Abstract
Mutations of the intracellular estrogen receptor alpha (ERα) is implicated in 70% of breast cancers. Therefore, it is of considerable interest to image various mutants (L536S, Y537S, D538G) in living cancer cell lines, particularly as a function of various anticancer drugs. We therefore developed a small (13 kDa) Affimer, which, after fluorescent labeling, is able to efficiently label ERα by traveling through temporary pores in the cell membrane, created by the toxin streptolysin O. The Affimer, selected by a phage display, predominantly labels the Y537S mutant and can tell the difference between L536S and D538G mutants. The vast majority of Affimer-ERαY537S is in the nucleus and is capable of an efficient, unrestricted navigation to its target DNA sequence, as visualized by single-molecule fluorescence. The Affimer can also differentiate the effect of selective estrogen receptor modulators. More generally, this is an example of a small binding reagent—an Affimer protein—that can be inserted into living cells with minimal perturbation and high efficiency, to image an endogenous protein.
Significance
It is difficult to design a probe which can specifically and efficiently bind to an intracellular native biomolecule, particularly those in the nucleus, while keeping the cell alive. Here, we demonstrate that a fluorescently labeled Affimer, a small ∼13 kDa protein probe, designed against a mutant of estrogen receptor alpha (ERα), can be delivered into live cancer cells, with high efficiency, by temporarily pretreating cells with a pore-forming toxin, streptolysin O. The Affimer was designed through a phage display and was sensitive to the mutant Y537S that is responsible for 70% of breast cancers and is resistant to the traditional endocrine therapy in breast cancer. The fluorescence is sufficiently strong such that it can be visualized at the single-molecule level.
Introduction
Methods to efficiently deliver fluorophores across the cell membrane are crucial for imaging the dynamics of intracellular proteins, including the Y537S and D538G mutants of the estrogen receptor alpha (ERα) that is implicated in 70% of breast cancers (1). Previously, we found that adding streptolysin O (SLO) can be used to create pores in the plasma membrane of living cells and enable fluorescently labeled antibodies or nanobodies to enter in a relatively harmless fashion with high specificity (approaching 90%) with a 10–30 min treatment time (2). However, the use of full antibodies (∼150 kDa) required fairly high concentrations (10–20 μM), similar to those used in other cytoplasmic techniques (3). Furthermore, because an antibody is larger than ∼50 kDa, it cannot enter the nucleus, where many cancer-related proteins act on DNA, including ERα. An antibody also tends to label the N- or C-terminus of the protein target, and is therefore unable to differentiate mutants in the protein’s ligand binding domain—including mutants such as ERα-Y537S and ERα-D538G, which have mutations around the estrogen binding site (1). In contrast, the use of nanobodies, which are ∼15 kDa, required only 200–600 nM, and can enter the nucleus—although this was only shown for (transfected) anti-GFP nanobodies (2).
Another small antibody-like protein is an Affimer, a ∼13 kDa protein that has been shown to have excellent specificity for its target (4,5). Affimers consist of four parallel β-sheets and one α-helix, have a high tolerance to the environmental acidity and temperature, and are functional inside the reducing environment of a cell (4, 5, 6, 7) Moreover, once produced from an established Affimer phage library, a large amount can be purified from E. coli in a cost-effective way compared with a traditional IgG-based antibody (5,8).
Here, we have isolated an Affimer against the ERα mutant Y537S in the presence of estradiol. Combined with dye conjugation and the SLO technique, we show that the Affimer reagent can enter a cancerous cell line (T47D) and fluoresce at the single-molecule level in the nucleus. We find that this anti-ERαY537S Affimer has a low affinity for another D538G mutant, which is found in approximately 20% of metastatic tumors (9, 10, 11). Even in the absence of estrogen, Y537S and D538G mutations are transcriptionally active and cause a decrease in sensitivity to endocrine therapies, such as aromastase inhibitors or tamoxifen, a potent selective estrogen receptor modulator (SERM) (12,13). Here, we show that the fluorescent response of the Affimer is a function of several different SERMs—4-hydroxytamoxifen (4-OHT) and 27-hydroxychloride (27HC), and also different to ERα mutants (including L536S as an inactive mutant, and Y537S and D538G as active mutants). Using this approach, it may be possible to develop new SERMs for patients expressing the mutants in a heterogeneous tumor with poor responses to endocrine therapy.
Materials and methods
Generation of anti-ERαY537S Affimers
A 6X-His-TEV-tagged ERαY537S ligand binding domain (LBD) was expressed in E. coli BL21 (DE3) and purified as described (11). The ERαY537S ligand binding domain was concentrated to 10 mg/mL and flash frozen in liquid nitrogen and stored at −80°C until used.
Affimer isolation
Purified ERαY537S was biotinylated using EZ-Link NHS-biotin (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Biotinylated ERαY537S was immobilized on a streptavidin-coated well in the presence of 10 mM 17β-estradiol (E2). Wells were washed and an Affimer phage library was applied for 2 h, then washed 27 times, and bound phage were eluted in a two-step elution with 0.2 M glycine (pH 2.2) followed by 0.1 M trimethylamine (pH 11.5). After three rounds of panning, 32 random clones were picked and tested for binding and specificity to Y537S treated with E2 ([Y537S + E2]) in a phage ELISA (4). Positive tested clones were sequenced, and three unique binders cloned into pET11 with free C-terminal cysteine (for labeling) were chosen.
Affimer production
Affimer proteins were produced in BL21 STAR (DE3) E. coli induced with 0.5 mM IPTG and grown overnight at 25°C at 150 rpm. Cells were harvested by centrifugation at 4816 × g for 15 min at 4°C and resuspended in 50 mM NaH2PO4, 500 mM NaCl, 30 mM imidazole, and 20% glycerol (pH 7.4), supplemented with EDTA-free protease inhibitor, 0.1 mg/mL lysozyme, 1% Triton X-100, and 10 U/mL benzonase nuclease. Proteins were purified from the supernatant by Ni-NTA chromatography and eluted in 50 mM NaH2PO4, 500 mM NaCl, 300 mM imidazole, and 20% glycerol (pH 7.4).
Affimer pull-down assay
Purified and dialyzed Affimer (25 μg) was incubated with 25 μL of washed Ni2+-NTA beads (Cytiva, Emeryville, CA, USA, cat. no. 28-9799-17) for 60 min at room temperature on a rotator. Affimer-loaded beads were washed and incubated with purified [Y537S + E2] for 1 h. Beads were washed and resuspended in 40 μL SDS sample buffer. Western blot analyses were conducted to determine the success of the pull-down assay using an anti-ERα antibody (Thermo Fisher Scientific).
Plasmid modification
ERαWT-GFP was purchased from Addgene, Watertown, MA, USA (plasmid no. 28230). Point mutations were made at corresponding L536S and Y537S sites, separately, using a Q5 site-directed mutagenesis kit (New England BioLabs, Ipswich, MA, USA, E0554S) following the manufacturer’s protocol to generate ERαY537S-GFP and ERαL536S-GFP. The resultant plasmids were then transfected to HeLa cells.
