Abstract
The energy cost of adaptive immune activation in endotherms is typically quantified from changes in resting metabolic rate following exposure to a novel antigen. An implicit assumption of this technique is that all variation in energy costs following antigenic challenge is due solely to adaptive immunity, while ignoring potential changes in the energy demands of ongoing bodily functions. We critically assess this assumption by measuring both basal metabolic rate (BMR) and exercise-induced maximal metabolic rate (MMR) in house sparrows before and after the primary and two subsequent vaccinations with either saline (sham) or two novel antigens (keyhole limpet haemocyanin and sheep red blood cells; KLH and SRBC, respectively). We also examined the effect of inducing male breeding levels of testosterone (T) on immune responses and their metabolic costs in both males and females. Although there was a moderate decrease in KLH antibody formation in T-treated birds, there was no effect of T on BMR, MMR or immunity to SRBC. There was no effect of vaccination on BMR but, surprisingly, all vaccinated birds maintained MMR better than sham-treated birds as the experiment progressed. Our findings caution against emphasizing energy costs or nutrient diversion as being responsible for reported fitness reductions following activation of adaptive immunity.
Keywords: adaptive immune costs, hypervaccination, basal metabolic rate, maximum metabolic rate, testosterone, immunocompetence handicap hypothesis
1. Introduction
The adaptive immune system of vertebrates is recognized as a major evolutionary advance in combating pathogenic infections [1]. The benefits of adaptive immunity are well established, but attempts to identify and measure the costs of its activation are far fewer and the results equivocal, with evidence both for [2–5] and against [6,7] reductions in fitness measures following adaptive immunity activation in endotherms. Reductions in fitness are typically ascribed to energy costs or nutrient diversion following immune activation, yet estimates of energy costs of peak antibody production following antigen exposure range from a 27% elevation in metabolic rate to a reduction of 13% [8,9]. While variations in protocols for measuring metabolic rates as well as the type, amount and number of antigenic challenges contribute to this disparity, changes in the energy demands of ongoing bodily functions may also occur. Evidence of rapid organ mass variation in rodents following novel antigen inoculation [10–12] contests the assumption that the energy demands of immune activation can be deduced from changes in basal metabolic rate (BMR) alone.
Unlike BMR, which reflects an endotherm's minimal or maintenance energy costs [13], an individual's exercise-induced maximum metabolic rate (MMR) requires the maximal performance of a suite of enzymes, organelles, cells, tissues, organs and organ systems [14], with redirection of resources from any of these organizational levels compromising attainment of maximal aerobic exertion. Thus, variation in MMR following immune activation can be assumed to reflect redistribution of energy and nutrient supply, as well as revealing its functional consequences.
Assessing the effects of antigenic challenges on MMR also permits testing assumptions underlying the provocative immunocompetence handicap hypothesis (ICHH; [15]). According to the ICHH, testosterone (T) suppresses immune responses in vertebrate males, yet females select mates based on the quality of their T-dependent sexual signals. Thus, males able to maintain superior condition and performance while experiencing a reduction in immunocompetence are likely to be those of superior genetic quality. A 2004 meta-analysis concluded that available data did not support the predictions of the ICHH [16], but the incorporation of more recent results from a broader range of studies found the reverse [17]. Nevertheless, the hypothesis does not consider potential fitness advantages that males might derive by reducing immunity-imposed demands for energy and nutrients during breeding.
Physical activities relying on aerobic fitness (e.g. repetitive songs and displays) are highly indicative of body condition and are the predominant male traits selected by females [18]. Thus, if T-induced immunosuppression improves availability of energy and nutrients for aerobically demanding activities in breeding males, then the presumed handicap imposed by elevated T would have to be reassessed. We experimentally tested this possibility by comparing MMR and BMR of male house sparrows before and after serial antigenic challenges, in T-treated and control birds. An equal number of females were used in this experiment to identify potential sex-related differences in metabolic responses to these treatments.
2. Material and methods
(a) . Experimental animals
We captured non-breeding, free-living house sparrows (Passer domesticus) during late winter in the Illawarra region of NSW, Australia. The 31 males and 31 females were distributed as mixed-sex flocks among four outdoor flight cages (4.5 × 3.6 × 2.5 m) exposed to ambient conditions. Birds had free access to commercial finch seed mix (Golden Cob, Mars Birdcare Australia), mineralized grit and water.
