Abstract
In humans, skin is a primary thermoregulatory organ, with vasodilation leading to rapid body cooling, whereas in Rodentia the tail performs an analogous function. Many thermodetection mechanisms are likely to be involved including transient receptor potential vanilloid-type 4 (TRPV4), an ion channel with thermosensitive properties. Previous studies have shown that TRPV4 is a vasodilator by local action in blood vessels, so here, we investigated whether constitutive TRPV4 activity affects Mus muscularis tail vascular tone and thermoregulation. We measured tail blood flow by pressure plethysmography in lightly sedated M. muscularis (CD1 strain) at a range of ambient temperatures, with and without intraperitoneal administration of the blood–brain barrier crossing TRPV4 antagonist GSK2193874. We also measured heart rate (HR) and blood pressure. As expected for a thermoregulatory organ, we found that tail blood flow increased with temperature. However, unexpectedly, we found that GSK2193874 increased tail blood flow at all temperatures, and we observed changes in HR variability. Since local TRPV4 activation causes vasodilation that would increase tail blood flow, these data suggest that increases in tail blood flow resulting from the TRPV4 antagonist may arise from a site other than the blood vessels themselves, perhaps in central cardiovascular control centres.
Keywords: thermoregulation, tail blood flow, ion channels, non-invasive blood pressure, 3Rs, TRPV4
1. Introduction
Thermoregulation is one of the defining homeostatic processes common to mammals; core body and brain temperatures are well maintained despite challenges such as changing ambient temperature and exercise to the degree that brain temperature rarely changes outside of a 3°C range [1–3]. Mammals detect temperatures at both central and peripheral sites and responses to changing temperatures can result both from local responses and central, hypothalamus-coordinated autonomic responses [4–6]. Typical thermogenic effector mechanisms include liver thermogenesis and skeletal muscle shivering whereas cooling mechanisms including behavioural changes and redistribution of blood from core to peripheral vessels [4,5,7]. Rodents use basal metabolic rate and non-shivering thermogenesis as their principle mechanisms for heat production, mainly because of their small size [8]. In terms of heat loss, transfer of excess heat to the environment is facilitated by so-called heat transfer zones, which are usually found at the body extremities, for example, in humans, typically, acute heat loss is mediated by redistributing blood to cutaneous vascular beds [5]. The location of critical heat transfer zones are somewhat species specific, so for example, the ear for elephants and rabbits [9,10], head vasculature in large dinosaurs [11,12] and the feet [13] and tail for rodents [14–16]. The tail of rodents is ideal as a heat transfer zone due to its glabrous nature [16]. It is thought that vasoconstriction rather than counter-current heat exchange provides the major barrier to core-to-tail heat flow [17].
In this work, we have investigated the role of Mus muscularis (mouse) transient receptor potential vanilloid-type 4 (TRPV4) in this homeostatic system using a potent and selective TRPV4 inhibitor, GSK2193874. TRPV4 is one of several temperature-sensitive ion channels and expressed in both the hypothalamus and the vasculature, in both smooth muscle and endothelial cells. Recently, there has been considerable interest in the immune, neuromodulatory, cardiovascular and thermoregulatory potential of small molecule TRPV4 modulatory drugs, such as GSK2193874 and HC-067047 [18–25].
TRPV4 is a relatively non-selective Ca2+ channel (PCa/PNa 6-10) that was first characterized as mechanosensory [26,27]; however, it is also activated by temperatures greater than 30°C, and so, at physiological temperatures, it would be expected to be constitutively active under basal conditions [28–30]. Activation of TRPV4 leads to vasodilation [31–34] and logically, therefore, transgenic elimination of TRPV4 (TRPV4-/- knock out) would be expected to increase blood pressure, but it does not [18,31].
The precise contribution of TRPV4 to thermosensing and thermoregulation in vivo remains unclear. No changes in escape latency from heat stimuli were observed in the hotplate challenge [35,36]. However, post-subcutaneous injection of capsaicin or carrageenan, TRPV4-/- mice showed longer escape latencies from the hot surface compared to wild-type [36]. In another study, it was shown that TRPV4 is required for normal thermal responsiveness in vivo; on a thermal gradient, TRPV4-/- mice selected warmer floor temperatures. In addition, TRPV4-/- mice also exhibited prolonged withdrawal latencies during acute tail heating [37].
In terms of pharmacological manipulations, the activation of TRPV4 with topological RN1747 decreased the core temperature of Rattus norvegicus and increased tail vasodilatation [38]. The effects of a TRPV4 inhibitor (HC067047), in the same study, were mixed with increases of core body temperature with ambients of 26 and 30°C, but not 22 and 32°C.
