Abstract
目的
研究动物处于不同行为状态时,纹状体听觉神经元对声音信息的特征表达是否被调控。
方法
以SPF级C57BL/6J清醒小鼠纹状体的听觉神经元为研究对象,通过搭建同步的在体电生理和运动记录系统,采用玻璃微电极贴附式记录方法长时间记录纹状体听觉神经元对噪声的听觉响应,并通过分析小鼠跑动速度将小鼠的行为状态分为安静状态和运动状态,分析动物处于两种行为状态下纹状体听觉神经元的自发活动和诱发响应。
结果
相对于安静状态,当动物处于运动状态时,纹状体听觉神经元的自发活动增高(37.06±12.02 vs 18.51±10.91,P < 0.001),而诱发响应下降(噪声强度=60 dB,3.45±2.99 vs 3.04±2.76,P < 0.001)。
结论
运动状态对纹状体的听觉响应具有显著的抑制作用,这可能是导致运动状态时声音信息的识别能力下降的重要原因。
Keywords: 运动调控, 纹状体, 听觉神经元, 听觉响应
Abstract
Objective
To explore whether the characteristic responses to sound stimulations of the auditory neurons in the striatum is regulated in different behavioral states.
Methods
The auditory neurons in the striatum of awake C57BL/6J mice were selected for this study. We recorded the auditory response of the striatum to noises over a long period of time by building a synchronous in vivo electrophysiological and locomotion recording system and using glass microelectrode attachment recording. By analyzing the running speed of the mice, the behavioral states of the mice were divided into the quiet state and the active state, and the spontaneous activity and evoked responses of the auditory neurons in the striatum were analyzed in these two states.
Results
Compared with those recorded in the quiet state, the spontaneous activity of the auditory neurons in the striatum of the mice increased significantly (37.06±12.02 vs 18.51±10.91, P < 0.001) while the auditory response of the neurons decreased significantly (noise intensity=60 dB, 3.45±2.99 vs 3.04±2.76, P < 0.001) in the active state.
Conclusion
Locomotion has a significant inhibitory effect on the auditory response of the striatum, which may importantly contribute to the decline of sound information recognition ability in the active state.
Keywords: locomotion modulation, striatum, auditory neurons, auditory response
运动和静止是两种典型的相互对立的大脑状态,现有研究显示动物处于运动状态时对听觉信息和视觉信息的表达受到不同程度的调节,动物从静止状态过渡到奔跑状态的时候,视觉皮层(VC)的大多数神经元表现出两倍以上的视觉诱发发放率,然而自发活动并没改变[1, 2]。与VC相反的是,当动物从安静状态过渡到活跃状态时,听觉皮层(AC)第2/3层的兴奋性神经元对于听觉刺激的听觉响应虽然频率调谐特性维持不变,但反应强度显著下降[3-7]。这些现象暗示运动状态可能对感觉信息的识别和感觉具有调控作用。
