BACKGROUND
Pediatric leukemias and lymphomas comprise the most common subset of pediatric cancers.1,2 The diagnosis of pediatric leukemia encompasses diverse clinical and biological diseases. While patients with pediatric acute lymphoblastic leukemia (ALL) have a greater than 80% chance of cure,3 other blood cancers have poorer prognoses. Novel therapies for acute myeloid leukemia (AML), infant ALL, adolescent/young adult ALL, and relapsed or refractory ALL are urgently needed. With advanced genetic and molecular profiling of these diseases, there is now the opportunity to develop targeted therapies with more favorable safety profiles and improved efficacy.
T-cell adoptive transfer is one type of targeted therapy that has had great success in the treatment of high-risk ALL. A multicenter clinical trial of treatment with the anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, tisagenlecleucel, demonstrated an overall remission rate within 3 months of 81%,4 leading to FDA-approval for therapy of relapsed pre-B ALL.5 Though this clinical success suggests promise for all pediatric blood cancers, expanded use of CAR-T cell therapy also has clear limitations. Treatment with CAR-T cells has the risk of severe toxicities including Cytokine Release Syndrome (CRS), Immune Effector Cell Associated Neurotoxicity Syndrome (ICANS), and CAR-associated Hemophagocytic Lymphohistiocytosis (carHLH).6,7 Further, tisagenlecleucel and all other commercial CAR-T cell products are derived from autologous hematopoietic cell collections, which can be challenging to perform in heavily pretreated patients with active disease. Per-patient CAR-T cell manufacture is also time-consuming and costly.8 While the collection of healthy donor T cells can be considered, the use of human leukocyte antigen (HLA)-mismatched allogeneic products carries the serious risk of graft-versus-host disease (GVHD).9 Natural Killer (NK) cells are alternate lymphocyte effector cells that are also potent killers. NK cells have the potential for ex vivo expansion and storage as an “off the shelf” therapy. While activated NK cells secrete cytokines that contribute to the inflammatory milieu, they do not directly cause graft versus host disease9,10 and have an overall less severe side effect profile than allogeneic T-cell products.11-15 Because of these favorable characteristics, there is expanded focus on the study and development of NK cells as immunotherapeutics targeted to cancer.
Natural Killer Cell Biology
NK cells are lymphocytes of the innate immune system that serve a critical function in host defense against viral infections and malignancy.16 They constitute 5% to 15% of circulating lymphocytes and are found in the peripheral blood as well as lymphoid and nonlymphoid organs such as the spleen, lung, and liver.15,17,18 NK cells arise from CD34+ hematopoietic stem cells and progress through developmental stages defined by the expression of surface receptors in response to cytokines including Interleukin (IL)-2, IL-7, and IL-15.15,19 NK cells in the peripheral blood are subdivided into two primary categories: CD56brightCD16dim/− cells are phenotypically less mature with the capability to produce higher levels of inflammatory cytokines, while CD56dimCD16+ NK cells are more mature with greater cytotoxic potential.15,18,19 Most NK cells in peripheral circulation fit the latter CD56dim profile, with the CD56bright subset representing <15% of the total circulating cell population,15,18 though recent single cell analyses have revealed additional diversity.20,21 NK cells perform their immune function through four major pathways: (1) secretion of cytokines and chemokines, (2) direct cytotoxicity, (3) target killing through induced apoptosis, and (4) antibody-dependent cellular cytotoxicity.22 NK cells are activated following the integration of stimulatory and inhibitory signals without the need for antigen processing and presentation or HLA matching.22,23 Major histocompatibility complex class I (MHC I) molecules are found on all healthy nucleated cells and provide protection from NK cell targeting through the ligation of the killer immunoglobulin-like (KIR) family of inhibitory NK cell receptors.15,24 Cells infected with virus and those that are malignant typically downregulate MHC I expression25-27 and upregulate NK cell activating ligands, thereby tipping the scale in favor of NK cell activation and killing.15,28
Unique Challenges to Natural Killer Cell Immunotherapy
Despite these promising attributes, there are challenges to the clinical translation of NK cell-based therapies. These include nonstandardized clinical-grade ex vivo expansion and historical reports of poor genetic engineering.15,29 Other challenges include limited in vivo persistence with a peripheral half-life of approximately 7 to 10 days,30-32 limited trafficking to and infiltration of the tumor microenvironment (TME),33 and tumor immunoevasion.34-36 Genetic modification of NK cells may overcome these challenges to optimize innate cytotoxicity.
STARTING MATERIAL FOR NATURAL KILLER CELL IMMUNOTHERAPY
NK cells for clinical application can be derived from multiple sources. Peripheral blood (PB) is the best-studied, given ease of collection. NK cells can be purified after blood apheresis with CD3+ cell depletion and/or CD56+ selection.37,38 Because NK cells represent only 10% to 15% of circulating PB cells, the expansion of peripheral blood-derived NK cells (PB-NKs) on a clinical scale is achieved by culture with supplemental cytokine and/or feeder cells.39-41 NK cell expansion using feeder cells was first described by Campana and colleagues,42 and consists of K562 cells engineered to express ligands that trigger NK cell activation (ex. membrane-bound IL15, IL21, and 4-1BBL).40,43,44 PB-NKs highly express a repertoire of activating receptors and are functionally mature, with cytotoxic capacity.45 PB-NKs also express higher levels of KIRs than other sources. This is particularly important for NK cell functionality, since KIR expression has a central role in NK cell education and licensing.46-48 One of the theoretic limitations to the use of PB-NKs as cancer immunotherapy is reportedly poor success with genetic modification.49,50 However, we and others have shown high levels of vector transduction of PB-NKs.37,38,51
NK cells can also be differentiated from induced pluripotent stem cells (iPSCs) understandardized culture conditions.52,53 The production of NK cells from iPSCs (iPSC-NKs) requires more expertise than peripheral blood NK cell selection.53 The iPSC platform produces a homogeneous NK cell population. NK cell iPSC derivation allows for multiple genetic modifications while cells are relatively undifferentiated, including CAR-engineering and genetic deletion.54,55 iPSC-NKs have a similar, but more immature phenotypic profile to PB-NKs, with higher expression of the inhibitory receptor NKG2A.52 Moreover, iPSC-NKs exhibit lower KIR expression.52 Nevertheless, iPSC-NKs have promise as anticancer immunotherapy54-56 and can be developed as “off-the-shelf” cellular banks, with well-defined NK cell therapy products generated on large scale, readily available for adoptive transfer.
