Abstract
Background and Aims
Ornamental flowering plant species are often used in managed greenspaces to attract and support pollinator populations. In natural systems, selection by pollinators is hypothesized to result in convergent multimodal floral phenotypes that are more attractive to specific pollinator taxa. In contrast, ornamental cultivars are bred via artificial selection by humans, and exhibit diverse and distinct phenotypes. Despite their prevalence in managed habitats, the influence of cultivar phenotypic variation on plant attractiveness to pollinator taxa is not well resolved.
Methods
We used a combination of field and behavioural assays to evaluate how variation in floral visual, chemical and nutritional traits impacted overall attractiveness and visitation by pollinator taxonomic groups and bee species to 25 cultivars of five herbaceous perennial ornamental plant genera.
Key results
Despite significant phenotypic variation, cultivars tended to attract a broad range of pollinator species. Nonetheless, at the level of insect order (bee, fly, butterfly, beetle), attraction was generally modulated by traits consistent with the pollination syndrome hypothesis. At the level of bee species, the relative influence of traits on visitation varied across plant genera, with some floral phenotypes leading to a broadening of the visitor community, and others leading to exclusion of visitation by certain bee species.
Conclusions
Our results demonstrate how pollinator choice is mediated by complex multimodal floral signals. Importantly, the traits that had the greatest and most consistent effect on regulating pollinator attraction were those that are commonly selected for in cultivar development. Though variation among cultivars in floral traits may limit the pollinator community by excluding certain species, it may also encourage interactions with generalist taxa to support pollinator diversity in managed landscapes.
Keywords: Salvia nemorosa, Rudbeckia spp, Echinacea spp, Nepeta spp, Agastache spp, pollinators, plant–pollinator interactions, pollination syndromes, ornamentals, bees, floral traits
INTRODUCTION
Human land-use has led to a global reduction in pollinator habitat and decline of high-quality foraging resources (Potts et al., 2010; Wagner et al., 2021). To mitigate these losses and support pollinator populations, there has been increased public interest in creating pollinator habitat in managed landscapes such as gardens, municipal parks and seminatural habitats (Campbell et al., 2017; Majewska and Altizer, 2020). Due to commercial availability and market preferences (Campbell et al., 2017), many of the flowering plant species used in these domestic environments are ornamental cultivars (Frankie et al., 2005; Burghardt et al., 2009). Certain cultivars have the potential to support pollinator communities, particularly in the context of highly modified urban and suburban landscapes (Garbuzov and Ratnieks, 2014; Rollings and Goulson, 2019; Erickson et al., 2020; Sponsler et al., 2020). However, ornamental cultivars result from a long history of breeding through hybridization and artificial selection (Horn, 2002; Wilde et al., 2015) with human preference as the primary selection pressure. Thus, these cultivars represent a range of floral phenotypes that are often distinct from parent wild types (Garbuzov and Ratnieks, 2015; Erickson et al., 2020). Despite their prevalence in anthropogenic landscapes and managed pollinator habitat, the mechanisms of pollinator community attraction to these varieties are not well understood.
Angiosperms are phenotypically diverse (Armbruster, 2014), and the complex structural, visual, chemical and nutritional traits of flowers are important determinants of pollinator foraging behaviour (Leonard et al., 2011; Willmer, 2011; Junker and Parachnowitsch, 2015). Pollinator taxa (e.g. bees, flies, butterflies and beetles) vary in their perception of and preference for suites of floral traits based on natural history, sensory abilities, learned associations and cognition (Chittka and Raine, 2006; Raine and Chittka, 2007; Willmer, 2011). Pollinator species’ behaviour and morphology influence their ability to access floral resources and serve as effective pollen vectors (Wang et al., 2017). Bee taxa, in particular, exhibit an impressive diversity of foraging behaviours and are especially variable in their preference for floral resources (Danforth et al., 2019). In co-evolved plant–pollinator communities, reciprocal adaptation of plants and pollinators has led to evolution of distinct floral phenotypes which attract the dominant or most effective pollinator taxonomic group (Willmer, 2011). In mutualistic plant–pollinator relationships, floral visual and olfactory cues – or ‘advertisements’ – are hypothesized to be generally honest signals of nutritional rewards (primarily pollen and nectar) to visiting pollinators (Wright and Schiestl, 2009; Schiestl and Johnson, 2013; Knauer and Schiestl, 2015). Honest floral signals facilitate pollinator learning (Knauer and Schiestl, 2015), which increases forager constancy and efficiency (Ortiz et al., 2021), even in highly opportunistic foragers.
Horticultural plant breeding results in significant variation in traits such as colour, morphology and nutritional reward among cultivars of the same genus or even species (Comba et al., 1999; Corbet et al., 2001; Garbuzov and Ratnieks, 2014, 2015; Erickson et al., 2020, 2021). Notably, some floral traits that are relevant to pollinator attraction, such as floral display size (Thompson, 2001), may also be influenced by cultural practices (e.g. pruning, planting design) as well as breeding. Since floral phenotypes in these cultivars have been selected by humans and not pollinators, floral advertisements and rewards may be uncoupled from pollinator preference. In extreme cases, ornamental plant breeding produces cultivars that are valueless or entirely inaccessible to pollinators, such as doubled varieties, or those that no longer offer nutritional rewards (Comba et al., 1999; Erickson et al., 2020). Nonetheless, many ornamental cultivars do have the capacity to attract and support pollinators (Garbuzov and Ratnieks, 2014, 2015; Erickson et al., 2020, 2021). However, several field studies have demonstrated significant variation in the overall and relative attractiveness to pollinator taxa among cultivars of single species (Garbuzov and Ratnieks, 2014; Erickson et al., 2020, 2021), indicating a potential interaction between floral phenotype and overall plant attractiveness.
To date, many studies on pollinator mediated selection on floral traits have focused on visitation by single species and primarily in specialized systems (Burger et al., 2010; Byers et al., 2014). However, generalization is ubiquitous in ecological communities (Danforth et al., 2019) and interactions between plants and pollinators exhibit a high degree of stochasticity and are rarely obligate (Willmer, 2011). Ornamental plants often attract generalized pollinators (Erickson et al., 2020, 2021). As they exhibit a high degree of variation in floral phenotype both within and across genera, these cultivars offer a powerful system to test the attractiveness of diverse floral traits to a generalized pollinator community. Understanding how pollinator taxa and communities respond to variation in floral traits is critical for untangling the role of generalist species or taxonomic groups as agents of selection (Waser et al., 2009; Gervasi and Schiestl, 2017).
Ornamental cultivars within genera often vary in a few measurable traits and probably have limited intracultivar genetic variability due to propagation methods. Thus, they present an opportunity to test the relative influence of single and multimodal floral traits on mediating pollinator species and community foraging behaviour in the context of a whole floral display. Floral advertisements are complex signals, and multiple traits often function synergistically or antagonistically upon multiple pollinator sensory modalities (hereafter referred to as ‘multimodal traits’) (Williams et al., 2010; Junker and Parachnowitsch, 2015; Russell et al., 2018). Furthermore, floral visual, chemical and nutritional traits can be pleiotropic at a genetic level (Smith, 2016), meaning that selection on one trait may influence the expression of another trait, which in turn influences pollinator attraction. Thus, there is a clear need for additional studies that can further elucidate the relationship between pollinator choice and floral traits against the backdrop of a multimodal phenotype (Leonard et al., 2011).
In this field and lab-based study, we use multivariate analyses to test how key floral traits (floral morphology, colour, scent, pollen and nectar rewards) of 25 ornamental plant cultivars across five herbaceous perennial plant genera influence pollinator visitation. For the first level of analysis, we examined how floral phenotypic traits across plant genera explained broad patterns of bee, fly, beetle and butterfly visitation. Because bees were found to visit all 25 cultivars and have sophisticated sensory systems that vary across species, we next investigated how trait variation among cultivars within plant genera structured floral preferences of bee species.
METHODS
Plant selection
Plants were selected to reflect variation in cultivar phenotype both among and within genera of commercially popular taxa. The five plant taxa used in this study were: Salvia nemorosa, Nepeta spp., Echinacea spp., Rudbeckia spp. and Agastache spp. The former two taxa are non-native to the Nearctic region while the latter three are native to this region. All taxa had significant wholesale value in the North American market and thus can be assumed to be widely used in home garden and landscaping applications (USDA—NASS, 2014). Plants were purchased from North Creek Nursery (Oxford, PA), Creek Hill Nursery (Leola, PA), Russell Gardens (Southampton, PA), Morningsun Farm (Vacaville, CA) and Bluestone Perennials (Madison, OH). Original plants were purchased in 2017 and were replaced annually as needed after assessing overwinter survival. Plants were never treated with neonicotinoid insecticides throughout the study, and glasshouse pest outbreaks were managed with insecticidal soap. Care was taken to ensure that no collections were done on plants immediately following treatment. We cannot account for how the plants were managed at all nurseries prior to our acquiring them (see Supporting Data for details on plug treatment at the nursery where most plant material was sourced). However, based on the time span between any potential application of any pesticides at the source nursery and when our data were collected, we expect that pollinator exposure in the field to any chemicals that would confound our results would be negligible (Cowles and Eitzer, 2017; Mach et al., 2018).
Pollinator preference assessments
As environmental context and ecological community dynamics can shape plant attractiveness to pollinators (Geslin et al., 2013; Kantsa et al., 2018), pollinator foraging preferences were assessed using both field collections and lab-based choice assays.
Field collections
The data used in these analyses were a subset of a data set used in a previous study (see Erickson et al., 2021 for details). Briefly, field data were collected across two independent sites in 2019 at the Penn State Russell E. Larson Agricultural Research Center in Pine Grove Furnace, PA (Site 1 = 40.704634, −77.973045, Site 2 = 40.712329, −77.933609). Due to poor soil conditions, plants at Site 1 were in pots whereas plants at Site 2 were planted directly in the ground. All potted plants were grown using Sungro MetroMix 830 (Agawam, MA, USA) media. Plants were fertilized at both sites in May 2019 to genus-specific levels with Osmocote Plus 7.5-g Tablets 15-8-11 (Scott’s Miracle-Gro, Marysville, OH, USA). Plants were arranged in a randomized complete block design with four blocks per plot and one replicate of each cultivar per block, for a total of 100 plants at each site. Within each block, plants were spaced 1 m apart and blocks 1.5 m apart.
All insect flower visitors to each plant replicate were collected for 5 min every other week from May 23 to September 23, 2019. For each sampling event, specimens were collected from each replicate once in the morning (09:00–13:00 h) and once in the afternoon (13:01–17:00 h) using an insect vacuum (Bioquip, Rancho Dominguez, CA, USA). Collections were performed in sets of two down the rows of the plot and the starting point alternated for each collection event. Specimens were euthanized in the field using dry ice then transferred to individual vials and stored in the laboratory at −20 °C until they could be processed and identified. Specimens were pinned and bees identified to species with assistance from S. Droege (USGS Patuxent Wildlife Research Center).
