Abstract
Background:
High-power short-duration radiofrequency ablation has improved lesion durability in pulmonary vein isolation. In this study, we investigate long-term clinical outcomes of high-power short-duration pulmonary vein isolation and posterior wall debulking as an initial treatment modality in all corner atrial fibrillation patients.
Methods:
This is a single-center, retrospective, observational study including all patients who have undergone high-power short-duration pulmonary vein and posterior wall debulking, regardless of atrial fibrillation type and/or duration. High-power short-duration power delivery protocol was defined as 45 W at all ablation sites. Clinical and electrocardiographic follow-up were performed in all patients.
Results:
One hundred forty-two patients were enrolled in this study. Paroxysmal atrial fibrillation was present in 88 (62%) of patients. The mean follow-up of this study was 36.9 months ± 12.2 months. During the follow-up period, 10 patients (11.4%) with a diagnosis of paroxysmal atrial fibrillation had recurrence, while recurrence in patients with persistent and long-standing persistent atrial fibrillation was slightly higher (15 patients (28.1%) and 5 patients (50%), respectively). No major life-threatening complications occurred.
Conclusion:
This study has demonstrated excellent arrhythmia-free outcomes in unselected, real world atrial fibrillation patients undergoing high-power short-duration pulmonary vein and debulking posterior wall isolations, however larger randomized trials are warranted.
Keywords: Atrial fibrillation, high-power short-duration, posterior wall ablation, pulmonary vein isolation, radiofrequency ablation
HIGHLIGHTS
Although pulmonary vein isolation is a mainstay strategy for atrial fibrillation ablation, there is a significant risk of recurrence, especially in non-paroxysmal patients.
Box isolation of posterior wall has been associated with mixed outcomes, in various studies.
Addition of posterior wall debulking to high-power short-duration pulmonary vein isolation has been associated with good outcomes in a long-term follow-up study.
Introduction
Despite being one of the most performed procedures in interventional electrophysiology, long-term outcomes of atrial fibrillation (AF) ablation suggest that current strategies are open for improvement. The observation that ectopic beats originating from pulmonary vein (PV)’s may initiate AF,1 and subsequent studies that demonstrated clinical benefit of electrical PV isolation (PVI) suggests that anatomical structures related to atria may contain foci that have a role in AF initiation. Non-PV triggers have been demonstrated in various structures, including posterior wall (PW) of left atrium (LA), left trial appendage, superior vena cava, vein of Marshal (VOM), coronary sinus ostium, or within areas of electrical scar.2–4 Despite being a common source of non-PV triggers, a common location of a scar, and having been demonstrated that PW isolation (PWI) has not been consistently associated with improved AF ablation outcomes.5,6 However, in up to 40% of patients undergoing redo procedures, PW reconnection is observed after PWI in the initial procedure.7 In this substantial proportion of patients, not only is PWI ineffective, it may lead to further arrhythmogenesis due to corridors of slow conduction, therefore yielding any potential benefit unrecognizable. Recently, high-power short-duration (HPSD) radiofrequency (Rf) ablation has been associated with improved lesion durability in PVI.8 In this study, we aim to investigate effects of posterior wall isolation, modified by applying HPSD and debulking technique (in lieu of box isolation).
Methods
This was a retrospective observational study of all consecutive patients undergoing atrial fibrillation catheter ablation in an experienced single center between January 1, 2016, and January 1, 2019. High-power short-duration PW debulking, in addition to PVI was performed in all patients included. Local Ethical Committee has approved and supervised conduction of this study. All patients provided their written informed consent for participation in this study.