Cancerous NK cell lines have also been established over the years as sources of NK cell therapy products (NK-92,57 NK-101,58 NK-YS,59 NKL,60 NKG,61 KHYG-1,62 and others). The NK-92 lymphoma cell line has been most widely used as a platform for genetic modification and subsequent adoptive transfer. NK-92 produce high levels of granzymes, perforins, and other death-inducing molecules (FAS-L, TRAIL) that exert potent cytotoxicity against cancer cells.63 NK-92 adoptive transfer (with or without CAR modification) has been shown to be safe, but with limited efficacy in the context of both hematologic64-66 and solid tumors.67,68 The use of aneuploid transformed cells with multiple cytogenetic abnormalities as anticancer therapy mandates preinfusion irradiation, which has the expected deleterious effect on in vivo expansion, persistence, and thus efficacy.69 Moreover, while NK-92 cells generally share a receptor repertoire with PB-NK cells, they lack CD16 (FcγRIII) and thus the capacity for antibody-dependent cellular cytotoxicity (ADCC).57,70 NK-92 also lack activating receptors, for example, NKp44 and almost all of the KIRs, with the exception of KIR2DL4.63
Umbilical cord blood (UCB) is another source for the generation of “off-the-shelf” NK cell products. UCB is relatively easy to isolate71 and contains a similar percentage of NK cells as PB.48,72 However, the small total blood volume in each CB unit makes acquiring satisfactory NK cell numbers for clinical use challenging. Similar to PB, UCB is heterogeneous, and NK cell purification is needed. Freshly isolated UCB-NKs are characterized by an immature immune phenotype with lower surface expression of NKG2C, CD57, adhesion molecules (CD2, CD11α, CD18, DNAM-1),73 CD62 L (LN homing receptor), and KIRs (CD158a, CD158b, and others) and higher expression of 2B4, CXCR4 (BM homing receptor), and NKG2A, compared to PB counterparts.48,74,75 Studies comparing granzyme and perforin expression in UCB- and PB-NK cells have reported contradictory results.75,76 The cytotoxic capacity of UCB-NK is lower than PB-NK, yet this difference can be partially mitigated with cytokine stimulation.48,75,76 Clinical trials testing adoptive transfer of unmodified or CAR-expressing UCB-NK as hematologic malignancy treatment have shown that this approach is safe, without GVHD or other toxicities, but with limited antitumor effect.77,78
METHODS OF GENETIC ENGINEERING IN NATURAL KILLER CELLS
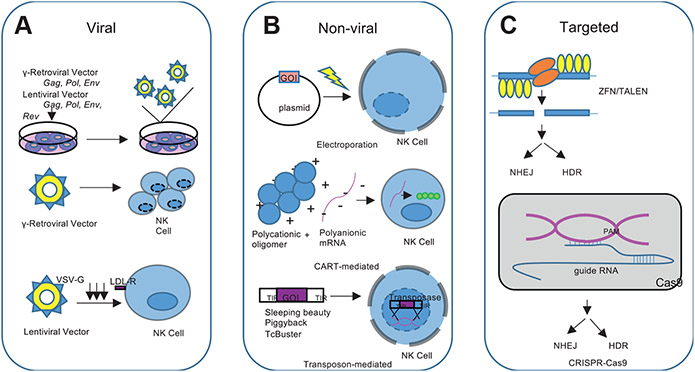
Though simple in concept, efficient genetic modification of NK cells is a challenge. Early attempts at NK cell engineering have reported low gene transfer efficiency using both viral-based and nonviral methods.29 Recent optimization of gene transfer strategies has been more successful with an improved safety profile (Fig. 1).79
Fig. 1.
Methods of Genetic Engineering in NK Cells. (A) Viral-mediated, including γ-retroviral and lentiviral vectors. (B) Nonviral mediated, including electroporation, Charge-altering releasable transporters, and transposon systems. (C) Targeted knockdown and knock-in, including zinc-finger nucleases (ZFN), transcription activator-like nucleases (TALENs), and CRISPR/Cas9 systems as NK cell engineering methods.
Viral-mediated natural killer cell engineering
NK cell modification with viral vectors is now associated with high-efficiency gene transfer. Viral vectors are readily manufactured and can stably integrate genetic material into the host genome.80 The Retroviridae family are the most commonly used vectors for gene therapy applications, including NK cell genetic modification.79 The Retroviridae family includes seven members: α-, β-, γ-, δ-, and ε-retroviruses, spumaviruses, and lentiviruses.79 Of these, γ-retroviruses and lentiviruses are most often used in cellular gene transfer for clinical application.79,80 Retroviral genetic modification includes the reverse transcription of the viral RNA genome into double-stranded DNA and permanent integration into the host genome facilitated by viral protein integrase encoded by the pol gene. Retroviral transduction has evolved to use replication-incompetent retroviruses as a safety mechanism to limit self-replication and infectivity.80
In γ-retrovirus vectors, the γ retroviral coding sequences are replaced by the transgene of interest and this recombinant plasmid as well as helper plasmids encoding viral capsid proteins, replication enzymes, and envelope glycoproteins (gag, pol, and env) are cotransfected into a virus packaging cell line where the recombinant plasmid is synthesized and packaged into viral particles. A limitation of successful γ-retroviral transduction is the requirement for a disrupted nuclear membrane in actively dividing target cells.80,81 Despite this obstacle, γ-retroviral vectors have demonstrated high rates of transduction and persistence of transgene expression in gene therapy applications, including the use of NK cells.
Lentivirus-based vectors similarly involve the replacement of essential viral genes with the transgene of interest and also use helper plasmids to encode gag, pol, and env. Unlike γ-retroviral vectors, lentivirus-based vectors additionally require the incorporation of the rev gene, which encodes the Rev protein and enhances nuclear export and expression of gag-pol transcripts through the binding of the rev responsive element (RRE).80,82 Lentivirus-based vectors further contain a central polypurine tract (cPTT)/central termination signal (CTS) that functions to facilitate nuclear import of the preintegration complex after target cell uptake of the virion.80,83 As a result, lentiviral vector transduction is not dependent on active cellular division, which notably expands the repertoire of potential target cells. Despite this reach, the use of lentiviral-based vectors for NK and other hematopoietic cell transduction has not resulted in high percentages of transduced cells, largely because of limited cellular entry. Lentivirus is standardly pseudotyped with vesicular stomatitis virus g-protein (VSV-G), which binds LDL-R on human cells. NK cells express LDL-R at low levels, making transduction efficiency low.84 The use of lineage-specific promotors and cell-type-specific lentiviral vectors using envelope proteins from viruses such as RD114 or baboon retrovirus are potential ways to improve the efficacy and specificity of lentiviral vectors.85-87
Though viral-based cell engineering can effectively achieve stable transgenic expression, without genomic targeting this method is inherently nonspecific and therefore associated with the risk of insertional mutagenesis. γ-retroviral vectors integrate with proximity to cellular gene promoters, transcription start sites, and CpG islands. This can result in neoplastic transformation if genomic insertion occurs at a protooncogenic site.80,88,89
Nonviral natural killer cell engineering
Nonviral NK cell modification can circumvent some potential pitfalls of viral engineering. The most used nonviral gene delivery method is electroporation, which is a simple and cost-effective strategy that can introduce nucleic acid into a target cell with high efficiency. Electroporation generates small, temporary pores in the cell and nuclear membrane by electrical pulsing, which allows for the transfer of charged nucleic acid molecules.90,91 The primary downside to electroporation is the toxicity that can lead to high rates of cell death.92 Successful NK cell transfection via electroporation has been described using primary NK cells with resultant efficient gene transfer.90,91,93
Alternative methods for the delivery of charged nucleotides across a nonpolar cell membrane include the use of cationic polymers, lipid nanoparticles, and more recently charge-altering releasable transporters. Charge-altering releasable transporters are multiblock polycationic oligomers that noncovalently complex with polyanionic mRNA to facilitate its delivery across the cellular membrane.94 Charge-altering releasable transporters have been used to transfect resting primary NK cells with mRNA to generate cytotoxic human anti-CD19-CAR NK cells with efficient transfection, viability, and preservation of the NK cell phenotype.95 A primary limitation to the use of charge-altering releasable transporter-mediated gene delivery is the transient nature of mRNA mediated cellular modification.95.
