Abstract
Background
Interventions to mitigate the COVID-19 pandemic may impact other respiratory diseases.
Aims
We aimed to study the course of pertussis in France over an 8-year period including the beginning of the COVID-19 pandemic and its association with COVID-19 mitigation strategies, using multiple nationwide data sources and regression models.
Methods
We analysed the number of French pertussis cases between 2013 and 2020, using PCR test results from nationwide outpatient laboratories (Source 1) and a network of the paediatric wards from 41 hospitals (Source 2). We also used reports of a national primary care paediatric network (Source 3). We conducted a quasi-experimental interrupted time series analysis, relying on negative binomial regression models. The models accounted for seasonality, long-term cycles and secular trend, and included a binary variable for the first national lockdown (start 16 March 2020).
Results
We identified 19,039 pertussis cases from these data sources. Pertussis cases decreased significantly following the implementation of mitigation measures, with adjusted incidence rate ratios of 0.10 (95% CI: 0.04–0.26) and 0.22 (95% CI: 0.07–0.66) for Source 1 and Source 2, respectively. The association was confirmed in Source 3 with a median of, respectively, one (IQR: 0–2) and 0 cases (IQR: 0–0) per month before and after lockdown (p = 0.0048).
Conclusions
The strong reduction in outpatient and hospitalised pertussis cases suggests an impact of COVID-19 mitigation measures on pertussis epidemiology. Pertussis vaccination recommendations should be followed carefully, and disease monitoring should be continued to detect any resurgence after relaxation of mitigation measures.
Keywords: Pertussis, epidemiology, COVID-19, lockdown
Introduction
Whooping cough or pertussis is a highly contagious respiratory disease transmitted from human to human via aerosolised respiratory droplets and is mainly caused by Bordetella pertussis. A resurgence of pertussis has been observed in many countries during the last decade despite widespread vaccine implementation [1]. Most pertussis morbidity and mortality are due to severe clinical forms in young infants that usually require admission to intensive care units. In 2014, the World Health Organization (WHO) estimated pertussis as the cause of 160,700 deaths in children younger than 5 years [2]. In Europe, the European Centre for Disease Prevention and Control (ECDC) reported an increase in pertussis cases between 2014 and 2016, followed by a slight decrease; there were 35,627 cases in 2018 [3].
Since December 2019, the world has been facing another infectious respiratory disease, the coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [4]. In France, a lockdown was ordered at the beginning of the first wave of the COVID-19 pandemic on 16 March 2020. Measures included school closures, workplace physical distancing and remote work, banning mass gatherings, travel restrictions and closure of public places; mandatory face covering was implemented on 11 May 2020 [5]. During the following months, the French government successively introduced several bundles of COVID-19 mitigation measures based upon the dynamics of SARS-CoV-2 infection. The French population initially faced difficulties of access to clinical and biological diagnosis and to public health services, with a subsequent delay of general childhood vaccinations despite the mandatory infant vaccinations since 2018 and booster vaccination guideline [6]. We hypothesised that such measures and their consequences might have impacted pertussis epidemiology as suggested for other transmissible airborne infectious diseases [7].
Thanks to a well-established surveillance system for pertussis cases through networks of outpatient laboratories and participating hospitals, and the French ambulatory surveillance for outpatient paediatric cases, we assessed the course of pertussis epidemiology in France between 1 January 2013 and 31 December 2020, and its potential association with COVID-19 mitigation strategies.
Methods
Data sources and case definitions
We performed a retrospective analysis using three nationwide data sources from pertussis surveillance systems in France from 2013 to 2020 (see Supplementary Figure S1 for the geographical distribution of these surveillance sites). Those include general population surveillance through two nationwide outpatient laboratories (Cerba, Laboratory 1 and Biomnis, Laboratory 2), which carry out more than 90% of the ambulatory testing for pertussis in mainland France (Source 1) [8], and the monitoring of severe paediatric cases through a nationwide network of 41 participating hospitals (Renacoq network, covering ca 30% of hospitalised paediatric pertussis cases) collecting hospitalised pertussis cases under the age of 1 year (Source 2) [9]. Santé publique France (SpF) and the French National Reference Centre (NRC) for Bordetella infections collect data from Source 1 every month, and twice a year from Source 2. For these two sources, a pertussis case was defined as a person with a positive result in a PCR targeting IS481 (simplex PCR) from nasopharyngeal swabs or aspirates. The quantitative PCR methods used for pertussis surveillance were quality-assessed by the French NRC for Bordetella infections [10].