Electrophysiologic Study
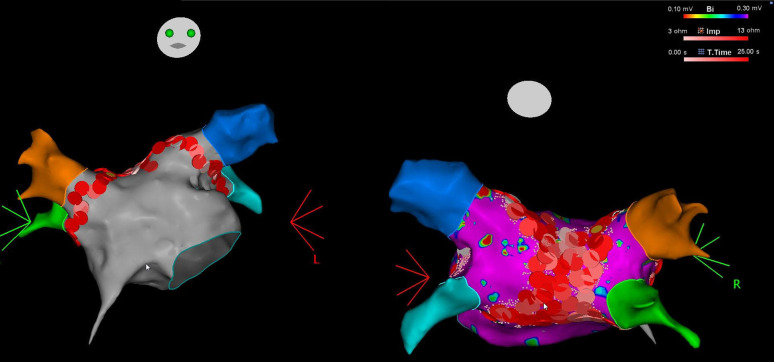
Patients were taken to the cardiac electrophysiology laboratory in a fasting state and the procedure was performed under general anesthesia or deep sedations. All patients continued oral anticoagulation regimen, peri-procedurally. Twelve lead surface electrocardiograms (ECGs) and intracardiac electrograms were recorded simultaneously by an electrophysiology system (EP Tracer, CardioTek B.V., Maastricht, The Netherlands or LABSYSTEM™ PRO, Boston Scientific, MA, USA). Following vascular access left atrial (LA), access was obtained using double trans-septal puncture. All patients then received intravenous heparin with target activated clotting time (ACT) >300. Electro-anatomic mapping was performed using 3D electro-anatomic mapping system (Carto 3, Biosense Webster, Diamond Bar, Calif, USA) and a Lasso decapolar circular mapping catheter or Pentaray (Biosense Webster) high-density mapping catheter. Point-by-point activation mapping was merged with computerized tomography (CT) scans to obtain more accurate LA and PV electro-anatomic maps. Pulmonary vein antral isolation was performed in all patients using irrigated-tip Rf ablation catheter. Pulmonary vein isolation simultaneously with debulking was initially started on posterior wall under continuous esophageal temperature monitoring, and power delivery was immediately terminated when esophageal temperature rapidly rose >0.5°C or whenever above 37.5°C. In that case, catheter preferentially was moved to anterior LA in order to ablate anterior PV antrum until esophagus temperature returns normal. Power delivery was set to 45 W at every site. An ablation index (AI) of 550 for anterior locations, and 350-400 for posterior sites was aimed for, according to CLOSE protocol.9 In case of catheter instability, rapid ventricular/atrial pacing was utilized to improve stability. Following successful isolation of all pulmonary veins (with wide area circumferential ablation, WACA technique), as assessed by entrance and exit block, adjacent lesions with 3-mm width lesion-tags were delivered at posterior LA between right and left pulmonary veins aiming for PW debulking/isolation (Figure 1. PWI was confirmed with entrance and exit block during PW pacing (10 mA/2 ms) and/or presence of dissociated electrograms in PW. Power delivery (HPSD) during PW ablation was limited to 45W/10 sec and/or an AI of 400 with the contact force was always kept under 15 g. In case of electrograms (EGMs) at PW remaining after initial ablation set, additional RF applications were also performed consecutively until total electrical silence with exit block was achieved.
Figure 1.
Left atrial map with complete lesion set around left and right pulmonary veins. On the right side, in the posterior view, posterior wall debulking lesion set is visible.
All ablations were preferentially performed during sinus rhythm. In case of atrial fibrillation resistant to 3 attempts of cardioversion before ablation, PVI and PWI were initially performed until AF terminated to SR or organized AT which was then re-mapped and ablated until SR was restored, or another DCCV was re-attempted. Low-voltage fibrotic areas were also targeted and homogenized whenever they were discovered and were always anchored to an electrically inert site (mitral annulus, posterior wall, or PVI lines). After a waiting period of 20-30 minutes, 20 µg of adenosine was also administered in order to discover dormant late reconnections. In case of reconnection in PVI or PWI, re-isolation was always performed. cavo-tricuspid isthmus (CTI) was performed in all cases until bilateral conduction block was demonstrated. All ablation procedures were performed by only 1 operator.
