Abstract
We describe a gonorrhoea case with ceftriaxone plus high-level azithromycin resistance. In April 2022, an Austrian heterosexual male was diagnosed with gonorrhoea after sexual intercourse with a female sex worker in Cambodia. Recommended treatment with ceftriaxone (1 g) plus azithromycin (1.5 g) possibly failed. Worryingly, this is the second strain in an Asian Neisseria gonorrhoeae genomic sublineage including high-level azithromycin-resistant strains that developed ceftriaxone resistance by acquisition of mosaic penA-60.001. Enhanced resistance surveillance and actions are imperative to prevent spread.
Keywords: Neisseria gonorrhoeae, extensively drug-resistant, XDR, ceftriaxone resistance, high-level azithromycin resistance
Multidrug- and extensively drug-resistant (XDR) Neisseria gonorrhoeae strains are major global public health concerns. In most countries worldwide, ceftriaxone (0.25–1 g) as monotherapy or combined with azithromycin (1–2 g) are recommended first-line gonorrhoea therapies, and resistance to ceftriaxone and azithromycin hampers therapy with these two last remaining treatment options [1-3]. We describe the second global XDR N. gonorrhoeae strain [4,5], with high-level resistance to azithromycin and resistance to ceftriaxone, cefixime, cefotaxime, ciprofloxacin and tetracycline, which caused a possible gonorrhoea treatment failure with ceftriaxone (1 g) plus azithromycin (1.5 g) in a case from Austria who was infected after condomless sexual contact in Cambodia.
Clinical case description and microbiology
In April 2022, a heterosexual male patient in his 50s consulted a urology department in Austria because of painful urination and urethral discharge. Five days before onset of symptoms, he had condomless heterosexual contact with a female commercial sex worker in Cambodia, who could not be traced. Based on sexual history, a urethral swab was taken, and N. gonorrhoeae culture (AT159 strain) verified the gonorrhoea diagnosis. The patient was treated with ceftriaxone (1 g intramuscularly) plus azithromycin (1.5 g single oral dose), according to European recommendations [1] but using a slightly adapted azithromycin dosing (1.5 g instead of 2 g). Approximately 2 weeks later at a follow-up visit, symptoms had resolved. Test of cure using culture of urethral, rectal and pharyngeal samples was negative, however, a PCR test (Allinity, Abbott, Chicago, Illinois, United States (US)) from the urethral swab culture sample was N. gonorrhoeae-positive. Because no post-treatment gonococcal isolates were available, the case was considered as a possible treatment failure [4,5]. Based on the antimicrobial susceptibility testing results (Table), additional treatment with amoxicillin-clavulanic acid (1 g) twice a day for 7 days was prescribed. At the test of cure after this second treatment, a urethral sample was N. gonorrhoeae culture-negative; unfortunately, no urine PCR sample was available. The patient was negative in Chlamydia trachomatis PCR (FluoroType CT, Bruker, Billerica, US) and Mycoplasma genitalium testing (MYCOPLASMA IST 3, bioMerieux SA, Marcy l’Etoile, France). The patient did not consent to HIV and syphilis testing.
Table. Antimicrobial minimum inhibitory concentration of the extensively drug-resistant Neisseria gonorrhoeae strain (AT159) causing a possible gonorrhoea treatment failure, Austriaa, April 2022 (n = 1 strain).
| Antimicrobial | MIC in mg/L | Interpretation (EUCAST v 12.0 [6]) |
|---|---|---|
| Ceftriaxone | 0.25 | Resistant |
| Cefixime | 1 | Resistant |
| Cefotaxime | 0.5 | Resistant |
| Azithromycin | > 256 | High-level resistant |
| Ciprofloxacin | 16 | Resistant |
| Tetracycline | 16 | Resistant |
| Penicillin G | 0.5 | Susceptible, increased exposure |
| Spectinomycin | 16 | Susceptible |
| Gentamicin | 4 | NA (Wild-type MIC) |
| Rifampicin | 0.125 | NA (Wild-type MIC) |
| Ertapenem | 0.016 | NA (Wild-type MIC) |
| Zoliflodacinb | 0.032 | NA (Wild-type MIC) |
| Lefamulin | 0.125 | NA (Wild-type MIC) |
EUCAST: European Committee on Antimicrobial Susceptibility Testing; MIC: minimum inhibitory concentration; NA: not applicable (due to lack of interpretative breakpoints).
a The patient reported having had sexual intercourse with a female sex worker in Cambodia.
b Pre-licensing international phase III randomised clinical trial is ongoing.
