Abstract
Introduction
Thyroid diseases in pregnancy are relatively common and are associated with adverse pregnancy and perinatal outcomes, increasing a neonate’s risk of admission to the neonatal intensive care unit (NICU). The aim of this study was to evaluate the indications for increased risk of NICU admission among the neonates of hypothyroid and hyperthyroid mothers.
Material and methods
The study data consisted of all singleton deliveries (n = 734 773) between 2004 and 2016 in Finland collected from the Finnish Medical Birth Register. The odds of NICU admission (with 95% confidence intervals) were compared between the neonates of hypothyroid or hyperthyroid mothers and of mothers without any thyroid diseases by specified neonatal characteristics and morbidities using logistic regression analysis. The studied neonatal characteristics were preterm birth (<37+0 gestational weeks), low birthweight (<2500 g), the rate of small‐ and large‐for‐gestational age infants, and eight disease‐specific neonatal outcomes: asphyxia, respiratory distress syndrome, meconium aspiration syndrome, pneumothorax, cardiovascular problems, infections, jaundice and hypoglycemia.
Results
The most common indications for NICU care were principally the same in the neonates of the mothers with and without thyroid disease: respiratory distress syndrome, infections, preterm birth, low birthweight and neonatal hypoglycemia. The preterm neonates, neonates with low birthweight, and large‐for‐gestational‐age infants had increased odds of NICU admission if their mother had hypothyroidism. Also neonates with cardiovascular problems, jaundice or hypoglycemia associated with maternal diabetes had increased odds of NICU admissions if their mother had hypothyroidism. Further, the preterm neonates, large‐for‐gestational‐age infants, and term infants with jaundice had increased odds of NICU admission if their mother had hyperthyroidism.
Conclusions
The most common indications for NICU care were similar for the neonates of the mothers with and without thyroid disease. However, the neonates of the mothers with thyroid diseases were more likely to need NICU care. The neonates of the mothers with thyroid diseases had higher odds of NICU treatment in cases of preterm birth, large for gestational age, and hypoglycemia.
Keywords: hyperthyroidism, hypothyroidism, neonatal intensive care, perinatal, thyroid
Maternal thyroid disease is associated with an increased need for neonatal intensive care. However, the reasons for neonatal intensive care unit treatment are similar for the infants of mothers with and without thyroid disease.

Abbreviations
- CI
confidence interval
- GDM
gestational diabetes
- ICD
International Statistical Classification of Diseases and Related Health Problems
- LGA
large for gestational age
- LT4
levothyroxine
- MBR
the Finnish Medical Birth Register
- NICU
neonatal intensive care unit
- OR
odds ratio
- SGA
small for gestational age
Key message.
Maternal thyroid disease is associated with an increased need for neonatal intensive care. However, the reasons for neonatal intensive care unit treatment are similar for the infants of mothers with and those without thyroid disease.
1. INTRODUCTION
Thyroid dysfunction in pregnancy is relatively common, affecting 3%–5% of pregnancies. 1 The prevalence of hypothyroidism in pregnancy is 2%–3%, and hyperthyroidism occurs in 0.4%–1.7% of all pregnancies. 2 , 3 Both overt hypothyroidism and hyperthyroidism have been associated with an increased risk of adverse obstetrical and neonatal events, such as fetal loss, gestational hypertensive disorders and preterm births. 1 , 2 , 4 In addition, thyroid autoimmunity and subclinical hypothyroidism have been associated with pregnancy and perinatal complications. 4 , 5 , 6 , 7 Since the fetus is dependent on maternal thyroid hormones, especially in the first trimester of pregnancy, maternal thyroid disease may also adversely affect neonatal health. 8 Thyroid hormones have a significant effect on fetal brain development, as they regulate many neurobiological processes, such as neurogenesis, the grave example of this being congenital cretinism due to lack of thyroid hormones during fetal development. 9
In our previous studies, maternal hypothyroidism 10 and maternal hyperthyroidism 11 were found to be associated with an increased need for neonatal intensive care unit (NICU) treatment—an association also observed in previous studies. 12 , 13 Preterm birth and low birthweight, which are both associated with maternal thyroid disease, 14 , 15 may partly explain this increased need. A recent study also reported increased odds of several specific neonatal morbidities among infants of women with thyroid diseases, including respiratory distress syndrome (RDS), sepsis and neonatal anemia. 12 However, the indications for NICU treatment among neonates of mothers with thyroid disease have not been examined previously.
The aim of this study was to evaluate the indications for increased admission to the NICU among neonates of hypothyroid and hyperthyroid mothers.
2. MATERIAL AND METHODS
2.1. Data collection
The data in this population‐based study were collected from Finnish nationwide registers and included all singleton live births (n = 734 773) between 2004 and 2016. The study period was chosen based on data consistency and availability. The data were obtained from the Finnish Medical Birth Register (MBR) and the Care Register for Health Care (HILMO), maintained by the Finnish Institute for Health and Welfare (THL) and the Special Refund Entitlement Register and the Prescription Register maintained by the Social Insurance Institution of Finland (Kela).