Follow-Up
Following the procedure, all patients received an anti-arrhythmic therapy (amiodarone or propafenone) for 3 months during the initial blanking period. Following this period, anti-arrhythmic drugs were discontinued unless there was a separate indication (such as history of ventricular tachycardia). All patients received a prophylactic gastrointestinal regimen consisting of proton-pump inhibitor, sucralfate and colchicine as described by our group in a previous report.10
Clinical status and rhythm follow-up were done with clinical visit, phone calls and 24-48-hour ambulator ECG monitoring, in following manner: patients had monthly visit for the first 3 months and 24-hour ambulatory ECG was performed at the visit. Thereafter, ambulatory ECG was performed bi-monthly in the first 12 months after the procedure, and subsequently in a 3-monthly schedule.
In case of a recurrence of arrhythmia and redo procedure, the second procedure was not included in this study as a separate entity.
Statistical Analysis
IBM Statistical Package for the Social Sciences Statistics (version 23, for Windows) was used for the statistical analyses. Continuous variables are expressed as the group mean ± 1 standard deviation and categorical variables were expressed as count and percentage. Normality test (Kolmogorov–Smirnov) was used for determination of normal distribution of data. Data were compared using the Fisher’s t-test or χ2 test for categorical variables and the Student’s t-test or Mann–Whitney U test for continuous variables, where appropriate. Kaplan–Meier plots were created to compare arrythmia-free survival between paroxysmal and non-paroxysmal AF.
Results
One hundred forty-two patients were enrolled in this study. The mean age was 56.2 ± 12.2 years and females comprised 51.7% of the population. Paroxysmal AF was present in 88 (62%) of patients, while non-paroxysmal AF was present in 54 (38%) patients. Most patients had a normal ejection fraction on echocardiogram (133 patients, 93.7%), and mean left atrial diameter was 42.8 mm ± 6.5 mm. Twenty-four (16.9%) patients were undergoing a repeat procedure. All other clinical characteristics are presented in Table 1.
Table 1.
Basic Clinical Characteristics
| Age | 56.2 ± 12.2 |
| Sex | |
| Male | 70 (49.3) |
| Female | 72 (51.7) |
| Type of atrial fibrillation | |
| Paroxysmal | 88 (62) |
| Persistent | 44 (31) |
| Long-standing persistent | 10 (7) |
| Diabetes Mellitus | 12 (8.5) |
| Hypertension | 77 (54.2) |
| Coronary artery disease | 10 (7) |
| Ejection Fraction | |
| >50% | 133 (93.7) |
| 40-50% | 4 (2.8) |
| >40% | 5 (3.5) |
| Prosthetic mitral valve | 5 (3.5) |
| Left atrium diameter | 42.8 ± 6.5 |
| Redo procedure | 24 (16.9) |
Procedural characteristics among de novo cases (Table 2).
Table 2.
Procedural Characteristics
| Procedural time (min) | 123.2 ± 20.6 |
| Radiofrequency duration (min) | 30.2 ± 12.2 |
| PVI success rate in de novo patients, -PVs (%) | 442 (100)/442 |
| PWI, n (%) | 142 (100)/142 |
| CTI, n (%) | 141 (99.3)/142 |
| Reconnected PVs in redo patients, -PVs (%) | 18 |
| Re-isolated PVs in redo patients, -PVs (%) | 18 (100) |
| Periprocedural complications, n (%) | |
| Access site complications | 4 (2.8) |
| Pericardial effusion | 2 (1.4) |
| Cardiac tamponade | 0 (0) |
| Stroke | 0 (0) |
| Systemic embolization | 0 (0) |
| Death | 0 (0) |
CTI, cavotricuspid isthmus; PV, pulmonary vein; PVI, pulmonary vein isolation; PWI, posterior wall isolation.
One hundred eighteen patients were undergoing de novo ablation. Mean radiation time was 30.2 min ± 12.6 min. There were totally 442 PVs, which were all isolated after WACA at the end of the procedures (100% PVI success). Furthermore, PWI was achieved in all patients after debulking. In all but 1 patient a bilateral CTI line block was achieved at the end of the procedure.
Procedural characteristics among redo cases (Table 2).
Twenty-four patients were undergoing a redo procedure. In this subgroup the mean radiation time was 30.2 min ± 10.3 min. There were 18 reconnected PVs, which were all re-isolated. Posterior wall was debulked in all patients and bilateral CTI block was achieved.