Another method for stable, nonviral genomic modification involves the use of DNA transposons, which are naturally occurring repetitive DNA sequences that can jump from one location in the genome to another.91,96 This natural phenomenon can be harnessed for gene editing by using a transposon vector containing the gene of interest flanked by terminal inverted repeats (TIRs), and a transposase enzyme that targets the TIRs to excise the gene of interest from the original site and stably reintegrate into chromosomal sites.91,97 DNA transposons have been effectively used to engineer human cells, including NK cells.91,97,98 The most extensively studied transposon systems for clinical gene transfer are Sleeping Beauty (SB), piggyback (PB), and TcBuster, which have been used as platforms for delivery of CARs into hematopoietic cells.91 Human iPSC-derived NK cells have been successfully engineered to express CARs using the PB transposon system with enhanced antigen-specific NK cell activity observed against mesothelin-expressing tumors in vitro and in vivo.55 PB and SB systems have also been used to successfully express a panel of mesothelin directed CARs in NK-92 cells.55 As with any nonspecific gene transfer, transposon-mediated chromosomal integration has the risk of causing insertional mutagenesis. A recent phase I clinical trial of donor-derived CD19-targeting CAR T-cells engineered using the PB transposon system reported the development of malignant CAR-expressing CD4+ T-cell lymphomas in 2 out of 10 treated patients.99,100 Transcriptomic analysis of malignant tissue confirmed transgene promoter-driven transcriptional upregulation of surrounding regions, background genomic copy number variations, and high transgene copy number per cell.100 This outcome highlights the need for careful surveillance and monitoring of patients receiving novel engineered cell therapy products, particularly when nontargeted gene engineering methodology is used.
Targeted knockdown and knock-in gene modification strategies
Targeted gene editing can also be achieved using zinc-finger nucleases (ZFN), transcription activator-like nucleases (TALENs), and (CRISPR)/CRISPR-associated protein 9 (Cas-9) systems, often in combination with adeno-associated virus (AAV) vectors. ZFN and TALEN manipulate protein-DNA interactions to target specific genomic loci for editing and require expertise in protein engineering.101 CRISPR-Cas9 uses RNA-guided DNA recognition, with guide RNA designed with homology to the genetic locus of interest.101 CRISPR-Cas9 can be used to reliably knockout target genes or to knock-in genetic modifications with targeted integration of a homologous recombination repair template.91,102 Efficient CRISPR-Cas9 mediated knockout of inhibitory signaling pathways in primary NK cells was recently described with efficiency reaching 90%.93 The CRISPR-Cas9 system was subsequently used in combination with recombinant AAV serotype 6 (rAAV6) for delivery of a homologous recombination template DNA for an efficient knock-in strategy.93 As an alternative, other groups have packaged Cas9 and guide RNAs into ribonucleoprotein (RNP) complexes for high gene-editing efficiency in NK cells.91,103 Related gene-editing techniques include Base editors (BEs) that fuse a catalytically inactive Cas9 protein to a DNA deaminase domain with the goal of precise introduction of a targeted single nucleotide change without double-strand breaks or DNA donor molecules.91,104
The development of successful NK cell gene-based therapies is rooted in an effective gene-editing strategy. Research investigating viral and nonviral delivery platforms with or without targeted knockdown or knock-in mechanisms aims to optimize the efficiency and safety profile of genetic engineering of NK cells. Regardless of the platform selected, each described genetic modification strategy has evidence of successful NK Cell modification and thus, can be considered to engineer enhanced effector cell function and immunotherapeutic efficacy.
THERAPEUTIC APPLICATIONS OF GENETICALLY MODIFIED NATURAL KILLER CELLS
Modulation of surface receptor expression
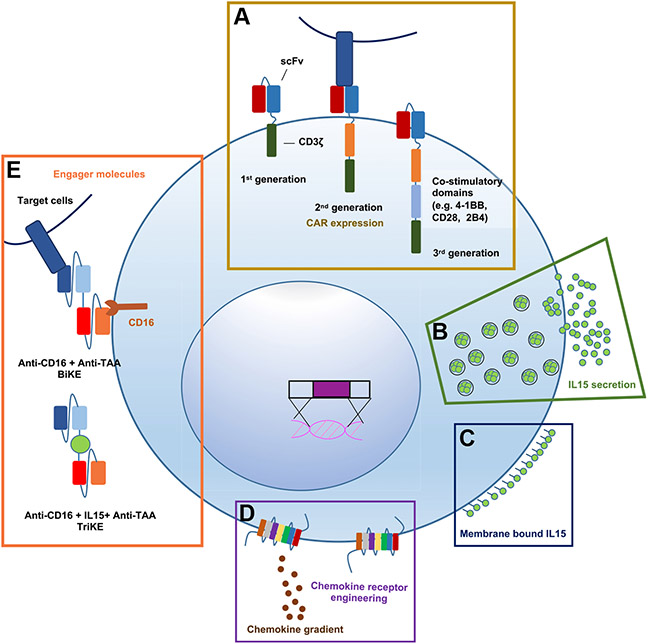
Chimeric antigen receptor-natural killer cells demonstrate target-specific activation
CAR-T cell therapy has revolutionized the treatment landscape for pediatric hematologic malignancies and broadened the developmental scope to include alternate tumor types and effector cell starting materials. NK cell CARs can be designed to target diverse antigens for specific NK cell activation by using unique extracellular single-chain variable fragments (scFvs) linked to combinations of intracellular costimulatory domains (Fig. 2A). A robust body of preclinical work demonstrates that NK cells can effectively be engineered to express CARs, and this has been comprehensively reviewed elsewhere.105-110 These preclinical investigations have laid the ground for clinical translation, and a number of CAR-NK cell therapy trials are underway. As of November 2021, 26 CAR-NK cell cancer treatment trials are registered on clinicaltrials.gov (Table 1). Two of these have published results. One example is an actively recruiting phase I/II trial (NCT03056339) led by the University of Texas MD Anderson Cancer Center. In this ongoing study, HLA-mismatched anti-CD19 UCB CAR-NK cells are administered to patients with relapsed or refractory CD19-positive hematologic malignancies. In a report describing the initial cohort of 11 patients, the treatment was well-tolerated without the development of CRS, ICANS, or GVHD. Lymphodepleting chemotherapy was given prior to CAR-NK cell infusion (1 × 105, 1 × 106, or 1 × 107 CAR-NK cells per kilogram of body weight), with 7 of 11 patients achieving a complete remission.78 A separate phase I clinical trial conducted in Suzhou, Jiangsu, China evaluated the safety of CD33 CAR-NK cells in patients with relapsed and refractory AML (NCT02944162). A third-generation lentiviral CAR construct (αCD33.CD28.4-1BB) was used for NK-92 cell transduction in this study. Three patients with relapsed and refractory AML treated with salvage chemotherapy were infused with three escalating doses of irradiated CD33-CAR NK-92 cells. No adverse effects or durable clinical efficacy were recorded.66
Fig. 2.
NK Cell Engineering for Therapeutic Application. Schematic depicting (A) Expression of CARs targeting TAA to enhance cytotoxicity and NK cell activation. (B) Constitutive secretion or (C) membrane-tethered cytokine can sustain NK cell activation and persistence. (D) Enhanced surface expression of chemokine receptors can mediate NK cell localization to tumor along chemokine gradients. (E) Engager molecules (BiKEs or TriKEs) can be combined with engineered CD16 to direct powerful ADCC of tumor cells. Incorporation of IL15 in the small molecule can further support persistence.
Table 1.