In this study, we had aimed to investigate whether the small molecule TRPV4 inhibitor, GSK2193874, would decrease tail vasodilation response to elevated ambient temperatures. As a surrogate for tail vasodilation, we used tail blood flow measured by volume plethysmography [39]. We also investigated frequency-domain heart rate variability (HRV). HRV is a sensitive tool that assesses the time difference between consecutive heart beats to evaluate autonomic nervous system modulation [40,41]. Accumulating data suggest that ultra-short-range HRV can be successfully derived from as low as 30 s of human ECG [42,43] and pulse rate variability (estimation of variation in heart rate (HR) from photoplethysmography) has recently been successfully measured from the rat tail [44]. Potentially, measurement of HRV from tail-cuffs would be a useful reduction, refinement or replacement (3Rs) laboratory animal welfare advancement, since surgery is not required. Therefore, we sought to, for the first time, (i) establish, empirically, the length of HR record necessary for HRV in mice and (ii) perform HRV from mouse tail volume plethysmography using the CODA apparatus. HRV reflects homeostasis in thermoregulation and blood pressure control and has been shown to be modulated by thermal stimuli in humans [45].
Surprisingly, we found the TRPV4 inhibitor increased tail blood flow when measured above mouse thermoneutrality, and we saw inhibitor dependent changes in ultra-short-range HRV raising the possibility that TRPV4 ion channels expressed outside of the vasculature, for example in the central nervous system, may also be involved with rodent thermoregulation.
2. Methods
Extended methods are included in the electronic supplementary material, information, but briefly:
(a) . Animals
Fourteen female adult CD1-mice (Charles River, UK) were used. All experimental procedures were ethically approved by the University's Animal Welfare Committee and performed under a UK Home Office Scientific Procedures licence (70/8746).
(b) . Volume pressure plethysmography recording
We used the CODA tail volume pressure plethysmography (VPR) system (Kent Scientific, Torrington, CT, USA) on control CD1-mice and mice that had received the selective TRPV4 antagonist GSK2193874. Full details of warming methodology and VPR methods are included in the electronic supplementary material. Note, all temperatures reported are ambient temperatures read from the thermocouple.
(c) . Statistical analyses
Blood pressure (MAP), HR and blood flow statistical comparisons were made with the nlme package in R, which incorporates a repeated-measures design. For HRV statistical comparisons, we used MANOVA in Minitab (PA, USA). p ≤ 0.05 was taken as significant.
(d) . Drugs
A sedative (midazolam 5 mg kg−1, i.p.) was supplied by our animal service unit and administered prior to recording. GSK2193874 (300 µg kg−1, i.p.) and DMSO were obtained from Sigma-Aldrich. GSK2193874 was dissolved in DMSO at 20 mg ml−1 stock then diluted 1 : 100 before i.p. injection (0.2 mg ml−1), following [23,34]. ‘Control’ includes 1% DMSO and volume of injection was dependent upon animal weight.
3. Results
We measured MAP, HR and blood flow (Flow) in 14 animals with and without GSK2193874 over the ambient temperature range of 31°C to 36°C. These are plotted in two-factor (treatment and temperature) format and analysed with a repeated-measures, mixed effects design. There was a statistically significant effect of temperature on all parameters measured, MAP (figure 1a: temperature F = 5.34, p ≤ 0.05; drug F = 0.38 p > 0.05, drug × temperature F = 0.17, p > 0.05), HR (figure 1b: temperature F = 7.37, p ≤ 0.05; drug F = 0.68 p > 0.05, drug × temperature F = 0.23, p > 0.05) and tail blood flow (figure 1c: temperature F = 13.21, p ≤ 0.005; drug F = 5.57, p ≤ 0.05, drug × temperature F = 14.00, p ≤ 0.0005). In the cases of HR and MAP, there was no significant effect of treatment with the TRPV4 antagonist (GSK2193874). However, with tail blood flow, there was both a significant increase with GSK2193874 treatment and a very highly significant interaction between temperature and GSK2193874 treatment.
Figure 1.
