Abstract
Many viruses induce shutoff of host gene expression (host shutoff) as a strategy to take over cellular machinery and evade host immunity. Without host shutoff activity, these viruses generally replicate poorly in vivo, attesting to the importance of this antiviral strategy. In this review, we discuss one particularly advantageous way for viruses to induce host shutoff: triggering widespread host messenger RNA (mRNA) decay. Viruses can trigger increased mRNA destruction either directly, by encoding RNA cleaving or decapping enzymes, or indirectly, by activating cellular RNA degradation pathways. We review what is known about the mechanism of action of several viral RNA degradation factors. We then discuss the consequences of widespread RNA degradation on host gene expression and on the mechanisms of immune evasion, highlighting open questions. Answering these questions is critical to understanding how viral RNA degradation factors regulate host gene expression and how this process helps viruses evade host responses and replicate.
Keywords: host shutoff, RNA decay, virus, ribonuclease, decapping enzymes
1. INTRODUCTION
For productive infection, a virus needs to hijack cellular machinery to express its proteins, replicate its genome, and assemble new virions without being blocked by the cell. To succeed in this takeover, many viruses globally reduce host protein production, a process called host shutoff. Host shutoff is thought to be advantageous for the virus for two reasons. First, reducing protein production is a way to block the cell-intrinsic antiviral response, especially because this response requires the induction of many antiviral effectors that are usually not expressed in uninfected cells. In addition, host shutoff may free up gene expression machinery, particularly ribosomes, and refocus cellular resources to making viral proteins (discussed in detail in Section 3.4), although a causal relationship has been difficult to test experimentally. Because of these advantages, many viruses have evolved mechanisms to block host gene expression, and thus, host protein production. There are examples of viruses interfering with every step of gene expression: transcription, RNA processing, nuclear RNA export, and translation. In this review, we focus on one common strategy used by multiple unrelated viruses to induce host shutoff: host RNA degradation.
Some viruses directly encode ribonucleases (RNases) to carry out host shutoff, although not all viral RNases are host shutoff factors. Indeed, viral RNases can have a variety of other roles, such as proofreading during viral transcription [e.g., coronavirus (CoV) nsp14/ExoN (1)], degradation of viral immunostimulatory RNA species [e.g., CoV EndoU (2)], or cleavage of the capped 5′ end of host RNAs to use as transcription primers (cap snatching) [e.g., the viral RNA polymerase of influenza viruses (3)]. Moreover, not all RNA degradation-based host shutoff mechanisms are triggered by a viral RNase. Some viruses indirectly induce RNA degradation by encoding factors that stimulate host pathways of degradation, including decapping enzymes that remove the 5′ cap from host messenger RNA (mRNA).
The goal of this review is to summarize and discuss our current understanding of the molecular mechanisms of host shutoff by viral proteins that trigger RNA degradation. We focus in particular on the viral RNases encoded by influenza A virus, α- β- and γ-herpesviruses, the viral decapping enzymes encoded by large DNA viruses such as vaccinia virus (VacV), and the CoV protein nonstructural protein 1 (nsp1) that induces an unknown cellular RNase to degrade RNA. After reviewing the specific mechanism of action for each of these factors, we discuss how these mechanisms trigger secondary consequences on cellular processes and influence the fight between host and virus (Figure 1).
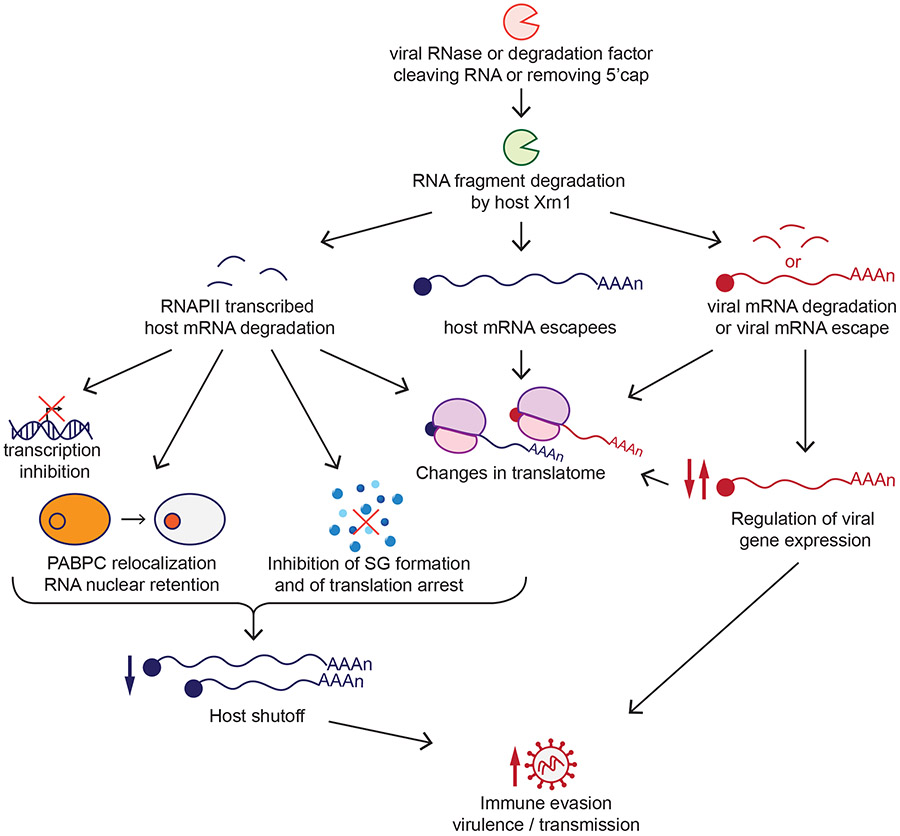
Figure 1.
Role of viral RNA degradation factors in host shutoff and the viral infectious cycle. Viral ribonuclease (RNase) or RNA degradation factors degrade host RNAs transcribed by RNA polymerase II (RNAPII). RNA degradation can then lead to host transcription inhibition, nuclear localization of the poly(A)-binding protein (PABPC) and nuclear retention of RNAs, inhibition of stress granule (SG) formation, and changes in the RNAs that are translated by the cell (translatome). The escape of some host messenger RNAs (mRNAs) from degradation and the presence of viral mRNAs also contribute to changes to the translatome. Viral mRNAs in some cases escape degradation, while in other viruses the degradation of viral mRNAs is integrated into the viral replication cycle as a mechanism of viral gene regulation. Overall, all these processes allow the virus to evade host immunity, affecting its virulence and transmission.
2. MECHANISM OF ACTION OF VIRAL RNA DEGRADATION FACTORS
Although viral RNA degradation factors are encoded by unrelated viruses and belong to different protein superfamilies, there are common themes in their mechanisms of host shutoff. Importantly, these common themes are all related to host RNA metabolism. Human cells use three different RNA polymerases (RNAPI, II, and III) to transcribe RNAs. While all three RNAPs transcribe noncoding RNAs, protein-coding mRNAs are synthesized solely by RNAPII. All the viral RNA degradation factors we discuss in this review have evolved to specifically target RNAs transcribed by RNAPII but not RNAPI or III (4-8), which makes sense if the goal of host shutoff is to reduce protein production. Exactly how these viral RNA degradation factors target RNAPII transcripts depends on the molecular mechanism of action of each factor, which also determines whether there is additional selectivity among RNAPII transcripts. Both mRNAs and noncoding RNAs synthesized by RNAPII are modified by RNAPII-associated processing factors. They have a 5′ methylguanosine cap and a poly(A)-tail, and they can be spliced by the splicing machinery if they contain introns. However, only mRNAs associate with translational machinery, including translation initiation factors and ribosomes. Which step of RNA metabolism, RNA structure, or host protein recruits the different viral RNA degradation factors determines whether they target all RNAPII transcripts, only mRNAs, or only mRNAs that are actively translated, as described below in detail for each factor. Of note, these different mechanisms also determine whether the viral RNA degradation factors can distinguish between host and viral mRNAs. Interestingly, despite these differences, a subset of RNAs escape degradation by different viral RNA degradation factors, as discussed in more detail in Section 3.4.
On top of targeting RNAPII specifically, all RNA degradation factors act either as endoribonucleases that cleave the RNA into fragments (4, 5) or as decapping enzymes that remove the 5′ methylguanosine cap from host mRNAs (6-8). Although the CoV RNA degradation factor nsp1 does not belong to either class, it also induces endoribonucleolytic cleavage of host mRNAs by an unknown host RNase (9). In addition, both endoribonucleolytic cleavage and decapping generate RNAs with a 5′ monophosphate that can then be degraded by Xrn1, a cellular exoribonuclease that degrades uncapped RNAs in a 5′–3′ direction (4, 6-10). In this section, we describe and compare the mechanisms of target selection and RNA degradation of known viral RNA degradation factor (Figure 2).
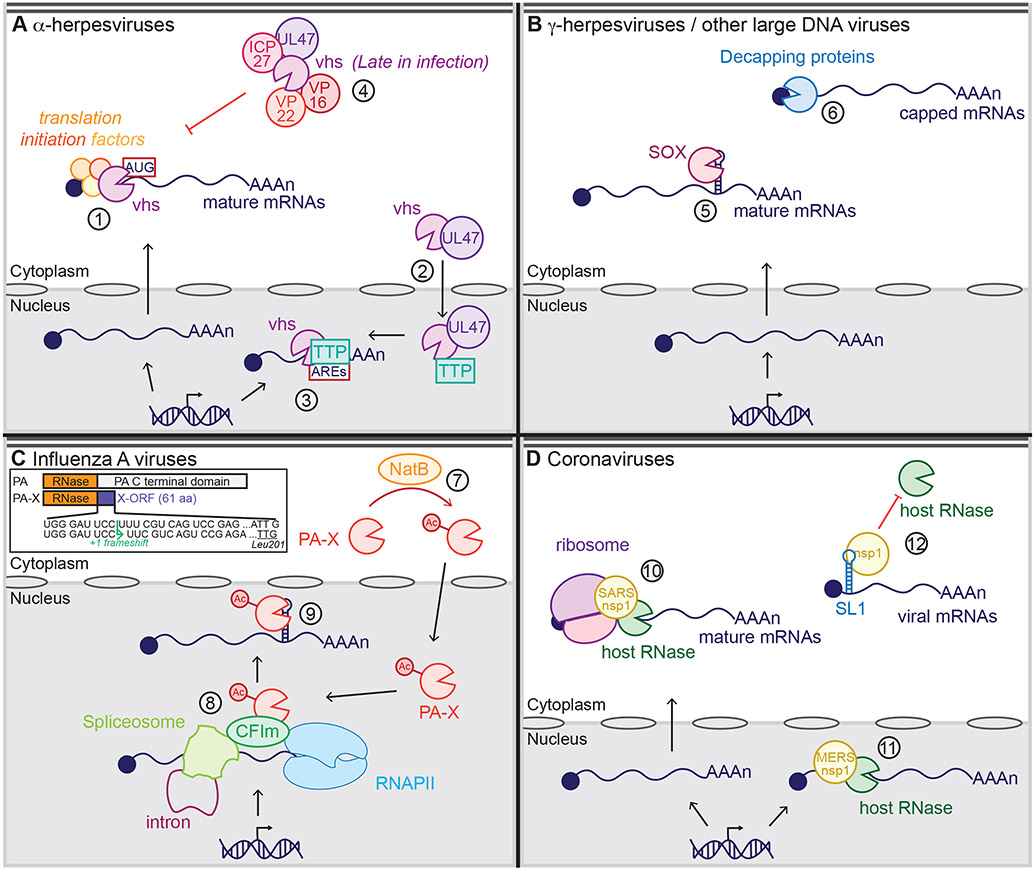
Figure 2.