We also included data from the French ambulatory surveillance of whooping cough for outpatient paediatric cases. Implemented in 2002 by the Association Clinique et Thérapeutique Infantile du Val de Marne (ACTIV) and the French NRC for Bordetella infections, this surveillance (primary care network pertussis surveillance, Source 3) does not aim to estimate the exhaustive number of pertussis cases but the duration of immunity conferred by pertussis vaccines in children in France [11]. From 2013 to 2020, 76 paediatricians from the ACTIV network throughout France reported patients with suspected pertussis (cough illness). All suspected patients were invited for biological confirmation of pertussis. Cases were defined as either ‘confirmed’ (i.e. positive in culture and/or PCR) or ‘epidemiological’ (i.e. acute cough lasting at least 14 days in a child or adult who was in contact with a confirmed case) [11].
Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were followed to report the study.
Statistical analysis
We conducted a quasi-experimental interrupted time series analysis relying on negative binomial regression models [12], with a time unit of 1 month, to analyse the changes in the incidence of positive Bordetella PCRs over time. All positive PCR results were included in the models. The models accounted for seasonality (using pairs of sine/cosine terms), secular linear trend, a binary variable to define periods before and after the first lockdown (this variable was coded 0 before 1 April 2020 and 1 thereafter; the actual lockdown started on 16 March 2020; Supplementary Figure S2 shows the timeline of implemented measures) and overdispersion of data. The models also included a dummy variable (with 36-month periods) to adjust for long-term cycles commonly observed in pertussis epidemiology [9]. In such models, an incidence rate ratio (IRR) greater than 1 indicates that the corresponding variable is associated with an increase in pertussis incidence.
Firstly, we carried out the analysis on our most comprehensive data source (Source 1) overall, and then stratified across three pre-specified age groups: pre-school children (0–5 years), primary and secondary school children (6–17 years) and adults (≥ 18 years). We further performed the following sensitivity analyses to assess the robustness of our findings: (i) fitting the data from Laboratory 1 and Laboratory 2 separately, (ii) including the negative samples when available (i.e. modelling the proportion of positive PCR results) and (iii) using a different modelling strategy relying on segmented linear regression with autoregressive errors to account for autocorrelation in the data [12]. Secondly, the analysis was carried out on data from Source 2, relying on the same negative binomial regression model used for Source 1. Thirdly, we did not include the data from Source 3 in the time series modelling because they were collected to evaluate the duration of vaccine-induced immunity and did not fulfil model requirements; instead, we carried out an analysis comparing the number of pertussis cases per month (all laboratory- or epidemiologically confirmed) before and after the lockdown. Differences between groups were assessed by the Mann–Whitney U test or the Student's t test, when appropriate. We used Stata/SE 15.0 (StataCorp LP, College Station, TX, United States) for all analyses.
Results
Outpatient laboratory pertussis surveillance (Source 1)
Over the study period, 17,912 pertussis cases were reported from the two private laboratories (Source 1). The median age of patients with a positive PCR was 20.0 years (interquartile range (IQR): 6.5–46.2), and the distribution among age groups was 23% 0–5 year-olds, 24% 6–17 year-olds and 53% ≥ 18 year-olds. A subset of cases aged 2–20 years between 2013 and 2019 has already been analysed elsewhere for other purposes [8]. Panel A of the Figure shows the evolution of overall pertussis cases for the 96-month study period. Before the lockdown (before 1 April 2020), the average number of cases per month was 204 (standard deviation (SD): 119), with a seasonal pattern (i.e. highest incidence in July months, mean = 315; SD: 180). Long-term epidemic cycles were observed: pertussis incidence was higher in 2013 and between 2017 and 2019, compared with the period 2014 to 2016. After the first lockdown, corresponding to the beginning of a sequence of several mitigation measures (please refer to Supplementary Figure S2 for the dates of implementation), the average number of cases was much smaller, reaching 14 cases per month (SD: 18; p < 0.001).