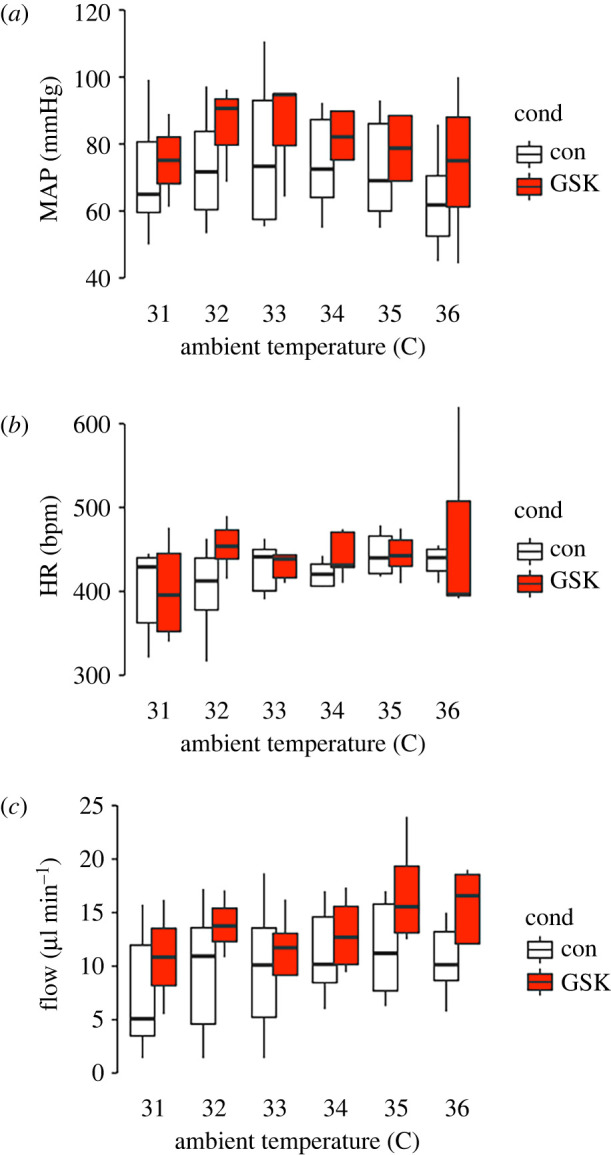
Effects of pharmacological inhibition of TRPV4 on blood pressure, HR and blood flow. (a) Mean arterial pressure at a range of ambient temperatures in control and GSK2193874, there was a significant association with temperature, but not drug. There was also no significant association between temperature and drug (mixed effects model: see main text for details). (b) Mean HR across a range of ambient temperature in control and GSK2193874, overall there was a significant change of HR with temperature, but no significant difference with drug and no significant association between drug and temperature (mixed effects model: see main text for details). (c) Tail blood flow across a range of ambient temperatures in control and GSK2193874. Overall, there was a statistically significant increase of blood flow with temperature and with GSK2193874 and a significant interaction (mixed effects model: see main text for details). Overall, n = 14 animals or for each temperature; 31°C n = (9,4) 32°C n = (10,4), 33°C n = (11,6), 34°C n = (10,7), 35°C n = (11,7) and 35°C n = (12,5). To investigate specific temperature points that were different to the 31°C value, we treated temperature as a factor and ran the estimated-marginal means method with the R-package Emmeans. This consists of 66 pairwise comparisons and we used Benjamini–Hochberg multiple comparison correction. In control, no individual flow is significantly greater than that at 31°C; however, in GSK2193874, blood flow at both 35°C and 36°C was significantly greater than at 31°C (p < 0.05).
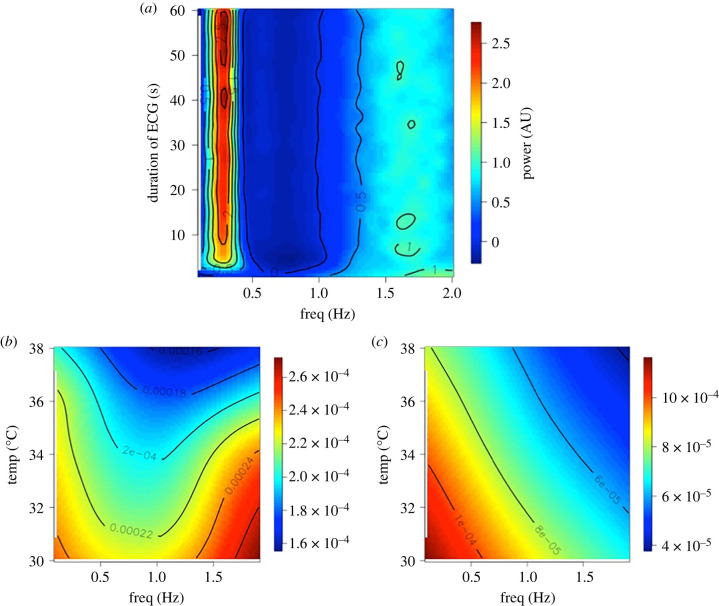
Since we were able to derive beat-by-beat HR records for several seconds (for example electronic supplementary material, figure S1), we investigated whether HRV could be captured over such short periods. To test whether this was feasible, we simulated mouse HR interval records of decreasing length using a modified version of McSharry et al. [46] and then measured HRV spectral powers using the Lomb–Scargle method [47,48] over 3000 simulations. Figure 2a shows that just a few seconds of ECG are sufficient to obtain a picture of the HRV in a mouse, in so far as, increasing the simulation duration beyond this does not greatly affect the HRV spectra. We therefore measured HRV power in the 0.1 to 1.9 Hz bands in our samples of control and GSK2193874 records (figure 2b,c) and compared these statistically with a MANOVA model, over a range of temperatures. There was no overall statistical difference with temperature; however, there was a statistically different set of spectra between control and GSK2193874-treated spectra. Furthermore, with univariate analyses, there was a significant difference between treatment and control at each individual frequency except the 0.5 Hz banding.