Lab-based choice assays
Cultivar choice and foraging behaviour of Bombus impatiens foragers on cultivars were recorded in the laboratory. Experiments were conducted using two custom built foraging arenas as in Russell et al. (2018) (see Supplementary Information Figure S1 for specifications). Bombus impatiens colonies were purchased from Koppert Biological Systems (Howell, MI, USA). Colonies were only included in choice assays if they were in the social phase of their life cycle – that is, they had not initiated gyne production and had few males (Amsalem et al., 2015). When not in use, colonies were stored in an incubator at 23 °C and maintained on 50 % (w/w) sugar syrup and honey bee-collected pollen according to standard practices (Velthuis and van Doorn, 2006). All colonies used in this study were flower naive – meaning they had never been previously exposed to real flowers or floral advertisements (ex. floral scent, colour). See Supplementary Information Table S1 for details on colony-level replication.
Before testing, colonies were attached to the training arena via a 12 × 5-cm plastic tube to allow foragers to acclimate. Each training arena included two feeders with 20 % (w/w) sucrose solution and a tray of honey bee-collected pollen. Feeders were painted black to avoid foragers associating colours found in test flowers with reward (Muth et al., 2015). Foragers were allowed to pass freely between the colony and the training arena for a minimum of 24 h and a maximum of 7 d before testing. Feeders were replenished daily to ensure consistent availability of food.
A separate arena was used to test forager preference for cultivars. Experimental trials were performed for within-individual plant genera, and individual foragers were allowed to choose between the five cultivars of either Agastache spp., Echinacea spp. and Rudbeckia spp. In each trial, three replicate inflorescences for each cultivar (grown in the glasshouse) were placed in water picks and mounted vertically on the long side of the arena. The source plant for these inflorescences was randomized across trials. Care was taken to select only inflorescences that were still producing nectar and/or pollen rewards and appeared healthy. Individual inflorescences were replaced after a successful foraging event or every 2 h if not visited to ensure freshness and presence of consistent floral scent and reward (Morrant et al., 2009; Ao et al., 2013). At the start of each trial, inflorescences were rearranged to prevent foragers from associating particular areas of the arena with rewards, and the locations of each flower were recorded (Raine and Chittka, 2007).
To test how cultivar phenotype influenced initial preferences, individual foragers that had not been previously tested, hereafter referred to as ‘naive foragers’, were collected from the training arena using an insect vacuum (Bioquip). Naive foragers were introduced to the training arena from the vacuum’s clear plastic collection canister. The testing trial started once the single forager entered the arena. Foragers were allowed to visit three inflorescences before being collected and removed from the arena. For each trial, time to initiate foraging (the time between entering the arena and initiating foraging on the first cultivar), cultivar choice (which cultivars the bee successfully foraged on), duration of foraging (length of time spent foraging on each of the three inflorescences), time between foraging events (interval between foraging events), and pollen and nectar collecting behaviour were recorded. Pollen foraging behaviour was characterized by the bee moving erratically over the anthers and brushing pollen onto the corbicula, while nectar foraging behaviour was characterized by the bee probing the corolla with its proboscis extended. Foragers that did not fly within 5 min or that flew but did not explore flowers (exploration being classified as a bee flying within ~5 cm of the flower and exhibiting signs of recognizing the flower such as circling or directional flight but ultimately not foraging) within 10 min were removed from trials.
Next, we used the successful foragers from the initial trial – hereafter referred to as ‘experienced foragers’ – to test whether floral visual and olfactory advertisements were a sufficiently honest signal of nutritional rewards (Wright and Schiestl, 2009; Knauer and Schiestl, 2015). Experienced foragers were held in isolation for 1 h in a plastic collection canister before reintroducing them for an ‘experienced’ trial and recording the same metrics. For each plant genus, there were between three and seven replicate trials per colony (most had six replicates) and either four or five colonies (see Supplementary Information for details of the data sets generated). All foragers that were introduced to the test arena, regardless of whether they had a successful foraging bout, were killed promptly to avoid their nestmates from gaining information on resource quality or floral cues (Molet et al., 2009).
Assessment of plant traits
Two additional replicates of each cultivar were grown in the glasshouse in 2018, 2019 and 2020 for collection of data on floral traits. Additionally, plants that had been housed outdoors in 2018 and 2019 and used for field data collections were brought into the glasshouse for use in choice assays and traits collections. All glasshouse plants were replaced in 2018. In 2019, all glasshouse plants were vernalized outdoors for reuse the following season. See Supplementary Information Table S2 for details on which plant sets were used for collection of different trait data, and associated number of replicates.
Floral area and plant height
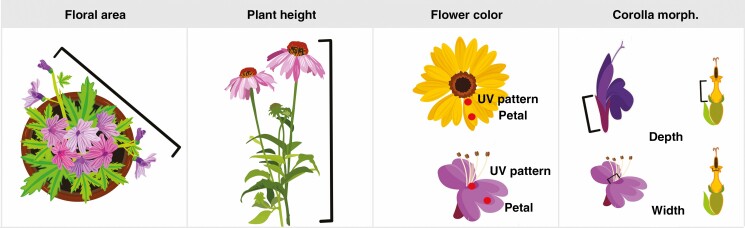
Total floral display area was measured every other week on each plant replicate in the field throughout the season in 2019 by photographing a single plant from above with a metre stick held at the level of the crown of the floral display. These photos were processed in Adobe Photoshop (22.3.0) by setting a unique measurement scale in pixels based on the metre stick and then selecting all flowers, based on colour, to calculate the total area in in bloom (Fig. 1). Additionally, the number of inflorescences per plant with at least 10 % of flowers or florets in anthesis was measured for each photo and was used to standardize visitor abundance measures.
Fig. 1.
Diagram of trait collections for floral area, plant height, flower colour and corolla morphology.
Plant height was measured from the ground to the crown of the plant for each replicate in the field once at peak maturity. For all plant taxa in this study, vertical growth was minimal after the initiation of flowering (Fig. 1).
Floral colour
Flower colour reflectance was measured for five technical replicate inflorescences from each of the two biological replicates (different plants) collected from the glasshouse using an Ocean Optics USB2000 + UV-VIS spectrometer and DH-2000BAL light source with a 200-μm reflectance probe (Ocean Optics, Winter Park, FL, USA). Before beginning collections, the spectrometer was first standardized using a white diffuse reflectance standard (Ocean Optics). Collections were all done indoors with an overhead LED as the sole ambient light source. For each flower, measurements were performed in triplicate at two points (petals and the mouth of the corolla) by holding the probe at a 90° angle 1 cm from the petal surface and then averaging measurements from for each point for analysis. For all measurements, the spectrometer was set to a 0.1-s integration time with 10 scans to average, and a boxcar width of 0. Petal measurements were used to represent flower colour for analyses, and reflectance measures at both points were used to detect the presence of ultraviolet patterns (Chittka et al., 1994; Horth et al., 2014).
Reflectance data were processed using Ocean View software (Ocean Optics, Version 4.1). Reflectance measures gathered between wavelengths of 300 and 700 nm were averaged across consecutive 5-nm segments for each replicate. Pollinators vary widely in their colour perception, so these raw spectral data were used for ordination analyses to prevent biasing results towards any particular taxa (Shrestha et al., 2013). For linear models, where a single value is needed for analysis, hue angle was calculated for each replicate based on trichromatic human colour space using the ‘pavo2’ (Maia et al., 2019) package in R. The presence of ultraviolet patterns, such as nectar guides (Leonard and Papaj 2011), was checked for each cultivar by identifying whether any measured wavelengths within 300–400 nm had reflectance values of at least 10 % intensity of the peak value (Chittka et al., 1994). A cultivar was considered to have ultraviolet pattern present if wavelengths in the UV spectrum were recorded in at least two replicate flowers (Fig. 1).
Floral scent
Headspace volatile organic compound (VOC) emissions were measured on glasshouse plants using dynamic headspace sampling. Individual intact inflorescences were enclosed with a nylon oven bag (Reynolds Consumer Products, Lake Forest, IL, USA) that had been baked for 24 h at 80 °C to remove any polymer VOCs (Stewart-Jones and Poppy, 2006) and secured at the stem with twist ties. VOCs were collected over 8 h from around 09:00 to 17:00 h at a flow rate of 250 mL min–1 over HayeSepQ filter traps (Sigma Aldrich, USA). Passive incoming air was purified over activated charcoal. VOCs were collected on single inflorescences from three biological replicates (individual plants) per cultivar.
Additional collections were performed to compare headspace VOCs on cut vs. intact flowers to determine whether cut flowers (used in behavioural assays) had altered profiles. The rate of VOC emissions and VOC diversity between glasshouse and cut flower collections were compared using ANOVA and the number and identity of unique compounds in cut vs. intact flowers for each cultivar was assessed (presence/absence). See SUpplementary Information for further details on these analyses.
Following collections, measured inflorescences were cut and dried at 37 °C for 48 h to then be weighed. Dry tissue weight was used to standardize emission rates. VOCs were eluted from filter traps using 150 µL dichloromethane and 5 µL of an internal nonyl acetate standard and stored at −80 °C until analysis. Samples were analysed using an Agilent 7890B gas chromatograph and 5977B mass spectrometer held at 250 °C. For each sample, 1 µL was injected and run through a column (HP-5MSUI, 30 m, 0.25 mm id, 0.25 μm, Agilent, USA) held at 40 °C. Compounds were classified based on Knudsen et al. 2006. See Supplementary Information for information on compound identification. The log sum emission rate (ng g–1 h–1), Simpson’s diversity (1/D) of all emitted compounds and the proportional emissions rate of each compound (the latter two measures based on non-log-transformed raw values) were calculated for each cultivar for statistical analyses.
Nectar
Nectar was collected from five florets per inflorescence at five replicate inflorescences per plant and on two replicates (individual plants) for each cultivar at both Site 1 in 2019 and Site 2 in 2018 and 2019, for a total of 10 total replicates per cultivar per site, using 1- to 10-µL glass microcapillary tubes. Plants were covered with fine mesh exclusion bags in the field for 24 h prior to collections to ensure adequate availability of nectar reward. Collections were only performed on healthy plants between 09:00 and 17:00 h. Nectar volume was estimated for each flower based on the height of the liquid in each capillary tube, measured using digital calipers (Mitutoyo 500-196-30).
Variation in nectar quantity and quality between sampling locations and years (for Site 2) was assessed using a linear mixed effects model with site or year and cultivar as fixed effects and the biological replicate identifier as the random effect (see Supplementary Information for results).
To assess nectar sugar composition, the cumulative nectar from a single inflorescence (five florets) was then expelled onto filter paper using a rubber bulb and samples were stored at −20 °C until analysis. Nectar volume per floret was estimated by averaging the cumulative nectar volume per inflorescence by the number of florets collected from. Nectar samples were washed from the filter paper using 20 µL to 1 mL of water (Morrant et al., 2009) (approximately a 1:100 volume dilution, which was accounted for in the final analysis) and then the eluted samples were cleaned for analysis using 0.45-μm spin filters in 1.5-mL microcentrifuge tubes, with centrifugation at 13000 r.p.m. for 10 min (Power et al., 2018). The fructose and glucose (monosaccharide) and sucrose (disaccharide) concentrations and the total concentration of the diluted nectar samples were analysed at the CSL Behring Fermentation Facility using a Thermo Scientific Vanquish UHPLC system (Waltham, MA, USA) with a 50 × 2.1-mm Thermo Scientific Hypersil Gold amino HPLC column for 1.9-μm particle size and a Refractomax 521 Refractive Index (RI) Detector. Due to column ageing, methods had to be adjusted between runs; however, a two-way ANOVA and Tukey post-hoc test confirmed no significant differences between methods in the final results (data not shown). In total, 2.5–3 µL of sample was run through the UHPLC in a mobile phase of either 80 % acetonitrile (ACN)/20 % water or 85% ACN/15% water at a flow rate of either 0.5 or 1 mL min–1. The RI Detectorwas held at 35 °C for all runs and the column compartment was held at either 40 or 35 °C (see Supplementary Information for specific methods). In cases where a single sample was analysed multiple times using different methods, values were averaged.