Cell culture and transfection
HeLa cells and wild-type (WT) T47D cells were subcultured in MEM supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies, Carlsbad, CA, USA) and 100 U/mL of penicillin plus 10 μg/mL of streptomycin (Corning, Corning, NY, USA). T47D cells with homozygous mutation Y537S and D538G established using the CRISPR technique were kind gifts from Professor David Shapiro (Biochemistry, University of Illinois, Urbana-Champaign, IL) (14). Cell lines were routinely tested for mycoplasma, and their identities confirmed by STR analysis.
Cells were cultured in MEM supplemented with 10% charcoal-stripped fetal bovine serum (Thermo Fisher Scientific) and 100 U/mL of penicillin plus 10 μg/mL of streptomycin (Corning). Triple-negative breast cancer MDA-MB-231 cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL of penicillin plus 10 μg/mL of streptomycin. When cell confluency reached 90–100%, they were plated on a 35 mm glass-bottomed dish with a 14 mm micro-well cover glass. Cells were ready for transfection after achieving 70–80% confluency. HeLa cells were usually transfected for 4 h using Lipofectamine 2000 transfection reagent (Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol.
Dye conjugation
The Affimer was conjugated with Alexa647 and Cy3B NHS ester dye following the manufacturer’s instructions. The labeling was done such that there was approximately one dye/Affimer as measured by the concentrations after conjugation.
Ligands treatment
Cells were serum starved in DMEM supplemented with 10% charcoal-stripped fetal bovine serum for 24 h before ligand treatment. Subsequently, cells were incubated with single ligand or two ligands together for 24 h. The final concentration for each ligand was 10 nM E2, 10 μM 4-OHT, and 10 μM 27HC.
Affimer labeling on fixed cells
Cells were washed with PBS and then fixed with 4% paraformaldehyde for 10 min. After that, they were permeabilized using 0.1% Triton X-100 for 5 min, followed by incubation with 3% BSA for 10 min to block nonspecific binding. Dye-conjugated probe was then diluted into 3% BSA and added to cells for 10 min. After washing the unbound probes away, cells were ready to image.
Intracellular labeling in live cells
Intracellular labeling of ERα in live cells using an Affimer conjugated with organic fluorophore follows the procedure described in previous publications with antibodies or nanobodies (2,15). Basically, cells were transiently permeabilized by SLO, a pore-forming protein extracted from bacteria, for 5–10 min at 37°C. After washing away SLO, the anti-ERα Affimer labeled with fluorophores was incubated with cells on ice for 5 min and then excess probes were washed away. Cells were then left to recover and repair their membrane in the normal culture medium for 20 min before imaging. For the single-particle tracking experiment, the imaging buffer contained 100 μL oxyrase (Oxyrase, Mansfield, OH, USA, EC-0005) and 20 mM sodium lactate, and cell dishes were sealed with black aluminum tape (ThorLabs, Newton, NJ, USA, T205-1.0) to minimize contact with oxygen in the environment.
Fluorescence imaging
All imaging was performed using a Nikon Ti Eclipse microscope equipped with a high N.A. objective lens (Nikon APO 100×, oil, N.A. = 1.49), a four-fiber-coupled laser system (405, 488, 561, and 640 nm, Agilent Technologies, Santa Clara, CA, USA, MLC400B), and an EMCCD camera (Andor, Belfast, UK, DU897). Bandpass emission filters (535/50, 600/50, 700/75) were used for fluorescence imaging. Single-particle tracking images were taken using 10 ms exposure times. All uncertainties are the standard deviation.
Time-resolved FRET assay
The time-resolved Förster resonance energy transfer (TR-FRET) assays were carried out as described previously (16), except that a coactivator peptide with the sequence FITC-LKEKHKILHRLLQDSSSPV (Thermo Fisher Scientific, cat no. PV4586) was used in place of the steroid receptor coactivator fragment. All uncertainties are the standard deviation.
Results
Anti-ERαY537S Affimer as a function of estradiol can be used to differentiate Y537S from L536S and WT in fixed HeLa cells
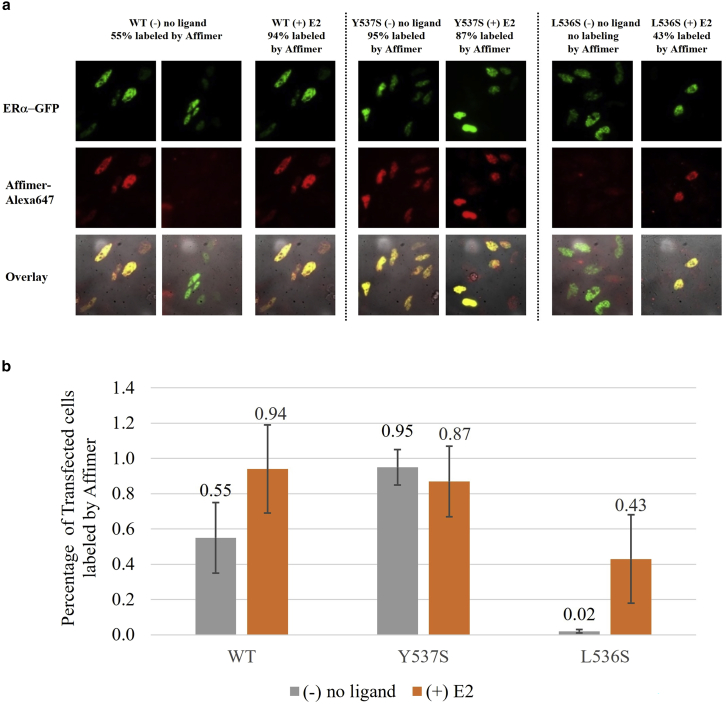
We first examined transfected ERα in an ERα-negative HeLa cell line (Fig. 1). We used three different human plasmids, all encoded with GFP: ERαWT (with variable activity, dependent on the E2 ligand), ERαY537S (constitutively active mutant), and ERαL536S (inactive mutant) (17,18). After fixation, permeabilization, and incubation with an Affimer-Alexa647, we recorded the fluorescence of the GFP and the Alexa647. Without the E2 ligand, we found the Affimer colocalized well with WT and Y537S in the nucleus, while showing little labeling to L536S (Fig. 1 a). Addition of E2 increases the amount of labeling with the WT and the L536S mutant, while the Y537S mutant, which was almost completely labeled even without E2, remained high (Fig. 1 b). The implication is that Affimer labeling to ERα can be affected by a conformational change caused by E2 ligand binding—whether or not it is wild-type without any mutations (WT) or an inactive mutant (L536S).
Figure 1.
a) HeLa cells were individually transfected with ERαWT-GFP, ERαY537S-GFP, or ERαL536S-GFP, and then fixed, permeabilized, and labeled with Affimer-Alexa647, either with no external ligand or with external E2. (b) The addition of E2 to the mutants. Error bars represent the standard deviation. To see this figure in color, go online.