(b) . Experimental protocol
All male birds were bilaterally castrated, under anaesthetic, 1 or 2 days after capture and returned to the flight aviaries. After birds adjusted to captivity for two weeks, we measured BMR and exercise-induced MMR (electronic supplementary material, figure S1). Blood was then sampled to characterize hormonal and immune status, and each bird implanted with either an empty or T-filled silastic tubule (see below). Metabolic evaluations and blood sampling were undertaken two weeks later to determine plasma T levels and ‘baseline’ metabolic and immune response parameters (below). Within each sex, birds were distributed randomly into one of the four experimental groups: antigenic injection/T implant (IC(immune challenge)/T), antigenic injection/control implant (IC/C), sham injection/T implant (S/T) and sham injection/control implant (S/C).
(c) . Testosterone and control implants
Under anaesthetic (methoxyflurane), birds were implanted subcutaneously with 6 mm lengths of medical-grade silastic tubing (1.47 mm inner diameter; 1.96 mm outer diameter; Dow Corning), sealed at each end with silastic glue (Dow Corning), via a small incision on one side of their thorax. These implants were either empty (control birds; C-implants) or filled with crystalline T (Sigma Chemical Co.; T-1500; T-implants). The resultant wounds were sealed with medical-grade cyanoacrylate adhesive (Vetbond).
(d) . Immune challenge through hypervaccination
Two novel antigens, keyhole limpet haemocyanin (KLH; Sigma-Aldrich) and sheep red blood cells (SRBC; Institute of Medical and Veterinary Science, Adelaide, South Australia) were administered concurrently to ensure stimulation of adaptive immunity in the immune-challenged groups. Inoculation of KLH was followed Hasselquist et al. [19], with 100 µl of 1 mg KLH ml−1 sterile water emulsified 1 : 1 with Freund's incomplete adjuvant (Sigma-Aldrich) injected into pectoral muscle. This was immediately followed by a 100 µl intra-abdominal injection of SRBC (10% by volume in phosphate-buffered saline (PBS)). Sham-treated birds were injected intra-muscularly with 100 µl sterile water emulsified 1 : 1 with Freund's incomplete immediately followed by an intra-abdominal injection of 100 µl PBS.
Injections were administered three times, two to three weeks apart, to achieve hypervaccination (electronic supplementary material, figure S1). Metabolic measurements and blood sampling occurred 12 days after the first injection and 6 days after the second and third injections in accordance with documented periods of peak KLH antibody titres after multiple injections in starlings ([19]; electronic supplementary material, figure S1).
(e) . Quantifying adaptive immunity
Following venepuncture, approximately 200 µl of blood was collected from a brachial vein in heparinized microhaematocrit tubes. Blood samples were immediately centrifuged at 6000 r.p.m. for 5 min and plasma stored at −20°C for hormonal (see electronic supplementary material) and immune analyses. Plasma concentrations of anti-KLH antibodies were determined using enzyme-linked immunosorbent assay (ELISA; [19,20]; see electronic supplementary material for details). Constitutive innate immunity and specific immunity to SRBC were determined by quantifying agglutination and lysis activity of plasma before and after exposure to SRBC, respectively, following Matson et al. [21], modified to use 1% suspension of SRBC instead of rabbit RBC.
(f) . Basal metabolic rate
Measurement of BMR was conducted when birds were post-absorptive, during the birds' rest-phase, and at temperatures within the thermoneutral zone for this species (29–31°C; [22]). At least 3 h after they had last fed, birds were placed individually in 2 l metal chambers fitted with a perch and supplied with room air at 500 ml min−1 (Tylan mass flow controllers FC-280S). Oxygen content of inlet and outlet air for each chamber was measured using a Sable Systems Oxzilla II oxygen analyser in combination with an electronic stream selector (Sable Systems Respirometer Multiplexer V 2.0; further details in [23]). Basal rates of oxygen consumption (BMR) were defined as the mean of the two lowest 5 min periods of oxygen uptake recorded during the last half of the 12 h measurement period, which ensured all birds were post-absorptive [24,25].