Pollen
Pollen samples were collected by clipping mature anthers from five inflorescences from two replicates of each cultivar at each field site and two replicates of each cultivar in the glasshouse. Plants in the field were covered for 24 h with a fine mesh exclusion bag before collections. Samples were stored at −20 °C until analysis. See Supplementary Material for details on pollen extraction from anthers.
Pollen protein content was measured using a Bradford assay following protocols from Vaudo et al. (2016) and the amount of reagent used was adjusted depending on sample weight (see Supporting Data). Total protein concentration in the sample was measured using a SpectraMax® 190 Microplate Reader (Molecular Devices, San Jose, CA, USA) and standardized using total sample mass. Due to variation in results and abiotic factors, only samples collected in the field and with at least 0.007 mg of tissue were ultimately included in statistical analyses on pollen protein.
Total pollen amount for each replicate was estimated by calculating the mass of the pollen sample for each cultivar replicate.
Corolla morphology
Corolla depth (from base to mouth) and width (diameter at mouth) was measured on glasshouse plants using adjustable digital calipers (Mitutoyo 500-196-30), as in Courcelles et al. (2013). Twenty-five replicates of each measurement were taken on two biological replicates per cultivar (Fig. 1).
Statistical analysis
All quantitative analyses were performed in the R statistical software version 4.0.3.
Phenotypic variation among cultivars and genera
The difference among cultivars (predictor variable) in individual floral trait states (response variables) was estimated using separate linear mixed effects models (LMMs) or linear models (LMs) (see Supplementary Information for model information) and comparisons among cultivars were calculated using Tukey post-hoc tests. Model fits were assessed using analysis of residuals.
Floral traits that predict overall abundance of pollinators in field assays
A generalized linear model (GLM) fit to a Gamma distribution was used to estimate which traits explained the overall attractiveness of a cultivar to pollinators in the field. The response variable for this analysis was ‘mean abundance of all visitors/5 min’ to each plant replicate in the field. The predictor variables were the mean values for individual floral traits measurements for each cultivar, except for floral display area and plant height, which were averaged for each cultivar at each site, to account for variation in growth patterns. Based on analysis of residuals, the predictor variables plant height, floral area and mean VOC emissions were log-transformed. Model fit was assessed using analysis of residuals. In addition, residuals were checked using the DHARMa package to check quantiles and outliers (Hartig 2021) (see Supplementary Information Fig. S2 for analysis of residuals).
Determining how floral traits across plant genera influence relative attraction of pollinator taxonomic groups in field assays
Mean abundance of visitors per inflorescence in 5 min from each pollinator taxonomic group (Anthophila, Coleoptera, Diptera, Lepidoptera) was calculated for each plant replicate in the field. To avoid ‘rare’ groups that may confound analyses, visitors from a taxonomic group that were collected fewer than five times at a cultivar across the whole season were removed.
Non-metric multi-dimensional scaling (NMDS) was used for the following analyses as this method allows the user to specify the similarity function. Separate ordinations were used to determine assembly of plant taxa in traits space for each set of traits. The separation of plant taxa in traits space was analysed using a PERMANOVA. For all PERMANOVAs, 999 permutations were used. Plant display size, based on plant height and floral display area, colour (binned reflectance values), corolla morphology, headspace VOCs emission rate and inverse Simpson’s diversity (1/D – used in place of raw VOC compound number to account for convergence errors due to variable scaling), and nectar and pollen qualities were analysed separately using a Euclidean dissimilarity matrix. As outlier observations receive disproportionate weight in a Euclidean dissimilarity matrix, all of the aforementioned trait measurements were log-transformed prior to ordination, with the exception of nectar monosaccharide percentage and colour as these values are on a discrete scale. A Bray–Curtis dissimilarity matrix, which can handle zero values, was used to analyse proportional emission rates of compounds. For traits collected in the field (plant height, floral display area), site was included as a fixed effect for PERMANOVAs (data not shown). For traits that were collected with repeated measures on biological replicates (colour, morphology, nectar, pollen), additional PERMANOVAs were conducted to account for variation within replicates (or, random effects) with the fixed effect of replicate and plant identifier set as the strata argument (Theodorou et al., 2020) (see Supplementary Information for results on these analyses). Distance-based analyses may be biased by data with high variance and heterogeneity (Warton et al., 2012), and so ultimately only ordinations with a PERMANOVA statistic (R2) of at least 0.15 were included in analyses. To visualize interactions between floral traits and pollinator preference, environmental vectors indicating the direction and strength of pollinator taxonomic group abundance in phenotypic space were fit to each floral trait ordination using the ‘vegan’ package (Oksanen et al., 2020).
Inter-cultivar variation in floral phenotypic traits and bee species’ visitation in field assays
How variation among cultivars within genera in floral phenotype impacts relative attractiveness and accessibility of a cultivar to pollinators was assessed at the pollinator species level. As Anthophila visitors were ubiquitous across cultivars and their natural history and functional traits are well resolved, these analyses were performed only for bee species.
The mean abundance of visitors/inflorescence/5 min to cultivars within bee species was calculated for each plant replicate across the growing season. Bee species with fewer than four specimens collected per cultivar were excluded from the analysis. The remaining species were then categorized based on functional traits that may be relevant to foraging behaviour in this system. The bee functional traits used for this study were intertegular distance (ITD), which is a proxy for body size (Normandin et al., 2017; Laha et al., 2020), tongue length, diet breadth, phenology and pollen transport system. The latter four traits were assessed for each species using previously published literature (Bartomeus et al., 2013; Normandin et al., 2017). ITD was measured on five randomly selected pinned specimens per bee species from this field study and then these measurements were averaged for each species. ITD measures were then converted to categorical data for analyses using 2 mm as the range of sizes included within each category that was relative to our data (ex. ‘small’) (see Supplementary Information Table S3 for classifications).
The mean abundance of bee visitors/inflorescence/5 min based on functional traits was calculated for each plant replicate in the field.
NMDS ordinations of bee species preference, based on abundance of individuals within each species with shared functional traits collected on each plant replicate, were performed for the five cultivars in each plant genus using a Bray–Curtis dissimilarity matrix. Dissimilarity of cultivars in bee preference space was assessed using PERMANOVA with site set for the strata argument.
Additional NMDS ordinations of cultivar floral functional traits (ex. colour, morphology) were performed within plant genera and the dissimilarity of cultivars in traits space was assessed using PERMANOVA. Mantel tests (with a Spearman correlation coefficient) were performed to assess whether dissimilarity of cultivars based on floral functional traits were correlated with dissimilarity of cultivars based on bee visitation, and when relevant to the trait, abundance of visitors by bee functional trait. Mantel tests with a significant correlation coefficient (R) below 0.15 were considered to have weak associations between preference and trait matrices (Johnson, 2013).
Validation of bee species preference to cultivars in laboratory choice assays
Community and environmental context can shape pollinator visitation to flowers (Cusser et al., 2019). To ensure that the patterns of bee species preference for cultivars used in our analyses were not simply an artifact of these variables, preferences of B. impatiens foragers to cultivars in the field and in laboratory choice assays were compared.
The probability of a cultivar being visited by a B. impatiens forager was modelled separately for lab and field assays and within plant genera. Generalized linear mixed models (GLMMs) were used to model field data, with individual plant replicate included as a random effect and number of inflorescences with flowers in anthesis and cultivar as predictor variables. Due to model singularity when choice assay data were run as GLMMs with colony as a mixed effect, GLMs were used. However, additional analyses (not shown) were run to confirm that colony did not have a significant effect on choice assay model results. Both field and choice assay models were fit to a binomial distribution. The estimated marginal means (EMMs) of the probability that a forager visited a cultivar were extracted from each model (Lenth 2020).
Within choice assays, the probability of a cultivar being the first or second choice and the probability of a cultivar being the first choice in a naive vs. experienced trial was compared using EMMs extracted from a binomial GLM and a Tukey post-hoc test (data not shown).
RESULTS
Phenotypic variation of cultivars
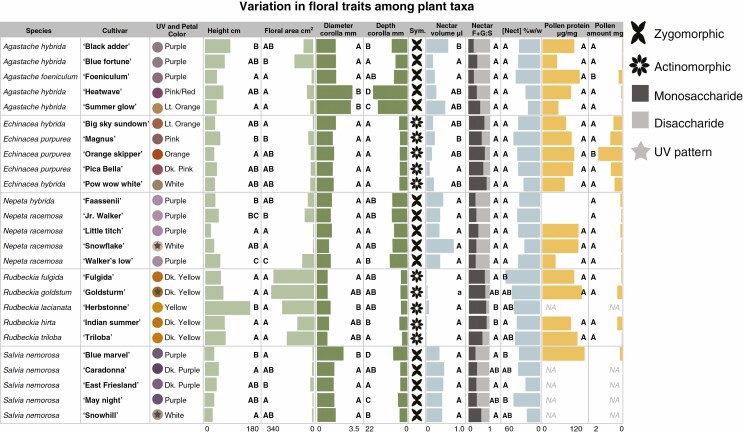
Within genera, cultivars exhibited significant variation in some floral phenotypic traits but not others (results are summarized in Fig. 2). All cultivars varied in petal colour (visualized by hue angle in human colour space), and ultraviolet pattern was detected in three cultivars in three separate genera. Cultivars varied significantly in at least one trait pertaining to plant size (area and height). Cultivars of all genera except Echinacea spp. differed from one another in at least one trait related to corolla morphology, with corolla depth being the most variable. All cultivars of Agastache spp., Nepeta spp. and S. nemorosa were zygomorphic while those of Echinacea spp. and Rudbeckia spp. were actinomorphic.
Fig. 2.
Means of trait measurements for each cultivar based on emmeans from LM and LMM regression analyses. Letters denote significant differences among cultivars within plant genera based on Tukey post-hoc test results at P < 0.05. Cultivars within all plant genera vary in some traits, with the greatest variation in flower colour, plant size and corolla morphology. Cultivars with a star over the colour point are those where a UV patten was detected in reflectance measures. ‘Sym’ refers to flower symmetry (either zygomorphic or actinomorphic). Nectar F + G:S refers to the nectar monosaccharide to disaccharide ratios. Pollen amount is measured by weighing dry tissue mass of collected samples before analysis.
There was significant variability among cultivars of Agastache spp. and Echinacea spp. in nectar volume and among cultivars of Rudbeckia spp. and S. nemorosa in nectar concentration and percent monosaccharide content (Fig. 2). Pollen protein content did not differ among cultivars of any genera, but the amount of available pollen varied significantly in Agastache spp., Echinacea spp. and Rudbeckia spp., with no pollen produced by R. lacianata ‘Herbstonne’. All cultivars of S. nemorosa except ‘Blue Marvel’ produced no pollen, and thus analyses of pollen traits on S. nemorosa were excluded from subsequent analyses.