Current models of the mechanisms of action of viral RNA degradation factors. (a) α-Herpesviruses and virion host shutoff (vhs): vhs binds translation initiation factors in the cytoplasm to access its target RNAs, then cleaves capped RNAs or RNAs containing an internal ribosome entry site (IRES) close to the translation start site (#1). The viral protein pUL47 also shuttles vhs to the nucleus (#2), where it binds the host protein tristetraprolin (TTP) to cleave short-lived messenger RNAs (mRNAs) containing AU-rich elements (AREs) (#3). Late in infection, vhs activity is dampened through interactions with the virion proteins (VPs) VP16, VP22, pUL47, and infected cell protein (ICP) 27 (#4). (b) γ-Herpesviruses and SOX; large DNA viruses and decapping proteins: SOX recognizes and cleaves a specific sequence and structure within cytoplasmic mRNAs associated with translation complexes (#5). The decapping proteins African swine fever virus (ASFV)-DP and L357 bind to the RNA to locate its 5′ cap, while D9 and D10 bind to both the RNA and the 5′ cap to locate the cap and cleave it (#6). (c) Influenza A viruses and polymerase acidic (PA)-X: The cellular N-terminal acetylase (Nat) complex NatB protein N-terminally acetylates PA-X (#7), leading to an active PA-X, which accumulates in the nucleus, where it binds the cleavage factor Im (CFIm) complex and RNA processing factors to access its target spliced RNAs transcribed by RNA polymerase II (RNAPII) (#8). PA-X then recognizes and cleaves a specific sequence and structure (#9). The inset shows the mechanism of PA-X production via ribosomal frameshifting during translation of the PA mRNA. PA-X thus has the same N terminal ribonuclease (RNase) domain as PA, but a unique C terminal domain termed the X-ORF. Panel c inset adapted from Reference (47). (d) Coronaviruses and nonstructural protein 1 (nsp1): Severe acute respiratory syndrome coronavirus (SARS-CoV) and SARS-CoV-2 nsp1 bind to the 40S ribosome subunit to target capped or IRES-containing mRNAs that are actively translated (#10), inducing RNA degradation by an unknown host RNase in the cytoplasm. Middle East respiratory syndrome coronavirus (MERS-CoV) nsp1 also induces RNA degradation, but within the nucleus and without binding to the ribosome (#11). SARS and MERS-CoV nsp1 bind to stem loop 1 (SL1) within the 5′ untranslated region (UTR) of viral mRNAs, leading to viral mRNA protection from degradation (#12).
2.1. Virus-Encoded RNases
α- β- and γ-herpesviruses, as well as influenza A viruses, directly encode an endoribonuclease to trigger RNA degradation in the infected cell. Here, we describe in more detail their molecular mechanism of action.
2.1.1. α-Herpesviruses.
Herpesviruses are large double-stranded DNA viruses that infect a wide range of animals. They are divided into the three subfamilies, α, β, and γ. The α-herpesviruses include human herpes simplex virus (HSV) 1 and 2, which cause oral and genital herpes. The best-studied host shutoff RNase among all viruses is the virion host shutoff (vhs) protein, encoded by the HSV UL41 gene. vhs belongs to the FEN1 nuclease family and acts as an endoribonuclease (11, 12). UL41 homologs in the animal α-herpesviruses pseudorabies virus (PrV) (12, 13), bovine herpesvirus 1 (BHV) (14), duck plague virus (15), and monkey B virus (16) also function as host shutoff factors. The other human α-herpesvirus, varicella zoster virus, also encodes a UL41 homolog, but this protein does not carry out host shutoff (17). Notably, β- and γ-herpesviruses do not encode UL41 homologs.
To access its target RNAs, HSV vhs binds the eukaryotic translation initiation factors (eIFs) eIF4H, eIF4AII, and eIF4F (18, 19), and these interactions are necessary for RNA degradation (20) (Figure 2a). These translation initiation factors bind capped mRNAs or in some cases mRNAs containing internal ribosome entry sites (IRESs). Therefore, HSV vhs specifically targets fully processed mRNAs in the cytoplasm, including viral mRNAs (21-23), but not pre-mRNAs in the nucleus or uncapped noncoding RNAs transcribed by RNAPI and RNAPIII (4). This preference is shared by its duck plague virus homolog (15). In addition, HSV vhs recognizes and degrades mRNAs that contain AU-rich elements (AREs) in their 3′ untranslated region (UTR) in the nucleus, presumably before they are exported to the cytoplasm, through a different protein-protein interaction. vhs traffics to the nucleus through interactions with the viral protein pUL47, where it binds the host ARE-binding protein tristetraprolin (TTP) (24, 25). TTP then recruits vhs to ARE-containing RNAs (24, 25) (Figure 2a).
In vitro, HSV and PrV vhs cleave mRNAs preferentially in the 5′ UTR and near the translation initiation site, which is consistent with recruitment by translation initiation factors (26). Moreover, mutations that alter the AUG site closest to a 5′ cap or IRES abolish cleavage at nearby sites (26, 27). However, analysis of vhs degradation patterns in cells transfected with HSV-1 vhs does not agree with the model that target RNAs are cleaved near the cap or IRES. Indeed, for the green fluorescent protein and DsRed mRNAs, we detected multiple degradation fragments that are shorter than the full-length mRNA (4). This result suggests that in vivo vhs may also cut RNAs at internal locations and not just near the 5′ cap (4). We were only able to visualize these fragments after depletion of the host 5′–3′ exoribonuclease Xrn1, indicating that RNA fragments generated by vhs are normally degraded by Xrn1 in cells (4). More studies are needed to clarify the cut site location of vhs in transfected and infected cells and its connection to recruitment by translation factors. mRNAs with AREs represent a special case, as vhs cleaves these RNAs near their AREs (28).
vhs is brought into cells by α-herpesvirus virions as a component of the tegument, the complement of proteins that line the space between the viral capsid and the viral envelope. It is thus active as soon as infection occurs (21). However, vhs also cleaves viral mRNAs, as they resemble host mRNAs. This degradation can inhibit viral gene expression and thus viral replication. Therefore, as the infection progresses and new vhs is synthesized, the viral proteins virion protein (VP) 16 (UL48), VP22 (UL49), UL47, and infected cell protein ICP27 bind to vhs and block its activity (25, 29-31) (Figure 2a). This late vhs inhibition promotes the switch from early to late viral gene expression. When early viral gene transcription stops and late gene transcription is induced, vhs degradation of early viral mRNAs helps remove the existing early gene transcripts, freeing the translation machinery to translate late gene mRNAs (21, 23, 32).
2.1.2. γ-Herpesviruses.
γ-Herpesviruses infect lymphocytes and a few other cell types and lead to tumor formation in immunocompromised individuals. This subfamily includes two human viruses, Kaposi’s sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV), and several animal viruses, including murine herpesvirus 68 (MHV68), commonly used to study γ-herpesvirus infection in small animal models. γ-Herpesviruses do not encode a UL41/vhs homolog but instead carry out host shutoff through a protein termed alkaline exonuclease, also called SOX/muSOX in KSHV and MHV68 [encoded by open reading frame (ORF)37] or BGLF5 in EBV (33-35). These proteins have a PD-D/E-X-K superfold, which is found in restriction enzymes and other nucleases (36, 37). The name alkaline exonuclease comes from a separate but poorly understood function as a deoxyribonuclease (DNase) during genome replication and from the fact that in vitro this DNase activity requires an alkaline pH. The DNase function is shared by SOX/muSOX/BGLF5 homologs from herpesviruses of all subfamilies, also called alkaline exonuclease and encoded by the UL12 gene in HSV and UL98 gene in the β-herpesvirus human cytomegalovirus. However, in γ-herpesviruses these proteins have evolved a second function as an endoribonuclease and host shutoff factor (33). One reason for the difference may be that SOX/muSOX/BGLF5 are localized to both nucleus and cytoplasm, whereas their α- and β-herpesvirus homologs are solely nuclear (35).
Like vhs, the γ-herpesvirus RNases target RNAPII transcripts that are competent for translation (i.e., capped and tailed, and in the cytoplasm) but not RNAPI and RNAPIII transcripts (4, 10) (Figure 2b). Additionally, SOX cosediments with the 40S ribosomal subunit in ribosome profiling experiments (10). However, active translation is not necessary, as a translation-competent mRNA with a 5′ hairpin that prevents ribosome scanning after cap binding is still downregulated by these proteins (4). It is unknown how the selectivity for translatable RNAs arises and whether the SOX proteins interact with specific cellular factors to access translation-competent mRNAs.
In contrast to vhs, KSHV SOX clearly cuts RNAs in a sequence-specific manner, as demonstrated both transcriptome-wide in cells overexpressing SOX and in vitro (38, 39) (Figure 2b). The fragments it generates are then degraded by Xrn1 (4). However, the cleavage site is complex and degenerate. SOX recognizes a stretch of three adenosines in the loop of a hairpin structure and cleaves the RNA a few nucleotides downstream of these sites (38, 39). EBV BGLF5 may also have sequence specificity, although this has not been rigorously tested (4).
Current results suggest γ-herpesvirus RNases are unable to discriminate between viral and host mRNAs. Indeed, the viruses may have adapted their replication cycle to the viral mRNA degradation, as in the absence of host shutoff, at least some of the viral proteins accumulate to higher levels and the protein composition of virions is altered (40, 41).
2.1.3. Influenza A virus.
RNA degradation-dependent host shutoff was first described in influenza A virus, a negative-sense single-stranded segmented RNA virus of the Orthomyxoviridae family, in 1982 (42). However, the protein responsible for this host shutoff, the RNase PA-X, was only discovered in 2012 (43). This is because PA-X is a low-abundance protein produced from the third influenza segment by +1 ribosomal frameshifting (43) (Figure 2c, inset). This segment encodes the polymerase acidic (PA) protein, a subunit of the viral RNA-dependent RNA polymerase, and the frameshifting for PA-X occurs after translation of amino acids 1–191 of PA (43). Therefore, PA and PA-X have the same N-terminal RNase domain, which belongs to the PD-D/E-X-K superfamily (44, 45), but different C-terminal domains (43). Despite this unusual frameshifting-based production mechanism, PA-X is encoded by all influenza A viruses (46). The PA RNase domain is used to snatch short stretches of capped host mRNAs to use as primers for viral RNA transcription (3). However, multiple studies have conclusively established that host shutoff is mediated by PA-X rather than by PA and cap snatching. Indeed, mutations that reduce frameshifting or truncate PA-X without altering the PA sequence also reduce host shutoff after transfection of the PA mRNA or infection with influenza A virus (43, 47, 48).
Like herpesvirus RNases, PA-X only targets RNAs transcribed by RNAPII. RNAs transcribed by RNAPI and III or the viral RNA polymerase are not degraded by PA-X (5). However, PA-X also targets noncoding RNAPII transcripts, indicating that its activity is not tied to translation (5). In fact, PA-X accumulates predominantly in the nucleus, and PA-X mutants that are fully cytoplasmic are not active (5, 48) (Figure 2c). Also, reports disagree on whether PA-X can degrade cytoplasm-restricted mRNAs, such as transfected mRNAs or T7 polymerase-synthesized RNAs (5, 48). Consistent with a predominantly nuclear activity, RNA downregulation by PA-X is linked to splicing (47). Spliced RNAs are more robustly downregulated by PA-X than intronless RNAs, and RNAs with more introns are downregulated to a greater extent by PA-X (47). Moreover, PA-X host shutoff appears to depend on interaction with Nudix hydrolase 21 (47), a subunit of the 3′ polyadenylation complex cleavage factor Im (CFIm) that has also been implicated in alternative splicing (49) (Figure 2c). Our study identified other cellular proteins that interact with PA-X, including mRNA processing factors (47), which we are currently investigating. Two additional studies also identified PA-X interacting proteins in chicken and human cells using proteomics approaches and PA-Xs from different influenza strains (50, 51). Although limited follow-up was done on these studies, ankyrin repeat domain 17 (Ankrd17) was identified as a cellular interactor that PA-X uses to dampen the immune response (50) (see Section 4.1). While cellular interacting proteins may recruit PA-X to spliced RNAs, more work is needed to uncover the full mechanism of PA-X RNA targeting.
Nuclear localization and protein interactions are likely mediated by the PA-X C-terminal domain, termed the X-ORF (5, 48) (Figure 2c, inset). While the X-ORF is dispensable for enzymatic activity in vitro (44, 45, 52), truncation of this domain and mutations within it reduce or abolish host shutoff in cells, during both viral infection and PA-X ectopic expression (5, 47, 48, 53-55). Moreover, almost all influenza A strains have stop codons for PA-X 61 or 41 amino acids after the frameshift, although there are other positions within the X-ORF that can accommodate a stop codon without disrupting the PA coding sequence (46). This suggests evolutionary pressure to retain the entire domain. Nonetheless, only the first 15 amino acids of the X-ORF are required for PA-X activity (47, 48, 53), suggesting that the rest have regulatory functions. Indeed, the last 20 amino acids of the X-ORF, which are absent in some influenza strains, can modulate replication and pathogenicity, and may contribute to species adaptation (46, 56, 57).
In terms of cleavage specificity, we previously concluded from northern blot analysis that PA-X cleavage occurs nonspecifically throughout the RNA (5). However, recent unpublished data from our laboratory suggest that PA-X preferentially cleaves RNA at a specific sequence and structure (L. Gaucherand, A. Iyer, I. Gilabert, C.H. Rycroft, M.M. Gaglia, unpublished article) (Figure 2c). Because SOX also recognizes a specific sequence and structure for cleavage (see Section 2.1.2), this may be an additional common feature of viral RNases. Why these viral RNases have evolved to recognize sequences and structures and how this relates to their function remain intriguing open questions.