Personnel at the delivery hospitals complete a structured form for the MBR that includes maternal and neonate data for all live births and stillbirths with a gestational age at birth of >22 weeks or birthweight of >500 g. The HILMO includes diagnosis information from all hospital wards at discharge and for specialized outpatient visits to public hospitals. The Special Refund Entitlement Register and the Prescription Register include information on chronic diseases, medication and reimbursement of medical expenses. The data linkage from the MBR to the other registers was performed using the unique personal identification codes assigned to all Finnish citizens and permanent residents, and these codes were encrypted before analysis. The Finnish registers used in this study are of good quality 16 , 17 and combining them increases their usefulness and validity. 18
We obtained information about hypothyroidism and hyperthyroidism diagnoses before and during pregnancy from the MBR, the HILMO and the Special Refund Entitlement Register using the International Statistical Classification of Diseases and Related Health Problems (ICD) codes. In addition to inpatient data, our study contained diagnoses from outpatient hospital clinics for specialized care, diagnoses from primary care in the MBR, information on women entitled for special reimbursement due to hypothyroidism, and information on drug purchases during pregnancy. The ICD‐8 or ICD‐9 code 244 or the ICD‐10 code E03 (with all digits) and the special reimbursement code 104, denoted hypothyroidism, and the ICD‐8 or ICD‐9 code 242 or the ICD‐10 code E05 (with all digits) denoted hyperthyroidism in our study. We obtained data on thyroid medication purchases 3 months prior to and during pregnancy from the Prescription Register, which contains information on all prescription‐only medication purchases and information related to the medication (the International Anatomic Therapeutic Chemical classification code and the time and number of purchases), which is collected from all Finnish pharmacies. Use of thyroid medication was defined as purchase of levothyroxine (H03AA01), propylthiouracil (H03BA02) or carbimazole (H03BB01). Our data did not include information on treatment duration or dosage.
Women were classified as hypothyroid if they had a diagnosis of hypothyroidism according to any of the registers or if they had purchased levothyroxine. They were classified as hyperthyroid if they had a recorded diagnosis of hyperthyroidism or antithyroid drug medication. The registers were also used to determine whether maternal diabetes was present. The ICD‐10 codes O24.4 or O24.9 or pathological results according to the oral glucose tolerance test were considered to denote gestational diabetes (GDM). The ICD‐8 or ICD‐9 codes 250 or 6480 or the ICD‐10 codes E10, E11, O24.0 and O24.1 and the special reimbursement codes 103, 171, 177, 215, 285 or 346 were considered to denote pre‐pregnancy diabetes. The HILMO data covered the years 1987–2016, and the Kela data for our cohort the years 2003–2016.
2.2. Outcome and covariates
The main outcome in our study was neonatal admission to an intensive care unit, as recorded in the MBR data. Background information on maternal demographics included age at delivery, smoking status, parity, body mass index, socioeconomic status, place of residence, diagnoses, hospitalization during pregnancy and mode of delivery. The newborn data included gestational age at birth, birthweight, diagnoses, treatment and hospitalization during the neonatal period until the age of 7 days. Gestational age at birth was primarily based on ultrasound and, when not available, on the date of the last menstrual period as registered in the MBR. If none of this information was available, the beginning of pregnancy was calculated by subtracting 280 days from the date of birth. The neonatal characteristics in this study included preterm birth <37 gestational weeks, low birthweight <2500 g, small‐for‐gestational‐age (SGA) infants (defined as birthweight less than two standard deviations of the gestational age‐adjusted mean), and large‐for‐gestational‐age (LGA) infants (defined as birthweight more than 2 SD of the gestational age‐adjusted mean). 19 The specific neonatal morbidities investigated in this study were asphyxia, RDS, meconium aspiration syndrome, pneumothorax, cardiovascular problems, infections, jaundice and hypoglycemia. Information on preterm birth, low birthweight, SGA and LGA, as well as information on specific neonatal morbidities was extracted from the MBR. We used the ICD‐10 codes P21 for asphyxia, P22 for RDS, P24.0 for meconium aspiration syndrome, P25.1 for pneumothorax, P29 for cardiovascular problems, P36 and P39.9 for infections, P59 for jaundice, and P70 for hypoglycemia. Neonatal jaundice was further classified as jaundice in preterm (<37 gestational weeks) newborns and jaundice in term newborns (≥37+0 gestational weeks). In this study, jaundice denotes non‐hemolytic jaundice leading to phototherapy or other medical treatment. Neonatal hypoglycemia was further classified as hypoglycemia related to GDM, pre‐pregnancy diabetes or unspecified causes. The categorization of the neonates is presented in Figure 1.
FIGURE 1.