Figure 2.
Short-range HRV analysis. (a) Stacked Lomb periodograms for 3000 simulated ECG inter-beat interval datasets. Y-axis is the duration of the simulated ECG record, x-axis is the frequency component of each Lomb–Scargle. The periodograms have been normalized and scaled therefore the power (colour bar on the right) is in arbitrary units (AU). Below are periodogram surfaces recorded at different temperatures under control (b), or after injection GSK2193874 (c). Power is given in the scale bars. MANOVA (Minitab) analyses show these two distributions are significantly different, Wilks lambda p < 0.05. Overall, n = 14 animals.
4. Discussion
In this work, we investigate the role of TRPV4 in mouse tail blood flow with a systemic inhibitor of TRPV4, GSK2193874. Surprisingly, we find that tail blood flow is increased by GSK2193874. There was also a detectable effect of GSK2193874 on HRV, but no significant change in blood pressure or HR.
(a) . Blood flow, heart rate and blood pressure effects
GSK2193874 is a small lipid-soluble inhibitor of TRPV4 [19] that crosses the blood–brain barrier well (brain : plasma ratio = 0.6, personal communication with Dr David Behm of GSK) and so there are several locations at which TRPV4 could potentially influence the control of blood flow in response to elevated temperatures. A non-exhaustive list of possible sites of action could include the vasculature or cardiovascular control neurons.
TRPV4 is expressed in both vascular smooth muscle and the endothelial cell lining [49]. Activation of these channels leads to vasodilatation. It is difficult to assess the mechanism of this vasodilatation without a full dose–response curve (DRC, see Limitations). However, it is likely to involve both endothelial and smooth muscle cells, potential release of endothelial relaxation or hyperpolarization factors and, ultimately, small local increases of Ca2+ activate potassium channels which hyperpolarize the muscle cells and allow relaxation/vasodilatation [31–33]. A TRPV4 inhibitor would therefore be expected to cause vasodilation (or have no effect if there was no constitutive TRPV4 activity) and so it seems unlikely the increase in tail blood flow we report in this study results from direct action on the vasculature. Furthermore, if the effect of GSK2193874 were primarily on blood vessels to cause dilation, we would have expected to see an overall drop in MAP and possibly then a reflex increase in HR since the baroreceptor loop features in established mechanisms of cardiovascular control as well as, specifically, thermoregulation [50,51]. We saw no change in blood pressure or HR, although multivariate analysis detected a small change in short-range HRV analysis. The potential for us to have missed such a baroreceptor-mediated effect due Type II errors is discussed in the Limitations section below.
A second location of TRPV4 channels that may be of relevance is the central nervous system, for example the hypothalamus [52]. It is known that other transient receptor potential channels influence the cardiovascular system via changes in sympathetic activity [53,54]. Our previous work shows that TRPV4 channels are located on pre-autonomic neurons of the hypothalamic paraventricular nucleus (PVN) and can influence cardiovascular control in response to osmotic challenge [55,56], and this effect was abolished with a TRPV4 inhibitor [55]. At the neuronal level, we have shown that the action current frequency of parvocellular PVN neurons is dramatically reduced when TRPV4 channels are inhibited [56]. To date, there have been no studies that have explored thermoregulatory roles for TRPV4 in central cardiovascular control neurons.
(b) . Heart rate variability effects
HRV analysis is an increasingly common method for cardiovascular assessment. In humans, for example, decreased HRV (i.e. a very steady pulse) is an independent predictor of cardiac mortality [57]. In animals too, it is proving increasingly useful in a range of contexts including phenotyping transgenic animals [58], investigating cardiovascular effects of drugs [59] and predicting arrhythmias [60]. While there are many papers analysing HRV in mice using radiotelemetry [61], we investigated here whether it was possible to do this with VPR and found that it was. It has previously been shown that relatively long photoplethysmography recordings could be used for HRV [44], with high accuracy, but the present study is the first to systematically analyse how long a recording needs to be. The derivation of this short-range HRV from non-invasive apparatus may prove a useful advance in 3Rs. In the electronic supplementary material, information, we compare (qualitatively) data with our previous telemetric study [62]. Since the average mouse HR is approximately eight times that of a human, an 8 s segment would be equivalent to the standard 1 min of recording necessary to detect higher frequency components of human ECG [41]. Here, simulation shows that periodograms from very short segments of ECG are similar to that of conventional 1 min records (figure 2a), and these data themselves and this approach may be of field interest. In terms of the response to temperature, we did not see an overall effect on HRV, probably because temperature typically affects the low-frequency powers, beyond the scope of ultra-short-range recording [45]; however, GSK2193874 did significantly alter overall frequency power curves.