In addition to its interactions with cellular proteins and potential target site specificity, PA-X activity is also regulated by N-terminal acetylation (Figure 2c) and rapid protein turnover (58, 59). N-terminal acetylation is a cotranslational modification that influences protein subcellular localization, stability, and protein-protein interactions (60). PA-X is modified by the N-terminal acetylase (Nat) complex NatB, and PA-X mutants that are acetylated by other Nat complexes are not active, although it is not clear why (58). Additionally, PA-X is very short lived. Its half-life ranges from 30 min to 3.5 h depending on the influenza strains tested (59), compared to 9 h for an average human protein (61). The X-ORF is important for turnover, as the different half-lives of PA-X from different strains are partly due to the X-ORF sequence, particularly whether PA-X amino acid 220 is an arginine or a histidine (59).
Finally, there is evidence for a functional interplay between PA-X and another influenza host shutoff protein, the nonstructural protein 1 (NS1) (62-65). In many influenza A strains, NS1 blocks RNA processing to reduce host gene expression (66). PA-X and NS1 seem to have coevolved in multiple influenza strains and have accumulated mutations in tandem that prevent excess host shutoff activity, which may be detrimental to the virus. This coevolution may regulate pathogenicity and viral fitness in vivo (62, 63, 65). However, it is currently unclear whether the coevolution is due to compensatory changes or a direct functional interaction between the two proteins.
2.2. Virus-Encoded Decapping Enzymes: Large DNA Viruses
Vaccinia virus (VacV) is a large double-stranded DNA virus of the Poxviridae family. VacV was used as a vaccine to eradicate the etiological agent of smallpox, variola virus, and is still used as a model for studies of poxviruses, as well as a potential vaccine vector (67). VacV encodes two decapping enzymes, D9 and D10 (6, 7), which remove the 5′ cap of mRNAs and thus trigger broad degradation of host mRNAs in the cytoplasm and host shutoff (68, 69). The reason why VacV encodes two decapping enzymes remains unclear, but their expression patterns point to separate roles in viral replication. During infection, D9 is expressed early, while D10 is expressed only after DNA replication (70, 71). Moreover, mutant viruses lacking D10 are more defective than viruses lacking D9 (71). D9 and D10 also bind RNAs and 5′ caps with different affinities in vitro (7). A recent article additionally saw some differences in target specificity between D9 and D10, with D10 being responsible for the downregulation of the majority of host transcripts (72).
In addition to VacV, decapping enzymes were identified in two other large DNA viruses. African swine fever virus (ASFV), a virus from the Asfaviridiae family that infects domestic pigs and boars, encodes ASFV-DP (also known as g5R or D250R) (8, 73), and mimivirus, a giant virus from the Mimiviridae family that infects Acanthamoeba species, encodes L375 (74).
Like cellular decapping enzymes, large DNA virus-encoded decapping enzymes contain a Nudix hydrolase domain, with the conserved Nudix motif GX5EX5[UA]XREX2EEXGU (75), where U is a hydrophobic amino acid and X any amino acid (73, 74, 76). They also have intrinsic decapping activity and convert m7GpppNm-capped RNAs to m7GDP and uncapped 5′ phosphorylated RNAs in vitro (6-8, 74).
Viral decapping enzymes need to bind capped RNAs to locate and cleave the cap (Figure 2b). In vitro assays indicate that these enzymes all recognize the RNA backbone, as they only act on caps attached to RNA and are inhibited by the addition of uncapped RNAs (6-8, 74). D9 and D10, but not ASFV-DP, are also inhibited in vitro by free methylated cap derivatives, suggesting that they interact with the cap structure as well (6-8). It is currently unknown whether any additional requirements are needed for RNA targeting by viral decapping enzymes. Moreover, it is unclear whether the decapping activity is limited to mRNAs, as RNAPII-transcribed noncoding RNAs also have caps. ASFV-DP interacts with the ribosomal protein RPL23a, but the role of this interaction in RNA decapping has not been investigated (77). A recent article reports that spliced RNAs are more robustly downregulated by the VacV decapping enzyme D10 than intronless RNAs (72), which is reminiscent of influenza A virus PA-X activity (47) (see Section 2.1.3) and suggests that D10 may interact with mRNA processing proteins. Finally, the viral decapping enzymes, like herpesviral RNases (see Sections 2.1.1 and 2.1.2), do not seem to discriminate between host and viral mRNAs, raising the question of how viral genes are expressed (77, 78). However, at least for D9 and D10, the effect on viral mRNAs may be weaker than that on host mRNAs, and late viral transcripts may mostly escape targeting, according to recent RNA sequencing results (72).
2.3. Host RNase Induced by Viral Protein: Coronaviruses
CoVs are single-stranded positive-sense RNA viruses that infect avian and mammalian hosts, usually causing respiratory or enteric infections. They are classified into four genera, α, β, γ, and δ. They include several human coronaviruses (HCoVs): α-CoVs HCoV-229E and HCoV-NL63 and β-CoVs HCoV-HKU1 and HCoV-OC43, all of which cause common colds, and β-CoVs Middle East respiratory syndrome coronavirus (MERS-CoV) and the two severe acute respiratory syndrome coronaviruses (SARS-CoV and SARS-CoV-2), which cause severe respiratory diseases and pandemics.
α- and β-CoVs, which encompass all HCoVs identified so far, encode the host shutoff protein nsp1 (Figure 2d). nsp1 proteins from α-CoVs and β-CoVs have similar biological functions, despite a lack of sequence similarity (79). This suggests an important role for nsp1 in infection. nsp1 inhibits host translation in all HCoVs tested and the swine transmissible gastroenteritis virus (TGEV), with some mechanistic differences (80, 81). All tested nsp1 homologs, except TGEV nsp1, also induce degradation of host mRNAs (9, 82-89). Yet, nsp1 does not resemble any known RNase in terms of sequence and structure, and cannot cleave RNA by itself in vitro but requires whole cell extracts to do so (90, 91), suggesting that nsp1 does not have intrinsic RNase activity. Because nsp1 binds ribosomes (see below), the dominant model is that nsp1 activates a host RNA quality control pathway linked to translation to trigger mRNA degradation. However, the host RNase responsible for the degradation is not yet known. One candidate that has been excluded is RNase L (84). A recent review suggested that the human homolog of the recently discovered yeast endonuclease Cue2 may be a good candidate, as it degrades mRNAs in which translation has stalled (92).
SARS-CoV and SARS-CoV-2 nsp1 bind the 40S ribosome subunit to gain access to mRNAs during translation and induce their degradation (9, 85, 88, 90) (Figure 2d). As a result, nsp1 specifically targets capped mRNAs transcribed by RNAPII (4, 86), as well as mRNAs with picornavirus type I and II IRES elements (9, 86, 88). Moreover, in in vitro assays using cellular extracts, SARS-CoV nsp1 induces endonucleolytic cleavage of the RNA within 30 nucleotides of the 5′ cap in capped RNAs or within the ribosome loading region of RNAs containing IRES elements, without any sequence preference (88). Similarly, in cells transfected with SARS-CoV nsp1, the RNA cleavage event induced by nsp1 occurs close to the 5′ cap, followed by degradation of the resulting RNA fragment by the host exoribonuclease Xrn1 (4).
Binding to the ribosome also enables nsp1 to inhibit translation by altering ribosome function and complex formation (93). Structural studies on SARS-CoV-2 nsp1 have shown that this inhibition is due to nsp1 inserting its C-terminal domain inside the 40S ribosomal mRNA channel to interfere with mRNA binding (94, 95). This action prevents the mRNA from entering the ribosome entry channel and likely marks the RNA for degradation (9). However, translation inhibition and RNA degradation are separate functions, as SARS-CoV and SARS-CoV-2 nsp1s with mutations at residues R124 and K125 still block translation but do not induce RNA decay (90, 96). R124 and K125 are positively charged and located on the molecular surface, so they may directly interact with RNA (91). Moreover, SARS-CoV nsp1 inhibits translation of uncapped mRNAs containing an IRES from encephalomyocarditis virus (EMCV), hepatitis C virus, or cricket paralysis virus but induces degradation of only those with EMCV IRESs (9). This specificity could stem from RNA elements in the target RNAs or from different requirements for translation initiation factors by different IRESs (9). Distinguishing between these possibilities will provide a more complete model of nsp1 action.
Viral mRNAs evade nsp1-induced RNA cleavage. The protection is conferred by their 5′ UTR, or leader sequence, which is identical in all genomic and subgenomic viral mRNAs (97, 98), and in particular the stem loop 1 (SL1) structure within it (88, 90, 95, 99-101). Interestingly, the protection may come from nsp1 itself binding to SL1 (102, 103) (Figure 2d). Yet, the mechanism for protection is still unclear. One study suggests that nsp1 binding to viral mRNA triggers structural changes in the ribosome to enable translation (90). Another study suggests that the viral leader protective sequence can change the structural conformation of nsp1, leading to its dissociation from the 40S ribosome subunit and allowing viral mRNA translation (99). However, other studies have found that SARS-CoV-2 nsp1 remains bound to the ribosome during viral mRNA translation (101).
MERS-CoV nsp1 also induces degradation of RNAPII transcripts and translation inhibition, and mutations can uncouple the RNA degradation and translation inhibition functions (86). Moreover, MERS-CoV nsp1 also binds to the 5′ UTR SL1 of viral mRNAs to protect them (82). However, MERS-CoV nsp1 uses a different mechanism to access its target mRNAs, as it does not bind the 40S ribosome subunit stably (86). Additionally, while SARS-CoV nsp1 is exclusively found in the cytoplasm, MERS-CoV nsp1 is located in both nucleus and cytoplasm. Moreover, MERS-CoV nsp1 does not degrade cytoplasm-restricted translated mRNAs such as electroporated mRNA or virus-like mRNAs synthesized in the cytoplasm using the Rift Valley fever virus cytoplasmic transcription machinery (86) (Figure 2d). More work is needed to determine the mechanism by which MERS-CoV nsp1 accesses its target RNAs and whether this leads to differences in host gene expression changes in MERS-CoV versus SARS-CoVs infection, which may in turn have implications for immune evasion by these viruses.
3. CONSEQUENCES FOR OTHER ASPECTS OF RNA METABOLISM
Interestingly, viral RNA degradation factors reduce gene expression not only directly by degrading or inducing degradation of host RNAs but also indirectly because the widespread reduction in cytoplasmic RNA levels triggers secondary changes in RNA metabolism (104, 105). These secondary changes include transcription inhibition and nuclear retention of RNAs, which further contribute to host shutoff, and inhibition of stress granule (SG) formation, which along with the decrease in host mRNAs promotes translation of viral mRNAs. In this section, we discuss these secondary effects (Figure 3).
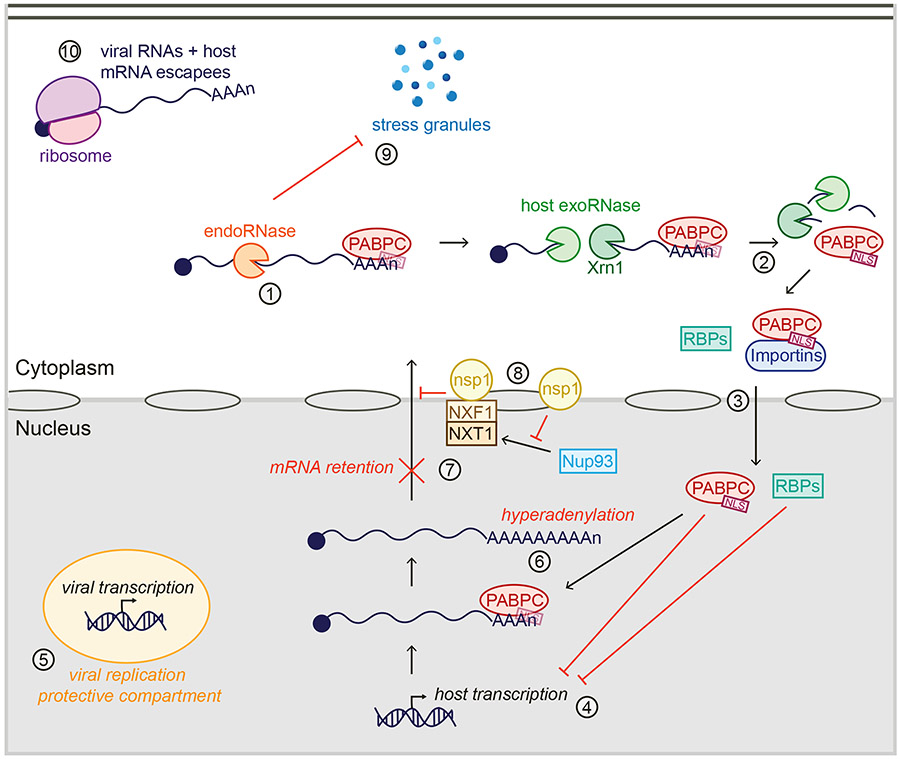
Figure 3.
