Abstract
The Cretaceous–Palaeogene (K–Pg) mass extinction was responsible for the destruction of global ecosystems and loss of approximately three-quarters of species diversity 66 million years ago. Large-bodied land vertebrates suffered high extinction rates, whereas small-bodied vertebrates living in freshwater ecosystems were buffered from the worst effects. Here, we report a new species of large-bodied (1.4–1.5 m) gar based on a complete skeleton from the Williston Basin of North America. The new species was recovered 18 cm above the K–Pg boundary, making it one of the oldest articulated vertebrate fossils from the Cenozoic. The presence of this freshwater macropredator approximately 1.5–2.5 thousand years after the asteroid impact suggests the rapid recovery and reassembly of North American freshwater food webs and ecosystems after the mass extinction.
Keywords: gar, K–Pg, Lilliput effect, Lepisosteidae, Fish
1. Introduction
The Cretaceous–Palaeogene (K–Pg) extinction is the most recent mass extinction in Earth's history and instigated a complete restructuring of terrestrial ecosystems to mammal-dominated communities [1,2]. This event was responsible for the loss of 70–80% of biodiversity [3–5], including the infamous demise of the non-avian dinosaurs [6,7]. At the same time, this extinction opened numerous ecological opportunities and is associated with adaptive radiations and prolonged diversifications in major extant lineages like neoavian birds [8–10], placental mammals [1,11], snakes [12,13], spiny-rayed fishes [14,15] and flowering plants [16]. Many lineages also experienced a pronounced decrease in average body size across the K–Pg boundary, a phenomenon known as the Lilliput effect [17–19].
The Lilliput effect is difficult to assess for any extinction because it requires an excellent fossil record that brackets the extinction event [19]. For this reason, the Lilliput effect has been best documented in marine groups like planktic foraminifera [20], mollusks [21] and lamniform sharks [22], but has also been reported in terrestrial environments [23]. While the incomplete terrestrial fossil record for many clades currently precludes an analysis of the Lilliput effect across end-Cretaceous ecosystems, it is well documented that large-bodied species go extinct at the K–Pg (e.g. [24–27]), leaving the earliest Palaeocene largely devoid of large-bodied animals. Recent discoveries document the tempo of increasing body size in the aftermath of the end-Cretaceous extinction [28]. Because many large-bodied animals require established food webs, body size is an important variable in assessing overall ecosystem health in the wake of mass disasters like the K–Pg event [29].
Here, we describe a nearly complete skeleton (figures 1 and 2) that represents a new, large-bodied species of fish in the clade Lepisosteidae. This lineage includes the seven extant species of gars and is characterized by low species diversity and morphological conservatism [30–33]. The new species, †Atractosteus grandei sp. nov., is among the largest known gars and holosteans, reaching a length of approximately 1.5 m. Yet, †A. grandei was recovered 18 cm above the K–Pg boundary and lived an estimated 1500–2500 years after the asteroid impact (see electronic supplementary material, table S1 for list of ages using different age models). The exceptional size of †A. grandei contrasts with the small size of most terrestrial vertebrates in the immediate aftermath of the K–Pg mass extinction and thus provides insights into the tempo of recovery and reassembly of Cenozoic North American freshwater ecosystems.