Periprocedural Complications
A steam-pop occurred in 1 patient at LA septum during the procedure. Two patients developed pericardial effusion, both of which were managed conservatively. There were no major complications, including cardiac tamponade requiring pericardiocentesis, stroke, systemic embolism, or death. Access site vascular hematoma occurred in three patients and pseudo-aneurysm occurred in 1 patient. One patient had severe gastrointestinal symptoms for which he underwent upper gastrointestinal endoscopy, which revealed mucosal erythema but no sign of ulceration or fistula formation.
Follow-up data
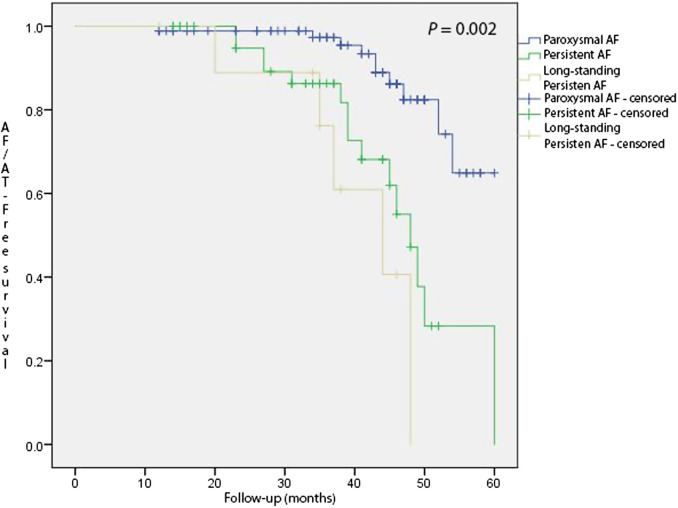
The mean follow-up of this study was 36.9 months ± 12.2 months. During the follow-up period 10 patients (11.4%) with a diagnosis of paroxysmal AF had recurrence, while recurrence in patients with persistent and long-standing persistent AF was slightly higher (15 patients (28.1%) and 5 patients (50%), respectively). Kaplan–Maier survival plot is presented in Figure 2, depicting significant difference between AF types and recurrence (P = .002). Among these patients 7 patients had recurrence as AT, 5 of which were septal ATs and 2 were peri-mitral AT. All other recurrences were documented as AF. During the follow-up period, 8 patients underwent a repeat procedure. There were no instances of roof-dependent AT, all PWs were isolated (Table 3).
Figure 2.
Kaplan–Maier arrhythmia-free survival plots for paroxysmal, persistent, and long-standing persistent atrial fibrillation patients.
Table 3.
Follow-up Data
| Mean follow-up (months) | 36.9 ± 12.2 |
| Recurrence during blanking period, n (%) | 19 (13.4) |
| Among Paroxysmal AF | 6 (6.8) |
| Among Persistent AF | 9 (20.4) |
| Among Longstanding persistent AF | 4 (40) |
| Isolated events during blanking period | 14 (9.8) |
| Recurrence Rates, n (%) | |
| Among Paroxysmal AF patients | 10 (11.4) |
| Among Persistent AF patients | 15 (28.1) |
| Among Longstanding Persistent AF patients | 5 (50) |
| Statistics | P < .001 |
| Recurrence as atrial tachycardia, n (%) | 7 (4.9) |
AF, atrial fibrillation.
Discussion
In this observational study including a wide range of AF subtypes which actually represented an all-comers real world data after a routine HPSD PVI and debulking PWI technique, acceptable mid-to-late term AF-free outcome was demonstrated. Furthermore, from a safety perspective, no major complication occurred in periprocedural and late follow-up period, suggesting that this technique may be applied to a wide spectrum of patients undergoing AF ablation.