In T47D cells, the Affimer binds to the WT and Y537S mutant, and significantly less to D538G, while the antibody binds to all three
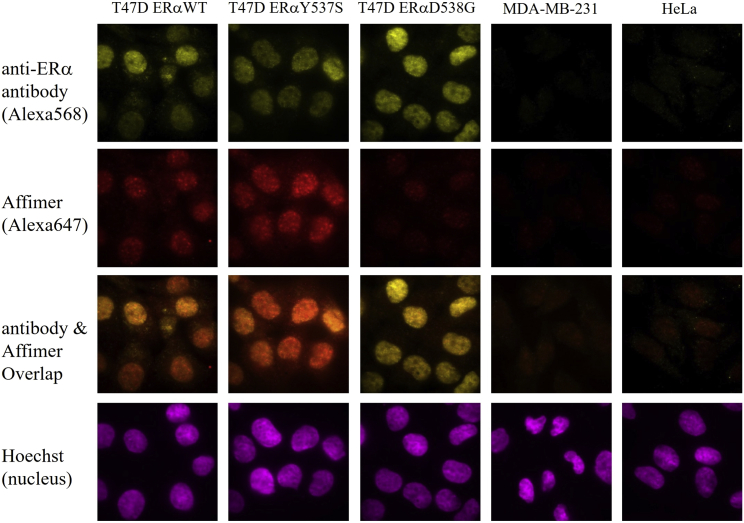
We then looked at the binding of the Affimer compared with that of an antibody to endogenous WT, Y537S, and D538G in T47D cells, an ERα-positive breast cancer cell line (Fig. 2). For Y537S and D538G, we used a CRISPR-modified T47D cells that was homozygous in Y537S or in D538G mutations. As negative controls, we used HeLa and MDA-MB-231 cell lines that lack ERα. In all cases, we labeled the ERα with both the fluorescent Affimer and a primary plus fluorescent secondary antibody, all on fixed cells, again looking for colocalization. The results (Fig. 2) showed that the Affimer and antibody were colocalized for the WT and Y537S, with a slightly enhanced signal for the Y537S. By contrast, the Affimer showed greatly reduced staining in cells harboring D538G ERα, while the antibody bound to all three of them. In the negative control cells, MDA-MB-231 and HeLa, there was little labeling, as expected. Overall, these results show that the anti-ERαY537S Affimer preferentially labels transfected and endogenous WT and Y537S mutant compared with D538G mutants.
Figure 2.
T47D cells with ERαWT, or CRISPR-established ERαY537S or ERαD538G were fixed, permeabilized, and labeled by Affimer-Alexa647 and anti-ERα primary antibody with anti-mouse secondary antibody-Alexa568, and Hoechst (as nucleus marker). MDA-MB-231 (triple-negative breast cancer cell) and HeLa cells (ERα-negative cells) were labeled the same way as the negative controls. To see this figure in color, go online.
In fixed T47D cells, the Affimer binds preferentially to ERα-ligand complexes that have an active conformation like Y537S + E2, but not to the active conformation of D538G; All cells are negatively regulated by 4-OHT, with 27HC having mixed results
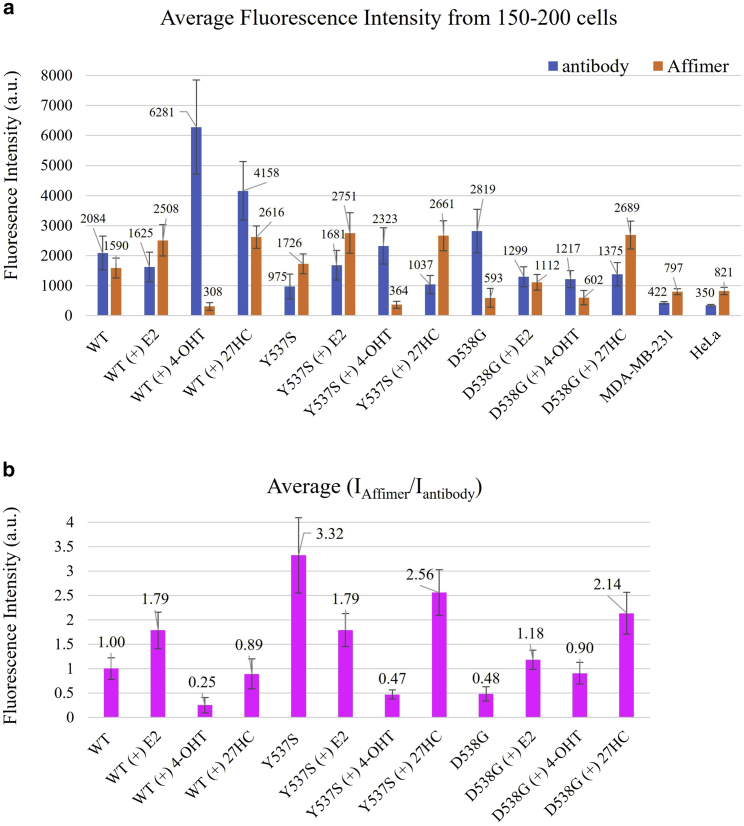
We next quantitatively examined the extent of Affimer labeling as a function of ERα-mutant and bound ligand, both of which can cause conformational changes. ERα included WT, Y537S, and D538G, and the ligands included E2, 4-OHT, or 27HC (Fig. 3). T47D cells were treated with different ligands, and then we fixed and labeled the cells with both the Affimer-Alexa647 and with a fluorescently labeled antibody pair (primary plus Alexa568 secondary) specific for the C-terminus (Fig. 3 a). The fluorescence arising from the antibody was used to normalize to the amount of ERα per cell, which may vary between mutants or ligands. The ratio of Affimer fluorescence divided by the antibody fluorescence was detected in each cell, and the average over 150–200 cells in each condition was taken to account for cell-to-cell heterogeneity. To study how the conformation may change as a function of ligand and mutant, the fluorescence ratio was set equal to one for the WT cells (Fig. 3 b). This final ratio yields the fluorescence of the Affimer in each condition divided by the fluorescence of Affimer for WT without any ligand, all normalized by the amount of ERα. We also used a different averaging method, using the ratio of averaged Affimer fluorescence divided by averaged antibody fluorescence over 150–200 cells (Fig. S1). Both methods showed consistent results.
Figure 3.
a) T47D cells with WT, Y537S, or D538G were treated with 10 nM E2, 1 μM 4-OHT, or 10 μM 27HC for 24 h separately, then fixed, permeabilized, and labeled with Affimer-Alexa647, anti-ERα primary antibody, anti-mouse secondary antibody-Alexa568, and Hoechst (nuclear stain). The absolute fluorescence intensity of Affimer and antibody in the nucleus labeled with Hoechst were recorded and averaged among around 150–200 cells in each sample. MDA-MB-231 and HeLa cells were treated in the same way as negative controls. (b) The average ratio of fluorescence intensity between Affimer and antibody in each single cell (average among 150–200 cells in each sample, normalized to T47D with WT). Error bars represent the standard deviation.To see this figure in color, go online.