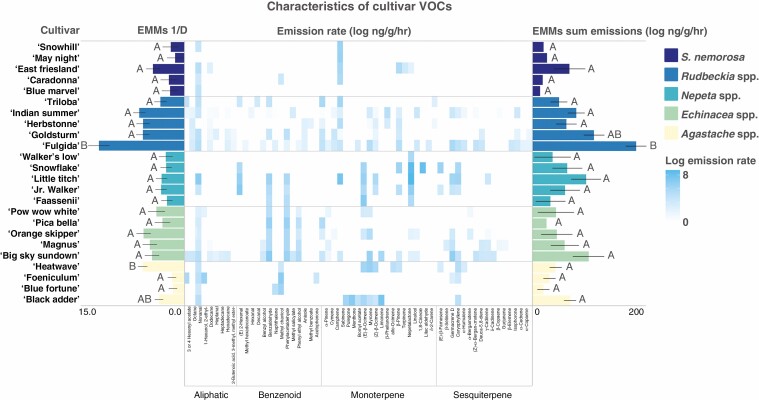
Only cultivars of Rudbeckia spp. varied significantly from one another in the total emission rate of headspace volatiles, with ‘Fulgida’ emitting the highest amount. Cultivars of Rudbeckia spp. also varied significantly in the diversity of floral headspace volatiles emitted, with ‘Fulgida’ exhibiting the most complex blend and with seven unique compounds for the genus (Fig. 3). The ‘Summer Glow’ cultivar of Agastache spp. did not produce a detectable level of VOCs and thus was excluded from analyses.
Fig. 3.
Simpson’s diversity, log emissions rate of each VOC compound, and total volatile log total emissions rate for each cultivar. Letters denote significant variation among cultivars within genera. Cultivars within some genera differ significantly in emissions rate and diversity, but most genera do not. Cultivars within many plant genera share similar VOC profiles, but some genera emit compounds unique from the rest.
Floral phenotype and total visitor abundance
GLM analyses revealed a significant relationship of total pollinator abundance with corolla depth, colour, nectar composition and floral display area, although the last had the greatest positive effect (t = 6.09, P < 0.01) on total visitor abundance (see Supplementary Information for model output and analysis of residuals).
Floral phenotype and pollinator taxonomic groups preference in the field
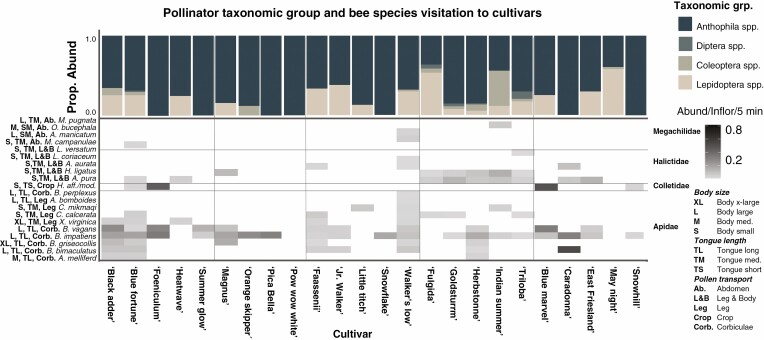
Across all cultivars and genera, visitors within the group Anthophila were the most abundant taxa collected at 23 out of 25 cultivars, and were the sole taxa collected on seven out of 25 cultivars. Mean abundance per inflorescence of insect taxonomic groups visiting a cultivar in a 5-min period ranged from 0.05 ± 0.01 to 0.59 ± 0.15 for Anthophila spp., 0.00 to 0.19 ± 0.11 for Coleoptera spp., 0.00 to 0.01 ± 0.01 for Diptera spp., and 0.00 to 0.46 ± 0.26 for Lepidoptera spp. (Fig. 4). Within Coleoptera, the pollen- and nectar-feeding species Chauliognathus pensylvanicus and C. marginatus were the most abundant on flowers (Riley 1892; Stugard 1931). The primary taxa within Diptera were also all flower-visiting taxa and were within the families Bombyliidae, Conopidae, Syrphidae and Tachinidae (Souza-Silva et al., 2001).
Fig. 4.
Abundance of pollinator visitors by taxonomic group and by bee species to cultivars across sites. Bee morphological traits are listed next to the species name. To avoid biasing results with ‘rare’ taxa, any taxonomic group with fewer than five total visitors to a cultivar across the whole season were removed and any bee species with fewer than four visitors to a cultivar across the whole season were removed. Bees are the primary visitors to cultivars, but cultivars vary in the bee taxa attracted and the functional traits of those species.
Analyses of individual floral functional traits and pollinator attraction across plant genera
Visual
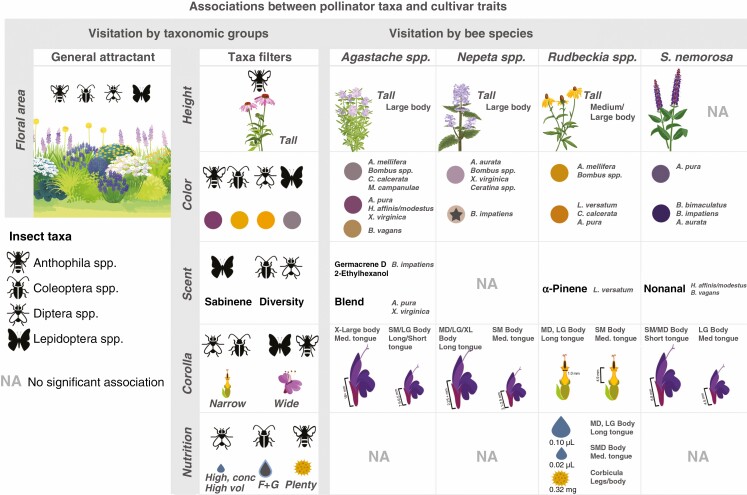
Ordination analyses of visitor abundance/inflorescence/5 min confirmed a positive significant relationship between plant display size (PERMANOVA R2 = 0.50, P < 0.01, Stress = 0.022) and pollinator preference – specifically, total abundance of Anthophila spp. increased with plant height (R2 = 0.14, P < 0.01). Abundance of Lepidoptera spp. decreased with plant height in multidimensional space (R2 = 0.09, P < 0.01) but not in the direction of any cluster of plant genera, and thus this relationship is inconclusive.
Variation across genera in colour traits space, based on raw spectral data, (PERMANOVA R2 = 0.58, P < 0.01, Stress = 0.06) was associated with visitation rate of all pollinator taxonomic groups. Visitation by Diptera spp. and Coleoptera spp. was positively correlated in the direction of yellow flower colours (R2 = 0.36, P < 0.01; R2 = 0.20, P < 0.01) while Lepidoptera spp. and Anthophila spp. were positively correlated in the direction of purple flower colour (R2 = 0.08, P < 0.01; R2 = 0.14, P < 0.01).
In corolla morphology space (PERMANOVA R2 = 0.71, P < 0.01, Stress = 0.00), visitation by Anthophila spp. and Lepidoptera spp. was positively correlated in the general direction of tubular corolla width (R2 = 0.17, P < 0.01; R2 = 0.01, P < 0.01), while visitation by Coleoptera spp. and Diptera spp. increased in the opposite direction (R2 = 0.04, P < 0.01; R2 = 0.28, P < 0.01), indicating that these taxa preferred flowers with narrower corollas (Table 1; Fig. 5).
Table 1.
Dissimilarity of cultivars in traits space and correlation with bee visitation
| Plant Size | Colour | Corolla morphology | Nectar quality | Pollen quality | Scent emissions | Scent composition | ||
|---|---|---|---|---|---|---|---|---|
| All genera | 0.50** | 0.58** | 0.71** | 0.35* | 0.40** | 0.18* | 0.33** | PERMANOVA R 2 |
| Agastachespp. | 0.15 o | 0.60** | 0.89** | 0.04 | 0.14 | 0.57o | 0.68** | |
| Echinaceaspp. | 0.16 | 0.45** | 0.04* | 0.12o | 0.07 | 0.06 | 0.13 | |
| Nepetaspp. | 0.52** | 0.23** | 0.31** | 0.06 | 0.13 | 0.28 | 0.29 | |
| Rudbeckiaspp. | 0.16* | 0.28** | 0.55** | 0.33** | 0.21** | 0.85** | 0.62** | |
| S. nemorosa | 0.16 | 0.61** | 0.87** | 0.18** | NA | 0.27 | 0.49** | |
| Agastachespp. | 0.28** | 0.42** | 0.56** | 0.01 | 0.01 | −0.13 | 0.16o | Bee species Mantel R |
| Echinaceaspp. | 0.63** | 0.28** | −0.02 | 0.05o | −0.01 | −0.12 | −0.02 | |
| Nepetaspp. | 0.48** | 0.15** | 0.21** | 0.08* | 0.04 | 0.08 | −0.02 | |
| Rudbeckiaspp. | 0.30** | 0.10** | 0.41** | 0.02 | 0.12* | 0.00 | 0.21o | |
| S. nemorosa | 0.13 | 0.37** | 0.50** | 0.06o | NA | −0.34 | 0.540 | |
| Body size | Body size | Pollen transport | ||||||
| Agastachespp. | 0.29* | 0.53** | 0.00 | 0.03 | Fun. traits Mantel R | |||
| Echinaceaspp. | 0.50* | −0.02 | 0.05o | −0.01 | ||||
| Nepetaspp. | 0.49** | 0.23** | 0.08* | 0.04 | ||||
| Rudbeckiaspp. | 0.41** | 0.42** | 0.05o | 0.15** | ||||
| S. nemorosa | 0.16o | 0.30** | 0.08* | NA | ||||
| Tongue length | ||||||||
| Agastachespp. | 0.49** | 0.02 | ||||||
| Echinaceaspp. | −0.02 | 0.05o | ||||||
| Nepetaspp. | 0.22** | 0.08* | ||||||
| Rudbeckiaspp. | 0.42** | 0.05o | ||||||
| S. nemorosa | 0.34** | 0.10* | ||||||
| PERMANOVA Mantel |
**P < 0.01 | *P ≤ 0.05 | ** P < 0.01 | *P ≤ 0.05 | o0.05 < P < 0.10 | P > 0.10 | ||
| R 2 ≥ 0.15 | R 2 ≥ 0.15 | R 2 ≤ 0.15 | R 2 ≤ 0.15 | R 2 ≥ 0.15 | ||||
| R ≥ 0.15 | R ≥ 0.15 | R ≤ 0.15 | R ≤ 0.15 | R ≥ 0.15 | ||||
PERMANOVA R2 values of dissimilarities of plant taxa in floral traits space and Mantel R values of correlations between floral traits and bee preference dissimilarity matrices. Values in bold black represent those that are statistically significant (P ≤ 0.05) and with a PERMANOVA R2 of at least 0.15 or a Mantel R of at least 0.15. Values in black, non-bold represent those that are statistically significant but that are below the determined cutoff for this study (0.15) or are close to statistically significant (0.05 < P< 0.10) and above the determined cutoff. Values in grey represent those that are statistically non-significant. Plant taxa vary across all plant genera in all traits except pollen nutritional qualities. Within plant genera, colour and corolla morphology are traits that are most variable across cultivars and this variation is correlated with bee species abundance and bee visitor abundance based on species’ functional traits.