Consequences of widespread RNA degradation by viral host shutoff factors on cellular and viral RNA metabolism. Poly(A)-binding protein (PABPC) usually binds RNAs in the cytoplasm. However, upon endonucleolytic cleavage of RNAs by viral ribonucleases RNases or the host RNase activated by nonstructural protein 1nsp1 (endoRNase) (#1) and subsequent RNA fragment degradation by Xrn1 and other host exoribonucleases (exoRNase) (#2), PABPC is released from the RNA, exposing its nuclear localization signal (NLS). PABPC thus interacts with nuclear import proteins and is shuttled to the nucleus (#3), along with other RNA-binding proteins (RBPs) that are also no longer RNA bound. In the nucleus, these proteins inhibit host transcription (#4), while viral transcription continues, likely in protective compartments (#5). PABPC accumulation in the nucleus leads to hyperadenylation (#6) and nuclear mRNA retention (#7). nsp1 also induces nuclear retention of RNAs independently of PABPC by preventing localization of Nup93 to the nuclear pore and interfering with the function of the NXF1-NXT1 complex (#8). In parallel, viral RNases prevent formation of stress granules (SGs) (#9), allowing translation of the viral mRNAs and host mRNAs that escape host shutoff (#10).
3.1. Transcriptional Inhibition
Global host mRNA decay triggers a feedback loop that inhibits transcription (104). Overexpression of the α- and γ-herpesvirus RNases and MHV68 and HSV-1 infections lead to a decrease in RNAPII recruitment to promoters and in mRNA synthesis (104, 106, 107). Evidence suggests that the transcriptional inhibition is triggered by the completion of RNA degradation by cellular machinery, as silencing of the cellular exonuclease Xrn1 and potentially other cellular factors involved in mRNA decay blocks the transcriptional feedback in cells overexpressing muSOX (104). Moreover, widespread RNA degradation during apoptosis triggers the same feedback mechanism in the absence of viral infection (108). However, Xrn1 depletion is not enough to rescue RNAPII promoter occupancy during MHV68 infection, suggesting that additional factors contribute to transcription inhibition during infection (106). These factors may include an increase in nuclear accumulation of RNA-binding proteins such as the poly(A)-binding proteins cytoplasmic poly(A)-binding protein (PABPC) and La ribonucleoprotein 4 (LARP4), which interfere with transcription through an unknown mechanism, and a reduction in nuclear levels of RNAPII subunits and/or the transcription initiation factor Gtf2B/TFIIB (106, 108-110). Interestingly, in MHV68 infection, RNAPII-transcribed viral RNAs are not subjected to transcription inhibition, possibly because they are transcribed in separate compartments (104, 106). Overall, these findings imply that simply measuring RNA levels does not appropriately capture RNA degradation during infection or even overexpression of viral RNA degradation-triggering factors, as both RNA degradation and transcription are altered during host shutoff. Consequently, assays that can separate RNA degradation from transcription should be used to draw conclusions about the degradation of specific RNAs by viral RNA degradation factors.
3.2. Nuclear Retention of RNAs
Another secondary consequence of widespread cytoplasmic RNA degradation is the nuclear retention of host RNAs. As mentioned above, widespread RNA degradation leads to changes in the nuclear-cytoplasmic distribution of RNA-binding proteins, including increased nuclear localization of PABPC in the presence of viral RNases and nsp1 (as far as we know, this has not been investigated during host shutoff due to decapping enzymes) (5, 111-115). PABPC is a nucleocytoplasmic shuttling protein, but it normally accumulates in the cytoplasm, where it binds to the poly(A) tail of mRNAs to stabilize them and enhance their translation (116). During cellular stress, such as heat shock, oxidative stress, transcriptional block, or host shutoff caused by viral infection or overexpression of viral host shutoff proteins, PABPC translocates to the nucleus (112, 117). This relocalization is due to a noncanonical nuclear localization signal (NLS) in PABPC RNA-binding domain (105). The NLS is normally masked when PABPC is bound to RNA but interacts with nuclear import machinery when PABPC is released upon RNA degradation during host shutoff (105). Importantly, nuclear PABPC accumulation leads to mRNA hyperadenylation and subsequent inhibition of mRNA export, which traps host RNAs in the nucleus and prevents them from being translated (112-114). In the case of viral infections, this may further intensify host shutoff.
Interestingly, widespread RNA degradation by HSV-1 vhs promotes nuclear retention of both host and viral mRNAs, but as VP22 and VP16 inhibit vhs activity later in infection, late viral transcripts are efficiently exported, allowing late protein translation (115).
In addition to this RNA degradation- and PABPC-dependent export regulation, SARS-CoV and SARS-CoV-2 nsp1 also block mRNA nuclear export directly by binding nucleoporins such as Nup93 and mRNA export factors such as NXF1-NXT1 and preventing their association with nuclear pores and/or RNA (118, 119).
3.3. Inhibition of Stress Granule Formation
A third effect of virus-induced mRNA decay is the inhibition of SG formation. SGs are stress-induced cytoplasmic aggregates of mRNAs, translation machinery, and mRNA-binding proteins (120). They form upon activation of kinases such as the double-stranded RNA-activated protein kinase R (PKR) that phosphorylate eIF2α and inhibit translation (120). SGs protect host mRNAs during stress so that their translation can resume once stress is relieved (120) but also have an antiviral function as they trap viral mRNAs and prevent their translation (121). To counteract this antiviral activity, HSV and influenza A virus use their RNases to inhibit SG formation. Evidence for vhs inhibition of SGs comes from disassembly of arsenite-induced SGs during infection with wild-type but not vhs-deficient HSV-2, as well as formation of SGs during infection with vhs-deficient HSV-1 and HSV-2, but not wild-type viruses, in the absence of other stimuli (122, 123). HSV-2 vhs can also localize to SGs, perhaps to degrade mRNAs in SGs (123). Additionally, vhs reduces accumulation of double-stranded RNA by degrading it, which prevents activation of PKR in infected cells (122, 124). Influenza A virus PA-X RNase activity also inhibits SG formation, but independently of eIF2α phosphorylation (114). The exact mechanism is not yet known, but it may simply be due to the lack of cytoplasmic mRNA to nucleate SGs.
3.4. Changes in the Host Translatome
The reduction in host cytoplasmic mRNAs and inhibition of SG formation ultimately help define the translatome, i.e., which mRNAs are translated during infection. Indeed, merely hours after infection with influenza A virus, SARS-CoV-2, HSV-1, or VacV, the majority of translated mRNAs are viral mRNAs (69, 125-127). In influenza A virus and SARS-CoV-2 infections, this shift to viral translation is likely a direct consequence of host mRNA depletion, as viral mRNAs are not translated more efficiently than host mRNAs (126, 127). Conversely, for VacV infections, mRNA depletion and direct enhancement of viral mRNA translation by D9 and D10 both contribute to the shift (69, 128).
Host shutoff may also help sculpt the host translatome, as some host mRNAs can escape host shutoff. Interestingly, mRNAs for genes involved in translation and oxidative phosphorylation escape RNA degradation and continue to be translated despite host shutoff after infection with multiple viruses, including influenza A virus and VacV, or after overexpression of SARS-CoV-2 nsp1 (69, 100, 127). Translation and oxidative phosphorylation are required for cell survival and viral replication, suggesting that protection of these mRNAs may be an intrinsic cellular mechanism (or an evolved viral mechanism) to promote cellular or viral survival. While the studies did not examine influenza PA-X or VacV D9/D10 mutant viruses, mRNAs involved in translation are enriched among transcripts that are not downregulated by PA-X activity, supporting the idea that these RNAs continue to be translated because they escape PA-X host shutoff (47). Of note, no enriched gene categories have been identified in RNAs escaping host shutoff by herpesviruses. However, specific RNAs do escape degradation by KSHV SOX, including the antiviral genes IL-6, GADD45B, and C19ORF66 (129-132). These mRNAs contain SOX resistance elements in their 3′ UTRs. These elements recruit a protective protein complex that includes the RNA-binding proteins AUF1, HuR, and nucleolin (130-134). At least for some of the RNAs, N6-adenosine methylation in the SOX resistance element is also required for protection from degradation (135). Interestingly, some of the SOX escapees are also resistant to degradation by the other viral RNases described in this review, such as vhs and PA-X, but not by cellular RNases such as the one induced by SARS-CoV nsp1 or the nonsense-mediated decay RNase SMG6 (130, 132, 133). These results suggest the potential existence of a common mechanism for RNA protection from viral RNase activity that could play a role in preserving expression of genes with key functions in viral replication or antiviral responses.
4. ROLE OF VIRAL RNASES IN IMMUNE EVASION AND VIRAL REPLICATION
Ultimately, the purpose of viral RNases is to promote viral replication, largely by facilitating immune evasion. The immune response is initiated by virus sensing by infected cells. All cells express proteins that can sense the presence of pathogen-associated molecules, including viral nucleic acids, triggering expression of antiviral genes to fight the virus and cytokines to alert neighboring immune and nonimmune cells of the infection (136). The best-studied early antiviral pathway is the type I and III interferon (IFN) pathway. Type I/III IFNs are transcribed when one of the nucleic acid sensors senses aberrant viral (and in some cases cellular) RNA or DNA species, leading to phosphorylation and nuclear import of the transcription factors interferon regulatory factor (IRF) 3 and 7 and nuclear factor-kappa B(NF-κB)(137, 138)(Figure 4). Secreted IFNs then signal in a paracrine fashion to neighboring cells and in an autocrine fashion back to the IFN-producing cell to activate tyrosine kinase 2 (TYK2) and Janus kinase (JAK), which phosphorylate and activate signal transducer and activator of transcription (STAT) 1 and 2. These transcription factors induce expression of the IFN-stimulated genes (ISGs), which sets up a potent antiviral state (137) (Figure 4). Viral RNA degradation factors contribute to reducing activation of type I/III IFN responses in infected cells by reducing the levels of the mRNAs that code for IFNs, ISGs, and signaling proteins in the IFN induction and response pathways. Consequently, mutant viruses that do not produce viral RNA degradation factors are often defective in vivo. In this section, we discuss the effect of viral RNA degradation factors on the antiviral response of the infected cell, viral replication in vivo and in cells, and disease (Figure 4).
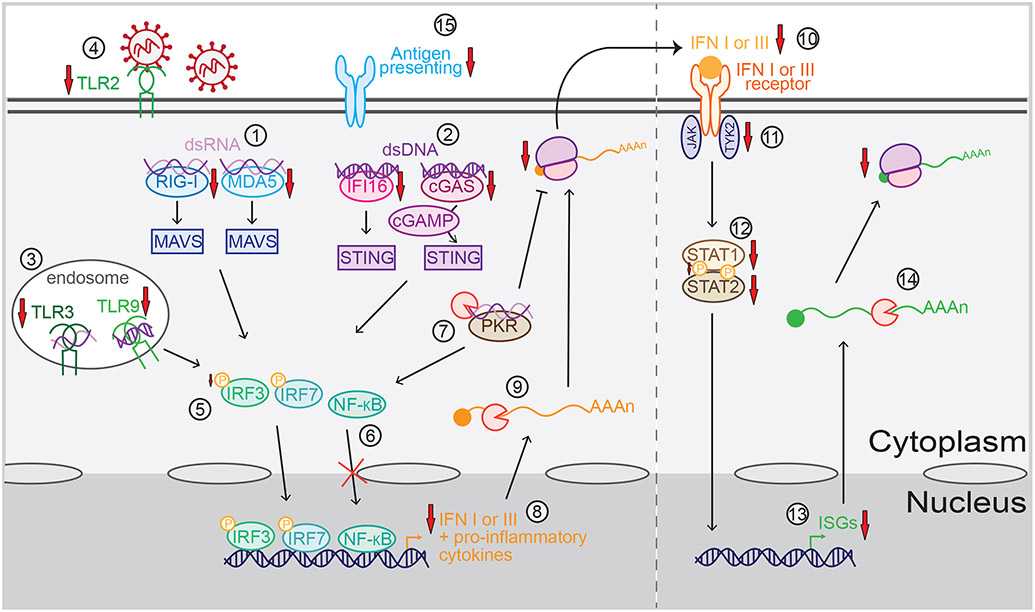
Figure 4.