Figure.
Association between COVID-19 pandemic and pertussis: time series analysis, France, 2013–2020 (n = 18,904)
Fitted values (bold line) as predicted by the negative binomial regression model. The red vertical line indicates the beginning of the first lockdown.
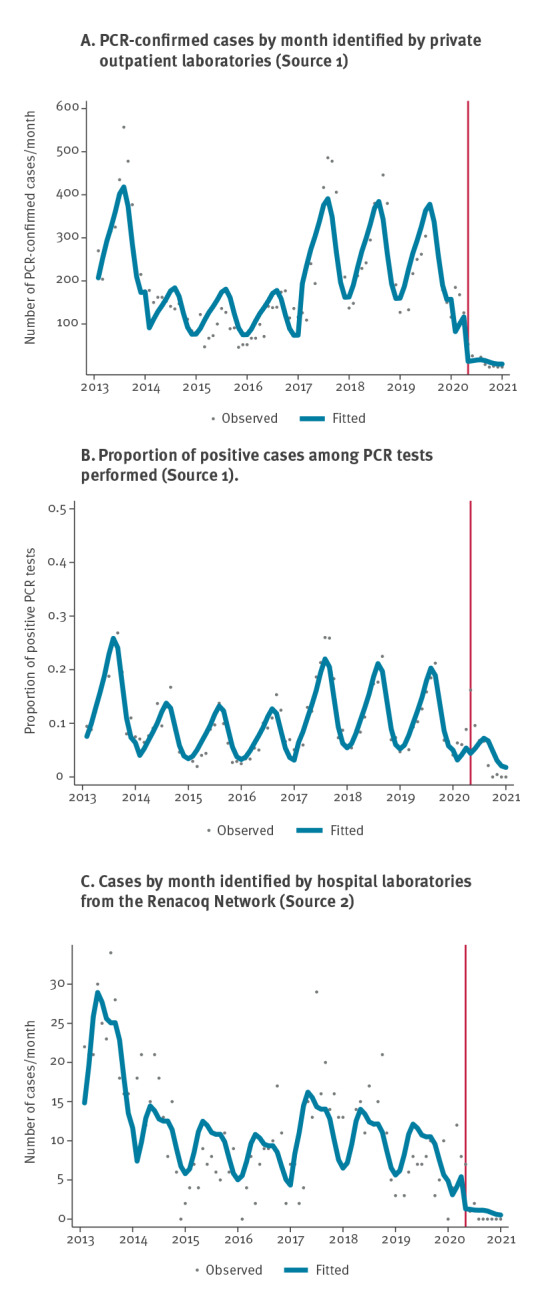
Through time series modelling, we observed a 89.8% decrease (95% confidence Interval (CI): 74.4–96.0) in pertussis cases after the lockdown, corresponding to an adjusted IRR (aIRR) of 0.10 (95% CI: 0.04–0.26) (Table 1). This significant decrease was confirmed in all age groups, with a sharper decrease in the 6–17 year-olds (−92.7%; 95% CI: −97.3 to −79.9) and the ≥ 18 year-olds (−93.6%; 95% CI: −97.5 to −83.5), as compared with the age group 0–5-years (−78.3%; 95% CI: −91.6 to −43.6 ).
Table 1. Association between COVID-19 pandemic and pertussis: interrupted time series analysis, France, 2013–2020 (n = 18,904).