Fig. 5.
Biotic associations between pollinator taxonomic groups, bee species and floral phenotypic traits based on interpretation of linear regression and NMDS analyses. Across all plant genera, floral area acts as a general and long-distance attractant to all pollinator taxa, while traits such as height, colour, corolla morphology, nutrition and scent operate across diverse spatial scales to shape the visiting pollinator community based on insect natural history, morphology and learned associations. While these associations can be broadly used as guidelines for creating pollinator habitat in gardens, there was a high degree of generalization across pollinator taxa, particularly among bee visitors. At the level of bee species, variation in cultivar phenotype led to novel associations with some bee species and excluded others, suggesting that cultivar development can influence the attractiveness or accessibility of these varieties to a pollinator community. Furthermore, pollinator species’ response to trait variation was specific to each plant genus, supporting previous studies and demonstrating the multimodal nature of floral visual and chemical traits.
Chemical
Dissimilarity of genera based on proportional abundance of all headspace volatile compounds (PERMANOVA R2 = 0.33, P = 0.01, Stress = 0.20) was relevant to abundance of Lepidoptera spp., which was positively correlated in the direction of the compounds nonanal and sabinene (R2 = 0.13, P = 0.01). Within phenotypic space based on floral scent emissions rate and diversity (PERMANOVA R2 = 0.18, P = 0.01, Stress = 0.00), visitation by Coleoptera spp. and Diptera spp. (R2 = 0.14, P = 0.02, R2 = 0.15, P < 0.01) was positively correlated in the direction of headspace volatiles 1/D and towards Rudbeckia spp. (Table 1; Fig. 5).
Nutrition
In nectar trait space across plant genera (PERMANOVA R2 = 0.35, P < 0.01, Stress = 0.000) (Table 1), visitation by Diptera spp. was correlated in the same direction as total nectar sugar concentration and mean nectar volume (R2 = 0.19, P < 0.01). Lepidoptera spp. were significantly correlated with nectar traits in traits space (R2 = 0.05, P< 0.01), but not in the direction of any particular traits, and thus this relationship is inconclusive in this system. Coleoptera spp. were correlated in the direction of increasing monosaccharide content (R2 = 0.09, P < 0.01). Abundance of Anthophila spp. was not correlated with any nectar traits in traits space.
Ordination analyses of pollen protein content and mass indicated significant dissimilarity of genera in traits space (PERMANOVA R2 = 0.40, P < 0.01, Stress = 0.018), largely driven by sample mass. Abundance of Anthophila spp. was correlated in the direction of pollen amount (R2 = 0.07, P < 0.01) while abundance of Diptera spp. and Lepidoptera spp. was correlated in the opposite direction of pollen amount (R2 = 0.05 P < 0.01; R2 = 0.03, P = 0.02) but not in the direction of any cluster of plant genera.
Bee species visitation and cultivar-level variation in floral phenotypic traits
Bee species preference and bee functional traits
Following removal of ‘rare’ taxa, 20 bee species across 13 genera and four families were collected on the cultivars in this study (Fig. 4). These bee species varied from one another in the three functional traits assessed in this study: body size (ITD), tongue length and pollen transportation location. These species did not differ from one another in other functional traits that may explain variation in foraging behaviour, including phenology (Normandin et al., 2017) or diet breadth, with the exception of M. pugnata, which is oligolectic on Asteraceae pollen (Fowler, 2016). Thus, these latter two traits were excluded from further analyses. Separate ordinations of Bray–Curtis dissimilarity matrices of the abundance of bee visitors collected at cultivars, based on taxonomic identity, revealed significant dissimilarity in bee pollinators attracted to cultivars for all genera except Echinacea spp. (Agastache spp. PERMANOVA R2 = 0.46, P < 0.01, Stress = 0.062; Nepeta spp. PERMANOVA R2 = 0.36, P < 0.01, Stress = 0.104; Rudbeckia spp. PERMANOVA R2 = 0.56, P < 0.01, Stress = 0.073; S. nemorosa PERMANOVA R2 = 0.37, P < 0.01, Stress = 0.070). As Echinacea spp. did not show any significant differences in pollinator attraction, these cultivars were excluded from subsequent analyses. Dissimilarities of cultivars based on ordinations of pollinator functional traits were overall consistent with dissimilarity based on species ordinations for all genera (Table 2).
Table 2.
Dissimilarity of cultivars in bee preference space
| Bee species | Body size | Tongue length | Pollen transport | ||
|---|---|---|---|---|---|
| Agastache spp. | 0.46** | 0.42** | 0.49** | 0.47** | PERMANOVA R 2 |
| Echinaceaspp. | 0.41 | 0.37 | 0.26 | 0.26 | |
| Nepetaspp. | 0.36** | 0.42** | 0.54** | 0.53** | |
| Rudbeckiaspp. | 0.56** | 0.61** | 0.62** | 0.60** | |
| S. nemorosa | 0.37** | 0.35** | 0.39** | 0.39** | |
|
**P
<
0.01
R 2 ≥ 0.15 |
P > 0.10 |
Values in bold black represent those that are statistically significant (P ≤ 0.05) and with and PERMANOVA R2 of at least 0.15. Values in grey represent those that are statistically non-significant. All plant genera except Echinacea spp. exhibited significant dissimilarity among cultivars in the bee taxa attracted, based on both species identity and functional traits.
Correlation of bee species, bee functional traits and cultivar phenotype
Mantel tests with a Spearman correlation were performed to compare traits and pollinator preference dissimilarity matrices.
Visual
Dissimilarity of cultivars across all five genera in plant size traits space (Table 1; Fig. 5) was correlated with bee visitor abundance based on forager body size in Rudbeckia spp. (Mantel R = 0.41, P < 0.01), Agastache spp. (Mantel R = 0.29, P = 0.01) and Nepeta spp. (Mantel R = 0.49, P < 0.01), with visitation by large and medium bodied bees, particularly Bombus spp., increasing with plant height (Fig. 5).
Cultivars of four out of five plant genera were significantly dissimilar in floral colour. Dissimilarity of cultivars in floral colour traits space was correlated with bee species visitation to all genera (Table 1). In the three genera with purple flowers, most visitors – primarily within Bombus and A. mellifera – preferentially visited purple flowers. Notable exceptions to this were in Agastache spp., where visitors of X. virginica, Hylaeus affinis/modestus and A. pura visited the pink ‘Heatwave’ cultivar and B. vagans visitors increased on the light orange ‘Summer Glow’ cultivar, and in Nepeta spp., where B. impatiens increased on the white coloured ‘Snowflake’ cultivar (Fig. 5).
Dissimilarity of cultivars within all genera in corolla morphology space was correlated with bee abundance based on both species’ taxonomic identity and functional traits, but the association between corolla morphology and pollinator species’ functional traits was generally specific to each plant genus. In Agastache spp., small and large bodied bee species with short or long tongues preferred cultivars with moderate to long corollas (7.5–9.5 mm), while very large bee species with medium length tongues (X. virginica) visited cultivars with very long corollas (18+ mm). In Nepeta spp., corolla depth was the primary driver of bee species visitation, with long tongued and large species increasing on cultivars with deeper (<1.00 mm) corollas and all other species increasing on slightly wider corollas (1.2 mm). In Rudbeckia spp., long tongued bees with medium to large body size also preferred cultivars with 1.00-mm-wide corollas while small bodied species increased on cultivars with deeper corollas (4.9 mm). Finally, in cultivars of S. nemorosa, we observed that small and medium sized short-tongued bees preferred cultivars with longer (8.8 mm), wider (2.4 mm) corollas and larger medium-tongued bees preferred cultivars with shorter (4.5 mm) narrower corollas (1.5 mm).
Chemical
Three plant taxa, Agastache spp., Rudbeckia spp. and S. nemorosa, showed significant dissimilarities of cultivars in VOC space, based on the PERMANOVAs. Visitation of certain bee species was significantly correlated with headspace VOC emission rate and composition in traits space for these genera. However, based on Mantel tests, there was no significant association between bee preference at the community level and headspace volatile properties for these plant taxa (Fig. 5). Specifically, visitation by Bombus impatiens to cultivars of Agastache spp. was correlated in the direction of the compounds germacrene D and 2-ethylhexanol (B. impatiens R2 = 0.77, P < 0.01) while visitation by X. virginica and A. pura was correlated in the direction of several compounds that differentiated A. hybrida ‘Heatwave’ (R2 = 0.76, P < 0.01; R2 = 0.69, P < 0.01). For cultivars within the genus Rudbeckia spp., visitation by L. versatum was correlated in the direction of α-pinene (R2 = 0.63, P = 0.02). For cultivars of S. nemorosa, visitation by H. affinis/modestus and B. vagans was correlated in the direction of nonanal (R2 = 0.84, P = 0.01; R2 = 0.85, P = 0.01).
Nutrition
In nectar traits space, only cultivars of Rudbeckia spp. and S. nemorosa were significantly dissimilar from one another (PERMANOVA R2 = 0.33, P < 0.00, Stress = 0.000; PERMANOVA R2 = 0.18, P < 0.01, Stress = 0.000), and compared to PERMANOVA statistics of analyses on other floral traits, separation of cultivars was relatively weak (Table 1). Similar to VOC analyses, Mantel tests of the correlation of cultivars in bee preference space and nectar traits space were not significant or were below our set threshold for these genera. However, we identified significant relationships using environmental vectors. Specifically, visitation of large and medium-sized long-tongued bees increased in cultivars with higher nectar volumes in Rudbeckia cultivars while medium-tongued, small species preferred cultivars with lower nectar volumes. In S. nemorosa, small and medium sized bees increased on high-volume, low-concentration nectars but not in the direction of any cluster of cultivars and thus this relationship was inconclusive (Table 1). Only cultivars of Rudbeckia were significantly dissimilar in pollen traits space (PERMANOVA R2 = 0.21, P = 0.02, Stress = 0.000), and were significantly correlated above our Mantel test statistic threshold with pollen-carrying mechanism, with species that carry pollen either in their corbicula (R2 = 0.18, P < 0.01) or on their legs/body (R2 = 0.22, P < 0.01) associated with larger pollen amounts (Table 1).
Validation of field data with B. impatiens choice assays
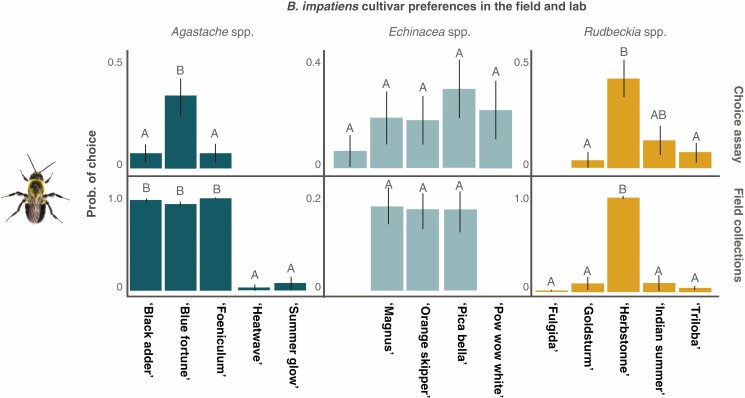
The probability that a single B. impatiens forager would select a cultivar as the first choice did not differ significantly between naive and experienced trials for all three genera tested genera except for Agastache spp., where experienced foragers were more likely to visit the cultivar ‘Foeniculum’ (Tukey post-hoc P = 0.01); however, overall preference for this cultivar was low.