Mechanisms of immune evasion by viral RNA degradation factors. Viral RNA degradation factors can block viral sensing by downregulating the levels of the double-stranded RNA (dsRNA) sensors retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) (#1) that signal through mitochondrial antiviral-signaling protein (MAVS), the double-stranded DNA (dsDNA) sensors interferon gamma-inducible protein 16 (IFI16) and cyclic GMP-AMP (cGAMP) synthase (cGAS) (#2) that signal through stimulator of interferon genes (STING), the endosomal RNA and DNA sensors Toll-like receptor (TLR) TLR3 and TLR9 (#3), and the surface receptor TLR2 (#4). These sensors signal to activate and translocate transcription factors nuclear factor-kappa B (NF-κB), interferon regulatory factor (IRF) 3 and IRF7 to the nucleus, although phosphorylation (represented by the yellow P) of IRF3 (#5) and translocation of NF-κB can also be blocked by some RNA degradation factors (#6). RNA degradation also dampens viral sensing through the RNA sensor protein kinase R (PKR) (#7), which activates NF-κB and inhibits translation. As a result of these signaling changes, transcription of messenger RNAs (mRNAs) for interferons (IFNs) and other proinflammatory cytokines is decreased (#8). Also, their mRNAs are degraded (#9), leading to less cytokine secretion (#10). Secreted type I and III IFNs are normally sensed by the IFN I and III receptors, respectively, which activate tyrosine kinase 2 (TYK2) and Janus kinase (JAK). Viral RNA degradation factors also decrease signaling downstream of the IFN receptors by downregulating the levels of TYK2 (#11) and signal transducer and activator of transcription (STAT) 1 and STAT2 proteins (#12), orinhibiting phosphorylation of STAT1 (#12). This leads to a decrease in IFN-stimulated gene (ISG) transcription (#13), in addition to direct degradation of ISG mRNAs (#14), which ultimately reduce ISG translation. Viral RNA degradation factors can also contribute to evasion of the adaptive immune response by downregulating antigen-presenting molecules (#15).
4.1. Decreased Antiviral Response
Several studies reported that HSV vhs reduces expression of nucleic acid sensors. HSV-1 vhs downregulates the mRNA for the DNA sensors cGMP-AMP synthase (cGAS) and interferon gamma-inducible protein 16 (139, 140). HSV-2 vhs downregulates the Toll-like receptors (TLRs) TLR2 and TLR3 and the RNA sensors retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) (141). Accordingly, HSV-1 and HSV-2 vhs strongly inhibit type I IFN production in murine embryonic fibroblasts (MEFs) (142, 143). Moreover, HSV-1 vhs prevents the induction of antiviral ISGs and proinflammatory cytokines (143-148). HSV-1 vhs also downregulates the mRNA for alpha thalassemia/mental retardation syndrome X-linked, an effector of intrinsic immunity (149). Although less studied, evidence of immune evasion was also observed in EBV BGLF5, which decreases expression of TLR9 and TLR2 (150, 151).
Although sensor downregulation has not been reported for influenza PA-X, this protein can dampen IFN activation independently of RNA degradation by binding Ankrd17, a protein that activates RIG-I to induce IFN-β expression (50, 152). PA-Xs from multiple human and avian strains also downregulate the mRNAs for type I IFNs, proinflammatory cytokines, ISGs, and other antiviral genes (47, 153-156). While this is likely mostly due to RNA degradation, PA-X may also restrict NF-κB p65 nuclear translocation and activity through an unknown mechanism, leading to changes in NF-κB target gene expression (157).
SARS-CoV and SARS-CoV-2 nsp1 also downregulate IFN-β, STAT2, and TYK2 expression both during infection and upon overexpression (83, 85, 99, 158-160). However, this downregulation likely comes not only from mRNA degradation but also from translation inhibition. nsp1s from HCoVs also block IRF3 and/or STAT1/2 phosphorylation, thereby dampening IFN signaling (87, 160-162). Inhibition of IRF3 phosphorylation by SARS-CoV nsp1 is abolished by mutations that block nsp1 RNA degradation but not translation inhibition, suggesting that mRNA degradation is involved, although the exact mechanism is unknown (159).
Viral RNA degradation factors also prevent activation of the antiviral response by degrading immunostimulatory viral nucleic acids. VacV D9 and D10 and HSV-1 vhs prevent the accumulation of double-stranded RNA that would otherwise be sensed by PKR (163, 164). However, sensing by PKR may not significantly contribute to IFN-β mRNA induction in HSV-1 infection (122).
In addition to inhibition of innate immune pathways, viral RNA degradation factors can also affect the adaptive immune response by altering expression of the major histocompatibility complex (MHC) proteins. EBV BGLF5, KSHV SOX, and HSV vhs downregulate MHC class I and II molecules, and BGLF5 downregulates CD1d, a nonclassical MHC protein that presents lipid antigens. By manipulating MHC proteins, these viral factors may promote escape of the infected cell from T cell recognition (34, 151, 165-167).
4.2. Consequences for Viral Replication and Disease
By interfering with antiviral responses, viral RNA degradation factors allow the virus to successfully evade host immunity and replicate. Indeed, mutant viruses that do not produce these factors often have replication defects, particularly in vivo where evading the host response is crucial, and cause milder disease. VacV mutants that encode catalytic mutant D9 and/or D10 replicate to lower titers in mouse lungs and cause less lung histopathology (78, 163). Similarly, defective viral replication and spread are observed in mice infected with nsp1-null mouse hepatitis virus (MHV), an in vivo model of CoV infection (168). The replication difference between wild-type and nsp1-deficient MHV disappears in mice that lack the type I IFN receptor, indicating that nsp1 is needed to evade the type I IFN pathway (168). vhs-deficient HSV-1 and HSV-2 also replicate to lower titers in mouse cornea, trigeminal ganglia, and brain, and cause less pathogenesis (169, 170). vhs-deficient HSV-2 is not attenuated in MEFs or mice lacking the type I IFN receptor but is attenuated in mice that lack mature immune B and T cells, indicating that HSV-2 vhs mediates escape from type I IFN responses but not adaptive immunity (142, 171). Interestingly, vhs-deficient HSV-1 is still attenuated in similar experiments in MEFs and mice lacking the type I IFN receptor (143, 171). This attenuation may reflect a need for vhs-mediated degradation of immediate early and early viral mRNAs during HSV-1 infection, to prevent their continued accumulation late in infection and promote translation of late viral mRNAs instead (32, 124).
Influenza A virus PA-X has a particularly striking function in immune regulation. PA-X-deficient viruses cause heightened immune and inflammatory responses in vivo, but these responses are often not protective. They do not reduce replication and instead lead to increased host morbidity and mortality. This phenomenon has been reported in mice, chickens, ducks, and pigs infected with several different influenza A strains, including the H1N1s from the 1918 and 2009 pandemics and highly pathogenic avian H5N1 strains (43, 155, 172-174). There is also increased recruitment of lymphocytes and neutrophils to the lungs of mice infected with a 2009 pandemic H1N1 virus lacking PA-X compared to wild-type virus (175). This strong effect on immune responses may explain the evolutionary pressure for influenza A virus strains to retain PA-X (46) and, perhaps, allows better transmission of the virus by preventing early death of the host. However, more studies that investigate the effect of PA-X on viral transmission are needed. Of note, some studies report no change or a decrease in virulence for mutant viruses that do not make PA-X, particularly with avian H9N2 and H7N9 strains in mice and avian H5N1 and H7N1 strains in eggs (176-178). The reasons for these strain- and model-dependent differences remain unclear, but they hint at a role for PA-X sequence variation in determining the virulence of different influenza A virus strains.
Like for vhs and late gene expression, there are also instances of host shutoff factors influencing replication independently of immune evasion. For example, host shutoff by MHV68 muSOX is needed for efficient viral trafficking to lymph nodes and establishment of a latent infection in vivo, even though it is dispensable for acute replication in mouse lungs (179). Moreover, the RNA degradation function of MERS-CoV nsp1 may play a role in assembly and/or budding of viral particles (180). What changes in gene expression mediate these functions remains unknown.
5. CONCLUDING REMARKS AND OUTSTANDING QUESTIONS
The field of viral RNases and RNA degradation-inducing factors keeps expanding as more viral proteins that induce host shutoff are discovered. Through viral RNA degrading factors, viruses from unrelated families with very different replication cycles have found a common way to modulate gene expression and tailor the cell to their needs while evading immunity. This convergent evolution of host shutoff mechanisms points to the efficiency of RNA degradation as a host shutoff and cellular takeover strategy. Virally induced RNA decay resembles cellular mRNA degradation, either basal decay or quality control pathways that are initiated by endonucleolytic cleavage, and uses some of the same machinery. Therefore, it may not be detected as aberrant (4). Nonetheless, widespread RNA degradation is still at some point sensed by the host, likely after enough RNA has been degraded to cause shuttling of PABPC and other RNA-binding proteins to the nucleus (109, 181).
In general, a key missing piece in understanding host shutoff is how the molecular activity and selectivity of the factors is linked to their role in immune evasion and/or the viral replication cycle. Even though these proteins also target innate immune genes, as we discussed in Section 4.1, often the changes in RNA levels are relatively small, which raises the question of how they lead to profound effects in vivo. Moreover, there is growing evidence that host shutoff processes are more selective than originally thought, although the molecular selectivity has not clearly been linked to functional consequences yet. It is also possible that the bulk of host shutoff is due to secondary effects on transcription and translation. Alternatively, immune signaling gene expression may be more robustly affected by host shutoff because these genes are minimally expressed before viral infection. Additional studies separating the contribution of direct RNA degradation from that of secondary effects on RNA metabolism and comparing RNA and protein changes will help resolve these questions.
Another important consideration is that host shutoff factors modulate the intrinsic responses of infected cells, which are often not innate immune cells. To understand their contribution to infection, we need to advance our understanding of how host shutoff-induced changes at the infected cell level alter recruitment of immune cells to the site of infection and host inflammation.
Lastly, much of the research has focused on how shutoff modulates immune responses, but for some of the proteins, evidence suggests that there are additional roles in viral replication that should be explored further.
Continued studies of the mechanism of action of host shutoff proteins coupled with investigation of the in vivo consequences of host shutoff will provide a complete picture of how viruses use RNA degradation factors to regulate the host and how we may be able to use this knowledge for therapeutic intervention.