The fluorescence of the 12 different ERα mutations and ligands can be divided into two zones, either ≤1, the ratio for the WT, or ≥1.79, the value of [Y537S + E2]. Since Affimer is screened against [Y537S + E2], we use this ratio as the threshold to define a “high” ratio. Among 12 conditions, 5 are high ([Y537S + none, E2, 27HC], [WT + E2], [DG538 + 27HC]); 6 are low ([WT + none, 4-OHT, 27HC], [Y537S + 4-OHT], [DG538 + none, 4-OHT), and only one [D538G + E2] falls outside of this range. A high ratio means that the conformation of the ERα mutants/ligands are similar to that of [Y537S + E2], which is a transcriptionally active conformation. A low ratio means its conformation is distinct from the Y537S + E2 mutant.
Grouping them in this way, the ligand 4-OHT bound to any of the three mutants is low, consistent with the antagonistic function of 4-OHT, which causes the inactive conformation (10). Adding E2 leads to an increase in the binding of the Affirmer for the D538G, but a decrease for Y537S. This again indicates that that D538G has a different structure than Y537S. The situation with 27HC is more complex. Adding 27HC to Y537S and D538G increases fluorescence significantly, but leads to a slight decrease with WT. 27HC is generally considered to be a partial agonist to the WT and having a different conformational change than both E2 (activating) or 4-OHT (downregulating) (19,20,21).
The Affimer labels transfected Y537S in living HeLa cells via the SLO technique
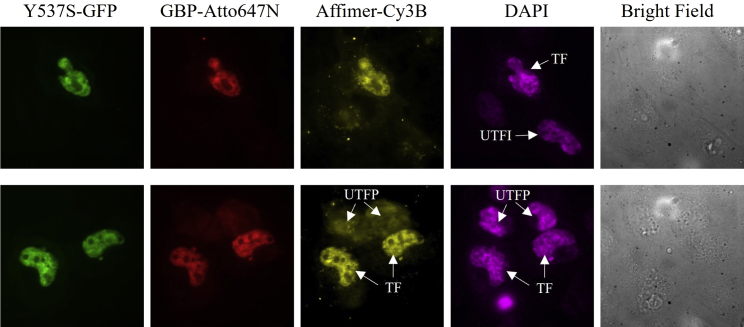
We next demonstrated that the Affimer binds specifically to Y537S in living cells (Fig. 4). We transfected Y537S-GFP (green) in HeLa cells (which does not have endogenous ERα) and then transiently permeabilized the cell membrane with SLO, followed by simultaneous labeling with three dyes: 1) an Atto647N-labeled GFP binding protein (called GBP), 2) Affimer-Cy3B, and 3) DAPI, a nuclear stain (Fig. 5). In our previous publication, we ran a cell viability assay to show that >90% cells can be successfully recovered after permeabilization (15). Since transfection and permeabilization are two sequential processes with independent efficiency, in Fig. 5 we show a combination of cells with different status (TF, UTFI, UTFP) in one field of view and compare them side by side.
Figure 4.
In vivo Y537S labeling in live HeLa cells via the SLO technique. External probes, GBP-Atto647N (red) and Affimer-Cy3B (yellow), were delivered into live HeLa cells to label transfected Y537S-GFP (green). A cell-permeable probe, DAPI (magenta), was used as a nucleus marker. Transfected (TF), untransfected and impermeabilized (UTFI), and untransfected and permeabilized (UTFP) cells are shown. To see this figure in color, go online.
Figure 5.
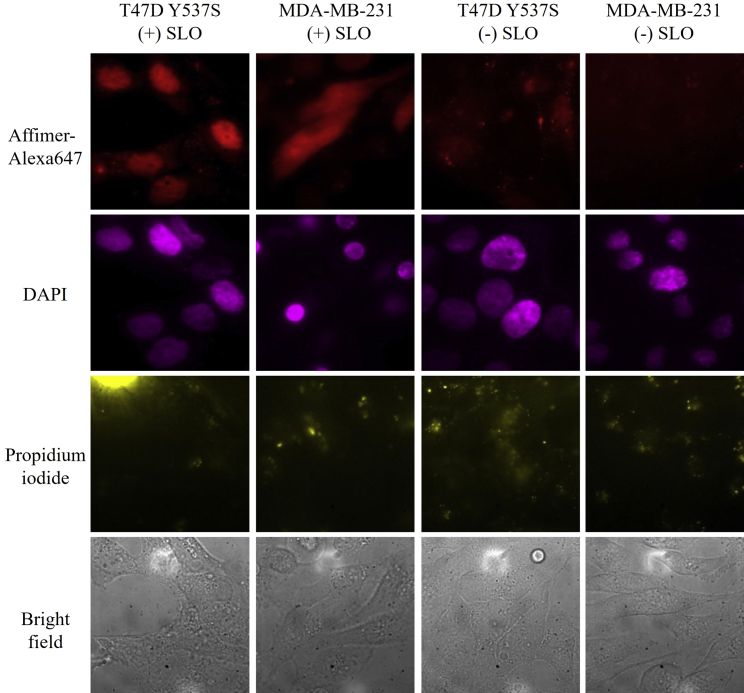
In vivo Y537S labeling in T47D Y537S cells and MDA-MB-231 cells via the SLO technique. External probe, Affimer-Alexa647 (red), was delivered into living T47D cells to label endogenous Y537S. DAPI (magenta) was used as nucleus marker. MDA-MB-231 cells were labeled in the same way as the negative control. After recovery, cells were stained with propidium iodide (yellow) to check the integrity of cell membrane. To see this figure in color, go online.
We found that the Affimer can bind to transfected Y537S in live HeLa cells. In transfected cells, the presence of GFP, confirms the transfection of Y537S. The GBP-Atto647N confirmed that the cells are permeabilized by SLO and the ordinarily cell-impermeable GBP-Atto647N can enter the nucleus of live cells. The Affimer-Cy3B colocalized with transfected Y537S-GFP, confirming that the Affimer can specifically stain the nuclear Y537S. As a control, in untransfected and impermeabilized cells—i.e., no DNA-plasmid inside the cell (UTFI), and the cell has not been treated with SLO—there is no fluorescence in the GFP, GBP-Atto647N, and Affimer-Cy3B because none of the dyes are present inside of the cell. The cells are, however, still intact, as can be seen by DAPI staining. In untransfected and permeabilized cells (UTFP)—which can be seen by labeling with DAPI—both GBP-Atto647N and Affimer-Cy3B are freely diffusing in the whole cell without specific binding to any target. Overall, the specificity of Affimer-Cy3B to transfected Y537S-GFP in living cells is consistent with the results from fixed transfected cells, indicating that the Affimer can enter the nucleus of living cells and maintain its specificity for Y537S.