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs) of antimicrobials were determined at the National Reference Laboratory for Gonorrhoea in Vienna, Austria and the WHO Collaborating Centre for Gonorrhoea and Other STIs in Örebro, Sweden by Etest (bioMerieux SA, Marcy l’Etoile, France), in accordance with the manufacturer’s instruction, or agar dilution (zoliflodacin and lefamulin). The MICs of 13 antimicrobials and, where available, interpretations using breakpoints according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) version 12.0 [6] are shown in the Table.
Briefly, AT159 showed high-level resistance to azithromycin (MIC > 256 mg/L), and resistance to ceftriaxone, cefixime, cefotaxime, ciprofloxacin, and tetracycline (Table). Accordingly, AT159 was XDR, according to international definitions [4,5]. However, the strain was susceptible to spectinomycin, ‘intermediate susceptible’ (i.e. susceptible, increased exposure) to benzylpenicillin and showed wild-type MICs of gentamicin, rifampicin, ertapenem and the new antimicrobials zoliflodacin and lefamulin (Table). AT159 did not produce beta-lactamase, according to a nitrocefin test (Becton Dickinson, Franklin Lakes, New Jersey, US). Only one gonococcal strain with ceftriaxone resistance combined with high-level azithromycin resistance (subsequently assigned as the WHO Q reference strain) has been previously described globally, which caused three gonorrhoea cases in the United Kingdom (UK) and Australia in 2018. These cases also had links to South East Asia [7,8].
Molecular investigation
The isolate AT159 was sequenced with NextSeq 550 (Illumina, San Diego, CA, US) and sequencing reads submitted to the European Nucleotide Archive (accession number PRJEB53054). Quality control, assembly and characterisation of molecular epidemiological sequence types (STs) and antimicrobial resistance determinants [9,10] were performed using a customised CLC Genomics Workbench (Qiagen), as previously described [11,12]. All sequences were also assembled using Spades and uploaded to the Pathogenwatch platform [9] for core genome multilocus sequence typing (cgMLST).
Briefly, AT159 was assigned to the novel MLST ST16406 and N. gonorrhoeae sequence typing for antimicrobial resistance (NG-STAR) type ST4465. The high-level azithromycin resistance was caused by the 23S rRNA A2059G target mutation (in all four 23S rRNA gene alleles) and the resistance to the extended-spectrum cephalosporins (ceftriaxone, cefixime and cefotaxime) by the mosaic penA-60.001 allele [7-10,13]. This mosaic penA-60.001 allele is also causing ceftriaxone resistance in the internationally spreading FC428 strain, first reported in Japan in 2015 [13] and subsequently identified in many countries globally including in Europe [14-18], although FC428 and AT159 are genomically widely different (data not shown). However, phylogenomic analysis of the draft AT159 genome sequence and the most closely related gonococcal genomes from several Asian countries [12], where many ceftriaxone-resistant strains appear to have emerged [7,8,13-18], showed that AT159 belongs to the same sublineage as WHO Q [7,8] (Figure), but differs by 313 alleles.
Figure.
Phylogeny of the most closely related Neisseria gonorrhoeae genome sequences from a recent study [12], Asia, 2011–2018 to the extensively drug-resistant N. gonorrhoeae strain (AT159) causing a possible gonorrhoea treatment failure, Austriaa, April 2022 (n = 71 genome sequences)
MLST: Multilocus sequence typing; UK: United Kingdom; WHO: World Health Organization.
a The patient reported having had sexual intercourse with a female sex worker in Cambodia.
WHO Q (purple star) was identified in the UK, but the infection was in Thailand [7]. Mutations or alleles conferring resistance to ceftriaxone (penA 60.001), azithromycin (23S rDNA A2059G) and ciprofloxacin (gyrA S91F) [9,10] are shown in red.
