade 1 | 40 (39.6%) | 19 (50.0%) | 6 (37.5%) | |
| Grade 2 | 3 (3.0%) | 2 (5.3%) | 2 (12.5%) | |
| Grade 3 | 0 (0%) | 0 (0%) | 2 (12.5%) | |
| Hepatotoxicity | 0.266 | |||
| Nil | 72 (71.3%) | 32 (84.3%) | 12 (75.0%) | |
| Grade 1 | 13 (12.9%) | 2 (5.3%) | 0 (0%) | |
| Grade 2 | 10 (9.9%) | 1 (2.6%) | 3 (18.8%) | |
| Grade 3 | 6 (5.9%) | 3 (7.9%) | 1 (6.3%) | |
| Pneumonitis | 1 (1.0%) | 2 (5.3%) | 0 (0%) | 0.044 |
TABLE 4.
Adverse effects in 155 NSCLC patients while on second‐line osimertinib
| Adverse effects while on second‐line osimertinib after first‐line EGFR‐TKIs | ||||
|---|---|---|---|---|
| Gefitinib then osimertinib | Erlotinib then osimertinib | Afatinib then osimertinib | p‐values | |
| Cutaneous adverse effects | 0.869 | |||
| Nil | 54 (53.5%) | 23 (60.5%) | 10 62.5%) | |
| Grade 1 | 45 (44.6%) | 14 (36.8%) | 6 (37.5%) | |
| Grade 2 | 2 (2.0%) | 1 (2.6%) | 0 (0%) | |
| Grade 3 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Gastrointestinal adverse effects | 0.938 | |||
| Nil | 72 (71.3%) | 30 (78.9%) | 12 (75.0%) | |
| Grade 1 | 26 (25.7%) | 7 (18.4%) | 4 (25.0%) | |
| Grade 2 | 2 (2.0%) | 1 (2.6%) | 0 (0%) | |
| Grade 3 | 1 (1.0%) | 0 (0%) | 0 (0%) | |
| Grade 4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hepatotoxicity | 0.938 | |||
| Nil | 99 (98.0%) | 37 (97.4%) | 15 (93.8%) | |
| Grade 1 | 1 (1.0%) | 1 (2.6%) | 1 (6.3%) | |
| Grade 2 | 1 (1.0%) | 0 (0%) | 0 (0%) | |
| Grade 3/4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Pneumonitis | 0 (0%) | 2 (5.3%) | 0 (0%) | 0.044* |
DISCUSSION
Our study concurred with previous findings that sequential use of afatinib followed by osimertinib could have survival benefit for patients with EGFR mutated NSCLC. These study findings added to the Giotag study about the potential role of afatinib followed by osimertinib in terms of survival benefit over gefitinib or erlotinib, which was not reported in the Giotag study19, 23 while for the pooled analysis of the LUX‐Lung 3, 6 and 7 trials, the most common subsequent therapy was platinum‐based chemotherapy with a minority of patients receiving osimertinib following afatinib due to the restricted availability of osimertinib at the time this study was undertaken. PFS1, PFS2 and OS were assessed in our study, which provided a comprehensive assessment of survival for the included patients. The current study findings would be more informative and more close to the current standard of care, with osimertinib largely replacing chemotherapy for suitable patients on detection of EGFR T790M mutation upon disease progression. Our real‐world data would be better able to address the issue of which type of therapy is preferred after disease progression with primary EGFR‐TKI in NSCLC patients with EGFR mutations.
Although osimertinib demonstrated superior OS and central nervous system disease control in the FLAURA study when compared with first‐generation EGFR‐TKIs, 15 , 24 first‐line treatment with osimertinib is limited by accessibility with its relative higher cost. As such, there is still a potential role for the use of first‐ and second‐generation EGFR‐TKIs in the first‐line setting, followed by the use of osimertinib in the second‐line setting if acquired T790M mutation is identified upon disease progression. Among first‐ and second‐generation EGFR‐TKIs, gefitinib, erlotinib and afatinib were commonly used, and they have slightly different adverse effect profiles. The choice of EGFR‐TKI usually depends on their demonstrated survival benefits and tolerability. The results from our study suggested that afatinib could be a reasonable choice of first‐line EGFR‐TKI among patients with sensitizing EGFR mutations and no brain metastasis. Given the high efficacy of CNS activity, osimertinib remains the optimal choice for patients with brain metastasis.
The potential benefits from afatinib could be related to it pharmacological property as an irreversible EGFR binder and inhibitor, as well as the coverage of HER2 inhibition. The possible drawback of using afatinib would be higher incidence of adverse effects. The results from our study concur with previous reports that afatinib use was associated with a higher chance of developing grade 3 or above adverse effects, especially cutaneous and gastrointestinal adverse effects. Appropriate dose adjustment might be needed to strike a balance between efficacy and tolerability. Pre‐emptive medications to handle the adverse effects, such as topical medications for cutaneous adverse effects, and antidiarrheal drugs might be beneficial for patients who are on afatinib to improve tolerability.
In this study, the PFS1 of 1.5 years, PFS2 of more than 3 years, as well as OS of more than 4 years with afatinib, were clinically meaningful. The sequential use of afatinib until definite disease progression, followed by osimertinib, could enhance survival benefits at a relatively lower cost compared to using one drug for the whole survival period. This regime would be attractive for patients who may have treatment cost concern, especially with osimertinib, as afatinib is now available for prescription in some health authority safety net systems in many places, including Hong Kong.