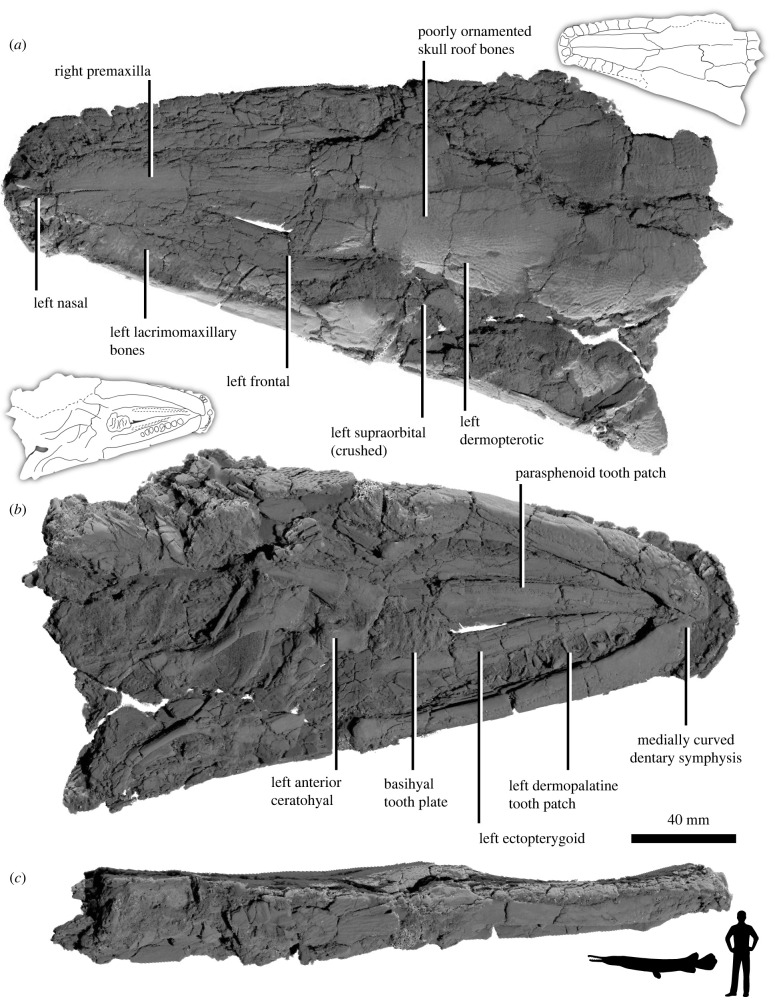
Figure 1.
Anatomy of †Atractosteus grandei sp. nov. Skull in (a) dorsal, (b) ventral and (c) lateral views. Inset shows size compared to a 1.85 m tall man.
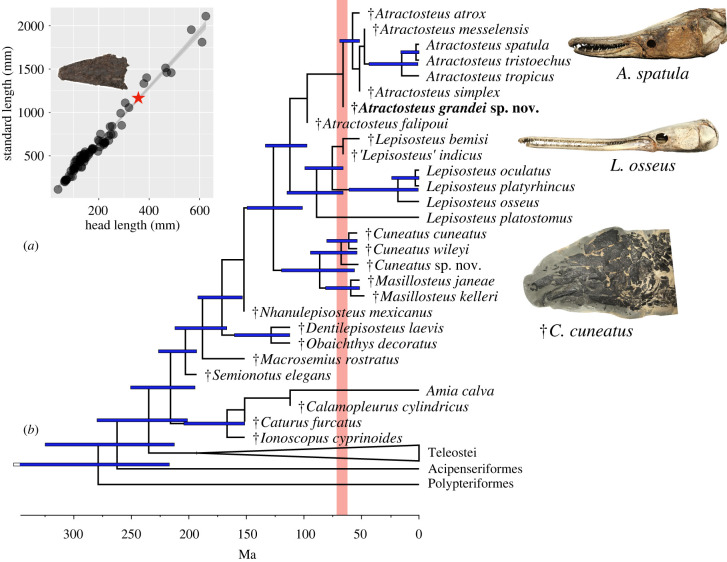
Figure 2.
Size and phylogenetic relationships of †Atractosteus grandei sp. nov. (a) Linear regression of HL against SL in a sample of gars, with red star indicating the position on the regression length corresponding to the length of the skull of †A. grandei sp. nov. (b) Time-calibrated maximum clade credibility tree from analysis of the morphological matrix in BEAST 2.6.6. Red line denotes the position of the K–Pg boundary/recovery period. Ma = millions of years.
2. Methods
(a) . Computed tomography
The skull of the holotype of †Atractosteus grandei, Denver Museum of Nature & Science (DMNH) EPV.128289, was scanned at the High-Resolution X-ray CT Facility of the University of Texas by Matthew Colbert. Scan parameters were: North Star Imaging scanner; Fein Focus High Power source, 200 kV, 0.17 mA, brass filter, source to object 378.661 mm, source to detector 733.437 mm, isometric voxel size = 103.5 µm, total slices = 3510. Resolution of the data was to 0.1035 mm. Sixteen-bit TIFF stacks of raw scan data were processed in VGStudio MAX v. 3.5.