Pulmonary vein reconnection is a common finding in patients undergoing re-do procedures, although prognostic-wise the presence of reconnection is a favorable finding.11 Nevertheless, durable PVI is a paramount of clinical success in AF ablation. Although contact-force sensing catheters and AI guided ablation has been shown to improve outcomes, PV reconnection is still present in a subset of patients.12,13 It is thought that AI predicts lesion quality better than conventional impedance measurement, the depth of lesion is mainly predicted by Rf power settings, and contact-force. Since increasing contact-force decreases operators’ control of catheter movement and “pushing” PW towards esophagus intuitively increases the potential complication risk, increasing power settings and/or duration of energy application remains the best option. While Yavin et al8 have demonstrated improved PVI durability with HPSD ablation, authors’ argue that less time necessary for catheter to be in a “stable” position is the reason of improved durability, rather than delivered energy. Authors also argue that increase in surface temperature and char-formation limit the ability of HPSD to create deep lesions. In our study, where energy delivery was continued until AI targets were reached (up to 25 s), no detrimental effects were observed, and it is reassuring that we have observed only 1 steam-pop during long duration ablation of a septal AT in a patient with highly scarred enlarged LA with thick interatrial septum. Furthermore, feasibility of very HPSD (70 W) ablation was demonstrated by Kottmaier et al14 actually leaving 50 W to be a relatively conservative HPSD strategy.
Rationale, Feasibility, and Technique of HPSD Posterior Wall Debulking
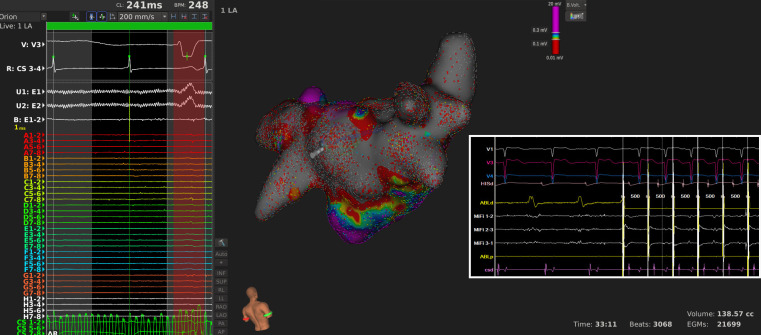
Studies and clinical observational data suggest that although effective, PVI still seems to be a not-always-sufficient target for AF ablation in all patients. Considerable risk of AF recurrence remains even with HPSD PVI, and progressively increases with duration of AF.15 PW has been implicated in AF arrhythmogenesis due to numerous characteristics. Embryologically, PW has similar precursors as PV antra, and both are anatomically and electrophysiologically distinct from other parts of LA.16 Moreover, PW is a frequent site of AF triggers as well as rotors.17 Initial attempts to isolate PW by linear lines (i.e., box isolation) have demonstrated feasibility yet in many studies outcomes have been either improved marginally or not at all.18–20 This may be explained by several factors. First, most of these studies employ a box technique of PW isolation, where durability is an important issue since reconnection has been reported in up to 40% of patients.7 Reconnection results from gaps in ablation lines, and these provide substrate for post-ablation ATs. These gaps preferentially occur at anatomically thick areas of atrial wall, such as septomarginal bundle on LA roof, where conventional energy settings led to limited lesions and thus creating a potential nidus for epicardial bridges using Bachmann bundle.21 Incomplete PW isolation with cryoballoon ablation seemed to improve outcomes when compared to PVI only in persistent AF patients, with follow-up studies demonstrating better durability of isolation.22,23 Second, even if durable endocardial PW isolation is achieved, lesions created with conventional ablation settings are not simply deep enough to affect epicardial tissue, therefore electrical activity can be present in epicardium in patients with silent posterior wall endocardium (Figure 3).24 Complex modeling studies have demonstrated that epicardial circuits have important role in AF mechanism, which may persist even after endocardial ablation with conventional energy settings.24,25
Figure 3.
A patient who had undergone a box posterior wall isolation and pulmonary vein isolation at outside hospital. Although posterior wall was endocardially silent (bipolar signal visible at E1-2 on the left, side), pacing from posterior wall revealed constant capture at 4 mA. After posterior wall debulking exit block was accomplished.