(g) . Maximum metabolic rate
Oxygen consumption rates (V̇O2) were measured during intense exercise within an enclosed 5 l drum with clear sides and carpet lining the inner rim [26]. These measurements took place in the morning following completion of the overnight BMR determinations, with all birds held in small cages with free access to food and water for 4 h prior to exercise measurements. A mass flow controller (Tylan Corp.) supplied air to the chamber at 5 l min−1 and the oxygen content of inlet and outlet ports was measured with an oxygen analyser (Sable Systems FC-1). A single bird was placed in the drum and, once settled, the cover was removed and the drum rotated. The Ping-pong balls within the drum encouraged birds to maintain a series of rapid take-offs and short-term flights interspersed with vigorous hopping. The V̇O2 data were adjusted with ‘instantaneous’ conversion procedures to account for gas mixing characteristics of the wheel and accurately resolve short-term oxygen content variation [23,26]. The highest continuous 60 s instantaneous oxygen consumption rate was designated as MMR.
(h) . Statistical analysis
We considered the performance (body mass, metabolic measures and immune responses) of animals two weeks after receiving their implant, but prior to the series of immune challenges (electronic supplementary material, figure S1) to represent individual baseline measures. We tested for differences in baseline performance measures between treatment groups across a set of hypothesis-driven candidate general linear models including fixed effects of T treatment (T/C), sex (M/F), immune challenge (IC/S—although no immune challenges had yet taken place) and their biologically relevant interactions, in R ([27]; electronic supplementary material, tables S1, S4, S7, S12 and S16).
To verify that immune-challenged individuals elicited adaptive immune responses, immune parameters after each injection were quantified as individual's change, positive or negative, in a given immune parameter (lysis, agglutination, KLH) compared to their ‘baseline’ for that immune parameter (measured two weeks post-implant; electronic supplementary material, figure S1). Across the study, the effects of injection stage (post-injection 1, 2 and 3) and experimental treatments on change in each immune parameter were assessed across a set of candidate linear mixed models including random effects for individual and fixed effects of immune treatment, T treatment, sex and their biologically relevant interactions, using ML estimation in the lme4 package in R ([27]; electronic supplementary material, tables S20, S23 and S25). Linear mixed models (with ML estimation in the lme4 package in R) were also used to verify T treatments elevated plasma T concentration, and that there were no effects of sex or immune treatments (electronic supplementary material, table S18). To test the effects of experimental treatments on change, in each metabolic parameter (BMR, MMR and body mass), as difference relative to baseline, a set of candidate linear mixed models were assessed in the lme4 package in R (with ML estimation), including random effects for individual and fixed effects for experimental treatments and stages (electronic supplementary material, tables S28, S31 and S33). Results are reported from all supported models, based on AICc comparisons across models. All data and code used in these analyses are publicly available through Dryad [28] and Zenodo [29], respectively.
3. Results
(a) . Evaluation of experimental treatments
Prior to the series of immune challenges, body mass and all immune measures were statistically indistinguishable between sexes, immune treatments and T treatments (electronic supplementary material, tables S1–S11). However, birds with T-implants had slightly lower BMR across all supported models (ca 1.5%; t = −2.36; p = 0.022; electronic supplementary material, tables S13–S15) and males had higher MMR (10.4%; t = 2.63; p = 0.011; electronic supplementary material, tables S16 and S17) than females. Plasma T content differed between implant groups (F1,135 = 90.019; p = <2.2 × 10−16; electronic supplementary material, table S18 and S19; figure S2), averaging 5.15 ± 0.36 and 0.56 ± 0.07 ng ml−1 in T-implanted and C-implanted birds, respectively, but was unaffected by sex, experimental stage or immune challenge (electronic supplementary material, table S18 and S19). All T-treated birds, including females, developed blackened bills, suggesting the elevated T in plasma was biologically effective [30,31]. Bills of all control birds retained a pale, non-reproductive colour throughout the experiment.