(b) . Length estimation
In order to compare the dimensions of the new Atractosteus to other lepisosteids, we collected measurements from the literature for the skull lengths of all seven extant and five crown-group fossil gar species (N = 79 individuals; [34]). Qualitative comparisons of meristic proportions have been used to estimate the sizes of gars (see [34]). A linear regression of head length (HL) versus standard length (SL) for gars produced the following equation relating HL to SL in crown Lepisosteidae: SL = 12.70393 + 3.19394*HL (adjusted R2 = 0.9775, F = 3431 on 1, 78 degrees of freedom, p < 2.2 × 10−16). A table with these data is included in the electronic supplementary material.
(c) . Phylogenetic analyses
We assessed the phylogenetic position of †Atractosteus grandei using the matrix of Grande [34] with the updates of [35] and [36]. This matrix consists of 26 taxa coded for 103 morphological characters and is included in the electronic supplementary material, data. Characters were treated as unordered and no implied weighting was used. Parsimony analysis was conducted in TNT v. 1.5 [37] with an initial Wagner search over 10 replicates with default parameters for ratchet, tree fuse, tree drift and sectorial search, followed by a round of traditional bisection–reconnection branch swapping over 100 000 trees. We also conducted a Bayesian analysis using the Fossilized Birth–Death model [38] and a relaxed lognormal clock in BEAST 2.6.6 [39], with dates based on the literature [34,36]. The analysis was run for 10 million generations with a 1.0 × 107 pre-burnin, and convergence was checked using v. Tracer 1.7.2 [40]. Trees were pooled and combined into maximum clade credibility topology in TreeAnnotator 2.6.4 with a 10% burnin [39].
3. Systematic palaeontology
Holostei [41]
Ginglymodi [42]
Lepisosteidae [43]
Atractosteus Rafinesque 1820
†Atractosteus grandei sp. nov.
-
(a)
Type specimen—DMNH EPV.128289, articulated skull and partial associated skeleton (figure 1).
-
(b)
Type locality and age—DMNH loc. 7251, Cedar Creek Anticline in the Williston Basin, Mud Buttes study area, Bowman County, North Dakota, USA, 29 km southeast of Marmarth North Dakota (qualified researchers can obtain more detailed locality information from the DMNH); Fort Union Formation, Danian, early Palaeocene; 18 cm above the K–Pg boundary clay and an estimated approximately 1500–2500 years after the K–Pg using different age models (see electronic supplementary material, table S1 for age derived from different age models). The skeleton was found at the base of a 30 cm thick grey, blocky siltstone interpreted to be a ponded water deposit (See electronic supplementary material for more geological and palaeoenvironmental details).
-
(c)
Etymology—The specific epithet, grandei, in honour of Lance Grande for his contributions to the study of holostean fishes.
-
(d)
Diagnosis—Distinguished from other Atractosteus species by the following: poorly ornamented skull roof bones; anterior triangular vomerine tooth plate with reduced fangs; vomerine teeth rows mediolaterally restricted to single line; low number of ectopterygoid teeth (nine instead of 12–13 in other species).
-
(e)
Remarks—†A. grandei is assignable to Atractosteus among lepisosteids based on the medially curved symphyses of the dentary and the shape of the vomerine heads approximately forming a triangle (figure 1; [34]). The skull is robust, resembling the largest two Atractosteus species A. spatula and †A. atrox and an unnamed form from the Cretaceous of Morocco [34,44] rather than A. tristoechus, A. tropicus, †A. simplex, †A. messelensis and †A. falipoui. The skull of †A. grandei also matches the crania of individuals of the largest extant species (A. spatula) in size and was likely approximately 400 mm when complete. This makes †A. grandei one of the largest extinct lepisosteids and holosteans currently recognized [34,45].