The Affimer labels nontransfected Y537S in a live T47D cell via the SLO technique
We also applied the Affimer in the presence SLO to native Y537S in T47D cells (Fig. 5, column 1). As a control, we used MDA-MB-231 with no ERα present (Fig. 5, column 2). We treated cells with a relatively high concentration of Affimer to enhance the fluorescence signal, although this inevitably caused some nonspecific binding. We made the conclusion about Affimer specificity not by absolute fluorescence intensity, but by comparing the labeling pattern between ERα-positive and -negative cells. Specifically, we observed Affimer accumulation in the nucleus of T47D cells, as well as colocalization with nuclear-staining DAPI; in MDA-MB-231 cells, the Affimer has a homogenous distribution in the whole cell without preferred binding to any (nuclear) targets. Column 3 and 4 of Fig. 5 are in the absence of SLO for T47D and MDA-MB-231 cells. Here, the Affimer cannot pass through the membrane and no labeling results. To demonstrate the integrity of cell membrane after SLO treatment, cells are incubated with propidium iodide, a dye that only labels the nucleus if the cytoplasmic cell membrane is disrupted (Fig. 5, row 3). In this case, propidium iodide is not able to enter the cells regardless of SLO treatment. This indicates that the cell membrane can fully recover after treatment with SLO. The results show that the Affimer is able to label not only transfected Y537S in live cells but native Y537S as well.
Single-molecule labeling of endogenous ERαY537S in live T47D cells
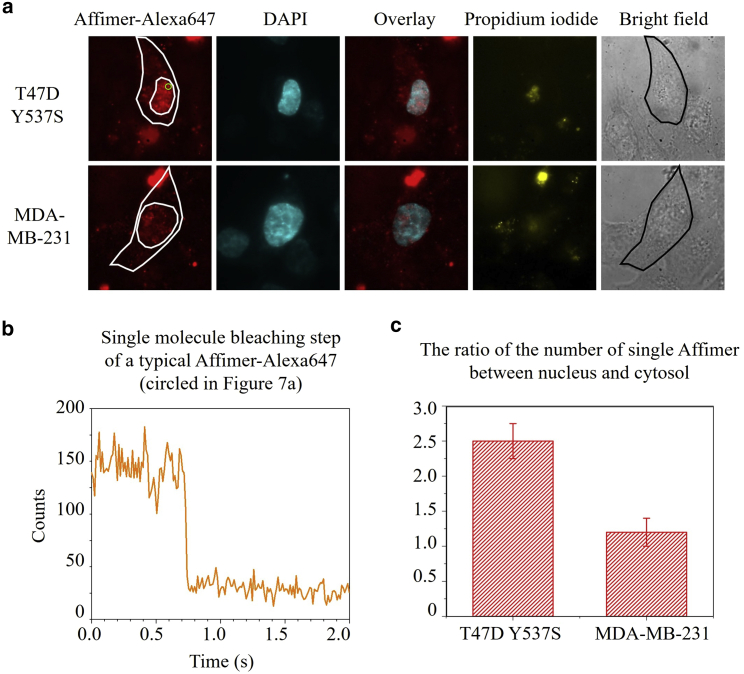
We next labeled native Y537S in T47D cells at the single-molecule level, still applying Affimer with the SLO technique, as reported previously. MDA-MB-231 cells without Y537S were also included as a control. To reduce the nonspecific labeling caused by the excess amount of Affimer shown in Fig. 5, we optimized the concentration of Affimer and achieved single-molecule labeling (Fig. 6 a). The single bleaching step of Affimer-Alexa647 is suggestive evidence that they are single molecules (Fig. 6 b). (Single-step photobleaching can, of course, occur with multiple fluorophores photobleaching together; however, that the average labeling is 1:1 by ensemble absorption measurement—see “Dye labeling” in the Materials and methods—and the predominance of the size of the fluorescence photobleaching in Fig. 6 b, indicates that it is most likely a single one.) We counted the number of Affimers in the nucleus and the cytosol, separately, and calculated the ratio between them (Fig. 6 c). In T47D Y537S cells, the ratio was around 2.5:1, indicating that the Affimer has ∼70% probability (2.5/(2.5 + 1)) for binding the nuclear Y537S, while in MDA-MB-231 cells the Affimer has an equal chance to diffuse in the nucleus and in the cytosol since there is no Y537S for binding. (From the antibody-labeling results on fixed cells, the cytoplasmic ERα is negligible.)
Figure 6.
In vivo single Y537S labeling in live T47D and MDA-MB-231 cells via the SLO technique. (a) T47D Y537S cells and MDA-MB-231 cells were labeled by Affimer-Alexa647 and DAPI. Propidium iodide was added to check cell viability after cell recovery. (b) A representative single bleaching step of Affimer-Alexa647 in T47D Y537S cells. (c) Bar graph of the number ratio of single Affimer-Alexa647 between nucleus and cytosol in T47D Y537S and MDA-MB-231 cells. Error bars represent the standard deviation.To see this figure in color, go online.
Diffusion of single Y537S decreases by 10× in the presence of estradiol, and undergoes a local search mechanism rather than 3D diffusion in a relatively efficient, unrestricted navigation to its target DNA sequence in the crowded nucleus
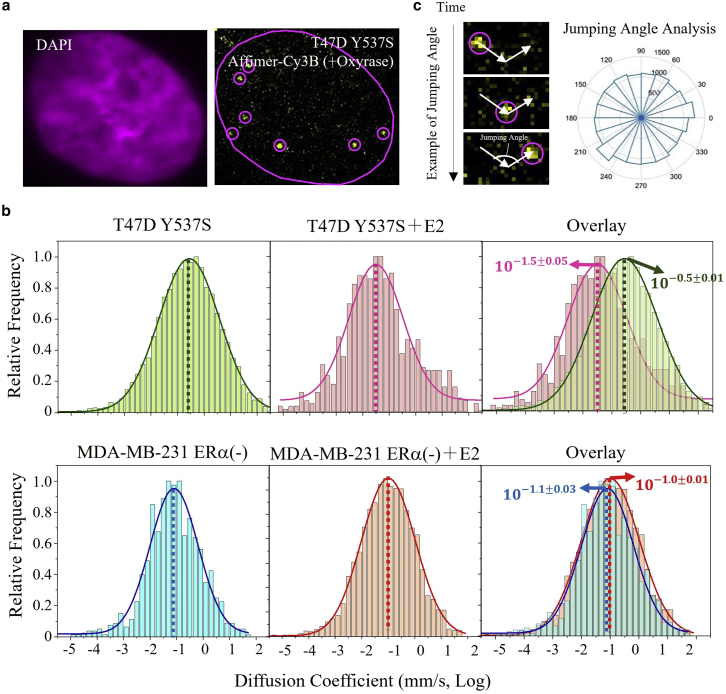
We then investigated the interaction between Y537S and its ligand, E2, and its interaction with the DNA in living cells (Fig. 7). To do so, we used the Affimer conjugated with Cy3B to observe the diffusion of single-particle Y537S, before and after E2 binding (Fig. 7 a, shown in the presence of E2). In the videos in the supporting material, one can see a series of labeled Affimers diffusing and their individual motion (Videos S1 and S2, shown with and without E2, look identical). To minimize the loss of fast diffusive Y537S, we set the time resolution to a minimum time (10 ms) and obtained a distribution of diffusion coefficients of each molecule in every frame (Fig. 7 b). By fitting the histogram with a Gaussian function, we obtained a diffusion coefficient of Y537S of μm2/s (∼0.25 μm2/s), without any ligand treatment. After treating with 10 nM E2 for 24 h, we observed that the diffusion coefficient shifted to μm2/s (∼0.03 μm2/s), or a factor of ∼10× less. In the MDA-MB-231 cells, no distinguishable shift was observed. To quantitatively evaluate the similarity between two distributions of diffusion coefficient before and after adding E2, we calculated the overlapping coefficient, defined as the overlapping area divided by the minimum of the two distributions (22). For T47D cells with Y537S, the overlapping coefficient was 69%, indicating that the distribution of diffusion coefficient had shifted by 31%, making [Y537S + E2] less diffusive than Y537S. By comparison, for MDA-MB-231 (without Y537S) the overlapping coefficient is >99%, which shows that cells without ERα are not be affected by E2. The reduction in the diffusion coefficient can be explained by the recruitment of cofactor proteins to Y537S driven by E2 binding. The resultant larger protein complex could cause a slower diffusion. The measurement of the shift of diffusion coefficient by single-particle tracking can also potentially be a way to validate the binding property of a new drug to Y537S.