| Outcome measures | Negative binomial modelling | p value | Segmented linear regression | p value | ||
|---|---|---|---|---|---|---|
| Adjusted IRRa | 95% CI | Change in levelb | 95% CI | |||
| Source 1 – Outpatient laboratories | ||||||
| Overall number of positive PCRs | 0.10 | 0.04 to 0.26 | < 0.001 | −242.2 | −348.3 to −136.2 | < 0.001 |
| Number of positive PCRs, by age | ||||||
| 0–5 years | 0.22 | 0.08 to 0.56 | 0.002 | −45.9 | −83.2 to −8.5 | 0.017 |
| 6–17 years | 0.07 | 0.03 to 0.20 | < 0.001 | −61.2 | −84.5 to −37.9 | < 0.001 |
| ≥ 18 years | 0.06 | 0.03 to 0.17 | < 0.001 | −133.6 | −184.2 to −83.1 | < 0.001 |
| Number of positive PCRs, by Laboratory | ||||||
| Laboratory 1 | 0.09 | 0.04 to 0.24 | < 0.001 | −103.5 | −152.9 to −54.1 | < 0.001 |
| Laboratory 2 | 0.11 | 0.04 to 0.27 | < 0.001 | −138.7 | −197.3 to −80.2 | < 0.001 |
| Proportion of positive PCRsc | 0.67 | 0.02 to 27.74 | 0.832 | −0.04 | −0.13 to −0.04 | 0.279 |
| Source 2 – Hospital laboratories (Renacoq network) | ||||||
| Number of positive PCRs in age group < 1 year | 0.22 | 0.07 to 0.66 | 0.007 | Not applicable | ||
CI: confidence interval; COVID-19: coronavirus disease; IRR: incidence rate ratio.
a The estimated IRR was adjusted for long-term cycles, seasonality (using pairs of sine/cosine terms) and secular trend.
b In contrast to the IRR that represents a relative change, the estimated change in level represents the absolute immediate change in the mean level of the outcome after the COVID-19 lockdown. The estimated change was adjusted for long-term cycles, seasonality (adjustment by calendar month), year and secular trend.
c Proportion calculated using Laboratory 1 data only for 2013–2015, and then data from Laboratory 1 and Laboratory 2 for 2016–2020, because of missing data on negative test results for Laboratory 2 for 2013–2015.
In a sensitivity analysis, our findings did not differ when data from Laboratory 1 and Laboratory 2 were fitted separately (Table 1). When including the negative samples, we observed a non-significant decrease in the proportion of positive PCR results (aIRR = 0.67; 95% CI: 0.02–27.74; p = 0.832) (Figure, panel B, Table 1). Whereas this proportion was 13.4% (SD: 7.6) before lockdown, it fell to 8.3% (SD: 5.2) during the next 5 months and to 0.1% (SD: 0.2) in the final 4 months of 2020 (September to December 2020) despite a relatively stable number of tests during the post-lockdown period (mean = 334 tests per month; SD: 60) (Table 2). Segmented linear regression models yielded results similar to negative binomial modelling, with a significant drp in the number of positive PCR results across all age groups (Table 1).
Table 2. Annual number of pertussis cases diagnosed across data sources, France, 2013–2020 (n = 19,039).
| Pertussis cases | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|
| Source 1 – IS481 PCR tests from outpatient laboratories | ||||||||
| Laboratory 1 | ||||||||
| Positive | 2,266 | 962 | 450 | 655 | 1,473 | 1,369 | 1,095 | 206 |
| Negative | 13,139 | 11,216 | 8,614 | 9,112 | 10,355 | 12,623 | 11,336 | 4,152 |
| Total number of tests | 15,405 | 12,178 | 9,064 | 9,767 | 11,828 | 13,992 | 12,431 | 4,358 |
| Laboratory 2 | ||||||||
| Positive | 1,601 | 666 | 552 | 784 | 1,881 | 1,864 | 1,688 | 400 |
| Negative | N/A | N/A | N/A | 9,372 | 11,296 | 13,069 | 13,819 | 5,366 |
| Total number of tests | 1,601 | 666 | 552 | 10,156 | 13,177 | 14,933 | 15,507 | 5,766 |
| Source 2 – Hospital laboratories (Renacoq network) | ||||||||
| Number of positive tests | 266 | 149 | 81 | 86 | 162 | 141 | 73 | 34 |
| Source 3 – Outpatient paediatric network (ACTIV) | ||||||||
| Total clinically suspected pertussis | 48 | 27 | 16 | 15 | 37 | 32 | 38 | 7 |
| Confirmed cases | 14 | 13 | 4 | 5 | 14 | 10 | 14 | 2 |
| Epidemiological cases | 7 | 4 | 4 | 4 | 13 | 6 | 17 | 4 |
N/A: not available.