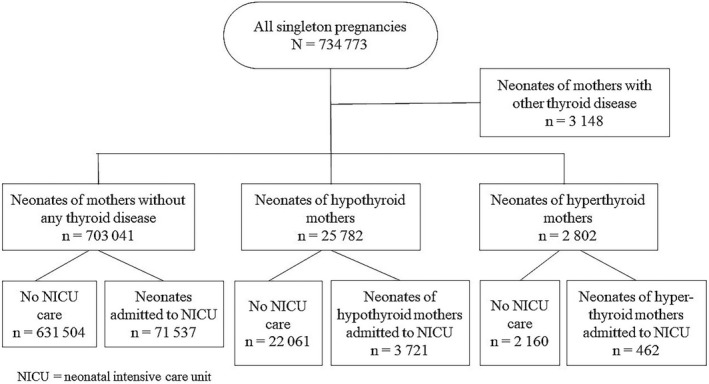
Flow chart of the study population.
2.3. Statistical analyses
The prevalence of perinatal problems and the proportion of admission to NICU care among neonates of women with or without thyroid diseases were counted by cross‐tabulation. The odds of NICU treatment (with 95% confidence intervals [CI]) were compared between the neonates of the hypothyroid or hyperthyroid mothers (exposure groups) and those of the mothers without any thyroid diseases (the reference group) for each specific neonatal feature using logistic regression analysis. The regression analyses therefore estimated, for instance, the total effect of hypothyroidism on NICU admissions on infants born preterm or on infants born with RDS. The analyses were adjusted for the following confounders: maternal age at delivery, pre‐pregnancy body mass index, smoking during pregnancy, parity, socioeconomic status based on maternal occupation during pregnancy and the catchment area of five tertiary hospitals in Finland. The missing data for explanatory categorical covariates were included as a separate category in the regression models. The children with missing data for birthweight, SGA and LGA outcomes were excluded from the analyses.
We performed a sensitivity analysis to compare the neonates of the mothers who had purchased levothyroxine (LT4) medication without a recorded diagnosis of hypothyroidism with the neonates of mothers who had purchased LT4 medication and a recorded diagnosis of hypothyroidism. This supplementary analysis was performed because these groups may differ substantially; that is, women with a recorded diagnosis of hypothyroidism and LT4 medication are more likely to have overt hypothyroidism, and those who had purchased LT4 medication but had no recorded diagnosis of hypothyroidism may have subclinical hypothyroidism or a diagnosis of hypothyroidism according to pregnancy thresholds. Likewise, we also performed a sensitivity analysis on the neonates of the hyperthyroid mothers with and without antithyroid drug purchases, assuming that hyperthyroid women on antithyroid drug medication are more likely to have active hyperthyroidism than those with a hyperthyroidism diagnosis but no antithyroid drug medication. Analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc.).
2.4. Ethical approval
The ethical board of the Northern Ostrobothnia Hospital District approved this study on October 13, 2014 (EETTMK: 80/2014). The Finnish Institute for Health and Welfare (THL/928/5.05.00/2014 and THL/408/5.05.00/2019) and Social Insurance Institution (Kela 75/522/2014 and Kela 25/522/2019) gave permissions to access the data from the aforementioned national health registers. This is a register‐based study where study subjects were not contacted.
3. RESULTS
In this study, 3.5% (n = 25 782) of the neonates were born to mothers with hypothyroidism and 0.4% (n = 2802) to mothers with hyperthyroidism. The mothers with thyroid disease were older and more often overweight or obese than mothers without any thyroid disease. The hypothyroid mothers smoked less and the hyperthyroid mothers were more often multiparous than the mothers without any thyroid disease (Table 1). The most common indications for NICU treatment both in study groups and in the reference group were RDS, infections, preterm birth, low birthweight and neonatal hypoglycemia. However, neonatal hypoglycemia associating with maternal diabetes was more common among the neonates born to mothers with hypothyroidism. In addition, infections were less common among the neonates born to mothers with thyroid diseases (Table 2).
TABLE 1.