Supporting the main thesis of this study are hybrid endocardial and surgical epicardial ablation, aiming to disrupt posterior epicardial circuits. In 1 small study where epicardial hybrid ablation was performed, a 79% arrhythmia-free survival was observed at 3 years follow-up, among both paroxysmal and persistent AF cases.26 In another multicenter European study which had a mean follow-up of 14 months with internal loop recorder, there was a 75% were in sinus rhythm during the last follow-up.27 In this study, the shortcomings of Rf ablation with conventional settings and linear lines are thought to be addressed by HPSD and PW debulking, simulating hybrid approach. There are other ongoing studies assessing hybrid surgical and catheter AF ablation with rationale similar to the rationale of our study.
We hypothesize that box isolation of PW using conventional energy settings is probably simply a technique with low effectiveness, leaving both gaps in lines and intact epicardial tissue in many cases and propose 2 changes in the technique. First, debulking the PW is less likely to lead to proarrhythmic gaps, and second, high power settings may improve the transmurality of lesions.
Posterior wall debulking was previously tested in 1 study enrolling persistent AF patients, where it improved outcomes.28 In this relatively small study Bai et al have performed extended PVI (lesions extending to septum and CS) in addition to PW isolation and superior vena cava isolation, yet the outcomes are much inferior to our findings. This may be explained by uniform HPSD ablation technique and the fact that we have also included paroxysmal AF patients as our pre-determined strategy for all-comers. yet we believe single procedure catheter ablation using HPSD may leave surgical approach obsolete in a significant number of patients.
Safety of HPSD Debulking
Among main concerns of HPSD ablation are effects of Rf to both local and nearby tissues (i.e., esophagus), and steam-pop formation. We have very seldomly observed dyspeptic concerns, and we did not encounter any serious complications.10 Steam-pop occurred only at 1 instance during a long-lasting RF application at LA septum with no consequence. Although esophageal temperature monitoring is not routinely recommended during ablation with conventional energy settings, this may not apply to HPSD settings although it has not been tested.29 In our study we have routinely monitored esophageal temperature during ablation in PW. During energy delivery, ablation was immediately stopped if temperature rose above 37.5°C mainly due to steep temperature curve rise, which contrasts to ablation with conventional low energy settings where temperature rise is more gradual. Adjacent lesions in PW were always created in interrupted fashion: for example, if a lesion in inferior portion of PW was created, the very next Rf application would be performed at least 20 mm away until whole posterior wall was debulked with overlapping lesions.
Incidence of Atrial Tachycardia Recurrences
Historically, the durability of PW isolation has been a major limitation after initial PW box isolation with conventional energy settings. Small gaps may create great nidus for ATs that are usually roof-dependent. Among the recurrent cases in our series, we have never observed roof-dependent tachycardias. The recurrences where either as AF, or when there was inducible AT, it was peri-mitral, localized reentry in anterior wall or PV tachycardias due to vein reconnections. Albeit the number of recurrent cases undergoing redo procedure was small, PWs where isolated at all instances.
Study Limitations
There are a few limitations that merit discussion in this relatively small, prospective albeit not randomized study. The study does not have a control group; however, a PVI-only control group would yield very limited additional information since the success rate of the later is well known. The follow-up consisted of clinical assessment and 24-hour Holter recordings obtained at predetermined intervals. Since continuous rhythm monitoring was not available, asymptomatic recurrences cannot be ruled out.
Posterior wall isolation durability cannot be determined with this study since very few patients underwent a redo procedure. A further limitation was that PW isolation was achieved and checked only endocardially. Also, routine catheterization of VOM was not performed and realistically, even if endocardially targeted lesions were delivered, VOM isolation was probably achieved in small number of cases. Finally, gastrointestinal complications occurred very infrequently, however routine endoscopic studies were not performed.
Conclusion
This study has demonstrated excellent arrhythmia-free outcomes in unselected, real world AF patients undergoing HPSD PVI and debulking PW isolations. This technique was both safe and effective as a first-line strategy, however, further randomized studies are warranted.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Ankara University (approval number: 235235).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.C., E.B., O.B.; Data analysis – B.C., E.B., O.B.; Statistics – B.C., T.S.T.; Drafting – E.B., T.A.; Critical revision – E.B., V.K., E.T.