Clinical trials testing the use of CAR-NK cells as anticancer immunotherapy
| Target | Agent | NCT | Year Posted |
Phase | Status | Tumor | NK Source | Sponsor | Vector |
|---|---|---|---|---|---|---|---|---|---|
| CD19 | CAR-NK019 | NCT04887012 | 2021 | I | Recruiting | B-cell NHL | iPSC | Second Affiliated Hospital, School of Medicine, Zhejiang University, China | Lentiviral |
| CD19 | Anti-CD19 CAR NK Cells | NCT04639739 | 2020 | Early I | Planned | • NHL | Unknown | Xinqiao Hospital of Chongqing, China | Unknown |
| CD22 | Anti-CD22 CAR-NK Cells | NCT03692767 | 2018 | Early I | Unknown | • Refractory B-cell Lymphoma | Unknown | Allife Medical Science and Technology Co., Ltd., China | Unknown |
| CD19 | Anti-CD19 CAR NK Cells | NCT03690310 | 2018 | Early I | Unknown | • Refractory B-cell Lymphoma | Unknown | Allife Medical Science and Technology Co., Ltd., China | Unknown |
| CD33 | Anti-CD33 CAR NK Cells | NCT05008575 | 2021 | I | Planned | • AML | Unknown | Xinqiao Hospital of Chongqing, China | Unknown |
| Mesothelin | Anti-Mesothelin CAR-NK Cells | NCT03692637 | 2018 | Early I | Unknown | • Epithelial Ovarian Cancer | Unknown | Allife Medical Science and Technology Co., Ltd., China | Unknown |
| NKG2D | CAR-NK cells targeting NKG2D ligands | NCT03415100 | 2018 | I | Unknown | • Metastatic Solid Tumors | PB-NK | The Third Affiliated Hospital of Guangzhou Medical University, China | mRNA electroporation |
| PSMA | Anti-PSMA CAR NK Cells | NCT03692663 | 2018 | Early I | Planned | Castration-Resistant Prostate Cancer | Unknown | Allife Medical Science and Technology Co., Ltd., China | Unknown |
| BCMA | Anti-BCMA CAR-NK Cells | NCT05008536 | 2021 | Early I | Planned | Refractory Multiple Myeloma | UCB-NK | Xinqiao Hospital of Chongqing, China | Unknown |
| ROBO1 | ROBO1 CAR-NK Cells | NCT03940820 | 2019 | I/II | Recruiting | Solid Tumors | NK-92 | Asclepius Technology Company Group (Suzhou) Co., Ltd., China | Lentiviral |
| BCMA | BCMA CAR-NK 92 Cells | NCT03940833 | 2019 | I/II | Planned | Multiple Myeloma | NK-92 | Asclepius Technology Company Group (Suzhou) Co., Ltd., China | Lentiviral |
| PD-L1 | PD-L1 t-haNK | NCT04847466 | 2021 | II | Planned | Gastroesophageal Junction Cancers; Advanced HNSCC | NK-92 | National Cancer Institute, United States | Unknown |
| CD19, CD22 | Anti-CD19/CD22 CAR NK CElls | NCT03824964 | 2019 | Early I | Unknown | Refractory B-Cell Lymphoma | Unknown | Allife Medical Science and Technology Co., Ltd., China | Unknown |
| CD19 | CAR-NK-CD19 Cells | NCT04796675 | 2021 | I | Recruiting | ALL, CLL, NHL | CB-NK | Wuhan Union Hospital, China | Retroviral |
| CD19 | NKX019 | NCT05020678 | 2021 | I | Recruiting | B-ALL, CLL, NHL | PB-NK | Nkarta Inc., United States | Unknown |
| CD33 | Anti-CD33 CAR-NK cells | NCT02944162 | 2016 | I/II | Unknown | AML | NK-92 | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | Lentiviral |
| CD19 | PCAR-119 | NCT02892695 | 2016 | I/II | Unknown | Leukemia, Lymphoma | NK-92 | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | Lentiviral |
| NKG2D | NKX101 | NCT04623944 | 2021 | I | Recruiting | AML, MDS | PB-NK | Nkarta Inc., United States | Unknown |
| ROBO1 | BiCAR-NK cells | NCT03941457 | 2019 | I/II | Recruiting | Pancreatic Cancer | NK-92 | Asclepius Technology Company Group (Suzhou) Co., Ltd., China | Lentiviral |
| CD19 | CAR.CD19-CD28-zeta-2A-iCasp9-IL15-transduced CB-NK cells | NCT03579927 | 2018 | I/II | Withdrawn | B-cell lymphoma | UCB, CB-NK | M.D. Anderson Cancer Center | Retroviral |
| CD19 | iC9/CAR.19/IL15-Transduced CB-NK Cells | NCT03056339 | 2017 | I/II | Active | B-Lymphoid Malignancies, ALL, CLL, NHL | UCB, CB-NK | M.D. Anderson | Retroviral |
| CD70 | CAR.70/IL15-transduced CB-NK cells | NCT05092451 | 2021 | I/II | Recruiting | B-cell Lymphoma, MDS, AML | CB-NK | M.D. Anderson | Retroviral |
| CD19 | FT596 | NCT04555811 | 2020 | I | Recruiting | NHL | iPSC | Masonic Cancer Center, University of Minnesota | Lentiviral |
| CD19 | FT596 | NCT04245722 | 2020 | I | Recruiting | B-cell lymphoma, CLL | iPSC | Fate Therapeutics | Lentiviral |
| ErbB2 | NK-92/5.28.z | NCT03383978 | 2017 | I | Recruiting | HER2-positive Glioblastoma | NK-92 | Johann Wolfgang Goethe University Hospital | Lentiviral |
| CD19 | CAR-ITNK | NCT04747093 | 2021 | I/II | Recruiting | B cell leukemia/lymphoma | T-cells | Nanfang Hospital of Southern Medical University, China | Unknown |
Abbreviations: ALL, acute lymphoblastic leukemia. acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; iPSC, induced pluripotent stem cells; MDS, myelodysplastic syndrome; NHL, Non-Hodgkin lymphoma; PB-NK, Peripheral blood-derived NK cells; UCB, CB-NK, umbilical, cord blood-derived CAR-engineered NK cells.
Downregulation of inhibitory receptors enhances natural killer cell activation
An alternative approach to cell surface receptor modulation is the downregulation of innate NK inhibitory receptors to bias NK cells toward activation. Cancer cells can block immune responses by targeting inhibitory receptors on the surface of NK cells. NKG2A is one such receptor that dimerizes with CD94 to bind HLA-E molecules on tumor cells, which dampens NK cell activity.111 Consequently, RNAi-mediated inhibition of NKG2A expression has improved NK cell activity in the preclinical study. NKG2A silencing can enhance NK cell cytotoxicity by up to 40%.112 Silencing of inhibitory receptors using CRISPR/Cas9, ZFN, or TALEN is likely to result in similarly amplified NK cell antitumor functionality.