虽然现有研究已显示运动抑制了AC对声音信息的表达,但运动进一步调节声音信息识别的神经机制目前还缺少相关研究。在听觉神经系统,我们前期的研究显示,虽然运动状态时AC第2/3层神经元被抑制,但第4层的神经元及其听觉信息输入脑区内侧膝状体腹侧核的听觉响应并没有改变[3]。这种运动状态对上行听觉神经通路的差异性调控机制暗示了运动状态可能进一步调控听觉输出神经环路来改变声音信息的识别。在下行听觉神经环路中,纹状体是AC的一个重要下行投射目标脑区[8-12],AC投射的轴突末端以一定的密度汇聚到单个纹状体神经元上,形成功能性谷氨酸能突触(即皮质纹状体突触)[13]。且现有研究显示,纹状体在听觉信息的识别中起到非常重要的作用[14-18],比如,动物经过训练后,可以识别不同的声音,这时如果用毒蝇蕈醇将纹状体抑制,动物对声音的识别会出现很大程度的偏差[18]。基于以上研究,我们猜想运动状态可能进一步通过调控AC下行目标脑区纹状体的听觉信息的表达来调控声音信息的识别,这对理解运动对于听觉环路的调控机制十分重要。为验证这一假设,本研究以小鼠为研究对象,通过比较其在不同状态的听觉响应来研究运动对纹状体声音信息表达的调节机制。
1. 材料和方法
1.1. 实验动物
实验所用动物为SPF级C57BJ/6J小鼠(南方医科大学实验动物中心),周龄6~8周,体质量18~20 g,雌雄不限。所有小鼠听力良好,检耳镜检查骨膜无异常,所有实验过程严格遵循南方医科大学实验动物保护和使用管理委员会制定的实验动物伦理准则。
1.2. 手术准备
在动物实验之前,通过腹腔注射戊巴比妥钠(60 mg/kg)的方式进行麻醉,等待动物完全进入麻醉状态后,将其置于小鼠适配器上,用手术剪刀剪开头皮暴露出颅骨,使用颅骨钻钻开颅骨,在前额骨下植入一根参比电极。随后使用牙科水泥将一个长约1.5 cm的刚性金属头钉粘合在颅骨前部正中位置,用于小鼠头部固定,之后依据小鼠脑图谱(第二版)定好纹状体脑区位置,在立体显微镜下用颅骨钻去除动物颅骨,暴露出记录区域。最后,将暴露在外边的脑组织均匀涂抹上凡士林。手术结束后小鼠被放回笼子恢复两天。正式记录前小鼠头部固定于刚性金属架,身体放置于可记录速度的转盘上进行训练,直至小鼠可以在转盘上自由跑动或静止,训练良好的小鼠将在屏蔽室实验台上进行电生理记录。
1.3. 系统搭建
为了同步记录神经元发放的电信号以及动物的运动状态,我们设计了一套基于Labview虚拟仪器控制的数据采集系统与给声系统,由Labview编辑的虚拟仪器来控制给声和记录系统,给声系统和记录系统由同一个触发信号触发以保证记录和给声同步进行。另外,系统可以在记录神经元发放状态的同时记录动物的运动状态,本系统使用速度编码器(US Digital)来计算转盘的转速,采用多通道记录系统(RHD2000,Intan Technologies),同时将电生理信号,声音的触发信号以及速度信号导入多通道记录系统中的3个通道,使得3种信号在同一时间轴上,这样便可以截取感兴趣时间段的数据进行分析处理,系统原理(图 1A)。
1.

运动对于纹状体神经元spontaneous的调制
Modulation of spontaneous firing of the neurons in the striatum by locomotion. A: Experimental setup (R, recording electrode; V, velocity. P, head-fixation post). B: Calculation model. C: Upper, spontaneous spikes recorded from a striatum cell (scale bar=0.1 s); Lower, concurrently recorded plate rotation speed (scale bar=10 cm/s). D: Summary of recordings from 21 striatum cells in quiet state (Q) vs active state (A). ***P < 0.001, paired t-test.
1.4. 声音刺激
本实验采用的刺激声音是通过自编Labview程序合成声音的波形,通过虚拟仪器控制,将合成的声音信号通过数据采集卡(NI, 6731)的信号输出口传入到TDT给声系统的喇叭驱动器(ED1),ED1再将信号输出给开放场的喇叭(ES1),随后ES1播放出刺激声音,所有的刺激声音均由精密的声音校正仪进行严格的校准。本实验涉及到的刺激声音为声长50 ms、不同强度(10~70 dB)的白噪声。
1.5. 电生理记录