There are some limitations of this study. While this study was done in a tertiary oncology center that received referrals throughout Hong Kong, it was a single centered study. Although the number of patients on afatinib was relatively small and the results did not reach statistical significance in all the outcomes, the results suggested the potential benefits from first‐line afatinib use and the results reflected real‐world experience in using EGFR‐TKI in advanced stage NSCLC patients with sensitizing EGFR mutations. The benefits of having a real‐world study is that it can provide data in an unselected setting. This could provide valuable information on patients that would be excluded from ordinary randomized control trials which have strict inclusion and exclusion criteria. A larger sample size study would be essential to confirm this observed association. This study did not include patients with uncommon and complex EGFR mutations with which afatinib was reported to have superior efficacy, compared to other first‐generation EGFR‐TKIs, for patients with uncommon EGFR mutations. Patients with brain metastases were excluded from this study, as osimertinib is the preferred agent for initial management for patients with EGFR‐mutated NSCLC and brain metastases.
In conclusion, first‐line use of afatinib, followed by osimertinib upon disease progression with acquired T790M mutation, offered significant longer overall survival in advanced stage EGFR mutated NSCLC patients.
FUNDING INFORMATION
This research project was supported partly with a research donation from Boehringer Ingelheim.
CONFLICT OF INTEREST
None declared.
Kwok WC, Ho JCM, Tam TCC, Ip MSM, Lam DCL. Survival benefits from afatinib compared with gefitinib and erlotinib among patients with common EGFR mutation in first‐line setting. Thorac Cancer. 2022;13(14):2057–2063. 10.1111/1759-7714.14528
Funding information Boehringer Ingelheim
REFERENCES
- 1. Yatabe Y, Kerr KM, Utomo A, Rajadurai P, Tran VK, Du X, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015;10(3):438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre‐Finn C, et al. Correction to: “metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up”. Ann Oncol. 2019;30(5):863–70. [DOI] [PubMed] [Google Scholar]
- 3. Kim T, Jang TW, Choi CM, Kim MH, Lee SY, Park CK, et al. Sequential treatment of afatinib and osimertinib or other regimens in patients with advanced non‐small‐cell lung cancer harboring EGFR mutations: results from a real‐world study in South Korea. Cancer Med. 2021;10(17):5809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12(8):735–42. [DOI] [PubMed] [Google Scholar]
- 5. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802). Ann Oncol 2015;26(9):1877–83. [DOI] [PubMed] [Google Scholar]
- 6. Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non‐small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116(5):568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urata Y, Katakami N, Morita S, Kaji R, Yoshioka H, Seto T, et al. Randomized phase III study comparing Gefitinib with Erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol. 2016;34(27):3248–57. [DOI] [PubMed] [Google Scholar]
- 8. Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR‐mutant NSCLC. Ann Oncol. 2018;29(suppl_1):i3–9. [DOI] [PubMed] [Google Scholar]
- 9. Zhao Y, Liu J, Cai X, Pan Z, Liu J, Yin W, et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non‐small cell lung cancer: systematic review and network meta‐analysis. BMJ. 2019;367:l5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. [DOI] [PubMed] [Google Scholar]
- 11. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. [DOI] [PubMed] [Google Scholar]
- 12. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34. [DOI] [PubMed] [Google Scholar]
- 13. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22. [DOI] [PubMed] [Google Scholar]
- 14. Paz‐Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: overall survival data from the phase IIb LUX‐lung 7 trial. Ann Oncol. 2017;28(2):270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113–25. [DOI] [PubMed] [Google Scholar]
- 16. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–66. [DOI] [PubMed] [Google Scholar]
- 17. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐lung 3 and LUX‐lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–51. [DOI] [PubMed] [Google Scholar]
- 18. Park K, Bennouna J, Boyer M, Hida T, Hirsh V, Kato T, et al. Sequencing of therapy following first‐line afatinib in patients with EGFR mutation‐positive non‐small cell lung cancer. Lung Cancer. 2019;132:126–31. [DOI] [PubMed] [Google Scholar]
- 19. Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC, et al. Sequential afatinib and osimertinib in patients with EGFR mutation‐positive non‐small‐cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019;15(25):2905–14. [DOI] [PubMed] [Google Scholar]
- 20. Ng WW, Lin CC, Cheng CY, Jiang JS, Kao SJ, Yeh DY. Real‐world outcomes of first‐ and second‐generation tyrosine kinase inhibitors first‐line in patients with epidermal growth factor receptor mutation‐positive non‐small cell lung cancer: a retrospective observational cohort study. PLoS One. 2021;16(6):e0253335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Popat S, Jung HA, Lee SY, Hochmair MJ, Lee SH, Escriu C, et al. Sequential afatinib and osimertinib in patients with EGFR mutation‐positive NSCLC and acquired T790M: a global non‐interventional study (UpSwinG). Lung Cancer. 2021;162:9–15. [DOI] [PubMed] [Google Scholar]
- 22. Health NIo . Common Terminology Criteria for Adverse Events (CTCAE)2018. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 23. Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC, et al. Sequential afatinib and osimertinib in patients with EGFR mutation‐positive non‐small‐cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16(34):2799–808. [DOI] [PubMed] [Google Scholar]
- 24. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with Osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. [DOI] [PubMed] [Google Scholar]