Enhancing natural killer cell activity through the modulation of cytokine signaling
Natural killer cell in vivo persistence can be sustained with cytokine stimulation
The success of adoptive NK cell transfer relies to a great extent on the persistence of the cells.11 Mature adoptively transferred NK cells have a limited lifespan in vivo with a peripheral half-life of about 7 to 10 days.30,32 This short lifespan has certain advantages including: decreased risk of prolonged on target, off-tumor effects,45 decreased immunogenicity, reduced risk of cytokine release syndrome and neurotoxicity,110 and the opportunity to safely redose NK cells for continued effect. Even so, clinical studies have demonstrated the important role of persistence and expansion for clinical efficacy.11,113-115 In order to prolong NK cell survival, exogenous cytokine administration with recombinant IL2, IL15, or IL15 “superagonists” has been used in clinical trials.116-121 However, cytokine infusion is associated with systemic toxicities that are dose- and therapeutic route-dependent.118-121 Localized cytokine delivery to support NK cell persistence may be safer, as first studied using NK-92 (Fig. 2B). An animal model of liver metastases paired with NK-92 treatment was used to show that NK-92 stably expressing and secreting IL2 had better antitumor control than NK-92 alone.122 Similarly, UCB CAR-NK cells engineered to secrete IL15 prolonged the survival of mice engrafted with CD19 positive tumors.123 Safety of IL15-secreting CAR-NK cells was tested in human patients in the MD Anderson phase I clinical trial (NCT03056339) using UCB-derived CD19-CAR.IL15 NK cells described above.78 The trial found that patients who had a response to therapy had a significantly higher early expansion of CAR-NK cells and that early expansion and low-level persistence of CAR-NK cells for at least 12 months after infusion was related to the inclusion of IL-15 in the CAR construct.78 Our group has also investigated transgenic IL15 in PB-NK cells designed for the treatment of hematological malignancies. As expected, constitutive IL15 secretion enhances NK and CAR-NK cell activation and supports in vivo persistence. However, we found that treatment with IL15-secreting NK cells caused lethal toxicity in one AML xenograft model.38 Membrane-bound IL15 or the IL15/IL15 R complex can as well augment NK cell persistence and may be a safer alternative to soluble IL15 (Fig. 2C).43 To illustrate, oncolytic virus-mediated local IL15/IL15 Rα (OV-IL15 C) secretion has been used to enhance PB-CAR-NK persistence and in vivo functionality.124 Similarly, the IL15/IL15 Rα fusion protein, a CD19-CAR, and a high-affinity, noncleavable CD16 (hnCD16) have together been expressed in iPSC-NKs, resulting in enhanced functionality in preclinical CD19+ malignancy models.125 This product, FT596, is now being investigated in a phase 1 clinical trial for the treatment of B-cell lymphoma and chronic lymphocytic leukemia (NCT04245722).
Natural killer cell modification can direct tumor site homing
Surface expression of chemokine receptors has been tested as a method to enhance NK cell tumor trafficking (Fig. 2D). CXCR2 expression in the YTS NK cell line and CXCR4 in PB-NKs can promote NK cell trafficking to renal cell carcinoma (RCC) and CXCL12-secreting glioblastoma (GB), respectively.126,127 Electroporation of primary human NK cells with gain-of-function CXCR4R334X mRNA upregulated CXCR4 on the NK cell surface and supported NK cell trafficking to bone marrow in a xenograft model.128 Engineered CCR7 expression on NK cells can promote migration toward CCL19 in lymph nodes.129 Primary NK cells transfected with mRNAs encoding for CXCR1 and an NKG2D-CAR can be efficiently directed toward IL8-secreting cancers.130 Moreover, combinational immunotherapy utilizing an oncolytic virus encoding CCL5 and NK cells engineered to express the CCR5 receptor can improve NK cell tumor infiltration.131
Manipulating natural killer cell interaction with small molecule engagers
BiKEs enhance natural killer cell activation and antibody-dependent cellular cytotoxicity
Preclinical and clinical studies support the use of bispecific small molecular engagers to stabilize the NK cell-target cell immunologic synapse and improve antitumor cytolysis (Fig. 2E). BiKEs that simultaneously target a tumor-specific antigen and CD16 (FcγRIII), the low-affinity Fc receptor,132,133 are capable of stimulating ADCC. For example, a CD16xCD33 BiKE tested in patients with myelodysplastic syndromes (MDS) can successfully activate primary patient NK cells with specific degranulation and cytokine production against CD33+ MDS targets and can reverse myeloid-derived suppressor cell (MDSC)-mediated immunosuppression of NK cells through targeted CD33+ MDSC lysis.134 Secondly, the use of a CD30xCD16 A BiKE (AFM13) targeting CD30 on tumor cells and the CD16 A isoform on NK cells has been extensively tested in patients with CD30+ malignancies with notable antitumor responses, a good safety profile, and dose-dependent NK cell CD69 upregulation.135-137
With these encouraging results, optimizing ADCC by combining BiKEs with genetically modified NK cells holds promise. Engineered expression of high-affinity Fc receptor isoforms to enhance ADCC is being studied by multiple groups.138-142 For example, FT516 is a CD19-targeted CAR-NK cell product derived from a clonal iPSC line, further engineered to include a high-affinity, noncleavable CD16.141 Preliminary results from a phase I trial of FT516 (NCT04023071) used in patients with relapsed/refractory B-cell lymphoma evaluated the safety of infusion in combination with the monoclonal CD20 antibody, rituximab. Patients received 3 days of lymphodepleting chemotherapy, followed by 1 dose of rituximab, and 3 weekly infusions of FT516 administered with systemic IL-2. Six patients with a median age of 65.5 years enrolled in the trial, all heavily pretreated, 5 of whom completed the therapy. Escalating doses were evaluated including 3 million cells/dose (2 patients), 90 million cells/dose (3 patients), and 300 million cells/dose (1 patients). No CRS, ICANS, GVHD, or other serious side effect were observed in 5 treated patients. Three patients treated at > 90 million cells/dose achieved an objective response.143
Trispecific killer engagers can complement antibody-dependent cellular cytotoxicity with cytokine driven stimulation
Similar to BiKEs, trispecific killer engagers (TriKEs) are composed of an antigen-specific scFv linked to the scFvs of two other antibodies of different specificities or to the scFv of one other antibody and a cytokine. TriKEs have also been designed to augment ADCC by engaging CD16 along with tumor-specific antigens.144 Newer generations of TriKEs incorporate the IL-15 cytokine to augment NK cell function. This, coupled with CD16 engineering modalities described above may further enhance the specificity and persistence of antitumor NK cell activation. Additional NK cell manipulations discussed above such as the downregulation of inhibitory receptors can be employed to further enhance TriKE immunotherapy, and combinatorial manipulation remains an area of active study.
SUMMARY
NK cells are a powerful tool for targeted immunotherapy. They harbor a range of innate cytotoxic mechanisms and can be genetically engineered to enhance their function. This can be conducted by modulating cell surface receptor expression, by manipulating stimulatory cytokines and chemokines, and by optimizing engagement with targeted small molecules (Table 2). NK cells are subject to genetic modification using viral and nonviral vectors and can be isolated or derived from different primary sources. This diversity leaves much option for further study and discovery. While early clinical experiences underline their promise, the clinical development of engineered NK cell therapy remains in its infancy with current reports limited to small cohorts of treated patients. There is a need for further discovery and subsequent translation into large-scale clinical trials to truly detail the role of these effector cells in the expand-ing armamentarium of immunotherapies for blood cancers. The success of genetically engineered NK cell therapy is dependent on the complete understanding of the factors that drive NK cell activation, immune synapse formation, and target cell killing. There remains much to be discovered given the potential for these remarkable innate effector cells to be effective anticancer immunotherapeutics.
Table 2.