Figure 7.
a) T47D cells with Y537S labeled with Affimer-Cy3B and DAPI by the SLO technique. (b) Diffusion coefficient distribution of single-molecule Affimer fitted by Gaussian function under different conditions. Left, T47D cells with WT, T47D cells with Y537S, and MDA-MB-231 cells without any ligands. Middle, the same cells with 10 nM E2 treatment for 24 h. Right, overlaid graph of no ligand (left) and E2 treatment condition (middle). The mean value of diffusion coefficient from Gaussian fitting in the left and middle graph are indicated by the arrows in the overlaid graphs. (c) Left, example of a jumping angle between two steps on the trajectory of a single molecule circled in Fig. 8a. Right, jumping angle distribution from all detected single-molecule Affimer. Uncertainties in the overlay represent on stabard deviation. To see this figure in color, go online.
Single Affimer-Cy3B (yellow fluorescence) inside the nucleus (cyan curve) is marked with magenta circles in each frame. The center position of single Affimer-Cy3B and its trajectory are shown in red. The video was recorded for 10 s at 10 ms/frame and plays at 50 fps.
Single Affimer-Cy3B (yellow fluorescence) inside the nucleus (cyan curve) is marked with magenta circles in each frame. The center position of a single molecule and its trajectory are shown in red. The video was recorded for 10 s at 10 ms/frame and plays at 50 fps.
Of course, the transcription factor Y537S must find its target DNA and/or its coactivator before it can activate the corresponding gene expression. Two protein searching mechanisms exist in the nucleus, the local search (with diffusion coefficient <0.5 μm2/s, the value of a free histone diffusion coefficient) and 3D diffusion (with diffusion coefficient >0.5 μm2/s) (23). Since the diffusion of Y537S is always slower than 0.5 μm2/s, we conclude that it undergoes a local search mechanism rather than 3D diffusion (Fig. 7 b). We studied the direction of motion between two steps in the trajectory of the Affimer’s movement and calculated the jumping angle distribution. There are two types of local searching in the nucleus, classified by the local search environment encountered in the target-search process: the restricted local search (skewed jumping angle distribution) and unrestricted local search (uniform jumping angle distribution) (23). The uniform distribution of jumping angle in all directions indicates that Y537S (with or without E2) labeled with Affimer tends to undergo a relatively efficient, unrestricted navigation to its target DNA sequence in the crowded nucleus (Fig. 7 c).
TR-FRET measurement of Affimer binding
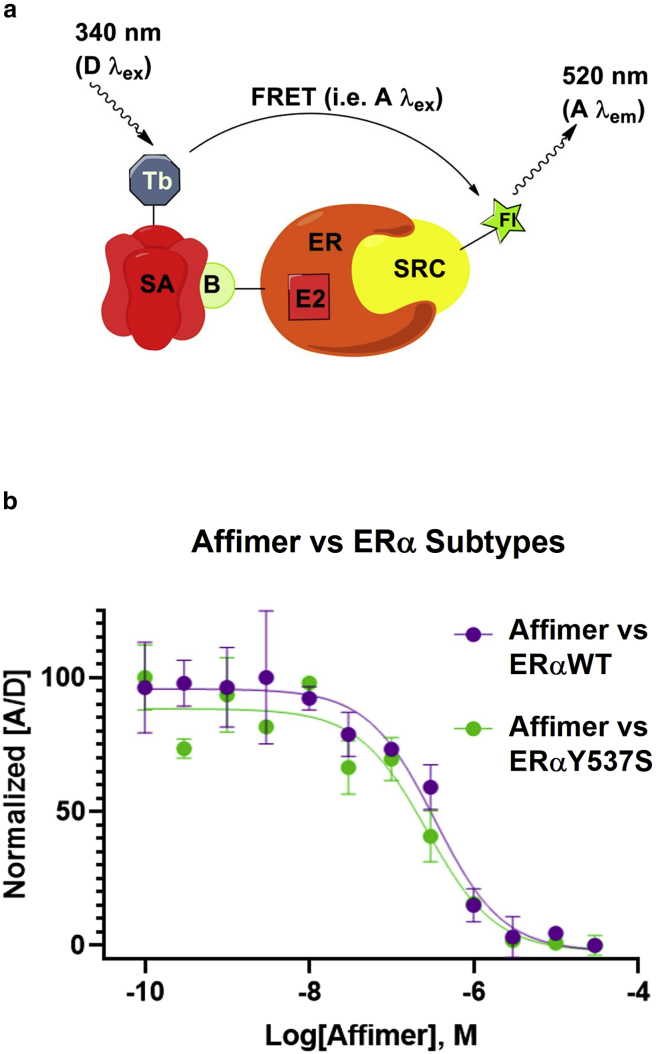
We conducted an in vitro TR-FRET assay to study the molecular basis of Affimer binding (16) (Fig. 8). In brief, an ERα ligand binding domain that is biotinylated at position C417 is labeled with streptavidin bound to a terbium-chelate donor fluorophore. A peptide derived from the SRC2 coactivator is labeled with a fluorescein acceptor and directly binds to ERα. The TR-FRET signal is detected to measure association of coactivator and ERα. Using this assay, we treated WT and Y537S with increasing concentrations of Affimer in the presence of constant E2, and observed a decreasing FRET signal in a concentration-dependent manner (Fig. 8 b). These data indicate that Affimer directly binds to both ERαWT and ERα-Y537S at a site that overlaps with the coactivator binding site, or results in a conformational change that obscures access to this site. The inhibition coefficient was not significantly different between WT and Y537S, indicating that it disrupts the interaction of SRC2 equally well with either ER subtype. The differential labeling to mutant ERα forms in the presence of different ligands suggests that context-specific differential cofactor recruitment may be able to disrupt Affimer binding.