电生理实验在听觉屏蔽室中进行。采用玻璃微电极贴附式记录纹状体神经元,在电生理记录过程中,用刚性金属固定杆将训练好的小鼠头部固定,在显微镜下去除记录脑区的凡士林薄膜,在拉制好的玻璃微电极内灌注含0.3%神经生物素的人工脑脊液(ACSF)并排出电极尖端气泡,将玻璃电极稳定安装在前置放大器探头上后,用推进器将玻璃微电极置于记录脑区正上方,通过压力表给予电极2个正压,滴加1滴ACSF在脑区表面,之后下降电极,使其与液体充分接触,直至有电生理信号出现,并测量电极电阻显示为6 MΩ左右,此时说明整个回路联通,可进行单细胞记录。之后将电极快速下降至记录脑区,将正压减半,用推进器缓慢移动电极寻找听觉神经元。与此同时,通过观察Labview软件中显示的电极电阻是否增大来判断电极尖端是否碰触到神经元,当观察到电极电阻忽然增大,且有诱发动作电位,此时撤掉电极正压,待电阻稳定不变后给予适当的负压吸引使神经元紧密贴附在电极上,最后给予小鼠noise声音刺激,判断是否为听觉神经元,若为听觉神经元,则长时间记录神经元发放的动作电位(spike)以及转盘的速度,同时每隔3000 ms给予不同强度的声音刺激,并使用高清摄像头进行录像。
1.6. 数据分析
数据分析通过自写的Matlab程序进行。首先读取多通道记录系统中的3个通道信息,在同一时间轴上获得声音信号、速度信号、电生理信号,最后通过分析转盘速度和全程录像将运动状态下神经元的发放状态和安静状态下神经元的发放状态分离开来。随后计算神经元不同行为状态下的spontaneous和诱发响应(evoked spikes)进行比较,具体计算方法为:记录到的动作电位利用Matlab程序设定阈值进行识别,首先计算神经元在noise刺激下动作电位在时间序列上的发放数,提取声音刺激开始前的一定时间窗口(T1)内的动作电位发放数(N1),神经元的spontaneous计算为N1 / T1,然后统计每次noise刺激后一段时间窗口(T2)内的放电总数(N2),T2长度的选择以声音刺激后有明显响应到响应恢复至声音刺激前的状态为准,evoked spikes计算为总动作电位数减去spontaneous动作电位数,即:N2-spontaneous×T2,该增量表示noise诱发的听觉响应,具体计算原理如图 1B。另外,本研究涉及的表现形式为散点图和刺激后时间直方图,本实验将多次noise刺激的响应数据在同一时间轴上按行排列起来,用一个点代表一个动作电位,组成散点图; 将记录的时间长度在时间轴上分为若干时间小段(bin),统计每个bin内的动作电位发放个数。
1.7. 统计学分析
采用SPSS 17.0进行统计分析,定量资料以均数±标准差表示,组间两两比较均采用配对t检验,P < 0.05为差异具有统计学意义。本研究一共记录到纹状体神经元21例,统计这些神经元在运动状态下和安静状态下的spontaneous和evoked spikes,并分析二者在动物处于不同行为状态(运动vs安静)时的差异性。
2. 结果
2.1. 纹状体听觉神经元不同行为状态下的spontaneous特性
本研究同时记录了小鼠在运动状态与安静状态下纹状体听觉神经元的响应(图 1A),分析神经元处于不同行为状态下的spontaneous与evoked spikes(图 1B)。实验首先研究了运动状态对于纹状体声响应神经元spontaneous的影响,结果显示,相比于安静状态,在小鼠处于运动状态时,听觉神经元的spontaneous剧烈上升,小鼠在安静状态下的spontaneous明显低于运动状态下的spontaneous(图 1C)。随后,对记录到的21例神经元的spontaneous情况进行统计,结果显示大部分神经元均表现出运动状态下spontaneous的增加,安静状态下的spontaneous为18.51 ± 10.91,运动状态下的spontaneous为37.06±12.02(P < 0.001,图 1D)。
2.2. 纹状体听觉神经元不同行为状态下的evoked spikes响应特性
当小鼠处于运动状态下,神经元的spontaneous明显高于小鼠安静时的spontaneous(图 2A、C)。当小鼠处于安静状态时,在给声区间内,神经元的evoked spikes十分清晰可见,且该神经元的听觉响应阈值为30 dB(图 2B),而小鼠处于运动状态时,神经元的evoked spikes明显下降,且在声音刺激为30 dB时已没有声音响应(图 2D)。
2.
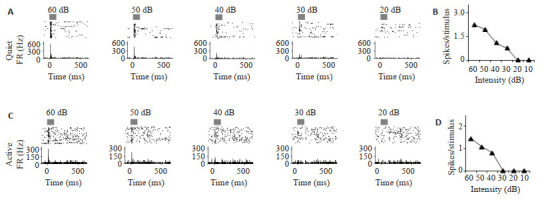
纹状体在运动状态下和安静状态下的声音响应对比
Acoustic responses in quiet state vs active state in the striatum. A: Responses of a neuron in the striatum to 60-20 dB noises in the quiet state. Gray Bar represents the duration (50 ms) of noise. B: Evoked-spikes vs intensity curve in the quiet state for the same neuron. C: Responses of the same neuron to 60-20 dB noises in the active state. D: Evoked-spikes vs intensity curve in the active state for the same neuron.