Demographic characteristics of the mothers with singleton pregnancies with and without thyroid diseases in Finland between 2004 and 2016
| Characteristic | Hypothyroid mothers, n = 25 782 (%) | P‐value | Hyperthyroid mothers, n = 2802 (%) | P‐value | Mothers without any thyroid disease, n = 703 041 |
|---|---|---|---|---|---|
| Maternal age, (years) | |||||
| <20 | 173 (0.7) | <0.001 | 14 (0.5) | <0.001 | 16 445 (2.3) |
| 20–34 | 18 204 (70.6) | 2045 (73.0) | 554 922 (78.9) | ||
| ≥35 | 7405 (28.7) | 743 (26.5) | 131 674 (18.7) | ||
| Smoking | |||||
| No smoking | 22 241 (86.3) | <0.001 | 2302 (82.2) | 0.798 | 579 034 (82.4) |
| Smoking during pregnancy | 2951 (11.4) | 430 (15.3) | 106 713 (15.2) | ||
| Data missing | 590 (2.3) | 70 (2.5) | 17 294 (2.5) | ||
| Parity | |||||
| Nulliparous | 10 317 (40.0) | <0.001 | 775 (27.7) | <0.001 | 294 551 (41.9) |
| Multiparous | 15 456 (59.9) | 2027 (72.3) | 407 933 (58.0) | ||
| Data missing | 9 (0.0) | — | 557 (0.1) | ||
| Body mass index | |||||
| <18.5 | 571 (2.2) | <0.001 | 112 (4.0) | 0.006 | 25 308 (3.6) |
| 18.5–24.9 | 13 131 (50.9) | 1604 (57.2) | 420 969 (59.9) | ||
| 25–29.9 | 6180 (24.0) | 620 (22.1) | 144 462 (20.5) | ||
| ≥30 | 5128 (19.9) | 357 (12.7) | 79 124 (11.3) | ||
| Data missing | 772 (3.0) | 109 (3.9) | 33 178 (4.7) | ||
| Socioeconomic status | |||||
| Upper white‐collar | 3658 (14.2) | <0.001 | 382 (13.6) | <0.001 | 105 425 (15.0) |
| Lower white‐collar | 7193 (27.9) | 773 (27.6) | 198 954 (28.3) | ||
| Blue‐collar | 2320 (9.0) | 303 (10.8) | 77 237 (11.0) | ||
| Student | 1752 (6.8) | 215 (7.7) | 63 642 (9.1) | ||
| Enterpreneur of freelancer | 508 (2.0) | 66 (2.4) | 12 437 (1.8) | ||
| Other | 842 (3.3) | 132 (4.7) | 24 625 (3.5) | ||
| Data missing | 9509 (36.9) | 931 (33.2) | 220 721 (31.4) | ||
| University hospital district | |||||
| Helsinki (reference) | 7205 (28.0) | <0.001 | 1073 (38.3) | <0.001 | 250 989 (35.7) |
| Turku | 4806 (18.6) | 432 (15.4) | 108 912 (15.5) | ||
| Tampere | 5568 (21.6) | 558 (19.9) | 137 683 (19.6) | ||
| Kuopio | 4614 (17.9) | 343 (12.2) | 95 110 (13.5) | ||
| Oulu | 3559 (13.8) | 389 (13.9) | 108 572 (15.4) | ||
| Data missing | 39 (0.1) | 7 (0.3) | 1775 (0.3) | ||
Note: The data are reported as number of mothers (%) unless stated otherwise.
TABLE 2.
Neonates with or without maternal thyroid disease admitted to NICU in Finland between 2004 and 2016
| Perinatal outcome | All live births, n = 734 773 | Neonates of women without thyroid disease admitted to NICU, n = 71 537 | Neonates of women with hypothyroidism admitted to NICU, n = 3721 | Neonates of women with hyperthyroidism admitted to NICU, n = 462 |
|---|---|---|---|---|
| Respiratory distress syndrome | 21 413 (2.9) | 16 755 (23.4) | 902 (24.2) | 100 (21.6) |
| Infections including sepsis | 21 143 (2.9) | 16 076 (22.5) | 640 (17.2) | 60 (13.0) |
| Preterm | 27 827 (3.8) | 15 327 (21.4) | 868 (23.3) | 104 (22.5) |
| Low birthweight | 22 225 (3.0) | 13 695 (19.1) | 674 (18.1) | 90 (19.5) |
| SGA | 25 883 (3.5) | 8057 (11.3) | 370 (9.9) | 49 (10.6) |
| LGA | 17 652 (2.4) | 3514 (4.9) | 354 (9.5) | 37 (8.0) |
| Hypoglycemia not otherwise specified | 14 295 (1.9) | 8030 (11.2) | 334 (9.0) | 45 (9.7) |
| Hypoglycemia with maternal GDM | 23 146 (3.2) | 5804 (11.2) | 354 (9.5) | 39 (8.4) |
| Asphyxia | 9113 (1.3) | 5491 (7.7) | 217 (5.8) | 15 (3.2) |
| Jaundice in preterm neonate | 8076 (1.1) | 5004 (7.0) | 255 (6.9) | 25 (5.4) |
| Jaundice In term neonate | 24 268 (3.3) | 4555 (6.4) | 287 (7.7) | 34 (7.4) |
| Hypoglycemia with maternal diabetes | 4764 (0.65) | 2609 (3.6) | 533 (14.1) | 42 (9.1) |
| Cardiovascular problems | 5873 (0.8) | 1668 (2.3) | 83 (2.2) | 7 (1.5) |
| Pneumothorax | 1925 (0.3) | 1533 (2.1) | 76 (2.0) | 6 (1.3) |
| Meconium aspiration syndrome | 1262 (0.2) | 1108 (1.5) | 34 (0.9) | 3 (0.6) |
Note: The data are reported as number of neonates (%) unless stated otherwise.
GDM, gestational diabetes; LGA, large for gestational age; NICU, neonatal intensive care unit; SGA, small for gestational age.
Altogether, 14.4% of the neonates of the women classified as hypothyroid needed NICU treatment compared with 10.2% of the neonates of the women without any thyroid disease (Table 2). Among the preterm neonates (OR [odds ratio] 1.35, 95% CI 1.20–1.52), the low‐birthweight neonates (OR 1.18, 95% CI 1.02–1.36) and the LGA infants (OR 1.92, 95% CI 1.67–2.20), the odds of NICU admission were increased if their mother had hypothyroidism, compared with the newborns of the women without any thyroid disease (Table 3). Infants with cardiovascular problems (OR 1.55, 95% CI 1.12–2.15), jaundice (preterm neonates: OR 1.36, 95% CI 1.06–1.73; term neonates: OR 1.34, 95% CI 1.16–1.54), hypoglycemia associated with GDM (OR 1.21, 95% CI 1.06–1.39) or hypoglycemia associated with pre‐pregnancy diabetes (OR 1.32, 95% CI 1.10–1.58) also had increased odds of NICU admissions if their mother had hypothyroidism (Table 3).