(b) . Immune responses to hypervaccination
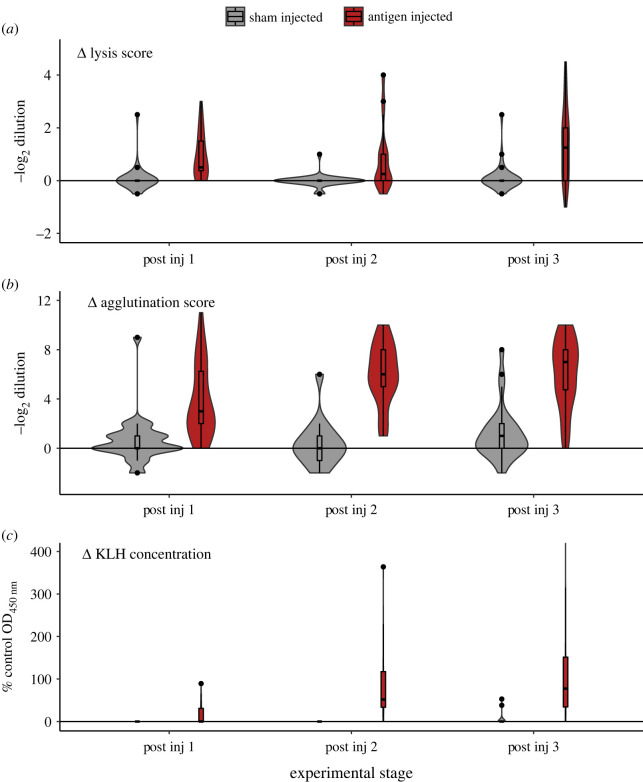
All birds injected with SRBC and KLH displayed significantly greater immune responses to these antigens than sham-injected individuals, with the extent of these differences becoming greater with each injection (figure 1), indicated by significant effects of stage (p = 0.036) and immune treatment (p < 0001) for lysis scores (electronic supplementary material, tables S21 and S22), and significant interactions between stage and immune treatment (p < 0.009) for agglutination (electronic supplementary material, table S24) and KLH (electronic supplementary material, tables S26 and S27). Neither body mass nor sex affected these results (electronic supplementary material, tables S21, S22, S24, S26 and S27) and T-treatment only had an effect on KLH response in one of the two supported models (electronic supplementary material, tables S26 and S27), whereby T-implants resulted in antigen-injected birds having 50% lower responses to KLH, on average, compared to C-implants (electronic supplementary material, table S26).
Figure 1.
Change in immune responses of antigen-injected (immune-challenged) (n = 32) and sham-injected (n = 30) birds following serial injections, in relation to pre-injection ‘baseline’ scores, where immune responses were measured as the highest twofold dilution of plasma producing lysis (a) or agglutination (b) to SRBC, and the optical density (at 450 nm) of plasma in an ELISA to KLH as a percentage of positive control (c). Immune responses of birds given a sham injection did not deviate from pre-injection values, whereas immune responses of vaccinated birds increased significantly after each injection.
(c) . Metabolic responses to hypervaccination
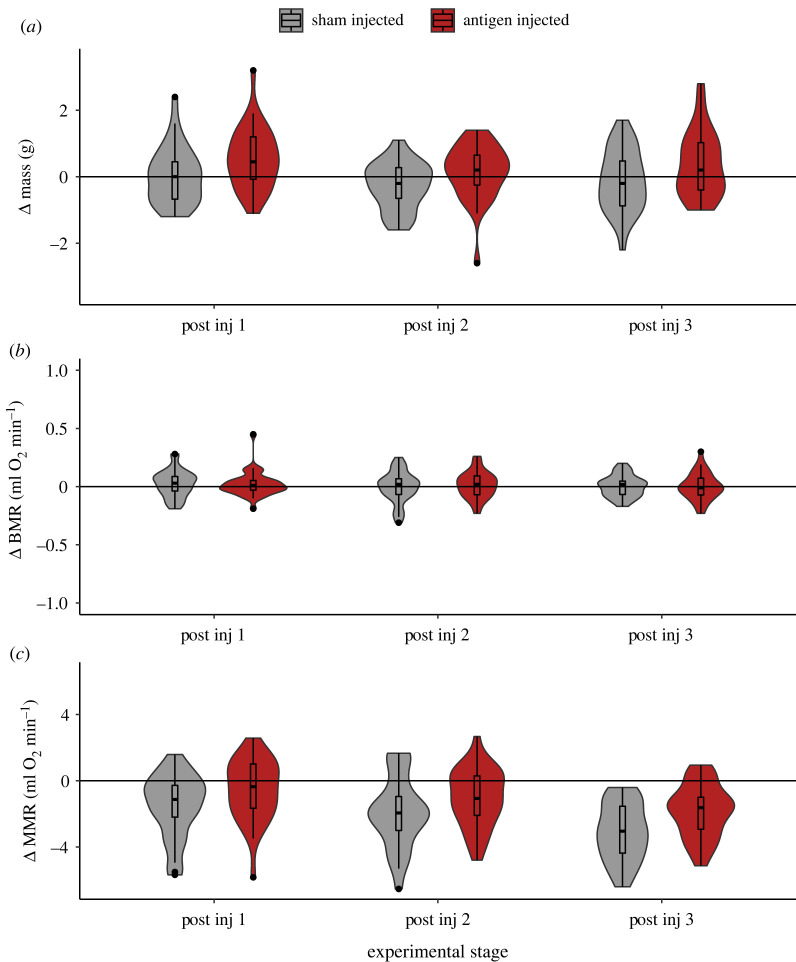
Throughout the experiment, BMR remained stable (figure 2b), and the supported model indicated individual change in BMR relative to baseline was unaffected by sex, plasma T content, immune challenge or measurement stage (electronic supplementary material, table S31 and S32). By contrast, individual changes in body mass were significantly higher in immune- compared to sham-challenged birds (p = 0.007, electronic supplementary material, tables S29 and S30; figure 2a). Similarly, although MMR decreased over the experimental period (as expected in captivity; figure 2c; ‘stage’ p < 0.001, electronic supplementary material, tables S34–S36), two of the three supported models included a significant interaction between injection type and stage (p < 0.05, electronic supplementary material, tables S34 and S36), indicating immune-challenged birds experienced significantly less decrease in their MMR over time compared to sham-injected birds (figure 2c).
Figure 2.
Change in body mass (a) and metabolic rates ((b) BMR; (c) MMR) of antigen-injected (immune-challenged) (n = 32) and sham-injected (n = 30) birds following serial injections, in relation to pre-injection ‘baseline’ scores. Body mass and BMR were unaffected by injection type and did not vary over time. By contrast, MMR declined in all treatment groups over the experimental period, but to a lesser extent in immune-challenged birds compared to sham-injected birds.