The poorly ornamented skull roof is composed of the paired nasals, frontals, parietals, dermopterotics and six extrascapulars. These bones are closely comparable to the same elements in other species of Atractosteus, and the frontals, premaxillae and parietals lack the elongation seen in longirostrine forms like Lepisosteus osseus, †'Lepisosteus’ indicus and †Herreraichthys coahuilensis [34,46]. The premaxillae and frontals are also not heavily abbreviated as in †Masillosteus spp. and †Cuneatus spp. [34,36]. The frontals compose the largest portion of this region and are slightly ornamented with ridges radiating outward from their centres onto the dermopterotics and parietals (figure 1a), as in Atractosteus spp. and all species of Lepisosteus except L. osseus [34]. Ornamentation on the premaxillae, parietals, dermopterotics and extrascapulars also primarily consists of ridges radiating from the ossification centres of each of these bones.
Anteriorly, small, subrectangular elements identifiable as the nasals are present. Laterally, there are seven lacrimomaxillary bones, which become progressively more elongated towards the posterior end of the rostrum. This is the condition in Atractosteus and Lepisosteus [34]. Due to the crushed nature of the posterior skull, the morphology of the lacrimals, suborbitals and other orbital rim bones are largely indiscernible.
Ventrally, the palate is preserved in exquisite detail (figure 1b). The widened vomers are lined with single plates of teeth with parallel margins. Anteriorly, these bear a triangular platform with fangs as in other Atractosteus [34]. However, the fangs are reduced in †A. grandei relative to most other Atractosteus, particularly A. spatula [34]. The ectopterygoids are widened and each bear nine large teeth, compared to 12–13 in other species of Atractosteus [34]. The mandibles show the plesiomorphic condition among lepisosteids retained in Atractosteus but absent in Lepisosteus wherein the dentary symphyses curve medially to contact the opposing element [34]. The basihyal tooth plate is positioned between the mandibles and appears as a rugose, elongated, rectangular element as in other lepisosteids [34]. All teeth exhibit the plicidentine condition.
In addition to the nearly complete articulated skull and lower jaws, the holotype of †A. grandei also includes a partial skeleton consisting of mostly articulated, but incomplete precaudal vertebrae (N = 21), partially dissociated fins, and abundant rhomboid-shaped ganoid scales. The anatomy of the body is difficult to examine, but the vertebrae show the opisthocoelus condition characteristic of the Lepisosteidae [34]. Additional description of †A. grandei is included in the electronic supplementary material.
(a) . Size estimation and phylogenetic analysis
The equation of the regression line relating HL to SL gave an estimated SL of 1.12 m for †A. grandei (HL = 346.5 mm). This SL, which implies a total length of 1.4–1.5 m (see [34]), is larger than all other fossil species of gars with the exception of the largest known gars in the species †Atractosteus atrox from the Eocene Green River Formation and all extant gars besides A. spatula and L. osseus [34]. This SL greatly exceeds those of other species of Lepisosteus and Atractosteus, as well as those of cuneatin and masillostein gars [34].
In the phylogenetic analysis of gar interrelationships using parsimony, †A. grandei nested within a polytomy formed by all species of Atractosteus, a relationship supported by low bootstrap values (35). This low level of support is probably due to the lack of information on the postcranial anatomy of A. grandei, which reduces the total number of characters coded for this taxon. Nonetheless, the position of this taxon as a species of Atractosteus is consistent across all most parsimonious trees. Bayesian phylogenetic analysis also found †A. grandei within Atractosteus as one node closer to the crown group than the Cenomanian species †A. falipoui [34].
4. Discussion
The discovery of †Atractosteus grandei directly above the K–Pg boundary provides a wealth of new information about the tempo of ecosystem change in the aftermath of the impact and the early evolution of crown-group gars. First, as the oldest definite member of the gar crown group from the Americas (approx. 5 million years older than Atractosteus sp. from the Paskapoo Formation; [34]), †A. grandei conclusively shows that crown lepisosteids have existed in North American river systems since the start of the Cenozoic. Second, †A. grandei displays the oldest case of large body size (greater than 1 m) in the Lepisosteidae.