ACKNOWLEDGMENTS
We apologize to the many contributors of the field whose work was not directly cited due to space limitations. Work on viral RNA degradation factors in the Gaglia laboratory was supported by National Institutes of Health (NIH) R01 AI137358, an American Lung Association COVID-19 & Respiratory Virus Research Award, and a donation to M.M.G. by Dr. Marie Rozan. L.G. was supported by NIH F31 AI154587.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Minskaia E, Hertzig T, Gorbalenya AE, Campanacci V, Cambillau C, et al. 2006. Discovery of an RNA virus 3’->5’ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc Natl Acad Sci U S A. 103(13):5108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hackbart M, Deng X, Baker SC. 2020. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc Natl Acad Sci US A. 117(14):8094–8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decroly E, Ferron F, Lescar J, Canard B. 2012. Conventional and unconventional mechanisms for capping viral mRNA. Nat Rev Microbiol. 10(1):51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaglia MM, Covarrubias S, Wong W, Glaunsinger BA. 2012. A Common Strategy for Host RNA Degradation by Divergent Viruses. Journal of Virology. 86(17):9527–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaperskyy DA, Schmaling S, Larkins-Ford J, McCormick C, Gaglia MM. 2016. Selective Degradation of Host RNA Polymerase II Transcripts by Influenza A Virus PA-X Host Shutoff Protein. PLoS Pathog. 12(2):e1005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrish S, Resch W, Moss B. 2007. Vaccinia virus D10 protein has mRNA decapping activity, providing a mechanism for control of host and viral gene expression. Proc Natl Acad Sci U S A. 104(7):2139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parrish S, Moss B. 2007. Characterization of a second vaccinia virus mRNA-decapping enzyme conserved in poxviruses. J Virol. 81(23):12973–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish S, Hurchalla M, Liu S-W, Moss B. 2009. The African swine fever virus g5R protein possesses mRNA decapping activity. Virology. 393(1):177–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamitani W, Huang C, Narayanan K, Lokugamage KG, Makino S. 2009. A two-pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat. Struct. Mol. Biol 16(11): 1134–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covarrubias S, Gaglia MM, Kumar GR, Wong W, Jackson AO, Glaunsinger BA. 2011. Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1. PLoS Pathogens. 7(10):e1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doherty AJ, Serpell LC, Ponting CP. 1996. The Helix-Hairpin-Helix DNA-Binding Motif: A Structural Basis for Non-Sequence-Specific Recognition of DNA. Nucleic Acids Research. 24(13):2488–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elgadi MM, Hayes CE, Smiley JR. 1999. The Herpes Simplex Virus vhs Protein Induces Endoribonucleolytic Cleavage of Target RNAs in Cell Extracts. J. Virol 73(9):7153–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H-W, Hsu W-L, Chang Y-Y, Jan M-S, Wong M-L, Chang T-J. 2010. Role of the UL41 protein of pseudorabies virus in host shutoff, pathogenesis and induction of TNF-α expression. J Vet Med Sci. 72(9): 1179–87 [DOI] [PubMed] [Google Scholar]
- 14.Hinkley S, Ambagala APN, Jones CJ, Srikumaran S. 2000. A vhs-like activity of bovine herpesvirus-1. Arch. Virol 145(10):2027–46 [DOI] [PubMed] [Google Scholar]
- 15.He T, Wang M, Cheng A, Yang Q, Jia R, et al. 2021. DPV UL41 gene encoding protein induces host shutoff activity and affects viral replication. Veterinary Microbiology. 255:108979. [DOI] [PubMed] [Google Scholar]
- 16.Black D, Ritchey J, Payton M, Eberle R. 2014. Role of the virion host shutoff protein in neurovirulence of monkey B virus (Macacine herpesvirus 1). Virol. Sin 29(5):274–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desloges N, Rahaus M, Wolff MH. 2005. The varicella–zoster virus–mediated delayed host shutoff: open reading frame 17 has no major function, whereas immediate–early 63 protein represses heterologous gene expression. Microbes and Infection. 7(15): 1519–29 [DOI] [PubMed] [Google Scholar]
- 18.Page HG, Read GS. 2010. The Virion Host Shutoff Endonuclease (UL41) of Herpes Simplex Virus Interacts with the Cellular Cap-Binding Complex eIF4F. J. Virol 84(13):6886–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng P, Everly DN, Read GS. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol 79(15):9651–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarma N, Agarwal D, Shiflett LA, Read GS. 2008. Small Interfering RNAs That Deplete the Cellular Translation Factor eIF4H Impede mRNA Degradation by the Virion Host Shutoff Protein of Herpes Simplex Virus. J. Virol 82(13):6600–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong AD, Frenkel N. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. U.S.A 84(7):1926–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oroskar AA, Read GS. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol 61(2):604–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oroskar AA, Read GS. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol 63(5):1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu M, Taddeo B, Roizman B. 2015. Tristetraprolin Recruits the Herpes Simplex Virion Host Shutoff RNase to AU-Rich Elements in Stress Response mRNAs To Enable Their Cleavage. J. Virol 89(10):5643–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu M, Taddeo B, Roizman B. 2013. The Nuclear-Cytoplasmic Shuttling of Virion Host Shutoff RNase Is Enabled by pUL47 and an Embedded Nuclear Export Signal and Defines the Sites of Degradation of AU-Rich and Stable Cellular mRNAs. J. Virol 87(24): 13569–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiflett LA, Read GS. 2013. mRNA Decay during Herpes Simplex Virus (HSV) Infections: Mutations That Affect Translation of an mRNA Influence the Sites at Which It Is Cleaved by the HSV Virion Host Shutoff (Vhs) Protein. J. Virol 87(1):94–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y-F, Tsai P-Y, Chulakasian S, Lin F-Y, Hsu W-L. 2016. The pseudorabies virus vhs protein cleaves RNA containing an IRES sequence. The FEBS Journal. 283(5):899–911 [DOI] [PubMed] [Google Scholar]
- 28.Esclatine A, Taddeo B, Roizman B. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. U.S.A 101(52): 18165–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam Q, Smibert CA, Koop KE, Lavery C, Capone JP, et al. 1996. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 15(10):2575–81 [PMC free article] [PubMed] [Google Scholar]
- 30.Taddeo B, Zhang W, Roizman B. 2010. Role of Herpes Simplex Virus ICP27 in the Degradation of mRNA by Virion Host Shutoff RNase. J. Virol 84(19):10182–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taddeo B, Sciortino MT, Zhang W, Roizman B. 2007. Interaction of herpes simplex virus RNase with VP16 and VP22 is required for the accumulation of the protein but not for accumulation of mRNA. PNAS. 104(29):12163–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dauber B, Saffran HA, Smiley JR. 2014. The Herpes Simplex Virus 1 Virion Host Shutoff Protein Enhances Translation of Viral Late mRNAs by Preventing mRNA Overload. J. Virol 88(17):9624–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaunsinger B, Ganem D. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13(5):713–23 [DOI] [PubMed] [Google Scholar]
- 34.Rowe M, Glaunsinger B, van Leeuwen D, Zuo J, Sweetman D, et al. 2007. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc. Natl. Acad. Sci. U.S.A 104(9):3366–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covarrubias S, Richner JM, Clyde K, Lee YJ, Glaunsinger BA. 2009. Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J. Virol 83(18):9554–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buisson M, Géoui T, Flot D, Tarbouriech N, Ressing ME, et al. 2009. A bridge crosses the active-site canyon of the Epstein-Barr virus nuclease with DNase and RNase activities. J. Mol. Biol 391(4):717–28 [DOI] [PubMed] [Google Scholar]
- 37.Dahlroth S-L, Gurmu D, Schmitzberger F, Engman H, Haas J, et al. 2009. Crystal structure of the shutoff and exonuclease protein from the oncogenic Kaposi’s sarcoma-associated herpesvirus. FEBS J. 276(22):6636–45 [DOI] [PubMed] [Google Scholar]
- 38.Gaglia MM, Rycroft CH, Glaunsinger BA. 2015. Transcriptome-Wide Cleavage Site Mapping on Cellular mRNAs Reveals Features Underlying Sequence-Specific Cleavage by the Viral Ribonuclease SOX. PLoS Pathog. 11(12):e1005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez AS, Vogt C, Bohne J, Glaunsinger BA. 2018. Site specific target binding controls RNA cleavage efficiency by the Kaposi’s sarcoma-associated herpesvirus endonuclease SOX. Nucleic Acids Research. 46(22): 11968–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abernathy E, Clyde K, Yeasmin R, Krug LT, Burlingame A, et al. 2014. Gammaherpesviral gene expression and virion composition are broadly controlled by accelerated mRNA degradation. PLoS Pathog. 10(1):e1003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feederle R, Bannert H, Lips H, Müller-Lantzsch N, Delecluse H-J. 2009. The Epstein-Barr Virus Alkaline Exonuclease BGLF5 Serves Pleiotropic Functions in Virus Replication. J. Virol 83(10):4952–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inglis SC. 1982. Inhibition of Host Protein Synthesis and Degradation of Cellular mRNAs During Infection by Influenza and Herpes Simplex Virus. Mol. Cell. Biol 2(12):1644–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jagger BW, Wise HM, Kash JC, Walters K-A, Wills NM, et al. 2012. An Overlapping Protein-Coding Region in Influenza A Virus Segment 3 Modulates the Host Response. Science. 337(6091):199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dias A, Bouvier D, Crépin T, McCarthy AA, Hart DJ, et al. 2009. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 458(7240):914–18 [DOI] [PubMed] [Google Scholar]
- 45.Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, et al. 2009. Crystal structure of an avian influenza polymerase PAN reveals an endonuclease active site. Nature. 458(7240):909–13 [DOI] [PubMed] [Google Scholar]
- 46.Shi M, Jagger BW, Wise HM, Digard P, Holmes EC, Taubenberger JK. 2012. Evolutionary Conservation of the PA-X Open Reading Frame in Segment 3 of Influenza A Virus. Journal of Virology. 86(22):12411–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaucherand L, Porter BK, Levene RE, Price EL, Schmaling SK, et al. 2019. The Influenza A Virus Endoribonuclease PA-X Usurps Host mRNA Processing Machinery to Limit Host Gene Expression. Cell Reports. 27(3):776–792.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi T, Chaimayo C, McGuinness J, Takimoto T. 2016. Critical Role of the PA-X C-Terminal Domain of Influenza A Virus in Its Subcellular Localization and Shutoff Activity. Journal of Virology. 90(16):7131–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scarborough AM, Flaherty JN, Hunter OV, Liu K, Kumar A, et al. 2021. SAM homeostasis is regulated by CFIm-mediated splicing of MAT2A. eLife. 10:e64930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Qi W, Chang Q, Chen R, Zhen D, et al. 2021. Influenza A virus protein PA-X suppresses host Ankrd17-mediated immune responses. Microbiology and Immunology. 65(1):48–59 [DOI] [PubMed] [Google Scholar]
- 51.Li Q, Yuan X, Wang Q, Chang G, Wang F, et al. 2016. Interactomic landscape of PA-X-chicken protein complexes of H5N1 influenza A virus. Journal of Proteomics. 148:20–25 [DOI] [PubMed] [Google Scholar]
- 52.Bavagnoli L, Cucuzza S, Campanini G, Rovida F, Paolucci S, et al. 2015. The novel influenza A virus protein PA-X and its naturally deleted variant show different enzymatic properties in comparison to the viral endonuclease PA. Nucleic Acids Research. 43(19):9405–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oishi K, Yamayoshi S, Kawaoka Y. 2015. Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity. J. Virol 89(16):8661–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oishi K, Yamayoshi S, Kawaoka Y. 2018. Identification of novel amino acid residues of influenza virus PA-X that are important for PA-X shutoff activity by using yeast. Virology. 516:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oishi K, Yamayoshi S, Kawaoka Y. 2019. Identification of Amino Acid Residues in Influenza A Virus PA-X That Contribute to Enhanced Shutoff Activity. Front. Microbiol 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X-H, Gong X-Q, Wen F, Ruan B-Y, Yu L-X, et al. 2020. The role of PA-X C-terminal 20 residues of classical swine influenza virus in its replication and pathogenicity. Veterinary Microbiology. 251:108916. [DOI] [PubMed] [Google Scholar]