Acknowledgments: None.
Declaration of Interests: The authors declare that they have no competing interest.
Funding: This study received no funding.
References
- 1. Haïssaguerre M, Jaïs P, Shah DC.et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659 666. 10.1056/NEJM199809033391003) [DOI] [PubMed] [Google Scholar]
- 2. Lin WS, Tai CT, Hsieh MH.et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non–pulmonary vein ectopy. Circulation. 2003;107(25):3176 3183. 10.1161/01.CIR.0000074206.52056.2D) [DOI] [PubMed] [Google Scholar]
- 3. Hayashi K, An Y, Nagashima M.et al. Importance of nonpulmonary vein foci in catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2015;12(9):1918 1924. 10.1016/j.hrthm.2015.05.003) [DOI] [PubMed] [Google Scholar]
- 4. Spragg D. Left atrial fibrosis: role in atrial fibrillation pathophysiology and treatment outcomes. J Atr Fibrillation. 2013;5(6):810. 10.4022/jafib.810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salih M, Darrat Y, Ibrahim AM.et al. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: a meta‐analysis. J Cardiovasc Electrophysiol. 2020;31(6):1394 1402. 10.1111/jce.14480) [DOI] [PubMed] [Google Scholar]
- 6. Lee JM, Shim J, Park J.et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5(11):1253 1261. 10.1016/j.jacep.2019.08.021) [DOI] [PubMed] [Google Scholar]
- 7. Markman TM, Hyman MC, Kumareswaran R.et al. Durability of posterior wall isolation after catheter ablation among patients with recurrent atrial fibrillation. Heart Rhythm. 2020;17(10):1740 1744. 10.1016/j.hrthm.2020.05.005) [DOI] [PubMed] [Google Scholar]
- 8. Yavin HD, Leshem E, Shapira-Daniels A.et al. Impact of high-power short-duration radiofrequency ablation on long-term lesion durability for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6(8):973 985. 10.1016/j.jacep.2020.04.023) [DOI] [PubMed] [Google Scholar]
- 9. Taghji P, El Haddad M, Phlips T.et al. Evaluation of a strategy aiming to enclose the pulmonary veins With contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: A Pilot Study. JACC Clin Electrophysiol. 2018;4(1):99 108. 10.1016/j.jacep.2017.06.023) [DOI] [PubMed] [Google Scholar]
- 10. Candemir B, Baskovski E, Mammadov M, Esenboga K, Altin T. Triple gastrointestinal prophylactic therapy following high-power short-duration posterior left atrial wall ablation. Indian Heart J. 2020;72(4):306 308. 10.1016/j.ihj.2020.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim TH, Park J, Uhm JS, Joung B, Lee MH, Pak HN. Pulmonary vein reconnection predicts good clinical outcome after second catheter ablation for atrial fibrillation. Europace. 2017;19(6):961 967. 10.1093/europace/euw128) [DOI] [PubMed] [Google Scholar]
- 12. Ioannou A, Papageorgiou N, Lim WY.et al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: an updated meta-analysis. Europace. 2020;22(11):1659 1671. 10.1093/europace/euaa224) [DOI] [PubMed] [Google Scholar]
- 13. Hussein A, Das M, Riva S.et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom From arrhythmia in persistent atrial fibrillation patients: The PRAISE Study Results. Circ Arrhythm Electrophysiol. 2018;11(9):e006576. 10.1161/CIRCEP.118.006576) [DOI] [PubMed] [Google Scholar]
- 14. Kottmaier M, Popa M, Bourier F.et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace. 2020;22(3):388 393. 10.1093/europace/euz342) [DOI] [PubMed] [Google Scholar]
- 15. Winkle RA, Mead RH, Engel G.et al. High-power, short-duration atrial fibrillation ablations using contact force sensing catheters: outcomes and predictors of success including posterior wall isolation. Heart Rhythm. 2020;17(8):1223 1231. 10.1016/j.hrthm.2020.03.022) [DOI] [PubMed] [Google Scholar]