The preservation of †A. grandei 18 cm (approx. 2–3 thousand years; see electronic supplementary material, table S1) above the K–Pg boundary in the Williston Basin of North Dakota shows that freshwater ecosystems able to support large, approximately 1.5 m-long apex predators like large gars either persisted across the K–Pg boundary or rapidly rebounded within a few thousand years after the K–Pg mass extinction. Freshwater ecosystems saw dramatically lower levels of extinction during the K–Pg event compared to that of marine ecosystems—10% in freshwater ecosystems [47] compared to 50% in marine ecosystems [48]. More recent studies have used high-resolution chronostratigraphic frameworks to analyse the pace of mammalian diversity [27] and maximum mammalian body mass through the first million years of the Cenozoic [28]. Larger (20 kg) mammals do not appear until approximately 300 kyr after the K–Pg mass extinction in the Denver Basin [28]. †A. grandei, living within thousands of years after the K–Pg, would have coexisted with small-bodied (body size less than 1 kg) mammalian ‘disaster’ faunas (e.g. [27]) and depauperate forests that represented an early wave of ecosystem succession in the early Palaeocene [49–53]. With an estimated total length of 1.4–1.5 m, the new gar is therefore one of the largest freshwater vertebrates to appear on the North American landscape directly after the impact. Further, †A. grandei evinces that some clades of freshwater vertebrates show little evidence of a heavy degree of size reduction consistent with an ecosystem-wide Lilliput effect.
In the thousands to hundreds of thousands of years following the extinction, large predatory gars like †A. grandei would have coexisted with a handful of relatively large, freshwater vertebrates, including the giant soft-shelled turtle Axestemys (approx. 1 m long carapace; [28,54,55]) and large-bodied salamanders [56]. The presence of larger vertebrates from disparate clades in freshwater ecosystems supports the idea that freshwater ecosystems functioned as refugia in the wake of the K–Pg extinction [47]. At the same time, the physiological agility of gars cannot be ignored. For example, extant lepisosteids can breathe air, a trait which allows gars to survive in deoxygenated water (e.g. [34,57]). In addition, extant species of the genus Atractosteus are able to tolerate a wide variety of salinities [58]. Finally, all extant gars are generalist predators (e.g. [34]). This suite of features undoubtedly helped gars persist and flourish as ‘disaster taxa’ in the wake of the destruction caused by the asteroid impact, helping this lineage establish a 65-million-year dynasty in North American river systems.
Acknowledgements
We thank Matthew Colbert at the University of Texas High-Resolution X-Ray CT Facility for scanning the skull of the holotype of †Atractosteus grandei, and Bhart Anjan-Bhullar at Yale University for access to the VGStudio Max software used to render scans. We thank Andrew Bentley for providing access to comparative material in the collections of the University of Kansas Museum of Natural History. We thank Tom Tucker for the preparation of the specimen. We thank R. Wicker for photography of the specimen. Y. Rollot and W. Bullard helped T.R.L. collect the specimen. We also thank the editor and two anonymous reviewers for their comments, which greatly improved the manuscript.
Data accessibility
All associated data are in the manuscript and electronic supplementary material [59].
Authors' contributions
C.D.B.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft and writing—review and editing; T.R.L.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.O'Leary MA, et al. 2013. The placental mammal ancestor and the post-K–Pg radiation of placentals. Science 339, 662-667. ( 10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 2.Hull P. 2015. Life in the aftermath of mass extinctions. Curr. Biol. 25, R941-R952. ( 10.1016/j.cub.2015.08.053) [DOI] [PubMed] [Google Scholar]