- 57.Gao H, Sun H, Hu J, Qi L, Wang J, et al. 2015. Twenty amino acids at the C-terminus of PA-X are associated with increased influenza A virus replication and pathogenicity. Journal of General Virology. 96(8):2036–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oishi K, Yamayoshi S, Kozuka-Hata H, Oyama M, Kawaoka Y. 2018. N-Terminal Acetylation by NatB Is Required for the Shutoff Activity of Influenza A Virus PA-X. Cell Reports. 24(4):851–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levene RE, Shrestha SD, Gaglia MM. 2021. The influenza A virus host shutoff factor PA-X is rapidly turned over in a strain-specific manner. J Virol. 95(8):e02312–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen KT, Mun S-H, Lee C-S, Hwang C-S. 2018. Control of protein degradation by N-terminal acetylation and the N-end rule pathway. Exp Mol Med. 50(7):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W, Smeekens JM, Wu R. 2016. Systematic study of the dynamics and half-lives of newly synthesized proteins in human cells. Chem. Sci 7(2):1393–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nogales A, Rodriguez L, DeDiego ML, Topham DJ, Martínez-Sobrido L. 2017. Interplay of PA-X and NS1 Proteins in Replication and Pathogenesis of a Temperature-Sensitive 2009 Pandemic H1N1 Influenza A Virus. J. Virol 91(17):e00720–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nogales A, Martinez-Sobrido L, Chiem K, Topham DJ, DeDiego ML. 2018. Functional Evolution of the 2009 Pandemic H1N1 Influenza Virus NS1 and PA in Humans. Journal of Virology. 92(19): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaimayo C, Dunagan M, Hayashi T, Santoso N, Takimoto T. 2018. Specificity and functional interplay between influenza virus PA-X and NS1 shutoff activity. PLOS Pathogens. 14(11):e1007465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nogales A, Villamayor L, Utrilla-Trigo S, Ortego J, Martinez-Sobrido L, DeDiego ML. 2021. Natural Selection of H5N1 Avian Influenza A Viruses with Increased PA-X and NS1 Shutoff Activity. Viruses. 13(9):1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levene RE, Gaglia MM. 2018. Host Shutoff in Influenza A Virus: Many Means to an End. Viruses. 10(9):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Dong L, Zhao C, Zheng P, Zhang X, Xu J. 2021. Vaccinia virus-based vector against infectious diseases and tumors. Human Vaccines & Immunotherapeutics. 17(6):1578–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burgess HM, Mohr I. 2015. Cellular 5’-3’ mRNA exonuclease Xrn1 controls double-stranded RNA accumulation and anti-viral responses. Cell Host Microbe. 17(3):332–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai A, Cao S, Dhungel P, Luan Y, Liu Y, et al. 2017. Ribosome Profiling Reveals Translational Upregulation of Cellular Oxidative Phosphorylation mRNAs during Vaccinia Virus-Induced Host Shutoff. J Virol. 91(5):e01858–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee-Chen GJ, Niles EG. 1988. Transcription and translation mapping of the 13 genes in the vaccinia virus HindIII D fragment. Virology. 163(1):52–63 [DOI] [PubMed] [Google Scholar]
- 71.Parrish S, Moss B. 2006. Characterization of a Vaccinia Virus Mutant with a Deletion of the D10R Gene Encoding a Putative Negative Regulator of Gene Expression. J Virol. 80(2):553–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ly M, Burgess HM, Shah SB, Mohr I, Glaunsinger BA. 2022. Vaccinia virus D10 has broad decapping activity that is regulated by mRNA splicing. PLoS Pathog. 18(2):e1010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cartwright JL, Safrany ST, Dixon LK, Darzynkiewicz E, Stepinski J, et al. 2002. The g5R (D250) gene of African swine fever virus encodes a Nudix hydrolase that preferentially degrades diphosphoinositol polyphosphates. J Virol. 76(3):1415–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kago G, Parrish S. 2021. The Mimivirus L375 Nudix enzyme hydrolyzes the 5’ mRNA cap. PLOS ONE. 16(9):e0245820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bessman MJ, Frick DN, O’Handley SF. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem. 271(41):25059–62 [DOI] [PubMed] [Google Scholar]
- 76.Koonin EV. 1993. A highly conserved sequence motif defining the family of MutT-related proteins from eubacteria, eukaryotes and viruses. Nucleic Acids Res. 21(20):4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quintas A, Pérez-Núñez D, Sánchez EG, Nogal ML, Hentze MW, et al. 2017. Characterization of the African Swine Fever Virus Decapping Enzyme during Infection. J Virol. 91(24):e00990–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu S-W, Wyatt LS, Orandle MS, Minai M, Moss B. 2014. The D10 Decapping Enzyme of Vaccinia Virus Contributes to Decay of Cellular and Viral mRNAs and to Virulence in Mice. J Virol. 88(1):202–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Connor RF, Roper RL. 2007. Unique SARS-CoV protein nsp1: bioinformatics, biochemistry and potential effects on virulence. Trends in Microbiology. 15(2):51–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Narayanan K, Ramirez SI, Lokugamage KG, Makino S. 2015. Coronavirus nonstructural protein 1: Common and distinct functions in the regulation of host and viral gene expression. Virus Research. 202:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan S, Balaji S, Lomakin IB, Xiong Y. 2021. Coronavirus Nsp1: Immune Response Suppression and Protein Expression Inhibition. Frontiers in Microbiology. 12:2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terada Y, Kawachi K, Matsuura Y, Kamitani W. 2017. MERS coronavirus nsp1 participates in an efficient propagation through a specific interaction with viral RNA. Virology. 511:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamitani W, Narayanan K, Huang C, Lokugamage K, Ikegami T, et al. 2006. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. U.S.A 103(34):12885–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burke JM, St Clair LA, Perera R, Parker R. 2021. SARS-CoV-2 infection triggers widespread host mRNA decay leading to an mRNA export block. RNA. 27(11):1318–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Narayanan K, Huang C, Lokugamage K, Kamitani W, Ikegami T, et al. 2008. Severe Acute Respiratory Syndrome Coronavirus nsp1 Suppresses Host Gene Expression, Including That of Type I Interferon, in Infected Cells. Journal of Virology. 82(9):4471–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lokugamage KG, Narayanan K, Nakagawa K, Terasaki K, Ramirez SI, et al. 2015. Middle East Respiratory Syndrome Coronavirus nsp1 Inhibits Host Gene Expression by Selectively Targeting mRNAs Transcribed in the Nucleus while Sparing mRNAs of Cytoplasmic Origin. Journal of Virology. 89(21):10970–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Shi H, Rigolet P, Wu N, Zhu L, et al. 2010. Nsp1 proteins of group I and SARS coronaviruses share structural and functional similarities. Infection, Genetics and Evolution. 10(7):919–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang C, Lokugamage KG, Rozovics JM, Narayanan K, Semler BL, Makino S. 2011. SARS coronavirus nsp1 protein induces template-dependent endonucleolytic cleavage of mRNAs: viral mRNAs are resistant to nsp1-induced RNA cleavage. PLoS Pathog. 7(12):e1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, Lokugamage KG, Rozovics JM, Narayanan K, Semler BL, Makino S. 2011. Alphacoronavirus transmissible gastroenteritis virus nsp1 protein suppresses protein translation in mammalian cells and in cell-free HeLa cell extracts but not in rabbit reticulocyte lysate. J Virol. 85(1):638–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mendez AS, Ly M, González-Sánchez AM, Hartenian E, Ingolia NT, et al. 2021. The N-terminal domain of SARS-CoV-2 nsp1 plays key roles in suppression of cellular gene expression and preservation of viral gene expression. Cell Reports, p. 109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Almeida MS, Johnson MA, Herrmann T, Geralt M, Wüthrich K. 2007. Novel beta-barrel fold in the nuclear magnetic resonance structure of the replicase nonstructural protein 1 from the severe acute respiratory syndrome coronavirus. J Virol. 81(7):3151–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodriguez W, Macveigh-Fierro D, Miles J, Muller M. 2021. Fated for decay: RNA elements targeted by viral endonucleases. Seminars in Cell & Developmental Biology. 111:119–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Setaro AC, Gaglia MM. 2021. All hands on deck: SARS-CoV-2 proteins that block early anti-viral interferon responses. Current Research in Virological Science. 2:100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan S, Peng L, Park JJ, Hu Y, Devarkar SC, et al. 2020. Nonstructural Protein 1 of SARS-CoV-2 Is a Potent Pathogenicity Factor Redirecting Host Protein Synthesis Machinery toward Viral RNA. Molecular Cell. 80(6):1055–1066.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schubert K, Karousis ED, Jomaa A, Scaiola A, Echeverria B, et al. 2020. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nature Structural & Molecular Biology. 27(10):959–66 [DOI] [PubMed] [Google Scholar]
- 96.Lokugamage KG, Narayanan K, Huang C, Makino S. 2012. Severe Acute Respiratory Syndrome Coronavirus Protein nsp1 Is a Novel Eukaryotic Translation Inhibitor That Represses Multiple Steps of Translation Initiation. Journal of Virology. 86(24):13598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H. 2020. The Architecture of SARS-CoV-2 Transcriptome. Cell. 181(4):914–921.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sola I, Almazán F, Zúñiga S, Enjuanes L. 2015. Continuous and Discontinuous RNA Synthesis in Coronaviruses. Annu Rev Virol. 2(1):265–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banerjee AK, Blanco MR, Bruce EA, Honson DD, Chen LM, et al. 2020. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell. 183(5):1325–1339.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rao S, Hoskins I, Tonn T, Garcia PD, Ozadam H, et al. 2021. Genes with 5′ terminal oligopyrimidine tracts preferentially escape global suppression of translation by the SARS-CoV-2 Nsp1 protein. RNA. 27(9):1025–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tidu A, Janvier A, Schaeffer L, Sosnowski P, Kuhn L, et al. 2021. The viral protein NSP1 acts as a ribosome gatekeeper for shutting down host translation and fostering SARS-CoV-2 translation. RNA. 27(3):253–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanaka T, Kamitani W, DeDiego ML, Enjuanes L, Matsuura Y. 2012. Severe Acute Respiratory Syndrome Coronavirus nsp1 Facilitates Efficient Propagation in Cells through a Specific Translational Shutoff of Host mRNA. Journal of Virology. 86(20):11128–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vankadari N, Jeyasankar NN, Lopes WJ. 2020. Structure of the SARS-CoV-2 Nsp1/5′-Untranslated Region Complex and Implications for Potential Therapeutic Targets, a Vaccine, and Virulence. J. Phys. Chem. Lett 11(22):9659–68 [DOI] [PubMed] [Google Scholar]
- 104.Abernathy E, Gilbertson S, Alla R, Glaunsinger B. 2015. Viral Nucleases Induce an mRNA Degradation-Transcription Feedback Loop in Mammalian Cells. Cell Host & Microbe. 18(2):243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar GR, Shum L, Glaunsinger BA. 2011. Importin α-Mediated Nuclear Import of Cytoplasmic Poly(A) Binding Protein Occurs as a Direct Consequence of Cytoplasmic mRNA Depletion. Mol. Cell. Biol 31(15):3113–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hartenian E, Gilbertson S, Federspiel JD, Cristea IM, Glaunsinger BA. 2020. RNA decay during gammaherpesvirus infection reduces RNA polymerase II occupancy of host promoters but spares viral promoters. PLoS Pathog. 16(2):e1008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedel CC, Whisnant AW, Djakovic L, Rutkowski AJ, Friedl M-S, et al. 2021. Dissecting Herpes Simplex Virus 1-Induced Host Shutoff at the RNA Level. J Virol. 95(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duncan-Lewis C, Hartenian E, King V, Glaunsinger BA. 2021. Cytoplasmic mRNA decay represses RNA polymerase II transcription during early apoptosis. eLife. 10:e58342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gilbertson S, Federspiel JD, Hartenian E, Cristea IM, Glaunsinger B. 2018. Changes in mRNA abundance drive shuttling of RNA binding proteins, linking cytoplasmic RNA degradation to transcription. eLife. 7:e37663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hartenian E, Glaunsinger BA. 2019. Feedback to the central dogma: cytoplasmic mRNA decay and transcription are interdependent processes. Critical Reviews in Biochemistry and Molecular Biology. 54(4):385–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horst D, Burmeister WP, Boer IGJ, van Leeuwen D, Buisson M, et al. 2012. The “Bridge” in the Epstein-Barr Virus Alkaline Exonuclease Protein BGLF5 Contributes to Shutoff Activity during Productive Infection. J. Virol 86(17):9175–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee YJ, Glaunsinger BA. 2009. Aberrant herpesvirus-induced polyadenylation correlates with cellular messenger RNA destruction. PLoS Biol. 7(5):e1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar GR, Glaunsinger BA. 2010. Nuclear Import of Cytoplasmic Poly(A) Binding Protein Restricts Gene Expression via Hyperadenylation and Nuclear Retention of mRNA. Mol. Cell. Biol 30(21):4996–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khaperskyy DA, Emara MM, Johnston BP, Anderson P, Hatchette TF, McCormick C. 2014. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS Pathog. 10(7):e1004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pheasant K, Möller-Levet CS, Jones J, Depledge D, Breuer J, Elliott G. 2018. Nuclear-cytoplasmic compartmentalization of the herpes simplex virus 1 infected cell transcriptome is co-ordinated by the viral endoribonuclease vhs and cofactors to facilitate the translation of late proteins. PLoS Pathog. 14(11):e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mangus DA, Evans MC, Jacobson A. 2003. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biology. 4(7):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma S, Bhattacharjee RB, Bag J. 2009. Expression of poly(A)-binding protein is upregulated during recovery from heat shock in HeLa cells. The FEBS Journal. 276(2):552–70 [DOI] [PubMed] [Google Scholar]
- 118.Zhang K, Miorin L, Makio T, Dehghan I, Gao S, et al. 2021. Nsp1 protein of SARS-CoV-2 disrupts the mRNA export machinery to inhibit host gene expression. Science Advances. 7(6):eabe7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gomez GN, Abrar F, Dodhia MP, Gonzalez FG, Nag A. 2019. SARS coronavirus protein nsp1 disrupts localization of Nup93 from the nuclear pore complex. Biochem. Cell Biol 97(6):758–66 [DOI] [PubMed] [Google Scholar]