在记录到的21例神经元中,大部分的神经元在运动状态下表现出和图 2所示神经元一样的听觉响应减弱。但在给予高强度noise时运动对于声响应的调制效果并不像低强度时那么强,noise强度为60 dB时,evoked spikes的听觉响应在运动状态的时候实际上并没有被严重减弱(图 2),但是随着noise强度的降低,运动状态对于听觉响应的调制作用似乎会越来越强。为排除个体因素的影响,本实验统计了这21例神经元在运动和安静状态下对不同强度noise的evoked spikes(图 3),结果显示,不管是给予高强度的noise,还是低强度的noise,安静状态下和运动状态下的声音响应差异都有统计学意义(P<0.001),但调制的幅度会随着声音刺激强度的减弱增大,特别是声强为40 dB的时候,调制的幅度达到最大(图 3D)。
3.

运动对于纹状体神经元的声响应调制
Modulation of acoustic responses of the neurons in the striatum by locomotion. A-C: Evoked-spikes by 60, 50 and 40 dB noises recorded from 21 striatum cells in the quiet (Q) state vs active (A) state, respectively. ***P < 0.001 by paired t-test. Error bars represent SD in all panels. D: Comparison of evoked-spikes vs intensity curves in the quiet state (black triangle) and the active state (red triangle). Error bars represent SD.
3. 讨论
当实验小鼠处于不同状态时,其感觉神经系统对感觉信息的表达存在显著的不同,这种现象广泛存在于不同的感觉系统,特别是感觉皮层。现有研究表明,在VC,动物在处于运动状态时,视觉神经元的evoked spikes增高而spontaneous不变[1, 19, 20],而AC(第2/3层)神经元在动物运动时表现为evoked spikes和spontaneous均下降[3],这些研究说明运动状态对听觉信息和视觉信息的表达有着不同的调节作用。针对听觉系统而言,显然目前的研究大都停留在运动对于AC听觉响应的调制,即运动对于AC表达声音信息具有抑制作用,仍没有关于运动是否会进一步调节声音信息识别的研究,但大量的经验显示当我们处于运动状态时,比如跑步或打篮球时,机体对于外界声音的识别能力显著下降,且纹状体在声音信息的识别中起到重要作用[15, 18],因此运动极大可能调节了纹状体的听觉响应进而使声音信息识别能力减弱。本研究在纹状体的电生理实验结果证实这一猜测。本研究结果显示:相对于安静状态,动物处于运动状态时,纹状体听觉神经元的evoked spikes会下降,而spontaneous会增高。以往的研究表明,听觉系统中的每个核团都有大量的神经元对声音信息的不同特征进行编码[21, 22],且听觉核团对于声音特征的编码主要表现为听觉神经元动作电位的发放形式[23]。另有研究表明,听觉神经元spontaneous与evoked spikes的大小可表征神经元对于声音信息的敏感特性[24],这表明神经元的spontaneous与evoked spikes改变可能意味着神经元对于声音信息处理的敏感性改变,因此从本研究的结果来看,运动对纹状体神经元的spontaneous与evoked spikes有调控作用,那么运动可能会改变纹状体神经元对于声音信息处理的敏感性,也即运动对纹状体的听觉响应具有显著的调控作用。此外,AC第2/3层的神经元在运动状态下听觉响应下降的直接原因是第1层的中间神经元起到了调节作用[3],而纹状体内的神经元绝大多数为中型多棘神经元,剩余的一小部分为中间神经元,且纹状体内中间神经元的主要投射目标是中型多棘神经元[25],基于此,我们猜测这样的实验现象很可能和纹状体内部中间神经元的调节作用有关。
另一方面,早有研究表明,听觉系统对于高声强声音刺激的听觉响应要显著高于其对低声强声音刺激的听觉响应,例如,当声音刺激的强度逐渐增加时,人类大脑中听觉神经束的神经元的平均放电率也逐渐增加[26],在声音频率保持不变的情况下,小鼠大脑中听觉神经元的听觉响应随着声音强度的增加而增加[22, 27, 28]。这些研究都暗示了听觉系统对于高声强声音刺激的敏感性是要高于其对低声强声音刺激的敏感性的,而且我们的结果进一步表明,即使运动对于高声强声音刺激的听觉响应有所调控,但是相较于对低声强声音刺激听觉响应的调控幅度而言,其调控作用并不十分明显,这说明动物即使在运动状态下,仍然在一定程度上保持了对于高声强声音的敏感性,但背后的神经机制究竟如何,还需进一步研究。
现有研究表明,VC和AC均会受到运动状态的调制,在VC,动物处于运动状态时,椎体神经元的发放率增加,而在AC,听觉响应在动物运动时减弱[1, 3]。在非感觉系统,例如像海马这样的深部脑区,动物处于运动状态时,神经元也显示出了明显的spontaneous增高[29, 30]。其它的一些将感觉信息处理与运动行为联系起来的研究表明,在运动的过程中一些特定核团表现出了对感觉刺激响应的减弱,如在运动期间,大鼠对于胡须刺激的响应也会相应下降[31]。这些研究可以得出结论,运动对大脑的调节广泛存在于各个脑区,而根据特定行为的需要去选择性获得不同感觉信息时候,运动可能对不同的大脑区域产生不同的影响。本研究在纹状体的结果进一步表明,运动状态时纹状体听觉响应受到的抑制可能是导致声音信息识别能力显著下降的重要原因。
综上所述,本研究通过在体电生理记录的方法发现运动对于纹状体听觉响应具有调控作用,为理解不同大脑状态下纹状体对听觉信息分辨力调控的神经机制提供了重要的理论支持。
Biography
黄威龙,在读硕士研究生,E-mail: 18530698105@163.com
Funding Statement
广东省杰出青年基金(2019B151502033)
Contributor Information
黄 威龙 (Weilong HUANG), Email: 18530698105@163.com.
梁 妃学 (Feixue LIANG), Email: lfx_2@hotmail.com.
References
- 1.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65(4):472–9. doi: 10.1016/j.neuron.2010.01.033. [Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex[J]. Neuron, 2010, 65(4): 472-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wongmassang W, Hasegawa T, Chiken S, et al. Weakly correlated activity of pallidal neurons in behaving monkeys. Eur J Neurosci. 