- 3.Raup DM, Sepkoski JJ Jr. 1982. Mass extinctions in the marine fossil record. Science 215, 1501-1503. ( 10.1126/science.215.4539.1501) [DOI] [PubMed] [Google Scholar]
- 4.Jablonski D, Raup DM. 1995. Selectivity of end-Cretaceous marine bivalve extinctions. Science 268, 389-391. ( 10.1126/science.11536722) [DOI] [PubMed] [Google Scholar]
- 5.Jablonski D. 2005. Mass extinctions and macroevolution. Paleobiology 31, 192-210. ( 10.1666/0094-8373(2005)031[0192:MEAM]2.0.CO;2) [DOI] [Google Scholar]
- 6.Brusatte SL, et al. 2015. The extinction of the dinosaurs. Biol. Rev. 90, 628-642. ( 10.1111/brv.12128) [DOI] [PubMed] [Google Scholar]
- 7.Chiarenza AA, et al. 2020. Asteroid impact, not volcanism, caused the end-Cretaceous dinosaur extinction. Proc. Natl Acad. Sci. USA 117, 17 084-17 093. ( 10.1073/pnas.2006087117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvis ED, et al. 2014. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320-1331. ( 10.1126/science.1253451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prum RO, et al. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569-573. ( 10.1038/nature15697) [DOI] [PubMed] [Google Scholar]
- 10.Field DJ, Bercovici A, Berv JS, Dunn R, Fastovsky DE, Lyson TR, Vadja V, Gauthier JA. 2018. Early evolution of modern birds structured by global forest collapse at the end-Cretaceous mass extinction. Curr. Biol. 28, 1825-1831. ( 10.1016/j.cub.2018.04.062) [DOI] [PubMed] [Google Scholar]
- 11.Álvarez-Carretero S, et al. 2022. A species-level timeline of mammal evolution integrating phylogenomic data. Nature 602, 263-267. ( 10.1038/s41586-021-04341-1) [DOI] [PubMed] [Google Scholar]
- 12.Grundler MC, Rabosky DL. 2021. Rapid increase in snake dietary diversity and complexity following the end-Cretaceous mass extinction. PLoS Biol. 19, e3001414. ( 10.1371/journal.pbio.3001414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein CG, et al. 2021. Evolution and dispersal of snakes across the Cretaceous–Paleogene mass extinction. Nat. Commun. 12, 1-9. ( 10.1038/s41467-020-20314-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman M. 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc. R. Soc. B 277, 1675-1683. ( 10.1098/rspb.2009.2177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghezelayagh A, et al. 2022 Prolonged morphological expansion of spiny-rayed fishes following the end-Cretaceous. bioRxiv 2021.07.12.452083. ( 10.1101/2021.07.12.452083) [DOI] [PubMed] [Google Scholar]
- 16.Magallón S, Sánchez-Reyes LL, Gómez-Acevedo SL. 2019. Thirty clues to the exceptional diversification of flowering plants. Ann. Bot. 123, 491-503. ( 10.1093/aob/mcy182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urbanek A. 1993. Biotic crises in the history of Upper Silurian graptoloids: a palaeobiological model. Hist. Biol. 7, 29-50. ( 10.1080/10292389309380442) [DOI] [Google Scholar]
- 18.Twitchett RJ. 2007. The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 132-144. ( 10.1016/j.palaeo.2006.11.038) [DOI] [Google Scholar]
- 19.Berv JS, Field DJ. 2018. Genomic signature of an avian Lilliput effect across the K–Pg extinction. Syst. Biol. 67, 1-13. ( 10.1093/sysbio/syx064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller G, Abramovich S. 2009. Lilliput effect in late Maastrichtian planktic foraminifera: response to environmental stress. Palaeogeogr. Palaeoclimatol. Palaeoecol. 510, 47-62. ( 10.1016/j.palaeo.2009.08.029) [DOI] [Google Scholar]
- 21.Łaska W, Rodríguez-Tovar FJ, Uchman A. 2017. Evaluating macrobenthic response to the Cretaceous–Palaeogene event: a high-resolution ichnological approach at the Agost section (SE Spain). Cretaceous Res. 70, 96-110. ( 10.1016/j.cretres.2016.10.003) [DOI] [Google Scholar]
- 22.Belben RA, Underwood CJ, Johanson Z, Twitchett RJ. 2017. Ecological impact of the end-Cretaceous extinction on lamniform sharks. PLoS ONE 12, e0178294. ( 10.1371/journal.pone.0178294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiest LA, Lukens WE, Peppe DJ, Driese SG, Tubbs J. 2018. Terrestrial evidence for the Lilliput effect across the Cretaceous–Paleogene (K–Pg) boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 491, 161-169. ( 10.1016/j.palaeo.2017.12.005) [DOI] [Google Scholar]