Genome assemblies of closely related Asian isolates (n = 70) [12] to AT159 (orange star) were uploaded and analysed on the Pathogenwatch platform [9,19] including a phylogenetic inference. Briefly, Pathogenwatch [9] provides a cgMLST defined by the 2016 Neisseria gonorrhoeae reference strains [20] and each assembly is linked to the closest reference by comparing the substitution score profiles to the reference core profiles excluding indels and non-ACTG characters. cgMLST profiles are then clustered by calculating distances between each assembly which shares a given cgMLST scheme. These distances are calculated as the number of different loci for the scheme, ignoring any missing loci. A clustering is performed using Single Linkage Clustering based on these calculated pairwise distances [9], see https://cgps.gitbook.io/pathogenwatch/technical-descriptions/cgmlst-clusters. A tree is finally constructed by writing all scaled pairwise scores to a matrix, and running a neighbour-joining implementation to construct a tree that is finally midpoint rooted [9], see https://cgps.gitbook.io/pathogenwatch/technical-descriptions/core-genome-tree.
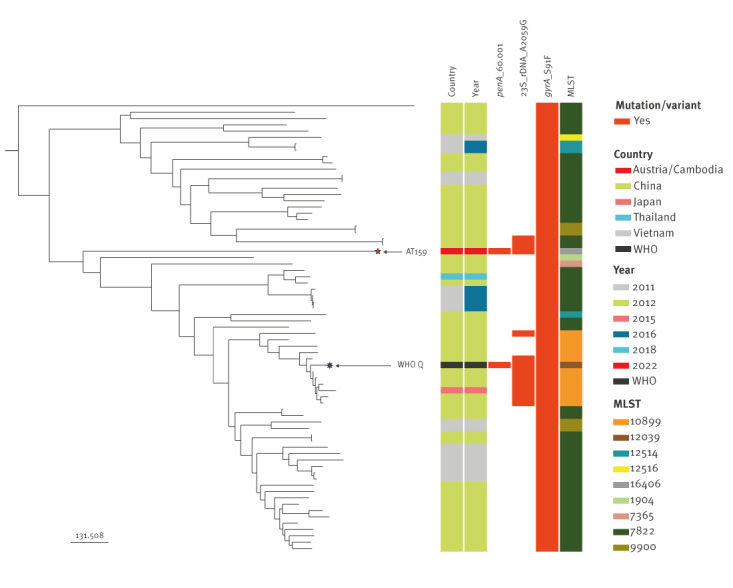
Concern is warranted given that many strains in this Asian N. gonorrhoeae sublineage show a high-level azithromycin resistance (because of 23S rRNA A2059G mutations) and that some of these strains, such as AT159 and WHO Q [7,8], appear to additionally develop ceftriaxone resistance by acquisition of mosaic penA-60.001 (Figure), most likely through transformation. All the strains in this sublineage are additionally resistant to ciprofloxacin (Figure).
Discussion
We describe the second global XDR gonococcal strain [4,5], with high-level resistance to azithromycin and resistance to ceftriaxone, cefixime, cefotaxime, ciprofloxacin, and tetracycline, which caused a possible gonorrhoea treatment failure with recommended ceftriaxone plus azithromycin therapy. The case from Austria reported about condomless sexual contact with a female sex worker in Cambodia 5 days before onset of symptoms. A limitation of our study is that the female sex worker could not be traced and, thus, no gonococcal isolate or other samples from this female were available to link to AT159. Notably, in 2019, another ceftriaxone-resistant gonococcal strain was reported in France, also after stating sexual contact with a female in Cambodia [21]. However, this strain belonged to the internationally spreading FC428 clone [13-18,21].