- 120.Anderson P, Kedersha N. 2008. Stress granules: the Tao of RNA triage. Trends in Biochemical Sciences. 33(3):141–50 [DOI] [PubMed] [Google Scholar]
- 121.McCormick C, Khaperskyy DA. 2017. Translation inhibition and stress granules in the antiviral immune response. Nat Rev Immunol. 17(10):647–60 [DOI] [PubMed] [Google Scholar]
- 122.Burgess HM, Mohr I. 2018. Defining the Role of Stress Granules in Innate Immune Suppression by the Herpes Simplex Virus 1 Endoribonuclease VHS. J Virol. 92(15):e00829–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Finnen RL, Zhu M, Li J, Romo D, Banfield BW. 2016. Herpes Simplex Virus 2 Virion Host Shutoff Endoribonuclease Activity Is Required To Disrupt Stress Granule Formation. J Virol. 90(17):7943–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dauber B, Poon D, Dos Santos T, Duguay BA, Mehta N, et al. 2016. The Herpes Simplex Virus Virion Host Shutoff Protein Enhances Translation of Viral True Late mRNAs Independently of Suppressing Protein Kinase R and Stress Granule Formation. J Virol. 90(13):6049–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rutkowski AJ, Erhard F, L’Hernault A, Bonfert T, Schilhabel M, et al. 2015. Widespread disruption of host transcription termination in HSV-1 infection. Nat Commun. 6:7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Finkel Y, Gluck A, Nachshon A, Winkler R, Fisher T, et al. 2021. SARS-CoV-2 uses a multipronged strategy to impede host protein synthesis. Nature. 594(7862):240–45 [DOI] [PubMed] [Google Scholar]
- 127.Bercovich-Kinori A, Tai J, Gelbart IA, Shitrit A, Ben-Moshe S, et al. 2016. A systematic view on influenza induced host shutoff. eLife. 5:e18311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cantu F, Cao S, Hernandez C, Dhungel P, Spradlin J, Yang Z. 2020. Poxvirus-encoded decapping enzymes promote selective translation of viral mRNAs. PLoS Pathog. 16(10):e1008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clyde K, Glaunsinger BA. 2011. Deep Sequencing Reveals Direct Targets of Gammaherpesvirus-Induced mRNA Decay and Suggests That Multiple Mechanisms Govern Cellular Transcript Escape. PLoS One. 6(5):e19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguez W, Srivastav K, Muller M. 2019. C19ORF66 Broadly Escapes Virus-Induced Endonuclease Cleavage and Restricts Kaposi’s Sarcoma-Associated Herpesvirus. J Virol. 93(12):e00373–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Glaunsinger B, Ganem D. 2004. Highly Selective Escape from KSHV-mediated Host mRNA Shutoff and Its Implications for Viral Pathogenesis. J Exp Med. 200(3):391–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muller M, Glaunsinger BA. 2017. Nuclease escape elements protect messenger RNA against cleavage by multiple viral endonucleases. PLoS Pathog. 13(8):e1006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muller M, Hutin S, Marigold O, Li KH, Burlingame A, Glaunsinger BA. 2015. A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases. PLoS Pathog. 11(5):e1004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hutin S, Lee Y, Glaunsinger BA. 2013. An RNA Element in Human Interleukin 6 Confers Escape from Degradation by the Gammaherpesvirus SOX Protein. J Virol. 87(8):4672–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Macveigh-Fierro D, Cicerchia A, Cadorette A, Sharma V, Muller M. 2022. The m6A reader YTHDC2 is essential for escape from KSHV SOX-induced RNA decay. Proc Natl Acad Sci U S A. 119(8):e2116662119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu J, Chen ZJ. 2014. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol 32:461–88 [DOI] [PubMed] [Google Scholar]
- 137.Lazear HM, Schoggins JW, Diamond MS. 2019. Shared and Distinct Functions of Type I and Type III Interferons. Immunity. 50(4):907–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santoro MG, Rossi A, Amici C. 2003. NF-kappaB and virus infection: who controls whom. EMBO J. 22(11):2552–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Orzalli MH, Broekema NM, Knipe DM. 2016. Relative Contributions of Herpes Simplex Virus 1 ICP0 and vhs to Loss of Cellular IFI16 Vary in Different Human Cell Types. J Virol. 90(18):8351–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su C, Zheng C. 2017. Herpes Simplex Virus 1 Abrogates the cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway via Its Virion Host Shutoff Protein, UL41. J Virol. 91(6):e02414–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yao X-D, Rosenthal KL. 2011. Herpes simplex virus type 2 virion host shutoff protein suppresses innate dsRNA antiviral pathways in human vaginal epithelial cells. J Gen Virol. 92(Pt 9):1981–93 [DOI] [PubMed] [Google Scholar]
- 142.Duerst RJ, Morrison LA. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology. 322(1):158–67 [DOI] [PubMed] [Google Scholar]
- 143.Pasieka TJ, Lu B, Crosby SD, Wylie KM, Morrison LA, et al. 2008. Herpes Simplex Virus Virion Host Shutoff Attenuates Establishment of the Antiviral State. J. Virol 82(11):5527–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang Z, Su C, Zheng C. 2016. Herpes Simplex Virus 1 Tegument Protein UL41 Counteracts IFIT3 Antiviral Innate Immunity. J Virol. 90(24):11056–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shen G, Wang K, Wang S, Cai M, Li M, Zheng C. 2014. Herpes simplex virus 1 counteracts viperin via its virion host shutoff protein UL41. J Virol. 88(20):12163–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.You H, Yuan H, Fu W, Su C, Wang W, et al. 2017. Herpes simplex virus type 1 abrogates the antiviral activity of Ch25h via its virion host shutoff protein. Antiviral Research. 143:69–73 [DOI] [PubMed] [Google Scholar]
- 147.Zenner HL, Mauricio R, Banting G, Crump CM. 2013. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J Virol. 87(24):13115–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Su C, Zhang J, Zheng C. 2015. Herpes simplex virus 1 UL41 protein abrogates the antiviral activity of hZAP by degrading its mRNA. Virol J. 12(1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jurak I, Silverstein LB, Sharma M, Coen DM. 2012. Herpes simplex virus is equipped with RNA- and protein-based mechanisms to repress expression of ATRX, an effector of intrinsic immunity. J Virol. 86(18):10093–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Gent M, Griffin BD, Berkhoff EG, van Leeuwen D, Boer IGJ, et al. 2011. EBV Lytic-Phase Protein BGLF5 Contributes to TLR9 Downregulation during Productive Infection. J Immunol. 186(3):1694–1702 [DOI] [PubMed] [Google Scholar]
- 151.van Gent M, Gram AM, Boer IGJ, Geerdink RJ, Lindenbergh MFS, et al. 2015. Silencing the shutoff protein of Epstein–Barr virus in productively infected B cells points to (innate) targets for immune evasion. Journal of General Virology. 96(4):858–65 [DOI] [PubMed] [Google Scholar]
- 152.Wang Y, Tong X, Li G, Li J, Deng M, Ye X. 2012. Ankrd17 positively regulates RIG-I-like receptor (RLR)-mediated immune signaling. European Journal of Immunology. 42(5):1304–15 [DOI] [PubMed] [Google Scholar]
- 153.Galvin HD, Husain M. 2019. Influenza A virus-induced host caspase and viral PA-X antagonize the antiviral host factor, histone deacetylase 4. J. Biol. Chem 294(52):20207–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hayashi T, MacDonald LA, Takimoto T. 2015. Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses. J. Virol 89(12):6442–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hu J, Mo Y, Wang X, Gu M, Hu Z, et al. 2015. PA-X decreases the pathogenicity of highly pathogenic H5N1 influenza A virus in avian species by inhibiting virus replication and host response. J. Virol 89(8):4126–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Narkpuk J, Jongkaewwattana A, Teeravechyan S. 2018. The avian influenza virus PA segment mediates strain-specific antagonism of BST-2/tetherin. Virology. 525:161–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hu J, Kong M, Cui Z, Gao Z, Ma C, et al. 2020. PA-X protein of H5N1 avian influenza virus inhibits NF-kappaB activity, a potential mechanism for PA-X counteracting the host innate immune responses. Veterinary Microbiology. 250:108838. [DOI] [PubMed] [Google Scholar]
- 158.Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, et al. 2020. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 369(6508):1249–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wathelet MG, Orr M, Frieman MB, Baric RS. 2007. Severe Acute Respiratory Syndrome Coronavirus Evades Antiviral Signaling: Role of nsp1 and Rational Design of an Attenuated Strain. Journal of Virology. 81(21):11620–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kumar A, Ishida R, Strilets T, Cole J, Lopez-Orozco J, et al. 2021. SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J Virol. 95(13):e0026621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xia H, Cao Z, Xie X, Zhang X, Chen JY-C, et al. 2020. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 33(1):108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shen Z, Yang Y, Yang S, Zhang G, Xiao S, et al. 2020. Structural and Biological Basis of Alphacoronavirus nsp1 Associated with Host Proliferation and Immune Evasion. Viruses. 12(8):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S-W, Katsafanas GC, Liu R, Wyatt LS, Moss B. 2015. Poxvirus decapping enzymes enhance virulence by preventing the accumulation of dsRNA and the induction of innate antiviral responses. Cell Host Microbe. 17(3):320–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dauber B, Saffran HA, Smiley JR. 2019. The herpes simplex virus host shutoff (vhs) RNase limits accumulation of double stranded RNA in infected cells: Evidence for accelerated decay of duplex RNA. PLoS Pathog. 15(10):e1008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tigges MA, Leng S, Johnson DC, Burke RL. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J Immunol. 156(10):3901–10 [PubMed] [Google Scholar]
- 166.Trgovcich J, Johnson D, Roizman B. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the gamma(1)34.5 and U(L)41 genes of herpes simplex virus 1. J Virol. 76(14):6974–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zuo J, Thomas W, van Leeuwen D, Middeldorp JM, Wiertz EJHJ, et al. 2008. The DNase of Gammaherpesviruses Impairs Recognition by Virus-Specific CD8+ T Cells through an Additional Host Shutoff Function. J. Virol 82(5):2385–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Züst R, Cervantes-Barragán L, Kuri T, Blakqori G, Weber F, et al. 2007. Coronavirus Non-Structural Protein 1 Is a Major Pathogenicity Factor: Implications for the Rational Design of Coronavirus Vaccines. PLOS Pathogens. 3(8):e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith TJ, Morrison LA, Leib DA. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol 76(5):2054–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strelow L, Smith T, Leib D. 1997. The Virion Host Shutoff Function of Herpes Simplex Virus Type 1 Plays a Role in Corneal Invasion and Functions Independently of the Cell Cycle. Virology. 231(1):28–34 [DOI] [PubMed] [Google Scholar]
- 171.Murphy JA, Duerst RJ, Smith TJ, Morrison LA. 2003. Herpes Simplex Virus Type 2 Virion Host Shutoff Protein Regulates Alpha/Beta Interferon but Not Adaptive Immune Responses during Primary Infection In Vivo. J. Virol 77(17):9337–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gao H, Sun Y, Hu J, Qi L, Wang J, et al. 2015. The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses. Sci Rep. 5:8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gong X-Q, Sun Y-F, Ruan B-Y, Liu X-M, Wang Q, et al. 2017. PA-X protein decreases replication and pathogenicity of swine influenza virus in cultured cells and mouse models. Veterinary Microbiology. 205:66–70 [DOI] [PubMed] [Google Scholar]
- 174.Hu J, Mo Y, Gao Z, Wang X, Gu M, et al. 2016. PA-X-associated early alleviation of the acute lung injury contributes to the attenuation of a highly pathogenic H5N1 avian influenza virus in mice. Med Microbiol Immunol. 205(4):381–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dunagan MM, Hardy K, Takimoto T. 2021. Impact of Influenza A Virus Shutoff Proteins on Host Immune Responses. Vaccines. 9(6):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao H, Xu G, Sun Y, Qi L, Wang J, et al. 2015. PA-X is a virulence factor in avian H9N2 influenza virus. J. Gen. Virol 96(9):2587–94 [DOI] [PubMed] [Google Scholar]
- 177.Hussain S, Turnbull ML, Wise HM, Jagger BW, Beard PM, et al. 2019. Mutation of Influenza A Virus PA-X Decreases Pathogenicity in Chicken Embryos and Can Increase the Yield of Reassortant Candidate Vaccine Viruses. Journal of Virology. 93(2):e01551–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kong M, Chen K, Zeng Z, Wang X, Gu M, et al. 2021. The virulence modulator PA-X protein has minor effect on the pathogenicity of the highly pathogenic H7N9 avian influenza virus in mice. Veterinary Microbiology. 255:109019. [DOI] [PubMed] [Google Scholar]
- 179.Richner JM, Clyde K, Pezda AC, Cheng BYH, Wang T, et al. 2011. Global mRNA degradation during lytic gammaherpesvirus infection contributes to establishment of viral latency. PLoS Pathog. 7(7):e1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nakagawa K, Narayanan K, Wada M, Popov VL, Cajimat M, et al. 2018. The Endonucleolytic RNA Cleavage Function of nsp1 of Middle East Respiratory Syndrome Coronavirus Promotes the Production of Infectious Virus Particles in Specific Human Cell Lines. J Virol. 92(21):e01157–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abernathy E, Glaunsinger B. 2015. Emerging roles for RNA degradation in viral replication and antiviral defense. Virology. 479–480:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]