To the Editor,
The devastating effect of coronavirus disease 2019 (COVID‐19) has led to rapid development and mass coverage of COVID‐19 vaccination worldwide to combat this deadly virus. 1 Presently, various vaccine options are available globally. In India, two major COVID‐19 vaccine, namely, ChAdOx1 nCoV‐19 Corona Virus Vaccine (Recombinant) COVISHIELD and Bharat Biotech BBV152 COVAXIN vaccine are licensed to use. COVISHIELD vaccination course includes two separate doses of 0.5 ml each. The second dose is administered between 4 to 12 weeks after the first dose. COVAXIN is an inactivated vaccine with a 2‐dose vaccination regimen given 28 days apart.
Lichen planus (LP) and lichenoid drug eruptions (LDEs) are uncommonly reported after vaccination. 2 In this study, we aim to characterize and describe the cases of new‐onset LP and lichenoid eruptions triggered by the COVID‐19 vaccine.
All the patients attending the LP clinic of our dermatology outpatient department were analyzed for history of COVID‐19 vaccination prior to onset of lesions. Patients with new onset of lesions with a positive history of vaccination were recruited for our study. A total for 58 patients were identified. Demographic characteristics, history of any concurrent disease or drug intake, clinical morphology, and histopathologicl features were analyzed. Time to disease onset was calculated from the dose of COVID‐19 vaccine administration.
Our study reported a female predominance of 63% in lichenoid eruptions post‐COVID‐19 vaccination. The mean duration of onset of cutaneous lesions following vaccination was 20.8 days with range being 10–42 days. The most common clinical variant observed was eruptive LP in 24.14% of cases (Table 1, Figure 1A,B).
TABLE 1.
Clinical characteristics of mucocutaneous lichenoid eruptions following COVID‐19 vaccination
| Characteristics | Participants |
|---|---|
| Total (n) | 58 |
| Age (years) | |
| Mean age | 42.72 |
| Age range | 18–71 |
| Gender (n, %) | |
| Female | 37 (63.8%) |
| Male | 21 (36.2%) |
| COVID‐19 vaccine administered | |
|
42 (72.41%) |
|
16 (27.59%) |
| Latency period (days) | |
| Mean duration | 20.8 |
| Duration range | 10–42 |
| Cutaneous distribution | |
|
04 (5.97%) |
|
26 (38.80%) |
|
15 (22.38%) |
|
03 (4.47%) |
|
05 (7.46%) |
|
03 (4.47%) |
|
09 (13.43%) |
|
02 (2.98%) |
| Clinical morphology | |
| Cutaneous lichen planus (LP) | |
| Eruptive LP | 14 (24.14%) |
| Linear LP | 05 (8.62%) |
| Annular LP | 03 (5.17%) |
| Hypertrophic LP | 04 (6.89%) |
| Ulcerative LP | 03 (5.17%) |
| Bullous LP | 01 (1.72%) |
| Lichen planus pigmentosus | 06 (10.34%) |
| Oral lichen planus | |
| Reticular | 04 (6.89%) |
| Erosive | 03 (5.17%) |
| Plaque type | 01 (1.72%) |
| Atrophic | 01 (1.72%) |
| Vulvovaginal LP | 02 (3.44%) |
| Nail lichen planus | 06 (10.34%) |
| Lichenoid drug eruptions | 05 (8.62%) |
| Clinical outcomes (n, %) | |
|
39 (67.24%) |
|
12 (20.69%) |
|
00 (00.00%) |
|
00 (00.00%) |
|
07 (12.10%) |
FIGURE 1.