In the absence of a gonococcal vaccine, early and effective diagnosis and antimicrobial treatment of gonorrhoea are essential [1,3,10]. However, N. gonorrhoeae has developed resistance to all classes of antimicrobials since introduction of antimicrobial treatment in the 1930s [1,7,8,10,13-18,21,22]. XDR N. gonorrhoeae strains, including those with resistance to all available treatment options, are a major global public health concern. They pose a risk of treatment failure and serious complications/sequelae on the individual level but also compromise the management and control of gonorrhoea on the public health level. Resistance or decreased susceptibility to ceftriaxone and azithromycin resistance in N. gonorrhoeae has been reported worldwide [22]. In recent years in Europe, the susceptibility to ceftriaxone has increased but, worryingly, the resistance to azithromycin rapidly increased [19,23]. Furthermore, sporadic ceftriaxone-resistant strains have been identified in several European countries [1,5,7,15,18,19,22,23]. However, the XDR strain described in the present paper is the second global gonococcal strain with ceftriaxone resistance combined with high-level azithromycin resistance, with relatively close relationship with WHO Q [7,8], although not the same subvariant (Figure). It is of concern that high-level azithromycin-resistant strains in an Asian N. gonorrhoeae genomic sublineage are able to develop ceftriaxone resistance by acquisition of mosaic penA-60.001. If such strains manage to establish a sustained transmission, many gonorrhoea cases might become untreatable. Promisingly, the XDR AT159 strain had wild-type MICs of the novel gonorrhoea antimicrobials lefamulin and zoliflodacin, which is in a phase 3 randomised clinical trial [10,12,19,22].
Conclusions
Ceftriaxone resistance combined with high-level azithromycin resistance in N. gonorrhoeae is a concern for future treatment of gonorrhoea and poses a major global public health threat. Improved prevention (including condom use), early and accurate diagnosis and effective, affordable and accessible treatment (ideally including test of cure and contact notification and treatment) of gonorrhoea are imperative. Furthermore, increased awareness of the spread of ceftriaxone-resistant strains and rapid identification and eradication of the sporadic XDR gonococcal strains are essential to avoid any clonal expansion and sustained transmission of these strains. Enhanced antimicrobial resistance surveillance, ideally including test of cure and whole-genome sequencing, nationally and internationally, particularly in Asia where many ceftriaxone-resistant strains appear to have emerged [5,7,8,13-18,21,22], is of highest importance. Ultimately, novel antimicrobials for effective treatment of gonorrhoea and/or a sufficiently effective gonococcal vaccine will be crucial.
Statements
Ethical statement: Ethical approval for the study was not necessary. Data were obtained from routine antimicrobial surveillance (standard care) and are published with a high-level of anonymisation. The patient gave consent to the publication of this case report.
Funding statement: The present work was funded by grants from the Örebro County Council Research Committee and the Foundation for Medical Research at Örebro University Hospital, Sweden.
Acknowledgements
The authors thank all additional people involved in laboratory work at the Austrian Agency of Health and Food Safety (Petra Hasenberger, Silke Stadlbauer, Ernst Amtmann).
Conflict of interest: None declared.
Authors’ contributions: SH clinically managed the gonorrhoea case. SP, FH, SS and SJ undertook the microbiological testing. DG performed the sequencing and bioinformatic analysis. MU and AI provided reference laboratory services. SP and MU wrote the first draft of the manuscript. All authors reviewed and approved the final version of the manuscript.