(c) . Limitations
We measured only ambient temperature and not core temperature. We felt that the loss of this important information was necessary to avoid the disturbance of using a rectal thermocouple on mice in the non-invasive recording equipment. Also, we used sedation that could influence the whole animal responses and only female mice, unlike many male only studies [23,34]. Furthermore, to keep the study manageable, we opted for a one antagonist dose study rather than a full in vivo DRC, which would have been useful. It is difficult to predict accurately the local concentration that an ion channel will ‘see’ when a drug given systemically will reach, but if we assume that GSK2193874 has a typical volume of distribution of between 1 and 10 l kg−1, our 300 µg kg−1 dose would translate to approximately 40 to 400 nM, in the order of the maximal dose for GSK2193874 on TRPV4 channels [23]. Although GSK2193874 is highly selective for TRPV4 compared to the other 200 + proteins, it has been assayed against [23], repeating our studies with TRPV4-/- lines [31] would be the only way to confirm with certainty that the true target was indeed TRPV4.
We encountered technical challenges too, e.g. recording VPR data below 30°C (ambient) was unreliable, so we report a relatively limited temperature range rather than strictly hot versus cold. These limitations could be addressed by a telemetric study, but large motivation for our current approach was to use a non-invasive blood pressure design, for 3Rs ethical reasons. Furthermore, as in many physiological studies, statistical power was an issue. Our initial design (see electronic supplementary material, information) included a power analysis for HR and blood pressure, which made a number of assumptions but passed 80% power with around eight or nine animals. We then used 14, however, we were not able to get all conditions for all animals and so the final statistical power could be below 80%. We have hypothesized that an increase of blood flow, by TRPV4 antagonist in the absence of significant changes in MAP/HR would be compatible with a central mechanism of vasodilation. However, if we simply missed changes due to a type II error, the vasodilation could result from baroreflex-mediated mechanisms. This could be addressed by either increasing animal numbers or by repeating similar experiments with surgical or pharmacological block of the baroreceptor reflex [63]. See electronic supplementary material, information for further discussion.
In conclusion, this whole animal study shows that a TRPV4 antagonist has a significant effect on tail blood flow in the context of thermoregulation, but its site of action, and the mechanism of such modulation remain to be determined. We also demonstrate non-invasive measurement of frequency-domain HRV analysis from very short-range data that may prove useful in future 3Rs friendly research.
Ethics
All experimental procedures were ethically approved by the University's Animal Welfare Committee and performed under a UK Home Office Scientific Procedures licence (70/8746).
Data accessibility
Data are available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.1rn8pk0vq [64].
The data are provided in the electronic supplementary material [65].
Authors' contributions
F.O.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing—original draft and writing—review and editing; C.A.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft and writing---review and editing; R.B.-J.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interest.
Funding
This project received funding from the BBSRC: BB/T002115/1, BB/T002115/1 BB/S008136/1 and BB/N003020/1 awarded to R.B.-J.
References
- 1.Fuller A, Moss DG, Skinner JD, Jessen PT, Mitchell G, Mitchell D. 1999. Brain, abdominal and arterial blood temperatures of free-ranging eland in their natural habitat. Pflugers Arch. 438, 671-680. ( 10.1007/s004249900105) [DOI] [PubMed] [Google Scholar]
- 2.Fuller A, Carter RN, Mitchell D. 1998. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J. Appl. Physiol. 84, 877-883. ( 10.1152/jappl.1998.84.3.877) [DOI] [PubMed] [Google Scholar]
- 3.Walters TJ, Ryan KL, Tate LM, Mason PA. 2000. Exercise in the heat is limited by a critical internal temperature. J. Appl. Physiol. 89, 799-806. ( 10.1152/jappl.2000.89.2.799) [DOI] [PubMed] [Google Scholar]
- 4.Tan CL, Knight ZA. 2018. Regulation of body temperature by the nervous system. Neuron 98, 31-48. ( 10.1016/j.neuron.2018.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemingway A, Price WM. 1968. The autonomic nervous system and regulation of body temperature. Anesthesiology 29, 693-701. ( 10.1097/00000542-196807000-00012) [DOI] [PubMed] [Google Scholar]