Hospital-based pertussis surveillance (Source 2)
Over the study period, 992 positive PCR tests were collected through the Renacoq hospital network (Source 2). The distribution among age groups was 61% 0–2 month-olds, 25% 3–5 month-olds and 14% 6–11 month-olds. We also observed a seasonal pattern (Figure, panel C). Before lockdown, the average number of pertussis cases per month was 11.3 (SD: 7.3). After the lockdown, a sharp decrease in the number of hospitalised pertussis cases was confirmed, with an average case number of 1.1 (SD: 2.3; p < 0.001). In time series modelling, there was a significant association between COVID-19 mitigation measures and pertussis cases, with a reduction of 78.4% (95% CI: 34.4–92.9; aIRR 0.22; 95% CI: 0.07–0.66; p = 0.007) (Figure, panel C and Tables 1 and 2). Between July 2020 and December 2020, no case of pertussis was notified.
Primary care network pertussis surveillance (Source 3)
From 2013 to 2020, the paediatric outpatient network (Source 3) reported 135 cases of pertussis. Confirmed and epidemiological cases accounted for 76 (56.3%) and 59 (43.7%) cases, respectively (Table 2). Among the 59 epidemiological cases, 25 were adults (family members) and 34 were children (siblings, family members, daycare centre or school). The mean age was 7.1 years (SD: 4.2) for confirmed cases and 22.4 years (SD: 20.1) for epidemiological cases. Before the lockdown, the median number of cases per month was one (IQR: 0–2). After the lockdown, no case of pertussis was identified in Source 3 (median = 0; IQ:R 0–0; p = 0.0048, Mann–Whitney U test).
Discussion
In this 8-year population-based study, we described the dynamics of pertussis epidemiology in France before and after the implementation of the first COVID-19 mitigation measures (16 March 2020, school closure and national lockdown; for all measures by date, see Supplementary Figure S2) [5]. All our data sources highlighted a long-term cyclical pattern commonly observed in pertussis [9], with two high-incidence periods: the year 2013, corresponding to the epidemic from 2011 to 2013 observed in many countries [8,13], and the years 2017 to 2019. Therefore, a decrease in pertussis incidence was to be expected in 2020, such as for the period 2014 to 2016. However, our interrupted time series analysis, accounting for this 36-month pertussis cycle and seasonality, showed an even sharper decrease in pertussis positive PCR tests, with evidence of its association with anti-COVID-19 measures. This drop was particularly marked, with almost no pertussis cases seen after the lockdown, a level never observed since the beginning of the French pertussis surveillance in 1996 [8].
The decrease in pertussis cases following the mitigation measures occurred in all age groups. However, it was less pronounced in the 0–5-year group tested by the two private laboratories. One explanation could be that the youngest children were more likely to visit their paediatrician despite lockdown measures. Another explanation could be that transmission might still have been active in this age group during the first months of 2020. We did not find a significant association between the proportion of positive PCR tests and the mitigation measures against SARS-CoV-2. Our data show that the overall number of PCR tests decreased right after the lockdown, while pertussis might still have circulated in households. However, despite a steady number of PCR tests performed during the whole post-lockdown period, the number of positive cases reached almost zero in the last 4 months of 2020; of note, access to clinical and biological diagnosis went back to normal during that period. All these findings suggest a significant decrease of pertussis circulation in France.
Previous studies have revealed a major impact of COVID-19 pandemic mitigation measures on transmissible diseases, such as, in the paediatric population, gastroenteritis, common cold, bronchiolitis and acute otitis media, but also measles [7,14]. However, a resurgence of some other common airborne infectious diseases has been observed in the setting of relaxed physical distancing recommendations, such as the delayed inter-seasonal respiratory syncytial virus (RSV) epidemic in France and in other parts of the world [15-17]. For now (December 2021), we have not observed a pertussis resurgence despite the end of the first lockdown and school fully reopening in France. Our data suggest that, unlike for RSV, slight relaxation of public health measures (with maintained physical distancing and mask wearing) had no impact on pertussis dynamics; the same was the case for the influenza virus for which no outbreak was noted in France and worldwide in 2020 [18-20]. We identified six studies, including two European studies from Italy and Sweden, describing the trends of several infectious diseases, including pertussis, before and after implementing their respective COVID-19 mitigation measures [21-24]. Although the power of these studies was much lower compared with ours (lower duration of surveillance and description of a maximum of ca 1,500 cases, vs 19,039 cases in our study), they found a decrease in pertussis cases following the implementation of mitigation measures, with reductions ranging from 28% to 87% [21-26].