TABLE 3.
Proportion and odds of neonates with or without maternal hypothyroidism admitted to NICU care in Finland between 2004 and 2016 by neonatal features
| Mothers without thyroid disease | Mothers with hypothyroidism | OR (95% CI) | |||
|---|---|---|---|---|---|
| Number of neonates | Number (%) of neonates admitted to NICU | Number of neonates | Number (%) of neonates admitted to NICU | ||
| Neonatal characteristics | |||||
| Preterm | 26 225 | 15 327 (58.4) | 1313 | 868 (66.1) | 1.35 (1.20–1.52) |
| Low birthweight a | 21 040 | 13 695 (65.1) | 965 | 674 (69.8) | 1.18 (1.02–1.36) |
| SGA b | 24 715 | 8057 (32.6) | 958 | 370 (38.6) | 1.14 (0.99–1.30) |
| LGA b | 16 461 | 3514 (21.3) | 1016 | 354 (38.8) | 1.92 (1.67–2.20) |
| Specific perinatal morbidities | |||||
| Asphyxia | 9113 | 5491 (60.1) | 319 | 217 (68.0) | 1.28 (1.00–1.66) |
| Respiratory distress syndrome | 20 126 | 16 755 (83.3) | 1067 | 902 (84.5) | 1.01 (0.85–1.20) |
| Meconium aspiration syndrome | 1220 | 1108 (90.8) | 38 | 34 (89.5) | 0.96 (0.32–2.87) |
| Pneumothorax | 1826 | 1533 (84.0) | 84 | 76 (90.5) | 1.51 (0.71–3.24) |
| Cardiovascular problems | 5643 | 1668 (29.6) | 192 | 83 (43.2) | 1.55 (1.12–2.15) |
| Infections including sepsis | 20 188 | 16 076 (79.6) | 791 | 640 (80.9) | 1.06 (0.88–1.27) |
| Jaundice in preterm neonate | 7655 | 5004 (65.4) | 353 | 255 (72.2) | 1.36 (1.06–1.73) |
| Jaundice in term neonate | 22 941 | 4555 (19.9) | 1113 | 287 (25.8) | 1.34 (1.16–1.54) |
| Hypoglycemia with maternal GDM | 23 146 | 5804 (25.1) | 1228 | 354 (28.8) | 1.21 (1.06–1.39) |
| Hypoglycemia with maternal diabetes | 3933 | 2609 (66.3) | 751 | 533 (71.0) | 1.32 (1.10–1.58) |
| Hypoglycemia not otherwise specified | 13 626 | 8030 (58.9) | 537 | 334 (62.2) | 1.15 (0.95–1.39) |
Note: The odds of NICU admission with 95% confidence intervals were estimated among mothers with hypothyroidism compared to mothers without thyroid disease.
GDM, gestational diabetes; LGA, large for gestational age; NICU, neonatal intensive care unit; SGA, small for gestational age.
622 children omitted from the analysis due to missing data.
623 children omitted from the analysis due to missing data.
Also, the neonates of the women with hyperthyroidism were admitted to the NICU more often than the neonates of the mothers without any thyroid disease (16.5% vs 10.2%, respectively) (Table 4). Among the preterm neonates (OR 1.45, 95% CI 1.04–2.02) and the LGA infants (OR 3.29, 95% CI 2.12–5.13), the odds of NICU admission were increased if their mothers had hyperthyroidism. Also among the term‐born neonates with jaundice, the odds of NICU care were increased if their mother had hyperthyroidism (OR 1.97, 95% CI 1.29–3.00). The number of neonates of hyperthyroid mothers was low in our study and the results did not reach statistical significance with regard to other specific neonatal morbidities (Table 4).
TABLE 4.