- 24.Archibald JD, Bryant LJ. 1990. Differential Cretaceous–Tertiary extinction of nonmarine vertebrates; evidence from northeastern Montana. Geol. Soc. Am. Special Paper 487, 549-562. ( 10.1130/SPE247-p549) [DOI] [Google Scholar]
- 25.MacLeod N, et al. 1997. The Cretaceous–Tertiary biotic transition. J. Geol. Soc. 154, 265-292. ( 10.1144/gsjgs.154.2.0265) [DOI] [Google Scholar]
- 26.Archibald JD. 2011. Extinction and radiation: how the fall of dinosaurs led to the rise of mammals. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- 27.Wilson GP. 2013. Mammals across the K/Pg boundary in northeastern Montana, USA: dental morphology and body-size patterns reveal extinction selectivity and immigrant-fueled ecospace filling. Paleobiology 39, 429-469. [Google Scholar]
- 28.Lyson TR, et al. 2019. Exceptional continental record of biotic recovery after the Cretaceous–Paleogene mass extinction. Science 366, 977-983. ( 10.1126/science.aay2268) [DOI] [PubMed] [Google Scholar]
- 29.Roopnarine PD. 2006. Extinction cascades and catastrophe in ancient food webs. Paleobiology 32, 1-19. ( 10.1666/05008.1) [DOI] [Google Scholar]
- 30.Darwin C. 1859. On the origin of species by means of natural selection, or, The preservation of favoured races in the struggle for life. London, UK: J. Murray. [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley SM. 1975. A theory of evolution above the species level. Proc. Natl Acad. Sci. USA 72, 646-650. ( 10.1073/pnas.72.2.646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiley EO, Schultze HP. 1984. Family Lepisosteidae (gars) as living fossils. In Living fossils, pp. 160-165. New York, NY: Berlin, Germany: Springer. [Google Scholar]
- 33.Lidgard S, Love AC. 2018. Rethinking living fossils. Bioscience 68, 760-770. ( 10.1093/biosci/biy084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grande L. 2010. An empirical synthetic pattern study of gars (Lepisosteiformes) and closely related species, based mostly on skeletal anatomy. The resurrection of Holostei. Copeia 10, 1-871. [Google Scholar]
- 35.Brito PM, Alvarado-Ortega J, Meunier FJ. 2017. Earliest known lepisosteoid extends the range of anatomically modern gars to the Late Jurassic. Sci. Rep. 7, 1-8. ( 10.1038/s41598-017-17984-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brownstein CD. 2022. Unappreciated Cenozoic ecomorphological diversification of stem gars revealed by a large new species. Acta Palaeontol. Polonica. [Google Scholar]
- 37.Goloboff PA, Catalano SA. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221-238. ( 10.1111/cla.12160) [DOI] [PubMed] [Google Scholar]
- 38.Gavryushkina A, Welch D, Stadler T, Drummond AJ. 2014. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comput. Biol. 10, e1003919. ( 10.1371/journal.pcbi.1003919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouckaert R, et al. 2019. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 15, e1006650. ( 10.1371/journal.pcbi.1006650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901-904. ( 10.1093/sysbio/syy032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller J. 1844. Über den Bau und die Grenzen der Ganoiden, und über das natürliche System der Fische. Bericht Akademie der Wissenschaften Berlin 1844, 416-422. [Google Scholar]
- 42.Cope ED. 1872. Observations on the systematic relations of the fishes. Proc. Am. Assoc. Adv. Sci. 20, 317-343. [Google Scholar]
- 43.Cuvier G. 1825. Recherches sur les ossemens fossils, our I’ on rétablit les characters des plusieurs animax don't les révolutions du globe ont détruit les espèces 3. Paris, France: G. Dufour et E. d'Ocagne. [Google Scholar]
- 44.Cooper SL, Martill DM, Beevor T, Gunn J. 2021. A large marine gar fish (Ginglymodi, Lepisosteiformes) from the Turonian Akrabou Formation of Asfla, Morocco. Cretaceous Res. 125, 104839. ( 10.1016/j.cretres.2021.104839) [DOI] [Google Scholar]