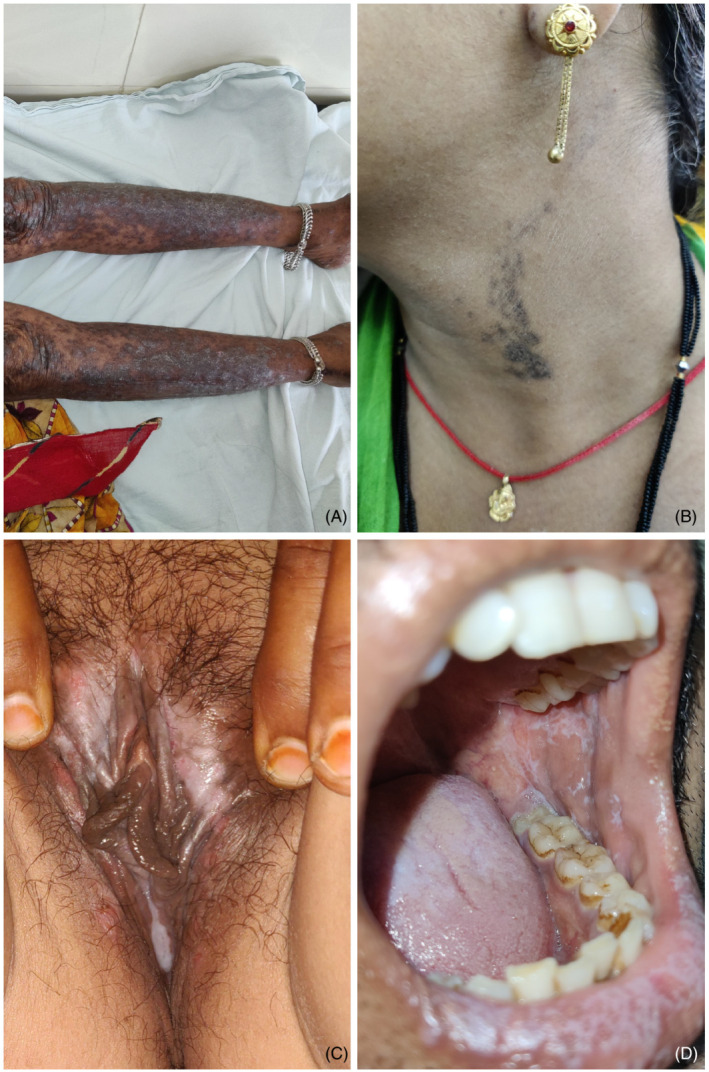
(A) Eruptive lichen planus 20 days following vaccination; (B) linear LP 10 days after vaccination; (C) vulvovaginal LP 1 month following vaccination; (D) reticular oral LP 14 days following vaccination
In oral LP, the most common subtype observed was reticular form followed by erosive form. One patient developed erosive lesions on gingival mucosa along with a reticulated white‐gray lacy pattern surrounding eroded epithelium on vulva, referred to vulvovaginal‐gingival syndrome (Figure 1C,D). Other unique presentation included LP‐lupus erythematosus overlap syndrome in one patient.
Among the six cases of nail LP, one case of nail LP triggered by COVID‐19 vaccine showed rapid progression resulting in pterygium formation, onycholysis, and nail loss.
There were five cases of LDEs that presented with psoriasiform lichenoid lesions without wickham's striae with generalized symmetrical or photo distribution and sparing the classical sites of LP.
Histologic analysis was available for 49 cases out of 58 patients. Features of lichenoid interface dermatitis were present in 73.47% cases. Histopathologic features typical for LP and its variants like circumscribed, wedge‐shaped hypergranulosis, hyperkeratosis, and irregular acanthosis of rete ridges, Civatte bodies, etc., were present in 63.26% cases. All the five cases of LDEs showed focal parakeratosis, localized degeneration of granular layer and dermal infiltrate consisting of eosinophils, neutrophils, and plasma cells on histopathology.
Another significant finding was that most of the cutaneous eruptions were mild to moderate and were treated adequately with topical medications only. However, most of the cases of oral and vulvovaginal LP required oral steroid therapy.
There is scarcity of literature on lichenoid eruptions as a cutaneous manifestation of COVID‐19 vaccination. 3 Only few cases of flare up of a pre‐existing LP has been reported following covid vaccine. 4
The COVID‐19 vaccination induces a Th1 response which subsequently increases the secretion of IL‐2, TNFa, and IFNc. These pro‐apoptotic cytokines are responsible for basal keratinocyte apoptosis and play a key role in the development of lichenoid lesions. 2 , 5 However, the exact pathogenesis is yet to be discovered.
AUTHOR CONTRIBUTIONS
A.A. and A.M. performed the research. A.A., A.M., B.C.G, and P.S. designed the research study. A.A., A.M., B.C.G, V.K.J., and R.D.M contributed essential reagents or tools. A.A. and A.M. analyzed the data. A.A. wrote the paper.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Farinazzo E, Ponis G, Zelin E, et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol. 2021;35:e548‐e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lai YC, Yew YW. Lichen planus and lichenoid drug eruption after vaccination. Cutis. 2017;100(6):E6‐E20. [PubMed] [Google Scholar]
- 3. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85:46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hiltun I, Sarriugarte J, Martínez‐de‐Espronceda I, et al. Lichen planus arising after COVID‐19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414‐e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Onn PY, Chang CL. Lichenoid cutaneous skin eruption and associated systemic inflammatory response following Pfizer‐BioNTech mRNA COVID‐19 vaccine administration. Respirol Case Rep. 2021;9(11):e0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