Proportion and odds of neonates with or without maternal hyperthyroidism admitted to NICU care in Finland between 2004 and 2016 by neonatal features
| Mothers without thyroid disease | Mothers with hyperthyroidism | OR (95% CI) | |||
|---|---|---|---|---|---|
| Number of neonates | Number (%) of neonates admitted to NICU | Number of neonates | Number (%) of neonates admitted to NICU | ||
| Neonatal characteristics | |||||
| Preterm | 26 225 | 15 327 (58.4) | 158 | 104 (65.8) | 1.45 (1.04–2.02) |
| Low birthweight a | 21 040 | 13 695 (65.1) | 128 | 90 (70.3) | 1.29 (0.88–1.89) |
| SGA b | 24 715 | 8057 (32.6) | 117 | 49 (41.9) | 1.42 (0.97–2.06) |
| LGA b | 16 461 | 3514 (21.3) | 82 | 37 (45.1) | 3.29 (2.12–5.13) |
| Specific perinatal morbidities | |||||
| Asphyxia | 9113 | 5491 (60.1) | 24 | 15 (62.5) | 1.06 (0.46–2.48) |
| Respiratory distress syndrome | 20 126 | 16 755 (83.3) | 114 | 100 (87.7) | 1.49 (0.85–2.65) |
| Meconium aspiration syndrome | 1220 | 1108 (90.8) | 3 | 3 (100) | — |
| Pneumothorax | 1826 | 1533 (84.0) | 6 | 6 (100) | — |
| Cardiovascular problems | 5643 | 1668 (29.6) | 17 | 7 (41.2) | 1.99 (0.69–5.78) |
| Infections including sepsis | 20 188 | 16 076 (79.6) | 74 | 60 (81.1) | 1.12 (0.62–2.02) |
| Jaundice in preterm neonate | 7655 | 5004 (65.4) | 36 | 25 (69.4) | 1.30 (0.63–2.67) |
| Jaundice in term neonate | 22 941 | 4555 (19.9) | 102 | 34 (33.3) | 1.97 (1.29–3.00) |
| Hypoglycemia with maternal GDM | 23 146 | 5804 (25.1) | 131 | 39 (29.8) | 1.44 (0.96–2.15) |
| Hypoglycemia with maternal diabetes | 3933 | 2609 (66.3) | 55 | 42 (76.4) | 1.82 (0.95–3.48) |
| Hypoglycemia not otherwise specified | 13 626 | 8030 (58.9) | 69 | 45 (65.2) | 1.48 (0.87–2.51) |
Note: The odds of NICU admission with 95% confidence intervals were estimated among mothers with hyperthyroidism compared to mothers without thyroid disease.
GDM, gestational diabetes; LGA, large for gestational age; NICU, neonatal intensive care unit; SGA, small for gestational age.
613 children omitted from the analysis due to missing data.
614 children omitted from the analysis due to missing data.
The neonates of the women with both a recorded diagnosis of hypothyroidism and levothyroxine purchases had increased odds of NICU treatment because of the same indications as described in the main analysis (Tabel S1). Additionally, they had increased odds of NICU care if they had asphyxia (OR 1.71, 95% CI 1.19–2.46). In contrast, only the preterm neonates and the term neonates with jaundice had increased odds of NICU admission when their mother had purchased LT4 medication without a recorded hypothyroidism diagnosis (Table S1).
Maternal hyperthyroidism without antithyroid drug medication was associated with increased odds of NICU care among the LGA infants (OR 3.78, 95% CI 2.29–6.24), the neonates with respiratory distress syndrome (OR 2.11, 95% CI 1.01–4.39) and the term neonates with jaundice (OR 1.98, 95% CI 1.21–3.24) but these associations were not observed in the hyperthyroid mothers on antithyroid medication. This was probably because only a few hyperthyroid mothers were on antithyroid medication. Maternal hyperthyroidism was associated with an increased risk of NICU admission in the preterm infants (OR 2.42, 95% CI 1.18–4.95) of the hyperthyroid mothers on antithyroid medication. Overall, the number of neonates included in the sensitivity analyses was small and our study was underpowered to investigate rare specific neonatal morbidities (Table S2).
4. DISCUSSION
In our previous studies, we found that the need for NICU care was higher in the neonates of mothers with thyroid diseases than in the neonates of mothers without thyroid disease. 10 , 11 Therefore, in this large, register‐based nationwide study, we wanted to evaluate the reasons for this difference. The main finding was that the most common indications for NICU care were principally the same in the neonates of the mothers with and without thyroid disease: preterm birth, low birthweight, respiratory distress syndrome and infections. Nevertheless, the neonates of the mothers with thyroid diseases were more likely to need NICU care.
Both maternal hypothyroidism and hyperthyroidism have been associated with somewhat increased risk of preterm births. 15 , 20 , 21 In our previous work, maternal hypothyroidism was associated especially with early preterm birth (<34 gestational weeks). 10 This may explain the observed association with the increased risk of NICU care among the preterm neonates with maternal hypothyroidism. Preterm birth is globally the second most common cause of death in children under 5 years of age, 22 and preterm birth is one of the main indications for NICU treatment. In the present study, the most common indications for NICU care were preterm birth and various neonatal problems associated with prematurity, such as RDS, low birthweight and hypoglycemia. In addition, our study found an association between increased NICU admission rates and maternal hypothyroidism among the neonates with cardiovascular problems. Cardiovascular problems, such as transitional hypotension, septic or cardiogenic shock and pulmonary hypertension, are reported to be more common in preterm neonates than in term neonates. 23 Preterm birth may partly explain this observed association, but we could not study it in detail due to the small number of cases.