Figure 8.
a) Schematic of TR-FRET measurement from a terbium-chelate donor excited at 340 nm, which transfers energy to a fluorescein-labeled steroid receptor coactivator (SRC2) peptide bound to ERα. Emission from fluorescein occurs at 520 nm (16). (b) FRET signal recorded in a TR-FRET assay to measure ERα-coactivator interaction in the presence of E2. ERα and coactivator are conjugated with a pair of FRET dyes, terbium donor (D) and fluorescein acceptor (A). When ERαWT and ERαY537S are treated with increasing concentrations of Affimer, the FRET signal is recorded to monitor the interaction between ERα and coactivator. Competition or conformational changes that obscure the SRC2-binding site result in a decrease in TR-FRET signal, as measured by the ratio of acceptor and donor emissions. Error bars represent the standard deviation. To see this figure in color, go online.
Discussion
Globally, breast cancer is the most common cancer in women, accounting for 12% of all new cancers in 2021 (24). Treating patients at the early stage of ERα-positive breast cancer with endocrine therapy, i.e., with SERMs or aromatase inhibitors, the 5-year relative survival rate is over 90% (25). SERMs inhibit ERα function by competing with estrogen in the ligand binding domain of ERα, leading to a conformational change, ultimately leading to the differential recruitment of coactivators and corepressors (26,27). However, resistance to endocrine therapy during long-term treatment, and tumor metastasis after the early stage of breast cancer, causes some patients to respond poorly (28,29). The most prevalent mutation occurs in ERα with Y537S accounting for ∼60% in breast cancer patients, and D538G occurring in approximately 20% (1).
Here, we used a small antibody mimic, a 13 kDa Affimer isolated against ERαY537 + E2, to label various ERα (WT, L536S, Y537S, and D538G mutants) in both fixed and living cancerous cells lines, as a function of different ligands. By creating temporary pores in the cell membrane with the toxin SLO, we found that the Affimer, labeled with the dye Alexa647 or Cy3B, goes into the nucleus (Figs. 4 and 5). This was done relatively efficiently, ∼ 60% of cells, compared with a few percent using a typical cell injection technique. The process is also done with relatively little harm (2,15).
Others have made significant efforts at making small antibody or antibody-like compounds to a wide variety of targets (30,31). For example, Koide et al. previously developed conformation-specific monobodies to the ERαWT-ligand complex (31). The continuing interest in Y537S requires a specific probe to Y537S, discriminating it from WT and other mutants, and to test it as a function of regulatory ligands, such as E2 and SERMs.
One advantage of the Affimer is its ability to bind different ERα mutants. In fixed cells, we find that a conventional antibody binds to all WT, Y537S, and D538G, while the Affimer reagent preferentially recognizes WT and Y537S over D538G (Fig. 2). The Affimer can also bind selectively to Y537S, rather than L536S and WT, as a function of E2 (Fig. 1). In a fixed breast cancer cell, the Affimer reagent tends to bind preferentially to ERα-ligand complexes that have an active conformation like [Y537S + E2], but not to the active conformation of D538G (Fig. 3). It reacts differently when a variety of regulators, such as E2, 4-OHT, or 27HC, is present.
The real advantage of the Affimer comes when using SLO, which enables ERα to be interrogated in living cells (Fig. 4) or in living cancer cell lines (Fig. 5). Furthermore, cells can easily be distinguished as to whether they have, or have not, been permeabilized by SLO, or are transfected or untransfected (Figs. 4 and 5). It is also clear from Figs. 4, 5, 6, and 7 that ERα is overwhelming in the nucleus (having a nuclear localization signal), whereas truncated versions can be in either the cytoplasm of the nucleus (32). We can also see, at the single-molecule level, that the Affimer bound to ERα diffuses in the nucleus. A straightforward analysis shows that the diffusion of single Y537S decreases by 10× in the presence of E2 and undergoes a local search mechanism, rather than 3D diffusion, in a relatively efficient, unrestricted navigation to its target DNA sequence in the crowded nucleus (Fig. 7). Finally, we measured the inhibition coefficient of the Affimer using a TR-FRET assay, indicating that the Affirmer does indeed bind ERα in a biologically meaningful way, disrupting binding of a coactivator protein (SRC2).
Conclusion
It is difficult to design a probe that can bind specifically and efficiently to an intracellular native biomolecule, particularly those in the nucleus—such as ERα—while keeping the cell alive. We have demonstrated that a fluorescently labeled Affimer, a ∼13 kDa protein probe, can be delivered with up to 60% efficiency into live cancerous cell lines by pretreating cells with a pore-forming toxin, SLO, and can distinguish Y537S mutant from WT and other mutants. In general, the use of an Affimer is highly advantageous because it is well characterized, is robust to environmental changes, and can be transported into the cell with high efficiency. The different affinity of the Affimer to ERα with different mutations and ligands enables us to distinguish the conformation of [Y537S + E2] from other conformations. Moreover, when the Affimer reagent is applied with SLO for live cell labeling, it is possible to study the movement of native Y537S mutant at the single-molecule level using an external fluorophore.
While, it would be ideal if the selected Affimer binds only to Y537 (or to D538G), the fact that it fluoresces to varying amounts with different ligands (or mutants) has advantages. Different regulators, whether they be coactivators, corepressors, and/or transcription, can be studied. By treating a cell with the range of ligands, one can get a sense of whether it reacts more like a negative regulator, like 4-OHT, or a positive regulator like E2, or acts like 27HC. Future efforts at designing new SERMs will benefit from the fluorescence assays developed here, as they are much quicker than efforts such as x-ray crystallography.
Author contributions
P.R. designed the research, performed and analyzed all the experiments, and wrote the manuscript. P.R.S. designed the research and analyzed and wrote the manuscript. G.L.G. and S.W.F. provided material for the Affimer screening. C.T., T.A., V.S., and D.T. isolated the Affimer reagents. C.C. and T.W.M. performed TR-FRET measurements. All members contributed to the research design and provided comments on the manuscripts.
Acknowledgments
This work was partially supported by grants NIH GM132392, NS097610, and NSF 1430124 (to P.R.S); NIH CA234025 and DoD W81XWH-20-BCRP-EOHS/BC200206 (to E.R.N.); Breast Cancer Now (to V.S. and D.T.); American Cancer Society RSG-21-037-01-CDD(to T.W.M.); Leverhulme Trust Research Project Grant (to D.T.); and Ludwig Fund for Cancer Research (to G.L.G.).
Declaration of interests
The authors declare no competing interests.
Editor: Vasanthi Jayaraman.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.06.028.