References
- 1. Unemo M, Ross J, Serwin AB, Gomberg M, Cusini M, Jensen JS. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2020;956462420949126. 10.1177/0956462420949126 [DOI] [PubMed] [Google Scholar]
- 2. St Cyr S, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911-6. 10.15585/mmwr.mm6950a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: WHO; 2016. Available from: https://www.who.int/publications/i/item/9789241549691 [PubMed]
- 4. Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009;7(7):821-34. 10.1586/eri.09.63 [DOI] [PubMed] [Google Scholar]
- 5. Cole MJ, Day M, Jacobsson S, Amato-Gauci AJ, Spiteri G, Unemo M, the European Gonorrhoea Response Plan Group. European Gonorrhoea Response Plan Group . The European response to control and manage multi- and extensively drug-resistant Neisseria gonorrhoeae. Euro Surveill. 2022;27(18). 10.2807/1560-7917.ES.2022.27.18.2100611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters; version 12.0, 1 Jan 2022. EUCAST; 2022. Available from: https://www.eucast.org/clinical_breakpoints
- 7. Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 2018;23(27):1800323. 10.2807/1560-7917.ES.2018.23.27.1800323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jennison AV, Whiley D, Lahra MM, Graham RM, Cole MJ, Hughes G, et al. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill. 2019;24(8):1900118. 10.2807/1560-7917.ES.2019.24.8.1900118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sánchez-Busó L, Yeats CA, Taylor B, Goater RJ, Underwood A, Abudahab K, et al. A community-driven resource for genomic epidemiology and antimicrobial resistance prediction of Neisseria gonorrhoeae at Pathogenwatch. Genome Med. 2021;13(1):61. 10.1186/s13073-021-00858-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unemo M, Seifert HS, Hook EW, 3rd, Hawkes S, Ndowa F, Dillon JR. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. 10.1038/s41572-019-0128-6 [DOI] [PubMed] [Google Scholar]
- 11. Golparian D, Harris SR, Sánchez-Busó L, Hoffmann S, Shafer WM, Bentley SD, et al. Genomic evolution of Neisseria gonorrhoeae since the preantibiotic era (1928-2013): antimicrobial use/misuse selects for resistance and drives evolution. BMC Genomics. 2020;21(1):116. 10.1186/s12864-020-6511-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golparian D, Kittiyaowamarn R, Paopang P, Sangprasert P, Sirivongrangson P, Franceschi F, et al. Genomic surveillance and antimicrobial resistance in Neisseria gonorrhoeae isolates in Bangkok, Thailand in 2018. J Antimicrob Chemother. 2022;dkac158. 10.1093/jac/dkac158 [DOI] [PubMed] [Google Scholar]
- 13. Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother. 2016;60(7):4339-41. 10.1128/AAC.00504-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis. 2018;24(4):735-40. 10.3201/eid2404.171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poncin T, Fouere S, Braille A, Camelena F, Agsous M, Bebear C, et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill. 2018;23(21):1800264. 10.2807/1560-7917.ES.2018.23.21.1800264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee K, Nakayama SI, Osawa K, Yoshida H, Arakawa S, Furubayashi KI, et al. Clonal expansion and spread of the ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, identified in Japan in 2015, and closely related isolates. J Antimicrob Chemother. 2019;74(7):1812-9. 10.1093/jac/dkz129 [DOI] [PubMed] [Google Scholar]
- 17. Chen SC, Yuan LF, Zhu XY, van der Veen S, Yin YP. Sustained transmission of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in China. J Antimicrob Chemother. 2020;75(9):2499-502. 10.1093/jac/dkaa196 [DOI] [PubMed] [Google Scholar]
- 18. Golparian D, Rose L, Lynam A, Mohamed A, Bercot B, Ohnishi M, et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill. 2018;23(47):1800617. 10.2807/1560-7917.ES.2018.23.47.1800617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sánchez-Busó L, Cole MJ, Spiteri G, Day M, Jacobsson S, Golparian D, et al. Europe-wide expansion and eradication of multidrug-resistant Neisseria gonorrhoeae lineages: a genomic surveillance study. Lancet Microbe. 2022;3(6):e452-63. 10.1016/S2666-5247(22)00044-1 [DOI] [PubMed] [Google Scholar]
- 20. Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, Ohnishi M, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71(11):3096-108. 10.1093/jac/dkw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poncin T, Merimeche M, Braille A, Mainardis M, Bebear C, Jacquier H, et al. Two cases of multidrug-resistant Neisseria gonorrhoeae related to travel in south-eastern Asia, France, June 2019. Euro Surveill. 2019;24(36):1900528. 10.2807/1560-7917.ES.2019.24.36.1900528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Unemo M, Lahra MM, Escher M, Eremin S, Cole MJ, Galarza P, et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe. 2021;2(11):e627-36. 10.1016/S2666-5247(21)00171-3 [DOI] [PubMed] [Google Scholar]
- 23. Day MJ, Jacobsson S, Spiteri G, Kulishev C, Sajedi N, Woodford N, et al. Significant increase in azithromycin "resistance" and susceptibility to ceftriaxone and cefixime in Neisseria gonorrhoeae isolates in 26 European countries, 2019. BMC Infect Dis. 2022;22(1):524. 10.1186/s12879-022-07509-w [DOI] [PMC free article] [PubMed] [Google Scholar]