- 6.Liedtke WB. 2017. Deconstructing mammalian thermoregulation. Proc. Natl Acad. Sci. USA 114, 1765-1767. ( 10.1073/pnas.1620579114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammel HT. 1968. Regulation of internal body temperature. Annu. Rev. Physiol. 30, 641-710. ( 10.1146/annurev.ph.30.030168.003233) [DOI] [PubMed] [Google Scholar]
- 8.Liu JS, Wang DH, Sun RY. 2004. Metabolism and thermoregulation in three species of rodent from northeastern China. J. Therm. Biol 29, 177-183. ( 10.1016/j.jtherbio.2004.02.003) [DOI] [Google Scholar]
- 9.Phillips PK, Heath JE. 1992. Heat exchange by the pinna of the african elephant (Loxodonta africana). Comp. Biochem. Physiol. Part A 101, 693-699. ( 10.1016/0300-9629(92)90345-Q) [DOI] [PubMed] [Google Scholar]
- 10.Conley KE, Porter WP. 1985. Heat loss regulation: role of appendages and torso in the deer mouse and the white rabbit. J. Comp. Physiol. B 155, 423-431. ( 10.1007/bf00684671) [DOI] [PubMed] [Google Scholar]
- 11.Porter WR, Witmer LM. 2020. Vascular patterns in the heads of dinosaurs: evidence for blood vessels, sites of thermal exchange, and their role in physiological thermoregulatory strategies. Anatomical Rec. 303, 1075-1103. ( 10.1002/ar.24234) [DOI] [PubMed] [Google Scholar]
- 12.Khamas WA, Smodlaka H, Leach-Robinson J, Palmer L. 2012. Skin histology and its role in heat dissipation in three pinniped species. Acta Vet. Scand. 54, 46. ( 10.1186/1751-0147-54-46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankenson FC, Marx JO, Gordon CJ, David JM. 2018. Effects of rodent thermoregulation on animal models in the research environment. Comp. Med. 68, 425-438. ( 10.30802/AALAS-CM-18-000049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen K. 1962. Heat exchange through the muskrat tail. Evidence for vasodilator nerves to the skin. Acta Physiol. Scand. 55, 160-169. ( 10.1111/j.1748-1716.1962.tb02428.x) [DOI] [PubMed] [Google Scholar]
- 15.Gordon CJ. 2017. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol. Behav. 179, 55-66. ( 10.1016/j.physbeh.2017.05.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant RT. 1963. Vasodilation and body warming in the rat. J. Physiol. 167, 311-317. ( 10.1113/jphysiol.1963.sp007151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young AA, Dawson NJ. 1982. Evidence for on-off control of heat dissipation from the tail of the rat. Can. J. Physiol. Pharmacol. 60, 392-398. ( 10.1139/y82-057) [DOI] [PubMed] [Google Scholar]
- 18.Sand CA, Starr A, Nandi M, Grant AD. 2015. Blockade or deletion of transient receptor potential vanilloid 4 (TRPV4) is not protective in a murine model of sepsis. F1000Res 4, 93. ( 10.12688/f1000research.6298.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung M, et al. 2017. Discovery of GSK2193874: an orally active, potent, and selective blocker of transient receptor potential vanilloid 4. ACS Med. Chem. Lett. 8, 549-554. ( 10.1021/acsmedchemlett.7b00094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon AH, et al. 2020. Pharmacologic inhibition of transient receptor channel vanilloid 4 attenuates abdominal aortic aneurysm formation. FASEB J. 34, 9787-9801. ( 10.1096/fj.202000251R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorneloe KS, Cheung M, Holt DA, Willette RN. 2017. Properties of the trpv4 agonist GSK1016790A and the TRPV4 antagonist GSK2193874. Physiol. Rev. 97, 1231-1232. ( 10.1152/physrev.00019.2017) [DOI] [PubMed] [Google Scholar]
- 22.Zhu YH, Pei ZM. 2018. GSK2193874 treatment at heatstroke onset reduced cell apoptosis in heatstroke mice. Cell. Mol. Biol. (Noisy-le-grand) 64, 36-42. ( 10.14715/cmb/2018.64.7.7) [DOI] [PubMed] [Google Scholar]
- 23.Thorneloe KS, et al. 2012. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 4, 159ra148. ( 10.1126/scitranslmed.3004276) [DOI] [PubMed] [Google Scholar]
- 24.Li M, Fang XZ, Zheng YF, Xie YB, Ma XD, Liu XT, Xia Y, Shao DH. 2019. Transient receptor potential vanilloid 4 is a critical mediator in LPS mediated inflammation by mediating calcineurin/NFATc3 signaling. Biochem. Biophys. Res. Commun. 513, 1005-1012. ( 10.1016/j.bbrc.2019.04.020) [DOI] [PubMed] [Google Scholar]
- 25.Dias FC, Alves VS, Matias DO, Figueiredo CP, Miranda ALP, Passos GF, Costa R. 2019. The selective TRPV4 channel antagonist HC-067047 attenuates mechanical allodynia in diabetic mice. Eur. J. Pharmacol. 856, 172408. ( 10.1016/j.ejphar.2019.172408) [DOI] [PubMed] [Google Scholar]