4. Discussion
Most studies examining the energy costs of antibody production are based on a single antigenic challenge. While these are valid measures, they miss the far greater antibody production that follows subsequent antigen exposures [32]. Our use of a hypervaccination protocol, paired with repeated metabolic evaluations, permitted us to evaluate multiple stages of adaptive immunity. As expected (e.g. [19]), immune measures increased substantially following sequential injections of novel antigens, as exemplified by the six- and sevenfold greater responses to the second and third injections of KLH compared with the first. (figure 1c).
All hormone-implanted birds had T levels corresponding to free-living male house sparrows during breeding (26), and approximately fivefold higher than in breeding free-living females [33]. Thus, although the T-treatment reduced responsiveness to KLH in all birds, this result in females stems from hyper-physiological T levels, that have been found to be immunosuppressive in females of other species [34,35]. By contrast, T-treatment had no effect on adaptive immune responses to serial SRBC vaccination. This calls into question the generality of T-mediated immunosuppression underlying the ICHH [17], particularly since our study incorporated all criteria associated with the highest effect sizes across previous studies of the ICHH, namely humoral immunity, experimental immune challenge, castrated males and animals sourced from free-living populations [17].
Our experimental design met all requirements for determining BMR [36] and clearly found that the immune challenges had no effect on the birds' maintenance energy requirements. These findings corroborate other studies reporting nil to negative metabolic costs of adaptive immune activation in endotherms [9,11,37–39]. If, however, immune activation promotes reallocation of resources among bodily functions, this effect is likely to be detected by evaluating changes in MMR. For instance, during avian moult, the protein demands of feather replacement are accompanied by increased rates of muscle protein turnover [40,41] and associated decreases in MMR, in direct proportion to the mass of feathers being replaced [23]. In our study, both immune-challenged and sham-treated birds showed a gradual decline in MMR over the duration of the experiment, likely a consequence of aerobic detraining due to reduced flight activities imposed by captivity [42,43]. Surprisingly, however, immune-challenged birds maintained significantly higher MMR than their unvaccinated counterparts as the study progressed, irrespective of their sex or T-treatment. Thus, the demands of antibody production did not constrain peak aerobic performance following immunization and, more remarkably, appeared to be protective of such activities in both sexes. Whether this was a consequence of differences between immune-challenged and sham-treatment groups in the extent of flight activity and/or in physiological processes forestalling aerobic decline requires further experimentation.