- 45.Grande L, Bemis WE. 1998. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. J. Vertebr. Paleontol. 18, 1-696. ( 10.1080/02724634.1998.10011114) [DOI] [Google Scholar]
- 46.Alvarado-Ortega J, Brito PM, Porras-Múzquiz HG, Mújica-Monroy IH. 2016. A Late Cretaceous marine long snout ‘pejelagarto’ fish (Lepisosteidae, Lepisosteini) from Múzquiz, Coahuila, northeastern Mexico. Cretaceous Res. 70, 19-28. ( 10.1016/j.cretres.2015.07.009) [DOI] [Google Scholar]
- 47.Sheehan PM, Fastovsky DE. 1992. Major extinctions of land-dwelling vertebrates at the Cretaceous–Tertiary boundary, eastern Montana. Geology 20, 556-560. () [DOI] [Google Scholar]
- 48.D'Hondt S. 2005. Consequences of the Cretaceous/Paleogene mass extinction for marine ecosystems. Annu. Rev. Ecol. Syst. 36, 295-317. ( 10.1146/annurev.ecolsys.35.021103.105715) [DOI] [Google Scholar]
- 49.Johnson KR. 2002. Megaflora of the Hell Creek and lower Fort Union Formations in the western Dakotas: vegetational response to climate change, the Cretaceous–Tertiary boundary event, and rapid marine transgression. Geol. Soc. Am. Special Paper 361, 329-392. ( 10.1130/0-8137-2361-2.329) [DOI] [Google Scholar]
- 50.Nichols DJ, Johnson KR. 2008. Plants and the KT boundary. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 51.Bercovici A, Pearson D, Nichols D, Wood J. 2009. Biostratigraphy of selected K/T boundary sections in southwestern North Dakota, USA: toward a refinement of palynological identification criteria. Cretaceous Res. 30, 632-658. ( 10.1016/j.cretres.2008.12.007) [DOI] [Google Scholar]
- 52.Peppe DJ. 2010. Megafloral change in the early and middle Paleocene in the Williston Basin, North Dakota, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 298, 224-234. ( 10.1016/j.palaeo.2010.09.027) [DOI] [Google Scholar]
- 53.Vajda V, Bercovici A. 2014. The global vegetation pattern across the Cretaceous–Paleogene mass extinction interval: a template for other extinction events. Glob. Planetary Change 122, 29-49. ( 10.1016/j.gloplacha.2014.07.014) [DOI] [Google Scholar]
- 54.Vitek NS. 2012. Giant fossil soft-shelled turtles of North America. Palaeontol. Electronica 15, 13A. ( 10.26879/299) [DOI] [Google Scholar]
- 55.Joyce WG, Brinkman DB, Lyson TR. 2019. A new species of trionychid turtle, Axestemys infernalis sp. nov., from the Late Cretaceous (Maastrichtian) Hell Creek and Lance formations of the Northern Great Plains, USA. Palaeontol. Electronica 22, 1-28. ( 10.26879/949) [DOI] [Google Scholar]
- 56.Wilson GP, DeMar DG, Carter G, Clemens WA, Horner Jr, Hartman JH. 2014. Extinction and survival of salamander and salamander-like amphibians across the Cretaceous-Paleogene boundary in northeastern Montana, USA. Through the end of the Cretaceous in the type locality of the Hell Creek Formation in Montana and adjacent areas. Geological Society of America Special Paper, 503, pp. 271-297.57. [Google Scholar]
- 57.Landolt JC, Hill LG. 1975. Observations of the gross structure and dimensions of the gills of three species of gars (Lepisosteidae). Copeia 1975, 470-475. [Google Scholar]
- 58.Schwarz DE, Allen PJ. 2014. Effects of salinity on growth and ion regulation of juvenile alligator gar Atractosteus spatula. Comp. Biochem. Physiol. A 169, 44-50. ( 10.1016/j.cbpa.2013.12.012) [DOI] [PubMed] [Google Scholar]
- 59.Brownstein CD, Lyson TR. 2022. Giant gar from directly above the K–Pg boundary suggests healthy freshwater ecosystems existed within thousands of years of the asteroid impact. FigShare. ( 10.6084/m9.figshare.c.6011615) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Brownstein CD, Lyson TR. 2022. Giant gar from directly above the K–Pg boundary suggests healthy freshwater ecosystems existed within thousands of years of the asteroid impact. FigShare. ( 10.6084/m9.figshare.c.6011615) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All associated data are in the manuscript and electronic supplementary material [59].