- 26.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. 2002. Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem. 277, 33 704-33 710. ( 10.1074/jbc.m204828200) [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, et al. 2002. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13 569-13 577. ( 10.1074/jbc.M200062200) [DOI] [PubMed] [Google Scholar]
- 28.Chung MK, Lee H, Caterina MJ. 2003. Warm temperatures activate TRPV4 in.mouse 308 keratinocytes. J. Biol. Chem. 278, 32 037-32 046. ( 10.1074/jbc.M303251200) [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. 2002. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem. 277, 47 044-47 051. ( 10.1074/jbc.M208277200) [DOI] [PubMed] [Google Scholar]
- 30.Shibasaki K, Suzuki M, Mizuno A, Tominaga M. 2007. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J. Neurosci. 27, 1566-1575. ( 10.1523/jneurosci.4284-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. 2009. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 297, H1096-H1102. ( 10.1152/ajpheart.00241.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell WB, Fleming I. 2010. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 459, 881-895. ( 10.1007/s00424-010-0804-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filosa JA, Yao X, Rath G. 2013. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 61, 113-119. ( 10.1097/FJC.0b013e318279ba42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pankey EA, Zsombok A, Lasker GF, Kadowitz PJ. 2014. Analysis of responses to the TRPV4 agonist GSK1016790A in the pulmonary vascular bed of the intact-chest rat. Am. J. Physiol. Heart Circ. Physiol. 306, H33-H40. ( 10.1152/ajpheart.00303.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki M, Mizuno A, Kodaira K, Imai M. 2003. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22 664-22 668. ( 10.1074/jbc.M302561200) [DOI] [PubMed] [Google Scholar]
- 36.Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. 2004. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J. Biol. Chem. 279, 35 133-35 138. ( 10.1074/jbc.M406260200) [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. 2005. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 25, 1304-1310. ( 10.1523/JNEUROSCI.4745.04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vizin RC, da Scarpellini CS, Ishikawa DT, Correa GM, de Souza CO, Gargaglioni LH, Carrettiero DC, Bicego KC, Almeida MC. 2015. TRPV4 activates autonomic and behavioural warmth–defence responses in Wistar rats. Acta Physiol. (Oxf.) 214, 275-289. ( 10.1111/apha.12477) [DOI] [PubMed] [Google Scholar]
- 39.Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. 2008. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am. J. Hypertens. 21, 1288-1291. ( 10.1038/ajh.2008.301) [DOI] [PubMed] [Google Scholar]
- 40.Barbieri R, Matten EC, Alabi AA, Brown EN. 2005. A point-process model of human heartbeat intervals: new definitions of heart rate and heart rate variability. Am. J. Physiol. Heart Circ. Physiol. 288, H424-H435. ( 10.1152/ajpheart.00482.2003) [DOI] [PubMed] [Google Scholar]
- 41.Malik M. 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043-1065. ( 10.1161/01.CIR.93.5.1043) [DOI] [PubMed] [Google Scholar]
- 42.Kim JW, Seok HS, Shin H. 2021. Is ultra-short-term heart rate variability valid in non-static conditions? Front. Physiol. 12, 596060. ( 10.3389/fphys.2021.596060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton RM, McKechnie PS, Macfarlane PW. 2004. Can cardiac vagal tone be estimated from the 10-second ECG? Int. J. Cardiol. 95, 109-115. ( 10.1016/j.ijcard.2003.07.005) [DOI] [PubMed] [Google Scholar]
- 44.Tochinai R, Sekizawa SI, Kobayashi H, Kuwahara M. 2021. Autonomic nervous activity in rats can be evaluated by blood photoplethysmography-derived pulse rate variability analysis. Transl. Reg. Sci. 3, 17-21. ( 10.33611/trs.2021-001) [DOI] [Google Scholar]
- 45.Fleisher LA, Frank SM, Sessler DI, Cheng C, Matsukawa T, Vannier CA. 1996. Thermoregulation and heart rate variability. Clin. Sci. (Lond) 90, 97-103. ( 10.1042/cs0900097) [DOI] [PubMed] [Google Scholar]
- 46.McSharry PE, Clifford GD, Tarassenko L, Smith LA. 2003. A dynamical model for generating synthetic electrocardiogram signals. IEEE Trans. Biomed. Eng. 50, 289-294. ( 10.1109/tbme.2003.808805) [DOI] [PubMed] [Google Scholar]
- 47.Lomb NR. 1976. Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 39, 447. ( 10.1007/BF00648343) [DOI] [Google Scholar]
- 48.Scargle JD. 1982. Studies in astronomical time series analysis. II. Statistical aspects of spectral analysis of unevenly spaced data. Astrophys. J. 263, 835-853. ( 10.1086/160554) [DOI] [Google Scholar]
- 49.White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. 2016. TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev. 96, 911-973. ( 10.1152/physrev.00016.2015) [DOI] [PubMed] [Google Scholar]