Even if the incidence of pertussis has strongly decreased since the introduction of booster vaccines, B. pertussis is still circulating in France, especially in older children, adults and elderly people, as we have previously shown [8,9,11]. This may be due to non-optimal vaccination coverage in these populations [27] and waning of immunity with the acellular pertussis vaccine [8,28]. The limited circulation of B. pertussis in the community may be a clear positive collateral effect of the measures imposed by the COVID-19 pandemic. However, this reduced bacterial circulation may decrease the herd immunity, with the subsequent risk of a larger epidemic in the coming years, after relaxation of all mitigation measures [29]. Most concerns are related to vaccine uptake; during the lockdown, paediatricians involved in data Source 3 declared that they followed vaccinations guidelines [30]. However, as in many countries, delays in vaccination have been noted in the general population in France since the beginning of the pandemic; they mostly concerned booster vaccinations for children and adults [6,31]. Such delays can be dangerous for infants under 6 months of age who are not or partially vaccinated, notably if there is no catch-up before a potential resurgence of pertussis.
Our study has limitations. Firstly, measurement bias is present in all data sources, as Sources 1 and 2 may have missed cases diagnosed by serology (sometimes prescribed even if not recommended) or cases diagnosed on clinical grounds, especially during the first lockdown (from mid-March to May 2020) when transportation was limited, office-based physicians could not be reached and private laboratories were overwhelmed by the implementation of large-scale SARS-CoV-2 testing. Secondly, we cannot determine whether the decrease in pertussis cases observed here was due to decreased pertussis circulation or reduced testing. However, the similar decrease observed in hospitalised cases in the youngest population does not favour the latter hypothesis, and access retrictions to outpatient testing were mostly limited to the first lockdown period. Thirdly, we may have lacked statistical power to detect a significant decrease in the proportion of positive cases, as the post-lockdown period was relatively short compared with the pre-lockdown period (9 months vs 87 months) and negative PCR tests results were not available from data Source 2. Finally, adherance to mitigation measures was not measured at the patient level, we cannot confirm that the observed drop in pertussis cases was a direct consequence of these measures.
Conclusion
This national-level study shows a strong association between the COVID-19 pandemic and the trend in pertussis incidence in France, with an unprecedented drop in pertussis cases. Pertussis should be closely monitored to detect any resurgence in the community when physical distancing restrictions are relaxed, as well as compliance with vaccination of infants, children and adults.
Acknowledgements
We thank the microbiologists and the paediatricians from the Renacoq network, as well as Annie Landier and Nathalie Armatys from the NRC, for their participation to PCR testings. We thank Martin Chalumeau for his methodological advice, and all the paediatricians of the French ambulatory surveillance of whooping cough. We thank Isabelle Ramay, Karin Lejeune, Aurore Prieur and Marine Borg for technical assistance.
Ethical statement
The data collections received approval by Institutional Review Boards (N° 2021 0608105701) for Source 1, and by the National Review Boards for Source 2 (N°449199) and Source 3 (N°1161396). Source 3 was registered at ClinicalTrials.gov (NCT04318431). All data processing and storage comply with the General Data Protection Regulation (GDPR) and ethical standards of the National Research Committee.
Data sharing
Anonymised database will be made available on reasonable request.
Supplementary Data
Conflict of interest: CL, RC, SBe (for the French ambulatory surveillance of whooping cough for outpatient paediatric cases by ACTIV network) received funding from GlaxoSmithKline, MSD and Sanofi Pasteur. The other authors did not declare any conflict of interest.