Maternal hypothyroidism has been associated with an increased risk of GDM and LGA in many previous studies. 10 , 24 , 25 In addition, women with type 1 diabetes have a higher prevalence of thyroid diseases, as both are autoimmune diseases. 26 Increased risk of pre‐pregnancy diabetes or GDM also explains the association between maternal hypothyroidism and neonatal hypoglycemia. In this study, maternal hypothyroidism and hyperthyroidism were also associated with increased risk of an LGA infant’s admission to the NICU. LGA infants are often born to mothers with GDM or pre‐pregnancy diabetes and, accordingly, they have an increased risk of hypoglycemia, which is one of the main indications for NICU treatment. In addition, maternal hypothyroidism was associated with increased odds of NICU admission in both the term and the preterm neonates with jaundice. Jaundice is associated with hypoglycemia, preterm birth and macrosomia, 27 which are also the most probable explanations for jaundice in neonates born to mothers with thyroid disorders.
When the analysis was restricted to the mothers with a recorded diagnosis of hypothyroidism and purchase of levothyroxine medication, maternal hypothyroidism was found to increase the risk of a neonate with asphyxia requiring NICU admission. This risk was not observed in the mothers on levothyroxine medication with no recorded diagnosis of hypothyroidism, as determined by the recorded disease codes. Mothers with a recorded diagnosis of hypothyroidism and levothyroxine use can be considered to have more active or more severe forms of hypothyroidism than those without a recorded diagnosis of hypothyroidism. Pregnant women with levothyroxine use with no recorded diagnosis of hypothyroidism may have subclinical hypothyroidism, which is often treated during pregnancy. This difference between these two sensitivity analysis groups may explain the observed difference concerning asphyxia.
To our knowledge, this is the first study to assess the risk of NICU admission for specific morbidities among neonates of mothers with thyroid dysfunction. This study is based on high‐quality data from large, nationwide health registers collected and maintained by law, so recall bias is unlikely. The MBR covers all births in Finland, since almost all mothers give birth in hospital. Information on planned home births and unplanned out‐of‐hospital births is collected separately, and information on missing cases is collected by other registers (the Central Population Register for live births and the Cause of Death Register for stillbirths and infant deaths). Combining the MBR data with the other registers increased our study’s reliability. Additionally, the large sample size enabled us to assess less frequently occurring outcomes. However, in the sensitivity analyses, the study was underpowered to investigate very rare neonatal morbidities such as meconium aspiration syndrome and pneumothorax.
This study has limitations, as it contains only information available on the registers. Despite the excellent quality of Finnish registers, there were some missing data. Another limitation of the present study was the absence of laboratory tests for the mothers’ thyroid hormone or antibody status. However, this might not be a major concern, as medical treatment is typically initiated after abnormal laboratory measurements. Additionally, we were unable to collect data concerning the exact dates on which the thyroid disease diagnoses were made. A neonate may have had multiple diagnoses, but comorbidity was not studied in detail. Finally, the etiology of prematurity (spontaneous/iatrogenic) could not be studied. Our logistic regression analyses were based on NICU admissions among children having each specific perinatal problem. However, there are multifactorial reasons for NICU admission and we could not determine the kind of role that each state played. In addition, we cannot fully exclude collider‐stratification bias 28 within our analyses, particularly on outcomes such as RDS, which is related to prematurity.
5. CONCLUSION
The neonates of the mothers with thyroid diseases were more likely to need NICU care, but the indications for NICU treatment were similar compared with the neonates of the mothers without any thyroid disease: preterm birth, low birthweight, RDS, hypoglycemia and infections. The neonates of the mothers with thyroid diseases had higher odds of NICU treatment in cases of prematurity, large for gestational age and hypoglycemia.
AUTHOR CONTRIBUTIONS
All authors contributed to the research conception and design and to data acquisition, analysis and interpretation. ST drafted the initial version of the manuscript. The other authors revised it critically for important intellectual content and approved the final version to be published. All authors agree to be accountable for all aspects of the work and to insure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FUNDING INFORMATION
This work was supported in part by the Northern Ostrobothnia Hospital District (Dr. Turunen), the Finnish Medical Society Duodecim (Dr. Turunen), the Drugs and Pregnancy project of the Finnish Institute for Health and Welfare (THL), the Finnish Medicines Agency (FIMEA) and the Social Insurance Institution of Finland (Kela) (Prof. Gissler and Dr. Leinonen).