Therapeutic applications for genetically modified NK cells
| Therapeutic Modality | Mechanism of Action | Clinical Translation |
|---|---|---|
| Modulation of Surface Receptor Expression | ||
| CAR-NK | CARs with specificity for tumor antigens are genetically introduced via viral or nonviral vectors for stable NK cell surface expression. | Several105-110 (see Table 1) |
| NKG2A Silencing | RNAi-mediated inhibition of NKG2A expression, an innate inhibitory NK cells receptor. Other applicable methods of gene silencing include CRISPR/Cas9, ZFN, and TALEN. | Preclinical112 |
| Modulation of Cytokine Signaling | ||
| IL2-secreting NK cells | NK-92 cells genetically engineered to stably secrete IL2, an important stimulatory cytokine for NK cell proliferation and functionality. | Preclinical122 |
| IL15-secreting CAR-NK cells | UCB CAR-NK cells genetically engineered to secrete IL15, an important stimulatory cytokine for NK cell proliferation and functionality. PB-NK cells genetically engineered to constitutively secrete transgenic IL15 to enhance NK cell activation and persistence. |
UCB-derived CD19-CAR.IL15 NK cells, phase I/II clinical trial (NCT03056339)78,123 Preclinical38 |
| IL15/IL15 R complex expressing CAR-NK cells | PB-NK cells genetically engineered to locally secrete IL15/IL15 Rα to enhance NK cell persistence and functionality. iPSC-NK cells genetically engineered to express the IL15/IL15 Rα fusion protein, a CD19-targeting CAR, and a high-affinity, noncleavable CD16 for enhanced functionality in CD19+ malignancies. |
Preclinical124 iPSC-derived CD19 CAR NK cell with a high-affinity, noncleavable CD16, and recombinant fusion of IL15 and IL15RF (FT596) as monotherapy or in combination with rituximab or obinutuzumab, phase I clinical trial (NCT04245722)125 |
| Chemokine receptor expression on NK cells (ex. CXCR2, CXCR4, CCR7, CXCR1, CCR5) | NK cells are engineered to express gain of function chemokine receptors to support NK cell tumor trafficking. | Preclinical126-131 |
| Small Molecule Engagers | ||
| BiKEs | Genetically modified NK cells engineered to express high-affinity CD16 Fc receptor isoform to enhance ADCC used in combination with BiKEs, targeting CD16 on NK cells and tumor antigens on target cells. | iPSC-derived CD19 CAR NK cell with a high-affinity, noncleavable CD16 (FT516) in combination with rituximab, phase I clinical trial (NCT04023071)138-143 |
| TriKEs | TriKEs incorporating IL-15 cytokine for enhanced NK cells function used together with NK cells genetically modified to express high-affinity CD16 Fc receptors, for further enhanced specify and persistence of antitumor NK cell activation. | Preclinical144 |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; BiKE, bispecific killer engager; iPSC, induced pluripotent stem cell; PB, peripheral blood; TALEN, transcription activator-like nucleases; TriKE, trispecifc killer engager; UCB, umbilical cord blood; ZFN, zinc-finger nucleases.
KEY POINTS.
NK cells are lymphocytes of the innate immune system with powerful intrinsic cytotoxic mechanisms that can be further enhanced by genetic engineering.
NK cells for therapeutic use are derived from numerous sources, including peripheral blood, cord blood, pluripotent stem cells, embryonic stem cells, and transformed NK cell lines.
Genetic modification of NK cells has been studied using viral and nonviral vectors for nontargeted and targeted genomic editing. Retroviral vectors have been optimized for safety and efficiency and are the preferred vehicle for ex vivo genetic engineering.
NK cell activity can be enhanced through the modification of cell surface receptors, manipulation of the inflammatory and suppressive cytokine milieu, and directed evasion of regulatory mechanisms.
Combination with small molecular engagers can strengthen NK cell targeting and activation.
CLINICS CARE POINTS.
NK cells are an option for “off-the-shelf” cellular therapy with a favorable safety profile and limited risk of graft-versus-host disease with allogeneic products.
NK cells can be genetically modified using both genomic targeted and nontargeted techniques. There is a risk of insertional mutagenesis without targeting, thus there is a need for close patient monitoring following engineered NK cell infusion.
NK-cell-based immunotherapy is made more effective by the presence of a tumor antigen for CAR or BiKE/TriKE targeting and mechanisms that promote NK cell persistence and activation.
Footnotes
DISCLOSURE
C.L. Bonifant and I. Christodoulou have pending patent applications describing the use of CAR-NK cells as therapeutics. C.L. Bonifant and I. Christodoulou have received research support from Merck Sharp and Dohme, Bristol-Myers Squibb, and Kiadis Pharma.
REFERENCES
- 1.Madhusoodhan PP, Carroll WL, Bhatla T. Progress and prospects in pediatric leukemia. Curr Probl Pediatr Adolesc Health Care 2016;46(7):229–41. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics. CA Cancer J Clin 2014;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 3.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a children’s oncology group report. Leukemia 2010;24(2):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Leary MC, Lu X, Huang Y, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory b-cell precursor acute lymphoblastic leukemia. Clin Cancer Res 2019;25(4):1142–6. [DOI] [PubMed] [Google Scholar]
- 6.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25(4):625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hines MR, Keenan C, Maron Alfaro G, et al. Hemophagocytic lymphohistiocytosis-like toxicity (carHLH) after CD19-specific CAR T-cell therapy. Br J Haematol 2021;194(4):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics 2016;3:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet 2009; 373(9674):1550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol 2003;21(4):149–61. [DOI] [PubMed] [Google Scholar]
- 11.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105(8):3051–7. [DOI] [PubMed] [Google Scholar]
- 12.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010;28(6):955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 1999;94(1):333–9. [PubMed] [Google Scholar]
- 14.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295(5562):2097–100. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Galat V, Galat Y, et al. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 2021;14(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mace EM, Orange JS. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol Rev 2019;287(1):202–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol 2016;16(5):310–20. [DOI] [PubMed] [Google Scholar]
- 18.Freud AG, Mundy-Bosse BL, Yu J, et al. The Broad spectrum of human natural killer cell diversity. Immunity 2017;47(5):820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abel AM, Yang C, Thakar MS, et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol 2018;9:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SL, Kennedy PR, Stacey KB, et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv 2020;4(7):1388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, Siebert JR, Burns R, et al. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat Commun 2019;10(1):3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knorr DA, Bachanova V, Verneris MR, et al. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol 2014;26(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Tian S, Zhang K, et al. Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Protein Cell 2017;8(12):861–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handgretinger R, Lang P, Andre MC. Exploitation of natural killer cells for the treatment of acute leukemia. Blood 2016;127(26):3341–9. [DOI] [PubMed] [Google Scholar]
- 25.Algarra I, Cabrera T, Garrido F. The HLA crossroad in tumor immunology. Hum Immunol 2000;61(1):65–73. [DOI] [PubMed] [Google Scholar]
- 26.Diefenbach A, Jamieson AM, Liu SD, et al. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol 2000;1(2):119–26. [DOI] [PubMed] [Google Scholar]
- 27.Cerwenka A, Bakker AB, McClanahan T, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000;12(6):721–7. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Cretney E, Kelly JM, et al. Activation of NK cell cytotoxicity. Mol Immunol 2005;42(4):501–10. [DOI] [PubMed] [Google Scholar]
- 29.Carlsten M, Childs RW. Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front Immunol 2015;6:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prlic M, Blazar BR, Farrar MA, et al. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med 2003;197(8):967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranson T, Vosshenrich CA, Corcuff E, et al. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 2003;101(12):4887–93. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004;22:405–29. [DOI] [PubMed] [Google Scholar]
- 33.Vitale M, Cantoni C, Pietra G, et al. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 2014;44(6):1582–92. [DOI] [PubMed] [Google Scholar]
- 34.Cekic C, Day YJ, Sag D, et al. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 2014;74(24):7250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Han Y, Guo Q, et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 2009;182(1):240–9. [DOI] [PubMed] [Google Scholar]
- 36.Trzonkowski P, Szmit E, Mysliwska J, et al. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 2004;112(3):258–67. [DOI] [PubMed] [Google Scholar]