- 50.Pires W, et al. 2010. Sinoaortic denervation prevents enhanced heat loss induced by central cholinergic stimulation during physical exercise. Brain Res. 1366, 120-128. ( 10.1016/j.brainres.2010.09.110) [DOI] [PubMed] [Google Scholar]
- 51.Zhang D, Ando M, Yamasaki F, Sato T. 2003. Carotid-sinus baroreflex modulation of core and skin temperatures in rats: an open-loop approach. Jpn J. Physiol. 53, 461-466. ( 10.2170/jjphysiol.53.461) [DOI] [PubMed] [Google Scholar]
- 52.Feetham CH, O'Brien F, Barrett-Jolley R. 2018. Ion channels in the paraventricular hypothalamic nucleus (PVN); emerging diversity and functional roles. Front. Physiol. 9, 760. ( 10.3389/fphys.2018.00760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alawi KM, Aubdool AA, Liang L, Wilde E, Vepa A, Psefteli MP, Brain SD, Keeble JE. 2015. The sympathetic nervous system is controlled by transient receptor potential vanilloid 1 in the regulation of body temperature. FASEB J. 29, 4285-4298. ( 10.1096/fj.15-272526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aubdool AA, et al. 2014. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 5, 5732. ( 10.1038/ncomms6732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feetham CH, Nunn N, Barrett-Jolley R. 2015. The depressor response to intracerebroventricular hypotonic saline is sensitive to TRPV4 antagonist RN1734. Front. Pharmacol. 6, 83. ( 10.3389/fphar.2015.00083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feetham CH, Nunn N, Lewis R, Dart C, Barrett-Jolley R. 2015. TRPV4 and KCa ion channels functionally couple as osmosensors in the paraventricular nucleus. Br. J. Pharmacol. 172, 1753-1768. ( 10.1111/bph.13023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thayer JF, Yamamoto SS, Brosschot JF. 2010. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 141, 122-131. ( 10.1016/j.ijcard.2009.09.543) [DOI] [PubMed] [Google Scholar]
- 58.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. 2000. Phenotypic screening for heart rate variability in the mouse. Am. J. Physiol. Heart Circ. Physiol. 279, H733-H740. ( 10.1152/ajpheart.2000.279.2.H733) [DOI] [PubMed] [Google Scholar]
- 59.Elghozi JL, Girard A, Laude D. 2001. Effects of drugs on the autonomic control of short-term heart rate variability. Auton. Neurosci. 90, 116-121. ( 10.1016/S1566-0702(01)00276-4) [DOI] [PubMed] [Google Scholar]
- 60.Thomsen MB, Volders PG, Beekman JD, Matz J, Vos MA. 2006. Beat-to-beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J. Am. Coll. Cardiol. 48, 1268-1276. ( 10.1016/j.jacc.2006.05.048) [DOI] [PubMed] [Google Scholar]
- 61.Thireau J, Zhang BL, Poisson D, Babuty D. 2008. Heart rate variability in mice: a theoretical and practical guide. Exp. Physiol. 93, 83-94. ( 10.1113/expphysiol.2007.040733) [DOI] [PubMed] [Google Scholar]
- 62.Nunn N, Feetham CH, Martin J, Barrett-Jolley R, Plagge A. 2013. Elevated blood pressure, heart rate and body temperature in mice lacking the XLαs protein of the Gnas locus is due to increased sympathetic tone. Exp. Physiol. 98, 1432-1445. ( 10.1113/expphysiol.2013.073064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shannon JR, Jordan J, Black BK, Costa F, Robertson D. 1998. Uncoupling of the baroreflex by N(N)-cholinergic blockade in dissecting the components of cardiovascular regulation. Hypertension 32, 101-107. ( 10.1161/01.hyp.32.1.101) [DOI] [PubMed] [Google Scholar]
- 64.O'Brien F, Staunton CA, Barrett-Jolley R. 2022. Data from: Systemic application of the transient receptor potential vanilloid-type 4 antagonist GSK2193874 induces tail vasodilation in a mouse model of thermoregulation. Dryad Digital Repository. ( 10.5061/dryad.1rn8pk0vq) [DOI]
- 65.O'Brien F, Staunton CA, Barrett-Jolley R. 2022. Systemic application of the transient receptor potential vanilloidtype 4 antagonist GSK2193874 induces tail vasodilation in a mouse model of thermoregulation. Figshare. ( 10.6084/m9.figshare.c.6026164) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- O'Brien F, Staunton CA, Barrett-Jolley R. 2022. Data from: Systemic application of the transient receptor potential vanilloid-type 4 antagonist GSK2193874 induces tail vasodilation in a mouse model of thermoregulation. Dryad Digital Repository. ( 10.5061/dryad.1rn8pk0vq) [DOI]
- O'Brien F, Staunton CA, Barrett-Jolley R. 2022. Systemic application of the transient receptor potential vanilloidtype 4 antagonist GSK2193874 induces tail vasodilation in a mouse model of thermoregulation. Figshare. ( 10.6084/m9.figshare.c.6026164) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.1rn8pk0vq [64].
The data are provided in the electronic supplementary material [65].