2021;53(7):2178–91. doi: 10.1111/ejn.14903. [Wongmassang W, Hasegawa T, Chiken S, et al. Weakly correlated activity of pallidal neurons in behaving monkeys[J]. Eur J Neurosci, 2021, 53(7): 2178-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou M, Liang FX, Xiong XR, et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci. 2014;17(6):841–50. doi: 10.1038/nn.3701. [Zhou M, Liang FX, Xiong XR, et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex[J]. Nat Neurosci, 2014, 17(6): 841-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513(7517):189–94. doi: 10.1038/nature13724. [Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex[J]. Nature, 2014, 513 (7517): 189-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuchibhotla KV, Gill JV, Lindsay GW, et al. Parallel processing by cortical inhibition enables context-dependent behavior. Nat Neurosci. 2017;20(1):62–71. doi: 10.1038/nn.4436. [Kuchibhotla KV, Gill JV, Lindsay GW, et al. Parallel processing by cortical inhibition enables context-dependent behavior[J]. Nat Neurosci, 2017, 20(1): 62-71.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliades SJ, Wang XQ. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 2003;89(4):2194–207. doi: 10.1152/jn.00627.2002. [Eliades SJ, Wang XQ. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations[J]. J Neurophysiol, 2003, 89(4): 2194-207.] [DOI] [PubMed] [Google Scholar]
- 7.Singla S, Dempsey C, Warren R, et al. A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat Neurosci. 2017;20(7):943–50. doi: 10.1038/nn.4567. [Singla S, Dempsey C, Warren R, et al. A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds[J]. Nat Neurosci, 2017, 20(7): 943-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hintiryan H, Foster NN, Bowman I, et al. The mouse cortico-striatal projectome. Nat Neurosci. 2016;19(8):1100–14. doi: 10.1038/nn.4332. [Hintiryan H, Foster NN, Bowman I, et al. The mouse cortico-striatal projectome[J]. Nat Neurosci, 2016, 19(8): 1100-14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakata S, Takemoto M, Song WJ. Differential cortical and subcortical projection targets of subfields in the core region of mouse auditory cortex. Hear Res. 2020;386:107876–85. doi: 10.1016/j.heares.2019.107876. [Nakata S, Takemoto M, Song WJ. Differential cortical and subcortical projection targets of subfields in the core region of mouse auditory cortex[J]. Hear Res, 2020, 386: 107876-85.] [DOI] [PubMed] [Google Scholar]
- 10.LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett. 1991;134(1):139–44. doi: 10.1016/0304-3940(91)90526-Y. [LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex[J]. Neurosci Lett, 1991, 134(1): 139-44.] [DOI] [PubMed] [Google Scholar]
- 11.Hunnicutt BJ, Jongbloets BC, Birdsong WT, et al. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife. 2016;5:e19103–9. doi: 10.7554/eLife.19103. [Hunnicutt BJ, Jongbloets BC, Birdsong WT, et al. A comprehensive excitatory input map of the striatum reveals novel functional organization[J]. eLife, 2016, 5: e19103-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo L, Weems JT, Walker WI, et al. Choice-selective neurons in the auditory cortex and in its striatal target encode reward expectation. J Neurosci. 2019;39(19):3687–97. doi: 10.1523/JNEUROSCI.2585-18.2019. [Guo L, Weems JT, Walker WI, et al. Choice-selective neurons in the auditory cortex and in its striatal target encode reward expectation[J]. J Neurosci, 2019, 39(19): 3687-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huerta-Ocampo I, Mena-Segovia J, Bolam JP. Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum. Brain Struct Funct. 