In conclusion, activation and reactivation of adaptive immunity did not provoke changes in BMR nor decrease maximal aerobic capacity. Plasma T levels had moderate, but inconsistent, effects on measured immune responses, but no effect on MMR. The absence of additional maintenance energy costs or reductions in maximum aerobic capability during peak antibody circulation following hypervaccination reinforces the notion that antibody production following adaptive immunity activation is a low-cost mechanism in the immunological armoury of vertebrates [44].
Acknowledgements
In memory of William R. Dawson: mentor, colleague, and friend.
Ethics
All aspects of animal care and their use in these experimental procedures were approved by the University of Wollongong Animal Ethics Committee (AE04/01).
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.4xgxd25br [28]. Statistical code used in this study is linked to the Dryad record, but also available directly from Zenodo: https://doi.org/10.5281/zenodo.5904580 [29].
The data are provided in the electronic supplementary material [45].
Authors' contributions
W.A.B.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization and writing—original draft; T.W.O.: data curation, investigation, methodology, project administration, validation and writing—review and editing; L.B.A.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation and writing—review and editing; K.C.K.: conceptualization, methodology, supervision, visualization and writing—review and editing; B.J.H.: data curation, formal analysis, investigation, project administration, software, validation, visualization, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was funded by the Australian Research Council grant DP0453021 awarded to W.A.B. and L.B.A.
References
- 1.Müller V, de Boer RJ, Bonhoeffer S, Szathmáry E. 2018. An evolutionary perspective on the systems of adaptive immunity. Biol. Rev. 93, 505-528. ( 10.1111/brv.12355) [DOI] [PubMed] [Google Scholar]
- 2.Graham JL, Mady RP, Greives TJ. 2017. Experimental immune activation using a mild antigen decreases reproductive success in free-living female dark-eyed juncos (Junco hyemalis). Can. J. Zool. 95, 263-269. ( 10.1139/cjz-2016-0131) [DOI] [Google Scholar]
- 3.Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. 2004. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. B 271, 925-930. ( 10.1098/rspb.2004.2678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nystrand M, Dowling DK. 2020. Effects of immune challenge on expression of life-history and immune trait expression in sexually reproducing metazoans—a meta-analysis. BMC Biol. 18, 17. ( 10.1186/s12915-020-00856-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilmonen P, Taarna T, Hasselquist D. 2000. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. B 267, 665-670. ( 10.1098/rspb.2000.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams TD, Christians JK, Aiken JJ, Evanson M. 1999. Enhanced immune function does not depress reproductive output. Proc. R. Soc. B 266, 753-757. ( 10.1098/rspb.1999.0701) [DOI] [Google Scholar]
- 7.Xu YC, Yang DB, Wang DH. 2012. No evidence for a trade-off between reproductive investment and immunity in a rodent. PLoS ONE 7, 11. ( 10.1371/journal.pone.0037182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demas GE, Chefer V, Talan MI, Nelson RJ. 1997. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6 J mice. Am. J. Physiol. Reg. Integr. Comp. Physiol. 273, R1631-R1637. ( 10.1152/ajpregu.1997.273.5.R1631) [DOI] [PubMed] [Google Scholar]
- 9.Mendes L, Piersma T, Hasselquist D. 2006. Two estimates of the metabolic costs of antibody production in migratory shorebirds: low costs, internal reallocation, or both? J. Ornithol. 147, 274-280. ( 10.1007/s10336-006-0070-8) [DOI] [Google Scholar]
- 10.Cai XQ, Yang M, Zhong WQ, Wang DH. 2009. Humoral immune response suppresses reproductive physiology in male Brandt's voles (Lasiopodomys brandtii). Zoology 112, 69-75. ( 10.1016/j.zool.2008.04.006) [DOI] [PubMed] [Google Scholar]