Authors’ contributions: Study conception: JT, CL, RC, DLB, NG, JFC, SBr. Data collection: SM, CF, CL, SBe, SG, FAEB, YS, JP, STP, VJ, JT. Statistical analysis: JFC. Data analysis: JFC, SM, CF, SBe, CL JT. Data interpretation: SM, JT, CL, JC, CF, SBe, SG, FAEB, YS, JP, DLB, NG, SBr, RC. Microbiology analyses: STP, VJ. Drafting the manuscript: SM, JFC, JT. Revising the manuscript: all authors. Approving the final version submitted: all authors. Study supervision: JT.
References
- 1. Domenech de Cellès M, Magpantay FM, King AA, Rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc Biol Sci. 2016;283(1822):283. 10.1098/rspb.2015.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974-80. 10.1016/S1473-3099(17)30390-0 [DOI] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control (ECDC). Pertussis - Annual epidemiological report for 2018. Stockholm: ECDC; 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/pertussis-annual-epidemiological-report-2018
- 4.World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Geneva: WHO; 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 5.République Française. Décret n° 2020-260 du 16 mars 2020 portant réglementation des déplacements dans le cadre de la lutte contre la propagation du virus covid-19. [Decree No. 2020-260 of 16 March 2020 regulating travel in the context of the fight against the spread of the covid-19 virus]. Journal Officiel de la République Française. Text 2. 2020. French. Available from: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000041728476
- 6.EPI-PHARE. Covid-19: usage des médicaments de ville en France. [Covid-19: use of community drugs in France]. Saint-Denis: EPI-PHARE; 2020. French. Available from: https://www.epi-phare.fr/rapports-detudes-et-publications/covid-19-usage-des-medicaments-de-ville-en-france-rapport4
- 7. Angoulvant F, Ouldali N, Yang DD, Filser M, Gajdos V, Rybak A, et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections-a time series analysis. Clin Infect Dis. 2021;72(2):319-22. 10.1093/cid/ciaa710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paireau J, Guillot S, Aït El Belghiti F, Matczak S, Trombert-Paolantoni S, Jacomo V, et al. Effect of change in vaccine schedule on pertussis epidemiology in France: a modelling and serological study. Lancet Infect Dis. 2022;22(2):265-73. 10.1016/S1473-3099(21)00267-X [DOI] [PubMed] [Google Scholar]
- 9. Tubiana S, Belchior E, Guillot S, Guiso N, Lévy-Bruhl D, Renacoq Participants . Monitoring the impact of vaccination on pertussis in infants using an active hospital-based pediatric surveillance network: results from 17 years’ experience, 1996-2012, France. Pediatr Infect Dis J. 2015;34(8):814-20. 10.1097/INF.0000000000000739 [DOI] [PubMed] [Google Scholar]
- 10. Guillot S, Guiso N. Follow-up of external quality controls for PCR-based diagnosis of whooping cough in a hospital laboratory network (Renacoq) and in other hospital and private laboratories in France. J Clin Microbiol. 2016;54(8):2169-71. 10.1128/JCM.00882-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guiso N, Levy C, Romain O, Guillot S, Werner A, Rondeau MC, et al. Whooping cough surveillance in France in pediatric private practice in 2006-2015. Vaccine. 2017;35(45):6083-8. 10.1016/j.vaccine.2017.09.072 [DOI] [PubMed] [Google Scholar]
- 12. Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ. 2015;350(jun09 5):h2750. 10.1136/bmj.h2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherry JD. Epidemic pertussis in 2012--the resurgence of a vaccine-preventable disease. N Engl J Med. 2012;367(9):785-7. 10.1056/NEJMp1209051 [DOI] [PubMed] [Google Scholar]
- 14.Santé Publique France. Rougeole: données annuelles 2020. Saint-Maurice: Santé Publique France; 2021. Available from: https://www.santepubliquefrance.fr/les-actualites/2021/rougeole-donnees-annuelles-2020