2014;219(5):1787–800. doi: 10.1007/s00429-013-0601-z. [Huerta-Ocampo I, Mena-Segovia J, Bolam JP. Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum[J]. Brain Struct Funct, 2014, 219(5): 1787-800.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Wang XX, Ge SY, et al. Medial geniculate body and primary auditory cortex differentially contribute to striatal sound representations. Nat Commun. 2019;10(1):418–25. doi: 10.1038/s41467-019-08350-7. [Chen L, Wang XX, Ge SY, et al. Medial geniculate body and primary auditory cortex differentially contribute to striatal sound representations[J]. Nat Commun, 2019, 10(1): 418-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponvert ND, Jaramillo S. Auditory thalamostriatal and corticostriatal pathways convey complementary information about sound features. J Neurosci. 2019;39(2):271–80. doi: 10.1523/JNEUROSCI.1188-18.2018. [Ponvert ND, Jaramillo S. Auditory thalamostriatal and corticostriatal pathways convey complementary information about sound features[J]. J Neurosci, 2019, 39(2): 271-80.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong QJ, Znamenskiy P, Zador AM. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature. 2015;521(7552):348–51. doi: 10.1038/nature14225. [Xiong QJ, Znamenskiy P, Zador AM. Selective corticostriatal plasticity during acquisition of an auditory discrimination task[J]. Nature, 2015, 521(7552): 348-51.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature. 2013;497(7450):482–5. doi: 10.1038/nature12077. [Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination[J]. Nature, 2013, 497 (7450): 482-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Walker WI, Ponvert ND, et al. Stable representation of sounds in the posterior striatum during flexible auditory decisions. Nat Commun. 2018;9(1):1534–42. doi: 10.1038/s41467-018-03994-3. [Guo L, Walker WI, Ponvert ND, et al. Stable representation of sounds in the posterior striatum during flexible auditory decisions[J]. Nat Commun, 2018, 9(1): 1534-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pakan JM, Francioni V, Rochefort NL. Action and learning shape the activity of neuronal circuits in the visual cortex. Curr Opin Neurobiol. 2018;52:88–97. doi: 10.1016/j.conb.2018.04.020. [Pakan JM, Francioni V, Rochefort NL. Action and learning shape the activity of neuronal circuits in the visual cortex[J]. Curr Opin Neurobiol, 2018, 52: 88-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadarlat MC, Stryker MP. Locomotion enhances neural encoding of visual stimuli in mouse V1. J Neurosci. 2017;37(14):3764–75. doi: 10.1523/JNEUROSCI.2728-16.2017. [Dadarlat MC, Stryker MP. Locomotion enhances neural encoding of visual stimuli in mouse V1[J]. J Neurosci, 2017, 37(14): 3764-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yildiz IB, Mesgarani N, Deneve S. Predictive ensemble decoding of acoustical features explains context-dependent receptive fields. J Neurosci. 2016;36(49):12338–50. doi: 10.1523/JNEUROSCI.4648-15.2016. [Yildiz IB, Mesgarani N, Deneve S. Predictive ensemble decoding of acoustical features explains context-dependent receptive fields[J]. J Neurosci, 2016, 36(49): 12338-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang FX, Li HF, Chou XL, et al. Sparse representation in awake auditory cortex: cell-type dependence, synaptic mechanisms, developmental emergence, and modulation. Cereb Cortex. 