- 11.Derting TL, Compton S. 2003. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiol. Biochem. Zool. 76, 744-752. ( 10.1086/375662) [DOI] [PubMed] [Google Scholar]
- 12.Ksiazek A, Konarzewski M. 2012. Effect of dietary restriction on immune response of laboratory mice divergently selected for basal metabolic rate. Physiol. Biochem. Zool. 85, 51-61. ( 10.1086/663696) [DOI] [PubMed] [Google Scholar]
- 13.Hulbert AJ, Else PL. 2004. Basal metabolic rate: history, composition, regulation, and usefulness. Physiol. Biochem. Zool. 77, 869-876. ( 10.1086/422768) [DOI] [PubMed] [Google Scholar]
- 14.Weibel ER, Taylor CR, Hoppeler H. 1991. The concept of symmorphosis—a testable hypothesis of structure–function relationship. Proc. Natl Acad. Sci. USA 88, 10 357-10 361. ( 10.1073/pnas.88.22.10357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstad I, Karter AJ. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603-622. ( 10.1086/285346) [DOI] [Google Scholar]
- 16.Roberts ML, Buchanan KL, Evans MR. 2004. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 68, 227-239. ( 10.1016/j.anbehav.2004.05.001) [DOI] [Google Scholar]
- 17.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. 2017. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. 92, 551-571. ( 10.1111/brv.12243) [DOI] [PubMed] [Google Scholar]
- 18.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 19.Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. 1999. Is avian humoral immunocompetence suppressed by testosterone? Behav. Ecol. Sociobiol. 45, 167-175. [Google Scholar]
- 20.Martinez J, Tomas G, Merino S, Arriero E, Moreno J. 2003. Detection of serum immunoglobulins in wild birds by direct ELISA: a methodological study to validate the technique in different species using antichicken antibodies. Funct. Ecol. 17, 700-706. ( 10.1046/j.1365-2435.2003.00771.x) [DOI] [Google Scholar]
- 21.Matson KD, Ricklefs RE, Klasing KC. 2005. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 29, 275-286. ( 10.1016/j.dci.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 22.Hudson JW, Kimzey SL. 1966. Temperature regulation and metabolic rhythms in populations of the house sparrow, Passer domesticus. Comp. Biochem. Physiol. 17, 203-217. ( 10.1016/0010-406X(66)90021-1) [DOI] [PubMed] [Google Scholar]
- 23.Buttemer WA, Bauer S, Emmenegger T, Dimitrov D, Peev S, Hahn S. 2019. Moult-related reduction of aerobic scope in passerine birds. J. Comp. Physiol. B 189, 463-470. ( 10.1007/s00360-019-01213-z) [DOI] [PubMed] [Google Scholar]
- 24.Benedict FG, Fox EL. 1933. Der Grundumsatz von kleinen Vögeln (Spatzen, Kanarienvögeln und Sittichen). Pflüger's Archiv für die gesamte Physiologie des Menschen und der Tiere 232, 357-388. ( 10.1007/BF01754796) [DOI] [Google Scholar]
- 25.Kendeigh SC. 1944. Effect of air temperature on the rate of energy metabolism in the English sparrow. J. Exp. Zool. 96, 1-16. ( 10.1002/jez.1400960102) [DOI] [Google Scholar]
- 26.Chappell MA, Bech C, Buttemer WA. 1999. The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J. Exp. Biol. 202, 2269-2279. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Buttemer WA, O'Dwyer T, Astheimer LB, Klasing KC, Hoye BJ. 2022. Data from: No evidence of metabolic costs following adaptive immune activation or reactivation in house sparrows. Dryad Digital Repository. ( 10.5061/dryad.4xgxd25br) [DOI] [PMC free article] [PubMed]
- 29.Buttemer WA, O'Dwyer T, Astheimer LB, Klasing KC, Hoye BJ. 2022. R Code for analyses within ‘No evidence of metabolic costs following adaptive immune activation or reactivation in house sparrows’. Zenodo ( 10.5281/zenodo.5904580) [DOI] [PMC free article] [PubMed]
- 30.Hegner RE, Wingfield JC. 1986. Behavioral and endocrine correlates of multiple brooding in the semicolonial house sparrow Passer domesticus. 1. Males. Horm. Behav. 20, 294-312. ( 10.1016/0018-506x(86)90039-5) [DOI] [PubMed] [Google Scholar]