- 15. Fourgeaud J, Toubiana J, Chappuy H, Delacourt C, Moulin F, Parize P, et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur J Clin Microbiol Infect Dis. 2021;40(11):2389-95. 10.1007/s10096-021-04323-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foley DA, Yeoh DK, Minney-Smith CA, Martin AC, Mace AO, Sikazwe CT, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin Infect Dis. 2021;73(9):e2829-30. 10.1093/cid/ciaa1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delestrain C, Danis K, Hau I, Behillil S, Billard MN, Krajten L, et al. Impact of COVID-19 social distancing on viral infection in France: A delayed outbreak of RSV. Pediatr Pulmonol. 2021;56(12):3669-73. 10.1002/ppul.25644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan SG, Carlson S, Cheng AC, Chilver MB, Dwyer DE, Irwin M, et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25(47):25. 10.2807/1560-7917.ES.2020.25.47.2001847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uyeki TM, Wentworth DE, Jernigan DB. Influenza activity in the US during the 2020-2021 season. JAMA. 2021;325(22):2247-8. 10.1001/jama.2021.6125 [DOI] [PubMed] [Google Scholar]
- 20. Rybak A, Levy C, Jung C, Béchet S, Batard C, Hassid F, et al. Delayed bronchiolitis epidemic in French primary care setting driven by respiratory syncytial virus: preliminary data from the Oursyn study, March 2021. Pediatr Infect Dis J. 2021;40(12):e511-4. 10.1097/INF.0000000000003270 [DOI] [PubMed] [Google Scholar]
- 21. Adegbija O, Walker J, Smoll N, Khan A, Graham J, Khandaker G. Notifiable diseases after implementation of COVID-19 public health prevention measures in central Queensland, Australia. Commun Dis Intell. 2018;2021:45. [DOI] [PubMed] [Google Scholar]
- 22. Belingheri M, Paladino ME, Piacenti S, Riva MA. Effects of COVID-19 lockdown on epidemic diseases of childhood. J Med Virol. 2021;93(1):153-4. 10.1002/jmv.26253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huh K, Jung J, Hong J, Kim M, Ahn JG, Kim JH, et al. Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. 2021;72(7):e184-91. 10.1093/cid/ciaa1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yun HE, Ryu BY, Choe YJ. Impact of social distancing on incidence of vaccine-preventable diseases, South Korea. J Med Virol. 2021;93(3):1814-6. 10.1002/jmv.26614 [DOI] [PubMed] [Google Scholar]
- 25. Kim DH, Nguyen TM, Kim JH. Infectious respiratory diseases decreased during the COVID-19 pandemic in South Korea. Int J Environ Res Public Health. 2021;18(11):18. 10.3390/ijerph18116008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falkenstein-Hagander K, Appelqvist E, Cavefors AF, Källberg H, Nilsson LJ, Silfverdal SA, et al. Waning infant pertussis during COVID-19 pandemic. Arch Dis Child. 2022;107(3):e19. 10.1136/archdischild-2021-323055 [DOI] [PubMed] [Google Scholar]
- 27. Marchal C, Belhassen M, Guiso N, Jacoud F, Van Ganse E, Le Pannerer M, et al. Vaccination coverage rates for diphtheria, tetanus, poliomyelitis and pertussis booster vaccination in France between 2013 and 2017: learnings from an analysis of national health system real-world data. Vaccine. 2021;39(3):505-11. 10.1016/j.vaccine.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 28. von König CHW. Acellular pertussis vaccines: where to go to? Lancet Infect Dis. 2018;18(1):5-6. 10.1016/S1473-3099(17)30613-8 [DOI] [PubMed] [Google Scholar]
- 29. Cohen R, Ashman M, Taha MK, Varon E, Angoulvant F, Levy C, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418-23. 10.1016/j.idnow.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.InfoVac-France. Point sur les consultations à maintenir durant la Pandémie COVID-19. [Update on the consultations to be maintained during the COVID-19 Pandemic]. InfoVac-France; 2020. French. Available from: https://www.infovac.fr/actualites/point-sur-les-consultations-a-maintenir-durant-la-pandemie-covid-19
- 31. Taine M, Offredo L, Drouin J, Toubiana J, Weill A, Zureik M, et al. Mandatory infant vaccinations in france during the COVID-19 pandemic in 2020. Front Pediatr. 2021;9:666848. 10.3389/fped.2021.666848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