- 37.Christodoulou I, Rahnama R, Ravich JW, et al. Glycoprotein Targeted CAR-NK Cells for the Treatment of SARS-CoV-2 Infection. Original Research. Front Immunol 2021;12:5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christodoulou I, Ho WJ, Marple A, et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J Immunother Cancer 2021;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciurea SO, Schafer JR, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood 2017;130(16):1857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009;69(9):4010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez Garcia LM, Escudero A, Mestre C, et al. Phase 2 Clinical Trial of Infusing Haploidentical K562-mb15-41BBL-Activated and Expanded Natural Killer Cells as Consolidation Therapy for Pediatric Acute Myeloblastic Leukemia. Clin Lymphoma Myeloma Leuk 2021. 10.1016/j.clml.2021.01.013. Jan 25. [DOI] [PubMed] [Google Scholar]
- 42.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005;106(1):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imamura M, Shook D, Kamiya T, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood 2014;124(7):1081–8. [DOI] [PubMed] [Google Scholar]
- 44.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 2012;7(1):e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marofi F, Saleh MM, Rahman HS, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem Cell Res Ther 2021;12(1):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005;436(7051):709–13. [DOI] [PubMed] [Google Scholar]
- 47.Saetersmoen ML, Hammer Q, Valamehr B, et al. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin Immunopathol 2019;41(1):59–68. [DOI] [PubMed] [Google Scholar]
- 48.Luevano M, Daryouzeh M, Alnabhan R, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol 2012;73(3):248–57. [DOI] [PubMed] [Google Scholar]
- 49.Boissel L, Betancur M, Lu W, et al. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma 2012;53(5):958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller S, Bexte T, Gebel V, et al. High cytotoxic efficiency of lentivirally and alpharetrovirally engineered cd19-specific chimeric antigen receptor natural killer cells against acute lymphoblastic leukemia. Front Immunol 2019;10:3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrera L, Santos S, Vesga MA, et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep 2019;9(1):18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med 2013;2(4):274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ni Z, Knorr DA, Kaufman DS. Hematopoietic and nature killer cell development from human pluripotent stem cells. Methods Mol Biol 2013;1029:33–41. [DOI] [PubMed] [Google Scholar]
- 54.Zhu H, Blum RH, Bernareggi D, et al. Metabolic Reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 2020;27(2):224–237 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Hermanson DL, Moriarity BS, et al. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018;23(2):181–92, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hermanson DL, Bendzick L, Pribyl L, et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells 2016;34(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994;8(4):652–8. [PubMed] [Google Scholar]
- 58.Yang HG, Kang MC, Kim TY, et al. Discovery of a novel natural killer cell line with distinct immunostimulatory and proliferative potential as an alternative platform for cancer immunotherapy. J Immunother Cancer 2019;7(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuchiyama J, Yoshino T, Mori M, et al. Characterization of a novel human natural killer-cell line (NK-YS) established from natural killer cell lymphoma/leukemia associated with Epstein-Barr virus infection. Blood 1998;92(4):1374–83. [PubMed] [Google Scholar]
- 60.Robertson MJ, Cochran KJ, Cameron C, et al. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol 1996;24(3):406–15. [PubMed] [Google Scholar]
- 61.Cheng M, Ma J, Chen Y, et al. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transpl 2011;20(11–12):1731–46. [DOI] [PubMed] [Google Scholar]
- 62.Yagita M, Huang CL, Umehara H, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia 2000;14(5):922–30. [DOI] [PubMed] [Google Scholar]
- 63.Maki G, Klingemann HG, Martinson JA, et al. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res 2001;10(3):369–83. [DOI] [PubMed] [Google Scholar]
- 64.Boyiadzis M, Agha M, Redner RL, et al. Phase 1 clinical trial of adoptive immunotherapy using "off-the-shelf" activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017;19(10):1225–32. [DOI] [PubMed] [Google Scholar]
- 65.Williams BA, Law AD, Routy B, et al. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017;8(51):89256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang X, Yang L, Li Z, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res 2018;8(6):1083–9. [PMC free article] [PubMed] [Google Scholar]
- 67.Arai S, Meagher R, Swearingen M, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008;10(6):625–32. [DOI] [PubMed] [Google Scholar]
- 68.Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013;15(12):1563–70. [DOI] [PubMed] [Google Scholar]
- 69.Tam YK, Maki G, Miyagawa B, et al. Characterization of genetically altered, interleukin 2-independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Ther 1999;10(8):1359–73. [DOI] [PubMed] [Google Scholar]
- 70.Matsuo Y, Drexler HG. Immunoprofiling of cell lines derived from natural killer-cell and natural killer-like T-cell leukemia-lymphoma. Leuk Res 2003;27(10):935–45. [DOI] [PubMed] [Google Scholar]
- 71.Munoz J, Shah N, Rezvani K, et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med 2014;3(12):1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kotylo PK, Baenzinger JC, Yoder MC, et al. Rapid analysis of lymphocyte subsets in cord blood. Am J Clin Pathol 1990;93(2):263–6. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka H, Kai S, Yamaguchi M, et al. Analysis of natural killer (NK) cell activity and adhesion molecules on NK cells from umbilical cord blood. Eur J Haematol 2003;71(1):29–38. [DOI] [PubMed] [Google Scholar]
- 74.Veluchamy JP, Heeren AM, Spanholtz J, et al. High-efficiency lysis of cervical cancer by allogeneic NK cells derived from umbilical cord progenitors is independent of HLA status. Cancer Immunol Immunother 2017;66(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Xu H, Zheng X, et al. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell Mol Immunol 2007;4(5):377–82. [PubMed] [Google Scholar]
- 76.Dalle JH, Menezes J, Wagner E, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res 2005;57(5 Pt 1):649–55. [DOI] [PubMed] [Google Scholar]
- 77.Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol 2017;177(3):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020;382(6):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J Immunol Res 2018;2018:4054815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan RA, Boyerinas B. Genetic modification of T cells. Biomedicines 2016;4(2). 10.3390/biomedicines4020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 2001;7(1):33–40. [DOI] [PubMed] [Google Scholar]
- 82.Matrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther 2010;18(3):477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zennou V, Petit C, Guetard D, et al. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 2000;101(2):173–85. [DOI] [PubMed] [Google Scholar]
- 84.Bari R, Granzin M, Tsang KS, et al. A Distinct Subset of Highly Proliferative and Lentiviral Vector (LV)-Transducible NK Cells Define a Readily Engineered Subset for Adoptive Cellular Therapy. Front Immunol 2019;10:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colamartino ABL, Lemieux W, Bifsha P, et al. Efficient and Robust NK-Cell Transduction With Baboon Envelope Pseudotyped Lentivector. Front Immunol 2019;10:2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandrin V, Boson B, Salmon P, et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 2002;100(3):823–32. [DOI] [PubMed] [Google Scholar]
- 87.Chockley P, Patil SL, Gottschalk S. Transient blockade of TBK1/IKKepsilon allows efficient transduction of primary human natural killer cells with vesicular stomatitis virus G-pseudotyped lentiviral vectors. Cytotherapy 2021;23(9):787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewinski MK, Bushman FD. Retroviral DNA integration–mechanism and consequences. Adv Genet 2005;55:147–81. [DOI] [PubMed] [Google Scholar]
- 89.Wu X, Li Y, Crise B, et al. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003;300(5626):1749–51. [DOI] [PubMed] [Google Scholar]
- 90.Deipolyi AR, Golberg A, Yarmush ML, et al. Irreversible electroporation: evolution of a laboratory technique in interventional oncology. Diagn Interv Radiol 2014;20(2):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robbins GM, Wang M, Pomeroy EJ, et al. Nonviral genome engineering of natural killer cells. Stem Cell Res Ther 2021;12(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmidt P, Raftery MJ, Pecher G. Engineering NK cells for CAR therapy-recent advances in gene transfer methodology. Front Immunol 2020;11:611163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pomeroy EJ, Hunzeker JT, Kluesner MG, et al. A genetically engineered primary human natural killer cell platform for cancer immunotherapy. Mol Ther 2020;28(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKinlay CJ, Vargas JR, Blake TR, et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc Natl Acad Sci U S A 2017;114(4):E448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilk AJ, Weidenbacher NL, Vergara R, et al. Charge-altering releasable transporters enable phenotypic manipulation of natural killer cells for cancer immunotherapy. Blood Adv 2020;4(17):4244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munoz-Lopez M, Garcia-Perez JL. DNA transposons: nature and applications in genomics. Curr Genomics 2010;11(2):115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tipanee J, VandenDriessche T, Chuah MK. Transposons: moving forward from preclinical studies to clinical trials. Hum Gene Ther 2017;28(11):1087–104. [DOI] [PubMed] [Google Scholar]