Despite potential differences in environmental conditions (ex. lighting, humidity, temperature, presence of other flowers and pollinators or lack thereof), the preferences of the bumble bee foragers were similar in both the field and laboratory assays, suggesting that the floral traits that drive these preferences are consistent, and perceived consistently, in both settings. The exception to this was in Agastache spp., where the cultivars ‘Black Adder’, ‘Blue Fortune’ and ‘Foeniculum’ were equally attractive in the field but ‘Blue Fortune’ was significantly more attractive than the rest in the choice assays (Fig. 6).
Fig. 6.
Emmeans of probability of Bombus impatiens visitation to cultivars in the field and in choice assays. Forager preferences in choice assays are consistent with those in the field, suggesting that variation in cultivar phenotype is relevant to species-level attraction even in the absence of community and landscape variables.
Comparisons of headspace volatiles 1/D revealed no significant differences between cut and intact flowers, but total emissions rate was significantly higher in intact vs. cut flowers. Cut and intact flowers did emit some unique compounds from one another, and a volatile (methyl jasmonate) associated with plant wounding was only found in one sample (data not shown).
DISCUSSION
Ornamental flowers as a tool for understanding plant–pollinator interactions
That broad patterns of visitation by pollinator taxa can be classified based on floral phenotype – or, ‘pollination syndromes’ – is a central theory in pollination ecology (Willmer, 2011). However, this hypothesis has been a topic of debate for more than a century, primarily due to the high degree of generalization in many plant–pollinator communities (Fenster et al., 2004; Ollerton et al., 2009). Most pollinator taxonomic groups in this study were found to be visiting plants with distinct floral phenotypes and across multiple genera, suggesting that most taxa were apparently generalized foragers (Fig. 4) and did not strictly converge on a floral trait character state (as would be expected in a more specialized relationship). Although many of the interactions between cultivars and flower visitors in this study were not apparently obligate, we still found that general patterns of visitation aligned with floral traits when considering the relative abundance of taxonomic groups on plants, probably based on pollinator physiology or natural history. For example, Coleoptera spp. lack the class of opsins that are sensitive to blue wavelengths (Sharkey et al., 2017), and thus are more able to perceive white and yellow flowers. Conversely, many fly species can perceive a wide range of wavelengths and can differentiate between different coloured flowers, suggesting that their preference in this study for pale and yellow flowers may be innate (Ellis et al., 2021). Thus, even in a generalized pollinator community where pollinator visitation to flowers is influenced by several biotic and abiotic factors, floral phenotype continues to be relevant in shaping the attractiveness of ornamental plants to pollinator taxonomic groups. These results demonstrate how floral phenotype and pollination syndromes may be utilized for plant selection and garden design to support a diverse pollinator community in managed greenspaces.
Our results suggest that in ornamental plants, certain floral cues, such as floral display area, may serve as general signals across taxa whereas others, such as plant height, are more taxon-specific (Fig. 5), even to the species level. For example, large-bodied bee species were positively associated with plant height in three plant genera (Fig. 5). Bee species present on these tall cultivars such as A. mellifera and certain Bombus spp. were not found to be visiting shorter varieties within these plant genera, despite being broad dietary generalists. However, tall cultivars did not preclude visitation by other bee taxa, and there were species (ex. B. impatiens) that were present across all or most varieties regardless of plant height (Fig. 4), suggesting that plant height may expand the visitor community to a cultivar (Thompson, 2001) (Fig. 5). Generalized opportunistic pollinator species often exhibit temporal and spatial specialization and floral constancy, particularly in communities with high plant diversity (Ebeling et al., 2011). In natural systems, this hierarchical trait specialization is thought to contribute to niche partitioning at the community level (Junker et al., 2013), and may be a driver of selection in generalist pollination systems. Multiple traits should be considered when developing a plant community comprising ornamental cultivars to encourage functional diversity and support temporal resource specialization across pollinator taxa.
The complex patterns of bee species visitation to cultivars illustrate that variation in floral traits at the cultivar level can influence how species interact with novel phenotypes. While in some cultivars, distinct floral traits such as plant height were related to expanding the visiting pollinator community (see above), in others, trait variation led to the exclusion of potential visitors. We observed that purple-coloured cultivars within genera were widely visited by many bee taxa, while non-purple cultivars supported only a limited subset of the bee pollinator community. Specifically, certain Bombus species were present on all or most cultivars within Nepeta spp. and Agastache spp. but were the sole visitors to N. racemosa ‘Snowflake’ (B. impatiens) and A. hybrida ‘Summer Glow’ (B. vagans, B. impatiens) (Fig. 4). Indeed, Bombus spp. are highly generalized and are quite adept at learning novel flower signals to exploit rewards (Gumbert, 2000), and therefore may be particularly flexible in overcoming the limitations of variation in cultivar colour. Additionally, we found that variation in corolla depth and width directly affected visitation of bee species to cultivars, indicating mechanical exclusion based on bee functional morphological traits (Wester et al., 2020) (Fig. 5). Namely, pollinator visitation to Agastache cultivars with very long corollas (‘Summer Glow’ and ‘Heatwave’) was correlated with bee tongue length. Within Agastache spp., the ‘Summer Glow’ corolla was on average 3 mm shorter (~18 mm) than that of ‘Heatwave’ (Fig. 2), and thus nectar rewards were probably accessible to long-tongued Bombus spp. Conversely, the sole visitors to ‘Heatwave’ (21-mm corolla) were large-bodied X. virginica spp., which were often observed to be nectar robbing, and small-bodied A. pura, which were observed to be accessing nectar rewards by travelling inside the corolla. Our results have implications for understanding both how cultivar breeding may influence plant utility to pollinators and how trait variation in wild plant populations can influence pollinator visitation (ex. Bradshaw and Schemske, 2003) and drive adaptive evolution in a generalized community.
The lack of an association between bee species attraction and plant nutritional traits to most taxa in this study was somewhat surprising, both because nectar properties were significant drivers of visitation rates across the broader taxonomic groups and pollen nutritional composition often promotes specialized foraging behaviour, even in generalist bee taxa (Roulston and Cane, 2000; Vaudo et al., 2016). These results suggest that the differences in nectar and pollen quality and quantities in the cultivars in our studies were not sufficiently large enough to drive differences in visitation patterns in the field (Heinrich, 1979; Keasar et al., 2008; Lemaitre et al., 2014; Nepi et al., 2018), though clearly breeding can reduce nutritional resource availability in many cultivars (Comba et al., 1999). Alternatively, other factors that were not evaluated in our analysis, such as floral microbial communities (Russell and McFrederick, 2021) or varying rates of evaporation due to daily variation in environmental conditions in the field, could have influenced nutritional quality and quantity (Pleasants, 1983).
Practical applications to plant breeding
The results of this study can assist ornamental flower breeders in identifying key heritable traits that affect pollinator attraction as well as those traits that are less significant. For example, flower colour, morphology and floral display area are all traits that respond readily to artificial selection and are commonly selected for in cultivar development (Mol et al., 1995; Comba et al., 1999; Yang et al., 2019), and were the traits that consistently were related to pollinator visitation in this study. Our results indicate that plant breeding and artificial selection can have direct effects on the attractiveness or accessibility of cultivars to pollinators, although further studies are needed to explore this hypothesis.
Floral traits, such as headspace volatiles, can evolve rapidly under selection (Zu et al., 2016), and the biosynthetic pathways that build these compounds are often located on the same chromosomes as those that regulate floral pigment deposition (Raguso et al., 2015) or flower structural characteristics such as corolla length (Smith, 2016). Thus, cultivar development and plant breeding may also influence plant attractiveness to pollinators through indirect selection on phenotypic traits. In this study, there was variation at the cultivar level in floral scent traits for some genera studied, in particular in the individual compounds produced (Fig. 2), which was probably not a product of direct artificial selection and breeding. Thus, this intercultivar variation in traits such as floral headspace volatiles may indicate pleiotropic consequences of direct selection on traits such as floral colour (Sobel and Streisfeld, 2013), morphology or plant stature (Zu and Schiestl, 2017). However, the genera with the greatest variation in floral VOCs were also those that comprised cultivars from distinct parent species, which may indicate that in this system, dissimilarity of cultivar VOC emissions and composition may largely reflect the parental genotypes of hybrid varieties (Stearn, 1950).
While it is assumed that cultivar development in ornamental flowers would uncouple floral traits from nutritional quality, these results suggested that the nutritional content and quality of the flowers in this study are apparently intact (Wright and Schiestl, 2009). In choice assays, naive and experienced B. impatiens foragers showed largely the same preferences among cultivars as we recorded in field studies. This suggests that for these ornamental genera, floral visual and chemical advertisements remain sufficiently honest despite variability in character state (Knauer and Schiestl, 2015), or that the nutritional rewards provided by the different cultivars were sufficiently equivalent and ‘average’ to support this generalist pollinator species (Fig. 4).
CONCLUSION
This study illustrates the value of ornamental cultivars as a model to explore the mechanisms of plant–pollinator interactions across multiple scales. Moreover, understanding how variability in cultivar phenotypic traits influences plant attractiveness, accessibility and nutritional quality to different pollinator taxa has valuable applications for stakeholders.
Identification of single traits or suites of floral traits that either exclude or encourage pollinator taxonomic groups can help inform plant selection in domestic pollinator habitats, from home gardens to urban greenspaces. We demonstrate that at the level of the total ecological community, increasing total flowering plant density is one of the most reliable methods for bringing diverse pollinator taxa, particularly generalist species, into a habitat (Laha et al., 2020; Staab et al., 2020). Furthermore, at the level of pollinator taxonomic group, associations between floral traits and pollinator preference were generally consistent with previously published studies of plant–pollinator interactions. Thus, at a broad scale, patterns of preference in generalist pollinators are largely conserved and floral phenotype may be used as a general guideline for informing cultivar selection for managed pollinator habitats. As pollinator species and taxonomic groups exhibited distinct patterns of attraction to floral visual, chemical and nutritional traits, managing a habitat with a broad selection of plants and phenotypes will be necessary for supporting a species-rich and functionally diverse pollinator community (Normandin et al., 2017; Kremen et al., 2018). Furthermore, while bee species are broadly attracted to these cultivars, there are clear species-level differences in their preferences to different floral traits. Thus, phenotypic variation among cultivars can predict differential attraction of bee taxa based on species identity and functional traits and lend insight into the implications of artificial selection and plant breeding.
Our results demonstrate that there is substantial flexibility in the role of traits for shaping pollinator attraction to ornamental plant cultivars. In some cases, cultivar development leads to novel interactions with pollinator taxa, meaning that cultivars may support niche differentiation in modified landscapes and increase community functional diversity. In other instances, cultivar development may exclude taxonomic groups, resulting in overall low utility of that variety to a community. Furthermore, direct artificial selection on floral phenotype may have pleiotropic effects on expression of other behaviourally significant traits, such as emissions of floral headspace volatiles, which should be considered when developing new cultivars to support pollinators. Therefore, the ornamental plant breeding industry has tremendous potential and power to develop cultivars that can support either specific groups of pollinators or a diversity of taxa.