- 31.Laucht S, Kempenaers B, Dale J. 2010. Bill color, not badge size, indicates testosterone-related information in house sparrows. Behav. Ecol. Sociobiol. 64, 1461-1471. ( 10.1007/s00265-010-0961-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough KC, Summerfield A. 2005. Basic concepts of immune response and defense development. ILAR J. 46, 230-240. ( 10.1093/ilar.46.3.230) [DOI] [PubMed] [Google Scholar]
- 33.Mazuc J, Bonneaud C, Chastel O, Sorci G. 2003. Social environment affects female and egg testosterone levels in the house sparrow (Passer domesticus). J. Exp. Zool. 6, 1084-1090. ( 10.1046/j.1461-0248.2003.00535.x) [DOI] [Google Scholar]
- 34.Duffy DL, Bentley GE, Drazen DL, Ball GF. 2000. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behav. Ecol. 11, 654-662. ( 10.1093/beheco/11.6.654) [DOI] [Google Scholar]
- 35.Zysling DA, Greives TJ, Breuner CW, Casto JM, Demas GE, Ketterson ED. 2006. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Horm. Behav. 50, 200-207. ( 10.1016/j.yhbeh.2006.03.004) [DOI] [PubMed] [Google Scholar]
- 36.Aschoff J, Pohl H. 1970. Rhythmic variations in energy metabolism. Fed Proc. 29, 1541. [PubMed] [Google Scholar]
- 37.Barbour K, McClune DW, Delahay RJ, Speakman JR, McGowan NE, Kostka B, Montgomery WI, Marks NJ, Scantlebury DM. 2019. No energetic cost of tuberculosis infection in European badgers (Meles meles). J. Anim. Ecol. 88, 1973-1985. ( 10.1111/1365-2656.13092) [DOI] [PubMed] [Google Scholar]
- 38.Pilorz V, Jackel M, Knudsen K, Trillmich F. 2005. The cost of a specific immune response in young guinea pigs. Physiol. Behav. 85, 205-211. ( 10.1016/j.physbeh.2005.04.008) [DOI] [PubMed] [Google Scholar]
- 39.Verhulst S, Riedstra B, Wiersma P. 2005. Brood size and immunity costs in zebra finches Taeniopygia guttata. J. Avian Biol. 36, 22-30. ( 10.1111/j.0908-8857.2005.03342.x) [DOI] [Google Scholar]
- 40.Buttemer WA, Addison BA, Klasing KC. 2020. The energy cost of feather replacement is not intrinsically inefficient. Can. J. Zool. 98, 142-148. ( 10.1139/cjz-2019-0170) [DOI] [Google Scholar]
- 41.Taruscio TG, Murphy ME. 1995. 3-Methylhistidine excretion by molting and non-molting sparrows. Comp. Biochem. Physiol. A 111, 397-403. ( 10.1016/0300-9629(95)00038-9) [DOI] [Google Scholar]
- 42.Buttemer WA, Warne S, Bech C, Astheimer LB. 2008. Testosterone effects on avian basal metabolic rate and aerobic performance: facts and artefacts. Comp. Biochem. Physiol. A 150, 204-210. ( 10.1016/j.cbpa.2006.06.047) [DOI] [PubMed] [Google Scholar]
- 43.Neufer PD. 1989. The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med. 8, 302-320. ( 10.2165/00007256-198908050-00004) [DOI] [PubMed] [Google Scholar]
- 44.Klasing K. 2004. The costs of immunity. Acta Zool. Sinica 50, 961-969. [Google Scholar]
- 45.Buttemer WA, O'Dwyer T, Astheimer LB, Klasing KC, Hoye BJ. 2022. Data from: no evidence of metabolic costs following adaptive immune activation or reactivation in house sparrows. FigShare. ( 10.6084/m9.figshare.c.6032397) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Buttemer WA, O'Dwyer T, Astheimer LB, Klasing KC, Hoye BJ. 2022. Data from: No evidence of metabolic costs following adaptive immune activation or reactivation in house sparrows. Dryad Digital Repository. ( 10.5061/dryad.4xgxd25br) [DOI] [PMC free article] [PubMed]
- Buttemer WA, O'Dwyer T, Astheimer LB, Klasing KC, Hoye BJ. 2022. R Code for analyses within ‘No evidence of metabolic costs following adaptive immune activation or reactivation in house sparrows’. Zenodo ( 10.5281/zenodo.5904580) [DOI] [PMC free article] [PubMed]
- Buttemer WA, O'Dwyer T, Astheimer LB, Klasing KC, Hoye BJ. 2022. Data from: no evidence of metabolic costs following adaptive immune activation or reactivation in house sparrows. FigShare. ( 10.6084/m9.figshare.c.6032397) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.4xgxd25br [28]. Statistical code used in this study is linked to the Dryad record, but also available directly from Zenodo: https://doi.org/10.5281/zenodo.5904580 [29].
The data are provided in the electronic supplementary material [45].