Supporting material
Document S2. Article plus supporting material
References
- 1.Clusan L., Le Goff P., et al. Pakdel F. A closer look at estrogen receptor mutations in breast cancer and their implications for estrogen and antiestrogen responses. Int. J. Mol. Sci. 2021;22:756. doi: 10.3390/ijms22020756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng K.W., Ishitsuka Y., et al. Selvin P.R. Labeling proteins inside living cells using external fluorophores for fluorescence microscopy. Elife. 2017;6:e25460. doi: 10.7554/eLife.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai W., Zhao P., Gao X. Cytosolic delivery of proteins by cholesterol tagging. Sci. Adv. 2020;6:eabb0310. doi: 10.1126/sciadv.abb0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiede C., Tang A.A.S., et al. McPherson M.J. Adhiron: a stable and versatile peptide display scaffold for molecular recognition applications. Protein Eng. Des. Sel. 2014;27:145–155. doi: 10.1093/protein/gzu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiede C., Bedford R., et al. Tomlinson D.C. Affimer proteins are versatile and renewable affinity reagents. Elife. 2017;6:e24903. doi: 10.7554/eLife.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes D.J., Tiede C., et al. Whitehouse A. Generation of specific inhibitors of SUMO-1–and SUMO-2/3–mediated protein-protein interactions using Affimer (Adhiron) technology. Sci. Signal. 2017;10:eaaj2005. doi: 10.1126/scisignal.aaj2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haza K.Z., Martin H.L., et al. Tomlinson D.C. RAS-inhibiting biologics identify and probe druggable pockets including an SII-α3 allosteric site. Nat. Commun. 2021;12:4045. doi: 10.1038/s41467-021-24316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tans R., van Rijswijck D.M., et al. van Gool A.J. Affimers as an alternative to antibodies for protein biomarker enrichment. Protein Expr. Purif. 2020;174:105677. doi: 10.1016/j.pep.2020.105677. [DOI] [PubMed] [Google Scholar]
- 9.Merenbakh-Lamin K., Ben-Baruch N., et al. Wolf I. D538G mutation in estrogen receptor-α: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 2013;73:6856–6864. doi: 10.1158/0008-5472.CAN-13-1197. [DOI] [PubMed] [Google Scholar]
- 10.Toy W., Shen Y., et al. Chandarlapaty S. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanning S.W., Mayne C.G., et al. Greene G.L. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife. 2016;5:e12792. doi: 10.7554/eLife.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu G., Tian L., et al. Fuqua S.A.W. Hormonal modulation of ESR1 mutant metastasis. Oncogene. 2021;40:997–1011. doi: 10.1038/s41388-020-01563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razavi P., Chang M.T., et al. Baselga J. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–438.e6. doi: 10.1016/j.ccell.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao C., Livezey M., et al. Shapiro D.J. Antiestrogen resistant cell lines expressing estrogen receptor α mutations upregulate the unfolded protein response and are killed by BHPI. Sci. Rep. 2016;6:34753. doi: 10.1038/srep34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng K.W., Ren P., Selvin P.R. Delivery of fluorescent probes using Streptolysin O for fluorescence microscopy of living cells. Curr. Protoc. Protein Sci. 2018;93:e60. doi: 10.1002/cpps.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore T.W., Gunther J.R., Katzenellenbogen J.A. Estrogen receptor alpha/co-activator interaction assay: TR-FRET. Methods Mol. Biol. 2015;1278:545–553. doi: 10.1007/978-1-4939-2425-7_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C., Koide A., et al. Skafar D.F. Mutation of Leu-536 in human estrogen receptor-alpha alters the coupling between ligand binding, transcription activation, and receptor conformation. J. Biol. Chem. 2003;278:27278–27286. doi: 10.1074/jbc.M303840200. [DOI] [PubMed] [Google Scholar]
- 18.Kriegel M., Wiederanders H.J., et al. Muller Y.A. A PROSS-designed extensively mutated estrogen receptor α variant displays enhanced thermal stability while retaining native allosteric regulation and structure. Sci. Rep. 2021;11:10509. doi: 10.1038/s41598-021-89785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzenellenbogen J.A., Mayne C.G., et al. Chandarlapaty S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance. Nat. Rev. Cancer. 2018;18:377–388. doi: 10.1038/s41568-018-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuSell C.D., Umetani M., et al. McDonnell D.P. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol. Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L., Wang L., et al. Nelson E.R. 27-Hydroxycholesterol acts on myeloid immune cells to induce T cell dysfunction, promoting breast cancer progression. Cancer Lett. 2020;493:266–283. doi: 10.1016/j.canlet.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inman H.F., Bradley E.L., Jr. The overlapping coefficient as a measure of agreement between probability distributions and point estimation of the overlap of two normal densities. Commun. Stat. Theor. Methods. 1989;18:3851–3874. doi: 10.1080/03610928908830127. [DOI] [Google Scholar]
- 23.Jain S., Shukla S., et al. Zhao H. TALEN outperforms Cas9 in editing heterochromatin target sites. Nat. Commun. 2021;12:606. doi: 10.1038/s41467-020-20672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung H., Ferlay J., et al. Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 25.Johnston S.R. New strategies in estrogen receptor–positive breast cancer. Clin. Cancer Res. 2010;16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 26.Dutertre M., Smith C.L. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J. Pharmacol. Exp. Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- 27.Komm B.S., Mirkin S. An overview of current and emerging SERMs. J. Steroid Biochem. Mol. Biol. 2014;143:207–222. doi: 10.1016/j.jsbmb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Fuqua S.A. The role of estrogen receptors in breast cancer metastasis. J. Mammary Gland Biol. Neoplasia. 2001;6:407–417. doi: 10.1023/a:1014782813943. [DOI] [PubMed] [Google Scholar]
- 29.Chang M.S. Tamoxifen resistance in breast cancer. Biomol. Ther. 2012;20:256–267. doi: 10.4062/biomolther.2012.20.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Y., Mukherjee S., et al. Melcher K. Structure of an AMPK complex in an inactive, ATP-bound state. Science. 2021;373:413–419. doi: 10.1126/science.abe7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koide A., Abbatiello S., et al. Koide S. Probing protein conformational changes in living cells by using designer binding proteins: application to the estrogen receptor. Proc. Natl. Acad. Sci. USA. 2002;99:1253–1258. doi: 10.1073/pnas.032665299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchhofer A., Helma J., et al. Rothbauer U. Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 2010;17:133–138. doi: 10.1038/nsmb.1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single Affimer-Cy3B (yellow fluorescence) inside the nucleus (cyan curve) is marked with magenta circles in each frame. The center position of single Affimer-Cy3B and its trajectory are shown in red. The video was recorded for 10 s at 10 ms/frame and plays at 50 fps.
Single Affimer-Cy3B (yellow fluorescence) inside the nucleus (cyan curve) is marked with magenta circles in each frame. The center position of a single molecule and its trajectory are shown in red. The video was recorded for 10 s at 10 ms/frame and plays at 50 fps.
Document S2. Article plus supporting material