SUPPLEMENTARY DATA
Supplementary information are available online at https://academic.oup.com/aob and consist of the following. Details on any relevant chemical treatments of study plants obtained from the nursery and information on vernalization of study plants. We outline further methods on VOC collection and identification, nectar and pollen analysis, and the Bombus foraging arena specifications for choice assays. We also include a detailed description of the model parameters used to analyse floral traits data. Figure S1 is an example of our foraging arenas and Table S1 has information on colony-level replication for choice assays. Table S2 contains information on replication and collection methods of floral traits. Table S3 contains information on bee functional traits and the categorical traits used in these analyses. We also include a section on statistical results, with data from PERMANOVA tests of repeated measures on biological replicates, model output from the GLM using all floral traits as predictors and total visitor abundance as the response variable, and DHARMa analysis of residuals (Fig. S2). Finally, we report which cultivars exhibited variation in nutritional traits across years and sites based on LMMs.
ACKNOWLEDGEMENTS
We thank Sam Droege for identification of bee specimens, Scott DiLoretto for providing glasshouse space and helping to maintain plants, and Heather Hines for providing access to the Ocean Optics UV-VIS spectrometer. We are grateful to Ashley Moak, Rachel Kaneshiki and Rachel Duke for assistance with data collection and specimen preparation. Pollinator icons were designed by G. Ionescu and Hasan through the Noun Project and all other images were accessed from iStock. Photos by Nick Sloff.
Contributor Information
E Erickson, Department of Entomology, Center for Pollinator Research, Huck Institutes of the Life Sciences, Pennsylvania State University, ASI Building University Park, PA, USA.
R R Junker, Evolutionary Ecology of Plants, Department of Biology, University of Marburg, 35043 Marburg, Germany; Department of Environment and Biodiversity, University of Salzburg, Salzburg, Austria.
J G Ali, Department of Entomology, Center for Pollinator Research, Huck Institutes of the Life Sciences, Pennsylvania State University, ASI Building University Park, PA, USA.
N McCartney, Department of Entomology, Center for Pollinator Research, Huck Institutes of the Life Sciences, Pennsylvania State University, ASI Building University Park, PA, USA.
H M Patch, Department of Entomology, Center for Pollinator Research, Huck Institutes of the Life Sciences, Pennsylvania State University, ASI Building University Park, PA, USA.
C M Grozinger, Department of Entomology, Center for Pollinator Research, Huck Institutes of the Life Sciences, Pennsylvania State University, ASI Building University Park, PA, USA.
FUNDING
This work was supported by a United States Department of Agriculture National Institute of Food and Agriculture Specialty Crop Research Initiative grant 2016-51181-235399 to H.M.P. and C.M.G., facilitated and administered in collaboration with the Interregional Research Project no. 4 grant 2015-34383-23710. R.R.J. was supported by German Research Foundation DFG (FOR 300/1). Additional support was provided by the Publius Vergilius Endowment to Penn State.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
- Amsalem E, Grozinger CM, Padilla M, Hefetz A.. 2015. The physiological and genomic bases of bumble bee social behaviour. Advances in Insect Physiology 48: 37–93. [Google Scholar]
- Ao M, Liu B, Wang L.. 2013. Volatile compound in cut and un-cut flowers of tetraploid Freesia hybrida. Natural Product Research 27: 37–40. doi: 10.1080/14786419.2011.647694. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. 2014. Floral specialization and angiosperm diversity: phenotypic divergence, fitness trade-offs and realized pollination accuracy. AoB PLANTS 6: plu003. doi: 10.1093/aobpla/plu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartomeus I, Ascher JS, Gibbs J, et al. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proceedings of the National Academy of Sciences of the United States of America 110: 4656–4660. doi: 10.1073/pnas.1218503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW.. 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426: 176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Burger H, Dötterl S, Ayasse M.. 2010. Host-plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Functional Ecology 24: 1234–1240. [Google Scholar]
- Burghardt KT, Tallamy DW, Shriver GW.. 2009. Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conservation Biology 23: 219–224. [DOI] [PubMed] [Google Scholar]
- Byers KJRP, Bradshaw HD, Riffell JA.. 2014. Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). Journal of Experimental Biology 217: 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B, Khachatryan H, Rihn A.. 2017. Pollinator-friendly plants, reasons for and barriers to purchase. American Society for Horticultural Science 27: 831–839. [Google Scholar]
- Chittka L, Raine NE.. 2006. Recognition of flowers by pollinators. Current Opinion in Plant Biology 9: 428–435. doi: 10.1016/j.pbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R.. 1994. Ultraviolet as a component of flower reflections, and the colour perception of hymenoptera. Vision Research 34: 1489–1508. [DOI] [PubMed] [Google Scholar]
- Comba L, Corbet SA, Barron A, et al. 1999. Garden flowers: insect visits and the floral reward of horticulturally-modified variants. Annals of Botany 83: 73–86. [Google Scholar]
- Corbet SA, Bee J, Dasmahapatra K, et al. 2001. Native or exotic? Double or single? Evaluating plants for pollinator-friendly gardens. Annals of Botany 87: 219–232. doi: 10.1006/anbo.2000.1322. [DOI] [PubMed] [Google Scholar]
- Courcelles DMM, Button L, Elle E.. 2013. Bee visit rates vary with floral morphology among highbush blueberry cultivars (Vaccinium corymbosum L.). Journal of Applied Entomology 137: 693–701. [Google Scholar]
- Cowles RS, Eitzer BD.. 2017. Residues of neonicotinoid insecticides in pollen and nectar from model plants. Journal of Environmental Horticulture 35: 24–34. doi: 10.24266/0738-2898-35.1.24. [DOI] [Google Scholar]
- Cusser S, Neff JL, Jha S.. 2019. Landscape context differentially drives diet breadth for two key pollinator species. Oecologia 191: 873–886. doi: 10.1007/s00442-019-04543-5. [DOI] [PubMed] [Google Scholar]
- Danforth BN, Minckley RL, Neff JL.. 2019. The solitary bees, Kalett A, Zodrow K, eds. Princeton, NJ: Princeton University Press. [Google Scholar]
- Ebeling A, Klein A-M, Tscharntke T.. 2011. Plant–flower visitor interaction webs: temporal stability and pollinator specialization increases along an experimental plant diversity gradient. Basic and Applied Ecology 12: 300–309. doi: 10.1016/j.baae.2011.04.005. [DOI] [Google Scholar]
- Ellis AG, Anderson B, Kemp JE.. 2021. Geographic mosaics of fly pollinators with divergent color preferences drive landscape-scale structuring of flower color in daisy communities. Frontiers in Plant Science 12: 617761. doi: 10.3389/fpls.2021.617761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson E, Adam S, Russo L, Wojcik V, Patch HM, Grozinger CM.. 2020. More than meets the eye? The role of annual ornamental flowers in supporting pollinators. Environmental Entomology 49: 178–188. doi: 10.1093/ee/nvz133. [DOI] [PubMed] [Google Scholar]
- Erickson E, Patch HM, Grozinger CM.. 2021. Herbaceous perennial ornamental plants can support complex pollinator communities. Scientific Reports 11: 17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD.. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35: 375–403. doi: 10.1146/annurev.ecolsys.34.011802.132347. [DOI] [Google Scholar]
- Fowler J. 2016. Specialist bees of the Northeast: host plants and habitat conservation. Northeastern Naturalist 23: 305. doi: 10.1656/045.023.0210. [DOI] [Google Scholar]
- Frankie GW, Thorp RW, Schindler M, Hernandez J, Ertter B, Rizzardi M.. 2005. Ecological patterns of bees and their host ornamental flowers in two Northern California cities. Journal of the Kansas Entomological Society 78: 227–246. doi: 10.2317/0407.08.1. [DOI] [Google Scholar]
- Garbuzov M, Ratnieks FLWW.. 2014. Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Functional Ecology 28: 364–374. [Google Scholar]
- Garbuzov M, Ratnieks FLW.. 2015. Using the British national collection of asters to compare the attractiveness of 228 varieties to flower-visiting insects. Environmental Entomology 44: 638–646. doi: 10.1093/ee/nvv037. [DOI] [PubMed] [Google Scholar]
- Gervasi DDL, Schiestl FP.. 2017. Real-time divergent evolution in plants driven by pollinators. Nature Communications 8: 14691. doi: 10.1038/ncomms14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geslin B, Gauzens B, Thébault E, Dajoz I.. 2013. Plant pollinator networks along a gradient of urbanisation. PLoS One 8: e63421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology 48: 36–43. doi: 10.1007/s002650000213. [DOI] [Google Scholar]
- Hartig F. 2021. DHARMa: Residual diagnostics for hierarchical (multilevel/mixed) regression. R package version 0.4.5. http://florianhartig.github.io/DHARMa/
- Heinrich B. 1979. ‘Majoring’ and ‘minoring’ by foraging bumblebees, Bombus vagans: an experimental analysis. Ecology 60: 245–255. doi: 10.2307/1937652. [DOI] [Google Scholar]
- Horn W. 2002. Breeding methods and breeding research. In: Vainstein, A. eds. Breeding for ornamentals: classical and molecular approaches. Dordrecht: Springer Netherlands, 47–83. [Google Scholar]
- Horth L, Campbell L, Bray R.. 2014. Wild bees preferentially visit Rudbeckia flower heads with exaggerated ultraviolet absorbing floral guides. Biology Open 3: 221–230. doi: 10.1242/bio.20146445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA. 2013. Are there pollination syndromes in the Australian epacrids (Ericaceae: Styphelioideae)? A novel statistical method to identify key floral traits per syndrome. Annals of Botany 112: 141–149. doi: 10.1093/aob/mct105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker RR, Blüthgen N, Brehm T, et al. 2013. Specialization on traits as basis for the niche-breadth of flower visitors and as structuring mechanism of ecological networks. Functional Ecology 27: 329–341. [Google Scholar]
- Junker RR, Parachnowitsch AL.. 2015. Working towards a holistic view on flower traits – how floral scents mediate plant–animal interactions in concert with other floral characters. Journal of the Indian Institute of Science 95: 43–68. [Google Scholar]
- Kantsa A, Raguso RA, Dyer AG, Olesen JM, Tscheulin T.Petanidou T.. 2018. Disentangling the role of floral sensory stimuli in pollination networks. Nature Communications 9: 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasar T, Sadeh A, Shmida A.. 2008. Variability in nectar production and standing crop, and their relation to pollinator visits in a Mediterranean shrub. Arthropod–Plant Interactions 2: 117–123. doi: 10.1007/s11829-008-9040-9. [DOI] [Google Scholar]
- Knauer AC, Schiestl FP.. 2015. Bees use honest floral signals as indicators of reward when visiting flowers. Ecology Letters 18: 135–143. doi: 10.1111/ele.12386. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B.. 2006. Diversity and distribution of floral scent. Botanical Review 72: 1–120. [Google Scholar]
- Kremen C, M’Gonigle LK, Ponisio LC.. 2018. Pollinator community assembly tracks changes in floral resources as restored hedgerows mature in agricultural landscapes. Frontiers in Ecology and Evolution 6: 170. [Google Scholar]
- Laha S, Chatterjee S, Das A, Smith B, Basu P.. 2020. Exploring the importance of floral resources and functional trait compatibility for maintaining bee fauna in tropical agricultural landscapes. Journal of Insect Conservation 24: 431–443. doi: 10.1007/s10841-020-00225-3. [DOI] [Google Scholar]
- Lemaitre AB, Pinto CF, Niemeyer HM.. 2014. Generalized pollination system: are floral traits adapted to different pollinators? Arthropod–Plant Interactions 8: 261–272. [Google Scholar]
- Lenth R. 2020. emmeans: estimated marginal means, aka least-squares means. R package version 1.5.3. https://github.com/rvlenth/emmeans.