- 98.Tipanee J, Chai YC, VandenDriessche T, et al. Preclinical and clinical advances in transposon-based gene therapy. Biosci Rep 2017;37(6). 10.1042/BSR20160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schambach A, Morgan M, Fehse B. Two cases of T cell lymphoma following Piggybac-mediated CAR T cell therapy. Mol Ther 2021;29(9):2631–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Micklethwaite KP, Gowrishankar K, Gloss BS, et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 2021;138(16):1391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morgan MA, Buning H, Sauer M, et al. Use of cell and genome modification technologies to generate improved "Off-the-Shelf" CAR T and CAR NK Cells. Front Immunol 2020;11:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naeimi Kararoudi M, Tullius BP, Chakravarti N, et al. Genetic and epigenetic modification of human primary NK cells for enhanced antitumor activity. Semin Hematol 2020;57(4):201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang X, Potter J, Kumar S, et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol 2015;208:44–53. [DOI] [PubMed] [Google Scholar]
- 104.Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 2017;169(3):559. [DOI] [PubMed] [Google Scholar]
- 105.Rafei H, Daher M, Rezvani K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. Br J Haematol 2021;193(2):216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia J, Minamino S, Kuwabara K. CAR-expressing NK cells for cancer therapy: a new hope. Biosci Trends 2020;14(5):354–9. [DOI] [PubMed] [Google Scholar]
- 107.Wang L, Dou M, Ma Q, et al. Chimeric antigen receptor (CAR)-modified NK cells against cancer: opportunities and challenges. Int Immunopharmacol 2019;74:105695. [DOI] [PubMed] [Google Scholar]
- 108.Daher M, Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK Cells: what lies beyond CAR-engineered T cells in the race against cancer. Cancer Discov 2021;11(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett 2020;472:175–80. [DOI] [PubMed] [Google Scholar]
- 110.Xie G, Dong H, Liang Y, et al. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 2020;59:102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamiya T, Seow SV, Wong D, et al. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest 2019;129(5):2094–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Figueiredo C, Seltsam A, Blasczyk R. Permanent silencing of NKG2A expression for cell-based therapeutics. J Mol Med (Berl) 2009;87(2):199–210. [DOI] [PubMed] [Google Scholar]
- 113.Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014;123(25):3855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grzywacz B, Moench L, McKenna D Jr, et al. Natural killer cell homing and persistence in the bone marrow after adoptive immunotherapy correlates with better leukemia control. J Immunother 2019;42(2):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woan KV, Miller JS. Harnessing natural killer cell antitumor immunity: from the bench to bedside. Cancer Immunol Res 2019;7(11):1742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Margolin K, Morishima C, Velcheti V, et al. Phase I Trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Cancer Res 2018;24(22):5552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romee R, Cooley S, Berrien-Elliott MM, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018;131(23):2515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conlon KC, Potter EL, Pittaluga S, et al. IL15 by continuous intravenous infusion to adult patients with solid tumors in a phase i trial induced dramatic NK-cell subset expansion. Clin Cancer Res 2019;25(16):4945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cooley S, He F, Bachanova V, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv 2019;3(13):1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller JS, Morishima C, McNeel DG, et al. A First-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res 2018;24(7):1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagashima S, Mailliard R, Kashii Y, et al. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood 1998;91(10):3850–61. [PubMed] [Google Scholar]
- 123.Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32(2):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma R, Lu T, Li Z, et al. An oncolytic virus expressing IL15/IL15ralpha combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res 2021;81(13):3635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goodridge JP, Mahmood S, Zhu H, et al. FT596: translation of first-of-kind multiantigen targeted off-the-shelf CAR-NK cell with engineered persistence for the treatment of B cell malignancies. Blood 2019;134(Supplement_1):301. [Google Scholar]
- 126.Muller N, Michen S, Tietze S, et al. Engineering NK Cells Modified With an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1alpha-secreting Glioblastoma. J Immunother 2015;38(5):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kremer V, Ligtenberg MA, Zendehdel R, et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer 2017;5(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levy E, Reger R, Segerberg F, et al. Enhanced bone marrow homing of natural killer cells following mRNA transfection with gain-of-function variant CXCR4(R334X). Front Immunol 2019;10:1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carlsten M, Levy E, Karambelkar A, et al. Efficient mRNA-based genetic engineering of human NK cells with high-affinity CD16 and CCR7 augments rituximab-induced ADCC against lymphoma and targets NK cell migration toward the lymph node-associated chemokine CCL19. Front Immunol 2016;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ng YY, Tay JCK, Wang S. CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncolytics 2020;16:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li F, Sheng Y, Hou W, et al. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J Immunother Cancer 2020;8(1). 10.1136/jitc-2019-000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Felices M, Lenvik TR, Davis ZB, et al. Generation of BiKEs and TriKEs to improve NK cell-mediated targeting of tumor cells. Methods Mol Biol 2016;1441:333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anegon I, Cuturi MC, Trinchieri G, et al. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med 1988;167(2):452–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gleason MK, Ross JA, Warlick ED, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014;123(19):3016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rothe A, Sasse S, Topp MS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 2015;125(26):4024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sasse SM J, Plütschow A, Hüttmann A, et al. AFM13 in patients with relapsed or refractory Hodgkin lymphoma: final results of an open-label, randomized, multicenter phase II trial. Blood 2020;136(Supplement 1):31–2. 10.1182/blood-2020-141250. Available at. [DOI] [PubMed] [Google Scholar]
- 137.Affimed announces presentation at AACR highlighting initial data from phase 1 study of cord blood-derived natural killer cells pre-complexed with innate cell engager AFM13. 2021. Available at: https://bit.ly/3m0nzJ9. Accessed September 26, 2021.
- 138.Repp R, Kellner C, Muskulus A, et al. Combined Fc-protein- and Fc-glycoengineering of scFv-Fc fusion proteins synergistically enhances CD16a binding but does not further enhance NK-cell mediated ADCC. J Immunol Methods 2011;373(1–2):67–78. [DOI] [PubMed] [Google Scholar]
- 139.Chen Y, You F, Jiang L, et al. Gene-modified NK-92MI cells expressing a chimeric CD16-BB-zeta or CD64-BB-zeta receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Oncotarget 2017;8(23):37128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Snyder KM, Hullsiek R, Mishra HK, et al. Expression of a recombinant high affinity IgG Fc receptor by engineered NK cells as a docking platform for therapeutic mAbs to Target cancer cells. Front Immunol 2018;9:2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu H, Blum RH, Bjordahl R, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 2020;135(6):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jing Y, Ni Z, Wu J, et al. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One 2015;10(3):e0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Strati P, Bachanova V, Goodman A, et al. Preliminary results of a phase I trial of FT516, an off-the-shelf natural killer (NK) cell therapy derived from a clonal master induced pluripotent stem cell (iPSC) line expressing high-affinity, non-cleavable CD16 (hnCD16), in patients (pts) with relapsed/refractory (R/R) B-cell lymphoma (BCL). J Clin Oncol 2021;39(15_suppl):7541. [Google Scholar]
- 144.Davis ZB, Vallera DA, Miller JS, et al. Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol 2017;31:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]