2018;29(9):3796–812. doi: 10.1093/cercor/bhy260. [Liang FX, Li HF, Chou XL, et al. Sparse representation in awake auditory cortex: cell-type dependence, synaptic mechanisms, developmental emergence, and modulation[J]. Cereb Cortex, 2018, 29(9): 3796-812.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino O. Enhanced sound perception by widespread- onset neuronal responses in auditory cortex. Neural Comput. 2007;19(12):3310–34. doi: 10.1162/neco.2007.19.12.3310. [Hoshino O. Enhanced sound perception by widespread- onset neuronal responses in auditory cortex[J]. Neural Comput, 2007, 19 (12): 3310-34.] [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Song C, Liang F. Evolution of auditory response signal- to- noise ratio in ascending auditory pathways. Nan Fang Yi Ke Da Xue Xue Bao. 2021;41(11):1712–8. doi: 10.12122/j.issn.1673-4254.2021.11.17. [Wang J, Song C, Liang F. Evolution of auditory response signal- to- noise ratio in ascending auditory pathways[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2021, 41(11): 1712-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–47. doi: 10.1146/annurev.neuro.051508.135422. [Kreitzer AC. Physiology and pharmacology of striatal neurons[J]. Annu Rev Neurosci, 2009, 32: 127-47.] [DOI] [PubMed] [Google Scholar]
- 26.王 聪, 张 巧丽, 赵 地, et al. 大脑听觉系统建模研究进展. https://www.cnki.com.cn/Article/CJFDTOTAL-JSJA2016S2001.htm. 计算机科学. 2016;43(S2):1-5, 15. [王聪, 张巧丽, 赵地, 等. 大脑听觉系统建模研究进展[J]. 计算机科学, 2016, 43(S2): 1-5, 15.] [Google Scholar]
- 27.Brecht EJ, Barsz K, Gross B, et al. Increasing GABA reverses age-related alterations in excitatory receptive fields and intensity coding of auditory midbrain neurons in aged mice. Neurobiol Aging. 2017;56:87–99. doi: 10.1016/j.neurobiolaging.2017.04.003. [Brecht EJ, Barsz K, Gross B, et al. Increasing GABA reverses age-related alterations in excitatory receptive fields and intensity coding of auditory midbrain neurons in aged mice[J]. Neurobiol Aging, 2017, 56: 87-99.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HF, Liang FX, Zhong W, et al. Synaptic mechanisms for ban-dwidth tuning in awake mouse primary auditory cortex. Cereb Cortex. 2019;29(7):2998–3009. doi: 10.1093/cercor/bhy165. [Li HF, Liang FX, Zhong W, et al. Synaptic mechanisms for ban-dwidth tuning in awake mouse primary auditory cortex[J]. Cereb Cortex, 2019, 29(7): 2998-3009.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villette V, Chavarha M, Dimov IK, et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell. 2019;179(7):1590–608. e23. doi: 10.1016/j.cell.2019.11.004. [Villette V, Chavarha M, Dimov IK, et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice[J]. Cell, 2019, 179(7): 1590-608. e23.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc Natl Acad Sci USA. 2012;109(40):E2726–34. doi: 10.1073/pnas.1210929109. [Varga C, Golshani P, Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice[J]. Proc Natl Acad Sci USA, 2012, 109 (40): E2726-34.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferezou I, Haiss F, Gentet LJ, et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56(5):907–23. doi: 10.1016/j.neuron.2007.10.007. [Ferezou I, Haiss F, Gentet LJ, et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice[J]. Neuron, 2007, 56(5): 907-23.] [DOI] [PubMed] [Google Scholar]