- Leonard AS, Dornhaus A, Papaj DR.. 2011. Why are floral signals complex? An outline of functional hypotheses. In: Patiny S, ed. Evolution of plant-pollinator relationships. Cambridge: Cambridge University Press, 279–300. [Google Scholar]
- Leonard AS, Papaj DR.. 2011. ‘X’ marks the spot: the possible benefits of nectar guides to bees and plants. Functional Ecology 25: 1293–1301. doi: 10.1111/j.1365-2435.2011.01885.x. [DOI] [Google Scholar]
- Mach BM, Bondarenko S, Potter DA.. 2018. Uptake and dissipation of neonicotinoid residues in nectar and foliage of systemically treated woody landscape plants. Environmental Toxicology and Chemistry 37: 860–870. doi: 10.1002/etc.4021. [DOI] [PubMed] [Google Scholar]
- Maia R, Gruson H, Endler JA, White TE.. 2019. pavo 2: new tools for the spectral and spatial analysis of colour in R. Methods in Ecology and Evolution 10: 1097–1107. [Google Scholar]
- Majewska AA, Altizer S.. 2020. Planting gardens to support insect pollinators. Conservation Biology 34: 15–25. [DOI] [PubMed] [Google Scholar]
- Mol J, Holton TA, Koes RE.. 1995. Floriculture: genetic engineering of commercial traits. Trends in Biotechnology 13: 350–355. [Google Scholar]
- Molet M, Chittka L, Raine NE.. 2009. How floral odours are learned inside the bumblebee (Bombus terrestris) nest. Naturwissenschaften 96: 213–219. doi: 10.1007/s00114-008-0465-x. [DOI] [PubMed] [Google Scholar]
- Morrant DS, Schumann R, Petit S.. 2009. Field methods for sampling and storing nectar from flowers with low nectar volumes. Annals of Botany 103: 533–542. doi: 10.1093/aob/mcn241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth F, Papaj DR, Leonard AS.. 2015. Colour learning when foraging for nectar and pollen: bees learn two colours at once. Biology Letters 11: 20150628. doi: 10.1098/rsbl.2015.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepi M, Grasso DA, Mancuso S.. 2018. Nectar in plant–insect mutualistic relationships: from food reward to partner manipulation. Frontiers in Plant Science 9: 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin E, Vereecken NJ, Buddle CM, Fournier V.. 2017. Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 5: e3051. doi: 10.7717/peerj.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet G, Friendly M, et al. 2020. vegan: community ecology package. R package version 2.5-7. https://github.com/vegandevs/vegan.
- Ollerton J, Alarcón R, Waser NM, et al. 2009. A global test of the pollination syndrome hypothesis. Annals of Botany 103: 1471–1480. doi: 10.1093/aob/mcp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz PL, Fernández-Díaz P, Pareja D, Escudero M, Arista M.. 2021. Do visual traits honestly signal floral rewards at community level? Functional Ecology 35: 369–383. [Google Scholar]
- Pleasants JM. 1983. Nectar production patterns in Ipomopsis aggregata (Polemoniaceae). American Journal of Botany 70: 1468–1475. doi: 10.1002/j.1537-2197.1983.tb10850.x. [DOI] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE.. 2010. Global pollinator declines: trends, impacts and drivers. Trends in Ecology & Evolution 25: 345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Power EF, Stabler D, Borland AM, Barnes J, Wright GA.. 2018. Analysis of nectar from low-volume flowers: a comparison of collection methods for free amino acids. Methods in Ecology and Evolution 9: 734–743. doi: 10.1111/2041-210X.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso RA, Thompson JN, Campbell DR.. 2015. Improving our chemistry: challenges and opportunities in the interdisciplinary study of floral volatiles. Natural Product Reports 32: 893–903. doi: 10.1039/c4np00159a. [DOI] [PubMed] [Google Scholar]
- Raine NE, Chittka L.. 2007. The adaptive significance of sensory bias in a foraging context: Floral colour preferences in the bumblebee Bombus terrestris. PLoS One 2: e556. doi: 10.1371/journal.pone.0000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley CV. 1892. Some notes on the Margined Soldier-Beetle (Chauliognathus marginatus). The Canadian Entomologist 24: 186–187. doi: 10.4039/ent24186-8. [DOI] [Google Scholar]
- Rollings R, Goulson D.. 2019. Quantifying the attractiveness of garden flowers for pollinators. Journal of Insect Conservation 23: 803–817. doi: 10.1007/s10841-019-00177-3. [DOI] [Google Scholar]
- Roulston TH, Cane JH.. 2000. Pollen nutritional content and digestibility for animals. Plant Systematics and Evolution 222: 187–209. doi: 10.1007/bf00984102. [DOI] [Google Scholar]
- Russell AL, Mauerman KB, Golden RE, Papaj DR.. 2018. Linking components of complex signals to morphological part: the role of anther and corolla in the complex floral display. Animal Behaviour 135: 223–236. doi: 10.1016/j.anbehav.2017.11.021. [DOI] [Google Scholar]
- Russell KA, McFrederick QS.. 2021. Elevated temperature may affect nectar microbes, nectar sugars, and bumble bee foraging preference. Microbial Ecology. doi: 10.1007/s00248-021-01881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD.. 2013. Pollinator-mediated evolution of floral signals. Trends in Ecology & Evolution 28: 307–315. doi: 10.1016/j.tree.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Sharkey CR, Fujimoto MS, Lord NP, et al. 2017. Overcoming the loss of blue sensitivity through opsin duplication in the largest animal group, beetles. Scientific Reports 7: 8. doi: 10.1038/s41598-017-00061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha M, Dyer AG.Burd M.. 2013. Evaluating the spectral discrimination capabilities of different pollinators and their effect on the evolution of flower colors. Communicative & Integrative Biology 6: e24000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD. 2016. Pleiotropy and the evolution of floral integration. New Phytologist 209: 80–85. [DOI] [PubMed] [Google Scholar]
- Sobel JM, Streisfeld MA.. 2013. Flower color as a model system for studies of plant evo-devo. Frontiers in Plant Science 4: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Silva M, Fontenelle JCR, Martins R.. 2001. Seasonal abundance and species composition of flower-visiting flies. Neotropical Entomology 30: 351–359. doi: 10.1590/S1519-566X2001000300002. [DOI] [Google Scholar]
- Sponsler DB, Shump D, Richardson RT, Grozinger CM.. 2020. Characterizing the floral resources of a North American metropolis using a honey bee foraging assay. Ecosphere 11: e03102. [Google Scholar]
- Staab M, Pereira-Peixoto MH, Klein A-M.. 2020. Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 194: 465–480. doi: 10.1007/s00442-020-04785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearn WT. 1950. Nepeta mussinii and N. x faassenii. Journal of the Royal Horticultural Society 75: 403–406. [Google Scholar]
- Stewart-Jones A, Poppy GM.. 2006. Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. Journal of Chemical Ecology 32: 845–864. doi: 10.1007/s10886-006-9039-6. [DOI] [PubMed] [Google Scholar]
- Stugard B. 1931. Biological notes on Chauliognathus pennsylvanicus DeGeer (Coleoptera, Cantharidae). Masters Thesis. University of Kansas. [Google Scholar]
- Theodorou P, Radzevičiūtė R, Lentendu G, et al. 2020. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nature Communications 11: 576. doi: 10.1038/s41467-020-14496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD. 2001. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126: 386–394. doi: 10.1007/s004420000531. [DOI] [PubMed] [Google Scholar]
- USDA - NASS, Census of Agriculture, 2014. Census of horticultural specialties. Washington, DC: U.S. Dept. of Commerce, Economics and Statistics Administration, Bureau of the Census. [Google Scholar]
- Vaudo AD, Patch HM, Mortensen DA, Tooker JF, Grozinger CM.. 2016. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proceedings of the National Academy of Sciences of the United States of America 113: E4035–E4042. doi: 10.1073/pnas.1606101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuis HHW, van Doorn A.. 2006. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 37: 421–451. [Google Scholar]
- Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D.. 2021. Insect decline in the Anthropocene: death by a thousand cuts. Proceedings of the National Academy of Sciences USA 118: e2023989118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao G-X, Wang L-L, Yang Y-P, Zhang Z-Q, Duan Y-W.. 2017. Evaluation of pollinator effectiveness based on pollen deposition and seed production in a gynodieocious alpine plant, Cyananthus delavayi. Ecology and Evolution 7: 8156–8160. doi: 10.1002/ece3.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton DI, Wright ST, Wang Y.. 2012. Distance-based multivariate analyses confound location and dispersion effects. Methods in Ecology and Evolution 3: 89–101. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J.. 2009. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Wester P, Cairampoma L, Haag S, Schramme J, Neumeyer C, Claßen-Bockhoff R.. 2020. Bee exclusion in bird-pollinated Salvia flowers: the role of flower color versus flower construction. International Journal of Plant Sciences 181: 770–786. doi: 10.1086/709132. [DOI] [Google Scholar]
- Wilde HD, Gandhi KJK, Colson G.. 2015. State of the science and challenges of breeding landscape plants with ecological function. Horticulture Research 2: 14069. doi: 10.1038/hortres.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Crone EE, Roulston TH, Minckley RL, Packer L, Potts SG.. 2010. Ecological and life-history traits predict bee species responses to environmental disturbances. Biological Conservation 143: 2280–2291. [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- Wright GA, Schiestl FP.. 2009. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Functional Ecology 23: 841–851. doi: 10.1111/j.1365-2435.2009.01627.x. [DOI] [Google Scholar]
- Yang F, Ignatieva M, Wissman J, Ahrné K, Zhang S, Zhu S.. 2019. Relationships between multi-scale factors, plant and pollinator diversity, and composition of park lawns and other herbaceous vegetation in a fast growing megacity of China. Landscape and Urban Planning 185: 117–126. doi: 10.1016/j.landurbplan.2019.02.003. [DOI] [Google Scholar]
- Zu P, Blanckenhorn WU, Schiestl FP.. 2016. Heritability of floral volatiles and pleiotropic responses to artificial selection in Brassica rapa. New Phytologist 209: 1208–1219. [DOI] [PubMed] [Google Scholar]
- Zu P, Schiestl FP.. 2017. The effects of becoming taller: direct and pleiotropic effects of artificial selection on plant height in Brassica rapa. The Plant Journal 89: 1009–1019. doi: 10.1111/tpj.13440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.