CONFLICT OF INTEREST
MG and MKL report grants from the Innovative Medicines Initiative (Building an ecosystem for better monitoring and communicating the safety of medicines’ use in pregnancy and breastfeeding: validated and regulatory endorsed workflows for fast, optimized evidence generation, IMI ConcePTION, grant agreement number 821520) while conducting the study. The other authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Table S1
Table S2
Turunen S, Vääräsmäki M, Marttila R, et al.. Indications for intensive care unit treatment among neonates born to mothers with thyroid disease: a population‐based cohort study. Acta Obstet Gynecol Scand. 2022;101:1093‐1101. doi: 10.1111/aogs.14413
REFERENCES
- 1. Dong AC, Stagnaro‐Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta‐analysis. Thyroid. 2019;29:278‐289. [DOI] [PubMed] [Google Scholar]
- 2. Negro R, Stagnaro‐Green A. Clinical aspects of hyperthyroidism, hypothyroidism, and thyroid screening in pregnancy. Endocr Pract. 2014;20:597‐607. [DOI] [PubMed] [Google Scholar]
- 3. Cooper DS, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1:238‐249. [DOI] [PubMed] [Google Scholar]
- 4. Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315‐389. [DOI] [PubMed] [Google Scholar]
- 5. Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth , TIM K, Derakhshan A, Taylor PN, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta‐analysis. JAMA. 2019;322:632‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sitoris G, Veltri F, Kleynen P, et al. The impact of thyroid disorders on clinical pregnancy outcomes in a real‐world study setting. Thyroid. 2020;30:106‐115. [DOI] [PubMed] [Google Scholar]
- 7. Lee SY, Cabral HJ, Aschengrau A, Pearce EN. Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J Clin Endocrinol Metab. 2020;105:e2015‐e2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549‐555. [DOI] [PubMed] [Google Scholar]
- 9. Chen ZP, Hetzel BS. Cretinism revised best practice & research. Clin Endocrinol Metab. 2010;24:39‐50. [DOI] [PubMed] [Google Scholar]
- 10. Turunen S, Vääräsmäki M, Männistö T, et al. Pregnancy and perinatal outcome among hypothyroid mothers: a population‐based cohort study. Thyroid. 2019;29:135‐141. [DOI] [PubMed] [Google Scholar]
- 11. Turunen S, Vääräsmäki M, Lahesmaa‐Korpinen AM, et al. Maternal hyperthyroidism and pregnancy outcomes: a population‐based cohort study. Clin Endocrinol (Oxf). 2020;93:721‐728. [DOI] [PubMed] [Google Scholar]
- 12. Männistö T, Mendola P, Reddy U, Laughon SK. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol. 2013;178:731‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lo JC, Rivkees SA, Chandra M, Gonzales JR, Korelitz JJ, Kuzniewicz MW. Gestational thyrotoxicosis, antithyroid drug use and neonatal outcomes within an integrated healthcare delivery system. Thyroid. 2015;25:698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luewan S, Chakkabut P, Tongsong T. Outcomes of pregnancy complicated with hyperthyroidism: a cohort study. Arch Gynecol Obstet. 2011;283:243‐247. [DOI] [PubMed] [Google Scholar]
- 15. Sheehan PM, Nankervis A, Araujo Júnior E, Da Silva CF. Maternal thyroid disease and preterm birth: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2015;100:4325‐4331. [DOI] [PubMed] [Google Scholar]
- 16. Teperi J. Multi method approach to the assessment of data quality in the Finnish medical birth registry. J Epidemiol Community Health. 1993;47:242‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40:505‐515. [DOI] [PubMed] [Google Scholar]
- 18. Gissler M, Louhiala P, Hemminki E. Nordic medical birth registers in epidemiological research. Eur J Epidemiol. 1997;13:169‐175. [DOI] [PubMed] [Google Scholar]
- 19. Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestational weeks. Ann Med. 2013;45:446‐454. [DOI] [PubMed] [Google Scholar]
- 20. Shinohara DR, Santos TDS, de Carvalho HC, et al. Pregnancy complications associated with maternal hypothyroidism: a systematic review. Obstet Gynecol Surv. 2018;73:219‐230. [DOI] [PubMed] [Google Scholar]
- 21. Andersen SL, Olsen J, Wu CS, Laurberg P. Low birth weight in children born to mothers with hyperthyroidism and high birth weight in hypothyroidism, whereas preterm birth is common in both conditions: a Danish national hospital register study. Eur Thyroid J. 2013;2:135‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Johnson HL, Cousens S, et al. Child health epidemiology reference group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. Erratum in: Lancet. 2012;380:1308. [DOI] [PubMed] [Google Scholar]
- 23. Rowcliff K, de Waal K, Mohamed AL, Chaudhari T. Noradrenaline in preterm infants with cardiovascular compromise. Eur J Pediatr. 2016;175:1967‐1973. [DOI] [PubMed] [Google Scholar]
- 24. Wikner BN, Sparre LS, Stiller CO, Källén B, Asker C. Maternal use of thyroid hormones in pregnancy and neonatal outcome. Acta Obstetr Gynecol Scand. 2008;87:617‐627. [DOI] [PubMed] [Google Scholar]
- 25. Hou J, Yu P, Zhu H, et al. The impact of maternal hypothyroidism during pregnancy on neonatal outcomes: a systematic review and meta‐analysis. Gynecol Endocrinol. 2016;32:9–13. Erratum in: Gynecol Endocrinol 2016;32:87. [DOI] [PubMed] [Google Scholar]
- 26. McCanlies E, O’Leary LA, Foley TP, et al. Hashimoto’s thyroiditis and insulin dependent diabetes mellitus: differences among individuals with and without abnormal thyroid function. J Clin Endocrinol Metab. 1998;83:1548‐1551. [DOI] [PubMed] [Google Scholar]
- 27. Van Haltren K, Malhotra A. Characteristics of infants admitted with hypoglycemia to a neonatal unit. J Pediatr Endocrinol and Metab. 2013;26:525‐529. [DOI] [PubMed] [Google Scholar]
- 28. Hernández‐Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164:1115‐1120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