- 16. Tahir KS, Mounsey JP, Hummel JP. Posterior wall isolation in atrial fibrillation ablation. J Innov Card Rhythm Manag. 2018;9(6):3186 3194. 10.19102/icrm.2018.090602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Della Rocca DG, Tarantino N, Trivedi C.et al. Non‐pulmonary vein triggers in nonparoxysmal atrial fibrillation: implications of pathophysiology for catheter ablation. J Cardiovasc Electrophysiol. 2020;31(8):2154 2167. 10.1111/jce.14638) [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Off MK, Solheim E, Schuster P, Hoff PI, Ohm OJ. Treatment of atrial fibrillation by silencing electrical activity in the posterior inter-pulmonary-vein atrium. Europace. 2008;10(3):265 272. 10.1093/europace/eun029) [DOI] [PubMed] [Google Scholar]
- 19. Tamborero D, Mont L, Berruezo A.et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol. 2009;2(1):35 40. 10.1161/CIRCEP.108.797944) [DOI] [PubMed] [Google Scholar]
- 20. Tokioka S, Fukamizu S, Kimura T, Takahashi M, Kitamura T, Hojo R. The effect of posterior wall isolation for persistent atrial fibrillation on recurrent arrhythmia. J Cardiovasc Electrophysiol. 2021;32(3):597 604. 10.1111/jce.14906) [DOI] [PubMed] [Google Scholar]
- 21. Pambrun T, Duchateau J, Delgove A.et al. Epicardial course of the septopulmonary bundle: anatomical considerations and clinical implications for roof line completion. Heart Rhythm. 2021;18(3):349 357. 10.1016/j.hrthm.2020.11.008) [DOI] [PubMed] [Google Scholar]
- 22. Aryana A, Baker JH, Espinosa Ginic MA.et al. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: a multicenter experience. Heart Rhythm. 2018;15(8):1121 1129. 10.1016/j.hrthm.2018.05.014) [DOI] [PubMed] [Google Scholar]
- 23. Aryana A, Di Biase L, Pujara DK.et al. Long-term durability of posterior wall isolation using the cryoballoon in patients with persistent atrial fibrillation: a multicenter analysis of repeat catheter ablations. J Interv Card Electrophysiol. 2021;62(1):161 169. 10.1007/s10840-020-00887-8) [DOI] [PubMed] [Google Scholar]
- 24. Jiang R, Buch E, Gima J.et al. Feasibility of percutaneous epicardial mapping and ablation for refractory atrial fibrillation: insights into substrate and lesion transmurality. Heart Rhythm. 2019;16(8):1151 1159. 10.1016/j.hrthm.2019.02.018) [DOI] [PubMed] [Google Scholar]
- 25. Parameswaran R, Kalman JM, Royse A.et al. Endocardial-epicardial phase mapping of prolonged persistent atrial fibrillation recordings: high prevalence of dissociated activation patterns. Circ Arrhythm Electrophysiol. 2020;13(8):e008512. 10.1161/CIRCEP.120.008512) [DOI] [PubMed] [Google Scholar]
- 26. Maesen B, Pison L, Vroomen M.et al. Three-year follow-up of hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg. 2018;53(suppl_1):i26 i32. 10.1093/ejcts/ezy117) [DOI] [PubMed] [Google Scholar]
- 27. Haywood GA, Varini R, Osmancik P.et al. European multicentre experience of staged hybrid atrial fibrillation ablation for the treatment of persistent and longstanding persistent atrial fibrillation. Int J Cardiol Heart Vasc. 2020;26:100459. 10.1016/j.ijcha.2019.100459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bai R, Di Biase L, Mohanty P.et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016;13(1):132 140. 10.1016/j.hrthm.2015.08.019) [DOI] [PubMed] [Google Scholar]
- 29. Calkins H, Hindricks G, Cappato R.et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33(5):369 409. 10.1016/j.joa.2017.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a